Abstract
Calcium signaling is used by neurons to control a variety of functions, including cellular differentiation, synaptic maturation, neurotransmitter release, intracellular signaling and cell death. This review focuses on one of the most important Ca2+ regulators in the cell, the plasma membrane Ca2+-ATPase (PMCA), which has a high affinity for Ca2+ and is widely expressed in brain. The ontogeny of PMCA isoforms, linked to specific requirements of Ca2+ during development of different brain areas, is addressed, as well as their function in the adult tissue. This is based on the high diversity of variants in the PMCA family in brain, which show particular kinetic differences possibly related to specific localizations and functions of the cell. Conversely, alterations in the activity of PMCAs could lead to changes in Ca2+ homeostasis and, consequently, to neural dysfunction. The involvement of PMCA isoforms in certain neuropathologies and in brain ageing is also discussed.
Keywords: Calcium, Brain, ATPase, Plasma membrane, Differentiation, Neurodegeneration
INTRODUCTION
Molecular systems in the plasma membrane and intracellular organelles contribute to the initiation of Ca2+ signals but also act as buffers for intracellular Ca2+ regulation. Among all systems involved, cells use Ca2+-ATPases as high affinity active transporters to pump Ca2+ ions through the plasma membrane (PMCA) or organelle membranes of sarco-endoplasmic reticulum (SERCA) and secretory pathway (SPCA). The resulting transmembrane Ca2+ gradients are used in a variety of signaling processes mediated by gated ion channels. PMCA shows a very high affinity for Ca2+ (kDa around 100 nmol/L)[1] and is directly involved in pumping Ca2+ out of the cell. PMCA hydrolyzes one mol of ATP in order to get the energy to transport one mol of Ca2+ from the cytoplasm to the extracellular media across the plasma membrane, with a Ca2+/H+ countertransport molar ratio of 1/1 and 1/0.6 for erythrocytes and brain PMCA, respectively[2,3]. This protein is about 130 kDa, contains 10 transmembrane domains and a characteristic carboxyl terminal tail responsible of the high regulation of PMCA activity, e.g. stimulation by calmodulin, phospholipids and kinases, controlled proteolysis (reviewed in[4]) and regulation by ethanol[5]. The high affinity of PMCA for calmodulin has been widely used to purify PMCA from brain[6] and cerebellum[7] and from other non-neural tissues[8], and have allowed an extensive characterization of PMCA proteins.
PMCAs belong to the P2B branch of the P-type ATPase superfamily, characterized by the formation of a phosphorylated intermediate during the reaction cycle[9]. Four different genes have been described (ATP2B1-4) that encode four PMCA isoforms (PMCA1, 2, 3 and 4). In addition, the primary gene transcripts can be alternatively spliced in two major regions (site A, close to a phospholipid-sensitive region in the first intracellular loop, and site C, in the regulatory region in the C-terminal tail) to give over 20 variants (reviewed in[10,11]). The isoforms are widely distributed in most eukaryotic cells, although the physiological role of such abundance of PMCAs is still far from clear.
Brain is the region with the highest quantity and diversity of PMCA isoforms, which must be related to the involvement of Ca2+ in many neuronal functions. Rest Ca2+ levels in neuronal cells increase from nmol/L to μmol/L range to activate a spectrum of cellular events, from synaptic transmission to neuronal plasticity, intracellular signaling, neurosecretion or gene expression[12]. Afterward, intracellular Ca2+ must be restored quickly to resting levels to avoid cell damage and PMCAs are one of the major transporters involved in Ca2+ clearance. This review will focus on the distribution and function of PMCAs in Ca2+ homeostasis and signaling in the central nervous system, from neural development to mature brain, as well as their involvement in neuropathologies and ageing.
PMCAs AND NEURAL DEVELOPMENT
Neuronal development is characterized by a sequence of events such as cell differentiation, migration, neurite outgrowth and synaptogenesis. Ca2+ signaling plays a central role in the organization and regulation of these events[13,14]. In these processes, PMCAs are considered major players for Ca2+ homeostasis. Independently of a prenatal or postnatal brain development, an upregulation of PMCA activity has been observed from the first developing stages on[15,16]. Moreover, the major increase of PMCA activity mostly occurs during the period of greatest synaptic development, when an increase in the content of several PMCAs activators, such as calmodulin, phosphatidylserine and protein kinase C, also occurs[17-19].
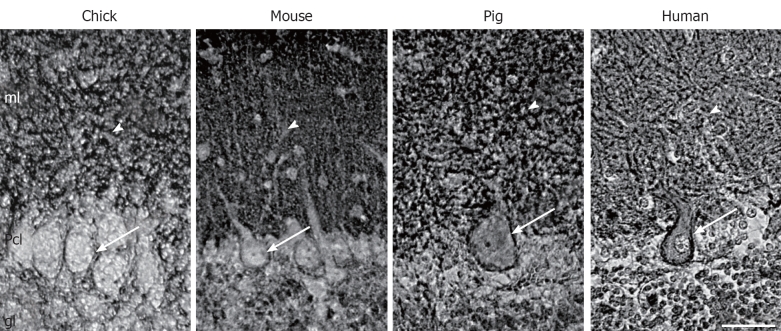
Although all PMCA isoforms are present in the adult brain, not all isoforms are expressed at the same time. In fact, maturation of brain is accompanied by changes of the expression and distribution of PMCA isoforms. This has been shown at mRNA[20,21], and protein levels[16,22,23]. The PMCA1b is early expressed, although it slowly decreases as PMCA1a expression increases with the development associated with synaptic maturation. The PMCA2 appears much later, and increases particularly in cerebellum. PMCA3 differs among species, being almost constitutively expressed during chick cerebellar development[22] and generally upregulated in rodents[16,20]. By contrast, there is a low expression of PMCA4 throughout development, and a high expression in adult nervous tissue[5,24-26]. The analysis of tissue sections confirms that all isoforms are associated with developing synaptic-enriched areas[7,15,22,27]. This suggests an involvement in synaptogenesis and maturation of neuronal electrophysiological properties, where the regulation of the magnitude and duration of Ca2+ spikes are critically required. In spite of this isoform coexpression in brain synaptic areas, cerebellum is the region with a more specific isoform-distribution pattern, different among species. In developing chick, PMCAs 1 and 3 has a restricted distribution in the soma and dendritic trunk of Purkinje cells, evolving according with cell maturation[22], while in mice these isoforms are more concentrated in the neuropil of the molecular layer from the first postnatal week[16]. However, PMCA2 is broadly and largely detected at the plasma membrane of the body cell and the whole dendritic arborization of Purkinje cells[16,22] (Figure 1). Therefore, these cells contain the highest diversity of PMCA isoforms since their morphogenesis. This fact points out at specific compartmental Ca2+ regulation, since Purkinje cells are also enriched in other Ca2+-binding proteins such as calbindin and parvalbumin[28] and also in other Ca2+ pumps, such as the endoplasmic reticulum localized-SERCA2[7,15,29] and the Golgi localized-SPCA1[30,31]. Other neural cell types seem to contain a specific combination of isoforms, e.g. chick cerebellar interneurons contain PMCAs 1 and 3, and granule cells only express PMCA2 at the latest developmental stages[22]. On the other hand, both in situ hybridization and immunocytochemical studies had failed to localize any of PMCA isoforms in glial cells of rat brain sections[32,33]. However, astrocytes in primary cultures showed expression of PMCA1 and PMCA4 at comparable levels to those seen in neurons, while PMCA2 was less abundant and PMCA3 was not found[34]. This discrepancy between tissue and cultured glia is still not solved.
Figure 1.
Localization of plasma membrane Ca2+-ATPase 2 in Purkinje cells from different species. Immunohistochemistry staining using an anti-plasma membrane Ca2+-ATPase 2 (PMCA2) antibody in para-sagittal sections of mature cerebella from chick, mouse, pig and human. PMCA2 is located at the plasma membrane of the soma (arrows) and in the dendritic arborization (arrowheads) of Purkinje cells. ml: Molecular layer; Pcl: Purkinje cell layer; gl: Granule layer. Scale bar: 40 μm.
The active participation of PMCAs in neural development has been also evidenced in differentiated PC12 cells containing a transient suppression of PMCA2 and 3, which in a slowing of neurite extension and survival reduction[35]. In contrast, similar suppression in stable-transfected undifferentiated PC12 induced a neuritogenesis-like process and an increase of PMCA4 when PMCA2 was blocked[36]. This suggests that a compensatory function among isoforms is possible under certain conditions.
PMCAs IN NEURAL FUNCTION
The high diversity of PMCAs in the brain and their overlapping localization in some brain areas does not imply functional redundancy, since isoforms show specific kinetic and regulatory differences. In fact, it has been reported a different rate of activation by Ca2+ of PMCA isoforms, PMCA3f and PMCA2a being the fastest ones, while PMCA4b is the slowest; on the other hand, alternative splicing at site C directly affects the calmodulin affinity, PMCA2b having the highest affinities (kDa around 2 nmol/L), followed by PMCA2a and 4b (5-10 nmol/L), and PMCA4a (50 nmol/L)[37]. Thus, diverse functional cell types or even distinct subcellular areas in the cell may use different PMCAs. Recent studies suggest that PMCA isoforms can be integrated in multiprotein complexes in membrane subdomains e.g. cholesterol-rich lipid rafts[38], which are emerging as important domains in cell Ca2+ signaling. In fact, we found that PMCA4, but not the other isoforms, is associated with lipid rafts isolated from synaptosomal plasma membranes[39]. On the other hand, lipid rafts prepared from the entire plasma membrane of primary cultured neurons contain all four PMCA isoforms and its activator calmodulin[40]. The discrepancy between both studies may be attributed to differences in the methodology and/or the existence of differences in the composition of rafts isolated from specific subcellular areas. The presence of PMCAs in rafts supports an active role of certain isoforms in cell signaling by facilitating their interaction with scaffolding proteins, such as some proteins from the membrane-associated guanylate kinase family (e.g. calcium/calmodulin-dependent serine protein kinase)[41], located at brain synapses, that interacts with PMCA4b or the Homer family [e.g. Ania-3[42], that couples N-methyl-D-aspartic acid (NMDA) and metabotropic glutamate receptors and could recruit PMCAs to domains close to Ca2+ influx channels]. Recently, it has been also reported an association of rat synaptosomal PMCA with tubulin, the main constituent of microtubules. This interaction resulted in the inhibition of PMCA, which can be reversed by calmodulin or ethanol[43]. Overall, the association of PMCA with different neural proteins may play a key control in neuronal Ca2+ signaling and regulation of synaptic activity.
Synapses can differ markedly in their efficacy and dynamics, in part due to differences in Ca2+ regulation. In this respect, the isoform PMCA2 has been particularly characterized since there is a restricted localization of variant PMCA2a in inhibitory presynaptic terminals throughout the adult rat brain[44], although a postsynaptic expression has also been observed in dendritic spines of cerebellar Purkinje cells[45]. Besides, PMCA2a shows a fast Ca2+ activation kinetics and high Vmax, being particularly suitable for the rapid clearance of Ca2+. This suggests that the isoform may play an important role in the control of local Ca2+ dynamics: this may be achieved by interacting - via its PDZ domain - with the post-synaptic PSD95 proteins and the NMDA glutamate receptor at the post-synapse, and with the soluble N-ethylmoleimide-sensitive fusion protein attachment protein receptors member syntaxin-1A at the pre-synapse[46]. The PMCA localization at the presynaptic terminal, clustered in the active zones[1], and its high Ca2+ affinity would allow to keep low Ca2+ levels, thereby controlling vesicular release following neural activity.
The function of PMCAs in brain is not only associated with neural activity. The pumps are also found in the choroid plexus, an epithelial tissue involved in the production and secretion of cerebrospinal fluid. All isoforms are expressed in developing chick[22] and mouse[21], although the PMCA3 seems to be most abundant in adult tissue[27,32,33]. This isoform could play a role in the transport required for tight regulation of Ca2+ in the cerebrospinal fluid[47].
PMCAs, NEURAL DYSFUNCTIONS AND BRAIN AGEING
The experimental use of animal models has already allowed a great progress correlating certain pathologies to the three types of Ca2+ pumps. Besides, several mutations of SERCA and SPCA result directly in human disorders such as Darier and Brody diseases for SERCA[48,49] and Hailey-Hailey diseases for SPCA[50,51]. However, only one human disease with genetic origin has been linked to PMCA defects, i.e. the PMCA2-attibuted hereditary deafness[52,53]. This has been found after observation of this dysfunction in a PMCA2 knockout mouse[54] and a defective mutant mouse named deafwaddler[55]. These mice presented for first time hearing problems and balance defects related to both abnormalities in Purkinje neurons[56] and the absence of calcium carbonate crystals in the otoconia of inner ear[54], another region largely enriched in PMCA2[57]. Interestingly, PMCA2 deficiency could not be rescued by the other isoforms.
Age-dependent alterations in intracellular Ca2+ are the leading causes of neurodegeneration and cell death. Consequently, Ca2+ regulating systems could be affected by age. In fact, the activity of synaptic PMCAs decreases during aging[58,59], with a parallel reduction of calmodulin[58]. These observations support the idea that PMCA function is linked to the levels of their modulators during development (as mentioned above) as well as during ageing. Changes in PMCA function may induce increased levels of intraneuronal Ca2+ and/or longer time periods of Ca2+ elevation following stimulation, which can enhance vulnerability of aged neurons to degeneration in situations of brain stress, such as hypoxia or ischemia. In fact, a decrease of PMCA protein level has also been observed in several brain regions of gerbils subjected to forebrain ischemia followed by prolonged reperfusion[60].
We have recently reported a putative role of PMCAs in human neurodegeneration caused in Alzheimer’s disease[24], a pathology directly related to ageing and hallmarked by accumulation of plaques of amyloid beta peptide and aberrant filaments of tau, presenilin mutations, and impairment of intracellular Ca2+ signaling in neurons[61-64]. By performing kinetic assays, we found a specific alteration of PMCA activity with respect to Ca2+-dependence in membranes of human brains affected by Alzheimer’s disease. This alteration may be caused by the enrichment of toxic amyloid beta in Alzheimer’s disease brain, because similar experiments, performed in the pig animal model, show an inhibition of synaptosomal PMCA activity by the peptide, at specific Ca2+ concentrations. Furthermore, the amyloid beta-mediated neurotoxicity is PMCA-isoform dependent and the toxic effect can be prevented by cholesterol[24]. Although the mechanism of amyloid-beta toxicity is still far from clear, the generation of reactive oxygen species (ROS) is also believed to occur. In this respect, oxidative stress is implicated in neuronal injury by modification of intracellular Ca2+ levels, and then Ca2+-regulatory proteins could act as targets for neurotoxic ROS. In vitro studies have shown that exposure of hippocampal neurons to hydrogen peroxide results in downregulation of PMCA protein[65]. Moreover, the activity of PMCA from synaptosomes is inhibited after exposure to ascorbate/iron-induced stress[66], by peroxyl radicals, hydrogen peroxide, and peroxynitrite[67]. Similar reduction of PMCA activity is shown in membranes from aged rats[67], where oxidative modification of PMCA can be a result of age-related chronic oxidative stress.
A relationship between other neurodegenerative disorders and PMCAs has been also reported, e.g. a downregulation of PMCA2 has been described in a screen for transcriptional dysfunctions in brains affected by Huntington’s disease in both mouse models and humans[68]. Thus, differences in PMCA2 expression may induce neuronal dysfunction by alteration of the homeostasis of Ca2+, which is widely considered to be an important factor in the pathogenesis of a number of neurodegenerative diseases.
Concerning the involvement of altered PMCAs in neural diseases, functional approaches have showed that the synaptosomal PMCA can interact with several drugs. This is the case of phenothiazine derivatives, such as thioridazine, chlorpromazine, and fluphenazine, which have been used in the treatment of schizophrenia and psychosis. These drugs interfere with the functioning of the synaptosomal PMCA, resulting a potent inhibitor of the ATPase activity while, under some conditions (i.e. in the presence of phosphate), it increases the rate of Ca2+ accumulation into synaptic plasma membrane vesicles[69]. This effect has been explained by the presence of an additional step in the catalytic cycle known as slippage pathway, which involves the release of bound Ca2+ from the phosphorylated intermediate to the cytoplasmic side of the membrane before its transport out of the cell. This pathway had been already observed in SERCA using chlorpromazine[70] or curcumine[71]. The slippage results in ATP hydrolysis uncoupled from Ca2+ transport, which favors heat production in mammals in physiological conditions, but appears to be a significant mechanism of organ dysfunction in disease conditions, such as ageing or ischemia[72].
The ethanol is another drug that significantly affects the central nervous system and produces stimulation or inhibition of synaptosomal PMCA activity in a dose-dependent manner. The stimulatory effect seems to involve its direct binding to a second autoinhibitory domain next to the calmodulin binding domain[5]. The sensitivity of synaptosomal PMCA (which is enriched in PMCA4) to ethanol is similar to that found for the erythrocyte PMCA4[73]. Accordingly, ethanol ingestion may overstimulate the PMCA activity, severely affecting the synaptic transmission.
Interaction networks emerging from these observations point out the role of PMCAs as potential targets of an increasing number of drugs acting upon the central nervous system.
CONCLUSION
There are many pieces of evidence suggesting that PMCAs play a key role in the nervous system. We begin to understand their role in neural Ca2+ homeostasis from neural development to mature brain, and also their involvement in neurological disorders. A more comprehensive understanding of the physiological function of the large diversity of PMCA isoforms in different areas of the cell, and in particular cell types, will be crucial for the future design of effective therapies for neurological diseases.
Footnotes
Supported by Grants No. BFU2008-00182 from MICINN, Fundaci¨®n Marcelino Botín, Spain (to Mata AM); Sepulveda MR received a Postdoctoral Fellowship from Programa de Reincorporaci¨®n de Doctores, Junta de Extremadura, Spain
Peer reviewer: Dr. Rafael Moreno-Sánchez, Department of Biochemistry, Instituto Nacional de Cardiologia, Juan Badiano No. 1, Seccion XVI, Mexico City 14080, Mexico
S- Editor Cheng JX L- Editor Negro F E- Editor Yang C
References
- 1.Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 2.Hao L, Rigaud JL, Inesi G. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J Biol Chem. 1994;269:14268–14275. [PubMed] [Google Scholar]
- 3.Salvador JM, Inesi G, Rigaud JL, Mata AM. Ca2+ transport by reconstituted synaptosomal ATPase is associated with H+ countertransport and net charge displacement. J Biol Chem. 1998;273:18230–18234. doi: 10.1074/jbc.273.29.18230. [DOI] [PubMed] [Google Scholar]
- 4.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 5.Sepúlveda MR, Mata AM. The interaction of ethanol with reconstituted synaptosomal plasma membrane Ca2+ -ATPase. Biochim Biophys Acta. 2004;1665:75–80. doi: 10.1016/j.bbamem.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Salvador JM, Mata AM. Purification of the synaptosomal plasma membrane (Ca(2+) + Mg(2+))-ATPase from pig brain. Biochem J. 1996;315(Pt 1):183–187. doi: 10.1042/bj3150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepúlveda MR, Hidalgo-Sánchez M, Mata AM. Localization of endoplasmic reticulum and plasma membrane Ca2+-ATPases in subcellular fractions and sections of pig cerebellum. Eur J Neurosci. 2004;19:542–551. doi: 10.1111/j.0953-816x.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- 8.Niggli V, Adunyah ES, Penniston JT, Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981;256:395–401. [PubMed] [Google Scholar]
- 9.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim Biophys Acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 10.Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann N Y Acad Sci. 2007;1099:226–236. doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 13.Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog Brain Res. 2009;175:443–452. doi: 10.1016/S0079-6123(09)17529-5. [DOI] [PubMed] [Google Scholar]
- 15.Sepúlveda MR, Hidalgo-Sánchez M, Mata AM. A developmental profile of the levels of calcium pumps in chick cerebellum. J Neurochem. 2005;95:673–683. doi: 10.1111/j.1471-4159.2005.03401.x. [DOI] [PubMed] [Google Scholar]
- 16.Marcos D, Sepulveda MR, Berrocal M, Mata AM. Ontogeny of ATP hydrolysis and isoform expression of the plasma membrane Ca(2+)-ATPase in mouse brain. BMC Neurosci. 2009;10:112. doi: 10.1186/1471-2202-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg M, Steinberg SF. Tissue-specific developmental regulation of protein kinase C isoforms. Biochem Pharmacol. 1996;51:1089–1093. doi: 10.1016/0006-2952(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 18.Manukian KH, Kirakosian LG. Proteolipids in developing rat brain. Neurochem Res. 1985;10:1533–1545. [PubMed] [Google Scholar]
- 19.Weinman J, Della Gaspera B, Dautigny A, Pham Dinh D, Wang J, Nojima H, Weinman S. Developmental regulation of calmodulin gene expression in rat brain and skeletal muscle. Cell Regul. 1991;2:819–826. doi: 10.1091/mbc.2.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt P, Neve RL. Expression of plasma membrane calcium-pumping ATPase mRNAs in developing rat brain and adult brain subregions: evidence for stage-specific expression. J Neurochem. 1992;59:1566–1569. doi: 10.1111/j.1471-4159.1992.tb08476.x. [DOI] [PubMed] [Google Scholar]
- 21.Zacharias DA, Kappen C. Developmental expression of the four plasma membrane calcium ATPase (Pmca) genes in the mouse. Biochim Biophys Acta. 1999;1428:397–405. doi: 10.1016/s0304-4165(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 22.Sepúlveda MR, Hidalgo-Sánchez M, Marcos D, Mata AM. Developmental distribution of plasma membrane Ca2+-ATPase isoforms in chick cerebellum. Dev Dyn. 2007;236:1227–1236. doi: 10.1002/dvdy.21131. [DOI] [PubMed] [Google Scholar]
- 23.Kip SN, Gray NW, Burette A, Canbay A, Weinberg RJ, Strehler EE. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus. 2006;16:20–34. doi: 10.1002/hipo.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrocal M, Marcos D, Sepúlveda MR, Pérez M, Avila J, Mata AM. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer's disease. FASEB J. 2009;23:1826–1834. doi: 10.1096/fj.08-121459. [DOI] [PubMed] [Google Scholar]
- 25.Zacharias DA, DeMarco SJ, Strehler EE. mRNA expression of the four isoforms of the human plasma membrane Ca(2+)-ATPase in the human hippocampus. Brain Res Mol Brain Res. 1997;45:173–176. doi: 10.1016/s0169-328x(97)00009-0. [DOI] [PubMed] [Google Scholar]
- 26.Stahl WL, Keeton TP, Eakin TJ. The plasma membrane Ca(2+)-ATPase mRNA isoform PMCA 4 is expressed at high levels in neurons of rat piriform cortex and neocortex. Neurosci Lett. 1994;178:267–270. doi: 10.1016/0304-3940(94)90775-7. [DOI] [PubMed] [Google Scholar]
- 27.Burette A, Rockwood JM, Strehler EE, Weinberg RJ. Isoform-specific distribution of the plasma membrane Ca2+ ATPase in the rat brain. J Comp Neurol. 2003;467:464–476. doi: 10.1002/cne.10933. [DOI] [PubMed] [Google Scholar]
- 28.Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. doi: 10.1080/14734220310022289. [DOI] [PubMed] [Google Scholar]
- 29.Plessers L, Eggermont JA, Wuytack F, Casteels R. A study of the organellar Ca2(+)-transport ATPase isozymes in pig cerebellar Purkinje neurons. J Neurosci. 1991;11:650–656. doi: 10.1523/JNEUROSCI.11-03-00650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepúlveda MR, Berrocal M, Marcos D, Wuytack F, Mata AM. Functional and immunocytochemical evidence for the expression and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in cerebellum relative to other Ca2+ pumps. J Neurochem. 2007;103:1009–1018. doi: 10.1111/j.1471-4159.2007.04794.x. [DOI] [PubMed] [Google Scholar]
- 31.Sepúlveda MR, Marcos D, Berrocal M, Raeymaekers L, Mata AM, Wuytack F. Activity and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol Cell Neurosci. 2008;38:461–473. doi: 10.1016/j.mcn.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Stahl WL, Eakin TJ, Owens JW Jr, Breininger JF, Filuk PE, Anderson WR. Plasma membrane Ca(2+)-ATPase isoforms: distribution of mRNAs in rat brain by in situ hybridization. Brain Res Mol Brain Res. 1992;16:223–231. doi: 10.1016/0169-328x(92)90229-5. [DOI] [PubMed] [Google Scholar]
- 33.Stauffer TP, Guerini D, Celio MR, Carafoli E. Immunolocalization of the plasma membrane Ca2+ pump isoforms in the rat brain. Brain Res. 1997;748:21–29. doi: 10.1016/s0006-8993(96)01282-6. [DOI] [PubMed] [Google Scholar]
- 34.Fresu L, Dehpour A, Genazzani AA, Carafoli E, Guerini D. Plasma membrane calcium ATPase isoforms in astrocytes. Glia. 1999;28:150–155. [PubMed] [Google Scholar]
- 35.Szemraj J, Kawecka I, Bartkowiak J, Zylińska L. The effect of antisense oligonucleotide treatment of plasma membrane Ca(+2)-ATPase in PC12 cells. Cell Mol Biol Lett. 2004;9:451–464. [PubMed] [Google Scholar]
- 36.Zylinska L, Kozaczuk A, Szemraj J, Kargas C, Kowalska I. Functional importance of PMCA isoforms in growth and development of PC12 cells. Ann N Y Acad Sci. 2007;1099:254–269. doi: 10.1196/annals.1387.008. [DOI] [PubMed] [Google Scholar]
- 37.Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 38.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 39.Sepúlveda MR, Berrocal-Carrillo M, Gasset M, Mata AM. The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes. J Biol Chem. 2006;281:447–453. doi: 10.1074/jbc.M506950200. [DOI] [PubMed] [Google Scholar]
- 40.Jiang L, Fernandes D, Mehta N, Bean JL, Michaelis ML, Zaidi A. Partitioning of the plasma membrane Ca2+-ATPase into lipid rafts in primary neurons: effects of cholesterol depletion. J Neurochem. 2007;102:378–388. doi: 10.1111/j.1471-4159.2007.04480.x. [DOI] [PubMed] [Google Scholar]
- 41.Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem. 2003;278:9778–9783. doi: 10.1074/jbc.M212507200. [DOI] [PubMed] [Google Scholar]
- 42.Sgambato-Faure V, Xiong Y, Berke JD, Hyman SE, Strehler EE. The Homer-1 protein Ania-3 interacts with the plasma membrane calcium pump. Biochem Biophys Res Commun. 2006;343:630–637. doi: 10.1016/j.bbrc.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monesterolo NE, Santander VS, Campetelli AN, Arce CA, Barra HS, Casale CH. Activation of PMCA by calmodulin or ethanol in plasma membrane vesicles from rat brain involves dissociation of the acetylated tubulin/PMCA complex. FEBS J. 2008;275:3567–3579. doi: 10.1111/j.1742-4658.2008.06502.x. [DOI] [PubMed] [Google Scholar]
- 44.Burette AC, Strehler EE, Weinberg RJ. "Fast" plasma membrane calcium pump PMCA2a concentrates in GABAergic terminals in the adult rat brain. J Comp Neurol. 2009;512:500–513. doi: 10.1002/cne.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burette A, Weinberg RJ. Perisynaptic organization of plasma membrane calcium pumps in cerebellar cortex. J Comp Neurol. 2007;500:1127–1135. doi: 10.1002/cne.21237. [DOI] [PubMed] [Google Scholar]
- 46.Garside ML, Turner PR, Austen B, Strehler EE, Beesley PW, Empson RM. Molecular interactions of the plasma membrane calcium ATPase 2 at pre- and post-synaptic sites in rat cerebellum. Neuroscience. 2009;162:383–395. doi: 10.1016/j.neuroscience.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy VA, Smith QR, Rapoport SI. Uptake and concentrations of calcium in rat choroid plexus during chronic hypo- and hypercalcemia. Brain Res. 1989;484:65–70. doi: 10.1016/0006-8993(89)90348-x. [DOI] [PubMed] [Google Scholar]
- 48.Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet. 1996;14:191–194. doi: 10.1038/ng1096-191. [DOI] [PubMed] [Google Scholar]
- 49.Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O'Donovan M, Craddock N, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- 50.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH Jr. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 51.Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, White J, Healy E, Dissanayake M, Larrègue M, Perrussel M, et al. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca(2+) pump. Hum Mol Genet. 2000;9:1131–1140. doi: 10.1093/hmg/9.7.1131. [DOI] [PubMed] [Google Scholar]
- 52.Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, et al. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA. 2007;104:1516–1521. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, et al. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- 54.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 55.Penheiter AR, Filoteo AG, Croy CL, Penniston JT. Characterization of the deafwaddler mutant of the rat plasma membrane calcium-ATPase 2. Hear Res. 2001;162:19–28. doi: 10.1016/s0378-5955(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 56.Kurnellas MP, Lee AK, Li H, Deng L, Ehrlich DJ, Elkabes S. Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons. Mol Cell Neurosci. 2007;34:178–188. doi: 10.1016/j.mcn.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21:5066–5078. doi: 10.1523/JNEUROSCI.21-14-05066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaidi A, Gao J, Squier TC, Michaelis ML. Age-related decrease in brain synaptic membrane Ca2+-ATPase in F344/BNF1 rats. Neurobiol Aging. 1998;19:487–495. doi: 10.1016/s0197-4580(98)00078-5. [DOI] [PubMed] [Google Scholar]
- 59.Michaelis ML, Bigelow DJ, Schöneich C, Williams TD, Ramonda L, Yin D, Hühmer AF, Yao Y, Gao J, Squier TC. Decreased plasma membrane calcium transport activity in aging brain. Life Sci. 1996;59:405–412. doi: 10.1016/0024-3205(96)00319-0. [DOI] [PubMed] [Google Scholar]
- 60.Lehotský J, Kaplán P, Racay P, Mézesová V, Raeymaekers L. Distribution of plasma membrane Ca2+ pump (PMCA) isoforms in the gerbil brain: effect of ischemia-reperfusion injury. Neurochem Int. 1999;35:221–227. doi: 10.1016/s0197-0186(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 61.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 62.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 63.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 64.Hernández F, Avila J. Tau aggregates and tau pathology. J Alzheimers Dis. 2008;14:449–452. doi: 10.3233/jad-2008-14414. [DOI] [PubMed] [Google Scholar]
- 65.Kip SN, Strehler EE. Rapid downregulation of NCX and PMCA in hippocampal neurons following H2O2 oxidative stress. Ann N Y Acad Sci. 2007;1099:436–439. doi: 10.1196/annals.1387.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira C, Ferreira C, Carvalho C, Oliveira C. Contribution of plasma membrane and endoplasmic reticulum Ca(2+)-ATPases to the synaptosomal [Ca2+]i increase during oxidative stress. Brain Res. 1996;713:269–277. doi: 10.1016/0006-8993(95)01554-x. [DOI] [PubMed] [Google Scholar]
- 67.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca(2+)-ATPase. Free Radic Biol Med. 1999;27:810–821. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 68.Kuhn A, Goldstein DR, Hodges A, Strand AD, Sengstag T, Kooperberg C, Becanovic K, Pouladi MA, Sathasivam K, Cha JH, et al. Mutant huntingtin's effects on striatal gene expression in mice recapitulate changes observed in human Huntington's disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum Mol Genet. 2007;16:1845–1861. doi: 10.1093/hmg/ddm133. [DOI] [PubMed] [Google Scholar]
- 69.Palacios J, Sepúlveda MR, Lee AG, Mata AM. Ca2+ transport by the synaptosomal plasma membrane Ca2+-ATPase and the effect of thioridazine. Biochemistry. 2004;43:2353–2358. doi: 10.1021/bi0359473. [DOI] [PubMed] [Google Scholar]
- 70.de Meis L. Fast efflux of Ca2+ mediated by the sarcoplasmic reticulum Ca2(+)-ATPase. J Biol Chem. 1991;266:5736–5742. [PubMed] [Google Scholar]
- 71.Logan-Smith MJ, Lockyer PJ, East JM, Lee AG. Curcumin, a molecule that inhibits the Ca2+-ATPase of sarcoplasmic reticulum but increases the rate of accumulation of Ca2+ J Biol Chem. 2001;276:46905–46911. doi: 10.1074/jbc.M108778200. [DOI] [PubMed] [Google Scholar]
- 72.Berman MC. Slippage and uncoupling in P-type cation pumps; implications for energy transduction mechanisms and regulation of metabolism. Biochim Biophys Acta. 2001;1513:95–121. doi: 10.1016/s0005-2736(01)00356-x. [DOI] [PubMed] [Google Scholar]
- 73.Cervino V, Benaim G, Carafoli E, Guerini D. The effect of ethanol on the plasma membrane calcium pump is isoform-specific. J Biol Chem. 1998;273:29811–29815. doi: 10.1074/jbc.273.45.29811. [DOI] [PubMed] [Google Scholar]