Abstract
Specialized microenvironments called niches keep stem cells in an undifferentiated and self-renewing state. Dedicated stromal cells form niches by producing a variety of factors that act directly on stem cells. The size and signaling output of niches must be finely tuned to ensure proper tissue homeostasis. Although advances have been made in identifying factors that promote niche cell fate, the mechanisms that restrict niche cell formation during development and limit niche signaling output in adults remain poorly understood. Here, we show that the histone lysine-specific demethylase 1 (Lsd1) regulates the size of the germline stem cell (GSC) niche in Drosophila ovaries. GSC maintenance depends on bone morphogenetic protein (BMP) signals produced by a small cluster of cap cells located at the anterior tip of the germarium. Lsd1 null mutant ovaries carry small germline tumors containing an expanded number of GSC-like cells with round fusomes that display ectopic BMP signal responsiveness away from the normal niche. Clonal analysis and cell type-specific rescue experiments demonstrate that Lsd1 functions within the escort cells (ECs) that reside immediately adjacent to cap cells and prevents them from ectopically producing niche-specific signals. Temporally restricted gene knockdown experiments suggest that Lsd1 functions both during development, to specify EC fate, and in adulthood, to prevent ECs from forming ectopic niches independent of changes in cell fate. Further analysis shows that Lsd1 functions to repress decapentaplegic (dpp) expression in adult germaria. The role of Lsd1 in regulating niche-specific signals may have important implications for understanding how disruption of its mammalian homolog contributes to cancer and metastasis.
Many adult tissues such as the skin, intestine, and hematopoietic system experience constant cell turnover. The homeostasis and function of these organs depend on the self-renewing capacity of stem cells. Adult stem cells are often maintained in specialized microenvironments called niches (1). The correct balance between stem cell self-renewal and stem cell daughter differentiation depends on the exquisite regulation of niche size and signaling output.
The germline stem cells (GSCs) of the Drosophila ovary have provided many insights into the functional relationships that exist between stem cells and their niches (2). Ovaries are composed of tube-like structures known as ovarioles. Two to three GSCs reside at the tip of each ovariole in a structure called the germarium (Fig. 1A). Within each germarium, five to seven somatic cap cells form the functional GSC niche. These cells produce Decapentaplegic (Dpp), a bone morphogenetic protein-like molecule, which initiates a signal transduction cascade within GSCs that serves to repress the transcription of the differentiation factor bag of marbles (bam) (3–5). Escort cells (ECs), also known as inner germarial sheath cells, lie adjacent to the cap cells and line the anterior region of germaria (6). These cells do not normally express niche signals and are thought to support the early differentiation of germline cysts (6). Ectopically expressing dpp throughout somatic cells blocks germline differentiation, resulting in the formation of GSC tumors (4). Therefore, limiting the number of cells that produce dpp appears essential for the normal functional output of the ovary.
Fig. 1.
Disruption of Lsd1 results in the formation of GSC-like tumors. (A) Illustration of a WT Drosophila germarium. The cap cells, which form the GSC niche, are located at the anterior tip of the germarium (dark blue). The fusome (yellow) changes from a predominantly round structure in GSCs to a highly branched structure in developing cysts. The ECs (red) line the anterior region of the germarium. (B and C) Germaria immunostained for Hts (green) and Vasa (red). (B) WT germaria contain two to three GSCs and five to seven cap cells. (C) Lsd1ΔN mutants display an expanded number of GSC-like cells with single round fusomes. WT (D) and Lsd1ΔN (E) homozygous germaria stained for phosphorylated histone H3 (red). Positive staining revealed that Lsd1 mutant GSC-like cells continue to divide. WT (F) and Lsd1ΔN (G) mutant germaria stained using an antibody against activated Caspase 3 (red). Cells undergoing cell death are rarely observed in WT germaria, but cell death occurs in Lsd1 mutant samples. In all panels, DNA is labeled with DAPI (blue). (Scale bars, 10 μm.)
Recent work has shown that ectopic expression of activated Notch within somatic cells results in a marked increase in the number of cap cells (7, 8). The increased number of cap cells subsequently leads to an expansion of the GSC population. Delta expressed by terminal filament cells of the developing gonad activates Notch in the adjacent somatic cells but not in the remaining somatic cells interspersed among the germ cells (7). Activation of Notch within adult ECs does not cause these cells to adopt a cap cell fate, whereas overexpression of dpp alone in adult ECs in the absence of expanded Notch signaling supports GSC maintenance (7). Thus, Notch controls cell fate decisions within the developing gonad.
Two additional pathways regulate dpp expression within adult ovaries. Disruption of the Janus kinase/Signal transducer and activator of transcription (Jak/Stat) pathway results in a GSC loss phenotype, whereas activation of the pathway within ECs leads to germline tumor formation marked by expanded Dpp responsiveness within germ cells (6, 9, 10). The epidermal growth factor (EGF) pathway also acts to regulate the signaling output of the niche. Stet, an EGF-processing molecule, acts in germline cysts to promote the production of EGF receptor (EGFR) ligands, including Spitz, Keren, and Gurken (11). These molecules activate the RAS-RAF-MEK-MAPK pathway within surrounding somatic cells, which, in turn, represses the transcription of dally, a factor that regulates Dpp transport and stability (12, 13). By repressing dally expression, the EGF pathway serves to restrict Dpp signaling to the most anterior region of the germarium (11). This pathway also plays a central role in a feedback loop that coordinates somatic cell survival and germ cell proliferation during development (14).
Alterations within local chromatin environments likely underlie the coordinated specification of cell fate programs within the developing gonad and may help to regulate the homeostatic function of ovarian cells in adulthood. Here, we show that absence of lysine-specific demethylase 1 [Lsd1/Su(var)3-3/CG17149] results in GSC tumor formation attributable to an expansion of niche signaling. Further results indicate that Lsd1 acts to control niche size both during development and in adulthood.
Results
We sought to identify chromatin-associated factors that regulate adult GSC behavior. The histone demethylase Lsd1 emerged as a likely candidate based on its role in various developmental processes. In humans, loss of Lsd1 has been linked to several high-risk cancers (15–17) and Lsd1 has recently been shown to regulate the transcription of TGF β1, a Dpp homolog, negatively (18). Previous work has shown that Drosophila Lsd1 mutants display male and female sterile phenotypes and defects in heterochromatin formation (19, 20). The earliest steps of germline cyst development appeared severely disrupted in Lsd1ΔN null allele homozygotes, resulting in the formation of small ovaries (19).
Lsd1 Mutants Display Small GSC Tumors.
To characterize the Lsd1 mutant ovarian phenotype further, we stained WT and Lsd1ΔN mutant ovaries with the germline marker Vasa and the fusome marker Hts (Fig. 1 B and C). The fusome is a specialized organelle that appears round in GSCs most of the time but becomes branched as GSC daughters move away from the niche and form multicellular cysts (21–23). In contrast to controls (average = 2, range: 1–3, n = 30 germaria), Lsd1 mutant ovaries contained an increased number of single undifferentiated GSC-like cells with round fusomes (average = 26, range: 6–79, n = 79 germaria). These single cells underwent cell division as indicated by phospho-histone H3 staining (Fig. 1 D and E). The average overall size of these Lsd1 mutant tumors did not increase over time, however, because of programmed cell death within the germline (Fig. 1 F and G).
Lsd1 Functions in a Nonautonomous Manner.
To begin to evaluate the function of Lsd1 in the germarium, we generated a polyclonal antibody to the N terminus of the Lsd1 protein. This antibody revealed ubiquitous Lsd1 expression throughout the germarium (Fig. 2A and Fig. S1). As expected for a histone demethylase, the protein predominantly localized to the nuclei of all cells examined. Interestingly, Lsd1 expression appeared highest in the GSCs but was also clearly present in the ECs and follicle cells of the germarium.
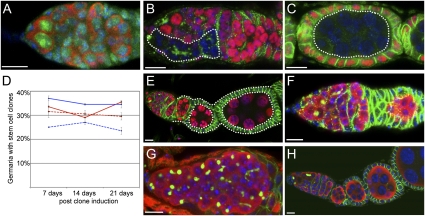
Fig. 2.
Lsd1 functions in a nonautonomous manner to regulate GSC numbers within the ovary. (A) WT germarium stained for Lsd1 (green), Vasa (red), and DNA (blue). (B and C) Negatively marked germline clones stained for GFP (red) and Hts (green). Lsd1ΔN homozygous germline clones (dotted lines) differentiate to form cysts with branched fusomes and morphologically normal egg chambers. (D) Graph shows the percentage of control and Lsd1 mutant stem cell clones maintained after clone induction. The solid red line refers to control germline clones, the dotted red line refers to Lsd1ΔN germline clones, the solid blue line refers to control follicle cell clones, and the dotted blue line refers to Lsd1ΔN follicle cell clones. (E) Lsd1ΔN homozygous follicle cells (dotted lines) do not exhibit a discernible phenotype. Germaria from UAS-Lsd1RNAi/+; hh-gal4/+ (F) and c587-Gal4/+; UAS-Lsd1RNAi/+ (G) females stained for Hts (green), Vasa (red), and DNA (blue). (H) Ovarian cells from c587-Gal4/+; UAS-Lsd1/+; Lsd1ΔN/Lsd1ΔN females stained for Hts (green), Vasa (red), and DNA (blue). (Scale bars, 10 μm.)
The GSC tumors within Lsd1 mutant ovaries could be caused by defects in the intrinsic programming of GSCs or by extrinsic defects in the surrounding somatic cells. We performed clonal analysis and cell-specific rescue experiments to distinguish between these possibilities. First, we induced negatively marked Lsd1 mutant clones in an otherwise heterozygous background in adults using FRT/FLP-mediated mitotic recombination (Fig. 2 B–E). Interestingly, we found that negatively marked Lsd1 mutant germline clones differentiated into morphologically normal egg chambers without any apparent block in differentiation, even over long periods of time (Fig. 2 B and C). Furthermore, Lsd1ΔN GSC clones were maintained at levels similar to control clones (Fig. 2D), demonstrating that Lsd1 was not required within the germline for GSC maintenance. Instead, these results suggest that Lsd1 acts within a different cell type (i.e., in a cell-nonautonomous manner) to control germline differentiation.
We then examined Lsd1 mutant follicle cell clones. These also appeared normal and were able to envelop germline cysts fully without any obvious defects (Fig. 2E). The absence of Lsd1 in the follicle cells did not result in an abnormal number of GSCs. Furthermore Lsd1ΔN follicle stem cell (FSC) clones were maintained over long periods of time (Fig. 2D). Together with the germline clone data, these experiments suggest that Lsd1 functions in either ECs or cap cells to limit the number of GSCs in the germarium. To distinguish between these two possibilities, we knocked down Lsd1 expression using RNAi in a cell-specific manner (24). Reducing Lsd1 levels in cap cells and terminal filament cells using Lsd1-RNAi in combination with hedgehog (hh)-gal4 (25, 26) (for expression, see Fig. 4C) did not disrupt the normal morphology of the germarium (Fig. 2F and Fig. S1). In contrast, Lsd1-RNAi driven by c587-Gal4, which expresses GAL4 in most somatic cells in the developing gonad but becomes largely restricted to ECs and early follicle cells in adults (27, 28) (for expression, see Fig. 4G), phenocopied Lsd1 mutants (Fig. 2G and Fig. S1), resulting in the formation of GSC-like tumors within all examined germaria. Furthermore, driving Lsd1 WT transgenes with c587-Gal4 rescued the Lsd1 null mutant phenotype so that the normal morphology of mutant germaria and ovarioles was fully restored in every female tested (Fig. 2H and Fig. S1). Given that reducing or eliminating Lsd1 function within follicle cells (Fig. 2 D and E) or cap cells (Fig. 2F) did not result in a phenotype, the knockdown and rescue experiments using c587-Gal4 strongly suggest that Lsd1 functions within ECs to limit the number of GSCs. Interestingly, the fully mature eggs produced by Lsd1 mutant females expressing rescuing transgenes remained sterile, suggesting that Lsd1 may also function outside of the ECs but in a manner unrelated to the expanded GSC phenotype. Perhaps Drosophila Lsd1 has an analogous function to its Caenorhabditis elegans homolog, which is required for germline maintenance over multiple generations (29). Regardless of this additional phenotype, the clonal loss-of-function and cell type-specific knockdown and rescue experiments presented here clearly demonstrate that Lsd1 is required in somatic cells (likely ECs) for nonautonomous control of the number of GSC-like cells within the germarium.
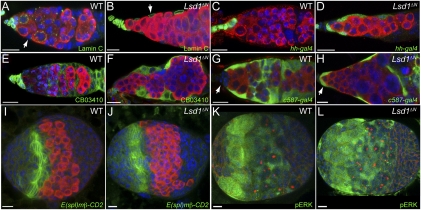
Fig. 4.
Loss of Lsd1 results in somatic cell fate changes. WT (A) and Lsd1ΔN homozygous (B) germaria stained for Lamin C (green). The arrows indicate ECs. UAS-GFP; hh-gal4 (C) and UAS-GFP; hh-gal4, Lsd1ΔN/Lsd1ΔN (D) germaria stained for the expression of GFP (green). CB03410 (E) and CB03410; Lsd1ΔN/Lsd1ΔN (F) germaria stained for the expression of GFP (green). c587-Gal4; UAS-GFP (G) and c587-Gal4; UAS-GFP; Lsd1ΔN/Lsd1ΔN (H) germaria stained for the expression of GFP (green) (arrows point to cap cells). Late larval WT (I and K) and Lsd1ΔN homozygous (J and L) female gonads stained for the E(spl)mβ-CD2 reporter (I and J) or pERK (K and L). Vasa labels the germline (red in A–J), and Hts stains the fusome (red in K and L). DNA is labeled with DAPI in all panels (blue). (Scale bars, 10 μm.)
Lsd1 Limits Dpp Signaling Within the Germarium.
We sought to understand further how Lsd1 regulated the differentiation of GSC daughters. GSCs express the translational repressor Nanos, a factor essential for GSC maintenance. On displacement away from the cap cell niche, GSC daughters express Bam, which represses the translation of nanos in differentiating cysts (30). Costaining control and Lsd1 mutant germaria for Nanos and Bam revealed that less than 1.5% of Lsd1 mutant germaria expressed detectable levels of Bam (Fig. 3B and Fig. S2). Instead, most germline cells continued to express Nanos, indicating that loss of Lsd1 prevents GSC daughters from differentiating into cystoblasts and multicellular cysts.
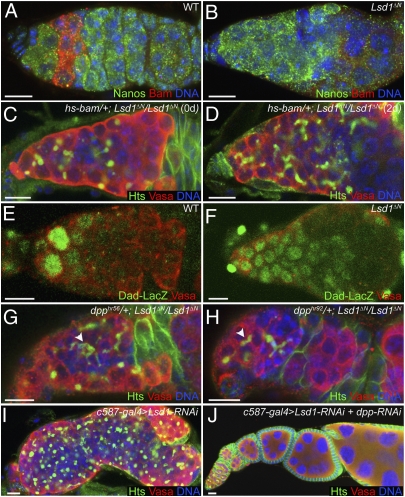
Fig. 3.
Loss of Lsd1 results in expanded Dpp signaling. WT (A) and Lsd1ΔN (B) homozygous mutant germaria immunostained for Nanos (green) and Bam (red). Only 1.5% of Lsd1ΔN mutant germaria (n = 244) express Bam. hs-bam/+; Lsd1ΔN/Lsd1ΔN germaria before heat shock (C) and 2 d after heat shock (D) stained for Hts (green) and Vasa (red). (E) High levels of Dad-LacZ (green) expression are normally restricted to GSCs in WT germaria. (F) Lsd1 mutants exhibit expanded Dad-LacZ expression in germline cells. dpphr56/+; Lsd1ΔN/Lsd1ΔN (G) and dpphr92/+; Lsd1ΔN/Lsd1ΔN (H) germaria stained for Hts (green) and Vasa (red). Both dpp mutant alleles dominantly suppress the Lsd1 phenotype, leading to the appearance of cysts with branched fusomes (arrowheads). Germaria from c587-Gal4/+; UAS-Lsd1-RNAi/+ (I) and c587-Gal4/+; UAS-Lsd1-RNAi/+; UAS-dpp-RNAi/+ (J) females dissected 1 d after eclosion stained for Hts (green) and Vasa (red). Reduction of dpp expression by RNAi dramatically suppressed the tumorous phenotype induced by Lsd1-RNAi. DNA is labeled with DAPI (blue in A–D and G–J). (Scale bars, 10 μm.)
To test whether Lsd1 mutant germline cells were capable of forming multicellular cysts, we expressed bam in Lsd1 mutant ovaries using an inducible transgene. Previous studies showed that bam expression is both necessary and sufficient for germ cell differentiation (31, 32). Expression of bam in Lsd1 mutants resulted in the formation of multicellular cysts that contained branched fusomes (Fig. 3D), indicating that Lsd1 mutant germline cells can undergo differentiation and form multicellular cysts. This result strongly suggests that the tumorous phenotype exhibited by Lsd1 mutants is caused by failure to initiate a proper differentiation program within GSC daughters.
Given the previous findings that Dpp signaling represses Bam expression in GSCs (3–5), we considered the possibility that ectopic Dpp pathway activity might account for the absence of Bam expression in Lsd1 mutants. To test this idea, we crossed a positive reporter of Dpp signaling, the Dad-LacZ enhancer trap, into the Lsd1ΔN mutant background. Normally, high levels of Dad-LacZ expression are limited to the two to three GSCs immediately adjacent to the cap cells (Fig. 3E). Although the overall levels of Dad-LacZ expression were not as high as in control GSCs, we found that the number of Dad-LacZ–positive cells was greatly expanded in Lsd1 mutant germaria (100%, n > 100 germaria) (Fig. 3F), suggesting that greater Dpp signaling accounts for the increased number of GSC-like cells in Lsd1 mutant ovaries. Consistent with this idea, two different dpp mutations partially suppressed the Lsd1 phenotype (Fig. 3 G and H), resulting in an increased number of germline cysts with branched fusomes and maturing egg chambers (Fig. S2). Furthermore, the expression of two different dpp-RNAi transgenes strongly suppressed the c587-Gal4 > Lsd1-RNAi-induced GSC tumor phenotype (Fig. 3 I and J and Fig. S2).
Lsd1 Functions During Development to Specify Somatic Cell Fate.
Loss of Lsd1 did not appear to result in changes in somatic cell numbers within developing gonads or adult germaria (Fig. S3). We considered the possibility that Lsd1 functioned in the somatic precursor cells of the developing gonad when ECs and cap cells are being specified. To test whether Lsd1 mutant somatic cells adopt inappropriate fates, we compared the expression of several cap cell-specific markers. Cap cells and terminal filament cells express high levels of Lamin C and hh (7, 27). CB03410, a previously identified protein trap line (33), is also expressed in adult cap cells and terminal filament cells. In Lsd1 mutants, the expression of all three markers expanded to most of the somatic cells within the germarium (Fig. 4 A–F), indicating that Lsd1 mutant ECs exhibit characteristics of cap cells. To determine whether Lsd1 mutant ECs completely switch their identity, we examined c587-Gal4 expression within WT and Lsd1 mutant adult ovaries (Fig. 4 G and H). Unlike WT adult germaria, which exhibited virtually exclusive expression of c587-Gal4 within ECs and early follicle cells, Lsd1 mutants also displayed c587-Gal4 expression in cap cells. Thus, Lsd1 mutant ECs and cap cells do not differentiate properly and display characteristics of both cell types in adult germaria.
Several signaling pathways have been implicated in the formation and regulation of the GSC niche (7–13). For example, Delta from newly formed terminal filament cells activates Notch signaling and induces cap cell identity within a small number of somatic cells in the developing gonad (7). The EGFR pathway has also been implicated in regulating the development of the ovary and niche output in adults (11, 14). We did not observe genetic interactions between Lsd1 and Notch (Fig. S3). Furthermore a transcriptional reporter of Notch activity, E(spl)mβ-CD2 (34, 35), exhibited a normal pattern of expression in Lsd1 pupal gonads, suggesting that loss of Lsd1 does not result in inappropriate derepression of Notch target genes within the developing gonad (Fig. 4 I and J). Likewise, we also found that activation of the EGFR-MAPK pathway does not change in Lsd1 mutant gonads or in adult ovaries, based on the expression of phosphorylated extracellular signal-regulated kinase (pERK) (Fig. 4 K and L and Fig. S3). Therefore, Lsd1 does not appear to interact with or influence the activity of these signaling pathways within developing gonads.
Lsd1 Functions During Development and in Adults to Limit Niche Size.
The role of Lsd1 in regulating EC fate does not preclude the possibility that this histone demethylase continues to restrict GSC number in adult ovaries independent of any developmental defects. To determine whether Lsd1 acts only during development, we took advantage of the temperature sensitivity of the gal4 system and performed a series of temperature shift experiments. In these experiments, Lsd1 expression was specifically knocked down in somatic cells using Lsd1 RNAi driven by c587-Gal4 (Fig. 5). Quantitation of phenotypes is shown in Fig. S4. First, females were raised at 29 °C during larval and pupal development and then either kept at 29 °C or shifted down to 18 °C for 7 d immediately after eclosion. Consistently, females raised and maintained at 29 °C exhibited a pronounced GSC tumor phenotype with no signs of proper egg chamber development (Fig. 5A and Fig. S4). Females shifted down to 18 °C for 7 d displayed signs of germline differentiation, however. Ovaries from these females often contained ovarioles with a number of developing germline cysts that were fully encapsulated by follicle cells (Fig. 5B and Fig. S4). These results indicate that restoration of Lsd1 function during adulthood can rescue underlying developmental defects that result from the absence of Lsd1 during larval and pupal development.
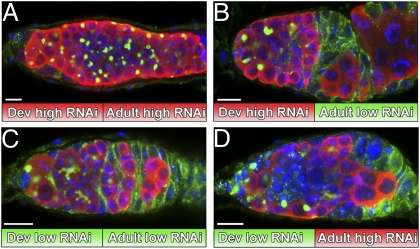
Fig. 5.
Lsd1 functions in adulthood to limit niche activity. (A–D) Germaria from c587-Gal4/+; UAS-Lsd1-RNAi/+ females raised at either 29 °C (high RNAi) or 18 °C (low RNAi) and shifted after eclosion stained for Hts (green), Vasa (red), and DNA (blue). (Scale bars, 10 μm.)
If Lsd1 functions to limit the size of the GSC niche in adults, knocking down Lsd1 expression in females specifically after they eclose would be predicted to result in a GSC expansion phenotype. To test this possibility, we raised c587-Gal4; UAS-Lsd1-RNAi females at 18 °C. Ovaries from these females appeared normal and did not exhibit an expanded number of GSCs, even after 7 d as adults (Fig. 5C). We observed a striking GSC tumor phenotype when we shifted these females to 29 °C for 7 d after eclosion, however (Fig. 5D). Lamin C staining showed that this phenotype was not accompanied by changes in EC identity (Fig. S4). These data demonstrate that Lsd1 functions to limit dpp signaling within germaria during adulthood.
Loss of Lsd1 Results in Increased dpp but Not dally Expression.
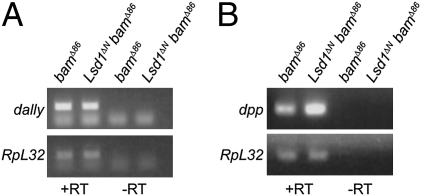
Although our analysis of pERK suggests that loss of Lsd1 does not lead to obvious changes in EGFR signaling (Fig. 4 and Fig. S3), this experiment does not rule out the possibility that Lsd1 influences the transcriptional output of the EGFR pathway. In adults, activation of the EGFR pathway limits Dpp signaling outside of the niche by repressing the transcription of dally (11), a glypican that facilitates Dpp transport and stability (12, 13). To determine whether Lsd1 specifically represses the expression of genes involved in promoting Dpp signaling, or perhaps dpp itself, we performed a number of RT-PCR–based experiments. To control for differences in the developmental state of the samples, we crossed the Lsd1ΔN mutation into a bamΔ86 mutant background. We observed no difference in dally mRNA levels between bamΔ86 and Lsd1ΔN bamΔ86 double-mutant germaria, further suggesting that Lsd1 functions independent of the EGFR pathway (Fig. 6A). In contrast, dpp mRNA levels were noticeably elevated in Lsd1 mutant adult germaria (Fig. 6B). We also found that ectopic expression of dpp driven by c587-Gal4 during development resulted in the expanded expression of the cap cell marker Lamin C (Fig. S5). These observations suggest that misregulation of dpp itself accounts for the phenotypes observed in Lsd1 mutants.
Fig. 6.
Lsd1 mutant germaria display elevated levels of dpp mRNA. Ethidium bromide-stained gel shows the products of a RT-PCR on RNA isolated from bamΔ86 and Lsd1ΔN bamΔ86 ovaries using dally- (A) or dpp- (B) specific primers. No difference in the levels of dally expression was observed, although dpp mRNA levels were clearly elevated in the absence of Lsd1. The presented gels are representative of three biological replicates.
Discussion
Previous work has focused on identifying signaling pathways that specify niche cell identity. Equally important in sculpting a fully functional stem cell microenvironment is preventing cells outside the normal niche from producing niche-specific signals in an inappropriate manner. The work presented here indicates that the conserved histone demethylase Lsd1 performs such a function in the somatic cells of the Drosophila ovary.
Lsd1 homologs regulate heterochromatin formation and gene expression across species (18–20, 29, 36–38). Our results indicate that Lsd1 acts nonautonomously to limit GSC numbers within the Drosophila ovary. These conclusions are based on several lines of experimental evidence. Clonal analyses demonstrate that Lsd1 does not function within the germline or in follicle cells in regard to the regulation of GSC number or GSC daughter differentiation. Furthermore Lsd1 mutant GSCs and FSCs are maintained for long periods of time, indicating that Lsd1 is not required within stem cells for their maintenance in the ovary. Strikingly, RNAi knockdown of Lsd1 within ECs phenocopies the expanded GSC phenotype of Lsd1 null mutants. In addition, independent Lsd1 transgenes rescue the Lsd1ΔN tumorous phenotype when expressed in the somatic cells that line the anterior region of the germarium. These data indicate that Lsd1 functions within the somatic cells of the germarium to limit the size of the functional GSC niche.
Previous results show that the Notch pathway helps to specify cap cells within pupal gonads. Delta expressed by terminal filament cells induces Notch activation within other adjacent somatic cells. Notch activation results in the specification of cap cell fate and the subsequent expression of dpp by these cells. The limited expression of Delta within the terminal filament cells of the developing gonad provides a simple mechanism for restricting the number of cap cells (7). We find that loss of Lsd1 results in the expanded expression of cap cell markers throughout the adult germarium, albeit at lower levels than typically observed in WT cap cells. This observation raises the possibility that additional factors help to repress these markers in ECs. We considered the possibility that Notch target genes become inappropriately derepressed far from the Delta-expressing terminal filament cells in the absence of Lsd1. A Notch transcriptional reporter displays a normal expression pattern in Lsd1 mutant gonads, however (Fig. 4). Furthermore, temporally restricted knockdown experiments suggest that Lsd1 limits niche signaling in adults independent of any role it has in the developing gonad (Fig. 5). Ectopic Notch activation within adult ECs does not result in expanded niche signaling (7). Based on these data, we conclude that Lsd1 functions independent of the Notch pathway in the Drosophila ovary.
Similarly, our data indicate that Lsd1 and the EGFR pathway do not directly cooperate to regulate niche size. Loss of Lsd1 does not alter pERK expression in the developing gonad or in the adult germarium. Furthermore, the mRNA levels of the EGFR target gene dally remain unchanged in the absence of Lsd1. Previous studies showed that the Jak/Stat pathway also regulates Dpp signaling in adults; however, unlike Lsd1, it does not have a developmental role in niche formation (9, 10). Despite these findings, we cannot rule out the possibility that Lsd1 interacts with the Jak/Stat pathway at some level in adult germaria. Our results suggest that Lsd1 directs EC cell formation and limits Dpp signaling through a previously unrecognized mechanism that does not involve the Notch and EGF pathways.
Strikingly, loss of Lsd1 results in elevated levels of dpp mRNA within adult ovaries. This finding is consistent with the observed expansion of Dad-LacZ expression in Lsd1 mutant germaria. Ectopic expression of dpp within somatic cells during gonad development results in a greater number of Lamin C-expressing somatic cells. Based on all the findings presented here, we favor a model in which Lsd1 represses dpp transcription outside the normal niche reiteratively during development and in adulthood. Within the developing gonad, loss of Lsd1 results in expanded dpp expression leading to perturbations in normal cap cell and EC development. In other tissues, dpp expression is maintained through autoregulatory mechanisms (39–41). Perhaps loss of Lsd1 results in low levels of inappropriate dpp expression that become reinforced through similar autoregulatory mechanisms. Restoration of normal Lsd1 activity in adults appears sufficient to block dpp activity outside of the normal niche caused by defects in EC differentiation, suggesting that Lsd1 function and the genes it targets for repression are likely to be the same in the developing gonad and in adults.
Recent studies show that mammalian Lsd1 directly targets TGF-β1 for transcriptional repression (18) and has cell-autonomous roles in cancer (15–18). Given the possible links between cancer and stem cells (42) and the observation that Lsd1 has a conserved role in regulating intercellular signaling molecules, it will be important to determine whether Lsd1 and other chromatin factors have additional nonautonomous functions that contribute to stem cell maintenance, tumorigenesis, and metastasis.
Methods
Drosophila Stocks.
Drosophila stocks were maintained at room temperature on standard cornmeal-agar medium unless specified otherwise. The following fly strains were used in this study: w1118 was used as a control; Lsd1ΔN and UAS-Lsd1 (19) were provided by N. Dyson (Massachusetts General Hospital Cancer Center, Charlestown, MA); hs-bam (32) and hs FLP; FRT2A histone GFP (43) were provided by D. McKearin (Howard Hughes Medical Institute, Chevy Chase, MD); hh-Gal4 (44) was provided by J. Jiang (University of Texas Southwestern, Dallas, TX); dpphr56(45), c587-Gal4 (28), Dad-LacZ (46), and CB03410 (33) were provided by A. Spradling (Carnegie Institute for Science, Baltimore, MD); and E(spl)mβ-CD2 (7, 47) was provided by D. Drummond–Barbosa (The Johns Hopkins School of Public Health, Baltimore, MD). dpphr92, N55e11, FRT2A, and UAS-GFP as well as UAS-dpp-RNAi (BL-31530 and BL-31531) lines were obtained from the Bloomington Stock Center. UAS-Lsd1-RNAi was obtained from the National Institute of Genetics, Japan.
Immunostaining.
Adult ovaries were dissected in Grace's medium and fixed in 4% (vol/vol) paraformaldehyde for 10 min. The ovaries were washed with PBT (PBS, 0.5% BSA, and 0.3% Triton-X 100) and stained with primary antibody overnight at 4 °C. The ovaries were washed and incubated in secondary antibody at room temperature for 5 h. Ovaries were then washed again and mounted in Vectashield containing DAPI (Vector Laboratories).
The following primary antibodies were used: mouse anti-1B1 (Hts) (1:20) (48) and mouse anti-Lamin C LC28.26 (1:20) (49) (Developmental Studies Hybridoma Bank), goat anti-VASA (1:200) (Santa Cruz Biotechnology), rabbit anti-VASA (1:500) (gift from A. Spradling), mouse anti-BamC A7 (1:20) (50) (gift from D. McKearin), rabbit anti-Nanos (gift from A. Nakamura, RIKEN, Kobe, Japan), rabbit anti-Spectrin (1:1,000) (51) (gift from R. Dubreuil, University of Illinois at Chicago), mouse anti-β-galactosidase (1:1,000) (Promega), rabbit anti-GFP (1:1,000) (Invitrogen), guinea pig anti-Lsd1 antibody (1:5,000) and mouse anti-CD2 (1:20) (AbD Serotec), rabbit antiphosphorylated ERK1/2 (1:100) and rabbit anti-cleaved Caspase-3 (1:250) (Cell Signaling Technology), guinea pig anti-Traffic Jam (1:5,000) (52) (gift from D. Godt, University of Toronto, Toronto, ON, Canada), and rabbit anti–phospho-histone H3 (1:250) (Upstate Cell Signaling Solutions). Fluorescence-conjugated secondary antibodies (Jackson Laboratories) were used at a dilution of 1:200. The Student t test was used (two-tail distribution and two-sample unequal variants; Microsoft Excel 2008) to compare the number of ovarioles with branched vs. round fusomes between genotypes.
Generation of Anti-Lsd1 Antibody.
A sequence corresponding to 1–150 amino acids of Lsd1 protein was cloned into PROEX (Invitrogen) to produce His6-tagged protein. The protein was expressed in Escherichia coli and purified with Ni-NTA agarose (Invitrogen). Polyclonal antisera were generated in guinea pigs (Covance).
Generation of GSC and FSC Clones.
FLP/FRT-mediated mitotic recombination was used to generate GSC and FSC clones (53). Adult females of the genotype hs-FLP; FRT2A histone GFP/FRT2A, Lsd1ΔN were heat-shocked at 37 °C for 1 h twice a day for 3 d. hs-FLP; FRT2A histone GFP/FRT2A flies were used as controls. The ovaries were dissected on days 7, 14, and 21 after induction of heat shock.
RNA Isolation and RT-PCR.
RNA was isolated from bamΔ86 and Lsd1ΔN bamΔ86 mutant ovaries using TRIzol (Invitrogen). The RNA was treated with DNase and subjected to RT-PCR reaction using the One Step RT-PCR kit (Qiagen). The primers used to amplify dpp and dally mRNA are as follows:
dpp forward: 5′-GGCTTCTACTCCTCGCAGTG
dpp reverse: 5′-TGCTTTTGCTAATGCTGTGC
dally forward: 5′-TGACTTGCACGAGGACTAC
dally reverse: 5′-TAATACGACTCACTATAGGGTGAGGAGATGCAGTTTGCAC
Supplementary Material
Acknowledgments
We thank N. Dyson, D. McKearin, A. Nakamura, A. Spradling, R. Dubreuil, D. Drummond–Barbosa, and D. Godt for providing reagents. A. Rothenfluh, P. R. Hiesinger, B. Ohlstein, and members of the M.B. laboratory provided comments on the manuscript. This work was funded, in part, by National Institutes of Health Grant GM086647 (to M.B.), March of Dimes Grant 5FY0910 (to M.B.), the E. E. and Greer Garson Fogelson Endowment (University of Texas Southwestern Medical Center), and by National Institutes of Health Grant T32GM008203 (to S.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015874108/-/DCSupplemental.
References
- 1.Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: Theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Kirilly D, Xie T. The Drosophila ovary: An active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 5.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 6.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: Similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 8.Ward EJ, et al. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 9.López-Onieva L, Fernández-Miñán A, González-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–3635. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2006;443:97–100. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- 15.Suikki HE, et al. Genetic alterations and changes in expression of histone demethylases in prostate cancer. Prostate. 2010;70:889–898. doi: 10.1002/pros.21123. [DOI] [PubMed] [Google Scholar]
- 16.Tsai WW, Nguyen TT, Shi Y, Barton MC. p53-targeted LSD1 functions in repression of chromatin structure and transcription in vivo. Mol Cell Biol. 2008;28:5139–5146. doi: 10.1128/MCB.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulte JH, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: Implications for therapy. Cancer Res. 2009;69:2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano L, Ji JY, Moon NS, Herr A, Dyson N. Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr Biol. 2007;17:808–812. doi: 10.1016/j.cub.2007.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudolph T, et al. Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell. 2007;26:103–115. doi: 10.1016/j.molcel.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 21.de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Dev Genet. 1995;16:6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 24.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 25.Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 26.Pan L, et al. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 28.Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579–2588. doi: 10.1242/dev.00499. [DOI] [PubMed] [Google Scholar]
- 29.Katz DJ, Edwards TM, Reinke V, Kelly WG. A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell. 2009;137:308–320. doi: 10.1016/j.cell.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc Natl Acad Sci USA. 2009;106:9304–9309. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKearin DM, Spradling AC. bag-of-marbles: A Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4(12B):2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- 32.Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 33.Buszczak M, et al. The carnegie protein trap library: A versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- 35.de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 1997;124:3241–3251. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- 36.Amente S, et al. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene. 2010;29:3691–3702. doi: 10.1038/onc.2010.120. [DOI] [PubMed] [Google Scholar]
- 37.Chosed R, Dent SY. A two-way street: LSD1 regulates chromatin boundary formation in S. pombe and Drosophila. Mol Cell. 2007;26:160–162. doi: 10.1016/j.molcel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, et al. LSD1-mediated epigenetic modification is required for TAL1 function and hematopoiesis. Proc Natl Acad Sci USA. 2009;106:10141–10146. doi: 10.1073/pnas.0900437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hepker J, Blackman RK, Holmgren R. Cubitus interruptus is necessary but not sufficient for direct activation of a wing-specific decapentaplegic enhancer. Development. 1999;126:3669–3677. doi: 10.1242/dev.126.16.3669. [DOI] [PubMed] [Google Scholar]
- 40.Chanut F, Heberlein U. Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development. 1997;124:559–567. doi: 10.1242/dev.124.2.559. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Hoppler S, Eresh S, Bienz M. decapentaplegic, a target gene of the wingless signalling pathway in the Drosophila midgut. Development. 1996;122:849–858. doi: 10.1242/dev.122.3.849. [DOI] [PubMed] [Google Scholar]
- 42.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 43.Maines JZ, Park JK, Williams M, McKearin DM. Stonewalling Drosophila stem cell differentiation by epigenetic controls. Development. 2007;134:1471–1479. doi: 10.1242/dev.02810. [DOI] [PubMed] [Google Scholar]
- 44.Tanimoto H, Itoh S, ten Dijke P, Tabata T. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol Cell. 2000;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- 45.Irish VF, Gelbart WM. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1987;1:868–879. doi: 10.1101/gad.1.8.868. [DOI] [PubMed] [Google Scholar]
- 46.Tsuneizumi K, et al. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- 47.Dobens L, Jaeger A, Peterson JS, Raftery LA. Bunched sets a boundary for Notch signaling to pattern anterior eggshell structures during Drosophila oogenesis. Dev Biol. 2005;287:425–437. doi: 10.1016/j.ydbio.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Ding D, Parkhurst SM, Lipshitz HD. Different genetic requirements for anterior RNA localization revealed by the distribution of Adducin-like transcripts during Drosophila oogenesis. Proc Natl Acad Sci USA. 1993;90:2512–2516. doi: 10.1073/pnas.90.6.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riemer D, et al. Expression of Drosophila lamin C is developmentally regulated: Analogies with vertebrate A-type lamins. J Cell Sci. 1995;108:3189–3198. doi: 10.1242/jcs.108.10.3189. [DOI] [PubMed] [Google Scholar]
- 50.McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 51.Lee JK, Coyne RS, Dubreuil RR, Goldstein LS, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 53.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.