Abstract
In conjunction with the construction of a diversity-oriented synthesis library of 10-membered ring “natural product-like” macrolides, the design, synthesis, and validation of a unique class of bifunctional linchpins, uniting benzyne reactivity initiated by type II anion relay chemistry (ARC) has been achieved, permitting access to diverse [2+2], [3+2], and [4+2] cycloadducts.
Keywords: benzyne cycloaddition, natural product-like macrolides
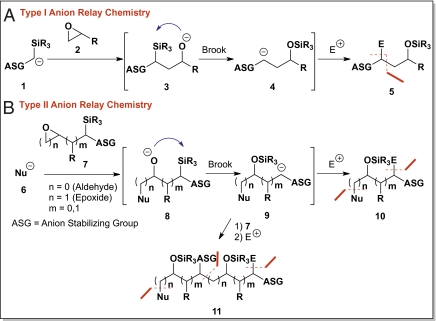
The discovery of chemical reactivity, coupled with innovative synthetic strategies, comprises the hallmark of complex molecule synthesis. Toward this end, we recently embarked on a research program to demonstrate the potential utility of anion relay chemistry (ARC) (1), a strategic reaction paradigm recently introduced by our laboratory for the iterative construction of architecturally complex natural and unnatural products (Scheme 1).
Scheme 1.
Type I and II ARC.
The driving force for this program was based on the recognition that although numerous elegant strategies, complete with exquisite stereochemical control, have been devised and implemented over the past several decades to access complex natural and unnatural products, the individual steps required for the multistep sequences often produce only minimal augmentation in structural complexity (2–5). In addition, the requisite purifications add time and costs, not to mention material loss and/or production of a significant waste stream. ARC, a multicomponent union tactic (6–8), holds the potential to alleviate, at least in part, these shortcomings. Particularly important is the potential to improve step efficiency, a critical aspect of complex molecule construction.
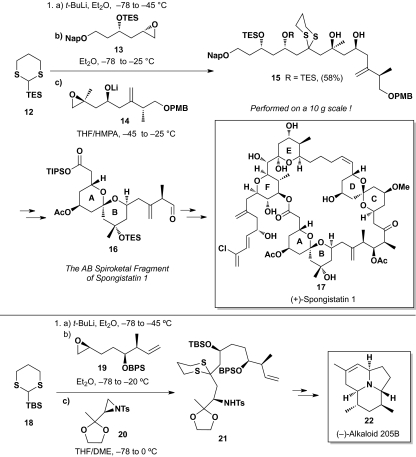
As defined, ARC entails orchestration of negative charge migration either through the bonding network of a molecule, as in the well recognized Michael or conjugate addition reaction, or alternatively transfer of charge “across space” by using a relay agent. An early example of the latter is the classic [1,2]-Brook rearrangement of α-trialkylsilyl alcohols (9, 10) initiated by strong base. Further analysis of the across space tactic reveals two subtypes (I and II), differentiated by the final locus of the reactive center after rearrangement. In Type I ARC (Scheme 1A), the negative charge is returned to the original carbon, permitting the reactive anion to serve as an effective tricomponent linchpin, a tactic (Scheme 2) that has served us well both during our 1-g synthesis of (+)-spongistatin 1 (17) (11) and the construction of alkaloid (–)-205B (22) (12).
Scheme 2.
Demonstration of ARC in total synthesis.
For the Type II protocol (Scheme 1B), the negative charge is transferred to a distal site, available for reaction either with simple terminating electrophiles, or for iterative reactions with a series of bifunctional linchpins, a process not dissimilar to “living polymerization” (13). It is the iterative reaction sequence, with diverse bifunctional linchpins that holds the greatest potential both for efficient construction of complex natural and unnatural products, as well as for diversity-oriented synthesis (DOS) (14). Of considerable significance, members of the DOS-derived libraries generated by this strategy would have a high ratio of sp3 to sp2 hybridized carbon atoms, an important structural feature missing in many existing screening collections (15, 16).
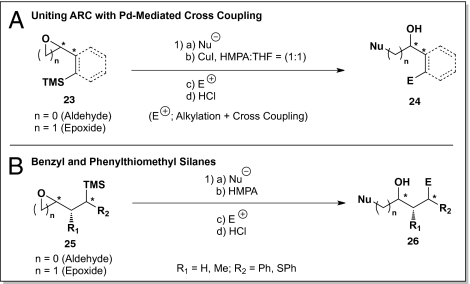
Early on, the synthetic potential of the Type II ARC protocol was limited by the lack of bifunctional linchpins. Fully convinced of the synthetic potential of the ARC concept, especially the iterative protocol, we set out to design, synthesize and validate effective new linchpins and then to demonstrate the potential of this tactic in DOS. Initial studies led to a series of bifunctional linchpins (Scheme 3A), comprising vinyl silanes, bearing β- or γ-electrophilic sites (aldehyde or epoxide). Application of carefully defined Brook rearrangement conditions [temperature, solvent polarity, and counter ion such as Li, K, and Cu(I)] to trigger the requisite [1,4]-C(sp2)→O silyl group migration permitted the development of multicomponent alkylation and cross-coupling reaction sequences (17). Equally effective, a series of three and four carbon benzyl and phenylthiomethyl silane bifunctional linchpins (Scheme 3B), bearing electrophilic sites either β or γ to a trialkylsilyl group, proved to be competent linchpins in multicomponent reactions (18).
Scheme 3.
Recently introduced bifunctional linchpins for Type II ARC.
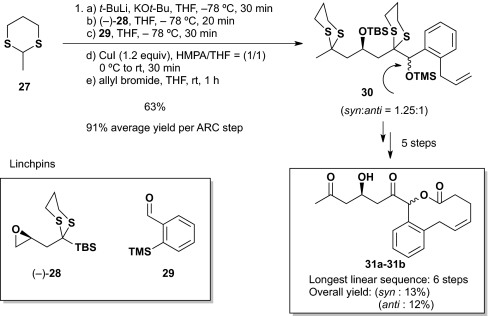
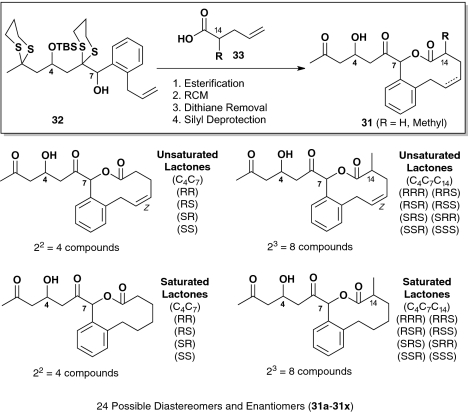
To demonstrate the potential of the iterative Type II ARC tactic for DOS, we selected our original epoxy silyl dithiane linchpin (–)-28 and the newly reported ortho-trimethylsilyl (TMS) benzaldehyde (29) to validate a proof of concept reaction sequence (19). The targets were syn and anti natural product-like macrolides 31a and 31b (Scheme 4). In the event, the reaction sequence proceeded in six steps, with an overall yield of 25% (i.e., 13 and 12%, respectively), which corresponds to an average yield of 80% per transformation.
Scheme 4.
Synthesis of macrocyclic natural product-like molecules.
Having achieved the reaction sequence proof of concept, we turned to the construction of a focused library of 10-membered ring macrolides based on 31 (Scheme 5), using several different acids (33) as coupling partners for Steglich esterification (DMAP/DCC) (20). We set as a goal a prospective tenet for DOS that we hope will become widely adopted by the DOS community: construction of all possible diastereomers and enantiomers of a chosen scaffold (21). Such a goal would, at least for some congeners, demand the development of innovative chemistry, as occurs in the field of natural product total synthesis. Pleasingly, we record here the successful completion of a library consisting of the 24 possible congeners of 31, including both saturated and unsaturated 10-membered ring macrolides, which has been submitted to the National Institutes of Health Molecular Libraries Small-Molecule Repository (MLSMR) for high-throughput screening (SI Appendix).
Scheme 5.
Natural product-like library synthesis using the ARC tactic.
Design, Synthesis, and Validation of an Effective Class of Bifunctional Linchpins Capable of Benzyne Reactivity
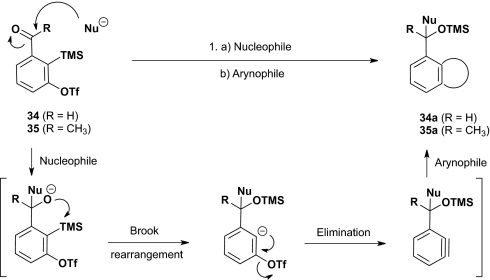
During the construction of the macrolide library (Scheme 5), we recognized the opportunity to develop a class of Type II ARC bifunctional linchpins based on the ortho-TMS benzaldehyde skeleton (29; Scheme 4) that held the promise of uniting ARC with the rich reactivity of benzyne. Specifically, by placing an effective leaving group ortho to the TMS group in either 29 or the corresponding ketone to furnish linchpins 34 and 35 (22), application of the now-validated conditions to trigger a [1,4]-Brook rearrangement with linchpin 29 [CuI and/or hexamethylphosphoramide (HMPA)] could be envisioned to furnish a benzyne intermediate capable of undergoing a wide variety of inter- or intramolecular [2+2], [3+2], and [4+2] cycloaddition reactions (Scheme 6). Critical for success in the multicomponent manifold would be the precise timing of the [1,4]-Brook rearrangement, required to access the benzyne intermediate, in conjunction with sufficiently dilute conditions to avoid possible intermolecular reactions (vide infra).
Scheme 6.
Potential benzyne reactivity initiated via the Type II ARC tactic.
Linchpin Synthesis and Initial Validation of Benzyne Reactivity
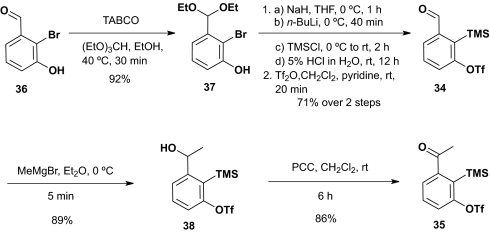
Bifunctional linchpins 34 and 35 (Scheme 7) required to explore this scenario were readily prepared from 2-bromo-3-hydroxy-benzaldehyde 36 (23), beginning by acetalization with (EtO)3CH in ethanol (1:2 vol/vol), by using 2,4,4,6-tetrabromo-2,5-cyclohexadienone (24) as catalyst. A five-stage sequence, involving metalation, followed by silylation, removal of the diethyl acetal, and triflation converted acetal 37 to 34, the requisite aldehyde linchpin. The corresponding ketone linchpin 35 was prepared by addition of MeMgBr to 34 followed by pyridinium chlorochromate (PCC) oxidation (25).
Scheme 7.
Synthesis of aldehyde and ketone linchpins 34 and 35.
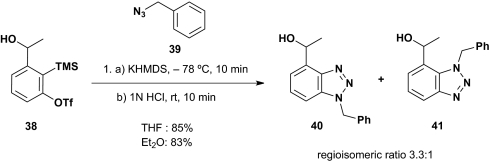
To demonstrate the feasibility of benzyne reactivity induced via the proposed [1,4]-Brook rearrangement, we initially used alcohol 38 (Scheme 8). For the arynophile, we selected benzyl azide 39. Surprisingly, unlike the conditions required to trigger silyl group migration ([1,4]-C(sp2)→O) with ortho-TMS benzaldehyde 29 (19), namely CuI in a polar solvent such as HMPA, the Brook rearrangement proceeded both rapidly and efficiently at low temperature in either Et2O or THF by using potassium hexamethyldisilazane (KHMDS) without addition of CuI. Removal of the TMS group (1 M HCl) furnished 40 and 41 as a mixture of regioisomers in 83–85% yield. Presumably silyl group migration, an equilibrium process (9, 10), is driven in this case by formation of the benzyne functionality, whereas for the parent linchpin 29, Cu(I) is required to facilitate the Brook rearrangement.
Scheme 8.
Demonstration of benzyne reactivity.
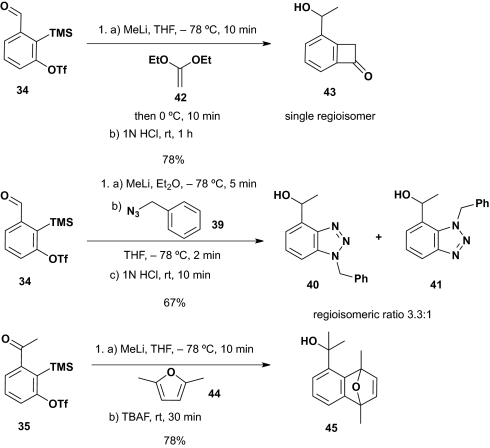
Encouraged by these results, we turned to explore the feasibility of intermolecular tricomponent [2+2], [3+2], and [4+2] cycloadditions to be achieved in a “single-flask” by using linchpins 34 and 35. For the Type II ARC process, MeLi was selected as the initiating nucleophile.
As illustrated in Scheme 9, addition of MeLi to 34 and 35 using 1,1-diethoxyethylene (42) and benzyl azide (39), respectively, as the arynophiles for 34 and 2,5-dimethylfuran (44) for 35, furnished the [2+2], [3+2], and [4+2] cycloadducts in 78, 67, and 78% yields. Solvent polarity, temperature, and reaction time with the timing of arynophile introduction proved critical. In the case of the intermolecular [2+2] and [4+2] cycloadditions, a mixture of linchpin and arynophile was treated with MeLi at −78 °C for 10 min in THF, followed, in turn, by warming to 0 °C for 10 min before the silyl group was removed. However, with benzylazide, an arynophile known to react with alkyl lithium (26), linchpin 34 was first reacted with MeLi in Et2O for 5 min at −78 °C. In turn, benzyl azide was then added in THF at −78 °C and allowed to react for 2 min exploiting a solvent, temperature, and reaction time known to initiate and complete the Type II ARC/benzyne reaction sequence. Treatment with 1 M HCl to remove the TMS group then furnished adducts 40 and 41 in 67%, again as a 3.3:1 mixture of regioisomers. Of note, the conversion of ketone 35 to 45 represents an example of a multicomponent Type II ARC process using a ketone-based linchpin.
Scheme 9.
Intermolecular [2+2], [3+2], and [4+2] benzyne cycloadditions.
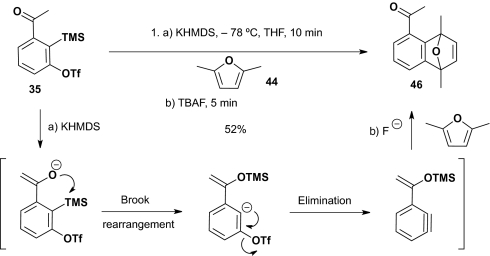
To expand further the utility of the ketone-based linchpin (35), we explored the possibility of using the oxygen anion of the derived enolate to trigger [1,4]-C(sp2)→O silyl group migration (for the first example demonstrating a ketone enolate in a Brook Rearrangement, see ref. 27) (Scheme 10). Elimination of the triflate group would then permit in situ benzyne formation to be followed by a [4+2] intermolecular cycloaddition with an appropriate arynophile.
Scheme 10.
Enolate initiation of the Type II ARC tactic leading to benzyne reactivity.
As predicted, treatment of 35 with KHMDS at −78 °C in the presence of 2,5-dimethylfuran furnished the [4+2] cycloadduct (46) in 52% yield, after removal of the TMS group using tetrabutylammonium fluoride (TBAF). Here again [1,4]-C(sp2)→O silyl group migration proceeded without the need of Cu(I), further supporting the suggestion that formation of the resulting benzyne intermediate drives the silyl group migration to the oxygen of the ketone enolate. This observation is the opposite of the Fallis’ observation (28), demonstrating that aryl anions generated via metal-halogen exchange of o-bromoacetophenone trimethylsilyl enol ether at −78 °C in THF undergo rapid [1,4]-O→C(sp2) retro-Brook rearrangement.
Turning next to the feasibility of the intramolecular cycloaddition manifold, we note that such cycloadditions have been achieved by using fixed cisoid dienes, such as furan and other cyclic dienes in natural product total synthesis (29–31). However, intramolecular [2+2], [3+2], or [4+2] benzyne cycloadditions with simple alkenes, azides, or acyclic dienes are rare. Indeed, to the best of our knowledge, only two examples have been reported, the first by Buszek (32), who demonstrated that treatment of aryl bromides possessing appropriate tethered dienes upon treatment with strong base afforded [4+2] cycloadducts, albeit in modest yield (20–28%). Later, Danheiser and coworkers (33) reported that treatment of o-(trimethylsilyl)aryl triflates with tetrabutylammonium triphenyldifluorosilicate (TBAT) undergo effective intramolecular [4+2] cycloadditions with conjugated enynes, arenynes, and dienes to generate highly condensed polycylic aromatic compounds. With these caveats in mind, we expanded our program to explore the feasibility of intramolecular [2+2], [3+2], and [4+2] cycloadditions exploiting benzyne intermediates generated via a [1,4]-C(sp2)→O Brook rearrangement.
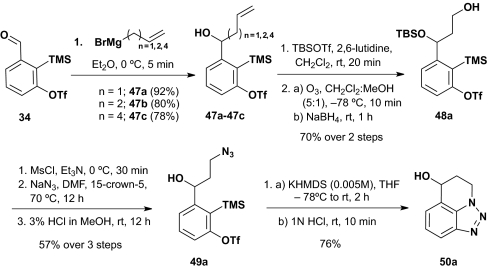
For the prospective intramolecular [2+2] and [3+2] cycloadditions, the substrate alcohols (47b, 47c, and 49a) were constructed as outlined in Scheme 11. Specifically, addition of the requisite Grignard reagents to 34 afforded 47b and 47c for the proposed intramolecular [2+2] cycloadditions. Azide 49a, substrate for a potential [3+2] intramolecular cycloaddition, was elaborated via a five-step sequence beginning with alcohol 47a. Protection of the hydroxyl as the t-butyldimethylsilyl (TBS) ether, followed by ozonolysis/reduction (NaBH4), mesylation, azide displacement, and TBS group removal led to 49a. Overall yields were good to excellent.
Scheme 11.
Intramolecular [3+2] benzyne cycloaddition.
Turning to intramolecular [2+2] cycloadditions induced via [1,4]-C(sp2)→O Brook rearrangement, we quickly realized that the terminal olefins in 47b and 47c were simply too unreactive to serve as a viable arynophile. Success, however, was achieved with the intramolecular [3+2] cycloaddition by using alcohol 49a (Scheme 11) when the reaction was carried out in a dilute THF solution (0.005 M) to avoid intermolecular reaction with KHMDS (1.1 equiv) to trigger the Brook rearrangement; the yield of 50a was 76%. To the best of our knowledge, this is the first example of an intramolecular [3+2] cycloaddition using a benzyne intermediate.
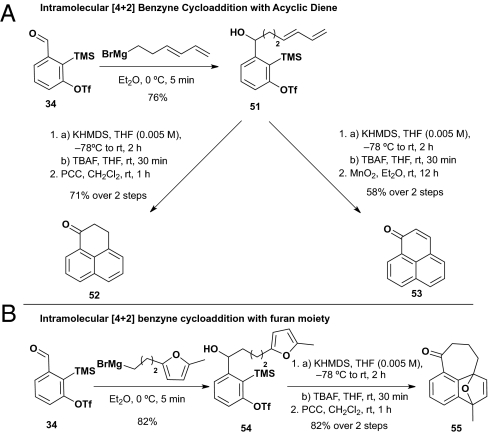
Substrate alcohols 51 and 54 for prospective intramolecular [4+2] cycloadditions were also readily prepared via addition of the corresponding Grignard reagents to aldehyde 34 (Scheme 12).
Scheme 12.
Intramolecular [4+2] benzyne cycloaddition.
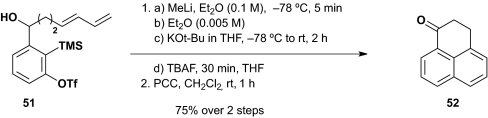
Validation of the intramolecular [4+2] cycloaddition process was first achieved upon treatment of alcohol 51 with KHMDS at –78 °C in a dilute THF solution (0.005 M), followed by PCC oxidation to furnish 52, a process that entailed oxidative aromatization after the initial [4+2] cycloaddition (Scheme 12A). The yield was 71%. Intramolecular cycloaddition using MnO2 as the terminal oxidant proceeded in similar fashion to provide phenalenone 53 in 58% over two steps (congeners of phenalenone 53 are of interest do to their bioactivity as the probes to analyze the role of DNA polymerase, see ref. 34). A third example entailed the intramolecular [4+2] cycloaddition of 54, carrying a pendent furan moiety; subsequent PCC oxidation furnished 55 in 82% yield for the two steps (Scheme 12B).
We turned next to the possibility of a two-component reaction by using the Type II ARC protocol to trigger an intramolecular [4+2] aryne cycloaddition. High dilution conditions would be required, given the anticipated competitive intermolecular reactivity of the benzyne intermediate. To overcome this issue, we first explored the use of concentration, solvent polarity (Et2O→THF) and counter ion exchange (Li+→K+) to trigger the [1,4]-C(sp2)→O Brook rearrangement leading to the benzyne intermediate, based on our earlier work demonstrating that such reaction conditions comprise competent Brook rearrangement triggers (35). Pleasingly, upon revisiting the conversion of 51 to 52 (Scheme 12A), decreasing the concentration with Et2O after addition of MeLi followed by addition of KOt-Bu in THF to trigger the Brook rearrangement was also effective. Oxidation with PCC furnished cycloadduct 52 in 75% yield for the two steps (Scheme 13).
Scheme 13.
Intramolecular [4+2] benzyne cycloaddition.
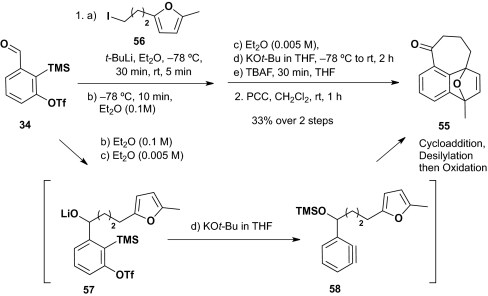
Encouraged by these results, we turned to validation of a “one-pot” two-component protocol, using linchpin 34 and the alkyl lithium derived from iodide 56 carrying a furan (Scheme 14), taking advantage of the conditions used above (Scheme 13). In the event, addition of the alkyl lithium derived from 56 in Et2O (0.1 M), followed in turn by dilution with Et2O to a substrate concentration of 0.005 M, transmetallation using KOt-Bu in THF, and PCC oxidation of the derived adduct pleasingly furnished cycloadduct 55, albeit in a modest 33% yield for the two steps.
Scheme 14.
One-pot intramolecular [4+2] benzyne cycloaddition.
Summary
During the development and application of an iterative Type II ARC strategy for DOS, that permitted construction of the 24 possible congeners of a focused library of 10-membered ring natural product-like macrolides, we designed, synthesized, and validated a unique class of bifunctional linchpins that led to the union of ARC with benzyne reactivity. This union greatly enhances the future potential of the ARC tactic by permitting access to diverse scaffolds that arise via inter- and intramolecular [2+2], [3+2], and [4+2] cycloaddition reactions. Particularly significant was the development of reaction conditions (temperature, solvent polarity, and counter ion) that permit the precise timing of the [1,4]-C(sp2)→O Brook rearrangement required to generate the benzyne intermediates for cycloaddition reactions.
Materials and Methods
General.
Unless otherwise indicated, all reactions were carried out under an argon atmosphere in flame- or oven-dried glassware, and solvents were freshly distilled or obtained from a solvent deoxygenation system.
Chemical Synthesis.
Details for the synthesis of linchpins, intermediates, and cycloadducts, as well as spectroscopic/analytical data for all new compounds, are available at SI Appendix.
Representative Procedure for ARC Tactic.
To a precooled (–78 °C) solution of 2-methyl-1,3-dithiane 27 (1.24 g, 9.2 mmol, 1.2 equiv) in THF (15 mL) was added KOt-Bu (1.0 M in THF, 9.7 mL, 9.7 mmol, 1.26 equiv) and t-BuLi (1.7 M in pentane, 5.7 mL, 9.7 mmol, 1.26 equiv). The resulting solution was stirred at –78 °C for 30 min, and a solution of epoxy silyl dithiane linchpin (–)-28 (2.24 g, 7.7 mmol, 1.0 equiv) in THF (15 mL) was added. The mixture was stirred for 20 min at –78 °C and then a solution of aldehyde 29 (1.64 g, 9.2 mmol, 1.2 equiv) in THF (15 mL) was added via cannula. After stirring for 30 min at –78 °C, the resulting solution was transferred via cannula to a mixture of CuI (1.75 g, 9.2 mmol, 1.2 equiv) and HMPA/THF (10 mL/10 mL) at 0 °C and then warmed to ambient temperature and stirred for 30 min. Allyl bromide (2.0 mL, 23.1 mmol, 3.0 equiv) was next added at ambient temperature and after 1 h, the reaction was quenched with saturated aqueous NH4Cl solution (100 mL). The resulting mixture was then extracted with Et2O (100 mL × 3) and the organic layers were combined, washed with brine (100 mL), dried over MgSO4, filtered, and concentrated in vacuo. Flash chromatography on silica gel (Et2O/hexane; 1/50) provided a 1.25:1 diastereomeric mixture of 30 (3.13 g, 4.87 mmol, 63%) as pale yellow oil. Rf = 0.8 (hexane/Et2O = 10/1).
Supplementary Material
Acknowledgments
Support for this research program was provided by the National Institutes of Health (Institute of General Medical Sciences) through Grants GM-081253 and 29028.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015265108/-/DCSupplemental.
References
- 1.Smith A-B, III, Wuest W-M. Evolution of multi-component anion relay chemistry (ARC): Construction of architecturally complex natural and unnatural products. Chem Commun (Camb) 2008:5883–5895. doi: 10.1039/b810394a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wender P-A, Verma V-A, Paxton T-J, Pillow T-H. Function-oriented synthesis, step economy, and drug design. Acc Chem Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 3.Wender P-A, Croatt M-P, Witulski B. New reactions and step economy: The total synthesis of (±)-salsolene oxide based on the type II transition metal-catalyzed intramolecular [4+4] cycloaddition. Tetrahedron. 2006;62:7505–7511. [Google Scholar]
- 4.Bertz S-H. The first general index of molecular complexity. J Am Chem Soc. 1981;103:3599–3601. [Google Scholar]
- 5.Bertz S-H, Sommer T-J. Rigorous mathematical approaches to strategic bonds and synthetic analysis based on conceptually simple new complexity indices. Chem Commun (Camb) 1997:2409–2410. [Google Scholar]
- 6.Dömling A, Ugi I., I Multicomponent reactions with isocyanides. Angew Chem Int Ed Engl. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Tietze L-F. Domino reactions in organic synthesis. Chem Rev. 1996;96:115–136. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]
- 8.Dömling A. In: Multicomponent Reactions. Zhu J, Bienaymé H, editors. Weinheim, Germany: Wiley-VCH; 2005. pp. 76–94. [Google Scholar]
- 9.Brook A-G. Isomerism of some α-hydorxysilanes to silyl ethers. J Am Chem Soc. 1958;80:1886–1889. [Google Scholar]
- 10.Brook A-G. Molecular rearrangements of organosilicon compounds. Acc Chem Res. 1974;7:77–84. [Google Scholar]
- 11.Smith A-B, III, et al. Spongipyran synthetic studies. Evolution of a scalable total synthesis of (+)-spongistatin 1. Tetrahedron. 2009;65:6489–6509. doi: 10.1016/j.tet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith A-B, III, Kim DS. Total synthesis of the neotropical poison-frog alkaloid (-)-205B. Org Lett. 2005;7:3247–3250. doi: 10.1021/ol0510264. [DOI] [PubMed] [Google Scholar]
- 13.Webster OW. Living polymerization methods. Science. 1991;251:887–893. doi: 10.1126/science.251.4996.887. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber S-L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 15.Clemons P-A, et al. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc Natl Acad Sci USA. 2010;107:18787–18792. doi: 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandapani S, Marcaurelle LA. Grand challenge commentary: Accessing new chemical space for ‘undruggable’ targets. Nat Chem Biol. 2010;6:861–863. doi: 10.1038/nchembio.479. [DOI] [PubMed] [Google Scholar]
- 17.Smith A-B, III, Kim WS, Tong R. Uniting anion relay chemistry with Pd-mediated cross coupling: Design, synthesis and evaluation of bifunctional aryl and vinyl silane linchpins. Org Lett. 2010;12:588–591. doi: 10.1021/ol902784q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith A-B, III, Tong R. Benzyl and phenylthiomethyl silanes: A new class of bifunctional linchpins for type II anion relay chemistry (ARC) Org Lett. 2010;12:1260–1263. doi: 10.1021/ol100130x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith A-B, III, Kim WS, Wuest W-M. Ortho-TMS benzaldehyde: An effective linchpin for type II anion relay chemistry (ARC) Angew Chem Int Ed Engl. 2008;47:7082–7086. doi: 10.1002/anie.200802301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steglich W, Neises B. Simple method for the esterification of carboxylic acids. Angew Chem Int Ed Engl. 1978;17:522–524. [Google Scholar]
- 21.Marcaurelle L-A, et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: Discovery of macrocyclic histone deacetylase inhibitors. J Am Chem Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himeshima Y, Sonoda T, Kobayashi H. Fluoride-induced 1,2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions. Chem Lett. 1983;12:1211–1214. [Google Scholar]
- 23.Stavrakov G, Keller M, Breit B. From central to axial to central chirality: Eanantioselective construction of the trans-4,5,9,10-tetrahydorxy-9,10-dihydrophenanthrene system. Eur J Org Chem. 2007;2007:5726–5733. [Google Scholar]
- 24.Firouzabadi H, Iranpoor N, Shaterian H-R. 2,4,4,6-Tetrabromo-2,5-cyclohexadienone (TABCO) as a versatile, efficient, and chemoselective catalyst for the acetalization and transacetalization of carbonyl compounds, the preparation of acetonides from epoxides and acylals (1,1-diacetates) from aldehydes. Bull Chem Soc Jpn. 2002;75:2195–2205. [Google Scholar]
- 25.Corey E-J, Suggs W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 1975;16:2647–2650. [Google Scholar]
- 26.Sieh D-H, Wilbur D-J, Michejda C-J. Preparation of trialkyltriazenes. A comparison of the N-N bond rotation in trialkyltriazenes and aryldialkyltriazenes by variable temperature 13C NMR. J Am Chem Soc. 1980;102:3883–3887. [Google Scholar]
- 27.Tsubouchi A, Matsuda H, Kira T, Takeda T. Silyl migration in conjunction with substitution on silicon in copper(I) t-butoxide-promoted coupling between o-silylphenyl ketones and organic halides. Chem Lett. 2009;38:1180–1181. [Google Scholar]
- 28.Comanita B-M, Woo S, Fallis A-G. A versatile tandem retro-[1,4]-Brook rearrangement-condensation reaction of o-bromoacetopheone silyl enol ethers. Tetrahedron Lett. 1999;40:5283–5286. [Google Scholar]
- 29.Best W-M, Wege D. Intramolecular Diels-Alder reactions of benzynes application to the total synthesis of mansonone E. Tetrahedron Lett. 1981;22:4877–4880. [Google Scholar]
- 30.Buszek K-R, Bixby D-L. Total synthesis of pseudopterosin A and E aglycon. Tetrahedron Lett. 1995;36:9129–9132. [Google Scholar]
- 31.Chen CL, Sparks S-M, Martin S-F. C-Aryl glycosides via tandem intramolecular benzyne-furan cycloadditions. Total synthesis of vineomycinone B2 methyl ester. J Am Chem Soc. 2006;128:13696–13697. doi: 10.1021/ja0652619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buszek K-R. First intramolecular benzyne diels-alder reaction with an acyclic diene. Unusual effect of diene geometry on the course of the reaction. Tetrahedron Lett. 1995;36:9125–9128. [Google Scholar]
- 33.Hayes M-E, Shinokubo H, Danheiser R-L. Intramolecular [4 + 2] cycloadditions of benzynes with conjugated enynes, arenynes, and dienes. Org Lett. 2005;7:3917–3920. doi: 10.1021/ol051372l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perpelescu M, et al. Novel phenalenone derivatives from a marine-derived fungus exhibit distinct inhibition spectra against eukaryotic DNA polymerases. Biochemistry. 2002;41:7610–7616. doi: 10.1021/bi020115a. [DOI] [PubMed] [Google Scholar]
- 35.Smith A-B, III, Xian M, Kim WS, Kim DS. The [1,5]-Brook rearrangement: An initial application in anion relay chemistry. J Am Chem Soc. 2006;128:12368–12369. doi: 10.1021/ja065033e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.