Abstract
The frog Xenopus, an important research organism in cell and developmental biology, currently lacks tools for targeted mutagenesis. Here, we address this problem by genome editing with zinc-finger nucleases (ZFNs). ZFNs directed against an eGFP transgene in Xenopus tropicalis induced mutations consistent with nonhomologous end joining at the target site, resulting in mosaic loss of the fluorescence phenotype at high frequencies. ZFNs directed against the noggin gene produced tadpoles and adult animals carrying up to 47% disrupted alleles, and founder animals yielded progeny carrying insertions and deletions in the noggin gene with no indication of off-target effects. Furthermore, functional tests demonstrated an allelic series of activity between three germ-line mutant alleles. Because ZFNs can be designed against any locus, our data provide a generally applicable protocol for gene disruption in Xenopus.
Frogs of the genus Xenopus have been an important model organism for cell and developmental biologists since the 1930s (1). Xenopus laevis is the standard model, but is allotetraploid and hence less suited for genetic approaches than the diploid Xenopus tropicalis, whose genome sequence has been determined (2). Whereas embryological manipulations and gain-of-function experiments are major strengths of Xenopus, reverse genetics is currently limited to the use of antisense reagents that provide transient and often incomplete gene knockdown (3). The ability to introduce targeted, heritable mutations that disrupt gene function has remained elusive.
Here, we provide a generally applicable solution to this problem: targeted gene disruption with designed zinc-finger nucleases (ZFNs). ZFNs are the fusion of the nonspecific cleavage domain of the type IIS restriction enzyme FokI to a zinc-finger protein (4, 5) that is engineered to bind a specific genomic locus to induce a targeted double-strand break (DSB). Pioneering studies in oocytes of X. laevis (6) and subsequent work in Drosophila (7) showed the mutagenic potential of a DSB induced by ZFNs (reviewed in refs. 10 and 11). Resolution of ZFN-induced DSBs via nonhomologous end joining (NHEJ) generates small insertions and deletions that often produce null or hypomorphic alleles (7, 9, 12).
In the present work, we set out to develop an effective protocol for gene disruption in X. tropicalis. Using ZFNs designed against a reporter transgene and the noggin locus, we optimized delivery and expression conditions and attained high frequencies of somatic and germ-line mutations that were transmissible to the next generation.
Results
Xenopus eggs are large and easily manipulated (13), offering the opportunity to deliver ZFNs via injection of mRNA, a method that has been successful in bringing about ZFN-driven gene disruption in other organisms (10, 11).
To develop conditions for gene disruption in X. tropicalis, we made use of transgenic animals carrying a single-copy GFP transgene (14). Wild-type X. tropicalis eggs were fertilized with sperm from a homozygous GFP transgenic male. The resulting heterozygous embryos were injected with mRNA-encoding ZFNs that target the eGFP coding region (15). Uninjected tadpoles express GFP robustly in the somites, lens, and head musculature (Fig. 1 A and B). Injection of 20 pg eGFP ZFN RNAs led to mosaic loss of fluorescence in otherwise healthy tadpoles (Fig. 1 E and F). At a higher dose of ZFNs, most cells had lost fluorescence, suggesting efficient somatic mutation of the transgene (Fig. 1 H and I).
Fig. 1.
Disruption of the eGFP transgene in X. tropicalis using ZFNs. (A–C) Uninjected tadpoles (U.C.). (D–F) Tadpoles injected with 20 pg of eGFP ZFN mRNA and 200 pg mCherry RNA (to monitor injection). (G–I) Heterozygous eGFP tadpoles injected with 50 pg eGFP ZFN mRNA and 200 pg mCherry RNA (tracer). (A, D, and G) Brightfield. (B, E, and H) eGFP expression of tadpoles in A, D, and G, respectively. (C, F, and I) Enlarged view of eGFP expression in B, E, and H, respectively. (J) Cel-1 digestion of eGFP amplicons. Bands migrating at 345 bp are full-length amplicons; Cel-1 cleavage products migrate at 246 and 99 bp. The fractions of modified chromatids detected by Cel-1 are quantified as percentage NHEJ. UC, uninjected control. (K) Sequence alignment of ZFN-induced mutant eGFP transgene alleles from tadpoles injected with 50 pg ZFN mRNA. Red nucleotides indicate insertions and dashes represent deletions. Horizontal bold lines at top indicate ZFN-binding sites.
To determine whether loss of fluorescence resulted from a ZFN-induced mutation in the eGFP transgene, we first genotyped the target locus using an assay based on the mismatch-sensitive endonuclease, Cel-1 (8). This analysis (Fig. 1J) demonstrated that ZFN-treated, but not control, tadpoles had acquired a DNA sequence alteration in the stretch targeted by the ZFNs. Sequencing subsequently revealed that individual tadpoles often carried multiple distinct indels ranging from 5 to 20 bp centered over the ZFN recognition site (Fig. 1K), a signature of mutagenic NHEJ. Taken together, these experiments show that ZFN mRNA injection into the two-cell embryo yields tadpoles without detectable developmental defects that exhibit both genetic and phenotypic mosaicism for the ZFN-targeted locus and trait, respectively.
To determine whether this approach can be used to disrupt an endogenous gene, we designed ZFNs that target the noggin locus. Noggin is a bone morphogenetic protein (BMP) antagonist that contributes to dorsal/ventral patterning during gastrulation in Xenopus (16). Although its function in later development has been studied in human patients (17) and mice (18–21), its developmental role in nonmammalian vertebrates remains poorly understood. Noggin is expressed at stages when knockdown via morpholinos is not effective. Therefore, mutant alleles of the endogenous noggin gene are required to probe its role throughout amphibian development.
We designed a panel of ZFNs targeting noggin, screened them in a budding yeast proxy system (22), and cloned the ZFNs into an expression construct that allows the synthesis of an efficiently translated mRNA with a stabilizing polyadenylation signal (23). Embryos were injected in the animal pole, and mRNA was deposited in the center of each blastomere at the two-cell stage and raised to stage 40, and DNA was isolated from tadpoles that exhibited broad mCherry (i.e., tracer) expression. Use of ZFNs carrying a wild-type FokI endonuclease domain yielded a significant fraction of embryos with developmental defects (Fig. 2A). However, this was alleviated by expressing the same zinc-finger DNA recognition domains fused to the obligate heterodimer forms of FokI, in which point mutations are made in the nuclease domain that prevent homodimers from forming a functional nuclease (8). These mutations, E490K, I538K and Q486E, I499L, are made in the FokI domain of the left and right ZFN, respectively (referred to as EL+KK). Even at the highest tested doses of such ZFN mRNA, more than 60% of the injected embryos developed normally (Fig. 2A).
Fig. 2.
Expression of ZFNs targeting noggin in X. tropicalis. (A) Optimization of ZFN delivery in X. tropicalis. EL+KK: ZFNs with the EL and KK modifications in the FokI domain (8); numbers represent different noggin ZFN pairs (Table S1). WT, ZFNs with wild-type Fok1 nuclease domains. PA−, ZFN transcripts lacking a polyadenylation signal. UC, uninjected control. (B) Comparison of different noggin ZFN pairs in the yeast activity assay and in injected tadpoles. Tadpoles were injected with 100 pg ZFN mRNA. Yeast activity values are represented as a percentage relative to ZFNs targeting the human CCR5 gene (9). Activity in tadpoles is calculated as the percentage of mutant amplicons sequenced from injected embryos. ND, no data. (C) Western blot for FLAG-tagged ZFN proteins.
To optimize the delivery of ZFNs, we also tested whether unstable, nonadenylated mRNA, which would be translated early and deliver a transient burst of ZFN, might be superior to the extended expression of ZFNs from transcripts that are cleaved and polyadenylated after injection (23). As expected, the nonadenylated transcripts led to expression of considerably less protein (Fig. 2C and Fig. S1), but even at higher doses did not induce noggin gene disruption at a frequency measurable by Cel-1. Thus, we conclude that the provision of ZFNs from stable polyadenylated mRNA is superior for effective genome editing in Xenopus.
Because it is difficult to predict a priori the extent to which ZFN overexpression may cause embryonic defects, we tested a panel of ZFNs in a yeast-based single-strand annealing assay (22) and in embryos (Fig. 2B and Table S1). Six ZFN pairs shown to have activity in the yeast assay were chosen for testing in Xenopus. These ZFNs were well tolerated in tadpoles and yielded efficient genome editing in Xenopus embryos as measured by sequencing noggin amplicons from injected tadpoles.
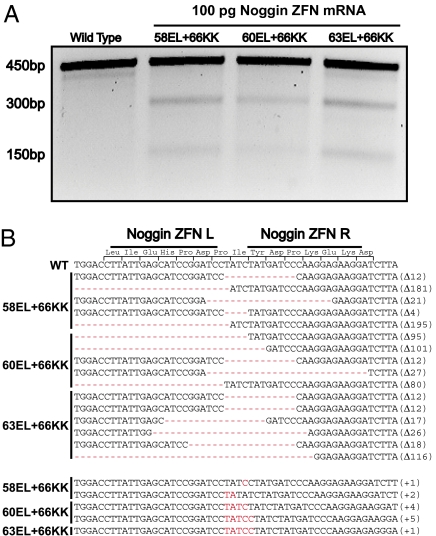
Because no Xenopus strains carrying noggin mutations exist, we were unable to screen for phenotypes on a heterozygous background (22) and instead screened the ZFNs for activity by genotyping the targeted region using the Cel-1 endonuclease (Fig. 3A). All of the ZFNs that were well tolerated produced targeted gene disruption. Direct sequencing of the nuclease-targeted region in tadpoles injected with ZFNs revealed a broad panel of insertions and deletions ranging in size from 5 to 195 bp (Fig. 3B), with frequencies of mutant amplicons from 10% to 47%. Such high rates of somatic mutagenesis suggested that tadpoles might also carry mutations in the germ line, allowing the establishment of lines carrying unique noggin alleles, a possibility we investigated next.
Fig. 3.
ZFN-driven editing of the noggin locus in X. tropicalis. (A) Somatic mutations in noggin detected by Cel-1. Bands migrating at 450 bp are full-length noggin amplicons. Bands migrating at 300 and 150 bp are Cel-1 digestion products. (B) Sequence alignment of noggin alleles induced by indicated ZFN pairs. Red nucleotides indicate insertions and dashes represent deletions. Horizontal bold lines at top indicate ZFN-binding sites. EL+KK, ZFNs with the EL and KK modifications in the FokI domain (8); numbers represent different noggin ZFN pairs (Table S1).
We injected wild-type embryos at the two-cell stage with 100 pg of mRNA encoding ZFNs targeting noggin that were tolerated by more than 60% of the injected embryos (Fig. 2B). Successful somatic genome editing in tadpoles and froglets was confirmed by isolating genomic DNA from tail or toe clips, respectively, and genotyping the noggin locus by Cel-1 (Table 1). Injected embryos were raised to sexual maturity and outcrossed to wild-type animals. Offspring from this cross were raised to tadpole stage 40, lysed, and analyzed via Cel-1 for mutations in the noggin locus. Genotyping offspring from a cross using a ZFN-treated male founder revealed that 3 of 18 tadpoles had inherited a ZFN-induced Δ12 allele of noggin (Fig. 4 A and B). A second male founder produced 6 of 50 embryos heterozygous for a ZFN-induced 3-bp insertion allele (Fig. 4 A and B), and a third male produced 12 of 50 tadpoles heterozygous for a 4-bp insertion. This ZFN-induced noggin frameshift allele results in a premature stop codon at position 55. This is likely to behave as a null allele because it lacks most of the BMP-binding residues and all of the residues required for dimerization (24).
Table 1.
Efficiency of noggin mutation induction and generation of germ-line mutants by zinc-finger nucleases in X. tropicalis
| Founder (males) | Noggin ZFN pair | Embryos injected in cohort | % mosaic embryos in cohort | Adults raised in cohort | Founder % germ-line mutagenesis | Mutation |
| 1 | 26EL+28KK | 300 | 16.1 | 4 | 16.7 | 12-bp deletion |
| 2 | 26EL+28KK | 568 | 60 | 17 | 12 | 3-bp insertion |
| 3 | 63EL+66KK | 116 | 100 | 2 | 24 | 4-bp insertion |
Percentage mosaic embryos in cohort was calculated by determining the percentage of siblings (10–34 tadpoles) found to be positive for mutations as detected by Cel-1 assays. Founder percentage germ-line mutagenesis was calculated by determining the percentage of heterozygous offspring produced by founders in an outcross.
Fig. 4.
Germ-line transmission of ZFN-induced noggin mutations. (A) Cel-1 digests of noggin amplicons from sibling heterozygous mutant and homozygous wild-type F1 tadpoles produced from three mutant line founders. Bands migrating at 450 bp are full-length noggin amplicons. Bands migrating at 300 and 150 bp are Cel-1 digest products. (B) Sequence alignments of the targeted noggin locus from Cel-1–positive F1 mutants. Genomic and translated sequences are shown for each mutant line. Asterisk indicates a stop codon. Red nucleotides and amino acids indicate insertions and dashes represent deletions. (C) Schematic of synthetic RNA injections into ventral vegetal blastomeres of four-cell-stage embryos to test functionality of the induced mutant noggin alleles. (D) Quantification of secondary axis induction following wild-type or mutant noggin RNA injection. Bars represent results of two (5 pg) or three (10 pg) independent experiments (±SD). Black bars show 5-pg RNA injections; white bars show 10-pg RNA injections. Two asterisks indicate significantly different (P < 0.01) from uninjected controls. (E, F, G, and G′) Uninjected control embryos. (H, I, J, and J′) Embryos injected with 10 pg of 4-bp insertion mutant noggin and 200 pg LacZ RNA. (K–M) Embryos injected with 10 pg of 12-bp deletion mutant noggin and 200 pg LacZ RNA. (N–P) Embryos injected with 10 pg of 3-bp insertion mutant noggin and 200 pg LacZ RNA. (Q–S) Embryos injected with 10 pg wild-type noggin and 200 pg LacZ RNA. (E, H, K, N, and Q) Dorsal view of stage 19. (F, I, L, O, and R) Dorsal view of stage 28. (G, J, M, P, and S) Embryos stained with 12/101 antibody. (G, J, and M) Lateral view. (G′, J′, P, and S) Dorsal view. Arrows show weak ectopic dorsal axis induction.
The recovery of these mutations in the offspring of adult animals raised from injected embryos demonstrates that ZFN-induced alleles of an endogenous gene can be transmitted to the next generation. Significantly, the parent and heterozygous tadpoles carrying mutant alleles were indistinguishable from wild-type siblings, indicating that this is an effective approach to establishing lines of animals carrying mutant alleles of investigator-specified genes. To test whether ZFN-induced mutagenesis caused off-target mutations, we made gynogenotes from noggin ZFN-injected females that lacked mutant noggin alleles in their germ line. These were siblings of the germ-line mutated males from clutches that showed a high frequency of somatic mutation. Gynogenesis diploidizes activated eggs by preventing the extrusion of the second polar body and serves to homozygose recessive mutations (25). Although off-target mutations would result in high frequencies of mutant embryos in gynogenotes produced from these females, we did not detect such mutations. Indeed, 87% of 192 gynogenotes were indistinguishable from wild-type tadpoles, and the remaining 13% had various mediolateral and dorsoventral defects, consistent with reported phenotypes and frequencies from young wild-type females (26). This result suggests that the potential for confounding off-target mutations in founder animals is negligible.
To test whether the ZFN-induced alleles result in loss of function, we cloned the full-length mutant alleles and synthesized corresponding mRNA for injection. Noggin induces ectopic dorsal tissue when expressed on the ventral side of Xenopus embryos (16), and this serves as an excellent test for noggin function. Amplified alleles were transcribed in vitro and 5 or 10 pg was injected into the ventral vegetal blastomeres of four-cell-stage X. laevis embryos (Fig. 4C). Embryos were cultured to stage 28 and scored for the presence or absence of an ectopic axis (Fig. 4D). The 4-bp insertion allele failed to induce any ectopic dorsal tissues in embryos (Fig. 4 H, I, J, and J′), consistent with a frameshift mutation. LacZ mRNA was used as a tracer and showed that injected cells populated the ventral posterior of injected tadpoles. The Δ12 allele induced ectopic axes in 8% and 16% of embryos when injected with 5 and 10 pg mutant noggin RNA, respectively. Interestingly, the induced axes in these embryos were underdeveloped, suggesting that this allele functions as a hypomorph (Fig. 4M, arrows). Finally, the 3-bp insertion allele was indistinguishable in activity from wild-type noggin (Fig. 4 N–P). These results demonstrate that specific loss-of-function mutant lines of X. tropicalis can be generated via targeted ZFN mutagenesis. Furthermore, the mutations in noggin form the basis of an allelic series that will be useful to probe noggin function in later development.
Discussion
Here we show that Xenopus can be added to the growing list of important model animals for which ZFN-encoding mRNA has allowed facile reverse genetics, including Drosophila (7), zebrafish (22, 27), and the rat (15, 28).
An important requirement for the use of ZFNs is a streamlined protocol to predict and implement effective gene disruption. We confirm that a proxy assay in budding yeast can identify ZFNs that function in the developing embryo. Furthermore, we have determined an expression vector architecture and dose of mRNA that enables high frequency gene disruption in both the soma and germ line of tadpoles and fertile adult animals. Remarkably, the injection of ZFN mRNA into a relatively small cohort of two-cell embryos was sufficient to raise adults carrying mutant alleles in the germ line. Our results demonstrate that, with optimal husbandry (26), homozygous mutants can be generated within 1 y of the initial mutagenesis and potentially within 7 mo if female founders are generated.
The Cys2-His2 zinc-finger protein (4, 5) is the most common DNA recognition motif in metazoa, and ZFNs can be engineered against any locus of interest (11). We show that ZFNs built using an archive of prevalidated two-finger modules targeting eGFP or noggin and carrying high-fidelity FokI endonuclease domains (8) induce mutations at a high rate when injected at doses that are well tolerated by the majority of injected embryos. Direct sequencing of both targets showed a variety of indels that are likely to result in null alleles. Indeed, we observed ZFN-induced loss of the eGFP fluorescence phenotype in injected tadpoles heterozygous for the eGFP transgene. The absence of a phenotype in founder noggin animals could be due to several factors, including the mosaic nature of the injected founders, non-autonomy of secreted Noggin, and a compensatory role of other BMP antagonists in the early embryo (29). However, artificial ventral expression of mRNAs encoding the ZFN-induced noggin mutants demonstrated that ZFNs induced both null and hypomorphic alleles of an endogenous X. tropicalis gene. The normal development of gynogenotes derived from females with high frequencies of somatic mutations in noggin (but no mutation in germ-line noggin) shows that off-target mutations in the germ line must be rare. Future analysis of homozygotes from noggin mutant lines will allow comparison of noggin function with that in the mouse.
Genome editing using ZFNs has the potential to enable numerous lines of experimentation that were previously impossible with existing Xenopus methodology. Permanent, heritable mutations will allow for the study of specific genes and later developmental processes without concern for the off-target or transient effects associated with morpholino oligonucleotides (3). Furthermore, the variety and size of ZFN-induced indels can be used to generate an allelic series of mutations. We note that several noggin alleles that we generated were deletions of substantial size (∼200 bp), indicating that ZFNs provide an attractive method not only for disrupting specific coding sequences, but also for targeting regulatory elements in the genome.
Due to the large size, abundance, and ready manipulation of their eggs and embryos, Xenopus has provided important insights in both cell biology (30) and embryology (31). Completion of the genome sequence of X. tropicalis brought this model system into the genomics age (2), but the genetic engineering tools essential for comprehensive study of biological mechanisms were lacking. Our work adds a robust method for genome editing that allows gene function to be analyzed across multiple developmental events.
Materials and Methods
Design and Screening of Zinc-Finger Nucleases.
Nucleases directed against the eGFP coding sequence were described previously (15). Nucleases against noggin were generated using an archive of prevalidated two-finger modules (32, 33), optimized as described (32), and used in their obligate-heterodimer form (8). The full amino acid sequence of the nucleases and their target recognition sequences are provided in SI Appendix. Nucleases were screened for activity in a budding-yeast proxy assay system as described (22).
Embryo Culture and Microinjection.
Female X. tropicalis were injected with 200 U human chorionic gonadotropin (HCG) (Chorulon), and males were injected with 100 U HCG. Collected embryos were dejellied in 3% cysteine. Embryos were injected into each blastomere at the two-cell stage with 2 nL maximum volume. Female X. laevis were injected with 500 U HCG, and eggs were fertilized with macerated testes and injected at the four-cell stage with 10 nL maximum volume. Diploid gynogenotes were generated according to previously described methods (25).
RNA Injection.
All ZFNs were cloned into CS108, a modification of the CS2 expression vector (23) (SI Appendix). Mutant noggin alleles were cloned using the sense primer 5′-GAATTCGGGCTCTGAACTTCCACTTG-3′ and the antisense primer 5′-GCGGCCGCTCAACATGAACATTTGCACTCA-3′. Amplicons were subcloned into CS-108 using EcoR1 and Not1. Synthetic mRNAs were transcribed using the mMessage mMachine kit (Ambion). Plasmids were linearized to produce transcripts with an SV40 polyadenylation signal (AscI) or without (NotI). ZFN RNAs were microinjected into both blastomeres of two-cell-stage X. tropicalis embryos along with 200 pg of mCherry RNA whose fluorescent product served to track successfully injected embryos and verify similar mRNA amounts injected. Embryos tend to orient with animal pole uppermost, so injection penetrated the animal hemisphere, but mRNA was deposited near the center of the blastomere. Broad inheritance of mRNA was confirmed by the distribution of RFP from injected mRNA. We also specifically tested inheritance of mRNA by prospective germ cells by injection of an RFP with a dead end 3′ UTR (34) and confirmed that fluorescence was effectively distributed to germ cells. Embryos were raised in 1/9 Marc's Ringer solution with gentamycin. Mutant noggin RNA was injected ventral-vegetally in four-cell embryos along with 200 pg LacZ RNA and cultured in 1/3 Marc's Ringer solution with gentamycin.
Cel-1 Assays.
Individual tadpoles were homogenized in lysis buffer with proteinase K and incubated at 55 °C overnight (13). After phenol/chloroform extraction and reprecipitation, a 450-bp fragment of the noggin locus was amplified using the sense primer 5′-GGGCTCTGAACTTCCACTTG-3′ and the antisense primer 5′-AGGGCTTCTGCCTAAGAAGG-3′. Primers were annealed at 60 °C and amplified using Accuprime polymerase (Invitrogen). Following PCR amplification, 9 μL from each 50-μL reaction (1/50 tadpoles equivalent) was denatured and reannealed by incubating at 94 °C for 5 min, followed by cooling at a rate of −2 °C/s to 85 °C then at a rate of −0.1 °C/s until the reaction reached 25 °C. One unit of Cel-1 (Transgenomics) was added to these annealed amplicons and incubated at 42 °C for 20 min. Digested products were resolved either by 10% polyacrylamide (Bio-rad) or 2% agarose gels and visualized after ethidium bromide staining.
Western Analysis.
Injected embryos were processed at stage 9 (13), and proteins were separated using 11% SDS/PAGE gels and blotted onto nitrocellulose membranes. An antibody recognizing the FLAG tag (Sigma F3165) diluted 1:1,000 and a HRP-conjugated donkey anti-mouse (Jackson Labs) diluted 1:5,000 were used to detect ZFN protein from injected embryo lysates.
Sequence Analysis.
Amplicons from targeted loci were cloned into pCR 2.1 TOPO T/A vectors (Invitrogen), and sequence was determined using either M13 forward or reverse primers.
Statistical Analysis:
Secondary axis induction assay means were compared using ANOVAs followed by pairwise Tukey post hoc comparisons.
Immunohistochemical Staining.
Embryos were fixed, dehydrated in methanol, and, after rehydration, bleached with hydrogen peroxide (13). Embryos were then blocked with 10% goat serum (Gibco) and incubated overnight in 1:1 dilution of 12/101 antibody (35) and 5% DMSO at 4 °C. Alexa 488 goat anti-mouse secondary (Molecular Probes) was used 1:250 with 5% DMSO. Embryos were cleared in benzyl benzoate/benzyl alcohol (2:1) and photographed with a Leica DFC 480 camera mounted on A Leica MZ FL II microscope. LacZ (tracer) staining was visualized as previously described (13).
Supplementary Material
Acknowledgments
We thank George Katibah for advice on Cel-1 assays, the Sangamo production group for technical support, Mustafa Khokha for the CS108 plasmid, and Paul Mead for the eGFP single-copy transgenic frog line. We also thank Andrea Wills and James Walker for additional microinjections. This work was supported by National Institutes of Health Grant HD054354.
Footnotes
*This Direct Submission article had a prearranged editor.
Conflict of interest statement: J.M.C., Y.D., I.A., F.M.F., A.H.L., C.N., D.Y.G., D.E.P., J.C.M., L.Z., E.J.R., P.D.G., F.D.U., and B.Z. are current or past employees of Sangamo Biosciences, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102030108/-/DCSupplemental.
References
- 1.Gurdon JB, Hopwood N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int J Dev Biol. 2000;44:43–50. [PubMed] [Google Scholar]
- 2.Hellsten U, et al. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisen JS, Smith JC. Controlling morpholino experiments: Don't stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- 4.Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavletich NP, Pabo CO. Zinc finger-DNA recognition: Crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 6.Bibikova M, et al. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 2001;21:289–297. doi: 10.1128/MCB.21.1.289-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JC, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 9.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll D. Progress and prospects: Zinc-finger nucleases as gene therapy agents. Gene Ther. 2008;15:1463–1468. doi: 10.1038/gt.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 12.Santiago Y, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 14.Hamlet MR, et al. Tol2 transposon-mediated transgenesis in Xenopus tropicalis. Genesis. 2006;44:438–445. doi: 10.1002/dvg.20234. [DOI] [PubMed] [Google Scholar]
- 15.Geurts AM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 17.Marcelino J, et al. Human disease-causing NOG missense mutations: Effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. Proc Natl Acad Sci USA. 2001;98:11353–11358. doi: 10.1073/pnas.201367598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 19.Bachiller D, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 20.Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- 21.McMahon JA, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 24.Groppe J, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 25.Khokha MK, et al. Rapid gynogenetic mapping of Xenopus tropicalis mutations to chromosomes. Dev Dyn. 2009;238:1398–46. doi: 10.1002/dvdy.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grammer TC, Khokha MK, Lane MA, Lam K, Harland RM. Identification of mutants in inbred Xenopus tropicalis. Mech Dev. 2005;122:263–272. doi: 10.1016/j.mod.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mashimo T, et al. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS ONE. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 31.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 32.Urnov FD, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 33.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koebernick K, Loeber J, Arthur PK, Tarbashevich K, Pieler T. Elr-type proteins protect Xenopus Dead end mRNA from miR-18-mediated clearance in the soma. Proc Natl Acad Sci USA. 2010;107:16148–16153. doi: 10.1073/pnas.1004401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kintner CR, Brockes JP. Monoclonal antibodies to the cells of a regenerating limb. J Embryol Exp Morphol. 1985;89:37–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.