Abstract
In October 2010, a virulent South Asian strain of El Tor cholera began to spread in Haiti. Interventions have included treatment of cases and improved sanitation. Use of cholera vaccines would likely have further reduced morbidity and mortality, but such vaccines are in short supply and little is known about effective vaccination strategies for epidemic cholera. We use a mathematical cholera transmission model to assess different vaccination strategies. With limited vaccine quantities, concentrating vaccine in high-risk areas is always most efficient. We show that targeting one million doses of vaccine to areas with high exposure to Vibrio cholerae, enough for two doses for 5% of the population, would reduce the number of cases by 11%. The same strategy with enough vaccine for 30% of the population with modest hygienic improvement could reduce cases by 55% and save 3,320 lives. For epidemic cholera, we recommend a large mobile stockpile of enough vaccine to cover 30% of a country's population to be reactively targeted to populations at high risk of exposure.
Keywords: infectious diseases, simulation modeling
After an absence of over 100 y, cholera has returned to Haiti (1, 2). By February 14, 2011, 234,303 cholera cases and 4,533 deaths were reported (3). Cholera is a waterborne disease that affects at least 3–5 million people annually, mostly in the developing world (4). The most recent example of Haiti shows that areas that have not seen cholera in decades can be vulnerable under the combination of poverty, lack of or destruction of infrastructure, weather, and natural disasters, conditions in which cholera thrives (4–8).
The cholera vaccine is safe, effective, and inexpensive but not widely used (9–11). Currently, two killed oral cholera vaccines could be made available. Dukoral is registered for use with the World Health Organization but is relatively expensive, whereas Shanchol (Shantha Biotechnics) is not yet registered but would be significantly cheaper and easier to administer than Dukoral (Crucell). It is believed that about one million doses of both vaccines together could be made available within the coming year. Most people would require two doses and small children would possibly require three doses to get optimal protection. So far, there has been reluctance to use the limited supplies of vaccine in Haiti because of the lack of a good strategy, logistical problems, and uncertainty about the size of the available supply (12). The benefits of cholera vaccination in emergency situations need to be weighed against that of other programs (11, 13–15). Through the use of a mathematical cholera transmission model (Fig. 1, Materials and Methods, and SI Appendix), we investigate various feasible vaccination strategies that could be effective in Haiti as well as other locations experiencing epidemic cholera.
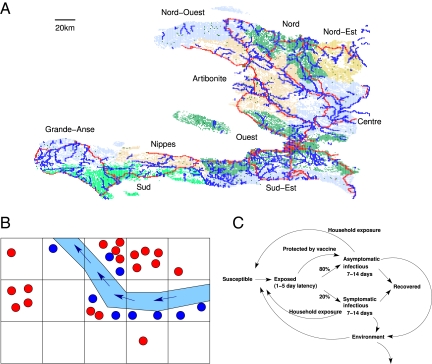
Fig. 1.
The cholera epidemic simulation model. (A) The population of Haiti in the simulation is divided into 1-km2 cells. The nine departments are indicated by different colors, and rivers and highways are superimposed in blue and red, respectively. Individuals may commute to nearby locations to work or occasionally travel longer distances. (B) Each 1-km2 cell is divided into communities (represented by dots) of ∼500 individuals. The river may be contaminated with V. cholerae, which can travel to downstream communities (indicated by the blue arrows). Only a limited number of communities in a cell can be in contact with the river, which is represented by the blue dots. (C) In the model's natural history of cholera, infected individuals shed V. cholerae into their communities, and susceptible individuals can be infected by this environmental source of V. cholerae. In addition, cholera is transmitted within households, which consist of 1–10 individuals. Infected residents living in a community on a river shed V. cholerae into both their community and the river.
Results
Cholera Epidemic in Haiti.
Simulated cholera epidemics begin with massive contamination of Vibrio cholerae on October 9, 2010, in the Artibonite River in the St. Marc, Petite Rivière d'Artibonite, Verrettes, and Mirebalais communes, where the first cholera cases were detected. Because the source and nature of the introduction of cholera to the region is not known, we did not model any events earlier than the first reported outbreaks along the Artibonite. We assume that these outbreaks were sparked by V. cholerae in the river at least 10 d before the first reported large outbreaks, but the cause of this contamination is beyond the scope of this study. In this baseline simulated scenario, 302,000 cumulative cases occur by the end of 6 mo (Table 1). We did not attempt to replicate the exact course of the epidemic, which was exacerbated by natural and political events, such as Hurricane Tomas, national elections, and possibly, uneven reporting rates that lie outside the scope of the model. However, the simulated departmental curves capture major features of the epidemic dynamics, including the initial sharp spike of cases in Artibonite in late October, the large wave of cases in Nord in November and Port-au-Prince in December, and the late arrival of the epidemic in the more remote departments of Grand-Anse and Nippes (Fig. 2 and Movie S1).
Table 1.
Number of simulated cholera cases in Haiti
| Strategy | Median cases | Attack rate (%) | Percentage of cases averted | Cases averted per 1,000 vaccinations | Deaths |
| Baseline | 302,000 | 3.3 | — | — | 6,040 |
| 5% prevaccination | 285,000 | 3.2 | 5.6 | 37.7 | 5,700 |
| 10% prevaccination | 265,000 | 2.9 | 12.2 | 40.7 | 5,310 |
| 30% prevaccination | 204,000 | 2.3 | 32.4 | 36.1 | 4,090 |
| 50% prevaccination | 154,000 | 1.7 | 48.9 | 32.7 | 3,090 |
| 70% prevaccination | 108,000 | 1.2 | 64.4 | 30.8 | 2,150 |
| 5% reactive mass vaccination | 290,000 | 3.2 | 3.9 | 26.2 | 5,810 |
| 10% reactive mass vaccination | 284,000 | 3.1 | 5.9 | 19.6 | 5,690 |
| 30% reactive mass vaccination | 236,000 | 2.6 | 22.0 | 24.5 | 4,710 |
| 50% reactive mass vaccination | 219,000 | 2.4 | 27.6 | 18.5 | 4,370 |
| +10% hygiene | 162,000 | 1.8 | 46.5 | 31.1 | 3,230 |
| +30% hygiene | 143,000 | 1.6 | 52.6 | 35.2 | 2,860 |
| 70% reactive mass vaccination | 205,000 | 2.3 | 32.1 | 15.3 | 4,100 |
| 5% reactive high-exposure vaccination | 270,000 | 3.0 | 10.5 | 70.5 | 5,410 |
| 10% reactive high-exposure vaccination | 244,000 | 2.7 | 19.2 | 64.1 | 4,880 |
| 30% reactive high-exposure vaccination | 168,000 | 1.9 | 44.5 | 49.6 | 3,350 |
| +10% hygiene | 136,000 | 1.5 | 54.9 | 61.2 | 2,720 |
| +30% hygiene | 127,000 | 1.4 | 57.9 | 64.5 | 2,550 |
| 50% reactive high-exposure vaccination | 163,000 | 1.8 | 46.0 | 30.8 | 3,260 |
| +10% hygiene | 125,000 | 1.4 | 58.6 | 39.2 | 2,500 |
| +30% hygiene | 113,000 | 1.3 | 62.5 | 41.8 | 2,260 |
| 70% reactive high-exposure vaccination | 161,000 | 1.8 | 46.6 | 22.3 | 3,220 |
The 5%, 10%, 30%, 50%, and 70% refer to having enough vaccine for that percentage of the general population. Fifty simulations were run per scenario, and the median number of cases (symptomatic individuals) is reported. We assume a 2% case fatality ratio. We also report the number and fraction of cases averted per 1,000 individuals vaccinated, assuming vaccinated individuals get both doses on time.
Fig. 2.
The timing of the cholera epidemic in Haiti in 2010–2011. (A) New daily hospitalizations by department (data from ref. 3). Port-au-Prince includes the communes of Carrefour, Cité Soleil, Delmas, Kenscoff, Petion Ville, Port-au-Prince, and Tabarre. Ouest does not include Port-au-Prince. (B) Simulated new cholera cases by department. The median numbers of cases in each department from 10 stochastic simulations are plotted using the same color scheme as in A.
We selected parameter values to be consistent with this broad pattern of epidemic spread. Significant departures from our parameter choices result in dynamics that do not match reported data from the epidemic. The timing of the regional epidemic, particularly in departments distant from Artibonite, was sensitive to parameters relevant to travel of individuals and the propagation of V. cholerae down rivers (SI Appendix, Figs. S10–S12). The magnitude of the epidemic peaks was sensitive to parameters relevant to transmission and the natural history of cholera (SI Appendix, Figs. S8, S9, S14, and S15) but less sensitive to within-household transmission of cholera (SI Appendix, Fig. S13).
Case for Vaccination.
We examined prevaccination strategies in which vaccination occurs well before the epidemic starts and reactive vaccination strategies in which vaccination begins after the epidemic has started. The results show that randomly prevaccinating a fraction of the population well before the epidemic begins can reduce the number of cases roughly in proportion to the number of individuals vaccinated and delay the epidemic peak (Fig. 3 A and B and Table 1). We measure the overall effectiveness of a vaccination strategy by the percentage of cases averted with respect to the baseline simulations in which vaccines were not used (16). To achieve 50% overall effectiveness, vaccine coverage of more than 50% of the population would be required.
Fig. 3.
Simulated effects of vaccination in Haiti. (A) The simulated total cumulative case incidence when 0%, 10%, 30%, 50%, and 70% of the population is prevaccinated. The median results from 50 simulations per scenario are plotted, and the vertical lines depict the minimum and maximum values. (B) Number of newly symptomatic individuals over time in simulations using different vaccination strategies. In reactive vaccination strategies, 50,000 people are vaccinated per day starting 21 d after the epidemic begins. In the mass vaccination campaigns, 70% of individuals in randomly selected 1-km2 cells are vaccinated each day. In the ring vaccination campaigns, cells are prioritized to be vaccinated 5 d after two residents of that cell become symptomatic. In the high-exposure campaigns, all cells along the rivers are prioritized to be vaccinated, regardless of the presence or absence of cholera. (C) The effectiveness of a simulated vaccination strategy is defined as the percentage of cases averted with respect to no vaccination. The points plot median estimates of the effectiveness of various strategies and amounts of vaccine using the bootstrap (50 simulation runs and 10,000 bootstrap trials), and the lines represent the 95% envelope from the bootstrap distribution. (D) The effect of adding a hygiene improvement component to reactive vaccination. Individuals in vaccinated regions may have 10% or 30% decreased exposure because of improved hygienic practices from outreach efforts.
Reactive Vaccination Strategies.
We present results for three different reactive vaccination strategies: reactive mass vaccination, reactive ring vaccination, and reactive high-exposure vaccination. By reactive, we mean that vaccine is not available until cases are detected in the country, but after that time, the vaccine could be deployed in regions where cholera cases have not yet appeared. For the reactive strategies, it takes 3 wk for a vaccine to confer maximum efficacy in an individual (SI Appendix, Fig. S1). For the preemptive strategy described above, all vaccinations occurred several weeks before the epidemic, and therefore, the vaccine was at maximum efficacy when the epidemic began. In all simulated reactive vaccination strategies, vaccine was not available until 21 d after the beginning of the epidemic, and 50,000 people were vaccinated per day after that point. In simulated reactive mass vaccination campaigns, randomly selected cells (1-km2 regions) were vaccinated such that 70% of the cell's residents were covered until available vaccine was depleted for the day. In a sensitivity analysis, we found that vaccinating a substantially smaller fraction of a cell's residents resulted in higher attack rates (SI Appendix, Fig. S6). For a given amount of vaccine, reactive mass vaccination was about one-half as effective as prevaccination (Fig. 3C and Table 1).
In simulated reactive ring vaccination campaigns, cells in which two or more cases appeared were prioritized for receiving vaccine after a 5-d delay. That is, local regions could receive vaccine only after cases appeared in them. Waiting for substantially more cases to appear or a longer delay after these cases appear seriously diminished the effectiveness of vaccination (SI Appendix, Fig. S7). Reactive ring vaccination was about as effective as reactive mass vaccination (Fig. 3C and Table 1).
High-Exposure Areas Should Be Vaccinated First.
In the 2010–2011 cholera epidemic in Haiti, the first cases of cholera occurred along the lower Artibonite River. In the model, individuals living on rivers have much higher exposure to cholera than those who do not. In addition, individuals who live along rivers in the model had the potential to infect many more individuals than those who do not live along rivers. A single simulated infectious individual would infect an average of 2.6 others in a fully susceptible population, and thus, our crude estimate of the basic reproductive number, R0, is 2.6 (SI Appendix, Fig. S5). By definition, R0 is the average number of secondary infections resulting from a typical infected person. The average is across the distribution of all of the transmission settings for the typical infected case. For example, an infected person living along a river would infect an average of 10.0 others, but one not living along a river would infect an average of 0.8 others. The published estimates of R0 for both epidemic and endemic cholera vary widely from 1.5 to 8.7 (7, 17), but our estimate of 2.6 falls within the published range.
We simulated the prioritization of vaccine to all regions along any major river in Haiti (Fig. 1A). After these regions with presumably high exposure to cholera were vaccinated, any remaining vaccine would go to the rest of the country. We refer to this vaccination strategy as reactive high-exposure vaccination. Although the simulated campaign does not start until 3 wk after the epidemic begins, prioritizing the high-risk regions along the rivers was even more effective than prevaccination if there was only enough vaccine to cover less than one-half of Haiti's population (Fig. 3C and Table 1).
A useful and necessary adjunct to a vaccination campaign is the promotion of improved hygiene and sanitation. We model the effect of a campaign to improve sanitation and promote better hygiene as a 10% or 30% reduction in exposure to cholera for all individuals in the regions where vaccination has taken place. A campaign that results in even a 10% reduction in exposure could result in a large reduction in cases (Fig. 3D, Table 1, and Movie S1).
From Fig. 3 and Table 1, the most efficient reactive vaccination strategy is to prioritize high-exposure regions for vaccine. It is even more effective when combined with an improvement in hygiene. If there is only enough vaccine to cover 50% of the entire population, then this strategy is more effective than a prevaccination strategy that does not target high-exposure regions. If enough vaccine is available for over 50% of the population, prevaccination is somewhat more effective. In addition, reactive high-exposure vaccination tends to be the most effective use of vaccine by providing the most cases averted per 1,000 people vaccinated. There are no situations where ring vaccination is effective.
Discussion
When vaccine is in limited supply, we have found, for epidemic cholera, that concentrating it to achieve coverage of 50–70% of the population in the high-exposure areas is the most effective intervention. In Haiti, this corresponds to enough vaccine for ∼30% of the general population. In addition, effort should be made to improve hygiene and reduce exposure to V. cholerae in combination with the vaccination efforts.
Our simulations suggest that it is more effective to first vaccinate areas at high risk of cholera, such as along rivers and other bodies of fresh water, than to wait for cases to appear in these areas. When cases appear in a region and local transmission is occurring, it may be too late to vaccinate for that season. Although it is difficult to know a priori the regions in an immunologically naïve population that are at highest risk, we may make informed decisions based on known risk factors from regions where cholera is endemic (18). For example, increases in environmental risk factors, such as the concentration of copepods and conductivity in local water bodies, proceed cholera outbreaks in rural Bangladesh by several weeks (19), and remote sensing technologies may help rapidly assess other risk factors (20). If similar risk factors could be found for epidemic cholera, then vaccine could be concentrated in those areas first. In Haiti, about 25% of the population lives along major rivers, a clear risk factor for epidemic cholera in Haiti. Because of the high transmissibility of epidemic cholera in our model of Haiti (SI Appendix, Fig. S10), there is little herd immunity induced by vaccination at the coverage levels that we consider. We would see larger herd effects for these coverage levels for endemic cholera where there is considerable population level immunity and much lower reproductive numbers (21, 22).
Sensitivity analyses of cholera models may help identify the most important gaps in our understanding of the disease. For example, our model is sensitive to the pathogenicity of the cholera strain, and therefore, estimating pathogenicity is important for accurate prediction of the epidemic size and the effect of interventions. Indeed, the pathogenicity of cholera may be significantly higher than the value that we used in our model (23). Conversely, if a model is insensitive to a parameter, this may identify a quantity that is less important to estimate or an intervention that is less likely to be effective. For example, if the model results indicate that the epidemic is not sensitive to frequency of travel, then travel restrictions might not be effective.
Because of the pervasive aquatic environment in Haiti coupled with poor sanitation, cholera will almost surely become endemic. Potentially facing annual epidemics, it may be necessary to revaccinate at-risk populations every 2 y given that the duration of vaccine protection is about 2.5 y (24). It may also be necessary to sustain educational campaigns to improve hygiene and sanitation to ensure continued compliance (25). As cholera becomes endemic in Haiti, large portions of the population may become partially immune because of natural infection, particularly adults, thus making vaccination of children a possible priority. The interplay of the aquatic environment and potential person to person transmission need to better understood and modeled to formulate more effective control strategies.
We believe there should a comprehensive global plan for the use of cholera vaccine for epidemic cholera (4, 6). Based on our results, we recommend that a large international mobile stockpile of oral cholera vaccine be created. This stockpile should be large enough to vaccinate reactively 50–70% of the population at risk for high exposure in the affected country. Such an intervention could cut epidemic cholera morbidity and mortality by roughly 50%. This would require the availability of the stockpile and knowledge of the important risk factors for transmission for each affected setting. In addition, emergency vaccination efforts could be coordinated with equally important activities such as training of personnel in the use of oral rehydration and stockpiling of oral rehydration salts packets.
Maintenance of a cholera stockpile for emergency use could be coordinated with production of cholera vaccine for general use in endemic areas (22). The two current killed oral cholera vaccines, Dukoral and Shanchol, have shelf lives of 3 and 2 y, respectively (11). If continuous production of these vaccines could be achieved, then the stockpiled vaccine that is not used within 2 y could be cycled out for routine vaccination in endemic areas. Because both vaccines can be made relatively cheaply, current cost of about $5 US for Dukoral and $1.50 US for Shanchol (11), an international investment case could be made to support production and distribution of these vaccines (4, 6). Vaccine distribution could be coordinated through global and regional public health organizations such as the World Health Organization and Pan American Health Organization. For endemic cholera, the routine biannual vaccination of 50–70% of at-risk populations would virtually eliminate cholera transmission from those regions (22). The rapid and repeated use of cholera vaccine could greatly reduce the burden of this disease in the developing world.
Materials and Methods
The form of the mathematical model is an individual-based stochastic model. A detailed description of the model is in SI Appendix. The model uses a synthetic population to represent the 9.5 million people of Haiti. The model incorporates population density data at a 1 km2 resolution and the locations of major rivers and highways (Fig. 1A). Susceptible people in the model can become infected by contact with V. cholerae in their local environment (Fig. 1B) or their households (Fig. 1C). After infected, people undergo a 1- to 5-d latent period, after which they become infectious (22). Twenty percent of infectious people are symptomatic and shed 10 times more V. cholerae into their local environments than asymptomatic individuals. We include a hyperinfectious state of freshly shed cholera that causes a burst of community-wide transmission (26). If an infectious individual lives or works near a river, that individual will also shed V. cholerae into the river, which transports V. cholerae downstream (Fig. 1B). People are placed into communities of ∼500, and these communities are spatially organized according to LandScan data, which estimates the population density at a 30° × 30° resolution (∼1 km2; http://www.ornl.gov/sci/landscan/, accessed November 11, 2010). Some people travel daily to nearby communities, where they can be exposed to or shed V. cholerae. People can also occasionally make long-distance trips within the country, with a higher probability if travel is along major highways.
In the simulation, people may be vaccinated, and it is assumed that vaccine reaches maximum efficacy after 3 wk (SI Appendix, Fig. S1); at this point, vaccinated infected people are 64% less likely to become symptomatic, VEP = 0.64 (27), and 50% less infectious than infected unvaccinated people, VEI = 0.50 (22). Vaccine provides some but less protection before 3 wk postvaccination. It is possible that vaccine also reduces the probability of infection given exposure to an infected source, VES. However, there are no estimates of this vaccine effect, because clinical disease with confirmed infection was the primary outcome from cholera vaccine trials. SI Appendix has further discussion of this point. The vaccination campaign may be accompanied by a hygiene awareness campaign, which lowers exposure to V. cholerae from the environment and the household by 10% or 30%.
Supplementary Material
Acknowledgments
We thank Jon Sugimoto for geographic information system data assistance and both Robyn Fisher and Lara Petusky Coger for their insights about Haiti. This work was partially supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study Grant U01-GM070749 and the National Institutes of Health Grant R01-A139129.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102149108/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention (CDC) Cholera outbreak—Haiti, October 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1411. [PubMed] [Google Scholar]
- 2.Chin CS, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministère de la Santé Publique et de la Population (MSPP) Rapport journalier MSPP du 14 février 2011. 2011. Available at http://www.mspp.gouv.ht/site/downloads/Rapport%20journalier%20MSPP%20du%2014%20fevrier%202011.pdf. Accessed February 21, 2011. [Google Scholar]
- 4.Waldor MK, Hotez PJ, Clemens JD. A national cholera vaccine stockpile—a new humanitarian and diplomatic resource. N Engl J Med. 2010;363:2279–2282. doi: 10.1056/NEJMp1012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair GB, et al. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol. 2006;44:4211–4213. doi: 10.1128/JCM.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S, et al. Public health. The cholera crisis in Africa. Science. 2009;324:885. doi: 10.1126/science.1173890. [DOI] [PubMed] [Google Scholar]
- 7.Siddique AK, et al. El Tor cholera with severe disease: A new threat to Asia and beyond. Epidemiol Infect. 2010;138:347–352. doi: 10.1017/S0950268809990550. [DOI] [PubMed] [Google Scholar]
- 8.Ivers LC, Farmer P, Almazor CP, Léandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 9.Clemens JD, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 10.Lopez AL, Clemens JD, Deen J, Jodar L. Cholera vaccines for the developing world. Hum Vaccin. 2008;4:165–169. doi: 10.4161/hv.4.2.5122. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Oral Cholera Vaccines in Mass Immunization Campaigns: Guidance for Planning and Use. Geneva: World Health Organization; 2010. [Google Scholar]
- 12.Cyranoski D. Cholera vaccine plan splits experts. Nature. 2011;469:273–274. doi: 10.1038/469273a. [DOI] [PubMed] [Google Scholar]
- 13.Legros D, et al. Mass vaccination with a two-dose oral cholera vaccine in a refugee camp. Bull World Health Organ. 1999;77:837–842. [PMC free article] [PubMed] [Google Scholar]
- 14.Jeuland M, et al. Cost-effectiveness of new-generation oral cholera vaccines: A multisite analysis. Value Health. 2009;12:899–908. doi: 10.1111/j.1524-4733.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaignat CL, Monti V. Use of oral cholera vaccine in complex emergencies: What next? Summary report of an expert meeting and recommendations of WHO. J Health Popul Nutr. 2007;25:244–261. [PMC free article] [PubMed] [Google Scholar]
- 16.Halloran ME, Longini IM, Jr, Struchiner CJ. Design and Analysis of Vaccine Studies. New York: Springer; 2010. [Google Scholar]
- 17.King AA, Ionides EL, Pascual M, Bouma MJ. Inapparent infections and cholera dynamics. Nature. 2008;454:877–880. doi: 10.1038/nature07084. [DOI] [PubMed] [Google Scholar]
- 18.Griffith DC, Kelly-Hope LA, Miller MA. Review of reported cholera outbreaks worldwide, 1995–2005. Am J Trop Med Hyg. 2006;75:973–977. [PubMed] [Google Scholar]
- 19.Huq A, et al. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantin de Magny G, et al. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci USA. 2008;105:17676–17681. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali M, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: A reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 22.Longini IM, Jr, et al. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris JB, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durham LK, et al. Estimation of vaccine efficacy in the presence of waning: application to cholera vaccines. Am J Epidemiol. 1998;147:948–959. doi: 10.1093/oxfordjournals.aje.a009385. [DOI] [PubMed] [Google Scholar]
- 25.Luby SP, Mendoza C, Keswick BH, Chiller TM, Hoekstra RM. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. Am J Trop Med Hyg. 2008;78:382–387. [PubMed] [Google Scholar]
- 26.Hartley DM, Morris JG, Jr, Smith DL. Hyperinfectivity: A critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3:e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black RE, et al. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987;55:1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.