Confocal microscopy can visualize cancer cell metastasis in the vicinity of a primary tumor site in animal models of cancer in real time. However, spatial resolution of current microscopes in vivo remains limited and subcellular structures cannot be readily imaged, reducing the molecular mechanistic insights that can be directly obtained from in vivo models of cancer metastasis. Moreover, therapeutic approaches that are successful in rodent models do not always translate into actual therapeutic approaches to human cancers. Hence, in vitro models remain useful not only to further our basic understanding of cancer cell biology and the metastatic cascade but also for clinical applications, such as high-throughput drug screening and testing. In particular, in vitro structures that acknowledge the three-dimensionality of the stromal space during invasions may have a significant impact on oncology in the near future (1).
In PNAS, Liu et al. (2) present a microfabricated construct to study in vitro cell invasion, a critical step in cancer metastasis, which is the primary cause of cancer-related death. This construct consists of vertical 320-μm-high and 100-μm-wide square microposts (Fig. 1), which the authors (2) call Tepuis in reference to Arthur Conan Doyle's novel The Lost World. High densities of cancer cells of different metastatic potential are deposited at the foot of the Tepuis and allowed to climb along the Tepuis until they reach their tops. Both the rate at which the Tepuis tops are reached and the fractions of Tepuis tops that are occupied are monitored (2).
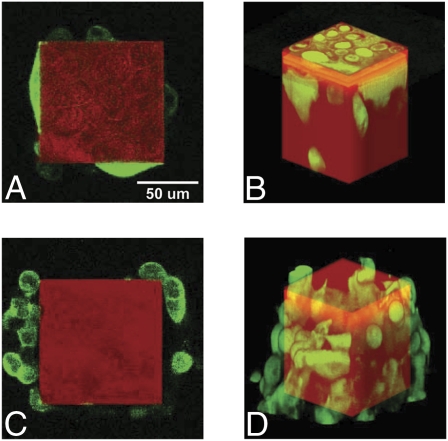
Fig. 1.
Confocal micrographs of GFP-tagged highly invasive PC-3 cells (A and B) and less-invasive LNCaP cells (C and D) climbing on the lateral surfaces of Tepuis. (Reprinted from ref. 2.)
Two cell lines are tested with this in vitro invasion assay: the well-characterized human prostate cancer cells (PC-3) that metastasize to the bone, and human lymph node carcinoma of the prostate cancer cells (LNACaP), which are phenotypically less invasive than PC-3. As expected, LNCaP cells show a lower invasion rate (i.e., longer time to reach the top of the Tepuis) than PC-3 cells (2). Less expected is the observed lower invasion efficiency of LNCaP cells compared with PC-3 cells (2) (i.e., a lower percentage of Tepui tops are occupied by LNCaP cells at steady state); 15% Tepui tops remain unoccupied by less-invasive LNCaP cells. Although small, this fraction is found to be highly reproducible. In particular, the fraction of unoccupied Tepuis remains unchanged at long times and therefore, is independent of the different proliferation rates of LNCaP and PC-3 cells. Interestingly, each Tepui top is either devoid or occupied by LNCaP cells, whereas PC-3 cells occupy all Tepui tops. These observations are consistent with contact inhibition (2).
Further demonstration of the importance of contact inhibition may come from molecular biological studies. Specifically, PC-3 cells do not express cell-surface adhesion molecule E-cadherin, which plays a critical role in contact inhibition and cancer transformation, whereas LNCaP cells express E-cadherin (3). Therefore, RNAi-based depletion of E-cadherin in LNCaP cells and, vice versa, forced expression of E-cadherin in PC-3 cells may lead to inversed phenotypes (i.e., E-cadherin–depleted LNCaP cells would display high invasion efficiency, whereas E-cadherin expressing PC-3 cells would show reduced invasion efficiency).
Are there alternative and/or complementary explanations for the observed lower occupancy of Tepui tops, and in particular, is this phenotype intrinsic to the two cell lines or because of the construct or both? Cell migration seems likely to play a minor role, if any, in setting occupancy rates of Tepui tops. However, lateral and top surfaces of the Tepuis are separated by a corner, which would not occur in 2D. This corner may serve as a filter, completely permissive to the invasion of PC-3 cells to the Tepui tops but less permissive to LNCaP cells. An untested alternative explanation may involve changes in the levels of expression and/or activation (i.e., phosphorylation) of proteins after an LNCaP cell accidentally makes it to the top of a Tepui. In particular, proteins that mediate cell–cell adhesion and associated shape changes from a mesenchymal shape configured for high motility back to a nonmotile epithelial phenotype (4) may play a critical role in controlling occupancy of Tepui tops, including α-catenin and E-cadherin (5, 6).
Tepuis constitute permanent wounds in the cell monolayer at the bottom of the dish. Indeed, cancer has been described as a wound that does not heal. Therefore, it would be interesting to determine whether traditional wound-healing assays, where a cell monolayer is wounded and rates of closure of the newly created empty space are recorded, lead to the same differential invasion efficiency as the Tepuis assay. It could also be informative to make use of microlithography to generate a flattened, 2D version of the 3D Tepuis by Liu et al. (2). This 2D version of the assay by Liu et al. (2) would determine whether the 3D geometric aspects of the assay actually influence occupancy of the Tepui tops. Cells would be grown on a large adhesive pattern connected to a 100-μm-wide × 320-μm-long rectangular pattern (corresponding to the sides of the Tepuis) and terminated by a 100- × 100-μm destination square (corresponding to the top of the Tepuis). Hence, this 2D version would preserve aspect ratios of the 3D Tepius by Liu et al. (2). However, unlike the 3D Tepuis, there would not be a discontinuity between the rectangular pattern and the destination square. If contact inhibition alone were the main mechanism underlying the binary occupation, then this planar assay would lead to binary occupancy of the destination squares.
What physiopathological aspects of the metastatic cascade are mimicked in the assay by Liu et al. (2)? Transformed cells of epithelial origin leave a primary tumor site by first crossing the basement membrane and then invading the underlying stromal, collagen I-rich dense meshwork containing cancer-associated fibroblasts and immune cells. Cancer cells invade the stroma and metastasize to blood vessels as singlets or undergo collective invasion to lymphatic vessels as small multiple-cell islets (7). This stromal invasion is followed by extravasation into and intravasation from the blood vessels and subsequent invasion of neighboring tissues. Invasive cells then reestablish distal colonies, which often enter a long dormancy period before recurrence. Clearly, the assay by Liu et al. (2) does not preserve many of these features. However, the distance that separates the foot from the top of the Tepuis is similar to the average distance between blood vessels in a tumor (8). Moreover, similar to the migration along the lateral surface of the Tepuis, intravital microscopy in mouse models suggests that the few cells that successfully leave the primary tumor site undergo rapid and persistent migration (9, 10). Finally, Tepui tops may resemble the bone, a preferential secondary site of metastasis for prostate cancer cells.
However, the width of the Tepuis and distance between Tepuis are orders of magnitude larger than the pore size of the ECM that surrounds and interpenetrates the primary tumor and hence, do not mimic per se the tumor-associated ECM. An important consequence of the geometric restriction caused by the tight pores of the cross-linked collagen matrix is illustrated by the critical role of cell surface- bound membrane type 1 matrix metalloprotease (MT1-MMP), whose substrate is collagen I. Biochemical blockade or RNAi-mediated depletion of MT1-MMP drastically inhibits migration of tumor cells that are fully embedded inside a highly tumor-associated cross-linked 3D collagen matrix (11, 12), whereas it plays no significant role in 2D motility and uncross-linked collagen I matrices (13).
Liu et al. present a microfabricated construct to study in vitro cell invasion, a critical step in cancer metastasis.
Based on micrographs in the article by Liu et al. (2) (Fig. 1), it appears that the spreading area of cells on the lateral surfaces of Tepuis is smaller than the width of the Tepuis (100 μm). In contrast, cancer cells in the stromal matrix are not in continuous contact with the ECM; rather, they form discrete adhesive contacts with ECM fibers that are much smaller than the size of cells. As a result, focal adhesions that contain clustered integrins and are believed to mediate cell migration on 2D surfaces are drastically reduced in size and number in 3D matrix (14). Micrographs of PC-3 cells migrating along the Tepuis (Fig. 1) suggest that these cells display the shape of vertically polarized cells commonly observed on culture dishes but not observed inside matrices (14), which suggests single-cell mesenchymal migration. In contrast, the shape of LNCaP cells does not conform to the surface of the Tepuis; rather, they are sometimes partially detached and also, form aggregates, which suggest collective migration.
Like all versatile in vitro assays, Tepuis by Liu et al. (2) open the possibility to test several important aspects of cancer metastasis. Future questions arise from the work by Liu et al. (2). (i) Do physical and topological properties of Tepuis influence the invasion rate and efficiency of Tepui tops, which could be addressed by manipulating the dimensions of the Tepuis, manipulating the cross-linkers density in gelatin to assess effects of substrate compliance, and switching to cylindrical posts (which better mimic ECM fibers) to assess effects of local curvature? (ii) Does differential occupancy of Tepuis occur in other types of prostate cancer cell lines and other types of cancer? (iii) Does the phenotypic difference between LNCaP cells that can and cannot completely occupy Tepui tops lead to genetic transformations (evolution) using serial passages through the Tepuis sieves? (iv) Does cell heterogeneity influence invasion rates and efficiency, which could be tested by using a mixture of cancer cells as opposed to pure cultures?
Tissue engineering has already had a tremendous impact on regenerative medicine and its clinical applications in personalized medicine (15). Tissue-engineering approaches, exemplified by the construct by Liu et al. (2), may have an equally significant impact on personalized cancer medicine (1).
Acknowledgments
Research in the authors’ laboratory is supported by Grants U54 CA143868 and R01 GM084204 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6853.
References
- 1.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, et al. Probing the invasivness of prostate cancer cells in a 3D microfabricated landscape. Proc Natl Acad Sci USA. 2011;108:6853–6856. doi: 10.1073/pnas.1102808108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran NL, Nagle RB, Cress AE, Heimark RL. N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am J Pathol. 1999;155:787–798. doi: 10.1016/S0002-9440(10)65177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Bajpai S, et al. α-Catenin mediates initial E-cadherin-dependent cell-cell recognition and subsequent bond strengthening. Proc Natl Acad Sci USA. 2008;105:18331–18336. doi: 10.1073/pnas.0806783105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajpai S, Feng Y, Krishnamurthy R, Longmore GD, Wirtz D. Loss of alpha-catenin decreases the strength of single E-cadherin bonds between human cancer cells. J Biol Chem. 2009;284:18252–18259. doi: 10.1074/jbc.M109.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giampieri S, et al. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatau SB, et al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci USA. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom RJ, George JP, Celedon A, Sun SX, Wirtz D. Mapping local matrix remodeling induced by a migrating tumor cell using three-dimensional multiple-particle tracking. Biophys J. 2008;95:4077–4088. doi: 10.1529/biophysj.108.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotary KB, et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 13.Wolf K, et al. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraley SI, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol. 2010;12:598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]