Abstract
Background
Observational studies suggest there are differences in adherence to antihypertensive medications in different classes. Our objective was to quantify the association between antihypertensive drug class and adherence in clinical settings.
Methods and Results
Studies were identified through a systematic search of English-language articles published from inception of computerized databases till February 1, 2009. Studies were included if they measured adherence to antihypertensives using medication refill data and contained sufficient data to calculate a measure of relative risk of adherence and its variance. An inverse-variance weighted random-effects model was used to pool results. Hazard ratios (HR) and odds ratios (OR) were pooled separately, and HRs were selected as the primary outcome. Seventeen studies met inclusion criteria. The pooled mean adherence by drug class ranged from 28% for beta-blockers to 65% for angiotensin II-receptor blockers (ARBs).There was better adherence to ARBs compared to angiotensin-converting enzyme inhibitors (ACEIs) (HR 1.33, 95%CI 1.13–1.57), calcium channel blockers (HR 1.57, 95% CI 1.38–1.79), diuretics (HR 1.95, 95%CI 1.73–2.20), and beta-blockers (HR 2.09, 95%CI 1.14–3.85). Conversely, there was lower adherence to diuretics compared to the other drug classes. The same pattern was present when pooling studies that used ORs. When accounting for publication bias, there were no longer significant differences in adherence between ARBs and ACEIs or between diuretics and beta-blockers.
Conclusion
In clinical settings, there are important differences in adherence to antihypertensives in separate classes with lowest adherence to diuretics and beta-blockers and highest to ARBs and ACEIs. Yet, adherence was suboptimal regardless of drug class.
Keywords: hypertension, medication adherence, meta-analysis
Introduction
Hypertension is the most common chronic illness in developed countries and one of the most important risk factors for cardiovascular disease.1 Numerous studies have shown that blood pressure lowering is associated with major reductions in coronary events, strokes, and mortality.2, 3 While counseling about lifestyle factors plays a role, prescribing antihypertensive medications remains the cornerstone of the medical management of hypertension.4
Clinical practice guidelines, such as the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of Hypertension (JNC7)5, have been developed to assist clinicians with their selection of antihypertensive medications. These guidelines rely heavily on data from clinical trials to inform their preferences for antihypertensive medications. Yet, as a result of selection bias, run-in periods, and behavior reinforcement through close follow-up, adherence to medication in clinical trial settings may not be representative of adherence in “real world” settings.6 For example, in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), the level of adherence to treatment at 1 year ranged from 83% to 88% depending on drug class.7 In observational studies, adherence to antihypertensive medications is typically much lower.8 Accordingly, clinical trials such as ALLHAT may underestimate the impact of differences in adherence to medications from separate drug classes in clinical settings.
The literature describing the association between antihypertensive drug class and adherence is characterized by a wide variety of patient populations, drug class comparisons, and definitions of adherence. This heterogeneity has made it difficult to draw conclusions about the clinical relevance of differences in adherence according to drug class. Quantifying the overall impact of drug class on adherence in observational settings would provide important evidence to guide the selection of blood pressure medications as well as to guide adherence monitoring. Accordingly, we performed a meta-analysis to determine the impact of antihypertensive drug class on adherence to blood pressure medications.
Methods
Data Sources and Searching
This study was performed as part of a larger systematic review of predictors of adherence to oral cardiovascular and diabetic medications. The research methodology was done in accordance with MOOSE guidelines.9 With the assistance of a medical librarian (L.F.), potentially relevant articles were identified by searching publicly available computerized databases. The search included all articles and abstracts (including unpublished doctoral theses) referenced from database inception to February 1, 2009 in MEDLINE, Database of Abstracts of Reviews of Effects, National Health Service Economic and Evaluation Database, Health Technology Assessment Database, EMBASE, and PsycINFO. All relevant subject heading and free text terms for adherence or compliance and for both the generic class of drug [e.g., beta-blocker (BB), angiotensin-converting enzyme inhibitor (ACEI)] and the individual names of antihypertensive medications, generic and proprietary, were included (Appendix 1). Due to the large volume of literature on this topic, a set of methodological filters was applied to the search strategies of the larger databases (MEDLINE, EMBASE and PsycINFO). Follow-up searches were performed by hand-searching bibliographies from selected articles.
Study Selection
Articles were eligible for inclusion if they were published in English and include community dwelling patients 18 years of age and older. Eligible study designs included observational cohorts in which adherence to antihypertensive medication was evaluated as an outcome. To reduce heterogeneity when pooling studies, studies were only eligible if they measured adherence using medication refill data. Articles also had to include data that compared adherence between at least two distinct antihypertensive drug classes, and had to report sufficient data to calculate a measure of relative risk of adherence and its variance.
A number of sociodemographic characteristics, medical and psychological conditions, and regimen complexity qualities have been shown to influence adherence.10, 11 Accordingly, studies were excluded if they did not consider at least one potentially confounding characteristic in their analyses of the association between drug class and adherence. Studies in which adherence was measured by determining whether patients were using a medication at a single time-point were also excluded as this was not equivalent to studying adherence with a regimen over time. For the remaining studies, we assigned a quality rating using a checklist adapted from the recommendations of the International Society of Pharmacoeconomics and Outcomes Research (ISPOR)(Appendix 2).12, 13
Two investigators (D.M., I.K.) independently reviewed all citations identified through the literature search using a predefined protocol. Articles that clearly did not meet inclusion criteria were excluded at the title and abstract level. The remaining articles were selected for full text review. When limited information was available from the abstract, full text was always obtained. Included articles underwent a quality assessment by two investigators (Z.S., I.K.). Disagreements regarding the selection and quality assessment of articles were resolved through discussion and full consensus was achieved at each stage of review.
Data Extraction
Two investigators (Z.S., I.K.) independently extracted data from selected studies using a standardized form. Information was collected regarding dates and sizes of the studies; types of patients enrolled; duration of follow-up; types of drug classes assessed; whether patients were concurrently taking antihypertensive medications from other drug classes; the proportion initiating ARBs; and whether the study had any pharmaceutical industry affiliation. Pharmaceutical affiliation was ascribed if the study received funding from a pharmaceutical company or if a study author was employed or served as a consultant for the industry. Adherence data pertaining to combination antihypertensive pills were not extracted.
Investigators also recorded the method used to define adherence; the mean adherence according to drug class; the measure of the relative risk of adherence between pairs of drug classes; and the types of covariates included in adjusted analyses. In accordance with ISPOR guidelines14, we defined adherence as an umbrella term that encompasses two related categories of pill-taking behavior: compliance and persistence. Adherence was categorized as compliance if it measured the proportion of days covered (PDC) with medication, calculated as the sum of the days’ supply for all prescriptions filled during the study time period divided by the total number of days in this time period. Individuals were then defined as compliant or non-compliant using a threshold of 80% for PDC. Adherence was categorized as persistence if it referred to either 1) a continuous measure of the number of days on a given antihypertensive from initiation of therapy to the end of the last supplied prescription in the study period before a significant gap in coverage with the medication or 2) a dichotomous variable in which patients were categorized as persistent or non-persistent depending on whether they had any significant gaps in coverage during the study period. Persistence studies were sub-categorized according to whether they defined persistence as medication persistence (time to discontinuation of a given medication) or therapy persistence (time to discontinuation of all antihypertensive medication).13 Dichotomous measures of adherence were used to calculate odds ratios (ORs) for adherence between two drug classes using logistic regression. Continuous measures of adherence were used to calculate hazard ratios (HRs) using Cox proportional hazards regression.
Data Synthesis and Analysis
Two pairs of studies15–18 included overlapping data, and hence, two studies were excluded from quantitative analysis.15, 17 The remaining studies were grouped for pooling according to comparisons of adherence between pairs of drug classes. Data were then subgrouped according to whether the measure of relative risk was an OR or HR. The pooled HR of adherence was selected as the primary outcome because 1) this was the most frequently used measure of adherence in the pooled studies and 2) the HR accounts for censoring and is thus the preferred measure of relative risk with prospective data. ARBs and diuretics were selected as the primary basis for comparison as they were the two drug classes with the most frequent pair-wise comparisons across studies. Individual estimates of log relative risks were pooled using random effects meta-analysis with inverse variance weighting in Stata, version 10 (College Station, TX). The i-squared statistic was used to estimate the percentage of variability across studies that is attributable to heterogeneity and was tested for deviation from zero. To test for sources of heterogeneity for our primary outcome, we performed sensitivity analyses that compared pooled HRs separately for articles in which adherence was defined as medication persistence and therapy persistence. Similarly, we performed sensitivity analyses by stratifying articles according to study size, study quality, country, and pharmaceutical affiliation. We used chi-square to test for significance in these analyses and log transformed HRs to approximate normality. To account for publication bias, we used the non-parametric “trim-and-fill” model.19 We used this model when there were at least 3 studies available for pooling.
As a secondary means of comparing adherence between drug classes, we pooled the mean percent adherence by individual drug class across studies that measured adherence as persistence. We weighted our calculations according to person-months of exposure.
Results
Qualitative analysis (n=17)
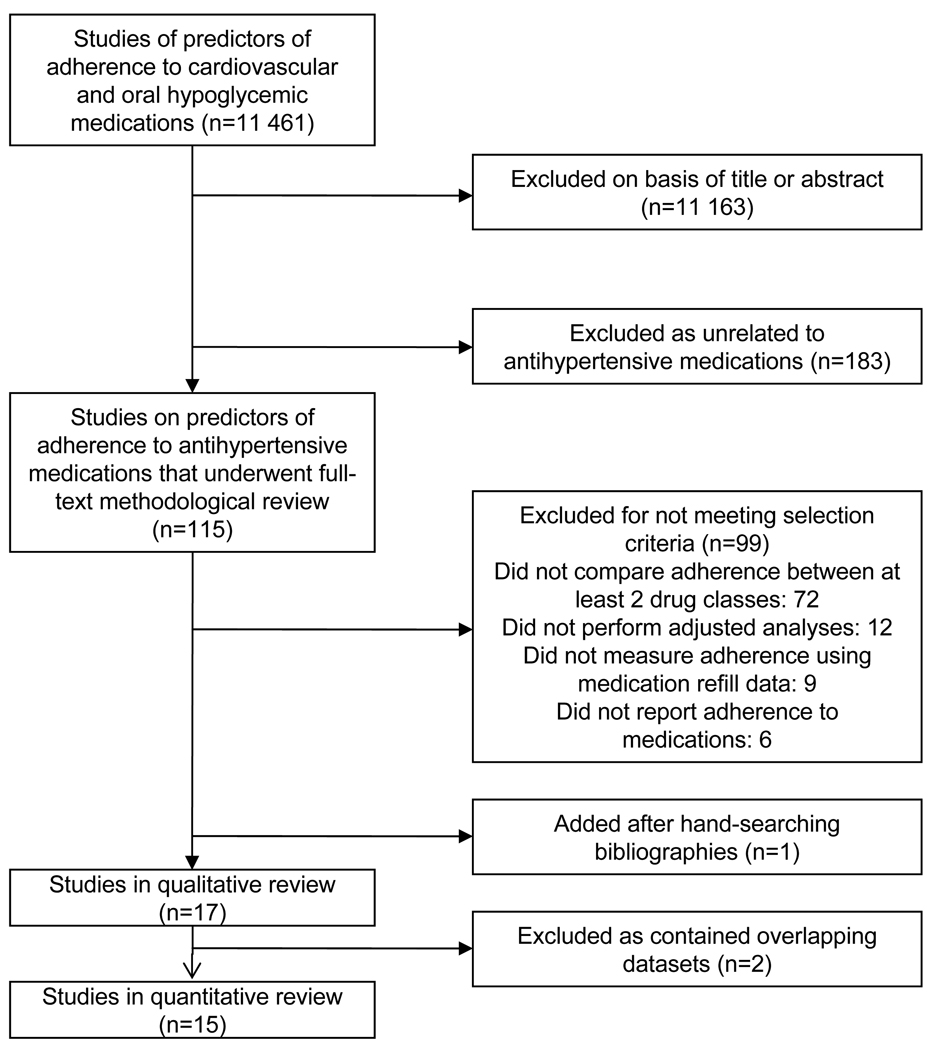
The comprehensive search yielded 115 unique articles related to predictors of adherence to antihypertensive medications.(Figure 1) Seventeen articles met inclusion criteria.15–18, 20–32(Table 1) Five studies were rated ‘excellent’ and satisfied all of the requirements on the quality checklist; twelve studies were rated ‘good’ and missed one or two items on the checklist. Included articles assessed adherence to antihypertensive medications between 1989 and 2004 for 935 920 patients. The studies measured adherence using medication refill data either from insurance claims or pharmacy refills in closed pharmacy systems in North American and Europe. Thirteen articles defined adherence as persistence (5 medication persistence; 8 therapy persistence) and four articles defined adherence as compliance. ARBs were the least likely drug class to be prescribed with rates from 2 – 23%.
Figure 1.
Literature search and selection
Table 1.
Summary of Included Studies of Association of Antihypertensive Drug Class and Adherence
| No | Reference | Country | Drug company affiliation |
Years enrolled |
Number of patients |
Dug classes evaluated |
Population | Percent initiating ARB |
Period observed |
Adherence definition |
Type of adherence measure |
Mean adherence rate |
Covariates | Quality rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bloom20 | United States |
Yes | 1995– 1996 |
21 723 | losartan, ACEI, CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
2.6% | 12 months |
Refilled first prescription on or within 3 months of the 1 year anniversary of first prescription |
Medication Persistence* |
64% ARB 58% ACE 50%CCB 43% BB 38% thiazide |
1, 2,9 | Good |
| 2 | Caro et al.21 | Canada | Yes | 1989– 1994 |
22 918 | ACEI CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
None | 12 months |
Last prescription covered period until the end of observation period |
Therapy Persistence† |
89% ACEI 86%CCB 85% BB 80% diuretics 84% overall (at 6 mo) |
1,2,9,10 | Good |
| 3 | Esposti et al. 200222 |
Italy | Yes | 1997 | 16 783 | losartan, ACEI, CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
1.9% | 12 months |
Days elapsed between first and last prescription |
Therapy Persistence |
58% ARB 40% ACEI 31% CCB 38% BB 30% diuretics 35% overall (>273 days elapsing between first and last prescription) |
1,2,6,7,10 | Good |
| 4 | Erkens et al.23 | Netherlands | Yes | 1997– 2001 |
2 243 | ARB, ACEI CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
19.9% | 12 months |
> 270 days elapsing between first and last prescription and additional prescription within 3 months after 1 year |
Medication Persistence |
62% ARB 60% ACE 35% CCB 35% BB 33% diuretics 44% overall |
1,8,10 | Good |
| 5 | Elliott et al. 24 | United States |
Yes | 2001– 2002 |
60 685 | valsartan lisinopril, amlodipine, HCTZ |
Initiating a new medication for HTN; eligible if continuing prior HTN medication |
15.3% | 12 months |
Days until lapse in therapy for ≥ 60 days |
Medication Persistence |
69%valsartan 65% lisinopril 60%amlodipine 56% HCTZ 62% overall No lapse in therapy for ≥ 60 days |
1,2,7,8,15 | Good |
| 6 | Mazzaglia et al.25 |
Italy | Yes, minor |
2000– 2001 |
13 303 | ARB, ACEI, CCB, BB, AB, diuretic |
Initiating a first medication for treatment of HTN other than comb- ination pills |
10.4% | 12 months |
Days until lapse in therapy for ≥ 60 days |
Therapy Persistence |
61% ARB 63% ACEI 64% CCB 58% AB 50% BB 47% diuretic 58% overall (estimated from bar graph) No lapse in therapy for ≥ 60 days |
1,2,6,7,14, 16 |
Excellent |
| 7 | Monane et al.26 |
United States |
No | 1982– 1988 |
8 643 | ACEI, CCB, BB, diuretic, other (no ARBs) |
Initiating a first medication for treatment of HTN; seniors (>65 years old) receiving Medicaid |
None | 12 months |
PDC ≥ 80% | Compliance | 20% overall | 1,2,3,15 | Excellent |
| 8 | Perrault et al.27 |
Canada | No | 1998– 2000 |
21 011 | ARB, ACEI CCB, BB, diuretic, combined therapy |
Initiating a first medication for treatment of HTN; adults 50– 64 years old; no prior history of CVD or secondary HTN |
13.0% | 12 months |
No lapse in therapy for ≥ 60 days |
Therapy Persistence |
73% ARB 71% ACEI 68% CCB 68% BB 61% diuretic 66% combo 67% overall |
1,2,4,5,6, 8,9,10 |
Excellent |
| 9 | Siegel et al.28 | United States |
No | 2002– 2003 |
40 492 | ARB, ACEI, CCB, BB, AB, diuretic |
Continuing or initiating a single medication for HTN; eligible if continuing prior HTN medication ; veterans only; at least 1 refill for drug class of interest during study period |
8.4% | 18 months |
PDC ≥ 80% | Compliance | 84% ARB 81% ACEI 83% CCB 80% BB 79% AB 78% diuretic (includes patients on multiple HTN medications) |
1,2,3,6,9 | Good |
| 10 | Taira et al. 200715‡ |
United States |
No | 1999– 2003 |
28 395 | ARB, ACEI CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
Not Reporte d |
3 years | PDC ≥ 80% | Compliance | Not reported | 1,2,5,6,13 | Good |
| 11 | Taira et al. 200616 |
United States |
Yes | 1999– 2004 |
114 232 | All drug classes |
Continuing or initiating medication treatment for HTN; eligible if continuing prior HTN medication |
Not Reporte d |
12 months |
PDC ≥ 80% | Compliance | Not reported | 1,2,3,6,11, 12 |
Good |
| 12 | Van Wijk et al. 200517* |
Netherlands | No | 1992– 2002 |
2 325 | ACEI, CCB, BB, AB, diuretic |
Initiating a first medication for treatment of HTN; no other indications for HTN medication (eg: CVD, migraine); at least 1 refill for drug class of interest during study period |
Not Reporte d |
10 years | Refilled first prescription at least two times per year |
Medication Persistence |
61% overall (includes patients who discontinued and restarted during 10 year period) |
1,2,12,13 | Good |
| 13 | Esposti et al. 200429 |
Italy | Yes | 2000 | 14 062 | ARB, ACEI CCB, BB, diuretic |
Initiating a first medication for treatment of HTN |
6.9% | 12 months |
Days elapsed between first and last prescription |
Therapy Persistence |
55% ARB, 43% ACEI, 35% CCB, 43% BB, 33% diuretics 40% overall > 273 days elapsed between first and last prescription |
1,6,7,10 | Good |
| 14 | Corrao et al. 30 | Italy | No | 1999– 2002 |
445 356 | All drug classes |
Initiating a first medication for treatment of HTN; at least 1 refill for drug class of interest during study period |
9% | 12 months |
Days until lapse in therapy for ≥ 60 days |
Therapy Persistence |
59% overall No lapse in therapy for ≥ 60 days |
1,2,8,15 | Excellent |
| 15 | Hoer et al.31 | Germany | Yes | 2000– 2003 |
62 754 | ARB, ACEI CCB, BB, diuretic |
Initiating a first medication for treatment of HTN other than combin- ation pills; no recent CVD related hospital- ization; at least 1 refill for drug class of interest during study period |
5.5% | 12 months |
Days until lapse in therapy for ≥ 60 days |
Medication Persistence |
53% ARBs 35% ACE 34% CCBs 14% BB 26% diuretics No lapse in therapy for ≥ 60 days |
1,2 | Good |
| 16 | Van Wijk et al. 200818 |
United States, Canada, and Netherlands |
No | 1998– 2004 |
9 664 United States 25 377 Canada 24 603 Nether- lands |
ARB, ACEI CCB, BB |
Initiating a first medication for treatment of HTN; seniors (>65 years old) |
9.9% US 1.6% Canada , 7.1% Nether- lands |
12 months |
No lapse in therapy for ≥180 days |
Therapy Persistence |
77% USA | 1, 2,3,4,6, 9,10 |
Good |
| 77% Canada | ||||||||||||||
| 76% Netherlands | ||||||||||||||
| 17 | Zhang et al.32 | United States |
No | 2004 | 1 351 | ARB, ACEI |
Initiating ARB or ACEI; eligible if continuing prior HTN medication |
22.8% | 6 months | PDC ≥ 80% | Compliance | 53% overall | 1,2,3,6,8, 9,10,11 |
Excellent |
Covariates: (1) age, (2) sex, (3) race/ethnicity, (4) income or receipt of social assistance, (5) residence, (6) comorbidity types, (7) comorbidity burden, (8) treatment with medication classes other than antihypertensives, (9) medication regimen characteristics (number of medications, frequency of dosing, number of pills per day), (10) health care utilization (prior hospitalizations, physician visits, use of other services), (11) out-of-pocket medication costs, (12) type of health insurance, (13) physician characteristics, (14) blood pressure at baseline, (15) year of first prescription, (16) family history of comorbidities
Abbreviations: ARB, angiotensin receptor blocker; ACEI, angiotensin converting enzyme inhibitor; CCB, calcium channel blocker; BB, beta-blocker; AB, alpha blocker; HCTZ, hydrochlorothiazide; PDC, proportion of days covered; HTN, hypertension; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease
Medication persistence means that patients were considered persistent if they continued the initial prescribed drug class, but not if they switched or combined a medication from another drug class or if they discontinued all antihypertensive medication therapy
Therapy persistence means that patients were considered persistent if they remained on any hypertension medications from the time of treatment initiation which could include continuing the initially prescribed drug class, combining with another drug class, or switching to a different drug class without a lapse in treatment.
Not included in pooled analysis due to overlapping data from other study
Articles had large differences in their selection of covariates for adjusted analyses. Covariates included demographics (age, race, gender, income, ethnicity), burden of disease (comorbidities), complexity of medication regimen (number of medications, frequency of dosing), health system barriers (insurance coverage, cost of care), health care utilization (numbers of hospitalizations and physician visits), and prescriber characteristics (type of physician).
Two articles provided data on overall compliance according to PDC.28, 32 In the study by Zhang et al., 53% were compliant to antihypertensives (either an ACEI or ARB) and in the study by Siegel et al., 78% to 84% were compliant. The mean overall persistence with antihypertensives, available from 12 studies, ranged from 35% to 84%.
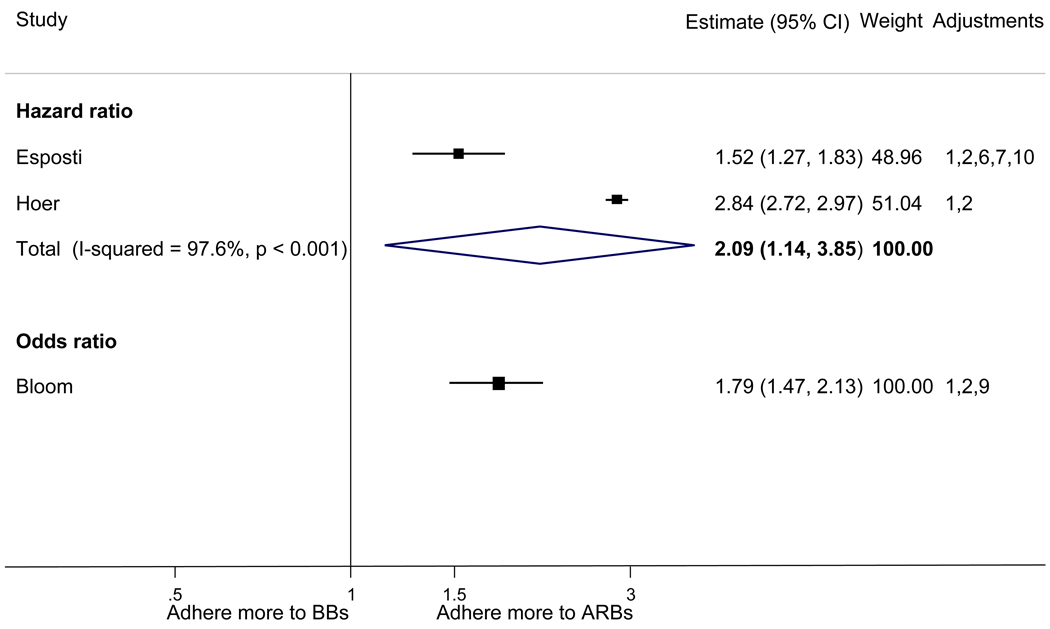
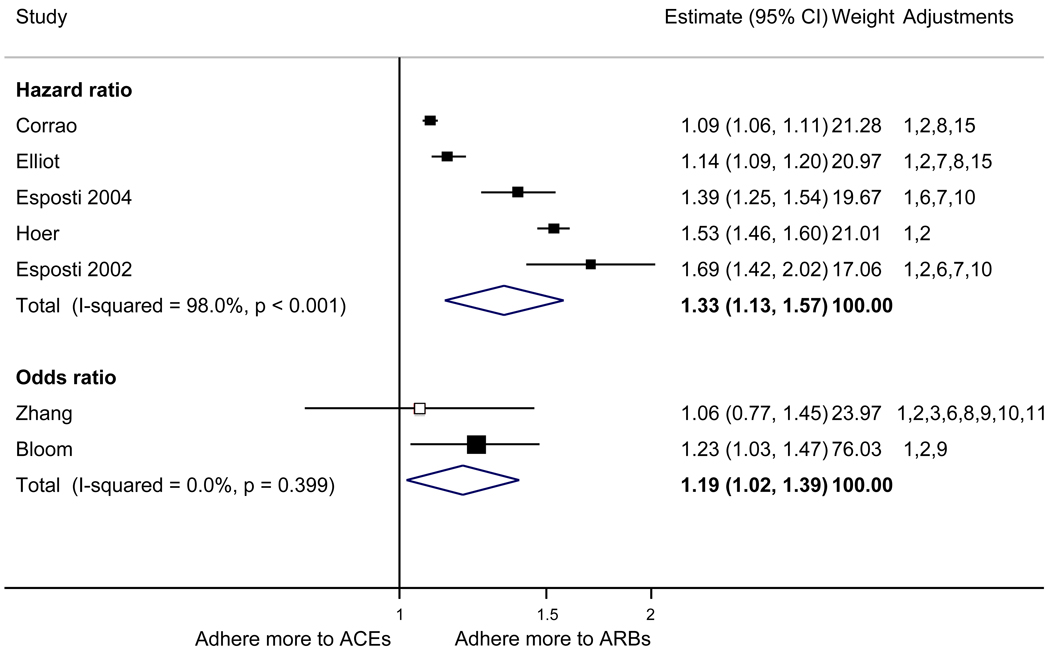
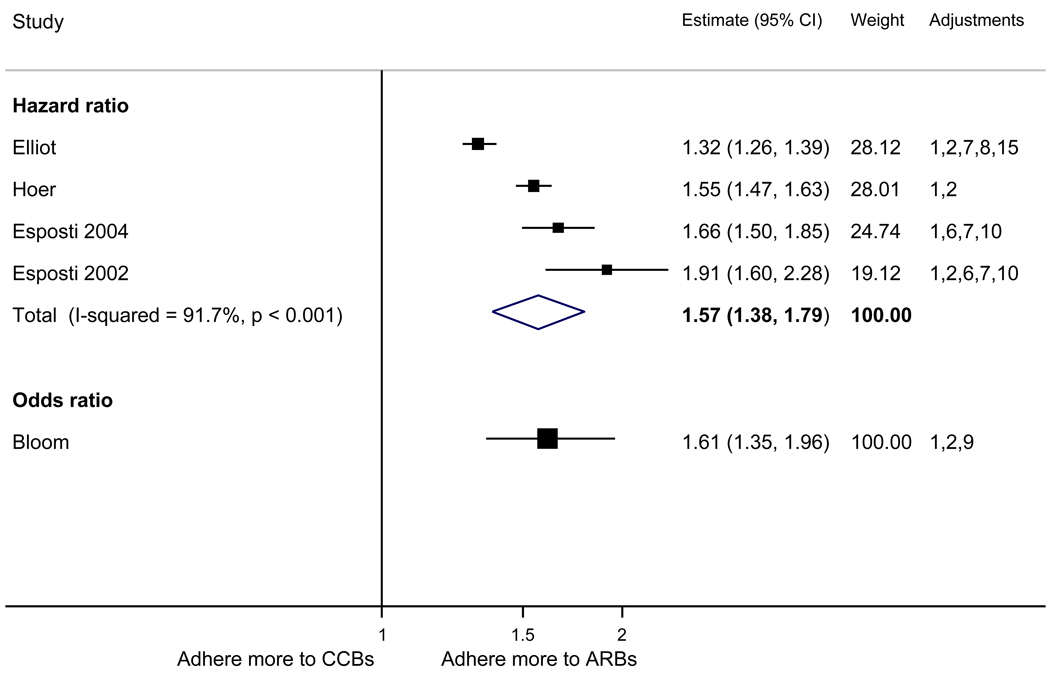
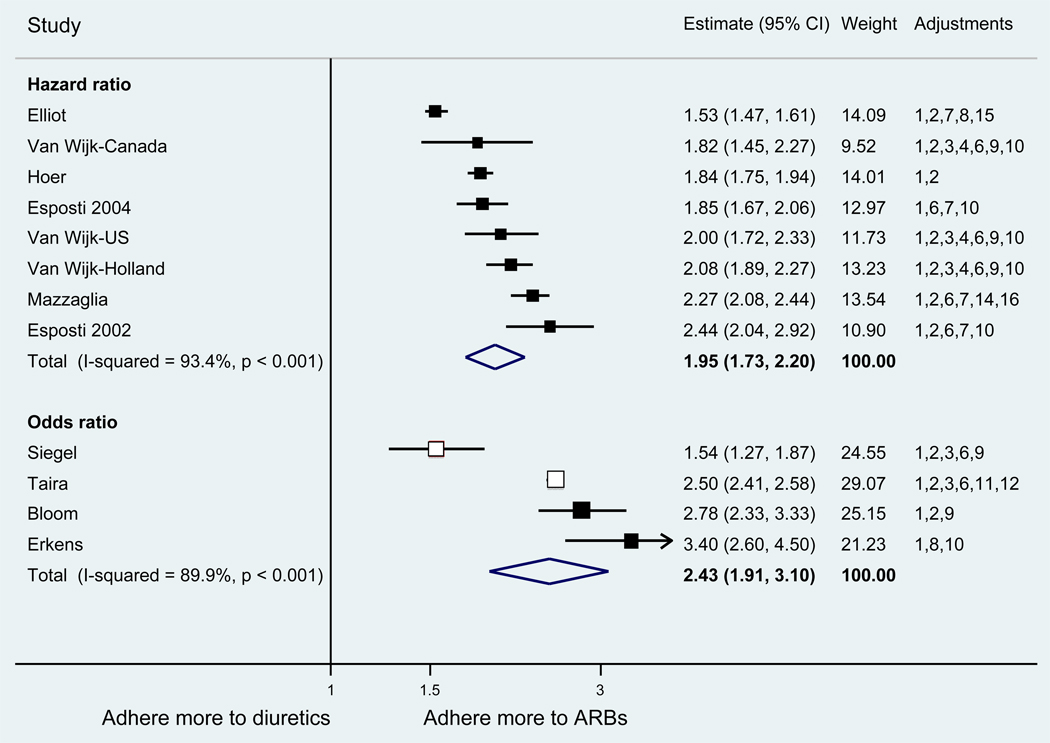
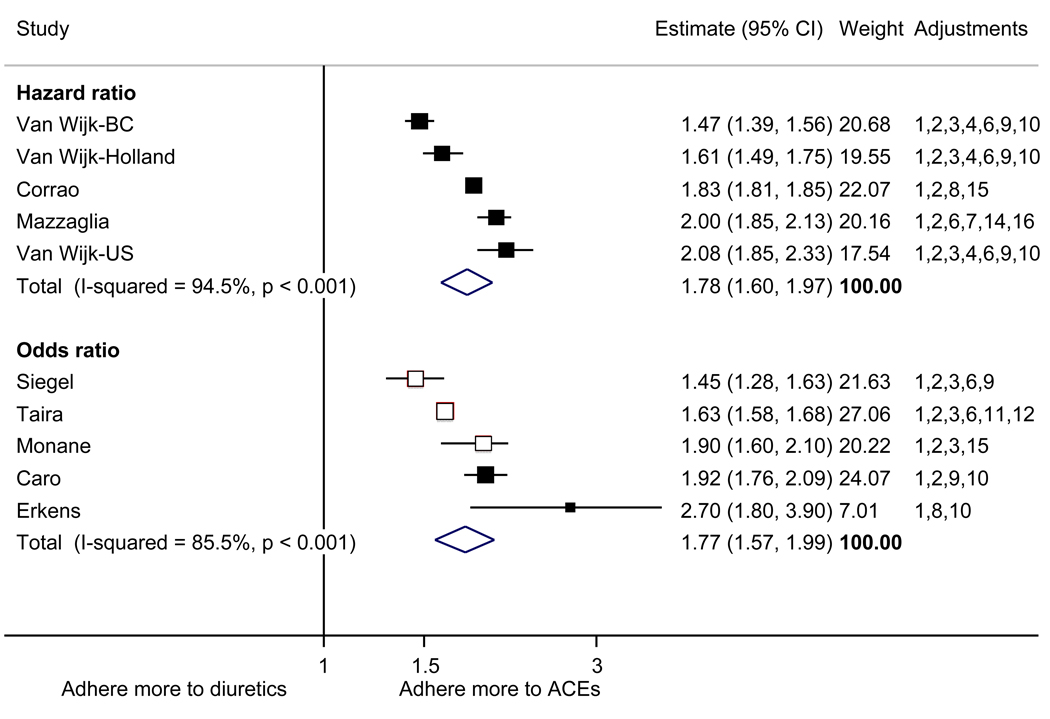
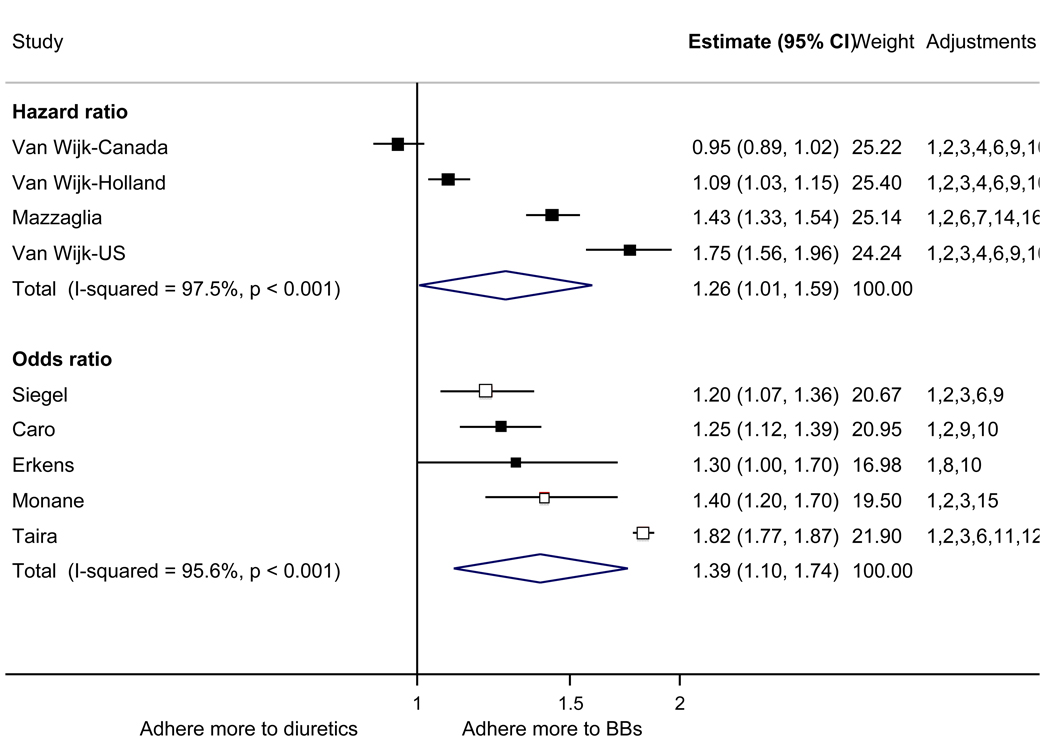
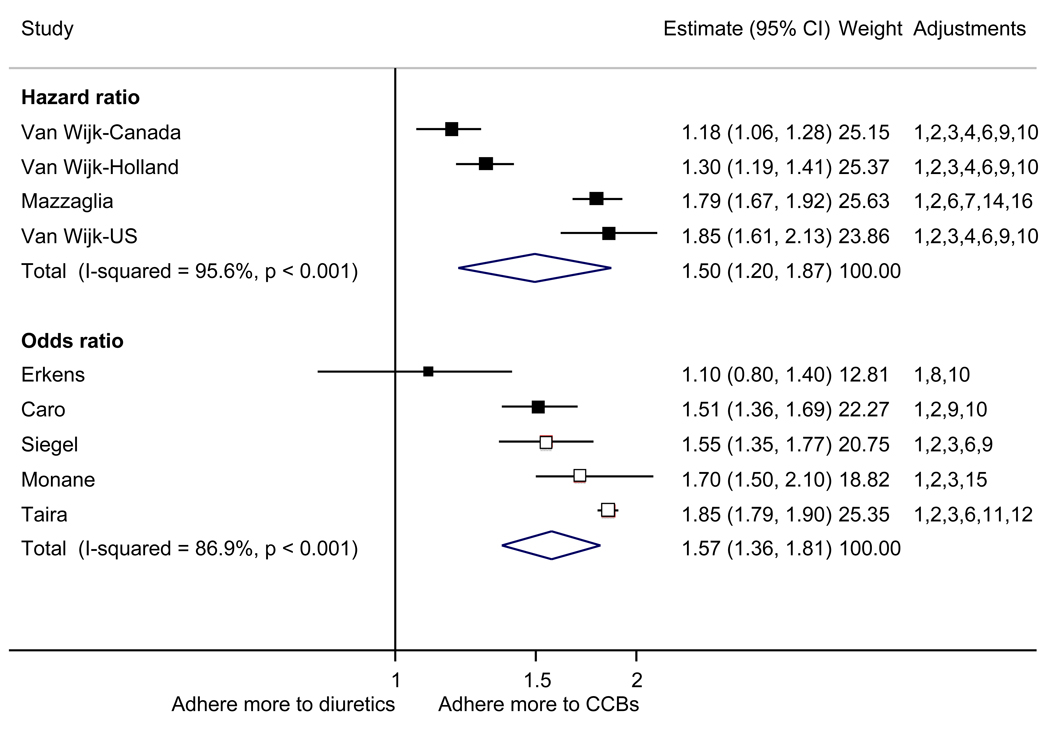
With respect to relative adherence, with one exception (Zhang et al.), there was better adherence (p<.05) to ARBs compared to other drug classes studied (ACEI, CCB, BB, diuretics), regardless of adherence definition.(Figure 2) Conversely, with two exceptions (van Wijk-BC., BB comparison; Erkens et al., CCB comparison), there was lower adherence to diuretics compared to other drug classes (p<.05).(Figure 3)
Figure 2.
Adherence to angiotensin receptor blockers compared to other antihypertensive drug classes: meta-analysis results. Hazard ratios and odds ratios with 95% confidence intervals on a logarithmic scale for individual or pooled study data for relative risk of adherence between pairs of classes of blood pressure medication. Black boxes refer to studies in which adherence is measured as persistence; red boxes refer to studies in which adherence is measured as compliance.
Adjustments: (1) age, (2) sex, (3) race/ethnicity, (4) income or receipt of social assistance, (5) residence, (6) comorbidity types, (7) comorbidity burden, (8) treatment with medication classes other than antihypertensives, (9) medication regimen characteristics (number of medications, frequency of dosing, number of pills per day), (10) health care utilization (prior hospitalizations, physician visits, use of other services), (11) out-of-pocket medication costs, (12) type of health insurance, (13) physician characteristics, (14) blood pressure at baseline, (15) year of first prescription, (16) family history of comorbidities
Abbreviations: ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; BB, beta-blocker; CCB, calcium-channel blocker; CI, confidence interval
Figure 3.
Adherence to diuretics compared to other antihypertensive drug classes: meta-analysis results. Hazard ratios and odds ratios with 95% confidence intervals on a logarithmic scale for individual or pooled study data for each pair-wise comparison for relative risk of adherence between pairs of classes of blood pressure medication. Black boxes refer to studies in which adherence is measured as persistence; red boxes refer to studies in which adherence is measured as compliance.
Adjustments: (1) age, (2) sex, (3) race/ethnicity, (4) income or receipt of social assistance, (5) residence, (6) comorbidity types, (7) comorbidity burden, (8) treatment with medication classes other than antihypertensives, (9) medication regimen characteristics (number of medications, frequency of dosing, number of pills per day), (10) health care utilization (prior hospitalizations, physician visits, use of other services), (11) out-of-pocket medication costs, (12) type of health insurance, (13) physician characteristics, (14) blood pressure at baseline, (15) year of first prescription, (16) family history of comorbidities
Abbreviations: ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; BB, beta-blocker; CCB, calcium-channel blocker; CI, confidence interval
Quantitative analysis (n=15)
The pooled mean age of patients was 61.7 years, and 53.1% were female. The pooled mean person-months of adherence observation across studies was 12.3 months. Using HR as the primary measure of relative adherence, there was an increased risk of adherence to ARBs compared to ACEIs (HR 1.33, 95%CI 1.13–1.57), CCBs (HR 1.57 95% CI 1.38–1.79), BBs (HR 2.09 95% CI 1.14–3.85), and diuretics (HR 1.95 95%CI 1.73–2.20) (Figure 2). In contrast, there was a lower risk of adherence to diuretics compared to all the other drug classes (Figure 3). As a secondary analysis, four studies measured the relative risk of adherence using ORs. The pattern of relative adherence remained the same using OR as the measure of relative adherence with highest adherence to ARBs and lowest to diuretics and beta-blockers (Figure 2, Figure 3).
Data were available for pooled estimates of adherence for individual drug classes from 9 studies, though 2 studies only contained data for 4 of the 5 drug classes which introduced some between-study bias. The mean (95% CI) persistence to medication followed the expected pattern with highest mean persistence to ARBs 64.9% (64.3% – 65.6%; and ACEIs 57.6% (57.2% – 57.9%); intermediate persistence to CCBs 52.0% (51.6% – 52.5%); and lowest to BBs 28.4% (28.1% – 28.8%); and diuretics 51.0% (51.4% – 51.8%).
Overall, other than the comparison between ACEIs and ARBs, there was significant heterogeneity when pooling data (i-squared statistic 91.7% to 98.0%). This supported the use of the random effects model to estimate the relative risk of adherence between pairs of drug classes. Given this substantial heterogeneity, we performed subgroup analyses for our primary outcome to assess the impact of different definitions of adherence, study size, quality, location, inclusion criteria, and pharmaceutical affiliation on the estimates of the relative risk of adherence by drug class. All i-squared values were either reduced or increased by less than 1% in subgroup analyses.
Subgroup analyses supported the robustness of the overall conclusions. First, we tested the impact of defining adherence as medication persistence or as therapy persistence. The definition of adherence did not change the overall pattern, with higher adherence to ARBs compared to other drugs regardless of definition; however, the difference between ARBs and ACEIs did not remain significant when restricting studies to those measuring adherence as therapy persistence. Similarly, subgroup analyses involving study size, location, and quality made no qualitative difference to the pattern of adherence with higher adherence to ARBs compared to other drugs and lower adherence to diuretics compared to all other drugs, though differences between diuretics and beta-blockers were not always statistically significant. Thirteen studies restricted their inclusion of patients to those who were initiating a first medication for hypertension and were not concurrently prescribed any other antihypertensives. There were no significant differences in the relationship between adherence and drug class when the meta-analysis was limited to these studies. Sufficient studies were available for testing the impact of pharmaceutical affiliation on relative adherence in two instances. The relative benefit of adherence to ARBs versus ACEIs was more pronounced (p =.006) in pharmaceutical-affiliated studies [pooled HR 1.41 (95% CI 1.17 – 1.70); 4 pooled studies] compared to non-pharmaceutical affiliated studies [HR1.09 (95%CI 1.06 – 1.11); 1 study]. There was no significant impact of pharmaceutical affiliation on the estimate of relative adherence between ARBs and diuretics.
Publication bias affected the estimate of relative adherence according to HR in two instances. There were no longer significant differences adherence between ARBs and ACEIs [HR 1.10 (95%CI 0.94 – 1.30)] or between BBs and diuretics [HR1.13 (95% CI 0.89–1.44)] after accounting for publication bias.
Discussion
We found that there was a significant relationship between adherence to antihypertensive medication and drug class. When compared to patients prescribed diuretics and BBs, the drug classes associated with the lowest adherence, patients prescribed ARBs were approximately twice as likely to have good adherence. Overall, ACEIs appeared to have the second best level of adherence, followed by CCBs, although insufficient data were available for definitive ranking of pair-wise comparisons and publication bias may have accounted for differences between ARBs and ACEIs.
There was a remarkable degree of consistency in the pattern of our results showing superior adherence to ARBs and ACEIs and inferior adherence to diuretics and beta-blockers. No single study dominated any of the pooled estimates and there was no substantial difference in the pattern regardless of the adherence definition, or the size, quality, and location of the study.
There are several possible reasons for these differences. Each of the drug classes is associated with distinct side-effects. Diuretics, for example, can cause urinary frequency, erectile dysfunction, fatigue, and muscle cramps.33 They can also produce metabolic and electrolyte abnormalities that may lead physicians to discontinue them.
Another possible etiology for the differences in adherence by drug class may be variation in provider and patient beliefs about medications. Perceived benefit of treatment is a core component of several health behavior models34 and has been associated with adherence in some studies of cardiovascular medications.35 Prior studies have shown differences in physician perceptions of the effectiveness and tolerability of antihypertensive medication in separate classes.36 Future studies might assess whether these differences mediate the relationship between adherence and drug class.
The higher rate of adherence to ARBs compared to diuretics suggests that drug cost plays a relatively minor role in antihypertensive adherence. It is possible that cost plays a more significant role in under-insured populations in which medication users are responsible for a significant portion of prescription costs; no articles were restricted to populations without prescription insurance or with low socioeconomic status.
There are several limitations to our conclusions. First, ARBs were prescribed at lower rates than are typical presently in developed countries. This may have biased the results comparing ARBs to other drug classes. There may have been factors related to selection of patients who are more adherent such that adherence to ARBs may be more related to patient selection factors rather than the properties of the drug class itself. Until this is assessed, it is premature to recommend ARBs as first line medications for clinicians interested in minimizing adherence problems. Second, these findings may not be generalizable to patients who are already started on one or more antihypertensive medications. In practice, a growing number of patients are on multiple BP medications and some guidelines even recommend starting with combinations of drug classes for patients with BP markedly above goal. Nevertheless, in our five studies which considered this question, the pattern of adherence between drug classes was the same. Third, these analyses involved multiple statistical tests, and thus the nominal type I error rate of 5% must be interpreted with caution. Fourth, there was significant heterogeneity when pooling data. Still, the pattern of relative adherence remained the same in subgroup analyses. Another limitation is that we excluded articles that assessed adherence by drug class but did not report adjusted analyses of relative risks. A qualitative review of these articles was consistent with the pattern of adherence found in our meta-analysis. Finally, we did not have resources to review non-English language publications nor to contact authors for unpublished data. Of note, one study of Korean patients published after we completed our systematic search was also consistent with our findings showing highest adherence to ACEIs and ARBs and lowest to diuretics.37
Implications
Although rates of blood pressure control are improving in the US, more than half of hypertensive Americans continue to have their blood pressure above recommended levels1 and poor adherence to antihypertensives remains an important cause of poor blood pressure control.38, 39 Lack of adherence to antihypertensive treatment has been associated with complications including increased cardiovascular events and health care costs.40, 41 Our findings remind us that it is important for clinicians to pay attention to adherence regardless of antihypertensive drug class as rates of adherence were suboptimal for all drug classes. Clinicians should pay special attention to adherence in patients who are prescribed diuretics and beta-blockers. Incorporating objective data from pharmacy refill claims or other sources may assist clinicians with assessing medication adherence and with optimizing their antihypertensive prescribing decisions.42 Clinical trials that simulate real-world settings as much as possible should be pursued to assess whether differences in adherence by drug class are associated with differences in blood pressure control and related clinical outcomes.
Clinical Summary.
More than half of US individuals with hypertension continue to have their blood pressure above recommended levels and poor adherence to antihypertensives remains an important cause of poor blood pressure control. Observational studies suggest that there may be important differences in adherence to antihypertensives in distinct drug classes. Yet, the literature describing the association between drug class and adherence is characterized by a wide variety of patient populations, drug class comparisons, and definitions of adherence. This heterogeneity has made it difficult to make firm conclusions about the clinical relevance of differences in adherence according to drug class. Accordingly, we performed a meta-analysis to determine the impact of antihypertensive drug class on adherence to blood pressure medications. We found that there was a significant relationship between adherence and antihypertensive drug class. When compared to patients prescribed diuretics and beta-blockers, the drug classes associated with the lowest adherence, patients prescribed angiotensin II receptor blockers were approximately twice as likely to have good adherence. Overall, angiotensin-converting enzyme inhibitors appeared to have the second best level of adherence, followed by calcium-channel blockers, although insufficient data was available for definitive rankings. Our findings demonstrate that it is important for clinicians to pay attention to adherence regardless of drug class as adherence was suboptimal for all drug classes, and that clinicians should pay special attention to adherence in patients who are prescribed diuretics and beta-blockers.
Supplementary Material
Acknowledgements
None
Funding Sources: No funders or sponsors were involved in the design or conduct of this study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript. Ms. Falzon is supported by grant HL04458-05 from the National Heart, Lung, and Blood Institute; Dr. Kronish is supported by grant 1K23HL098359 from the National Heart, Lung, and Blood Institute; and Dr. Mann is supported by grant 1K23DK081665 from the National Institute of Diabetes, Digestive, and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Egan BM, Zhao Y, Axon RN. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, Casiglia E, Kerlikowske K, Coope J. Antihypertensive drugs in very old people: A subgroup meta-analysis of randomised controlled trials. Indana group. Lancet. 1999;353:793–796. doi: 10.1016/s0140-6736(98)08127-6. [DOI] [PubMed] [Google Scholar]
- 3.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the jnc vi risk stratification. Hypertension. 2000;35:539–543. doi: 10.1161/01.hyp.35.2.539. [DOI] [PubMed] [Google Scholar]
- 4.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Andrade SE, Walker AM, Gottlieb LK, Hollenberg NK, Testa MA, Saperia GM, Platt R. Discontinuation of antihyperlipidemic drugs--do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 7.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 8.Fitz-Simon N, Bennett K, Feely J. A review of studies of adherence with antihypertensive drugs using prescription databases. Ther Clin Risk Manag. 2005;1:93–106. doi: 10.2147/tcrm.1.2.93.62915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Organization WH. Adherence to long-term therapies - evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 11.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 12.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 13.Halpern MT, Khan ZM, Schmier JK, Burnier M, Caro JJ, Cramer J, Daley WL, Gurwitz J, Hollenberg NK. Recommendations for evaluating compliance and persistence with hypertension therapy using retrospective data. Hypertension. 2006;47:1039–1048. doi: 10.1161/01.HYP.0000222373.59104.3d. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: Terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 15.Taira DA, Gelber RP, Davis J, Gronley K, Chung RS, Seto TB. Antihypertensive adherence and drug class among asian pacific americans. Ethnicity & Health. 2007;12:265–281. doi: 10.1080/13557850701234955. [DOI] [PubMed] [Google Scholar]
- 16.Taira DA, Wong KS, Frech-Tamas F, Chung RS. Copayment level and compliance with antihypertensive medication: Analysis and policy implications for managed care. Am J Manag Care. 2006;12:678–683. [PubMed] [Google Scholar]
- 17.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens. 2005;23:2101–2107. doi: 10.1097/01.hjh.0000187261.40190.2e. [DOI] [PubMed] [Google Scholar]
- 18.van Wijk BLG, Shrank WH, Klungel OH, Schneeweiss S, Brookhart MA, Avorn J. A cross-national study of the persistence of antihypertensive medication use in the elderly. J Hypertens. 2008;26:145–153. doi: 10.1097/HJH.0b013e32826308b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther. 1998;20:671–681. doi: 10.1016/s0149-2918(98)80130-6. [DOI] [PubMed] [Google Scholar]
- 21.Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: The importance of actual practice data. CMAJ. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- 22.Esposti LD, Esposti ED, Valpiani G, Di Martino M, Saragoni S, Buda S, Baio G, Capone A, Sturani A. A retrospective, population-based analysis of persistence with antihypertensive drug therapy in primary care practice in italy. Clin Ther. 2002;24:1347–1357. doi: 10.1016/s0149-2918(02)80039-x. [DOI] [PubMed] [Google Scholar]
- 23.Erkens JA, Panneman MMJ, Klungel OH, van den Boom G, Prescott MF, Herings RMC. Differences in antihypertensive drug persistence associated with drug class and gender: A pharmo study. Pharmacoepidemiology & Drug Safety. 2005;14:795–803. doi: 10.1002/pds.1156. [DOI] [PubMed] [Google Scholar]
- 24.Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. JABFM. 2007;20:72–80. doi: 10.3122/jabfm.2007.01.060094. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaglia G, Mantovani LG, Sturkenboom MC, Filippi A, Trifiro G, Cricelli C, Brignoli O, Caputi AP. Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: A retrospective cohort study in primary care. J Hypertens. 2005;23:2093–2100. doi: 10.1097/01.hjh.0000186832.41125.8a. [DOI] [PubMed] [Google Scholar]
- 26.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: Results from a population-based study in the elderly. Am J Hypertens. 1997;10:697–704. doi: 10.1016/s0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 27.Perreault S, Lamarre D, Blais L, Dragomir A, Berbiche D, Lalonde L, Laurier C, St-Maurice F, Collin J. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39:1401–1408. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- 28.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the department of veterans affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Esposti LD, Di Martino M, Saragoni S, Sgreccia A, Capone A, Buda S, Esposti ED. Pharmacoeconomics of antihypertensive drug treatment: An analysis of how long patients remain on various antihypertensive therapies. J Clin Hypertens (Greenwich) 2004;6:76–84. doi: 10.1111/j.1524-6175.2004.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, Cesana G, Mancia G. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: A population-based study in Italy. J Hypertens. 2008;26:819–824. doi: 10.1097/HJH.0b013e3282f4edd7. [DOI] [PubMed] [Google Scholar]
- 31.Hoer A, Gothe H, Khan ZM, Schiffhorst G, Vincze G, Haussler B. Persistence and adherence with antihypertensive drug therapy in a german sickness fund population. J Hum Hypertens. 2007;21:744–746. doi: 10.1038/sj.jhh.1002223. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Carlson AM, Gleason PP, Schondelmeyer SW, Schommer JC, Dowd BE, Heaton AH. Relationship of the magnitude of member cost-share and medication persistence with newly initiated renin angiotensin system blockers. J Manag Care Pharm. 2007;13:664–676. doi: 10.18553/jmcp.2007.13.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger MH. Diuretics and their side effects. Dilemma in the treatment of hypertension. Hypertension. 1988;11:II16–II20. doi: 10.1161/01.hyp.11.3_pt_2.ii16. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 35.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 36.Ubel PA, Jepson C, Asch DA. Misperceptions about beta-blockers and diuretics: A national survey of primary care physicians. J Gen Intern Med. 2003;18:977–983. doi: 10.1111/j.1525-1497.2003.20414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung SK, Lee SG, Lee KS, Kim DS, Kim KH, Kim KY. First-year treatment adherence among outpatients initiating antihypertensive medication in korea: Results of a retrospective claims review. Clin Ther. 2009;31:1309–1320. doi: 10.1016/j.clinthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 41.McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a medicaid population. Med Care. 1994;32:214–226. doi: 10.1097/00005650-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When more is not better: Treatment intensification among hypertensive patients with poor medication adherence. Circulation. 2008;117:2884–2892. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.