Although meiosis is extremely important not only for sexual reproduction but also for creating diversity, very little is known about meiotic regulation. Five APC/C coactivators have been found in the fission yeast genome. This study investigates how all the coactivators are involved in the meiosis I/meiosis II transition.
Abstract
Meiosis is a specialized form of cell division generating haploid gametes and is dependent upon protein ubiquitylation by the anaphase-promoting complex/cyclosome (APC/C). Accurate control of the APC/C during meiosis is important in all eukaryotic cells and is in part regulated by the association of coactivators and inhibitors. We previously showed that the fission yeast meiosis-specific protein Mes1 binds to a coactivator and inhibits APC/C; however, regulation of the Mes1-mediated APC/C inhibition remains elusive. Here we show how Mes1 distinctively regulates different forms of the APC/C. We study all the coactivators present in the yeast genome and find that only Slp1/Cdc20 is essential for meiosis I progression. However, Fzr1/Mfr1 is a critical target for Mes1 inhibition because fzr1Δ completely rescues the defect on the meiosis II entry in mes1Δ cells. Furthermore, cell-free studies suggest that Mes1 behaves as a pseudosubstrate for Fzr1/Mfr1 but works as a competitive substrate for Slp1. Intriguingly, mutations in the D-box or KEN-box of Mes1 increase its recognition as a substrate by Fzr1, but not by Slp1. Thus Mes1 interacts with two coactivators in a different way to control the activity of the APC/C required for the meiosis I/meiosis II transition.
INTRODUCTION
The ubiquitin-proteasome pathway is one of the fundamental regulatory systems and controls many cellular processes including the cell cycle, signal transduction, stress response, and neuronal differentiation. Ubiquitylation is accomplished through the cooperation of three enzymes—E1, E2, and E3—by which ubiquitin molecules are covalently attached to the lysine residues of the target proteins. Subsequently, the polyubiquitin chains are recognized and degraded to short peptides by the 26S proteasome (Hershko and Ciechanover, 1998). In this process the E3 ubiquitin ligases play a critical role in recognizing the right targets as well as transferring ubiquitins at the right time. One of the major ubiquitin ligases in the cell cycle is the anaphase-promoting complex/cyclosome (APC/C) (Peters, 2006; Thornton and Toczyski, 2006; Morgan, 2007; Pesin and Orr-Weaver, 2008). The APC/C is a 1.5-MDa protein complex, consisting of >11 conserved subunits, which triggers two essential events in mitosis: sister chromatid separation and mitotic exit via ubiquitylation of securin/Cut2/Pds1 and cyclin B/Cdc13/Clb2, respectively. The APC/C activity is elaborately regulated during the cell cycle. The critical factor for this regulation is the Fizzy/Cdc20 family of coactivators, which recognizes target substrates via its C-terminal WD40 repeat domain (Morgan, 2007; Yu, 2007). There are two types of coactivator: Fizzy/Cdc20/Slp1, which is required for the APC/C activity in anaphase, and Fizzy-related/Cdh1/Ste9, which maintains its activity during late mitosis and G1 (Peters, 2006; Thornton and Toczyski, 2006; Morgan, 2007). Moreover, the coactivators have recently been shown to have an additional role in the activation of ubiquitylation reactions toward recruited substrates through their C-box (Kimata et al., 2008a). Thus the Fizzy/Cdc20 family of coactivators is essential for APC/C-dependent ubiquitylation.
The mitotic regulation of APC/C activity has been extensively studied; however, its regulation in meiosis remains largely elusive. Meiosis is a specialized form of cell division in which diploid germ cells give rise to haploid gametes such as eggs, sperms, or spores. Defective meiotic progression results in aneuploidy, which is associated with birth defects and many infertility diseases. Meiosis differs from mitosis in several aspects. First, meiosis comprises a single round of DNA replication followed by two consecutive rounds of chromosome segregation, called meiosis I and meiosis II, without an intervening DNA replication (Petronczki et al., 2003; Morgan, 2007). Second, in mitosis, proteolysis of cyclin B (inactivation of Cdk) is required for mitotic exit and subsequent DNA replication, whereas in meiosis, complete proteolysis of cyclin B during meiosis I prevents direct entry into meiosis II, which is essential for producing haploid gametes (Iwabuchi et al., 2000). Hence the APC/C requires additional layers of regulation in meiosis. In several organisms, meiosis-specific coactivators and inhibitors of the APC/C have been identified. In budding yeast, a stoichiometric subunit of the APC/C, Apc15/Mnd2, exclusively inhibits the activity of the APC/C associated with a meiosis-specific coactivator, Ama1, to prevent precocious loss of securin (Oelschlaegel et al., 2005; Penkner et al., 2005). In Drosophila, the Fizzy/Cdc20 family proteins Cort and Fzr2, expressed in female and male germ lines, respectively, seem to regulate meiotic progression (Jacobs et al., 2002; Swan and Schupbach, 2007; Pesin and Orr-Weaver, 2008). In mouse and frog oocytes, Erp1/Emi2 inhibits APC/C activity to establish the semipermanent cell cycle arrest as a component of the cytostatic factor (Rauh et al., 2005; Shoji et al., 2006). However, the detailed mechanism underlying the meiosis I/meiosis II transition remains to be elucidated.
In the fission yeast Schizosaccharomyces pombe, a meiosis-specific APC/C inhibitor, Mes1, is required to prevent complete cyclin B proteolysis during exit from meiosis I (Izawa et al., 2005). We recently showed that Mes1 is not merely an APC/C inhibitor but also an APC/C substrate that binds to coactivators and competes with the binding of other substrates by forming a ternary inhibitory complex with APC/C. Because ubiquitylation of Mes1 dissociates it from the complex, the APC/C becomes partially active during anaphase I (Kimata et al., 2008b). However, in the S. pombe genome, in addition to mitotic Slp1 and Ste9, three more Fizzy/Cdc20 family members exist that are exclusively expressed in meiosis. One of them, Fzr1/Mfr1, has been shown to be required for meiosis II exit and subsequent sporulation (Asakawa et al., 2001; Blanco et al., 2001). However, the roles for the other coactivators as well as their interactions with Mes1 remain unclear. Here we demonstrate that Mes1 preferentially suppresses Fzr1/Mfr1 rather than Slp1 to prevent the hyperactivation of the APC/C–Fzr1/Mfr1 during meiosis I exit. While Slp1 escapes complete inhibition as a result of the ubiquitylation of Mes1, Fzr1/Mfr1 is kept inactive because of its inability to induce efficient Mes1 ubiquitylation.
RESULTS
Roles of five coactivators of the APC/C in meiosis in fission yeast
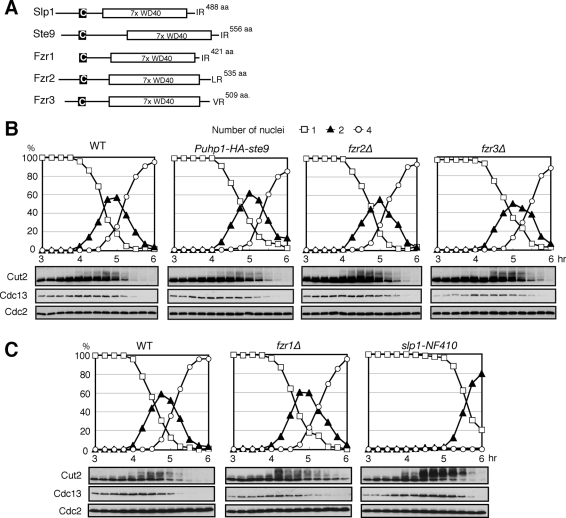
In the fission yeast S. pombe genome, in addition to the mitotic coactivators Slp1 and Ste9, there are three other putative APC/C coactivators—Fzr1/Mfr1, Fzr2 (SPAC13G6.08), and Fzr3 (SPCC1620.04c)—expressed exclusively in meiosis (Figure 1A) (Asakawa et al., 2001; Blanco et al., 2001; Yamamoto et al., 2008). To investigate a role for the individual coactivators, we monitored meiotic progression in the diploid cells of null mutants (including a temperature-sensitive mutant of an essential gene, slp1) of each coactivator. Meiosis was synchronously induced by thermal inactivation of the Pat1 kinase in prestarved homozygous (h−/h−) diploid cells. We first analyzed the mutants of Ste9, Fzr2, and Fzr3, whose meiotic roles have not been examined. Because Ste9 is essential for nitrogen starvation–induced G1 arrest, a prerequisite for meiotic entry, we created Puhp1-HA-ste9 mutants, in which the expression of HA-tagged Ste9 is under the control of the uhp1 promoter and is repressed in meiosis. Puhp1-HA-ste9 diploids were able to arrest in G1 phase upon nitrogen starvation, although the expression levels of Ste9 were much lower than in the wild type (WT) and almost undetectable until late meiosis II (see Supplemental Figure S1). We examined both the number of nuclei in these cells and the protein levels of the APC/C substrates Cut2/securin and Cdc13/cyclin B. In Puhp1-HA-ste9 diploid cells and fzr2Δ and fzr3Δ diploids, we did not observe any significant effect on meiotic progression, except a slight delay at the end of meiosis (Figure 1B). Notably, Ste9 appeared as slow-migrating bands during most of meiosis in WT cells, suggesting that Ste9 is highly phosphorylated and thus inactive until the end of meiosis (see Supplemental Figure S1, top). Immunoblotting analysis revealed that Fzr2 was induced after 5.5 h at the late stage of meiosis II (Supplemental Figure S2). Furthermore, we created the double mutant fzr2Δfzr3Δ diploid cells but still found that there is no significant defect in meiotic progression (Supplemental Figure S3). Thus neither Ste9, Fzr2, nor Fzr3 appears to be involved in the transition between meiosis I and meiosis II, although they might partially or redundantly contribute to the exit from meiosis.
FIGURE 1:
Roles for fission yeast APC coactivators in meiotic progression. (A) Schematic diagrams representing the domains of five Fizzy/Cdc20 family APC/C coactivators in fission yeast. All of them share a C-box motif (C in the black box) and seven tandem repeats of WD40 domain (7x WD40) and terminate at either IR, LR, or VR dipeptides. (B) Synchronized meiosis by pat1-114 inactivation in WT, Puhp1-HA-ste9, fzr2Δ, and fzr3Δ diploid cells. Meiotic progression was examined microscopically for the number of nuclei in a cell. Protein levels were analyzed by immunoblotting using specific antibodies at indicated time points. (C) Same as B, but meiotic progression in WT, fzr1Δ, and slp1-NF410 mutant diploid cells upon pat1-114 inactivation was examined.
Next we addressed the meiotic roles of Slp1 and Fzr1/Mfr1. The fzr1Δ diploid cells showed almost no delay in meiosis I progression but exhibited a delay in nuclear division and clear accumulation of Cut2 and Cdc13 in meiosis II, consistent with a previous report (Figure 1C, middle) (Blanco et al., 2001). To analyze Slp1 function, we made a new temperature-sensitive slp1-NF410 allele by PCR-based mutagenesis that exhibits tight metaphase arrest at 33°C and 36°C. The slp1-NF410 mutant diploid cells showed a significant delay in anaphase I onset and stabilization of Cut2 and Cdc13 in the early stage of meiosis accompanied by accumulation of highly phosphorylated Cut2 (Figure 1C, right).
In meiosis, Fzr1/Mfr1 is partly active and can trigger anaphase I
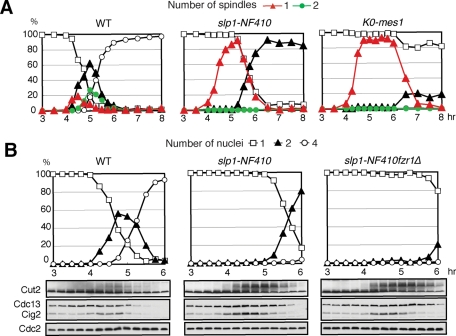
We noticed that in the slp1-NF410 mutant allele, despite a significant delay, >80% of the cells finally underwent anaphase I after 5 h and formed two nuclei by the end of meiosis (Figure 2A). In contrast, in a K0-mes1 mutant strain, which expresses lysine-less Mes1 and thus completely inhibits APC/C, anaphase I barely took place and most of the cells carried a single nucleus even at the end of meiosis (Figure 2A) (Kimata et al., 2008b). We speculated that in the slp1-NF410 mutant, some residual APC/C activity might remain and initiate anaphase I. We also noticed that although fzr1Δ did not appear to affect meiosis I progression, Fzr1/Mfr1 protein levels started to accumulate from 4.5 h after meiotic induction, which corresponds to anaphase I onset (Supplemental Figure S4). To investigate whether the delayed onset of anaphase I in the slp1-NF410 mutant is due to Fzr1/Mfr1 activity, we analyzed the slp1-NF410 fzr1Δ double mutant. The double mutant cells indeed showed further delay in the onset of anaphase I and accumulated more Cut2 and Cdc13 than the slp1-NF410 single allele (Figure 2B). These data suggest that Fzr1/Mfr1 is responsible for the delayed onset of anaphase I in the slp1-NF410 mutant and is also capable of triggering anaphase I.
FIGURE 2:
Fzr1/Mfr1 activates APC/C in anaphase I in the absence of Slp1 activity. (A) Synchronized meiosis in WT, slp1-NF410, and K0-mes1 diploid cells. Meiotic progression was examined microscopically for the number of nuclei per cell as well as the number of short spindles stained by anti-tubulin antibody. Protein levels were analyzed by immunoblotting using specific antibodies. (B) As in Figure 1B, meiotic progression in WT, slp1-NF410, and slp1-NF410fzr1Δ diploids was monitored.
Fzr1/Mfr1 is an important target of Mes1 inhibitory function during meiosis I
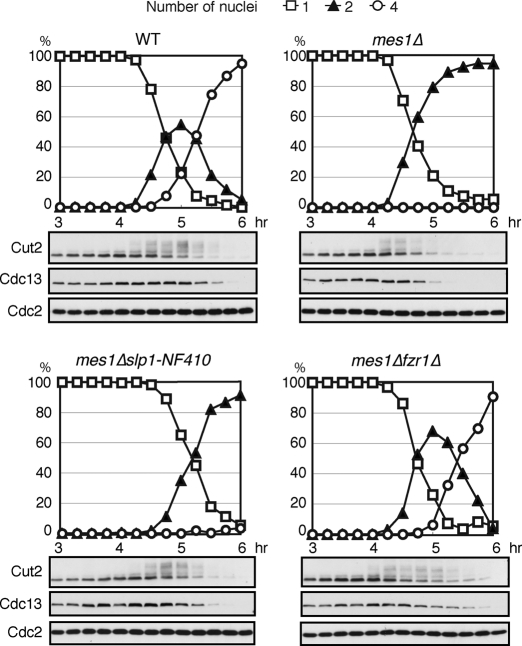
To understand the functional relationship between Mes1 and the coactivators active in the meiosis I/meiosis II transition, we next investigated meiotic progression in double mutants of mes1Δ and slp1-NF410 or fzr1Δ. Consistent with our previous results, mes1Δ cells failed to enter meiosis II, showing earlier degradation of Cut2 and Cdc13 than WT cells (Figure 3). To our surprise, unlike the previous report using the slp1-362 allele (Izawa et al., 2005), almost no cells could enter meiosis II in mes1Δslp1-NF410 double mutant cells. On the contrary, mes1Δfzr1Δ double mutant cells completed meiosis in a similar manner to WT, apart from a subtle delay in the exit from meiosis II, presumably caused by the lack of Fzr1/Mfr1 function (Figure 3). Thus Fzr1/Mfr1, rather than Slp1, seems to be a critical target for inhibition by Mes1 during meiosis I. It is noteworthy that anaphase I occurs earlier in mes1Δ slp1-NF410 cells than in slp1-NF410 cells (compare Figures 3 and 2B), suggesting that Mes1 counteracts Fzr1/Mfr1 function.
FIGURE 3:
Mes1 inhibits Fzr1/Mfr1 during meiosis I. Meiosis was induced by thermal inactivation of Pat1, and meiotic progression was monitored in WT, mes1Δ, mes1Δslp1-NF410, and mes1Δfzr1Δ mutant diploid cells. Samples were examined microscopically for the number of nuclei in a cell to monitor progression through meiosis and analyzed by immunoblotting using specific antibodies at indicated time points.
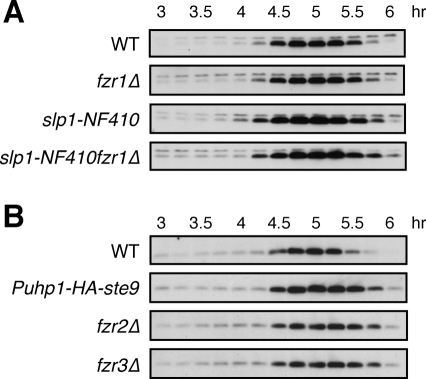
We previously showed that D-box and KEN-box mutations strongly stabilized Mes1, suggesting that its stability is completely under APC/C control (Kimata et al., 2008b). In an attempt to investigate which APC/C coactivator might target Mes1 for destruction in meiosis, we analyzed Mes1 levels during pat1-114–synchronized meiosis in the different coactivator mutants. As shown in Figure 4A, Mes1 was clearly stabilized during anaphase I in the slp1-NF410 allele, whereas no such stabilization was observed in fzr1Δ, compared with WT cells. The Mes1 stability was not enhanced by combining the slp1-NF410 mutation with fzr1Δ, suggesting that Fzr1/Mfr1 is unlikely to target Mes1 for destruction in meiosis I. We also examined whether the rest of the APC/C coactivators are involved in Mes1 destruction (Figure 4B). In all the mutants (ste9, fzr2Δ, and fzr3Δ), Mes1 levels were significantly increased at the end of meiosis II (5.5–6 h) rather than in early meiosis, pointing to their roles in Mes1 destruction in meiosis II. To investigate their redundant role in Mes1 destruction, we made fzr2Δfzr3Δ double and fzr1Δfzr2Δfzr3Δ triple mutant strains. Mes1 stability in those mutant cells was similar to that in fzr2Δ or fzr3Δ, although Mes1 was slightly stabilized in early meiosis (Figure 4 and Supplemental Figure S5). Because Mes1 was ultimately degraded at the end of meiosis II even in fzr1Δfzr2Δfzr3Δ triple mutant cells, Ste9 or Slp1 might also be involved in Mes1 stability. All together, these results suggest that Mes1 destruction might be regulated by different APC/C coactivators at different stages of meiosis.
FIGURE 4:
Roles for coactivators in Mes1 turnover during meiosis. (A) pat1-114–induced synchronization of meiosis was performed in WT, fzr1Δ, slp1-NF410, and slp1-NF410fzr1Δ mutant diploid strains expressing HA-tagged Mes1 under the native promoter. HA-Mes1 levels were analyzed by immunoblotting using anti-HA antibody. The levels of HA-Mes1 did not show any change by fzr1Δ but were significantly increased by slp1-NF410 mutations. (B) HA-tagged Mes1 levels were analyzed in synchronized meiosis in WT, Puhp1-HA-ste9, fzr2Δ, and fzr3Δ strains using anti-HA antibody.
Reconstitution of different coactivator-dependent ubiquitylation and proteolysis
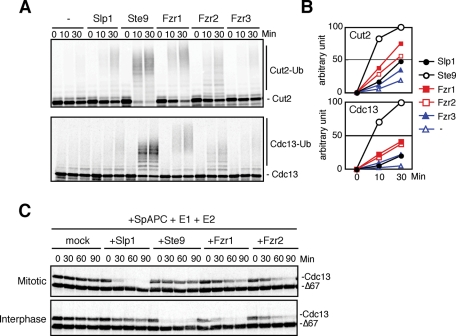
To further investigate the interaction and interdependency between Mes1 and the APC/C coactivators, we reconstituted APC/C-dependent ubiquitylation reactions in vitro. S. pombe APC/C was purified from a lid1+-TAP strain (Yoon et al., 2002) using a tandem affinity purification (TAP) strategy. Lid1/Cut20 is the fission yeast orthologue of Apc4. We assessed our cell-free ubiquitylation assay in two ways. First, we asked whether the purified S. pombe APC/C ubiquitylated the canonical APC/C substrates Cut2 and Cdc13 only in the presence of E2 enzymes and a coactivator (Supplemental Figure S6A). Then, we examined whether the purified APC/C exhibited a D-box dependency for ubiquitylation of substrates (Supplemental Figure S6B). Because our cell-free ubiquitylation assay gave positive results for both criteria, we decided to use this cell-free system. We compared the activity of the five APC/C coactivators for ubiquitylation of Cut2 and Cdc13 (Figure 5, A and B). Ste9 showed the most robust stimulation of ubiquitylation for both substrates. Among the meiosis-specific coactivators, Fzr1/Mfr1 and Fzr2 showed significant activation, if weaker than Ste9, whereas no activation was observed with Fzr3, which is probably caused by inefficient in vitro translation of Fzr3 (Supplemental Figure S7). Unexpectedly, Slp1 showed only weak stimulation (Figure 5, A and B). We reasoned that this might be due to the use of APC/C purified from asynchronously growing cells (mainly in G2 phase). However, this is not the case because when we purified APC/C from metaphase-arrested cells such as mts3-1 or slp1-362 at the restrictive temperature, we were still unable to observe efficient activation by Slp1 (unpublished data).
FIGURE 5:
APC/C-dependent ubiquitylation and destruction by different coactivators. (A) In vitro ubiquitylation assays using S. pombe APC/C and coactivators. 35S-labeled Cut2 and Cdc13 were used as substrates. Samples were taken at indicated time points and analyzed by SDS–PAGE followed by autoradiography. (B) The intensity of the signals corresponding to the ubiquitinated substrates in A was quantified and presented in the graph. (C) Destruction assays of Cdc13 in S. pombe APC/C, E1, E2, and coactivators supplemented Xenopus egg extracts from which endogenous APC/C and Fizzy/Cdc20 had been depleted.
To reconstitute APC/C function activated by different coactivators, we immunodepleted endogenous Xenopus APC/C and Fizzy/Cdc20 from Xenopus egg extracts, then added back purified S. pombe APC/C, in vitro translated S. pombe coactivators, and analyzed the destruction of APC/C substrates. In this system, Cdc13 and Mes1 were destroyed upon the addition of S. pombe APC/C and Slp1 (Supplemental Figure S8). We also asked whether the cell-free system reflected the cell-cycle dependent regulation of coactivators. As expected, Slp1, but not Ste9, was able to support Cdc13 destruction in mitotic extracts (Figure 5C, top), consistent with the established notion that CDH1 orthologues are inhibited by Cdk1-dependent phosphorylation in mitosis. In contrast, in interphase egg extracts, Slp1 was unable to activate Cdc13 destruction whereas Ste9 stimulated Cdc13 destruction efficiently (Figure 5C, bottom). Intriguingly, Fzr1/Mfr1 showed stronger APC/C activation for Cdc13 in interphase extracts than in mitotic extracts, whereas Fzr2 showed only weak activation in both extracts (Figure 5C). We observed similar results with a different substrate, Cut2 (unpublished data). Collectively, these data suggest that Slp1, Ste9, Fzr1/Mfr1, and Fzr2 can all activate APC/C and ubiquitylate cyclin B for destruction, although their activities seem to be regulated differently during the cell cycle.
APC/C–Fzr1/Mfr1 poorly ubiquitylates Mes1
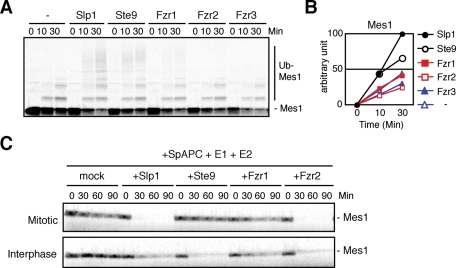
Next we wished to investigate how Mes1 ubiquitylation and destruction are regulated by different coactivators. As shown in Figure 6, both Slp1 and Ste9 efficiently ubiquitylate Mes1 and support S. pombe APC/C-dependent Mes1 destruction in Xenopus egg extracts. Slp1 could activate APC/C and destroy Mes1 even in interphase extracts (Figure 6C, bottom). In contrast, although Fzr1/Mfr1 efficiently ubiquitylated Cut2 and Cdc13, it hardly ubiquitylated Mes1 (compare Figures 5A and 6A). Consistently, Fzr1/Mfr1 barely activated S. pombe APC/C and destroyed Mes1 in a cell-free system (Figure 6C). Fzr2 proficiently induced Mes1 destruction in egg extracts despite its inefficiency in Cdc13 destruction (Figure 6C). Therefore Mes1 is ubiquitylated by APC/C–Slp1, APC/C–Ste9, and APC/C–Fzr2 but might not be a favorable substrate for APC/C–Fzr1/Mfr1.
FIGURE 6:
Fzr1/Mfr1 cannot activate APC/C for Mes1 ubiquitylation and destruction. (A, B) In vitro ubiquitylation assay of Mes1 was performed as in Figure 5A except using Mes1 as a substrate. The intensity of ubiquitylated Mes1 signals was quantified and shown in the graph. (C) Destruction assays were performed as in Figure 5C apart from using Mes1 as a substrate.
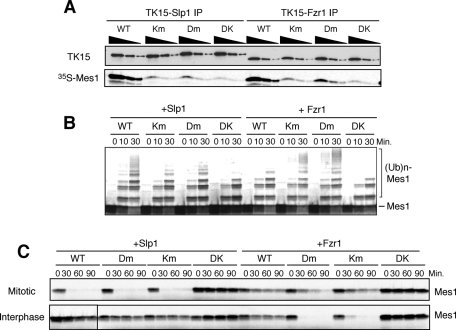
To understand why Fzr1/Mfr1 cannot induce Mes1 ubiquitylation, we first compared the binding affinity between Mes1 and Slp1 or Fzr1/Mfr1. Fzr1/Mfr1 bound Mes1 in a D-box– and KEN-box–dependent manner with an affinity comparable to Slp1 in vitro, and mutation of the D-box and KEN-box completely abolished the interaction (Figure 7A). Even a single mutation of the D-box or KEN-box significantly decreased its binding with Mes1. Next we investigated a role for the D-box and KEN-box of Mes1 in its ubiquitylation. Double mutations of the D-box and KEN-box abolished Mes1 ubiquitylation as well as its destruction induced by Slp1, in agreement with the D-box and KEN-box dependency of the interaction between Mes1 and Slp1 (Figure 7, A–C). However, to our surprise, a single mutation of the D-box or KEN-box showed completely opposite effects on Mes1 ubiquitylation and destruction by Slp1 and Fzr1/Mfr1. APC/C–Slp1 poorly ubiquitylated Mes1 with the single D-box or KEN-box mutation, whereas APC/C–Fzr1/Mfr1 ubiquitylated the single D-box/KEN-box mutant of Mes1 better than WT (intact D-/KEN-boxes) (Figure 7, B and C). Depending on coactivators, the optimal binding and dissociation rates for supporting ubiquitylation of substrates might be different. It is also possible that the bipartite interactions between Mes1 and Slp1 or Fzr1/Mfr1 via its D-box and KEN-box might position Mes1 differently, relative to the active site of the APC/C.
FIGURE 7:
Different relationship between Mes1 and Slp1 or Fzr1/Mfr1 toward Mes1 ubiquitylation and destruction. (A) In vitro binding between coactivators (TK15-tagged Slp1 or TK15-tagged Fzr1) and Mes1 was analyzed. The coactivators and 35S-labeled Mes1 were incubated together, and the activators were immunoprecipitated using anti-TK15 beads. Three dilutions for each immunoprecipitate were analyzed by SDS–PAGE, and copurified Mes1 was determined by autoradiography. KEN-box mutant (Km), D-box mutant (Dm), and KEN-box and D-box double mutant (DK) of Mes1 were used as controls. Similar amounts of WT Mes1 were bound to Slp1 and Fzr1/Mfr1 in a D-box– and KEN-box–dependent manner. (B) Slp1- or Fzr1/Mfr1-dependent ubiquitylation assays were performed using WT, KM, DM, and DK of Mes1 as a substrate. Dm or Km Mes1 protein was more efficiently ubiquitylated than WT Mes1 by S. pombe Fzr1/Mfr1–APC/C. (C) Destruction assays of Mes1 in Xenopus egg extracts from which endogenous APC/C was predepleted and S. pombe APC/C and Slp1 or Fzr1/Mfr1 were supplemented. Fzr1/Mfr1–APC/C destroyed Dm or Km more progressively than WT Mes1.
Slp1 alleviates Mes1 inhibition through ubiquitylation
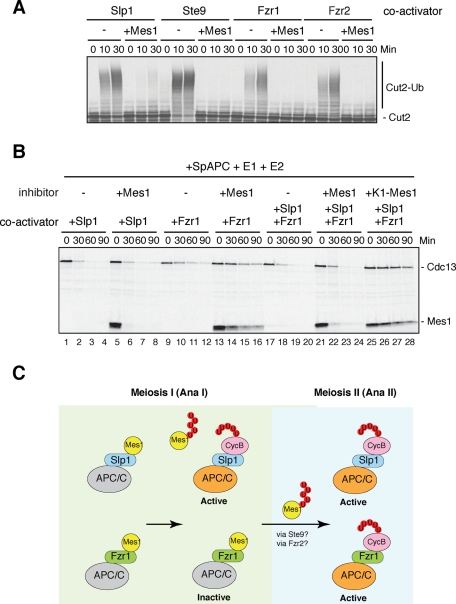
To understand why Mes1 appears to preferentially inhibit Fzr1/Mfr1, we assessed the inhibitory activity of Mes1 toward Slp1 and Fzr1/Mfr1. First, we performed in vitro ubiquitylation assays with Mes1 as an inhibitor. Unlike the in vivo situation, Mes1 equally inhibited ubiquitylation of Cut2 induced by the APC/C with Slp1 and Fzr1/Mfr1 as well as the other coactivators (Figure 8A). In our previous report we showed that expression of the K0- or K1-Mes1 mutant, in which all the ubiquitin acceptor lysine residues (in the case of K1-Mes1, except the one in the KEN-box) are mutated to alanine, arrested cells at metaphase I, suggesting that Mes1 ubiquitylation is required for Slp1-dependent APC/C activation during meiosis I (Figure 2A) (Kimata et al., 2008b). To assess the effect of Mes1 ubiquitylation, we reconstituted the cyclin destruction assay with Mes1 as an inhibitor as well as a substrate. We used Xenopus egg extracts in which endogenous APC/C and Fizzy/Cdc20 had been depleted and S. pombe APC/C, coactivators, E1, and E2s were supplemented. As shown in Figure 8B, we found that Mes1 inhibited Fzr1/Mfr1 more potently than Slp1. In agreement with Figure 6, Mes1 is reasonably stable in the presence of Fzr1/Mfr1 but not in the presence of Slp1 (Figure 8B, compare lanes 5–8 and 13–16). When a cell-free system included both Slp1 and Fzr1/Mfr1, which reflects the conditions of meiosis I, Mes1 failed to inhibit Cdc13 destruction, whereas K1-Mes1 completely inhibited Cdc13 destruction (Figure 8B, compare lanes 21–24 and 25–28). Taken together, our data suggest that while Fzr1/Mfr1 is kept mostly inactive due to its poor ability to stimulate Mes1 ubiquitylation, Slp1 alleviates Mes1 inhibition through its ubiquitylation, leading to a suitable level of APC/C activation for the meiosis I/meiosis II transition.
FIGURE 8:
Slp1, but not Fzr1/Mfr1, overcomes Mes1 inhibition via ubiquitylation. (A) In vitro ubiquitylation assays were performed with 35S-labeled Cut2 as a substrate and Mes1 as an inhibitor. Samples were taken at indicated time points and analyzed by SDS–PAGE followed by autoradiography. Mes1 efficiently inhibited Cut2 ubiquitylation induced by Slp1, Ste9, Fzr1/Mfr1, and Fzr2. (B) Destruction assays were performed using Cdc13 as a substrate as described in Figure 5C except that the coactivator(s), Slp1 and/or Fzr1/Mfr1, and an inhibitor WT or K1-Mes1 were simultaneously added into the extracts. Samples were taken at the indicated time points and analyzed by SDS–PAGE followed by autoradiography. K1-Mes1 has an intact KEN-box in the construct. (C) A model for ubiquitylation-dependent regulation of Slp1 and Fzr1/Mfr1 by Mes1 in meiosis. Mes1 binds both Slp1 and Fzr1/Mfr1 and initially inhibits them as a competitive substrate. Slp1 is able to ubiquitylate Mes1 and escape from complete inhibition, whereas Fzr1/Mfr1 is kept inactive during anaphase I (meiosis I) due to its inability to ubiquitylate Mes1. In meiosis II, when Mes1 is ubiquitylated and degraded, both Slp1 and Fzr1/Mfr2 become active and complete anaphase II (meiosis II).
DISCUSSION
In this article we show that among five APC/C coactivators in fission yeast, Slp1 is solely required for anaphase I onset in meiosis. On the other hand, Fzr1/Mfr1 appears to play a role mainly in meiosis II exit and, possibly, in the sporulation process. We also show that Fzr1/Mfr1 is a critical target of Mes1 and is inhibited to prevent premature APC/C activation during anaphase I. Unlike Slp1–APC/C, Fzr1/Mfr1–APC/C hardly ubiquitylates Mes1, although it is capable of ubiquitylating cyclin B efficiently. Thus Mes1 binds and initially inhibits both Slp1 and Fzr1/Mfr1 as a competitive substrate; however, Slp1 overcomes the inhibition by inducing Mes1 ubiquitylation, whereas Fzr1/Mfr1 remains bound to Mes1 and inactive. Thus we propose that Mes1 is working as a pseudosubstrate inhibitor for Fzr1–APC/C, although Mes1 is a competitive inhibitor for Slp1–APC/C, as we reported before (Kimata et al., 2008b). This distinctive regulation of these two coactivators by Mes1 achieves a proper APC/C activation required for the meiosis I/meiosis II transition (Figure 8C).
Roles of the APC/C coactivators in meiosis
In several eukaryotic cells, meiosis-specific APC/C coactivators have been identified: Fzr1/Mfr1 in S. pombe, Ama1 in Saccharomyces cerevisiae, and Cort and Fzr2 in Drosophila (Cooper et al., 2000; Asakawa et al., 2001; Blanco et al., 2001; Chu et al., 2001). In fission yeast, Fzr1/Mfr1 has been described to play an important role in the exit from meiosis II (Asakawa et al., 2001; Blanco et al., 2001). However, there are two other candidates for APC/C coactivators, Fzr2 (SPAC13G6.08) and Fzr3/Mug55 (SPCC1620.04c), expressed specifically in meiosis (Mata et al., 2002). Consistent with Yamamoto and colleagues (2008), we did not observe significant effects on meiotic progression in fzr2Δ or fzr3Δ strains, although we found a slight delay in the destruction of Mes1 in meiosis II (Figures 1B and 4B). Yet the expression profile of Fzr2 in the synchronized meiosis and the ability of Fzr2 to target Mes1 for destruction in vitro imply that Fzr2 might be a coactivator specialized for eliminating Mes1 (Supplemental Figure S2 and Figure 6C). It is also possible that Fzr2 and Fzr3 have redundant functions toward Mes1 and APC/C substrate proteolysis. In fact, both Mes1 and Cdc13 were more stable in fzr1Δfzr2Δfzr3Δ than in the fzr2Δ or fzr3Δ single deletion strain (Figure 1 and Supplemental Figures S3 and S5). We also analyzed the function of Ste9/Cdh1 in meiosis for the first time in fission yeast. We found that Ste9 was highly phosphorylated from the beginning of meiosis to late meiosis II, suggesting that Ste9 is dissociated from the APC/C for most of the time in meiosis (Supplemental Figure S1). We observed no clear effects on meiotic progression in the Ste9-shutoff strains. Thus, although it has been suggested that its orthologue Cdh1 plays a role in meiosis I progression in mouse oocytes, Ste9 seems not to play a major role in the progression of meiosis in fission yeast (Reis et al., 2007).
What is the role of meiosis-specific APC/C coactivators? We have shown that Fzr1/Mfr1 contributes to the delayed anaphase onset in slp1-NF410 mutants (Figure 2). Hence, although Fzr1/Mfr1 appears dispensable for the meiosis I/meiosis II transition under normal situations, it might be important for the APC/C activation during perturbed conditions so that cells can complete the meiotic program within a certain time frame to produce gametes. In agreement with this notion, Yamamoto et al. (2008) suggested that Fzr1/Mfr1 accelerates anaphase II onset when the spindle checkpoint was activated in meiosis I by a rec12 mutation. Alternatively, meiosis-specific coactivators might play additional roles in meiosis II exit or later processes for gamete production. The fzr1Δ showed a delay in cyclin B/Cdc13 destruction, chromosome segregation in meiosis II exit, and subsequent spore formation (Figure 1) (Asakawa et al., 2001; Blanco et al., 2001). Fzr1/Mfr1 might be required for higher APC/C activity or for the destruction of a subpopulation of cyclin B/Cdc13, which resides in a different cellular location and thus escapes targeting by Cdc20/Slp1, for complete inactivation of Cdc2 during meiosis II exit. It has been suggested that in budding yeast Ama1p targets Ssp1p for prospore membrane closure and is involved in the activation of the mitogen-activated protein kinase Smk1 (McDonald et al., 2005; Diamond et al., 2009). Further understanding of the roles for the meiosis-specific coactivators will require identification of their specific target(s).
The meiosis-specific regulation of the APC/C by its coactivators and inhibitors
The mes1Δfzr1Δ diploid cells went through the meiosis I/meiosis II transition almost as efficiently as WT cells (Figure 3). Mes1 probably inhibits Fzr1/Mfr1 to prevent the premature activation of the APC/C in meiosis I. This antagonism between Mes1 and Fzr1/Mfr1 is similar to that of Mnd2/Apc14 and Ama1 in budding yeast, where Mnd2 specifically inhibits the activity of APC/C–Ama1 and meiosis progresses almost normally in the cells with both genes deleted (Oelschlaegel et al., 2005; Penkner et al., 2005). Because Fzr1/Mfr1 is required for meiosis II exit and subsequent sporulation, Mes1 hinders Fzr1/Mfr1 during meiosis I. We observed that Fzr1/Mfr1 was less active in mitotic extracts than in interphase extracts (Figure 5C), suggesting that Mes1 destruction and the dephosphorylation of Fzr1/Mfr1 might form a more robust mechanism for the rapid APC/C activation required for meiosis II exit. It has been found that the meiosis-specific inhibitor Emi2 inhibits Cdc20 to establish metaphase arrest in mice and frogs. In flies, there exist meiotic coactivators, Cort and Fzr2, which are exclusively expressed and regulate chromosome segregation in oocytes and testis, respectively (Chu et al., 2001; Jacobs et al., 2002). It might be of interest to investigate whether similar antagonistic mechanisms between a specific coactivator and inhibitor exist in metazoans.
Although Mes1 clearly plays an important role in meiosis I progression, it appears that another layer of regulation is also present. For example, our data with mes1Δfzr1Δ imply the existence of other regulatory mechanisms besides Mes1, by which Slp1 function is partly inhibited and thus residual Cdk activity is preserved for the meiosis I/meiosis II transition, even in the absence of Mes1. The activity of the APC/C may be controlled via its localization during the transition. Alternatively, rather than APC/C, Cdk activity might be up-regulated by the suppression of Cdc2 inhibitory phosphorylation, which has been suggested in Xenopus oocytes (Iwabuchi et al., 2000). On the other hand, cells lacking Slp1 function were able to complete meiosis I, although it was delayed, suggesting that Mes1 cannot entirely inhibit Fzr1/Mfr1 function. At present, we do not know the exact identity of this mechanism, but the ability of Mes1 to inhibit Fzr1/Mfr1 may be regulated in some other way.
Different modes of interaction between substrates and APC/C coactivators
In our previous report, we showed that the nonubiquitylatable form of Mes1, K0-Mes1 or K1-Mes1, causes metaphase I arrest by inhibiting Slp1 function, suggesting that Mes1 ubiquitylation is required for anaphase I onset (Kimata et al., 2008b). In this article, we have shown that Slp1 induces Mes1 ubiquitylation more efficiently than Fzr1/Mfr1, which releases Slp1 from Mes1 inhibition but keeps Fzr1/Mfr1 inactive during the meiosis I exit (Figures 6 and 8). Mes1 binds and inhibits Slp1 and Fzr1/Mfr1 to a similar extent in vitro, and both coactivators can induce ubiquitylation of cyclin B/Cdc13 and securin/Cut2 (Figure 5). Notably, Fzr1/Mfr1 disfavors the presence of both the D-box and KEN-box of Mes1 for ubiquitylation; thus mutation in the D-box or KEN-box of Mes1 increases its recognition as a substrate by Fzr1/Mfr1 (Figure 7). A similar observation has been reported with Acm1 in budding yeast. The KEN-box and the third D-box of Acm1 are required for binding to Cdh1, but this suppresses Acm1 ubiquitylation by APC/C–Cdh1, which makes Acm1 a pseudosubstrate inhibitor (Choi et al., 2008; Enquist-Newman et al., 2008; Ostapenko et al., 2008). However, the difference between Mes1 and Acm1 is that Mes1 binds and inhibits Slp1 as well as Fzr1/Mfr1 (Figure 8A), whereas Acm1 cannot bind or inhibit Cdc20 efficiently. This evidence suggests that the efficiency of substrate ubiquitylation by the APC/C is not determined solely by the binding affinity between the coactivators and the substrates. Rather, the position of the substrates relative to the active site of the APC/C or the dynamics or the location of the interaction between a substrate-loaded coactivator and the APC/C core might make a difference in ubiquitylation efficiency. Furthermore, it is noteworthy that Fzr1/Mfr1 has an extremely short N-terminal domain compared with other Fizzy/Cdc20 family members (Figure 1A). Although the C-box is unambiguously the vital element, the N-terminal domain might have an as yet unidentified function in stimulating ubiquitylation of substrates such as Mes1, which Fzr1/Mfr1 might have lost during evolution, thus preventing complete destruction of cyclin B during meiosis I. The roles of the Fizzy/Cdc20 family of coactivators are clearly a key to understanding the APC/C-dependent ubiquitylation of the right substrates at the right time. This is a future topic for investigation.
MATERIALS AND METHODS
Yeast genetics and molecular biology
Methods of handling S. pombe were as described previously (Moreno et al., 1991). All the strains used in this study are listed in Supplemental Table S1. The slp1-NF410, Puhp1-HA-ste9, and fzr1Δ strains were created by PCR-directed methods described previously (Bähler et al., 1998; Ors et al., 2009). Also, fzr2Δ and fzr3Δ were as described previously (Yamamoto et al., 2008).
Synchronous meiosis in pat1-114 diploid cells
The methods of creating zygotic diploids and inducing synchronous meiosis in pat1-114 diploid cells were as described previously (Kitajima et al., 2003). Aliquots of cells were collected and either fixed with 70% ethanol for DNA staining or lysed in 20% trichloroacetic acid for immunoblotting at 15-min intervals during a time course.
Antibodies
Synthetic peptides CLFNKKPKEESTLIR and CMLEGIDGKIDSHLR, which correspond to the C-terminal amino acid sequences of Fzr1/Mfr1 and Fzr2, respectively, were coupled to keyhole limpet hemocyanin (KLH) and injected into rabbits to make anti-Fzr1 (HY81) and anti-Fzr2 (HY84) polyclonal antibodies (Murex Biotech, Kent, UK). Specificity was confirmed by immunoblot analysis of different coactivators prepared by a coupled transcription/translation in reticulocyte lysate (TNT; Promega). To make a monoclonal antibody (mAb) against Ste9 (HY100), a peptide corresponding to the C-terminal Ste9 (CSTMSSPFDPTMKIR) was coupled to KLH and injected into BALB/c mice. Hybridoma cells produced from the spleen cells from a hyperimmune mouse were fused with Sp/0 myeloma cells using standard procedures. Positive clones were identified using immunoadsorbent assays and immunoblotting of S. pombe extracts. The mAb HY100 gave the strongest signal in these assays. Antibodies were used as follows: anti-Ste9 (HY100, 1:1), Fzr1 (HY81, 1:100), Fzr2 (HY84, 1:20), anti-Cdc2 (mAb Y100, 1μg/ml), anti-Cdc13 (1:200), anti-TAT1 (1:400; a gift from Keith Gull, William Dunn School of Pathology, Oxford, UK), anti-PSTAIRE (1:10,000; a gift from Y. Nagahama, National Institute for Basic Biology, Aichi, Japan), and anti-HA (12CA5 or 16B12, 1:500).
Destruction and ubiquitylation assays using purified fission yeast APC/C
S. pombe APC/C was purified from asynchronously growing apc4/lid1+–TAP mts3–1mad2Δmad3Δ strains using a TAP method (Yoon et al., 2002). Substrates and APC/C coactivators were prepared by a coupled transcription/translation in reticulocyte lysate (TNT; Promega). S. pombe maltose-binding protein–tagged E1 (Uba1) and His-tagged E2s (Ubc1, Ubc4, and Ubc11) were expressed in bacteria and purified using amylose resin (New England Biolabs) or Ni-NTA beads (Qiagen) as described by the suppliers. In vitro ubiquitylation assays were performed as described previously (Ors et al., 2009). For in vitro destruction assays, Xenopus cytostatic factor egg extracts were prepared essentially as described (Murray et al., 1989). Xenopus APC/C and Fizzy/Cdc20 were first immunodepleted using anti-Apc3 antibody (mAb, AF3.1) and anti-Fizzy (FZYB50) (Hayes et al., 2006); then purified S. pombe APC/C, E1, and E2 enzymes were added into the predepleted extracts and incubated 10 min before the coactivators were added. The reaction was started by adding substrates, and samples were taken every 30 min for analysis.
Mes1 binding assays
Slp1 and Fzr1/Mfr1 tagged with TK15 peptides (EDTTLDEEFK) at the N-terminus were prepared in coupled transcription/translation in reticulocyte lysates (TNT; Promega). Mes1 was translated and labeled with [35S]methionine in reticulocyte lysates. Slp1 or Fzr and Mes1 were mixed and incubated in binding buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% NP-40, 50 mM NaF, 60 mM glycerophosphate, and 0.1 mM Na3VO4) at 23°C for 10 min. Magnetic beads (Dynabeads Protein A; Invitrogen) coupled with anti-TK15 antibodies were added in the sample and incubated for a further 30 min. The beads were collected by a magnet and washed with binding buffer three times, and the bound proteins were eluted by boiling in 2× sample buffer containing 2% SDS. The samples were run in a dilution series and analyzed by immunoblotting and autoradiography.
Supplementary Material
Acknowledgments
The authors thank Kathy Gould, Yoshitaka Nagahama, and Keith Gull for providing strains and antibodies; Kayoko Tanaka and Yoshinori Watanabe for Puhp1-HA plasmids; Masamitsu Sato and Takashi Toda for pCR2.1-nat plasmid and helpful advice; and Cancer Research UK (CR-UK) support service for making anti-Ste9 mAb. We also thank Ayumu Yamamoto and members of the Yamano laboratory for helpful discussions and critical reading of the manuscript. Y.K. is a recipient of a postdoctoral fellowship for research abroad from the Japan Society for the Promotion of Science. This work was supported by Marie Curie Cancer Care.
Abbreviations used:
- APC/C
anaphase-promoting complex/cyclosome
- KLH
keyhole limpet hemocyanin
- mAb
monoclonal antibody
- TAP
tandem affinity purification
- WT
wild type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-09-0774) on March 9, 2011.
REFERENCES
- Asakawa H, Kitamura K, Shimoda C. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol Genet Genomics. 2001;265:424–435. doi: 10.1007/s004380000429. [DOI] [PubMed] [Google Scholar]
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blanco MA, Pelloquin L, Moreno S. Fission yeast mfr1 activates APC and coordinates meiotic nuclear division with sporulation. J Cell Sci. 2001;114:2135–2143. doi: 10.1242/jcs.114.11.2135. [DOI] [PubMed] [Google Scholar]
- Choi E, Dial JM, Jeong DE, Hall MC. Unique D box and KEN box sequences limit ubiquitination of Acm1 and promote pseudosubstrate inhibition of the anaphase-promoting complex. J Biol Chem. 2008;283:23701–23710. doi: 10.1074/jbc.M803695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Henrion G, Haegeli V, Strickland S. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/Fizzy protein family. Genesis. 2001;29:141–152. doi: 10.1002/gene.1017. [DOI] [PubMed] [Google Scholar]
- Cooper KF, Mallory MJ, Egeland DB, Jarnik M, Strich R. Ama1p is a meiosis-specific regulator of the anaphase promoting complex/cyclosome in yeast. Proc Natl Acad Sci USA. 2000;97:14548–14553. doi: 10.1073/pnas.250351297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond AE, Park JS, Inoue I, Tachikawa H, Neiman AM. The anaphase promoting complex targeting subunit Ama1 links meiotic exit to cytokinesis during sporulation in Saccharomyces cerevisiae. Mol Biol Cell. 2009;20:134–145. doi: 10.1091/mbc.E08-06-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist-Newman M, Sullivan M, Morgan DO. Modulation of the mitotic regulatory network by APC-dependent destruction of the Cdh1 inhibitor Acm1. Mol Cell. 2008;30:437–446. doi: 10.1016/j.molcel.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M, Ohsumi K, Yamamoto TM, Sawada W, Kishimoto T. Residual Cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J. 2000;19:4513–4523. doi: 10.1093/emboj/19.17.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa D, Goto M, Yamashita A, Yamano H, Yamamoto M. Fission yeast Mes1p ensures the onset of meiosis II by blocking degradation of cyclin Cdc13p. Nature. 2005;434:529–533. doi: 10.1038/nature03406. [DOI] [PubMed] [Google Scholar]
- Jacobs H, Richter D, Venkatesh T, Lehner C. Completion of mitosis requires neither fzr/rap nor fzr2, a male germline-specific Drosophila Cdh1 homolog. Curr Biol. 2002;12:1435–1441. doi: 10.1016/s0960-9822(02)01074-6. [DOI] [PubMed] [Google Scholar]
- Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008a;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Kimata Y, Trickey M, Izawa D, Gannon J, Yamamoto M, Yamano H. A mutual inhibition between APC/C and its substrate Mes1 required for meiotic progression in fission yeast. Dev Cell. 2008b;14:446–454. doi: 10.1016/j.devcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Miyazaki Y, Yamamoto M, Watanabe Y. Rec8 cleavage by separase is required for meiotic nuclear divisions in fission yeast. EMBO J. 2003;22:5643–5653. doi: 10.1093/emboj/cdg527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Lyne R, Burns G, Bahler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet. 2002;32:143–147. doi: 10.1038/ng951. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Cooper KF, Winter E. The Ama1-directed anaphase-promoting complex regulates the Smk1 mitogen-activated protein kinase during meiosis in yeast. Genetics. 2005;171:901–911. doi: 10.1534/genetics.105.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morgan DO. The Cell Cycle: Principles of Control. London, UK: New Science Press; 2007. [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Oelschlaegel T, Schwickart M, Matos J, Bogdanova A, Camasses A, Havlis J, Shevchenko A, Zachariae W. The yeast APC/C subunit Mnd2 prevents premature sister chromatid separation triggered by the meiosis-specific APC/C-Ama1. Cell. 2005;120:773–788. doi: 10.1016/j.cell.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Ors A, Grimaldi M, Kimata Y, Wilkinson CR, Jones N, Yamano H. The transcription factor Atf1 binds and activates the APC/C ubiquitin ligase in fission yeast. J Biol Chem. 2009;284:23989–23994. doi: 10.1074/jbc.M109.018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapenko D, Burton JL, Wang R, Solomon MJ. Pseudosubstrate inhibition of the anaphase-promoting complex by Acm1: regulation by proteolysis and Cdc28 phosphorylation. Mol Cell Biol. 2008;28:4653–4664. doi: 10.1128/MCB.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkner AM, Prinz S, Ferscha S, Klein F. Mnd2, an essential antagonist of the anaphase-promoting complex during meiotic prophase. Cell. 2005;120:789–801. doi: 10.1016/j.cell.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Siomos MF, Nasmyth K. Un ménage à quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Reis A, Madgwick S, Chang HY, Nabti I, Levasseur M, Jones KT. Prometaphase APCcdh1 activity prevents nondisjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192–1198. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Schupbach T. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development. 2007;134:891–899. doi: 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BR, Toczyski DP. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20:3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Kitamura K, Hihara D, Hirose Y, Katsuyama S, Hiraoka Y. Spindle checkpoint activation at meiosis I advances anaphase II onset via meiosis-specific APC/C regulation. J Cell Biol. 2008;182:277–288. doi: 10.1083/jcb.200802053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HJ, Feoktistova A, Wolfe BA, Jennings JL, Link AJ, Gould KL. Proteomics analysis identifies new components of the fission and budding yeast anaphase-promoting complexes. Curr Biol. 2002;12:2048–2054. doi: 10.1016/s0960-9822(02)01331-3. [DOI] [PubMed] [Google Scholar]
- Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.