Non–SCF-type F-box protein Roy1 interacts with the Rab5-like small GTPase Ypt52. Skp1 is indispensable for the interaction of Roy1 and Ypt52. Roy1 binds to GDP and the nucleotide-free form of Ypt52 and inhibits the formation of GTP-bound, active Ypt52. Roy1 negatively modulates cell viability and intracellular transport by suppressing Ypt52.
Abstract
Skp1/Cul1/F-box (SCF)–type F-box proteins are a component of the Cullin-RING SCF ubiquitin E3 ligase, which is involved in numerous cellular processes. However, the function of non–SCF-type F-box proteins remains largely unknown. The Rab5-like small guanosine 5′-triphosphatase Vps21/Ypt51 is a key regulator of intracellular transportation; however, deletion of its isoforms, Ypt52 and Ypt53, results in only a modest inhibition of intracellular trafficking. The function of these proteins therefore remains largely elusive. Here we analyze the role of a previously uncharacterized non–SCF-type F-box protein, Roy1/Ymr258c, in cell growth and intracellular transport in Saccharomyces cerevisiae. Roy1 binds to Ypt52 under physiological conditions, and Skp1 is indispensable for the association of Roy1 with Ypt52. The vps21Δ yeast cells exhibit severe deficiencies in cell growth and intracellular trafficking, whereas simultaneous deletion of roy1 alleviates the defects caused by deletion of vps21. However, additional disruption of ypt52 in roy1Δvps21Δ cells largely suppresses the cell growth and trafficking observed in roy1Δvps21Δ cells. We demonstrate that Roy1 interacts with guanosine 5′-diphosphate–bound and nucleotide-free Ypt52 and thereby inhibits the formation of guanosine 5′-triphosphate–bound, active Ypt52. These results thus indicate that Roy1 negatively modulates cell viability and intracellular transport by suppressing Ypt52.
INTRODUCTION
The attachment of ubiquitin to intracellular proteins is a key mechanism in the regulation of many cellular processes. Ubiquitination is an enzymatic cascade sequentially catalyzed by ubiquitin-activating enzyme E1, conjugating enzyme E2, and ligase E3 (Hershko and Ciechanover, 1998; Deshaies, 1999; Joazeiro and Weissman, 2000). Cullin-RING SCF E3 ligase, which widely participates in protein posttranslational modification by polyubiquitination, is assembled by Skp1, Cdc53/Cul1, RING-finger domain–containing Rbx1/Roc1/Hrt1, and F-box proteins (Bai et al., 1996; Feldman et al., 1997; Skowyra et al., 1997; Kamura et al., 1999). In the SCF E3 ligase complex, F-box protein binds to the adaptor protein Skp1 and to the scaffold protein Cdc53/Cul1 and recognizes and recruits substrate to the ubiquitin-loaded E2 to facilitate the transfer of ubiquitin to substrate. Unlike SCF-type F-box proteins, non–SCF-type F-box proteins like Rcy1 possess a conserved F-box domain in the N-terminal region, which can interact with Skp1 but cannot form a complex with Cdc53 and Rbx1 (Willems et al., 2004; Petroski and Deshaies, 2005). Compared with the widely studied SCF-type F-box proteins, the functions of non–SCF-type F-box proteins remain largely unknown.
In eukaryotic cells, endocytosis is a basic process by which extracellular molecules are internalized by specific and highly efficient transportation pathways between intracellular vesicles. Secretory proteins transit between intracellular organelles, from the endoplasmic reticulum (ER) to the Golgi complex, between the Golgi cisternae, and from the Golgi to the cell surface (Mellman, 1995; Wendland et al., 1998). In most cases, both endocytosis and secretion utilize a vesicle-mediated transport system that delivers the specific content to a defined subcellular location. Similar to the lysosome of animal cells, the vacuole of the yeast cell Saccharomyces cerevisiae functions as the major intracellular protein degradation compartment (Bryant and Stevens, 1998). Internalized proteins are delivered to the vacuole to be degraded. Functional enzymes for vacuolar degradation are sorted from those intended for secretory pathways in the late Golgi. These proteins then travel through the late endosome, where they merge with the endocytic pathway to be transferred to the vacuole. Additionally, some internalized receptors recycle back to the plasma membrane (PM), allowing multiple rounds of ligand binding and internalization (Galan et al., 2001).
Guanosine 5′-triphosphatases (GTPases) belonging to the Ypt/Rab family are key regulators of various steps of these transportation pathways (Segev, 2001a, 2001b; Stenmark and Olkkonen, 2001), and the specific subcellular localization of each Rab implies its involvement in a particular transport step. Rabs switch between the inactive guanosine 5′-diphosphate (GDP)–bound and the active, guanosine 5′-triphosphate (GTP)–bound states. Several factors are involved in the spatial and temporal control of Rab activity. Rab escort protein or Rab GDP dissociation inhibitor (Rab GDI) delivers geranylgeranylated Rab to its target membrane, where Rab is activated by specific guanine nucleotide exchange factors. Activated GTP-bound Rab recruits downstream effectors to the membrane (Pereira-Leal and Seabra, 2001; Stenmark, 2009). GTPase-activating protein catalyzes the GTP hydrolysis and returns Rab to its inactive state. GDP-bound Rab is susceptible to extraction from the membrane by Rab GDI, and they form a stable cytosolic complex that serves as a cytoplasmic reservoir of Rab protein. Rab undergoes multiple cycles of the GDP-GTP switch, with corresponding membrane association and disassociation (Ullrich et al., 1994; Delprato et al., 2004; Pfeffer, 2005).
In mammalian systems, there are three isoforms of Rab5 (a, b, and c), and they organize the early steps of endocytic pathways (Bucci et al., 1995). Several specific roles have been attributed to the different isoforms (Carney et al., 2006). Among the 11 Rab proteins in the yeast S. cerevisiae, three Ypts (Vps21/Ypt51, Ypt52, and Ypt53) show strong homology with Rab5 (Singer-Kruger et al., 1994; Bock et al., 2001). Like Rab5, Vps21 is indispensable in both the endocytic and vacuolar protein sorting pathways. Defects in Vps21 result in severe consequences on cell growth and intracellular trafficking; however, defects in two other Rab isoforms, Ypt52 and Ypt53, do not significantly impact cell growth and trafficking. The underlying role of these functionally dormant proteins in cell growth and intracellular transport remains largely elusive (Singer-Kruger et al., 1994). In this study, we demonstrate that a non–SCF-type F-box protein, Ymr258c, renamed Roy1 (repressor of Ypt52), regulates cell viability and intracellular trafficking by inhibiting Ypt52, which may provide an explanation for the dormancy of Ypt52.
RESULTS
F-box protein Roy1 interacts with the small GTPase Ypt52
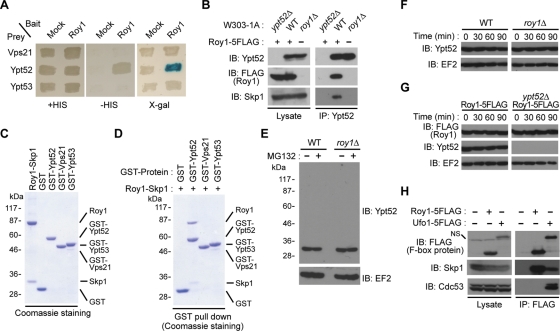
To investigate the function of SCF E3 ligase in S. cerevisiae, we performed a yeast two-hybrid assay using the F-box protein Roy1 as the bait. The GTPase Ypt52 was identified as a candidate substrate, consistent with a previous high-throughput yeast two-hybrid study (Yu et al., 2008). Ypt52, Ypt51/Vps21, and Ypt53 were shown to be homologues of mammalian Rab5 in budding yeast (Singer-Kruger et al., 1994). However, in our yeast two-hybrid assay, the bait protein Roy1 specifically bound to Ypt52 rather than to Vps21 or Ypt53 (Figure 1A).
FIGURE 1:
Roy1 interacts with Ypt52 under physiological conditions. (A) The interaction of the indicated small GTPases with Roy1 was determined with a two-hybrid assay by the expression of the HIS3 gene or the LacZ reporter gene. (B) The indicated yeast cells were lysed using glass beads and a multibead shocker. Lysates were subjected to IP with anti-Ypt52 antibodies, and the resulting precipitates and total cell lysates were subjected to IB analysis with indicated antibodies. (C) The indicated recombinant proteins purified from Sf21 insect cell lysates or E. coli BL21 (DE3) cell lysates were analyzed by SDS–PAGE and visualized with Coomassie staining. The positions of recombinant proteins are indicated. (D) The indicated recombinant proteins were mixed and incubated at 30°C for 30 min. Binding of Ypt52 and the Roy1–Skp1 complex was detected by GST pull-down analyses. (E) The indicated cells were cultured to an OD600 = 0.6 and then incubated in the presence of 0.25% DMSO or 50 μM MG132 for 2 h. Cells were harvested and lysed by the TCA lysis method. Extracts were subjected to IB with indicated antibodies. (F and G) The indicated cells were cultured to an OD600 = 0.8 and then incubated in the presence of 200 μg/ml cycloheximide. Cells were harvested and lysed by the TCA lysis method at the indicated time intervals. Extracts were subjected to IB with the indicated antibodies. (H) The indicated lysates were prepared as described in B and subjected to IP with anti-FLAG antibodies. The resulting precipitates and total cell lysates were subjected to IB with indicated antibodies. NS: nonspecific band.
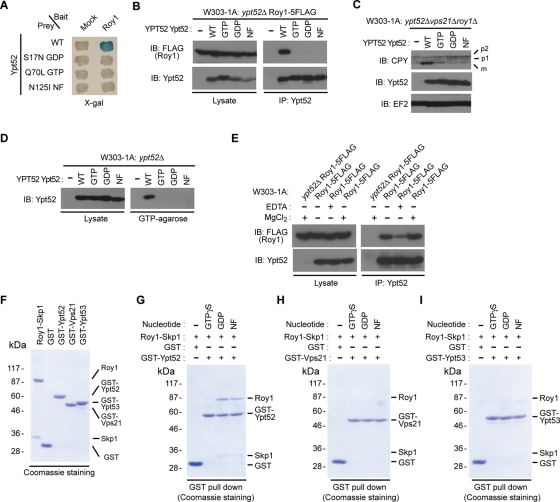
We next explored the interaction between Roy1 and Ypt52 in physiological conditions. Roy1 was tagged with five copies of FLAG at its C-terminus and expressed in wild-type yeast cells. The Roy1-5FLAG construct was fully functional, as the vps21Δ strain with Roy1-5FLAG was indistinguishable from the parent vps21Δ strain with respect to temperature-sensitive (ts) growth and carboxypeptidase Y (CPY) sorting (Supplemental Figure S1, A and B). We also generated rabbit polyclonal antibodies specific for Ypt52, as no band was detected in ypt52Δ cell lysate (Figure 1B). Immunoprecipitation (IP) using anti-Ypt52 antibodies and immunoblot (IB) analyses revealed that endogenous Roy1 interacted with native Ypt52 and Skp1 (Figure 1B). However, in roy1 null mutant cells, no interaction between Ypt52 and Skp1 was detected, indicating that Ypt52 binds to Skp1 through Roy1. To investigate the association between the Roy1–Skp1 complex and Ypt52 in vitro, we mixed recombinant Roy1–Skp1 complex purified from insect cells with recombinant glutathione S-transferase (GST) fusion proteins purified from Escherichia coli and examined the binding of these proteins by GST pull-down assays (Figure 1, C and D). Consistent with the in vivo findings, the Roy1–Skp1 complex specifically bound to GST-Ypt52 but not to GST-Vps21, r GST-Ypt53, or GST alone.
Roy1 is a non–SCF-type F-box protein
As an F-box protein–interacting partner, Ypt52 was thought to be degraded by the ubiquitin-proteasome system via SCFRoy1. Unexpectedly, the addition of MG132 to the culture medium did not up-regulate Ypt52 or result in its ubiquitination (Figure 1E). Furthermore, deletion of roy1 had no effect on the turnover of Ypt52 (Figure 1F). Likewise, Ypt52 did not affect the stability of Roy1 (Figure 1G). These results raised the question of whether Roy1 formed a Cullin-RING SCF E3 ligase. As reported previously (Ivantsiv et al., 2006), the endogenous SCF-type F-box protein Ufo1 interacted with Skp1 and Cdc53, while a larger amount of endogenous Roy1 bound to Skp1 but not to Cdc53 (Figure 1H). These findings indicate that the F-box protein Roy1 does not form an SCF-type E3 complex.
Skp1 is required for the interaction of Roy1 and Ypt52
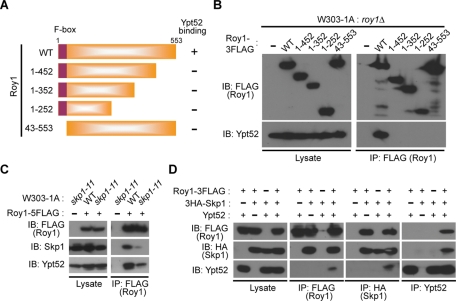
To determine which region of Roy1 interacted with Ypt52, we generated roy1Δ cells that expressed C-terminally 3FLAG-tagged Roy1, wild-type, and a series of F-box domain deletion and C-terminus–deficient mutants from inducible GAL1 promoter (depicted in Figure 2A). Only wild-type Roy1 associated with Ypt52 (Figure 2B). Loss of the F-box domain abolished the binding of Roy1 to Ypt52, which raised two possibilities: Either Roy1 bound directly to Ypt52 via the F-box domain, or Skp1 acted as an intermediary linking Roy1 and Ypt52. In the skp1-11 ts yeast strain, Skp1 is unable to bind F-box adaptors at the restrictive temperature (Siergiejuk et al., 2009). We utilized skp1-11 ts cells to monitor the impact of Skp1 on the binding of Roy1 and Ypt52. Disruption of the interaction between Skp1 and Roy1 clearly decreased the association of Ypt52 with Roy1 by IP assay (Figure 2C). We next transfected mammalian 293T cells with various combinations of Roy1, Ypt52, and Skp1 (Figure 2D). In the absence of Ypt52, Roy1 still bound to Skp1; however, the absence of Skp1 abolished the interaction between Roy1 and Ypt52. On the basis of these observations, we conclude that Skp1 is indispensable for the association of Roy1 with Ypt52 and that both the F-box domain and the C-terminus of Roy1 are required to form the Roy1–Ypt52–Skp1 complex.
FIGURE 2:
Interaction of Skp1 with Roy1 is required for its binding to Ypt52. (A) Schematic representation of Roy1 deletion mutants generated in the present study. (B) Yeast cells were cultured in YPD medium, and then the expression of Roy1 was induced in YPG medium for 12 h. Lysates were subjected to IP with anti-FLAG antibodies, and the resulting precipitates and total cell lysates were subjected to IB with the indicated antibodies. (C) The indicated cells were cultured in YPD medium to an OD600 = 0.5 at 25°C and then at 37°C for 2 h. Lysates were subjected to IP and IB as described in B. (D) At 48 h after transfection with the expression vectors for the indicated proteins, 293T cells were harvested. Lysates were subjected to IP with the indicated antibodies, and the resulting precipitates and total cell lysates were subjected to IB with the indicated antibodies.
The combined deletion of Roy1, Ypt52, and Vps21 influences yeast cell growth
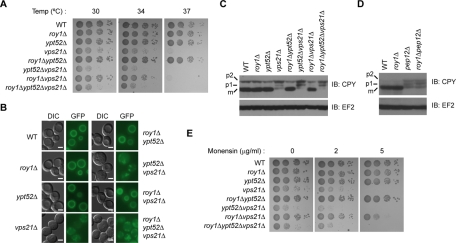
Previous reports have shown that disruption of vps21 results in severe growth defects, while deletion of ypt52 inhibits cell growth only weakly (Singer-Kruger et al., 1994). We therefore wanted to determine whether Roy1 was involved in regulating cell viability via Ypt52 and Vps21. We compared the cell growth rates of the indicated mutants on complete yeast peptone dextrose (YPD) plates at different temperatures (Figure 3A). No clear growth difference was observed among wild-type, roy1Δ, and ypt52Δ cells. Consistent with the previous report (Singer-Kruger et al., 1994), vps21Δ exhibited a significant growth deficiency, especially at high temperatures. The roy1Δypt52Δ cells had similar viability to wild-type cells. However, growth was suppressed in the ypt52Δvps21Δ cells to a slightly larger extent than in the vps21Δ single mutant cells. Surprisingly, deletion of roy1 in the roy1Δvps21Δ cells considerably rescued the growth defect observed in vps21Δ cells at 30°C and 34°C. Additional deletion of the ypt52 gene in roy1Δvps21Δ resulted in an extreme growth deficiency, indicating that Ypt52 can considerably complement the growth-promoting function of Vps21, but only in the absence of Roy1.
FIGURE 3:
Genetic interactions of roy1, ypt52, and vps21 on cell growth and intracellular trafficking. (A) The indicated yeast cells were grown to exponential phase in YPD medium. Cells were harvested and resuspended in YPD medium to an OD600 of 0.4. Three microliters of 10-fold serial dilutions were spotted onto YPD plates, incubated for 36 h at the indicated temperature, and photographed. (B) The indicated yeast cells were stained with FM2-10 as described in Materials and Methods and analyzed by fluorescence microscopy. Bar, 2 μm. (C and D) The indicated yeast cells were harvested and lysed by the TCA lysis method. Extracts were subjected to IB with the indicated antibodies. (E) The growth of the yeast strains was compared as described in A in the presence of the indicated concentration of monensin at 30°C for 36 h.
Roy1, Ypt52, and Vps21 affect the endocytic and vacuolar protein sorting pathways
Mutants of vps21 and ypt52 display delays in both fluid-phase and receptor-mediated endocytosis. Additionally, these small GTPases influence vacuolar protein sorting pathways (Singer-Kruger et al., 1994). We wondered whether Roy1 also affected intracellular trafficking pathways through Ypt52 and Vps21. We first tested whether Roy1, Ypt52, and Vps21 affected the endocytosis of lipophilic FM2-10 from the PM into the vacuole utilizing a series of gene deletion yeast strains. As shown in Figure 3B, in wild-type cells, after 25 min of incubation at 30°C, FM2-10 reached the vacuole, which appears as a fluorescently stained circle. The delivery of FM2-10 from the PM to the vacuole in the roy1Δ or ypt52Δ strains was similar to that observed in wild-type cells. In vps21Δ cells, the dye appeared in dot-like structures rather than in circles, indicating that the endocytic pathway was blocked and the dye remained within the membrane-enclosed vesicles and did not travel to the vacuole. The roy1Δypt52Δ cells exhibited a phenotype similar to the wild-type cells. Consistent with the deficient cell growth at high temperatures, the ypt52Δvps21Δ cells showed more serious defects than vps21Δ in the transport of FM2-10; the dye diffused and irregularly accumulated as spots. However, in the roy1Δvps21Δ cells, the deficiency noticed in the vps21Δ mutant was largely rescued. This rescue was not seen in roy1Δypt52Δvps21Δ cells, suggesting that Ypt52 is responsible for the restoration of endocytosis. The phenotypes of these mutants with respect to endocytosis were consistent with the growth tendencies shown in Figure 3A.
On the basis of the reports that Vps21 was essential for the maturation of the soluble vacuolar marker hydrolase CPY (Graham and Emr, 1991; Stack et al., 1995; Gerrard et al., 2000), we monitored the possible role of Roy1, Ypt52, and Vps21 on vacuolar protein sorting. In the wild-type cells, mainly mature CPY (mCPY) was detected (Figure 3C). Consistent with our previous observations regarding cell growth and FM2-10 transportation, mutant cells demonstrated deficiencies in CPY maturation. The roy1Δ cells, the ypt52Δ cells, and the roy1Δypt52Δ cells displayed nearly normal CPY maturation. In the vps21Δ cells, the total amount of CPY drastically decreased; furthermore, the ratio of mCPY to total CPY dramatically declined, consistent with previous reports that a deficiency in Vps21 caused the missorting of CPY into the extracellular space. Deletion of Ypt52 and Vps21 slightly aggravated the effects of Vps21 deletion on CPY delivery, as the ratio of the p1 form of CPY increased, suggesting that the transport of CPY is inhibited at the early step from the ER to the Golgi complex. However, it is possible that the aberrantly clipped form of p2, which has similar mobility to p1, may increase the ratio of p1. The maturation pathway of CPY, which was disrupted in the vps21Δ mutant, was considerably restored in the roy1Δvps21Δ cells. Consistent with the effects of the triple deletion on growth and FM endocytosis, roy1Δypt52Δvps21Δ cells exhibited severe CPY maturation defects.
To investigate whether the Ypt52-dependent suppression of Roy1 is specific to Vps21, we generated double mutants with a deletion of roy1 and another class D gene, pep12, and examined the ability of these cells to sort CPY. As shown in Figure 3D, CPY delivery was severely inhibited in pep12Δ cells, and the additional deletion of roy1 could not rescue the CPY maturation defect observed in pep12Δ cells. Together, these findings suggest that Ypt52 functions in place of Vps21 in the absence of Roy1.
Given that yeast cells deficient in the trans-Golgi and post-Golgi compartments are highly sensitive to monensin (Muren et al., 2001), we utilized monensin to further confirm the roles of Roy1, Ypt52, and Vps21 in protein trafficking. The growth defects observed in YPD plates at restrictive temperatures were reproduced in plates with 2 and 5 μg/ml monensin (Figure 3E). The poor cell viability caused by blocked intracellular transport, which was observed in both the single mutant vps21Δ cells and the triple mutant roy1Δypt52Δvps21Δ cells, was reversed in the roy1Δvps21Δ cells. These observations suggest that the effects of Ypt52 on endocytosis and vacuolar protein sorting pathways are largely down-regulated by Roy1.
Roy1 negatively modulates the function of Ypt52
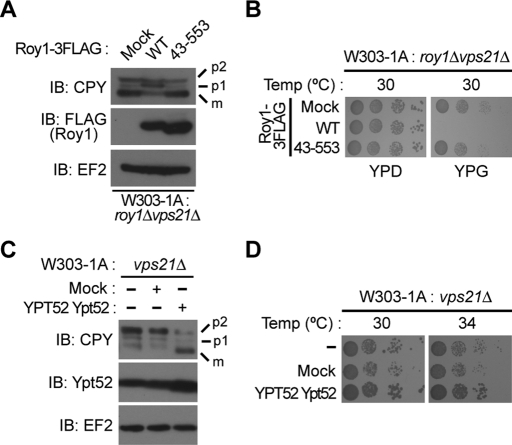
In Figure 3, we showed that deletion of roy1 partly rescued the intracellular transport deficiencies caused by deletion of vps21. To further confirm the function of Roy1 on CPY maturation and cell growth, we generated roy1Δvps21Δ cells that expressed C-terminally 3FLAG-tagged Roy1, wild-type, and an F-box domain deletion mutant, all driven from the GAL1 promoter. As shown in Figure 4A, the introduction of wild-type Roy1 reversed the effects of Roy1 deletion and perturbed the sorting and maturation of CPY. However, the expression of the F-box domain deletion mutant of Roy1 did not alter CPY maturation, which is probably due to the inability of Roy1 lacking the F-box domain to interact with Ypt52. We observed a similar pattern in the cell growth assay (Figure 4B).
FIGURE 4:
Roy1 negatively modulates the function of Ypt52. (A) The indicated cells were cultured in YPD medium and then in YPG for an additional 12 h. Yeast cells were then harvested and lysed by the TCA method. Extracts were subjected to IB with the indicated antibodies. (B) The indicated yeast cells were prepared as described in Figure 3A and spotted onto a YPD plate or a YPG plate, incubated for 48 h at 30°C, and photographed. (C) Cell extracts were subjected to IB with the indicated antibodies. (D) The growth of the indicated yeast cells was compared as described in Figure 3A at the indicated temperatures for 36 h.
If Roy1 inhibits Ypt52, we would predict that overexpression of Ypt52 would alleviate the deficiencies caused by deletion of vps21. Indeed, approximately twofold overexpression of Ypt52 increased the ratio of mCPY in vps21Δ cells (Figure 4C). In addition, overexpression of Ypt52 suppressed the abnormally high temperature sensitivity of vps21Δ cells (Figure 4D). Together with the data presented in Figure 3, these results demonstrate that Roy1 represses the endocytic and vacuolar protein sorting pathways by blocking the function of Ypt52. We next wanted to elucidate the mechanism by which Roy1 inhibits Ypt52.
Ypt52 affects the intracellular localization of Roy1
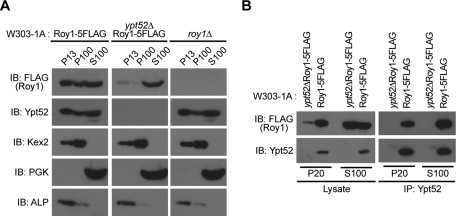
Because a previous report showed that membrane association is necessary for small GTPases to regulate intracellular trafficking (Stenmark, 2009), we hypothesized that Roy1 might affect the dynamic association of Ypt52 to vesicle membranes. We performed fractionation assays to determine the subcellular localizations of Roy1 and Ypt52. The fractions were probed by immunoblotting for Roy1 and Ypt52 together with the maker proteins alkaline phosphatase (ALP) (vacuole), Kex2 (trans-Golgi network), and phosphoglycerate kinase (PGK) (cytosol) (Gerrard et al., 2000) (Figure 5A). As expected, in wild-type cells, ALP and Kex2 were detected predominantly in the P13 and P100 membrane fractions, while PKG was detected primarily in the S100 cytosolic fraction. Similar localization patterns were observed in ypt52Δ and roy1Δ cells. Roy1 and Ypt52 from wild-type cells distributed in the P13, P100, and S100 fractions at a ratio of 2:1:7 and 3.5:1.5:5, respectively. Loss of Roy1 did not cause any difference in the localization of Ypt52; however, loss of Ypt52 reduced the amount of Roy detected in the P13 and P100 membrane fractions. These findings suggest that the ability of Roy1 to associate with membrane is mostly dependent on Ypt52.
FIGURE 5:
Ypt52 affects the subcellular localization of Roy1. (A) The indicated yeast cells were cultured in YPD medium, collected in the exponential growth phase, and subjected to subcellular fractionation to yield P13, P100, and S100 fractions as described in Materials and Methods. The amount of the indicated proteins in each fraction was monitored by IB with anti-FLAG, anti-Ypt52, anti-Kex2, anti-PGK, and anti-ALP. (B) The indicated fractions were subjected to IP with anti-Ypt52 antibodies, and the resulting precipitates and total cell lysates were subjected to IB with indicated antibodies.
Roy1 interacts with Ypt52 in both the membrane and cytosolic fractions
The observation that Roy1 and Ypt52 are localized to both the membrane and the cytoplasm prompted us to examine whether Roy1 could associate with Ypt52 in both fractions. P20 membrane and S100 cytosolic fractions were subjected to IP with antibodies to Ypt52. As shown in Figure 5B, Ypt52 immunoprecipitated a large amount of Roy1 in the S100 cytosolic fraction and slightly less Roy1 in the P20 membrane fraction, suggesting that Roy1 may not completely inhibit the function of Ypt52 in the membrane. These findings are consistent with our observation that additional deletion of ypt52 slightly enhances the defects in cell growth and intracellular trafficking observed in vps21Δ cells (Figure 3).
Roy1 binds to GDP-bound and nucleotide-free Ypt52
Small GTPases switch between active, GTP-bound and inactive, GDP-bound forms. To elucidate the mechanism by which Roy1 inhibits Ypt52, we wanted to determine whether Roy1 interacted with GTP- or GDP-bound Ypt52. We evaluated the interaction between Roy1 and wild-type Ypt52 or three Ypt52 mutants (the inactive, GDP-bound form; the active, GTP-bound form; and a nucleotide-free form) by yeast two-hybrid assay (Figure 6A) and IP (Figure 6B). Roy1 bound to wild-type Ypt52 but not to any of the three mutants. Next, we monitored the effects of the three mutants on CPY maturation (Figure 6C) and binding to GTP-agarose (Figure 6D). Similar to previous observations, wild-type Ypt52 recovered the CPY maturation defects observed in roy1Δypt52Δvps21Δ cells and was affinity purified by GTP-agarose beads. Unexpectedly, the GTP-bound mutant of Ypt52 could not rescue the CPY sorting defects or bind to GTP-agarose beads, indicating that the construct is not functional, probably due to the conformational defects. We thus concluded that these mutants were unable to be used for further experiments.
FIGURE 6:
Roy1 specifically interacts with the GDP and nucleotide-free (NF) forms of Ypt52. (A) The interaction of the indicated Ypt52 wild-type and mutants with Roy1 was monitored with a yeast two-hybrid assay by the expression of the LacZ reporter gene. (B) The indicated cell lysates were subjected to IP with anti-Ypt52, and the resulting precipitates and total cell lysates were subjected to IB with the indicated antibodies. (C) The indicated yeast cells were harvested and lysed by the TCA lysis method. Extracts were subjected to IB with the indicated antibodies. (D) The indicated cell lysates were subjected to GTP-agarose pull-down analyses, and the affinity-purified proteins and lysates were subjected to IB with anti-Ypt52. (E) The indicated cells were lysed and subjected to IP with anti-Ypt52 in the presence of 3 mM EDTA or 5 mM MgCl2, and the resulting precipitates and total cell lysates were subjected to IB with the indicated antibodies. (F) GST fusion proteins were purified in the presence of 10 mM EDTA, and binding to the Roy1–Skp1 complex was analyzed as described in Figure 1C. (G–I) The indicated GST proteins were incubated with 1 mM GTPγS, 1 mM GDP, or no nucleotide at 30°C for 30 min and then mixed with Roy1–Skp1 complex at 30°C for 30 min. Binding of GST proteins and the Roy1–Skp1 complex was detected by GST pull-down analyses.
To clarify the requirement of nucleotide on the association of Roy1 with Ypt52, we carried out cell lysis and IP analyses in the presence of EDTA or magnesium (Figure 6E). The addition of EDTA drastically decreased the binding between Roy1 and Ypt52, while the interaction of Roy1 to Ypt52 was clearly enhanced by the addition of magnesium. These observations suggest that binding of GTP or GDP to Ypt52 increases the association of Ypt52 with Roy1. To further verify the nucleotide preference for the binding of these proteins in vitro, GST fusion proteins were purified from E. coli in the presence of 10 mM EDTA; preloaded with GTPγS, GDP, or no nucleotide (NF); and then incubated with recombinant Roy1–Skp1 complex. The binding of these proteins was analyzed by GST pull-down experiments (Figure 6, F–I). Roy1 associated with a large amount of GDP-bound Ypt52 and slightly less nucleotide-free Ypt52, but GTP-bound Ypt52 interacted with Roy1 very little. We did not detect the interaction of Roy1 with any form of Vps21 or Ypt53, indicating that Ypt52 is a specific interacting partner of Roy1.
Roy1 inhibits the formation of active, GTP-bound Ypt52
The observation that Roy1 associates with the GDP-bound and nucleotide-free forms of Ypt52 raised the possibility that Roy1 might affect the binding of GTP to Ypt52. To investigate this, GST-Ypt52 or GST was incubated with bovine serum albumin (BSA) or Roy1–Skp1 complex and then mixed with [γ-32P] GTP. As shown in Figure 7, the GTP binding to Ypt52 was reduced ∼50% by the addition of Roy1. Together with the data presented in Figure 6, these data demonstrate that Roy1 interacts with GDP-bound and nucleotide-free Ypt52, decreasing the ratio of GTP-bound, active Ypt52, which in turn inhibits the function of Ypt52.
FIGURE 7:
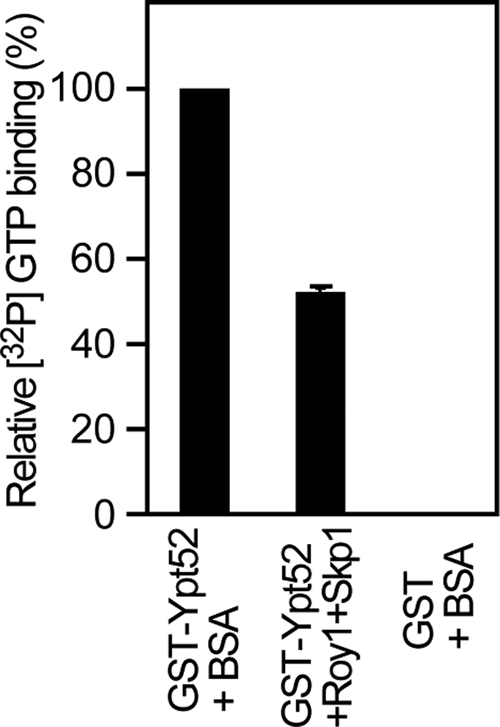
Roy1 inhibits the formation of active, GTP-bound Ypt52. GST-Ypt52 or GST was incubated with BSA or Roy1–Skp1 complex at 30°C for 30 min and then mixed with [γ-32P]GTP at 30°C for 5 min. The amount of protein-bound GTP was analyzed as described in Materials and Methods. Data are expressed as a percentage of the [32P]GTP binding of GST-Ypt52 + BSA and are the means ± SD from three independent experiments.
DISCUSSION
In this study, we demonstrate that Roy1, a previously uncharacterized non–SCF-type F-box protein, interacts with the GDP-bound and nucleotide-free forms of the Rab5-like small GTPase Ypt52, thereby decreasing the formation of GTP-bound, active Ypt52. Thus Roy1 functions as a negative modulator of Ypt52 by interrupting the GDP-GTP cycle of Ypt52. It is interesting to note that Roy1 is partially related to Rab GDI, which manages the recycling of Rab protein by extracting inactive Rab from membrane and delivering it to new transport intermediates. S. cerevisiae has one Rab GDI, GDI1/SEC19, which associates with the GDP form of multiple Rabs, including Vps21 and Ypt7. GDI1 is localized in both cytoplasm and membrane, and its membrane association is Rab independent (Luan et al., 1999). Roy1 preferentially interacts with GDP-bound and nucleotide-free Ypt52, which blocks the access of new GTP and decreases the formation of GTP-bound, active Ypt52. This, in turn, would interrupt the cycling of GDP-bound and GTP-bound Ypt52. Furthermore, the membrane association of Roy1 is strongly dependent on Ypt52. Based on these observations, Roy1 may function as a Ypt52-specific GDI1 inhibitor.
The association between Roy1 and Ypt52 is similar to the association between another non–SCF-type F-box protein, Rcy1, and the Rab11-like small GTPases Ypt31 and Ypt32 (Ypt31/32). As a downstream effector of Ypt31/32, Rcy1 interacts with GTP-bound Ypt31/32 and regulates endocytic membrane traffic and protein recycling (Wiederkehr et al., 2000; Galan et al., 2001; Chen et al., 2005). Additionally, deficiency in Ypt31/Ypt32 decreases the stability of Rcy1. However, Roy1 binds to the GDP-bound and nucleotide-free forms of Ypt52, which indicates that Roy1 does not directly participate in the transport of membrane-coated vesicles as a downstream effector of Ypt52. In addition, Ypt52 does not affect the stability of Roy1 and vice versa. We therefore propose that Roy1 regulates membrane trafficking in a manner different from Rcy1.
This study sheds light on the role of Skp1 and non–SCF-type F-box protein. It is well known that in the SCF complex, Skp1 serves as the adaptor between the F-box protein/substrate and Cdc53/Rbx1. However, in a non-SCF complex, the role of Skp1 remains largely unknown, although one previous report indicates that Skp1 is necessary for Rcy1 in recycling (Galan et al., 2001). To clarify the function of Skp1 in Roy1 binding to Ypt52, we first constructed an F-box domain deletion mutant of Roy1 to disrupt the interaction between Skp1 and Roy1. Inhibition of Roy1–Skp1 binding abolishes the association of Roy1 with Ypt52. We also utilized skp1-11 cells to perturb the interaction between Skp1 and Roy1. Disrupting the binding between Skp1 and Roy1 prevented Ypt52 from coprecipitating with Roy1. Finally, we further confirmed these findings using a mammalian cell system, in which the absence of Skp1 completely inhibited the interaction of Roy1 and Ypt52. These results suggest that Skp1 is indispensable for the binding of the non–SCF-type F-box protein Roy1 to Ypt52. The underlying mechanism for this interaction could be that Skp1 interacting with the F-box domain of Roy1 causes a conformational alteration, which allows Ypt52 to bind to the Roy1–Skp1 complex. The details of this interaction remain to be elucidated by future structural analyses.
The overall homology of Vps21/Ypt51, Ypt52, and Ypt53 is very similar to the corresponding mammalian Rab5 proteins and similar to the Rab5 proteins; these three Ypts have clearly different roles in cell growth and intracellular transportation. Among mutants of the three genes, cells deficient in vps21 display the most severe defects in cell growth and endocytic and vacuolar protein sorting, whereas disruption of ypt52 or ypt53 barely influences these cellular processes. However, the overexpression of Ypt52 could clearly rescue the deficiencies caused by deletion of vps21, suggesting that the function of Ypt52 partially overlaps with that of Vps21. This notion was also supported by the fact that roy1 deletion in vps21Δ cells resulted in the activation of Ypt52 and partially rescued the defective phenotypes caused by vps21 deletion. However, a question remains about the physiological role of Roy1 in inhibiting the function of Ypt52. It is possible that, under normal conditions, Roy1 inhibits nonspecific actions of Ypt52, which would otherwise result in adverse effects in related cellular processes. When cells require increased protein transport through the secretory and/or endocytic pathway, Roy1 would “unlock” and activate Ypt52 to maintain cellular homeostasis. It is essential to determine how the activity of Roy1 function is regulated in response to extra- and/or intracellular signals. We also speculate that the function of another Rab5-like small GTPase, Ypt53, may be modulated by unknown molecules in a manner similar to Ypt52.
Is the function of Roy1 restricted to the modulation of Ypt52 activity? As we observed in cell growth assays on YPD plates at high temperatures and on monensin plates, deletion of roy1 in the triple mutant roy1Δypt52Δvps21Δ cells alleviates the growth deficiencies observed in the double mutant ypt52Δvps21Δ cells. Thus it is possible that Roy1 has other functions besides inhibiting Ypt52. In addition, as shown in Figure 5A, a small amount of Roy1 localizes in the membrane fraction even in the absence of Ypt52. Whether Roy1 has an intrinsic ability to bind directly to the lipid bilayer and whether an unknown factor helps recruit Roy1 to the membrane fraction remain to be investigated. Regardless of the unidentified roles of Roy1, our findings show an expanded function for Roy1 in regulating cell growth and intracellular trafficking by controlling the function of Ypt52.
MATERIALS AND METHODS
Yeast strains and growth conditions
Yeast strains used in this study are listed in Supplemental Table S1. All strains except for L40 were derived from W303-1A. The strains were constructed with the use of standard genetic techniques and grown in either YPD-rich medium (1% yeast extract, 1% peptone, and 2% glucose), YPG-rich medium (1% yeast extract, 1% peptone, and 2% galactose), or SD standard minimal medium (0.67% yeast nitrogen base with 2% glucose and lacking the appropriate amino acids) (Burke et al., 2000).
Antibodies
Polyclonal antibodies to Ypt52 were generated in a rabbit by standard procedures with recombinant Ypt52 (residues 1–234) as an antigen. The mouse monoclonal antibody (mAb) to hemagglutinin (HA) (12CA5) was obtained from Roche (Mannheim, Germany). The mouse mAb to FLAG (M2) was from Sigma-Aldrich (St. Louis, MO). The mouse mAb to Kex2 was from Abcam (Cambridge, UK). Mouse mAbs to ALP and PGK were from Invitrogen (Carlsbad, CA). Rabbit polyclonal antibodies to Cdc53 (y-300) and goat polyclonal antibodies to Skp1 (yC-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies to EF2 were described previously (Inada and Aiba, 2005). Rabbit polyclonal antibodies to CPY were a gift from T. Endo (Nagoya University, Nagoya, Japan).
Plasmid construction
The pLexAKM bait vector was constructed as described previously (Liu et al., 2009). The pRS306GAL1 vector was generated by subcloning the PCR fragment of Gal1 promoter into the SacI-XbaI sites of pRS306. The following vectors were commercially obtained: pJG4-5 (Origene, Rockville, MD), pCI-neo (Promega, Fitchburg, WI), pGEX-6P-1 (GE Healthcare, Little Chalfont, UK), pFastBac HT C (Invitrogen), and pFastBac 1 (Invitrogen). Wild-type or deletion mutants of Roy1 (1–455, 1–352, 1–252, and 45–553) were tagged at their C-termini with 3FLAG and subcloned into the pRS306GAL1 vector. Roy1 tagged with 3FLAG at the N-terminus was subcloned into the pFastBac HT C vector. Skp1 was N-terminally tagged with 3HA before subcloning into the pCI-neo vector. Point mutations of Ypt52 included S17N, Q70L, and N125I.
Yeast two-hybrid system
The yeast strain L40 was transformed with the plasmid pLexA-KM encoding the F-box protein Roy1 and with a budding yeast DNA library in the pJG4-5 vector. The cells were then streaked either on plates lacking histidine (to detect the interaction-dependent activation of HIS3) or on plates containing X-gal (to detect the interaction-dependent expression of β-galactosidase).
Yeast extracts
Yeast cells grown in YPD were collected in the exponential growth phase. Total cell extracts were prepared by the trichloroacetic acid (TCA) lysis method (Avaro et al., 2002). Briefly, cells were resuspended in 20% TCA and homogenized with glass beads. A twofold volume of 5% TCA was then added, and the extracts were centrifuged at 15,000 × g for 10 min at room temperature. The pellets were resuspended in 5% SDS, 40 mM Tris-HCl (pH 8.8), 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, and 10% 2-mercaptoethanol.
IP and IB analyses
Yeast cells were harvested and lysed in a solution containing 40 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM dithiothreitol (DTT), 0.5% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A, and 5 μg/ml aprotinin, using glass beads and a multibead shocker (Yasui Kikai, Osaka, Japan). Lysates were incubated with the indicated antibodies and protein A–Sepharose for 1 h at 4ºC. The beads were then washed three times with a solution containing 40 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM DTT, and 0.5% Triton X-100. Immunoprecipitated proteins were separated by SDS–PAGE, transferred to Hybond-P membranes (GE Healthcare), and subjected to IB analysis. Immune complexes were detected with SuperSignal West Pico or West Dura chemiluminescence reagents (Thermo Scientific, Rockford, IL).
To assess the requirement of nucleotide binding on the association of Roy1 with Ypt52, cell lysis and IP were carried out with the above lysis buffer in the presence of 3 mM EDTA or 5 mM MgCl2.
Transfection in mammalian cells
293T cells were transiently transfected with the indicated plasmids using polyethylenimine (Polysciences, Warrington, PA). At 48 h after transfection, cells were harvested, lysed, and subjected to IP and IB analysis as described above.
FM2-10 staining
An endocytosis assay using the fluorescent dye FM2-10 (Invitrogen) was performed using a modification of a protocol described previously (Vida and Emr, 1995; Wiederkehr et al., 2000). Yeast cells were grown to optical density (OD)600 = 1.0, harvested, and resuspended in cold YPD media. FM2-10 (16 mM in dimethyl sulfoxide [DMSO]) was diluted in H2O and added to 5 OD600 of cells to a final concentration of 40 μM. The cells were then incubated with the dye for 10 min on ice. After internalization, yeast cells were washed with cold YPD media three times to remove surface-bound dye. Cells were further incubated for 25 min at 30°C. Stained cells were collected by centrifugation and mounted with 2.6% low–melting temperature agarose. Cells were placed on slides and visualized with a W-PI 10×/23 objective and a green fluorescent protein filter set (Axio Observer.Z1; Carl Zeiss). Images were analyzed with Axio Vision 4.6.
Subcellular fractionation
Cells were cultured with YPD medium (50 ml), harvested at OD600 = 1, and resuspended in 6 ml of 50 mM Tris-HCl (pH 8.0) containing 1% 2-mercaptoethanol for 10 min at 30ºC. Cells were lysed with 1.5 ml of a lysis buffer containing 0.2 M sorbitol, 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A, and 5 μg/ml aprotinin using glass beads and a multibead shocker. Unbroken cells were removed by centrifugation for 5 min at 500 × g at 4°C. One milliliter of whole cell extract was subjected to centrifugation at 13,000 × g for 10 min at 4°C to yield a P13 (pellet) fraction. The resulting supernatant fraction was subjected to centrifugation at 100,000 × g for 30 min at 4°C, yielding P100 (pellet) and S100 (supernatant) fractions. The P13 and P100 pellets were resuspended in 300 μl of 8 M urea, 5% SDS, 40 mM Tris-HCl (pH 6.8), 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, and 10% 2-mercaptoethanol. Proteins in the S100 fraction were precipitated with 10% TCA and resuspended in 300 μl of 8 M urea, 5% SDS, 40 mM Tris-HCl (pH 6.8), 0.1 mM EDTA, 0.4 mg/ml bromphenol blue, and 10% 2-mercaptoethanol.
To yield the fractions P20 and S100 for the IP, 100 OD of cells were lysed in 3 ml of the lysis buffer described previously in this article, using glass beads and a multibead shocker. Unbroken cells were removed by centrifugation for 5 min at 500 × g at 4°C. Then 2 ml whole cell extract was subjected to centrifugation at 20,000 × g for 20 min at 4°C to yield the P20 (pellet) fraction. The resulting supernatant fraction was subjected to centrifugation at 100,000 × g for 30 min at 4°C. The S100 (supernatant) fraction was collected and adjusted to 0.2 M sorbitol, 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, and 0.5% Triton X-100. The P20 pellet was resuspended in 2 ml of 0.2 M sorbitol, 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, and 0.5% Triton X-100.
GTP-agarose pull-down assay
Yeast cells were harvested and lysed in a solution containing 40 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.5% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A, and 5 μg/ml aprotinin, using glass beads and a multibead shocker (Yasui Kikai). Lysates were incubated with GTP-agarose (Sigma-Aldrich) for 1 h at 4ºC. The beads were then washed three times with a solution containing 40 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 0.5% Triton X-100. Affinity-purified proteins were subjected to IB analysis as described above.
In vitro binding assay
The Roy1–Skp1 complex was purified by Ni2+-agarose chromatography (Qiagen, Hilden, Germany) from lysates of Sf21 cells that had been coinfected with baculoviruses encoding His6-3FLAG-Roy1 and Skp1. GST-Ypt52, GST-Vps21, GST-Ypt53, or GST was expressed in E. coli BL21 (DE3) and purified by glutathione-Sepharose (GE Healthcare) affinity chromatography. For the in vitro binding experiments between GST fusion proteins and the Roy1–Skp1 complex, 3 μg GST-protein was mixed with 7 μg Roy1–Skp1 complex in a 25-μl reaction containing 40 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100, and 10% (vol/vol) glycerol for 30 min at 30°C and then subjected to affinity purification with glutathione-Sepharose. To examine the requirement for nucleotide binding on the association of GST-protein to the Roy1–Skp1 complex, GST-protein was purified from E. coli BL21 (DE3) in the presence of 10 mM EDTA to dissociate the nucleotide. After dialysis with a solution containing 40 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10% (vol/vol) glycerol, 3 μg GST-protein was incubated at 30°C for 30 min in a 25-μl reaction containing 40 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100, 10% (vol/vol) glycerol, and 1 mM GTP, GDP, or no nucleotide. The protein was then mixed with 7 μg Roy1–Skp1 complex at 30°C for 30 min.
GTP binding assay
Three micrograms of GST-Ypt52 or GST were incubated with 7 μg BSA or Roy1–Skp1 complex in a 25-μl reaction containing 40 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100, and 10% (vol/vol) glycerol for 30 min at 30°C. The protein was then mixed with 66 μM [γ-32P] GTP (8.8 × 105 cpm/μl) for 5 min at 30°C. The reactions were filtered through nitrocellulose membranes (0.45-μm pore), which were then washed three times with a 5 ml solution containing 40 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 5 mM MgCl2. The amount of radioactivity associated with the membranes was measured using a liquid scintillation counter.
Supplementary Material
Acknowledgments
We thank Mike Tyers and Yoshiko Kikuchi for yeast strains, Toshiya Endo for antibodies to CPY, Tohru Yoshihisa for technical advice for the GTP-binding assay, and Mie Hirano for help in preparation of the manuscript. This work was supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan; the Sumitomo Foundation; the Uehara Foundation; and the Saibou Kagaku Foundation.
Abbreviations used:
- ALP
alkaline phosphatase
- BSA
bovine serum albumin
- CPY
carboxypeptidase Y
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- ER
endoplasmic reticulum
- GDI
GDP dissociation inhibitor
- GDP
guanosine 5′-diphosphate
- GST
glutathione S-transferase
- GTP
guanosine 5′-triphosphate
- GTPase
guanosine 5′-triphosphatase
- HA
hemagglutinin
- IB
immunoblot
- IP
immunoprecipitation
- mAb
monoclonal antibody
- mCPY
mature CPY
- OD
optical density
- PGK
phosphoglycerate kinase
- PM
plasma membrane
- Roy1
repressor of Ypt52
- TCA
trichloroacetic acid
- ts
temperature sensitive
- YPD
yeast peptone dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-08-0716) on March 9, 2011.
Y. Liu, K. Nakatuskasa, M. Kotera, T. Kishi, S. Mimura, and T. Kamura conceived and designed experiments. Y. Liu, K. Nakatuskasa, M. Kotera, A. Kanada, T. Nishimura, T. Kishi, and T. Kamura performed the experiments. Y. Liu, K. Nakatuskasa, M. Kotera, and T. Kamura analyzed data. Y. Liu, K. Nakatuskasa, and T. Kamura wrote the article.
REFERENCES
- Avaro S, Belgareh-Touze N, Sibella-Arguelles C, Volland C, Haguenauer-Tsapis R. Mutants defective in secretory/vacuolar pathways in the EUROFAN collection of yeast disruptants. Yeast. 2002;19:351–371. doi: 10.1002/yea.838. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Lutcke A, Steele-Mortimer O, Olkkonen VM, Dupree P, Chiariello M, Bruni CB, Simons K, Zerial M. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. doi: 10.1016/0014-5793(95)00477-q. [DOI] [PubMed] [Google Scholar]
- Burke D, Dawson D, Stearns T. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Carney DS, Davies BA, Horazdovsky BF. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol Biol Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprato A, Merithew E, Lambright DG. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell. 2004;118:607–617. doi: 10.1016/j.cell.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ. SCF and Cullin/RING H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Galan JM, Wiederkehr A, Seol JH, Haguenauer-Tsapis R, Deshaies RJ, Riezman H, Peter M. Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol Cell Biol. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard SR, Bryant NJ, Stevens TH. VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol Biol Cell. 2000;11:613–626. doi: 10.1091/mbc.11.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Inada T, Aiba H. Translation of aberrant mRNAs lacking a termination codon or with a shortened 3′-UTR is repressed after initiation in yeast. EMBO J. 2005;24:1584–1595. doi: 10.1038/sj.emboj.7600636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivantsiv Y, Kaplun L, Tzirkin-Goldin R, Shabek N, Raveh D. Unique role for the UbL-UbA protein Ddi1 in turnover of SCFUfo1 complexes. Mol Cell Biol. 2006;26:1579–1588. doi: 10.1128/MCB.26.5.1579-1588.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Kamura T, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- Liu Y, Mimura S, Kishi T, Kamura T. A longevity protein, Lag2, interacts with SCF complex and regulates SCF function. EMBO J. 2009;28:3366–3377. doi: 10.1038/emboj.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan P, Balch WE, Emr SD, Burd CG. Molecular dissection of guanine nucleotide dissociation inhibitor function in vivoRab-independent binding to membranes and role of Rab recycling factors. J Biol Chem. 1999;274:14806–14817. doi: 10.1074/jbc.274.21.14806. [DOI] [PubMed] [Google Scholar]
- Mellman I. Molecular sorting of membrane proteins in polarized and nonpolarized cells. Cold Spring Harb Symp Quant Biol. 1995;60:745–752. doi: 10.1101/sqb.1995.060.01.080. [DOI] [PubMed] [Google Scholar]
- Muren E, Oyen M, Barmark G, Ronne H. Identification of yeast deletion strains that are hypersensitive to brefeldin A or monensin, two drugs that affect intracellular transport. Yeast. 2001;18:163–172. doi: 10.1002/1097-0061(20010130)18:2<163::AID-YEA659>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Structural clues to Rab GTPase functional diversity. J Biol Chem. 2005;280:15485–15488. doi: 10.1074/jbc.R500003200. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol. 2001a;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/Rab GTPases: regulators of protein trafficking. Sci STKE. 2001b;2001:re11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Siergiejuk E, Scott DC, Schulman BA, Hofmann K, Kurz T, Peter M. Cullin neddylation and substrate-adaptors counteract SCF inhibition by the CAND1-like protein Lag2 in Saccharomyces cerevisiae. EMBO J. 2009;28:3845–3856. doi: 10.1038/emboj.2009.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Kruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo JS, Gallwitz D, Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2:REVIEWS3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Emr SD, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Avaro S, Prescianotto-Baschong C, Haguenauer-Tsapis R, Riezman H. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J Cell Biol. 2000;149:397–410. doi: 10.1083/jcb.149.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems AR, Schwab M, Tyers M. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Yu H. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.