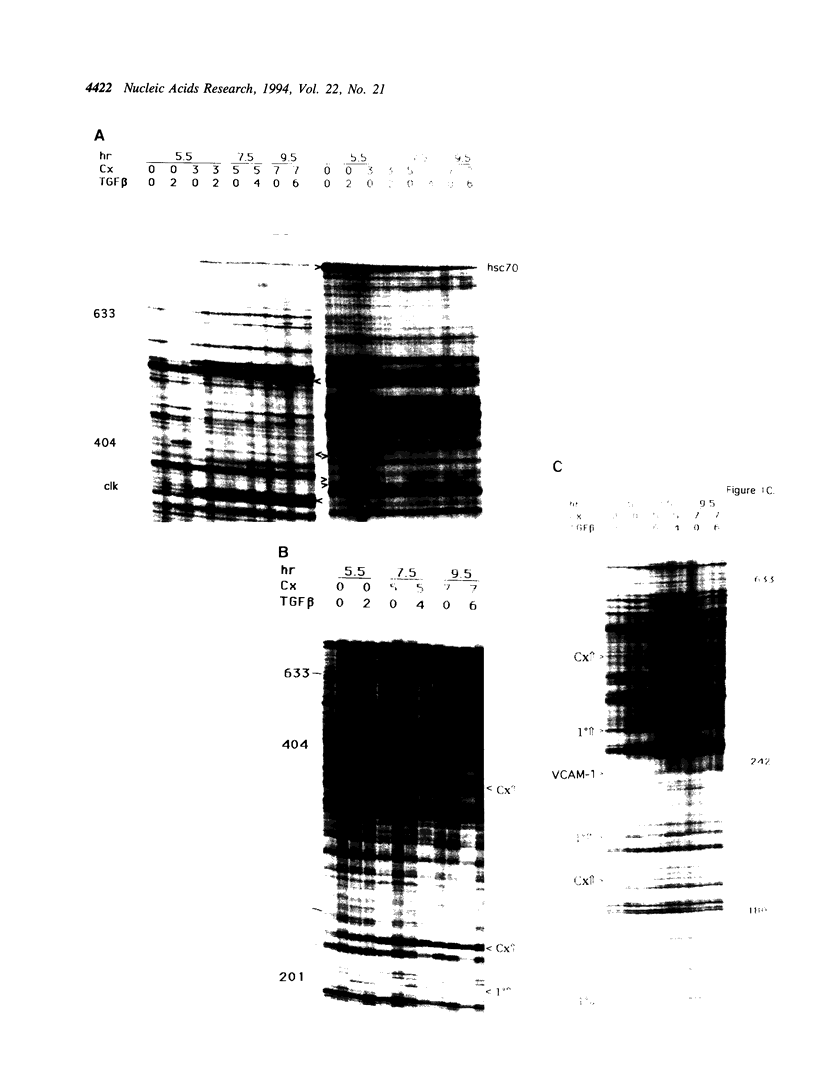
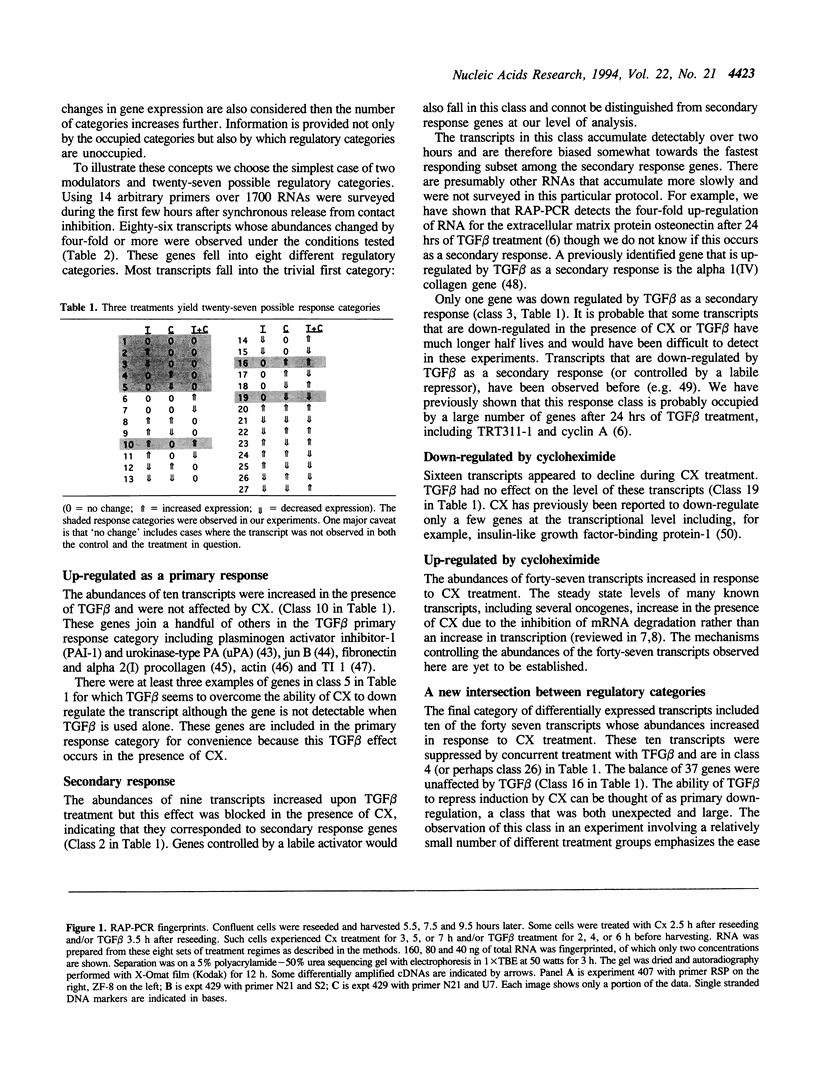
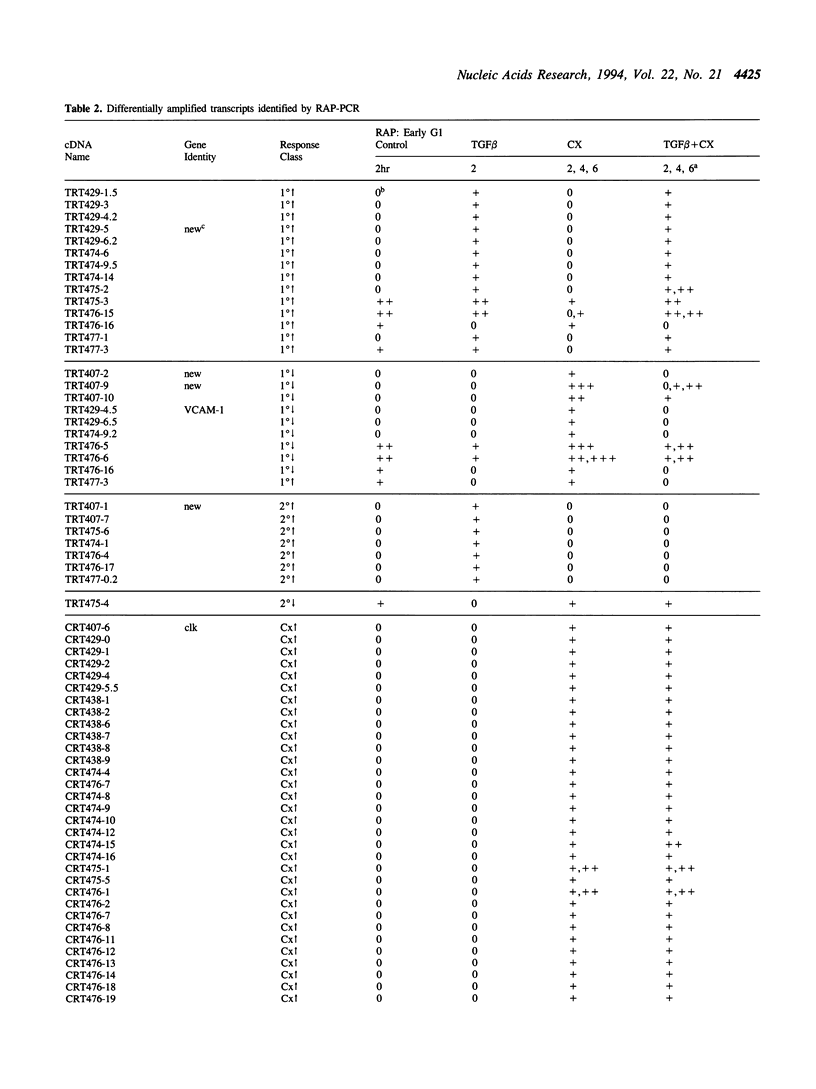
Abstract
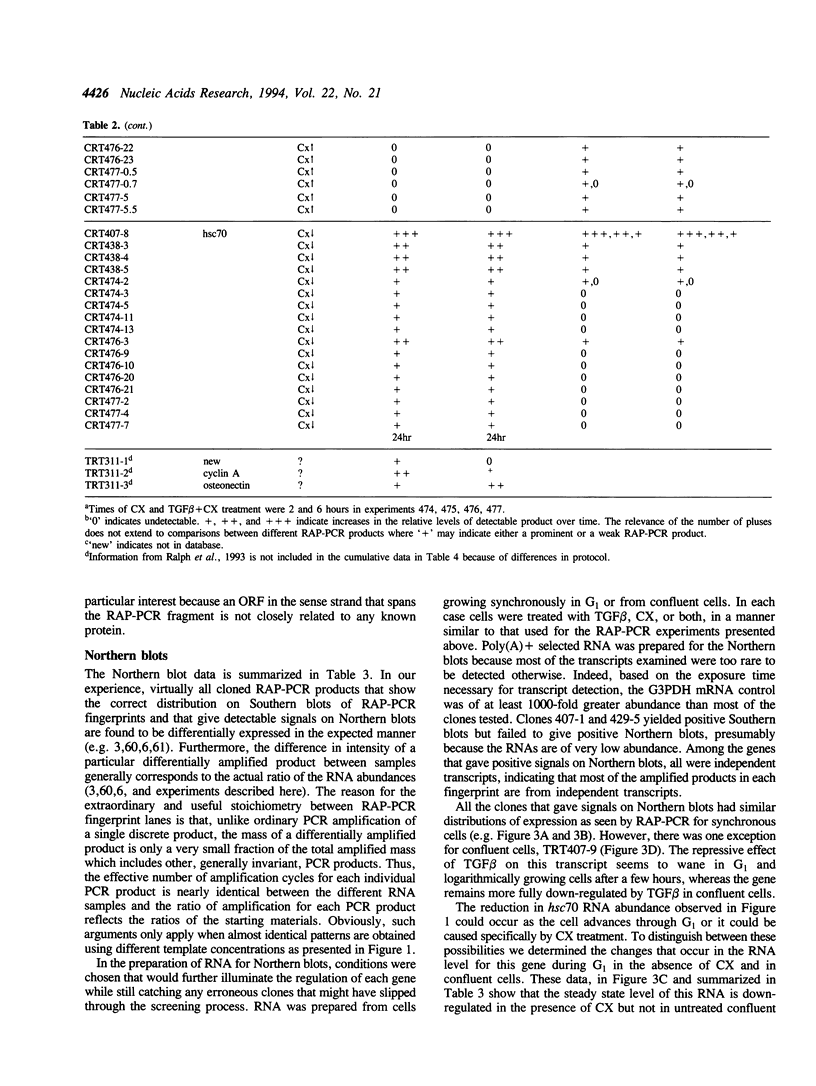
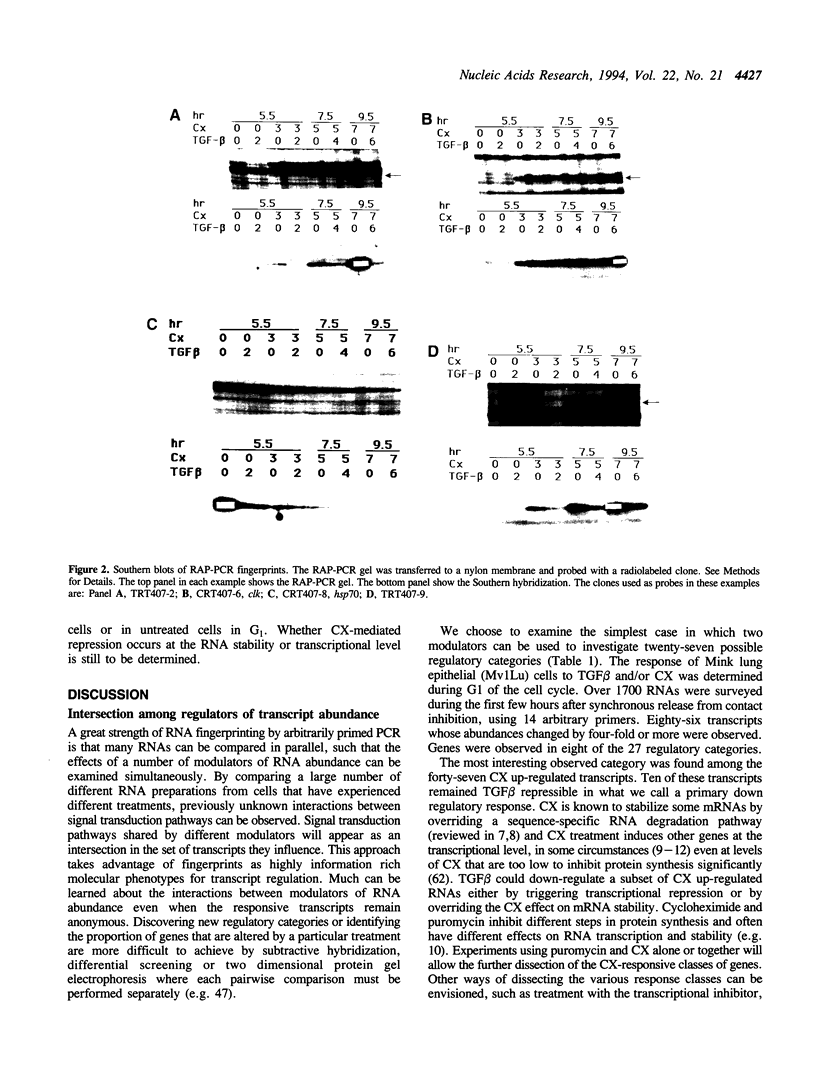
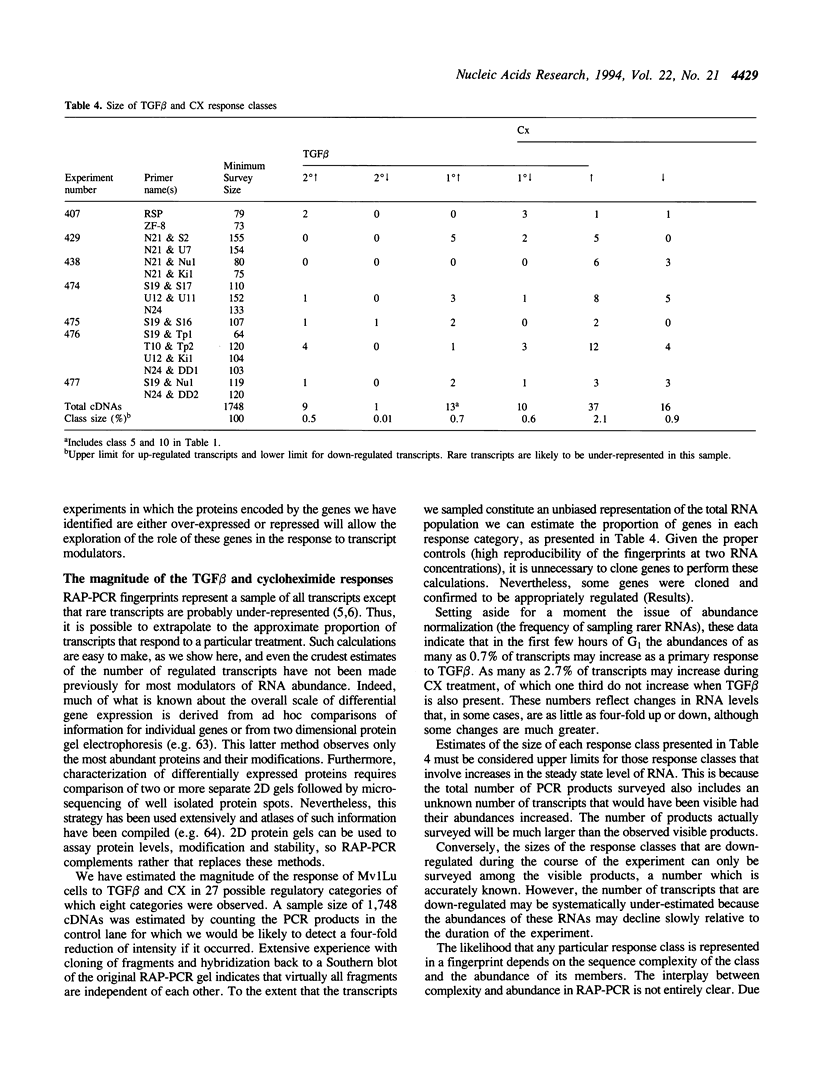
Using RNA fingerprinting by arbitrarily primed PCR it is possible to infer convergent transcript regulatory pathways from the coordinate behavior of subsets of anonymous transcripts without cloning any genes. The number of transcripts in each response category can be estimated. The same may be true for differential display. We demonstrate these claims by treating a cell line with two known modulators of RNA abundance, transforming growth factor-beta (TGF beta) and cycloheximide (CX), used together and alone. The responses of over 1700 anonymous transcripts were monitored under these three conditions and in an untreated control. Eight of the twenty-seven [3(3)] possible transcript response categories were observed among 86 differentially expressed transcripts. For example, CX stabilizes or induces as many as 2.7% of transcripts of which about one third do not accumulate when TGF beta is also present. This intersection may reflect CX stabilization or induction of an important class of RNAs that otherwise usually have short half-lives. We predict that RNAs in this class constitute the majority of transcripts targeted for rapid down regulation in response to TGF beta and perhaps most other natural transcriptional modulators.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. H., Zeheb R., Gelehrter T. D., Heaton J. H., Fukumoto S., Yee J. A., Martin T. J. Transforming growth factor beta inhibits plasminogen activator (PA) activity and stimulates production of urokinase-type PA, PA inhibitor-1 mRNA, and protein in rat osteoblast-like cells. J Cell Physiol. 1991 Oct;149(1):34–43. doi: 10.1002/jcp.1041490106. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. D., Sheng H. M., Alam T., Townsend C. M., Jr, Papaconstantinou J. Posttranscriptional regulation of albumin and alpha-fetoprotein messenger RNA by transforming growth factor-beta 1 requires de novo RNA and protein synthesis. Mol Endocrinol. 1992 Nov;6(11):1789–1796. doi: 10.1210/mend.6.11.1282669. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Letwin K., Tannock L., Bernstein A., Pawson T. A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 1991 Feb;10(2):317–325. doi: 10.1002/j.1460-2075.1991.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereta J., Bereta M., Cohen S., Cohen M. C. Regulation of VCAM-1 expression and involvement in cell adhesion to murine microvascular endothelium. Cell Immunol. 1993 Apr 1;147(2):313–330. doi: 10.1006/cimm.1993.1072. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Rasmussen H. H., Olsen E., Madsen P., Leffers H., Honoré B., Dejgaard K., Gromov P., Hoffmann H. J., Nielsen M. The human keratinocyte two-dimensional gel protein database: update 1993. Electrophoresis. 1993 Nov;14(11):1091–1198. doi: 10.1002/elps.11501401178. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Couffinhal T., Duplàa C., Moreau C., Lamazière J. M., Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ Res. 1994 Feb;74(2):225–234. doi: 10.1161/01.res.74.2.225. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Dittel B. N., McCarthy J. B., Wayner E. A., LeBien T. W. Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1). Blood. 1993 May 1;81(9):2272–2282. [PubMed] [Google Scholar]
- Dogra S. C., Hahn C. N., May B. K. Superinduction by cycloheximide of cytochrome P4502H1 and 5-aminolevulinate synthase gene transcription in chick embryo liver. Arch Biochem Biophys. 1993 Jan;300(1):531–534. doi: 10.1006/abbi.1993.1073. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Khew-Goodall Y., Vadas M. A. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J Immunol. 1993 May 15;150(10):4494–4503. [PubMed] [Google Scholar]
- Gonnet G. H., Cohen M. A., Benner S. A. Exhaustive matching of the entire protein sequence database. Science. 1992 Jun 5;256(5062):1443–1445. doi: 10.1126/science.1604319. [DOI] [PubMed] [Google Scholar]
- Grande J., Melder D., Zinsmeister A., Killen P. Transforming growth factor-beta 1 induces collagen IV gene expression in NIH-3T3 cells. Lab Invest. 1993 Oct;69(4):387–395. [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Howe P. H., Draetta G., Leof E. B. Transforming growth factor beta 1 inhibition of p34cdc2 phosphorylation and histone H1 kinase activity is associated with G1/S-phase growth arrest. Mol Cell Biol. 1991 Mar;11(3):1185–1194. doi: 10.1128/mcb.11.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z. W., Hoffman B. B. Cycloheximide induces the alpha 1B adrenergic receptor gene by activation of transcription in DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1993 Dec;44(6):1105–1112. [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. W., Smith K. A. Molecular cloning of a novel human cdc2/CDC28-like protein kinase. J Biol Chem. 1991 Feb 25;266(6):3402–3407. [PubMed] [Google Scholar]
- Kallin B., de Martin R., Etzold T., Sorrentino V., Philipson L. Cloning of a growth arrest-specific and transforming growth factor beta-regulated gene, TI 1, from an epithelial cell line. Mol Cell Biol. 1991 Oct;11(10):5338–5345. doi: 10.1128/mcb.11.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Kramer I. M., Koornneef I., de Vries C., de Groot R. P., de Laat S. W., van den Eijnden-van Raaij A. J., Kruijer W. Phosphorylation of nuclear protein is an early event in TGF beta 1 action. Biochem Biophys Res Commun. 1991 Mar 29;175(3):816–822. doi: 10.1016/0006-291x(91)91638-s. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Landesman Y., Pagano M., Draetta G., Rotter V., Fusenig N. E., Kimchi A. Modifications of cell cycle controlling nuclear proteins by transforming growth factor beta in the HaCaT keratinocyte cell line. Oncogene. 1992 Aug;7(8):1661–1665. [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McClelland M., Welsh J. RNA fingerprinting by arbitrarily primed PCR. PCR Methods Appl. 1994 Aug;4(1):S66–S81. doi: 10.1101/gr.4.1.s66. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Nilsen-Hamilton M. Transforming growth factor-beta and its actions on cellular growth and differentiation. Curr Top Dev Biol. 1990;24:95–136. [PubMed] [Google Scholar]
- Oguchi S., Weisz A., Esumi H. Enhancement of inducible-type NO synthase gene transcription by protein synthesis inhibitors. Activation of an intracellular signal transduction pathway by low concentrations of cycloheximide. FEBS Lett. 1994 Feb 7;338(3):326–330. doi: 10.1016/0014-5793(94)80293-9. [DOI] [PubMed] [Google Scholar]
- Ooi G. T., Brown D. R., Suh D. S., Tseng L. Y., Rechler M. M. Cycloheximide stabilizes insulin-like growth factor-binding protein-1 (IGFBP-1) mRNA and inhibits IGFBP-1 transcription in H4-II-E rat hepatoma cells. J Biol Chem. 1993 Aug 5;268(22):16664–16672. [PubMed] [Google Scholar]
- Osipovich O. A., Fegeding K. V., Misuno N. I., Kolesnikova T. S., Savostin I. K., Sudarikov A. B., Voitenok N. N. Differential action of cycloheximide and activation stimuli on transcription of tumor necrosis factor-alpha, IL-1 beta, IL-8, and P53 genes in human monocytes. J Immunol. 1993 Jun 1;150(11):4958–4965. [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ralph D., McClelland M., Welsh J. RNA fingerprinting using arbitrarily primed PCR identifies differentially regulated RNAs in mink lung (Mv1Lu) cells growth arrested by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10710–10714. doi: 10.1073/pnas.90.22.10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. H., van Damme J., Puype M., Gesser B., Celis J. E., Vandekerckhove J. Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes. Electrophoresis. 1992 Dec;13(12):960–969. doi: 10.1002/elps.11501301199. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Sensel M. G., Binder R., Lazier C. B., Williams D. L. Reactivation of apolipoprotein II gene transcription by cycloheximide reveals two steps in the deactivation of estrogen receptor-mediated transcription. Mol Cell Biol. 1994 Mar;14(3):1733–1742. doi: 10.1128/mcb.14.3.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Stone B., Wharton W. Targeted RNA fingerprinting: the cloning of differentially-expressed cDNA fragments enriched for members of the zinc finger gene family. Nucleic Acids Res. 1994 Jul 11;22(13):2612–2618. doi: 10.1093/nar/22.13.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka I. M., Hightower L. E. Regulation of chicken Hsp70 and Hsp90 family gene expression by transforming growth factor-beta 1. J Cell Physiol. 1993 Apr;155(1):54–62. doi: 10.1002/jcp.1041550108. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Welsh J., Chada K., Dalal S. S., Cheng R., Ralph D., McClelland M. Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res. 1992 Oct 11;20(19):4965–4970. doi: 10.1093/nar/20.19.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Genomic fingerprinting using arbitrarily primed PCR and a matrix of pairwise combinations of primers. Nucleic Acids Res. 1991 Oct 11;19(19):5275–5279. doi: 10.1093/nar/19.19.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Petersen C., McClelland M. Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res. 1991 Jan 25;19(2):303–306. doi: 10.1093/nar/19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K., McClelland M. Stress-inducible gene of Salmonella typhimurium identified by arbitrarily primed PCR of RNA. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):639–643. doi: 10.1073/pnas.91.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Wuthrich R. P., Jenkins T. A., Snyder T. L. Regulation of cytokine-stimulated vascular cell adhesion molecule-1 expression in renal tubular epithelial cells. Transplantation. 1993 Jan;55(1):172–177. doi: 10.1097/00007890-199301000-00032. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Zentella A., Weis F. M., Ralph D. A., Laiho M., Massagué J. Early gene responses to transforming growth factor-beta in cells lacking growth-suppressive RB function. Mol Cell Biol. 1991 Oct;11(10):4952–4958. doi: 10.1128/mcb.11.10.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]