Abstract
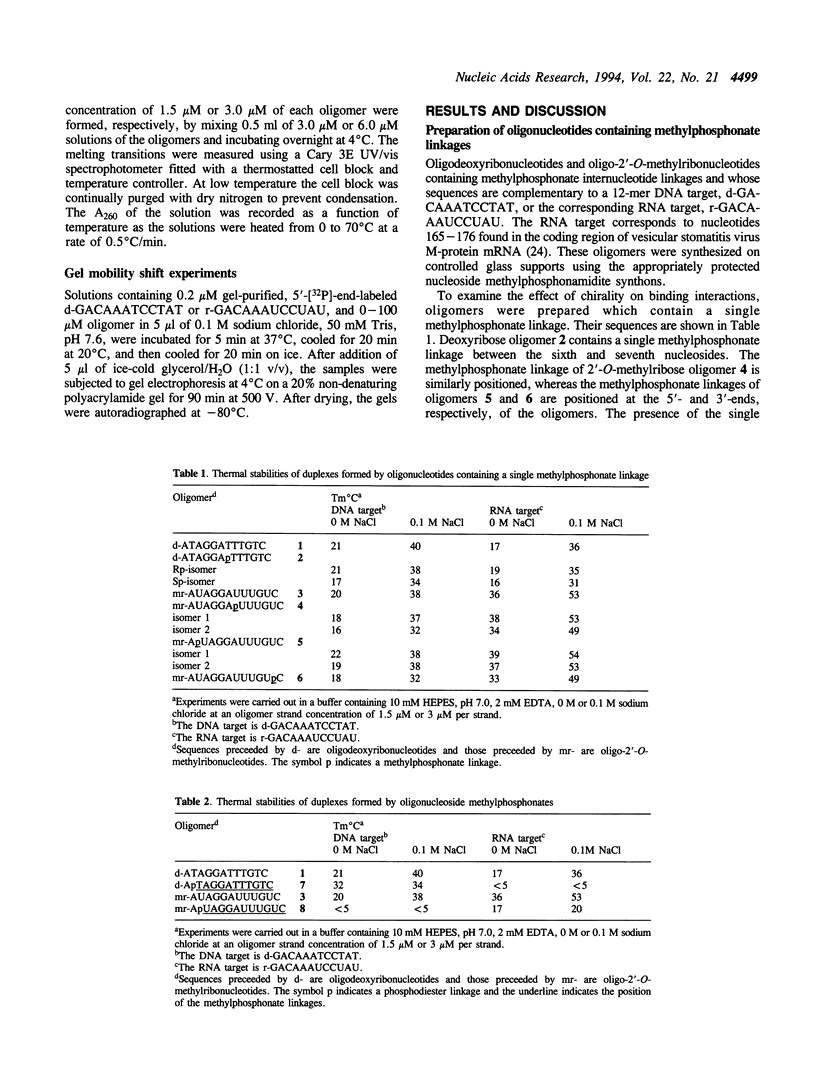
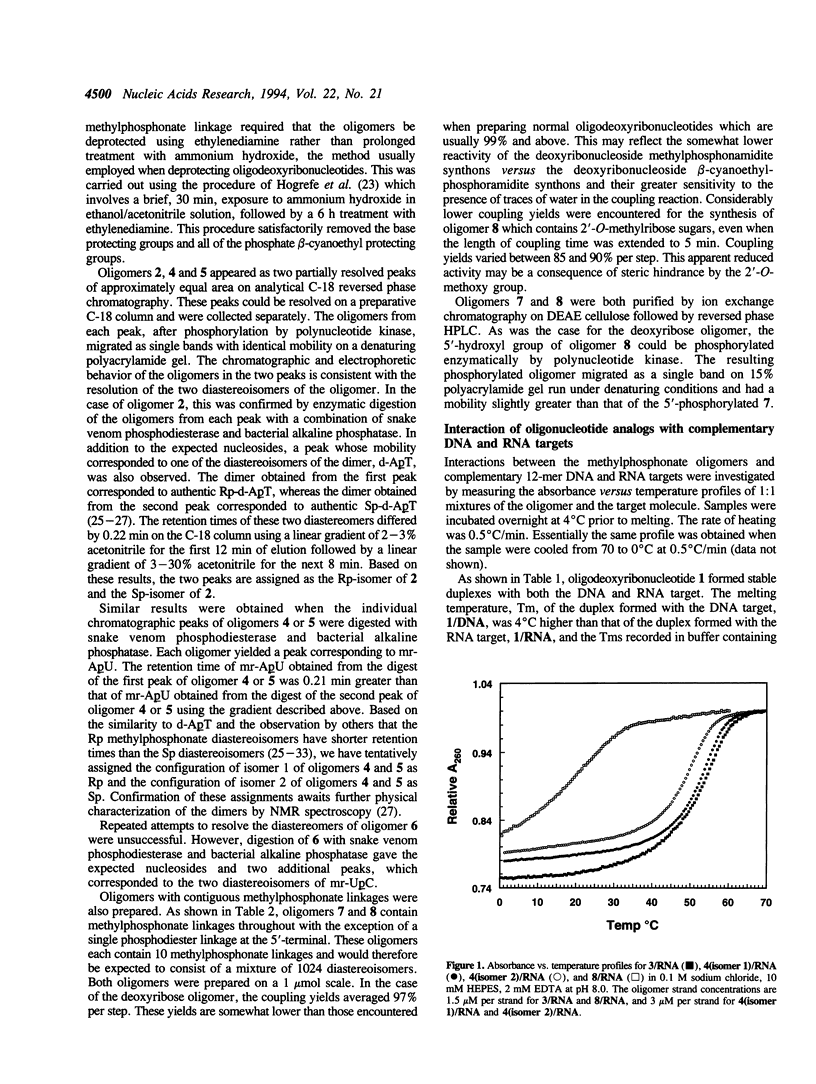
The interactions of oligonucleotide analogs, 12-mers, which contain deoxyribo- or 2'-O-methylribose sugars and methylphosphonate internucleotide linkages with complementary 12-mer DNA and RNA targets and the effect of chirality of the methylphosphonate linkage on oligomer-target interactions was studied. Oligomers containing a single Rp or Sp methylphosphonate linkage (type 1) or oligomers containing a single phosphodiester linkage at the 5'-end followed by 10 contiguous methylphosphonate linkages of random chirality (type 2) were prepared. The deoxyribo- and 2'-O-methylribo- type 1 12-mers formed stable duplexes with both the RNA and DNA as determined by UV melting experiments. The melting temperatures, Tms, of the 2'-O-methylribo-12-mer/RNA duplexes (49-53 degrees C) were higher than those of the deoxyribo-12mer/RNA duplexes (31-36 degrees C). The Tms of the duplexes formed by the Rp isomers of these oligomers were approximately 3-5 degrees C higher than those formed by the corresponding Sp isomers. The deoxyribo type 2 12-mer formed a stable duplex, Tm 34 degrees C, with the DNA target and a much less stable duplex with the RNA target, Tm < 5 degrees C. In contrast, the 2'-O-methylribo type 2 12-mer formed a stable duplex with the RNA target, Tm 20 degrees C, and a duplex of lower stability with the DNA target, Tm < 5 degrees C. These results show that the previously observed greater stability of oligo-2'-O-methylribonucleotide/RNA duplexes versus oligodeoxyribonucleotide/RNA duplexes extends to oligomers containing methylphosphonate linkages and that the configuration of the methylphosphonate linkage strongly influences the stability of the duplexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bower M., Summers M. F., Powell C., Shinozuka K., Regan J. B., Zon G., Wilson W. D. Oligodeoxyribonucleoside methylphosphonates. NMR and UV spectroscopic studies of Rp-Rp and Sp-Sp methylphosphonate (Me) modified duplexes of (d[GGAATTCC])2. Nucleic Acids Res. 1987 Jun 25;15(12):4915–4930. doi: 10.1093/nar/15.12.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko K. K., Lindner K., Saenger W., Miller P. S. Molecular structure of deoxyadenylyl-3'-methylphosphonate-5'-thymidine dihydrate, (d-ApT x 2H2O), a dinucleoside monophosphate with neutral phosphodiester backbone. An X-ray crystal study. Nucleic Acids Res. 1983 May 11;11(9):2801–2814. doi: 10.1093/nar/11.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M., Maurizot J. C., Asseline U., Barbier C., Thuong N. T., Hélène C. Oligothymidylates covalently linked to an acridine derivative and with modified phosphodiester backbone: circular dichroism studies of their interactions with complementary sequences. Nucleic Acids Res. 1989 Mar 11;17(5):1823–1837. doi: 10.1093/nar/17.5.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Vickers T. A., Bruice T. W., Freier S. M., Jenison R. D., Manoharan M., Zounes M. Pseudo--half-knot formation with RNA. Science. 1992 Aug 14;257(5072):958–961. doi: 10.1126/science.1502560. [DOI] [PubMed] [Google Scholar]
- Han F., Watt W., Duchamp D. J., Callahan L., Kézdy F. J., Agarwal K. Molecular structure of deoxycytidyl-3'-methylphosphonate (RP) 5'-deoxyguanidine, d[Cp(CH3)G]. A neutral dinucleotide with Watson-Crick base pairing and a right handed helical twist. Nucleic Acids Res. 1990 May 11;18(9):2759–2767. doi: 10.1093/nar/18.9.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe R. I., Reynolds M. A., Vaghefi M. M., Young K. M., Riley T. A., Klem R. E., Arnold L. J., Jr An improved method for the synthesis and deprotection of methylphosphonate oligonucleotides. Methods Mol Biol. 1993;20:143–164. doi: 10.1385/0-89603-281-7:143. [DOI] [PubMed] [Google Scholar]
- Hogrefe R. I., Vaghefi M. M., Reynolds M. A., Young K. M., Arnold L. J., Jr Deprotection of methylphosphonate oligonucleotides using a novel one-pot procedure. Nucleic Acids Res. 1993 May 11;21(9):2031–2038. doi: 10.1093/nar/21.9.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B. S. Antisense oligonucleotides made of 2'-O-alkylRNA: their properties and applications in RNA biochemistry. FEBS Lett. 1993 Jun 28;325(1-2):123–127. doi: 10.1016/0014-5793(93)81427-2. [DOI] [PubMed] [Google Scholar]
- Lesnik E. A., Guinosso C. J., Kawasaki A. M., Sasmor H., Zounes M., Cummins L. L., Ecker D. J., Cook P. D., Freier S. M. Oligodeoxynucleotides containing 2'-O-modified adenosine: synthesis and effects on stability of DNA:RNA duplexes. Biochemistry. 1993 Aug 3;32(30):7832–7838. doi: 10.1021/bi00081a031. [DOI] [PubMed] [Google Scholar]
- Lesnikowski Z. J., Jaworska M., Stec W. J. Octa(thymidine methanephosphonates) of partially defined stereochemistry: synthesis and effect of chirality at phosphorus on binding to pentadecadeoxyriboadenylic acid. Nucleic Acids Res. 1990 Apr 25;18(8):2109–2115. doi: 10.1093/nar/18.8.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnikowski Z. J., Jaworska M., Stec W. J. Stereoselective synthesis of P-homochiral oligo(thymidine methanephosphonates). Nucleic Acids Res. 1988 Dec 23;16(24):11675–11689. doi: 10.1093/nar/16.24.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löschner T., Engels J. W. Diastereomeric dinucleoside-methylphosphonates: determination of configuration with the 2-D NMR ROESY technique. Nucleic Acids Res. 1990 Sep 11;18(17):5083–5088. doi: 10.1093/nar/18.17.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Bhan P., Cushman C. D., Trapane T. L. Recognition of a guanine-cytosine base pair by 8-oxoadenine. Biochemistry. 1992 Jul 28;31(29):6788–6793. doi: 10.1021/bi00144a020. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Dreon N., Pulford S. M., McParland K. B. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones. Synthesis and physical studies. J Biol Chem. 1980 Oct 25;255(20):9659–9665. [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Matteucci M. D., Martin J. C. Current concepts in antisense drug design. J Med Chem. 1993 Jul 9;36(14):1923–1937. doi: 10.1021/jm00066a001. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Vyazovkina E. V., Rife J. P., Lebedev A. V., Wickstrom E. Preparation of trimers and tetramers of mixed sequence oligodeoxynucleoside methylphosphonates and assignment of configurations at the chiral phosphorus. Nucleic Acids Res. 1993 Dec 25;21(25):5957–5963. doi: 10.1093/nar/21.25.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J., Kan L. S., Ts'o P. O. A simple method of the preparation of 2'-O-methyladenosine. Methylation of adenosine with methyl iodide in anhydrous alkaline medium. Biochim Biophys Acta. 1980 Apr 17;629(1):178–183. doi: 10.1016/0304-4165(80)90276-7. [DOI] [PubMed] [Google Scholar]