Abstract
Background
Several genetic aberrations with prognostic impact in first-line therapy have been described in patients with acute myeloid leukemia and normal karyotype. However, little is known about the influence of these aberrations on outcome after relapse. This study aimed to identify clinical and molecular risk factors for patients with relapsed acute myeloid leukemia with normal karyotype.
Design and Methods
We analyzed 94 patients with acute myeloid leukemia and normal karyotype after first relapse for clinical and molecular risk factors for survival. All patients had received first-line treatment and follow-up within two prospective, multicenter trials. Leukemic blasts were analyzed at diagnosis for genetic aberrations in the FLT3, NPM1, CEBPA, WT1, IDH1 and IDH2 genes by polymerase chain reaction and/or direct sequencing.
Results
A second complete remission was achieved in 52% of patients who received re-induction therapy. The presence of an FLT3-internal tandem duplication, duration of first complete remission less than 6 months and age above the median of 47 years were associated with a significantly lower rate of second complete remission. The median survival after relapse was 11 months and the 6-year survival rate was 28%. In multivariate analysis, FLT3-internal tandem duplication and age above the median were the only independent negative prognostic factors for survival. The 6-year survival rate of patients with none of these factors was 56%, whereas it was significantly inferior in patients with one or both of these factors (15% and 6%, respectively). This was also true for patients who underwent allogeneic stem cell transplantation after relapse.
Conclusions
FLT3-internal tandem duplication and age are the major prognostic factors in patients with relapsed acute myeloid leukemia with a normal karyotype. Patients with at least one of these risk factors have a dismal outcome and might be considered for investigational treatment approaches after relapse. (ClinicalTrials.gov Identifier: NCT00209833)
Keywords: prognostic factors, FLT3-ITD, CN-AML, second complete remission
Introduction
Approximately 50% of patients with acute myeloid leukemia (AML) have a cytogenetically normal (CN) form of the disease; that is, no chromosomal aberrations can be detected by standard cytogenetics. In recent years, a wide variety of somatic gene mutations has been described in these patients.1,2 The most frequent of these mutations affect the nucleophosmin gene, NPM1, and are present in approximately 50% of cases of CN-AML.3 Internal tandem duplications (ITD) in the cytokine receptor gene FLT3 are also very common, occurring in approximately 20–30% of cases of CN-AML. In addition, loss-of-function mutations in the Wilms’ tumor gene, WT1, the CEBPA gene which encodes for the myeloid transcription factor C/EBPα and mutations in the isocitrate dehydrogenase genes 1 and 2 (IDH1 and IDH2) (all present in approximately 10% of CN-AML) have been described.4–8
The prognostic impact of these aberrations in CN-AML has been analyzed at initial diagnosis and was found to be very strong. Patients with an NPM1 mutation in the absence of an FLT3-ITD and patients with a CEBPA mutation have a significantly better prognosis than patients who either harbor an FLT3-ITD or are negative for all three genetic aberrations.9,10 While these findings have been confirmed in several studies, analyses of the prognostic impact of mutations in WT1, IDH1 and IDH2 have produced conflicting results.6–8,11,12 In contrast, little is known about the prognostic value of gene mutations in patients with relapsed disease. Relapse occurs in approximately 50–60% of patients with CN-AML who achieve a complete remission and there is no clearly defined therapeutic standard for these patients.13,14 The spectrum of therapeutic options in these patients ranges from intensive re-induction therapy combined with allogeneic stem cell transplantation to experimental approaches or best supportive care. The identification of easily accessible prognostic factors for survival after relapse is needed since these might be able to serve as a basis for risk stratification and choice of therapy in these patients. We, therefore, performed a comprehensive analysis of 94 patients with relapsed CN-AML. These patients had been treated first-line with a uniform protocol within two successive multicenter trials.15 Pretreatment samples from the patients had been extensively analyzed for gene mutations and polymorphisms with known prognostic impact.
Design and Methods
Patients’ characteristics
All patients analyzed were treated within the multicenter treatment trials AML SHG 295 (February 1995 to May 1999) or AML SHG 0199 (ClinicalTrials Identifier NCT00209833, June 1999 to September 2004). These trials included adult patients (aged 17–60 years at initial diagnosis, median age 47 years) with de novo or secondary AML (French-American British [FAB] classification M0–M2, M4–M7). Both treatment protocols included intensive, response-adapted double induction and consolidation therapy and details have been previously reported.15,16 Patients for this analysis were selected according to the following criteria: (i) normal karyotype on cytogenetic analysis at diagnosis; (ii) relapse after achievement of a complete remission according to accepted criteria; (iii) intensive salvage treatment after relapse; and (iv) availability of bone marrow or peripheral blood samples from the time of initial diagnosis for molecular analysis. These criteria were fulfilled by 94 patients (28 from the AML SHG 295 trial and 66 from the AML-SHG 0199 trial). Written informed consent to participation in the study was obtained from the patients according to the Declaration of Helsinki, and the study was approved by the institutional review board of Hannover Medical School.
Cytogenetic and molecular analyses
Pretreatment samples from all patients were studied centrally by G- or R-banding analysis. At least 20 metaphases were analyzed. Chromosomal abnormalities were described according to the International System for Human Cytogenetic Nomenclature. Various genes were assessed for frequently occurring mutations and expression, as previously described (i.e. FLT3-ITD,4 NPM1,10 CEBPA,5 IDH1 [mutations and IDH1 SNP rs11554137],7 IDH2,8 and WT1 [mutations, WT1 SNP rs16754 and WT1 expression]17).
Statistical analysis
Complete remission and remission duration were defined according to recommended criteria.18 The median follow-up time for survival after relapse was calculated according to the method of Korn.19 The primary end-point of the analysis was survival after relapse, measured from the date of documented relapse until death (treatment failure) or last follow-up for those still alive (censored). Pairwise comparisons of variables for exploratory purposes were performed using the Mann-Whitney-test or the χ2-test. The Kaplan-Meier method and log-rank test were used to estimate the distribution of survival after relapse, and to compare differences between survival curves, respectively. Mutations/polymorphisms in the analyzed genes (FLT3, NPM1, WT1, CEBPA, IDH1, and IDH2) were used as categorical variables. Expression of WT1 mRNA was used either as a continuous variable or dichotomized at the median expression value (median normalized copy number, gene transcripts per ABL transcripts). The duration of first complete remission was dichotomized into less than 6 months and 6 months or more. For multivariate analysis, a Cox proportional hazards model was constructed for survival after relapse. Variables with P values of 0.1 or less in univariate analysis were included in the model. A conditional backwards-elimination procedure was used to exclude redundant or unnecessary variables. A logistic regression model was used to analyze associations between variables and response to re-induction therapy. To provide quantitative information on the relevance of results, 95% confidence intervals (95% CI) of odds ratios (OR) and hazard ratios (HR) were computed. Multivariate analysis of survival after relapse was the primary analysis, all other analyses were considered exploratory. Statistics were performed with the SPSS version 16.0 software package.
Results
Patients’ characteristics and molecular aberrations
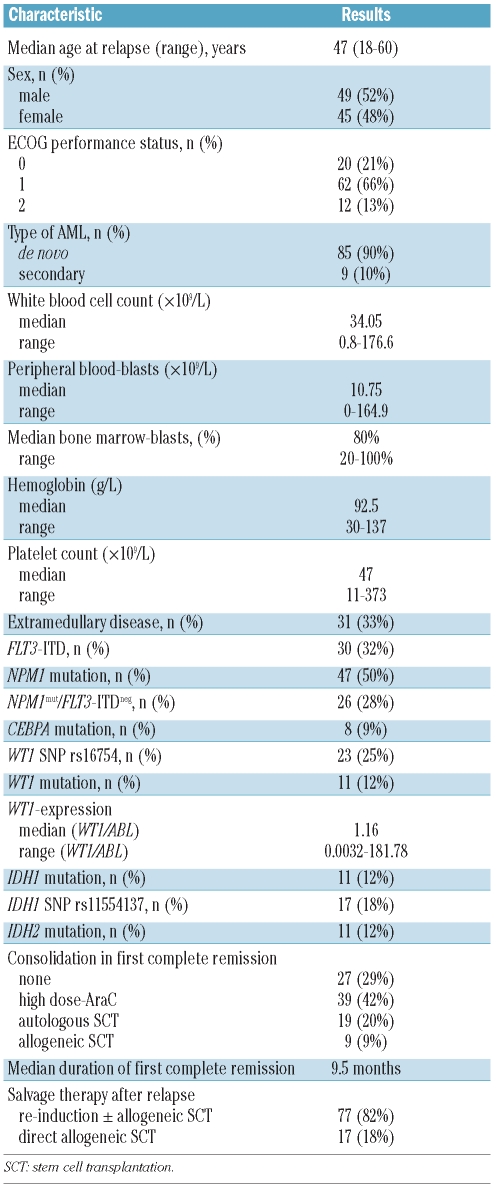
Out of 149 relapsed patients, 94 fulfilled the selection criteria. The outcome and clinical characteristics of these patients were not significantly different from those of the relapsed patients not included in this analysis because specimens were not available (n=51, data not shown). The clinical characteristics and results of the molecular analysis of the analyzed patients are presented in Table 1. The frequency of the different molecular aberrations was 50% for NPM1 mutations, 30% for FLT3-ITD, 12% for IDH1 mutations, 12% for IDH2 mutations, 12% for WT1 mutations and 9% for CEBPA mutations.
Table 1.
Patients’ characteristics.
Response to salvage treatment
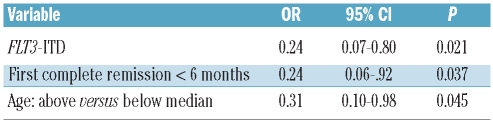
Of the 94 relapsed patients, 77 received intensive, cytarabine-based re-induction chemotherapy and 17 patients underwent allogeneic stem cell transplantation directly, without prior re-induction (5 patients received grafts from matched, related donors and 12 patients received grafts from unrelated donors). Of the 77 patients who underwent re-induction treatment, 40 (52%) achieved a second complete remission. In univariate analysis the presence of an FLT3-ITD (second complete remission rate 22% versus 64%, P=0.001), NPM1/FLT3-high risk status (second complete remission rate 41% versus 73%, P=0.022), duration of first complete remission less than 6 months (second complete remission rate 18% versus 65%, P<0.0001) and age above the median (second complete remission rate 38% versus 69%, P=0.012) had a negative impact on the achievement of a second complete remission. There was also a trend towards a lower second complete remission rate in patients with high WT1-expression (second complete remission rate 40% versus 63%, P=0.06). The other clinical and molecular parameters listed in Table 1 did not influence the second complete remission rate. In multivariate analysis, the presence of an FLT3-ITD, duration of first complete remission and age were the only independent risk factors for the achievement of second complete remission (Table 2).
Table 2.
Significant factors for the achievement of a second complete remission in multivariate analysis.
Survival after relapse
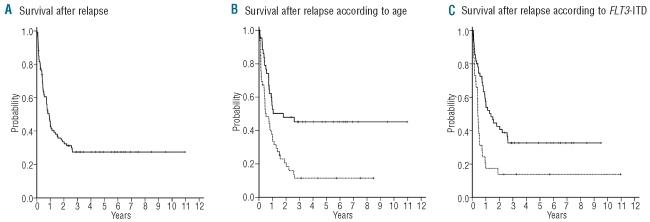
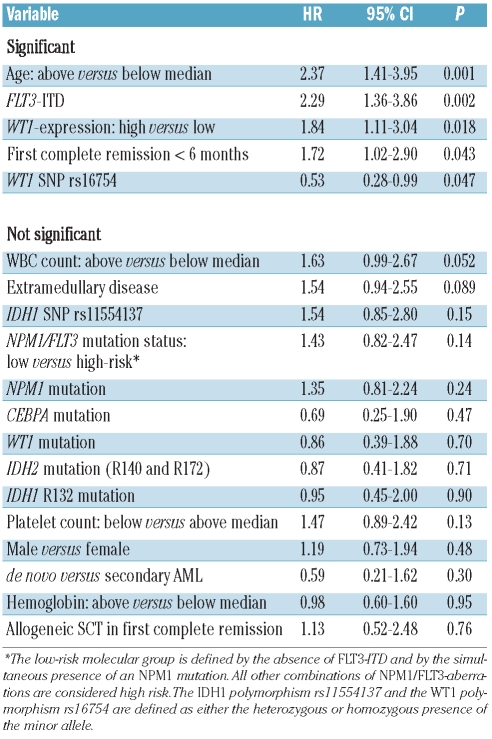
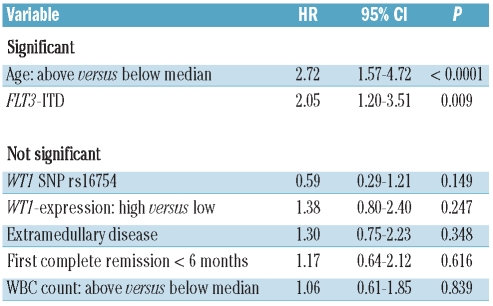
The median follow-up for survival after relapse was 65 months (95% CI, 46 – 86 months). Of the 94 patients studied, 65 died resulting in a median survival after relapse of 11 months and a 6-year rate of survival after relapse of 28% (95% CI, 23% – 33%; Figure 1A). Univariate analysis revealed that FLT3-ITD, age, WT1-expression, duration of first complete remission and the WT1 SNP rs16754 were significant prognostic factors for survival after relapse. Other clinical or molecular parameters had no effect on survival after relapse (Table 3). In multivariate analysis, age and the presence of an FLT3-ITD were the only independent risk factors for survival after relapse (Table 4). This was also true when only the 85 patients who did not receive an allogeneic stem cell transplantation in first complete remission were included in the analysis (data not shown). The univariate Kaplan-Meier analysis for these risk factors is shown in Figure 1B and C.
Figure 1.
(A) Survival after relapse for all patients. (B) Survival after relapse according to age. Solid line: patients <47 years (n=46); dotted line: patients ≥ 47 years (n=48); P=0.001. (C) Survival after disease according to FLT3-ITD. Solid line: patients without FLT3-ITD (n=64); dotted line: patients with FLT3-ITD (n=30); P=0.001.
Table 3.
Univariate analysis for survival after relapse.
Table 4.
Multivariate analysis for survival after relapse.
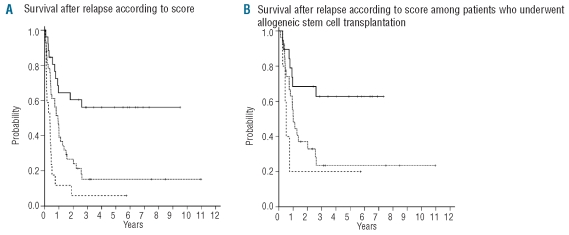
When these two risk factors were combined into a risk score, three groups of patients with significantly different survival after relapse could be separated: the median survival after relapse of patients without any of these characteristics has not been reached and the 6-year survival after relapse rate is 56%; patients with one of these factors had a median survival after relapse of 11 months and a 6-year survival rate after relapse of 15%, while patients with both risk factors had a median survival after relapse of 4.5 months and a 6-year survival rate after relapse of 6% (Figure 2A). The outcome of patients with none of these risk factors was still significantly better than that of patients with one or two risk factors when only patients who received re-induction chemotherapy (data not shown) or patients who underwent allogeneic stem cell transplantation after relapse (Figure 2B) were considered.
Figure 2.
(A) Survival after relapse according to risk score. Solid line: patients without any risk factor (n=30); dotted line: patients with one risk factor (n=45); dashed line: patients with both risk factors (n=19); P<0.0001. (B) Survival after relapse according to risk score for patients who underwent allogeneic stem cell transplantation after relapse. Solid line: patients without any risk factor (n=19); dotted line: patients with one risk factor (n=27); dashed line: patients with both risk factors (n=5); P=0.022.
Discussion
Prospective data on prognostic factors in patients with relapsed AML are limited.14 In previous studies, age at relapse, duration of first complete remission, stem cell transplantation in first complete remission and cytogenetics at diagnosis were associated with outcome after relapse.20 However, little is known about the impact of gene mutations and polymorphisms in patients with relapsed AML and a normal karyotype. We, therefore, investigated whether some of these mutations are of prognostic value not only at initial diagnosis but also after relapse. We analyzed a cohort of 94 patients with relapsed CN-AML. These patients were treated within two consecutive multicenter trials and prospective follow-up continued after relapse. All analyzed patients received intensive re-induction therapy and/or underwent allogeneic stem cell transplantation after relapse. Thus, the prognostic impact of molecular aberrations could be evaluated in the context of intensive salvage therapy.
Fifty-two percent of the patients who received re-induction therapy achieved a second remission. This is within the expected range for this population of patients.20–22 Prognostic factors for the achievement of a second complete remission were age of the patient, the duration of first complete remission and – as the only molecular marker - the presence of an FLT3-ITD at initial diagnosis. Similar results were reported by Ravandi et al.21 and Boissel et al.23 However, in both studies molecular aberrations apart from FLT3-ITD were not taken into account and the analysis by Boissel et al. also included patients with other karyotypes. The significantly inferior second complete remission rate of FLT3-ITD-positive patients is of interest since in several large studies the presence of an FLT3-ITD was not associated with an inferior complete remission rate after first-line induction therapy.4,24,25 Thus, compared to cells from other subgroups of CN-AML, leukemic cells harboring an FLT3-ITD might be especially prone to acquire chemoresistance during the course of disease, e.g. by an increase in the ratio of mutant-to-wild-type alleles.26
In addition to the second complete remission rate, we also analyzed the effect of clinical characteristics and molecular markers on long-term outcome of the patients. As the primary endpoint, we chose survival from the time of relapse to exclude an effect of the duration of first complete remission on the analysis.20 In multivariate analysis, age and the presence of an FLT3-ITD were the only factors with an impact on survival. Other molecular markers with an accepted impact on prognosis at initial diagnosis such as CEBPA mutations or combined NPM1/FLT3 mutational status had no impact on survival after relapse.
Somatic mutations in CN-AML show different degrees of stability during disease progression: NPM1 and CEBPA mutations seem to be stable genetic events.27,28 Data on the stability of IDH1/2 mutations are limited. In six patients with IDH1/2 mutations for whom we could analyze paired samples taken at diagnosis and at relapse, the same mutation was present at relapse and in none of 16 IDH1/2 wild-type patients was a mutation acquired at relapse (data not shown). In contrast, the mutational status of WT1 or FLT3 might be different at initial diagnosis and at relapse in some patients.26,29–31 It is, therefore, compelling that the presence of an FLT3-ITD at initial diagnosis was still the only molecular aberration with a prognostic impact after relapse in our analysis. Thus, in addition to its own role in leukemogenesis, FLT3-ITD might also be a surrogate marker for high-risk characteristics of leukemic cells which persist, although the ITD is no longer detectable in the bulk leukemic population.
When we combined age and FLT3-ITD status in a prognostic score, three groups of patients with significantly different survival could be separated: the 6-year survival rate of patients with none of these risk factors was 56%, whereas the prognosis of patients with one or two of these risk factors was dismal with the patients’ 6-year survival rate being 15% and 6%, respectively. Importantly, this was also true when only patients who underwent allogeneic stem cell transplantation after relapse were considered. Thus it appears that the antileukemic effect of allogeneic stem cell transplantation might not be able to overcome the adverse impact of FLT3-ITD and/or age. The numbers of patients with one or two risk factors were small in the subset of patients who had undergone allogeneic stem cell transplantation after relapse. However, if this finding is confirmed in further trials, it challenges the role of standard allogeneic stem cell transplantation in the salvage therapy of these patients. Collectively, our data show that FLT3-ITD and age were major prognostic factors in our cohort of patients with relapsed CN-AML. Younger FLT3-ITD-negative patients had a high chance of successful salvage after re-induction chemotherapy and allogeneic stem cell transplantation. In contrast, the outcome of FLT3-ITD-positive patients - especially if they were older - was dismal and such patients might be considered candidates for investigational approaches. Of special interest for the FLT3-ITD-positive patients in this respect are novel tyrosine kinase inhibitors which have shown promising activity in early trials although their optimal schedule for single agent application and combination with chemotherapy has yet to be defined.32–36 These agents might be able to improve the outcome of certain subgroups of patients in the future.
Acknowledgments
We thank Elvira Lux, Sylvia Horter and Diana Dudacy for their excellent technical support. We also thank all participating centers of the AML SHG Hannover for inclusion of patients and data acquisition.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Funding: this study was supported by grant n. DJCLS H 06/04v from the Deutsche-José-Carreras Leukämie-Stiftung e.V. (to A.G. and JK); grants n. 01GI0378 (Kompetenznetz “Akute und chronische Leukämien”) and 01KG0605 from the Bundesministerium für Bildung und Forschung (to AG and JK) and the Dieter-Schlag-Stiftung (to KW)
References
- 1.Fröhling S, Scholl C, Gilliland DG, Levine R. Genetics of myeloid malignancies: pathogenetic and clinical implications. J Clin Oncol. 2005;23(26):6285–95. doi: 10.1200/JCO.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Dohner K, Dohner H. Molecular characterization of acute myeloid leukemia. Haematologica. 2008;93(7):976–82. doi: 10.3324/haematol.13345. [DOI] [PubMed] [Google Scholar]
- 3.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 4.Fröhling S, Schlenk RF, Breituck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 5.Frohling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22(4):624–33. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 6.Gaidzik VI, Schlenk RF, Moschny S, Becker A, Bullinger L, Corbacioglu A, et al. Prognostic impact of WT1 mutations in cytogenetically normal acute myeloid leukemia: a study of the German-Austrian AML Study Group. Blood. 2009;113(19):4505–11. doi: 10.1182/blood-2008-10-183392. [DOI] [PubMed] [Google Scholar]
- 7.Wagner K, Damm F, Gohring G, Gorlich K, Heuser M, Schafer I, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010;28(14):2356–64. doi: 10.1200/JCO.2009.27.6899. [DOI] [PubMed] [Google Scholar]
- 8.Thol F, Damm F, Wagner K, Gohring G, Schlegelberger B, Hoelzer D, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 2010;116(4):614–6. doi: 10.1182/blood-2010-03-272146. [DOI] [PubMed] [Google Scholar]
- 9.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 10.Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106(12):3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 11.Paschka P, Marcucci G, Ruppert AS, Whitman SP, Mrozek K, Maharry K, et al. Wilms’ tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B study. J Clin Oncol. 2008;26(28):4595–602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 13.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89(8):998–1008. [PubMed] [Google Scholar]
- 15.Heil G, Krauter J, Raghavachar A, Bergmann L, Hoelzer D, Fiedler W, et al. Risk-adapted induction and consolidation therapy in adults with de novo AML aged </= 60 years: results of a prospective multicenter trial. Ann Hematol. 2004;83(6):336–44. doi: 10.1007/s00277-004-0853-z. [DOI] [PubMed] [Google Scholar]
- 16.Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27(18):3000–6. doi: 10.1200/JCO.2008.16.7981. [DOI] [PubMed] [Google Scholar]
- 17.Damm F, Heuser M, Morgan M, Yun H, Göhring G, Schlegelberger B, et al. A single nucleotide polymorphism in the mutational hotspot of WT1 predicts a favorable outcome in cytogenetically normal acute myeloid leukemia patients. J Clin Oncol. 2010;28(4):578–85. doi: 10.1200/JCO.2009.23.0342. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Korn EL. Censoring distributions as a measure of follow-up in survival analysis. Stat Med. 1986;5:255–60. doi: 10.1002/sim.4780050306. [DOI] [PubMed] [Google Scholar]
- 20.Breems DA, van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, Kantarjian H, Faderl S, Garcia-Manero G, O’Brien S, Koller C, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34(6):752–6. doi: 10.1016/j.leukres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95(11):1857–64. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boissel N, Cayuela JM, Preudhomme C, Thomas X, Grardel N, Fund X, et al. Prognostic significance of FLT3 internal tandem repeat in patients with de novo acute myeloid leukemia treated with reinforced courses of chemotherapy. Leukemia. 2002;16(9):1699–704. doi: 10.1038/sj.leu.2402622. [DOI] [PubMed] [Google Scholar]
- 24.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 25.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 26.Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100(7):2387–92. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 27.Falini B, Martelli MP, Mecucci C, Liso A, Bolli N, Bigerna B, et al. Cytoplasmic mutated nucleophosmin is stable in primary leukemic cells and in a xenotransplant model of NPMc+ acute myeloid leukemia in SCID mice. Haematologica. 2008;93(5):775–9. doi: 10.3324/haematol.12225. [DOI] [PubMed] [Google Scholar]
- 28.Shih LY, Liang DC, Huang CF, Wu JH, Lin TL, Wang PN, et al. AML patients with CEBPalpha mutations mostly retain identical mutant patterns but frequently change in allelic distribution at relapse: a comparative analysis on paired diagnosis and relapse samples. Leukemia. 2006;20(4):604–9. doi: 10.1038/sj.leu.2404124. [DOI] [PubMed] [Google Scholar]
- 29.Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100(7):2393–8. doi: 10.1182/blood-2002-02-0420. [DOI] [PubMed] [Google Scholar]
- 30.Hou HA, Huang TC, Lin LI, Liu CY, Chen CY, Chou WC, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: stability during disease evolution and implication of its incorporation into a survival scoring system. Blood. 2010;115 (25):5222–31. doi: 10.1182/blood-2009-12-259390. [DOI] [PubMed] [Google Scholar]
- 31.Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia. 2006;20(7):1217–20. doi: 10.1038/sj.leu.2404246. [DOI] [PubMed] [Google Scholar]
- 32.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114 (14):2984–92. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–45. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortes J, Foran J, Ghirdalaadze D, DeVetten MP, Zodelava M, Holman P, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study [abstract] Blood. 114(22):264. [Google Scholar]
- 35.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurinib for FLT3 mutant AML patients in first relapse [abstract] Blood. 114(22):325–6. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–62. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]