Abstract
Branched actin assembly is critical for a variety of cellular processes that underlie cell motility and invasion, including cellular protrusion formation and membrane trafficking. Activation of branched actin assembly occurs at various subcellular locations via site-specific activation of distinct WASp family proteins and the Arp2/3 complex. A key branched actin regulator that promotes cell motility and links signaling, cytoskeletal and membrane trafficking proteins is the Src kinase substrate and Arp2/3 binding protein cortactin. Due to its frequent overexpression in advanced, invasive cancers and its general role in regulating branched actin assembly at multiple cellular locations, cortactin has been the subject of intense study. Recent studies suggest that cortactin has a complex role in cellular migration and invasion, promoting both on-site actin polymerization and modulation of autocrine secretion. Diverse cellular activities may derive from the interaction of cortactin with site-specific binding partners.
Key words: cortactin, migration, invasion, lamellipodia, invadopodia, cancer, actin, actin assembly, scaffold, membrane trafficking, secretion
Introduction
Cell movement is a critical cellular process that contributes to embryonic development, immune defense and wound healing. The actin cytoskeleton has long been known to be critical for various aspects of this process, including polarization, leading edge protrusion and cellular contraction (Fig. 1). Myosin-based contraction of unbranched actin filaments is closely connected to cellular traction formation and speed, and is critical for forward cell movement.1,2 By contrast, dynamic branched actin assembly nucleated by the Arp2/3 complex is critical for other aspects of cell motility, including formation of protrusive motility structures and membrane trafficking to promote directional cell motility and secretion of extracellular factors (Fig. 1).
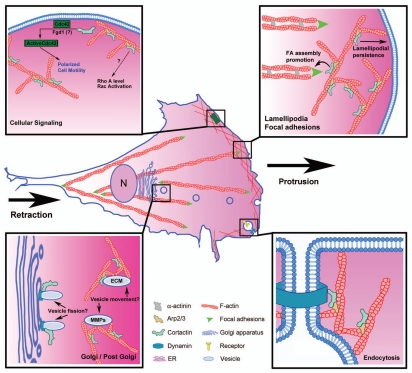
Figure 1.
Regulation of cellular motility by branched actin and cortactin. Cell motility requires coordination of several processes, including protrusion of the leading edge lamellipodium, adhesion, contraction of actin bundles, and retraction of the rear of the cell. Depicted in the zoomed panels are mechanisms by which cortactin may regulate motility, including: promoting lamellipodial persistence, focal adhesion assembly, cellular signaling and secretion of autocrine factors
The identification of branched actin networks at the leading edge of migrating cells, along with the discovery of the Arp2/3 protein complex that is essential for nucleation of those networks,3–6 led to a great deal of excitement in the cell motility field. Indeed, Arp2/3 activation by WAVE2 was found to be required for the first step of canonical cell motility: formation of leading edge protrusions known as lamellipodia.7–10 Concurrently, the Src kinase substrate cortactin was shown to bind Arp2/3 complex,11 serve as a cofactor for Arp2/3 activation, and to stabilize branched actin networks after they are formed.12,13 In cells, cortactin localizes at sites of dynamic actin assembly and is favored as a marker for actin-rich motility protrusions such as lamellipodia and invadopodia.14–16 Interestingly, in addition to Arp2/3 complex, cortactin binds to a large number of signaling, cytoskeletal and membrane trafficking proteins (Table 1 and Fig. 2) and links them to dynamic actin networks. Because of this linkage and the general role that cortactin plays in stabilizing branched actin networks,13 a number of studies have examined the role of cortactin in migration and invasion. Overall, cortactin appears to be a strong promoter of cellular invasiveness, with multiple potential mechanisms.
Table 1.
Table of cortactin binding partners
| Cortactin binding proteins | Localization | Function | Binding site | References |
| Arp2/3 | Located at branch points of actin filaments network | Actin nucleation | NTA | 4, 11 |
| Actin filaments | Cell cytoskeleton | Cytoskeletal polymer | Repeat regions | 15 |
| HDAC6 | Cytoplasm | Deacetylase | Repeat region | 36, 151 |
| SIRT1 | Cytoplasmic and nuclear | Deacetylase | Repeat region | 37 |
| Caldesmon | Filamentous distribution, lamella and lamellipodia | Actin binding protein, contraction | N-terminus | 152 |
| p120 catenin | Cell-cell junction, nucleus, membrane ruffles, actin halos associated with endocytic vesicles | Cell-cell adhesion via cadherin stability & trafficking | N-terminus | 81, 153–155 |
| Grb2 | Cytoplasm, plasma membrane, lipid rafts, perinuclear region | Signaling adaptor | N-terminus | 52, 156, 157 |
| Met | Plasma membrane, dorsal ruffles, early endosomes and late endosomes | Receptor tyrosine kinase | ? | 52, 158 |
| K+ channel Kv1.2 | Cortical cytoskeleton | Ion channel | ? | 159 |
| PTP1B | Cytoplasmic face of endoplasmic reticulum | Tyrosine phosphatase | Tyr446 | 160, 161 |
| Nck1 | Cytoplasmic, cell periphery, podosomes, invadopodia | Signaling adaptor | phospho-Y421, 466 | 162–164 |
| Syk | Nucleus, cytoplasm, perinuclear region, plasma membrane at cell-cell contacts | Tyrosine kinase | ? | 53, 58, 165 |
| Src family kinases (Src, Fer) | Cytoplasmic, plasma membrane, focal adhesions, podosomes, invadopodia | Tyrosine kinase | phospho-Y421, 466, 482 | 55, 56, 70, 71, 166, 167 |
| ERK1/2 | Nucleus, cytoplasm | Serine/Threonine kinase | S405, 418 | 57, 168–170 |
| PAK1 | Cytoplasm, plasma membrane, focal adhesions | Serine/Threonine kinase | S113 | 59, 170–173 |
| CBP90 | Cytosol, membrane and synaptic vesicles | ? | SH3 | 33 |
| ZO-1 | Cell-cell junction | Tight junction adaptor | SH3 | 32 |
| BPGAP1 | Cytoplasm, plasma membrane | RhoA-GAP | SH3 | 174, 175 |
| Hip1R | Present at all clathrin patches | Membrane trafficking | SH3 | 176 |
| BK channels | Plasma membrane | Membrane excitability | SH3 | 177 |
| ASAP1/AMAP1 | Recycling endosomes, focal adhesions, invadopodia, podosomes | ARF6 GAP | SH3 | 129, 178–181 |
| Abl/Arg | Cytoplasm, nuclear, plasma membrane | Tyrosine kinase | SH3 | 45, 60, 182, 183 |
| N-WASp | Golgi, Podosomes and invadopodia. | Actin assembly | SH3 | 97, 184, 185 |
| Dynamin2 | Plasma membrane, trans-Golgi network, cell cortex, cortical ruffles | GTP ase, Membrane trafficking | SH3 | 186–189 |
| CortBP1/SHANK2 | Within secretory granules (cytoplasm), membrane ruffles, neuronal growth cones, lipid rafts | Synaptic plasticity, adaptor protein, regulates Na+/H+ exchanger 3 | SH3 | 190–195 |
| FGD1 | Cytoplasm, Golgi, cell cortex and membrane ruffles | Cdc42-GEF | SH3 | 196, 197 |
| WIP | Perinuclear region, membrane ruffles | Adaptor protein, Actin binding/assembly, WASp stabilization | SH3 | 68, 198–200 |
| Non-muscle myosin light chain kinase | Actin stress fibers, lamellipodia | Contraction | SH3 | 47, 201, 202 |
| Missing in metastasis (MIM) | Plasma membrane, actin bundles, stress fibers, cytoplasm | Adaptor protein, Actin binding and regulation | SH3 | 203, 204 |
| CD2AP | Cell membrane, endosomes, immune synapse (T cells) | Endocytosis (binds to Rab4 & c-Cbl) | SH3 | 205–210 |
List of Cortactin binding proteins
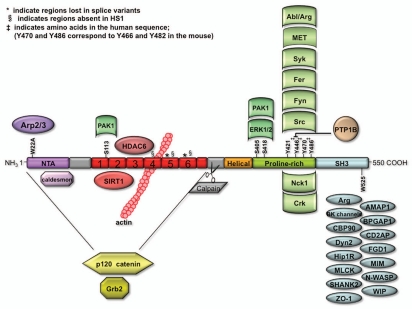
Figure 2.
Cortactin domain structures. Schematic diagram of key cortactin domains and binding partners. The following abbreviations are used: NT A, N-terminal acidic domain and SH3, Src homology 3 domain. Proteins whose interaction with cortactin has been narrowed down to a particular domain are represented in the same color as the domain on cortactin. Interacting proteins shown in yellow bind the amino terminus of cortactin, which constitute the NT A + repeats domains. Amino acids that are essential for the interaction with key cortactin binding proteins, including W22 for interaction with Arp2/3 and W525 for interactions within the SH3 domain, are shown. The kinases known to phosphorylate cortactin are found above the respective sites they have been shown (or hypothesized) to phosphorylate.
General Features of Cortactin
The gene encoding cortactin, CTTN (previously denoted EMS1), is located on the long arm of chromosome 11, in the 11q13 region that is frequently amplified in a number of cancer types.17 Cortactin is ubiquitously expressed, except in most hematopoietic cells that instead express the homolog hematopoietic specific 1 (HS1).18 Osteoclasts are a notable exception to this rule, expressing both HS1 and cortactin.19 The mechanisms controlling cortactin expression are not well understood; however, an increase in cortactin mRNA has recently been shown to be downstream of hyaluronan (HA) binding to its receptor, CD44, through the activation of the NFκB pathway.20 In addition, phospho-Stat3 was recently shown to bind the CTTN promoter and upregulate transcription.21 In cancer, cortactin is frequently overexpressed, both as a consequence of gene amplification and by additional unidentified mechanisms.17,22–25
Cortactin contains the following key domains: an amino-terminal acidic domain, a tandem repeat domain, a carboxy-terminal proline-rich region that contains a number of phosphorylation sites and an SH3 domain (Fig. 2). The N-terminus of cortactin is critical for regulating branched actin assembly, via conserved interactions with the branched actin-nucleating Arp2/3 protein complex and with filamentous actin (F-actin) at the acidic and repeats domains, respectively.11,26 Interestingly, recent structural studies found that cortactin alters the lateral and longitudinal contacts of actin subunits within an actin filament, suggesting that by changing the local conformation of filamentous actin cortactin might promote the exposure of new binding sites for Arp2/3 complex and thereby indirectly increase the affinity of Arp2/3 complex for the side of a mother actin filament.27,28 The C-terminus instead allows cortactin to function as a scaffolding protein, since many cytoskeletal, membrane trafficking and signaling proteins bind to the C-terminal SH3 domain (Fig. 2 and Table 1) and can be bridged to the actin cytoskeleton through cortactin.29
Cortactin is evolutionarily conserved with members identified in a diverse array of species from sponges to mammals.18 Although no cortactin gene exists in yeast, the protein ABP1 is thought to be a functional homolog based on its role in endocytosis and its ability to induce weak activation of Arp2/3 complex through interactions with both F-actin and Arp2/3 complex.30,31 While orthologs exist in a number of species, they differ in the number of tandem F-actin binding repeats they contain, similar to the splice variants (discussed below). For example, Drosophila cortactin contains only four repeats.32
Cortactin function is altered through several different mechanisms including alternative splicing, phosphorylation and acetylation. The three major splice variants of cortactin, A, B and C, respectively contain 6.5, 5.5 and 4.5 of the cortactin repeats domains.33,34 Loss of the repeat domains via alternative splicing leads to both diminished binding affinity for F-actin, decreased localization to cellular cortical actin and decreased motility.33–35 Acetylation can also occur within the tandem repeats region and regulates both F-actin-binding and cell motility.36,37 A recent paper is suggestive for cortactin deacetylation being important in invadopodia function, as the cortactin deacetylase HDAC6 regulates both invadopodia activity and protein acetylation at invadopodia.38
Cortactin was originally identified as a substrate for Src tyrosine kinase (at Y421, Y470 and Y486 in the human sequence); however, it is a substrate for many different kinases (reviewed in refs. 39 and 40). An increase in phosphorylation of tyrosine, serine and/or threonine residues of cortactin is seen upon stimulation by numerous sources, including fibroblast growth factor (FGF),41,42 epidermal growth factor (EGF),43,44 platelet-derived growth factor (PDGF;45), thrombin,46 sphingosine-1-phosphate,47 homophilic ligation of E-cadherin,48 bacterial phagocytosis49 and integrin activation.50 The downstream kinases involved in the phosphorylation of cortactin by these pathways include Src family kinases (Fer, Fyn, Syk and Src), tyrosine kinases (Abl and Arg, ErbB2 and c-Met), as well as serine/threonine kinases extracellular regulated kinase 1/2 (ERK1/2; at S405 and S418), p21 activated kinase 1 (PAK1; at S405/418) and protein kinase D (PKD; at S298).16,45,51–59 Phosphorylation has been shown to be important for enhancing cortactin function in migration and invasion by altering the complement of proteins associated with cortactin.43,44,60,61
Many of the phosphorylation sites occur within the proline-rich domain, and may regulate binding to the adjacent SH3 domain (Fig. 2). In particular, Src kinase phosphorylation has been shown to inhibit accessibility of the SH3 domain,57 although this may be opposed by the binding of SH2-domain containing proteins, such as Nck1, to the phosphorylated tyrosine.62,63 Indeed, in cells, tyrosine phosphorylation of cortactin has been shown to increase the binding affinity of the SH3 domain binding partner Dynamin 2.64 By contrast, ERK phosphorylation increases accessibility of the SH3 domain resulting in increased N-WASp binding to cortactin,57 which may account for Erk-regulation of cell motility and lamellipodial dynamics.43 Likewise, PAK1 phosphorylation of the same sites in cortactin was shown to increase N-WASp binding to cortactin without affecting the Arp3- or actin-binding properties of cortactin.54 It is likely that the Erk and Src phosphorylation events are not mutually exclusive in cells,43 which may account for diverging models from in vitro biochemical experiments57 and cellular studies.64 In addition, a number of novel phosphorylation sites were identified by mass spectrometry,65 including many in the amino-terminus; the regulatory kinases and functions of those novel sites remain largely unknown. Taken together, these data suggest that cortactin phosphorylation regulates the affinity and combination of binding proteins associated with cortactin.
Cortactin and the Actin Cytoskeleton
Virtually all of the cellular activities of cortactin, including cell migration and invasion, as well as localization, require association with Arp2/3 complex and the actin cytoskeleton.11,12,35,66,67 Through this association, cortactin has been shown in vitro to regulate branched actin assembly by many mechanisms, including activation of Arp2/3 complex, stabilization of actin branches, enhancing activation of Arp2/3 complex by Wiskott-Aldrich Syndrome protein (WASp) family proteins and scaffolding of other actin regulators, such as N-WASp and WIP.12,13,26,68 A function that is unique to cortactin and is thought to be important for regulation of actin dynamics is prevention of the de-branching of actin filament networks.13 This function is likely to be particularly important in newly polymerized networks in cellular protrusions, since cortactin strongly localizes to such actin-rich structures and also has a high affinity for ATP-bound and ADP-Pi-bound actin.66 Indeed, a recent study showed faster turnover of actin networks in cortactin-null cells compared with controls, as measured by fluorescence recovery after photobleaching (FRAP).69 Recruitment of cortactin to sites of new protrusions and dynamic actin assembly occurs in response to many signals, including Rac activation,70 and requires the presence of binding sites for the Arp2/3 complex and (to a lesser extent) F-actin.11,12,66 In aggregate, these data suggest a role for cortactin in the regulation of newly polymerizing actin networks.
Cortactin in Cell Motility
The prominent localization of cortactin to the leading edge of migrating cells sparked an early interest in its potential function in cell migration. Indeed, numerous studies have demonstrated an important role for cortactin in the motility of diverse cell types, including fibroblasts, endothelial cells and a variety of carcinoma cell lines. Overexpression of cortactin has been shown to increase cell motility in transwell, scratch assays and single cell random motility experiments.66,71–73 Likewise, knockdown of cortactin using si/shRNA approaches has been shown to decrease cell motility.20,66,74,75 Recently, two groups generated and analyzed cortactin-null mouse embryonic fibroblasts (MEFs) generated from embryos containing FLOX-ed cortactin alleles and reported divergent results. One group reported a similar effect of cortactin knockout to the shRNA studies, with decreased migration in wound closure and single cell motility assays in cortactin-null MEFs, compared to controls.69 By contrast, Tanaka et al. reported that cortactin loss did not affect MEF motility in wound closure and transwell migration assays.76 It is unclear why no effect was evident in the latter study; however it is possible that the requirement for cortactin in efficient cell migration depends on the microenvironment. Indeed, in Drosophila, loss of the single cortactin gene diminishes border cell migration.77
The mechanism by which cortactin affects migration is not entirely clear (Fig. 1). Although cortactin is a prominent marker of lamellipodia, it is not essential for their formation.66,69,75,78–80 Instead, cortactin affects the characteristics of lamellipodia, including their stability or persistence,66,81 actin dynamics within the lamellipodia,66,69 whether a dominant lamellipodium or multiple smaller protrusions are formed,78 and PDGF-induced membrane ruffling69 (Fig. 1). Furthermore, inhibition of lamellipodia formation by other mechanisms does not necessarily lead to decreased cell motility speed,82 suggesting that lamellipodia may be more important for cell directionality rather than to drive cell motion.
A second potential mechanism by which cortactin might affect cell motility is via regulation of adhesion dynamics. In fibrosarcoma cells, cortactin was found to affect the rate of assembly of focal complexes.66 Likewise, Lai et al. found that cortactin-null cells treated with PDGF had more prominent focal adhesions.69 Interestingly, Boguslavsky et al. found that the cortactin-binding partner, p120-catenin, regulates both the assembly rate of focal adhesions and lamellipodial persistence, similar to cortactin.66,81 Those similarities suggest both a partnership of the two molecules and a linkage between lamellipodial stability and adhesion formation. Cortactin has also been shown to affect the rate of cell spreading, an adhesion-dependent process.60,81 As adhesions have been closely tied to cell motility speed,83,84 and shown to be necessary for lamellipodial stability,85–87 cortactin regulation of adhesions seems a likely mechanism of motility regulation.
A third, and not mutually exclusive, mechanism by which cortactin has been postulated to regulate cell motility is via activation of cellular signaling. Although generally cortactin has been thought to act as an effector of cellular signaling proteins, Lai et al. recently demonstrated a constitutive defect in cdc42 signaling and a defect in PDGF-induced Rac activity in cortactin-null MEFs.69 Cortactin was also found to affect both the expression and activity of RhoA in head and neck squamous carcinoma cells (HNSCC).88 Alteration in Rho GTPase activity could indeed affect multiple steps of motility reported to be regulated by cortactin, including adhesion dynamics (via Rho A) and lamellipodial activity (via Rac1). Alterations in cdc42 activity could affect secretion of extracellular motility factors,89 including matrix metalloproteinases (MMPs),90,91 and extracellular matrix (ECM).92
Cortactin in Invasion-Extracellular Matrix Degradation: Invadopodia and Podosomes
While migration allows for lateral movement, invasion involves degradation of ECM to create space for tumor cell growth and movement. Dynamic changes in the actin cytoskeleton allow for the formation of specialized organelles used in ECM degradation: invadopodia and podosomes.93,94 Invadopodia are actin-rich protrusions with associated concentrated proteolytic activity found on the basal surface of invasive carcinoma cells. Podosomes are similar structures, that are primarily found in normal cells that need to cross tissue barriers or remodel ECM, such as macrophages and osteoclasts. Although the two structures contain similar molecular machinery and have common functions of ECM degradation and motility,93,94 recent studies have identified distinguishing features of invadopodia and podosomes, including the importance of Grb2 for podosome but not invadopodia assembly95–97 and different dynamics of membrane activity between the two structures.98
Invadopodia are thought to form in stages, with actin assembly being triggered at basal membrane sites by growth factor and integrin-induced signaling,97,99,100 followed by stabilization and matrix degradation97,101 (Fig. 3). Cortactin is a key component of both invadopodia and podosomes, and is frequently used as a marker of those structures. Live cell imaging studies of invadopodia have found that cortactin is either recruited simultaneously with61 or a few minutes before101 recruitment of the transmembrane metalloproteinase MT1-MMP. Within 1–2 min after MT1-MMP recruitment, ECM degradation occurs, indicating rapid progression through these stages. It is currently unknown whether actin assembly occurs concurrently with or prior to cortactin recruitment.
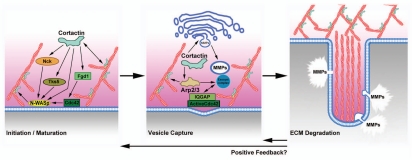
Figure 3.
Model of cortactin function at invadopodia. Cortactin is thought to contribute to two major processes in invadopodia: (1) actin polymerization for initiation and/or maturation of invadopodia via activation of N-WASp via Nck, activation of cdc42 via Fgd1, and coactivation of Arp2/3 complex and (2) vesicular trafficking of matrix metalloproteinases to invadopodia via either regulation of post-Golgi trafficking or vesicle capture at invadopodia. Once ECM-degradation is established at invadopodia, they may become longer-lived due to positive feedback.
An early study showed that neutralizing antibodies against cortactin block ECM degradation at invadopodia.14 Numerous subsequent studies have reported that cortactin regulates both the number and activity of invadopodia and podosomes.44,61,67,79,90,91,101–105 Mechanistically, there are two major processes by which cortactin is thought to regulate invadopodia: (1) by facilitating actin assembly at invadopodia initiation sites; and (2) by regulating membrane trafficking for the recruitment of ECM-degrading proteinases to invadopodia (Fig. 3). A role for cortactin in actin assembly at invadopodia is likely based on its general role in regulating Arp2/3 activity, as well as the potential to provide positive feedback through direct binding to the Arp2/3 activator N-WASp and upstream regulators including the cdc42 GEF, Fgd1 and Nck1.63,97,106,107 Consistent with that idea, two recent papers demonstrated that cells expressing cortactin molecules with non-phosphorylatable mutations at the Src phosphorylation sites have reduced N-WASp activity, Nck1 recruitment and barbed end polymerization.44,61 In addition, the phospho-mutant cortactin affected the lifetime of invadopodia, suggesting a role for cortactin in invadopodia maturation.61
Membrane trafficking is also a critical contributing process to invadopodia, as its function in ECM degradation relies on delivery of proteinases94,108 (Fig. 3). In fact, “mature” invadopodia are often defined as those associated with ECM degradation.61,101 Our laboratory identified a specific role for cortactin in regulating the secretion, cell-surface expression and localization to invadopodia of the matrix metalloproteases (MMPs) MT1-MMP, MMP–2 and MMP-9.90,91 Consequently, the importance of cortactin in protein trafficking likely accounts for the larger defect in ECM degradation than invadopodia numbers in cells lacking cortactin.90,91 This statement is supported by the observation that the degradation defect in cortactin-deficient cells could not be overcome by overexpression of MT1-MMP, suggesting a block in secretion when cortactin is absent.90,109 Similarly, in osteoclast podosomes, loss of cortactin was found to lead to selective inhibition of proteinase recruitment to actin-rich podosomes, and a block in formation of the mature sealing ring.103 However, this point is controversial, as a previous study found a loss of the actin-rich podosomes themselves.79
Interestingly, the impact of cortactin loss on invadopodia, complete block in invadopodia-associated ECM degradation and reduction in invadopodia numbers, is similar to that of MMP inhibition by GM6001, TIMP2 or MT1-MMP siRNA.91,101,108 At this point it is unclear whether the reduction in invadopodia numbers in cortactin-KD cells is the result of inhibition of actin assembly at invadopodia initiation sites or a decrease in invadopodia lifetime due to abolished positive feedback from ECM degradation. Live cell imaging using markers other than cortactin will be required to answer this question, if indeed these two functions are separable.
Cortactin in Membrane Trafficking
As noted above, one mechanism by which cortactin might regulate motility and invasion is through augmentation of membrane trafficking, via direct effects on actin polymerization and/or bridging membrane trafficking proteins to the actin cytoskeleton (Figs. 1 and 3). Generally, actin polymerization is thought to be critical for fission of vesicles, although fusion and tethering functions have also been noted.110–112 Of note, cortactin and several cortactin binding proteins have been shown to be important for protein trafficking to and from the cell surface. For example, many studies have shown that cortactin regulates both clathrin-dependent and -independent endocytosis.54,64,113–118 Interaction with SH3 binding partners, such as the Arp2/3 activator N-WASp and the membrane pinchase Dynamin 2, along with the actin cytoskeleton appears to be necessary and is regulated by kinases such as PAK1 and Src.54,113 Of particular interest for cancer cell motility and invasion, cortactin expression levels were shown to affect ligand-induced internalization and downregulation of EGFR levels in HNSCC cells. Thus, cortactin-overexpressing cancers are likely to have increased EGFR levels via regulation of turnover.119 However, as with many cortactin phenotypes, some studies have found no effect of cortactin expression changes on endocytosis69,120 indicating that cellular context (either microenvironmental or cell-type) may dictate whether cortactin is essential for regulation of specific phenotypes.
With regard to exocytosis, fewer studies have been performed. We demonstrated that cortactin regulates the secretion of the gelatinases MMP-2 and MMP-9, MT1-MMP and apolipoprotein A1 from cancer cells. However, it is unknown at this point whether the block in proteinase secretion seen in cortactin-KD cells occurs secondary to defective transport from the Golgi121 or post-Golgi carriers, or from lack of recruitment of vesicular carriers to invadopodia sites.122 Both MMP9 and MT1-MMP have been localized to late endocytic/lysosomal compartments108,123 and trafficking of MT1-MMP to invadopodia depends on the late endocytic v-SNARE VAMP7,108 suggesting a potential point of regulation. However, at least in glial cells, MMP2 appears to reside in separate vesicles123 from MMP9 and may therefore derive from a separate compartment. Furthermore, overexpression of cortactin that cannot bind SH3 partners leads to a block in trafficking from the trans-Golgi compartment,121 suggesting another site where cortactin may be required for MMP trafficking. Finally, cortactin is considered to be an important scaffolding protein in dendritic spines and links to the exocyst protein Sec8/EXOC4 through its binding partner SHANK2 and PSD-95.124,125 The exocyst complex has been shown to mediate tethering of post-Golgi vesicles to the plasma membrane126 and regulate both cellular migration and invadopodia formation.112,127 In addition, the cortactin binding partners N-WASp, Dynamin 2, FGD1 and ASAP1 all regulate both membrane trafficking and invadopodia function,97,106,107,128,129 and are likely candidates to mediate the effects of cortactin at one or more membrane trafficking compartments (Table 1).
Cortactin in Cancer
Much of the interest in cortactin has stemmed from the early finding that the cortactin gene, CTTN, was amplified in HNSCC and breast cancers130 as part of an amplification of the 11q13.3 region. Subsequently, cortactin overexpression has been found in many cancer types, including melanomas, ovarian, gastric, hepatic, colorectal and esophageal.25,131–136 In 11q13-amplified cancer cell lines, cortactin expression is increased parallel with gene copy number, indicating that gene copy number and protein expression levels are “coupled.”74 In addition to gene amplification, cortactin expression is increased in many tumors by alternative means,23,25 although the exact mechanism remains to be determined. The frequent, non-random increase in cortactin expression suggests that it provides a selective advantage to developing or progressing tumors.
Although a number of candidate genes exist in the 11q13 region, including several FGF family members and FADD,137 cortactin and cyclin D1 have received the most attention. Cyclin D1 is a well known oncogene that is deregulated in many cancers and has been particularly associated with tumorigenesis in breast cancer.138 Consistent with its role in cell migration and invasion, cortactin overexpression has been associated with tumor aggressiveness, regional and distant metastasis, poor patient prognosis and decreased patient survival. In HNSCC tumors, Rodrigo et al. reported that in the rare cases with independent amplification of cortactin and cyclin D1, cortactin amplification correlated most significantly to decreased patient survival.24 Subsequent studies confirmed this finding at the protein expression level, finding that cortactin expression in laryngeal cancer predicts disease-specific mortality independent of cyclin D1 and FADD expression.22,139 Furthermore, cortactin expression in HNSCC was found to predict local recurrence, disease-free survival and overall survival independent of EGFR expression status.140,141 The fact that EGFR and cortactin expression are independent predictors of disease-free survival suggests that regulation of EGFR by cortactin119,142 is not the only mechanism by which cortactin promotes cancer aggressiveness. In other cancers, including hepatic,25 breast,143 esophageal,133 ovarian,132 melanoma,136 gastric,134,135 and colorectal,131 cortactin expression and/or amplification has also been strongly associated with poor prognosis, often as an independent predictor of disease recurrence.
Experimental studies using mouse models have largely confirmed the prediction that cortactin promotes tumor aggressiveness. Unlike cyclin D1, transgenic expression of cortactin in the mouse mammary gland does not induce hyperplasias or tumors.144 By contrast, overexpression of cortactin in established human carcinoma cell lines leads to aggressive in vivo behavior for multiple tumor types. In experimental metastasis assays, cortactin overexpression in breast and esophageal squamous carcinoma (ESCC) cells led to enhanced metastasis to the bone and lungs, respectively.133,145 Likewise, cortactin-overexpression in hepatocellular carcinoma cells led to intrahepatic metastasis from orthotopic injection sites.146 Using a semiorthotopic tumor model for HNSCC, our laboratory found that cortactin expression regulated invasiveness across a tracheal boundary in vivo and invasive behavior in vitro.109 In addition to effects on cell motility and invadopodia activity, a mechanism by which cortactin might promote cancer aggressiveness is through regulation of cell-cell adhesions. However, since cortactin appears to promote rather than inhibit cell-cell junction formation and strength,48,147 inactivation of cortactin may be required for promotion of epithelial-mesenchymal transition.148
In addition to regulating invasiveness, we also found that cortactin expression affected the size of HNSCC tumors.109 For ESCC, but not breast or hepatocellular carcinoma, cortactin was also found to affect tumor size.133,145,146 We speculate that tumor type or its local microenvironment may dictate whether cortactin only affects invasiveness or also tumor size. Removal of space constraints via proteolytic activity and altered angiogenesis have been postulated as mechanisms for the effects of other invadopodia proteins on tumor size, raising the possibility that cortactin may function similarly.149,150 Alternatively, cortactin has also been shown to affect anchorage- and serum-independent growth109,133 and to regulate cell cycle inhibitor levels88 in squamous carcinoma cells. The mechanism by which cortactin alters these tumorigenic properties is a current area of investigation, but at least for serum independence it appears to be associated with the role that cortactin plays in autocrine secretion.109 Regardless, it is clear that cortactin expression induces aggressive behavior in multiple cancer types, and in human cancers is a strong and independent prognostic marker of poor outcome. Future studies should focus on a better understanding of the molecular and cellular mechanisms by which cortactin influences tumor growth and metastasis.
Summary
Actin assembly serves a pivotal role in cell migration and invasion. Dynamic branched actin networks, nucleated by the Arp2/3 complex, provide the force for the formation of many cellular protrusions, including lamellipodia and invadopodia. They also serve as platforms for the assembly of signaling and membrane trafficking proteins at sites of vesicle formation and other branched actin-rich structures. The branched actin regulator, cortactin, may be particularly important in the latter process as it links the Arp2/3 complex to a variety of binding partners. Challenges for the future include identification of relevant protein complexes that regulate different cortactin-dependent cellular processes as well as determination of how tissue-specific contexts determine the outcome of cortactin and cortactin-binding partner interactions.
Acknowledgements
Funding was provided by NIH grant 1R01GM075126 and ACS RSG-118085 to A.M.W., 5T32 CA009592-23 support of K.C.K., and AHA predoctoral fellowship 10PRE4030003 to S.S.
References
- 1.Jay PY, Pham PA, Wong SA, Elson EL. A mechanical function of myosin II in cell motility. J Cell Sci. 1995;108:387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machesky LM, Atkinson SJ, Ampe C, Vandekerckhove J, Pollard TD. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, et al. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikawa T, Yamaguchi H, Itoh T, Kato M, Ijuin T, Yamazaki D, et al. PtdIns(3,4,5)P3 binding is necessary for WAVE2-induced formation of lamellipodia. Nat Cell Biol. 2004;6:420–426. doi: 10.1038/ncb1125. [DOI] [PubMed] [Google Scholar]
- 9.Suetsugu S, Miki H, Yamaguchi H, Takenawa T. Requirement of the basic region of N-WASP/WAVE2 for actin-based motility. Biochem Biophys Res Commun. 2001;282:739–744. doi: 10.1006/bbrc.2001.4619. [DOI] [PubMed] [Google Scholar]
- 10.Suetsugu S, Yamazaki D, Kurisu S, Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 11.Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, et al. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uruno T, Liu J, Zhang P, Fan Yx Y, Egile C, Li R, et al. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 13.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, et al. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 14.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Parsons JT. Cortactin, an 80/85 kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol Cell Biol. 1993;13:2891–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rossum AG, Schuuring-Scholtes E, van Buuren-van Seggelen V, Kluin PM, Schuuring E. Comparative genome analysis of cortactin and HS1: the significance of the F-actin binding repeat domain. BMC Genom. 2005;6:15. doi: 10.1186/1471-2164-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiura K, Lim SS, Little SP, Lin S, Sato M. Differentiation dependent expression of tensin and cortactin in chicken osteoclasts. Cell Motil Cytoskeleton. 1995;30:272–284. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- 20.Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 21.Du XL, Yang H, Liu SG, Luo ML, Hao JJ, Zhang Y, et al. Calreticulin promotes cell motility and enhances resistance to anoikis through STAT3-CTTN-Akt pathway in esophageal squamous cell carcinoma. Oncogene. 2009;28:3714–3722. doi: 10.1038/onc.2009.237. [DOI] [PubMed] [Google Scholar]
- 22.Gibcus JH, Mastik MF, Menkema L, de Bock GH, Kluin PM, Schuuring E, et al. Cortactin expression predicts poor survival in laryngeal carcinoma. Br J Cancer. 2008;98:950–955. doi: 10.1038/sj.bjc.6604246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greer RO, Jr, Said S, Shroyer KR, Marileila VG, Weed SA. Overexpression of cyclin D1 and cortactin is primarily independent of gene amplification in salivary gland adenoid cystic carcinoma. Oral Oncol. 2007;43:735–741. doi: 10.1016/j.oraloncology.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo JP, Garcia LA, Ramos S, Lazo PS, Suarez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:3177–3182. [PubMed] [Google Scholar]
- 25.Yuan BZ, Zhou X, Zimonjic DB, Durkin ME, Popescu NC. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn. 2003;5:48–53. doi: 10.1016/S1525-1578(10)60451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 27.Pant K, Chereau D, Hatch V, Dominguez R, Lehman W. Cortactin binding to F-actin revealed by electron microscopy and 3D reconstruction. J Mol Biol. 2006;359:840–847. doi: 10.1016/j.jmb.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 28.Shvetsov A, Berkane E, Chereau D, Dominguez R, Reisler E. The actin-binding domain of cortactin is dynamic and unstructured and affects lateral and longitudinal contacts in F-actin. Cell Motil Cytoskeleton. 2009;66:90–98. doi: 10.1002/cm.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 30.Goode BL, Rodal AA, Barnes G, Drubin DG. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J Cell Biol. 2001;153:627–634. doi: 10.1083/jcb.153.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olazabal IM, Machesky LM. Abp1p and cortactin, new “hand-holds” for actin. J Cell Biol. 2001;154:679–682. doi: 10.1083/jcb.200105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–29677. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 33.Ohoka Y, Takai Y. Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells. 1998;3:603–612. doi: 10.1046/j.1365-2443.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- 34.van Rossum AG, de Graaf JH, Schuuring-Scholtes E, Kluin PM, Fan YX, Zhan X, et al. Alternative splicing of the actin binding domain of human cortactin affects cell migration. J Biol Chem. 2003;278:45672–45679. doi: 10.1074/jbc.M306688200. [DOI] [PubMed] [Google Scholar]
- 35.Katsube T, Togashi S, Hashimoto N, Ogiu T, Tsuji H. Filamentous actin binding ability of cortactin isoforms is responsible for their cell-cell junctional localization in epithelial cells. Arch Biochem Biophys. 2004;427:79–90. doi: 10.1016/j.abb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 38.Rey M, Irondelle M, Waharte F, Lizarraga F, Chavrier P. HDAC6 is required for invadopodia activity and invasion by breast tumor cells. Eur J Cell Biol. 2011;90:128–135. doi: 10.1016/j.ejcb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lua BL, Low BC. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 2005;579:577–585. doi: 10.1016/j.febslet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 41.Zhan X, Hu X, Hampton B, Burgess WH, Friesel R, Maciag T. Murine cortactin is phosphorylated in response to fibroblast growth factor-1 on tyrosine residues late in the G1 phase of the BALB/c 3T3 cell cycle. J Biol Chem. 1993;268:24427–24431. [PubMed] [Google Scholar]
- 42.Zhan X, Plourde C, Hu X, Friesel R, Maciag T. Association of fibroblast growth factor receptor-1 with c-Src correlates with association between c-Src and cortactin. J Biol Chem. 1994;269:20221–20224. [PubMed] [Google Scholar]
- 43.Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One. 2010;5:13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa K, Kashiwada K, Takahashi M, Sobue K. Translocation of cortactin (p80/85) to the actin-based cytoskeleton during thrombin receptor-mediated platelet activation. Exp Cell Res. 1995;221:197–204. doi: 10.1006/excr.1995.1367. [DOI] [PubMed] [Google Scholar]
- 47.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 48.Ren G, Helwani FM, Verma S, McLachlan RW, Weed SA, Yap AS. Cortactin is a functional target of E-cadherin-activated Src family kinases in MCF7 epithelial monolayers. J Biol Chem. 2009;284:18913–18922. doi: 10.1074/jbc.M109.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehio C, Prevost MC, Sansonetti PJ. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 51.Eiseler T, Hausser A, De Kimpe L, Van Lint J, Pfizenmaier K. Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J Biol Chem. 2010;285:18672–18683. doi: 10.1074/jbc.M109.093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crostella L, Lidder S, Williams R, Skouteris GG. Hepatocyte Growth Factor/scatter factor-induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene. 2001;20:3735–3745. doi: 10.1038/sj.onc.1204474. [DOI] [PubMed] [Google Scholar]
- 53.Gallet C, Rosa JP, Habib A, Lebret M, Levy-Toledano S, Maclouf J. Tyrosine phosphorylation of cortactin associated with Syk accompanies thromboxane analogue-induced platelet shape change. J Biol Chem. 1999;274:23610–23616. doi: 10.1074/jbc.274.33.23610. [DOI] [PubMed] [Google Scholar]
- 54.Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances Cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010;11:1079–1091. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 55.Kapus A, Di Ciano C, Sun J, Zhan X, Kim L, Wong TW, et al. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. The role of Fyn and FER kinases. J Biol Chem. 2000;275:32289–32298. doi: 10.1074/jbc.M003172200. [DOI] [PubMed] [Google Scholar]
- 56.Kim L, Wong TW. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J Biol Chem. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maruyama S, Kurosaki T, Sada K, Yamanashi Y, Yamamoto T, Yamamura H. Physical and functional association of cortactin with Syk in human leukemic cell line K562. J Biol Chem. 1996;271:6631–6635. doi: 10.1074/jbc.271.12.6631. [DOI] [PubMed] [Google Scholar]
- 59.Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–519. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamura H, Resh MD. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J Biol Chem. 1995;270:26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- 63.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci USA. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Yu D, Zeng XC, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem. 2007;282:16086–16094. doi: 10.1074/jbc.M701997200. [DOI] [PubMed] [Google Scholar]
- 65.Martin KH, Jeffery ED, Grigera PR, Shabanowitz J, Hunt DF, Parsons JT. Cortactin phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2006;119:2851–2853. doi: 10.1242/jcs.03034. [DOI] [PubMed] [Google Scholar]
- 66.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 67.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 68.Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, et al. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr Biol. 2003;13:384–393. doi: 10.1016/s0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 69.Lai FP, Szczodrak M, Oelkers JM, Ladwein M, Acconcia F, Benesch S, et al. Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol Biol Cell. 2009;20:3209–3223. doi: 10.1091/mbc.E08-12-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, et al. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 72.Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- 73.Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- 74.Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 75.van Rossum AG, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp Cell Res. 2006;312:1658–1670. doi: 10.1016/j.yexcr.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka S, Kunii M, Harada A, Okabe S. Generation of cortactin floxed mice and cellular analysis of motility in fibroblasts. Genesis. 2009;47:638–646. doi: 10.1002/dvg.20544. [DOI] [PubMed] [Google Scholar]
- 77.Somogyi K, Rorth P. Cortactin modulates cell migration and ring canal morphogenesis during Drosophila oogenesis. Mech Dev. 2004;121:57–64. doi: 10.1016/j.mod.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Kempiak SJ, Yamaguchi H, Sarmiento C, Sidani M, Ghosh M, Eddy RJ, et al. A neural Wiskott-Aldrich Syndrome protein-mediated pathway for localized activation of actin polymerization that is regulated by cortactin. J Biol Chem. 2005;280:5836–5842. doi: 10.1074/jbc.M410713200. [DOI] [PubMed] [Google Scholar]
- 79.Tehrani S, Faccio R, Chandrasekar I, Ross FP, Cooper JA. Cortactin has an essential and specific role in osteoclast actin assembly. Mol Biol Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Unsworth KE, Way M, McNiven M, Machesky L, Holden DW. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol. 2004;6:1041–1055. doi: 10.1111/j.1462-5822.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 81.Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, et al. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci USA. 2007;104:10882–10887. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 85.Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res. 2005;302:83–95. doi: 10.1016/j.yexcr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 86.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, et al. Regulation of lamellipodial persistence, adhesion turnover and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Croucher DR, Rickwood D, Tactacan CM, Musgrove EA, Daly RJ. Cortactin modulates RhoA activation and expression of Cip/Kip cyclin-dependent kinase inhibitors to promote cell cycle progression in 11q13-amplified head and neck squamous cell carcinoma cells. Mol Cell Biol. 30:5057–5070. doi: 10.1128/MCB.00249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–1279. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 90.Clark ES, Weaver AM. A new role for cortactin in invadopodia: Regulation of protease secretion. Eur J Cell Biol. 2008;87:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 92.Wu X, Quondamatteo F, Brakebusch C. Cdc42 expression in keratinocytes is required for the maintenance of the basement membrane in skin. Matrix Biol. 2006;25:466–474. doi: 10.1016/j.matbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 95.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oser M, Dovas A, Cox D, Condeelis J. Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur J Cell Biol. 2011;90:181–188. doi: 10.1016/j.ejcb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASPArp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Artym VV, Matsumoto K, Mueller SC, Yamada KM. Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur J Cell Biol. 2011;90:172–180. doi: 10.1016/j.ejcb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adh Migr. 2009;3:288–292. doi: 10.4161/cam.3.3.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 102.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, et al. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma T, Sadashivaiah K, Chellaiah MA. Regulation of sealing ring formation by L-plastin and cortactin in osteoclasts. J Biol Chem. 285:29911–29924. doi: 10.1074/jbc.M109.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: roles of the protein interaction domains. Exp Cell Res. 2006;312:760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 105.Webb BA, Jia L, Eves R, Mak AS. Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur J Cell Biol. 2007;86:189–206. doi: 10.1016/j.ejcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, et al. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is upregulated in prostate and breast cancer. Cancer Res. 2009;69:747–752. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi H, Miki H, Takenawa T. Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion and tubulogenesis of epithelial cells. Cancer Res. 2002;62:2503–2509. [PubMed] [Google Scholar]
- 108.Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol. 2008;18:926–931. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 109.Clark ES, Brown B, Whigham AS, Kochaishvili A, Yarbrough WG, Weaver AM. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–444. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 111.Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 112.Liu J, Yue P, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell. 2009;20:3763–3771. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol. 2010;30:781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Engqvist-Goldstein AE, Zhang CX, Carreno S, Barroso C, Heuser JE, Drubin DG. RNAi-mediated Hip1R silencing results in stable association between the endocytic machinery and the actin assembly machinery. Mol Biol Cell. 2004;15:1666–1679. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 117.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of {gamma}c cytokine receptor. J Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 119.Timpson P, Lynch DK, Schramek D, Walker F, Daly RJ. Cortactin overexpression inhibits ligand-induced downregulation of the epidermal growth factor receptor. Cancer Res. 2005;65:3273–3280. doi: 10.1158/0008-5472.CAN-04-2118. [DOI] [PubMed] [Google Scholar]
- 120.Barroso C, Rodenbusch SE, Welch MD, Drubin DG. A role for cortactin in Listeria monocytogenes invasion of NIH 3T3 cells, but not in its intracellular motility. Cell Motil Cytoskeleton. 2006;63:231–243. doi: 10.1002/cm.20119. [DOI] [PubMed] [Google Scholar]
- 121.Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, et al. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- 122.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sbai O, Ould-Yahoui A, Ferhat L, Gueye Y, Bernard A, Charrat E, et al. Differential vesicular distribution and trafficking of MMP-2, MMP-9 and their inhibitors in astrocytes. Glia. 2009;58:344–366. doi: 10.1002/glia.20927. [DOI] [PubMed] [Google Scholar]
- 124.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 125.Riefler GM, Balasingam G, Lucas KG, Wang S, Hsu SC, Firestein BL. Exocyst complex subunit sec8 binds to postsynaptic density protein-95 (PSD-95): a novel interaction regulated by cypin (cytosolic PSD-95 interactor) Biochem J. 2003;373:49–55. doi: 10.1042/BJ20021838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 127.Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006;8:1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]
- 128.Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, et al. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- 131.Cai JH, Zhao R, Zhu JW, Jin XL, Wan FJ, Liu K, et al. Expression of cortactin correlates with a poor prognosis in patients with stages II-III colorectal adenocarcinoma. J Gastrointest Surg. 14:1248–257. doi: 10.1007/s11605-010-1247-2. [DOI] [PubMed] [Google Scholar]
- 132.Lin CK, Su HY, Tsai WC, Sheu LF, Jin JS. Association of cortactin, fascin-1 and epidermal growth factor receptor (EGFR) expression in ovarian carcinomas: correlation with clinicopathological parameters. Dis Markers. 2008;25:17–26. doi: 10.1155/2008/284382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–11699. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 134.Wang X, Cao W, Mo M, Wang W, Wu H, Wang J. VEGF and cortactin expression are independent predictors of tumor recurrence following curative resection of gastric cancer. J Surg Oncol. 102:325–330. doi: 10.1002/jso.21644. [DOI] [PubMed] [Google Scholar]
- 135.Xie HL, Li ZY, Gan RL, Li XJ, Zhang QL, Hui M, et al. Differential gene and protein expression in primary gastric carcinomas and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. J Dig Dis. 11:167–175. doi: 10.1111/j.1751-2980.2010.00432.x. [DOI] [PubMed] [Google Scholar]
- 136.Xu XZ, Garcia MV, Li TY, Khor LY, Gajapathy RS, Spittle C, et al. Cytoskeleton alterations in melanoma: aberrant expression of cortactin, an actin-binding adapter protein, correlates with melanocytic tumor progression. Mod Pathol. 23:187–196. doi: 10.1038/modpathol.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gibcus JH, Menkema L, Mastik MF, Hermsen MA, de Bock GH, van Velthuysen ML, et al. Amplicon mapping and expression profiling identify the Fasassociated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin Cancer Res. 2007;13:6257–6266. doi: 10.1158/1078-0432.CCR-07-1247. [DOI] [PubMed] [Google Scholar]
- 138.Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–727. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Rodrigo JP, Garcia-Carracedo D, Garcia LA, Menendez S, Allonca E, Gonzalez MV, et al. Distinctive clinicopathological associations of amplification of the cortactin gene at 11q13 in head and neck squamous cell carcinomas. J Pathol. 2009;217:516–523. doi: 10.1002/path.2462. [DOI] [PubMed] [Google Scholar]
- 140.Fantozzi I, Grall D, Cagnol S, Stanchi F, Sudaka A, Brunstein MC, et al. Overexpression of cortactin in head and neck squamous cell carcinomas can be uncoupled from augmented EGF receptor expression. Acta Oncol. 2008;47:1502–1512. doi: 10.1080/02841860802089801. [DOI] [PubMed] [Google Scholar]
- 141.Hofman P, Butori C, Havet K, Hofman V, Selva E, Guevara N, et al. Prognostic significance of cortactin levels in head and neck squamous cell carcinoma: comparison with epidermal growth factor receptor status. Br J Cancer. 2008;98:956–964. doi: 10.1038/sj.bjc.6604245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Timpson P, Wilson AS, Lehrbach GM, Sutherland RL, Musgrove EA, Daly RJ. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res. 2007;67:9304–9314. doi: 10.1158/0008-5472.CAN-07-0798. [DOI] [PubMed] [Google Scholar]
- 143.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 144.van Rossum AG, van Bragt MP, Schuuring-Scholtes E, van der Ploeg JC, van Krieken JH, Kluin PM, et al. Transgenic mice with mammary gland targeted expression of human cortactin do not develop (premalignant) breast tumors: studies in MMTV-cortactin and MMTV-cortactin/-cyclin D1 bitransgenic mice. BMC Cancer. 2006;6:58. doi: 10.1186/1471-2407-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y, Tondravi M, Liu J, Smith E, Haudenschild CC, Kaczmarek M, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–6911. [PubMed] [Google Scholar]
- 146.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, et al. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–636. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 147.Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, et al. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang K, Wang D, Song J. Cortactin is involved in transforming growth factor-beta1-induced epithelial-mesenchymal transition in AML-12 cells. Acta Biochim Biophys Sin (Shanghai) 2009;41:839–845. doi: 10.1093/abbs/gmp070. [DOI] [PubMed] [Google Scholar]
- 149.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 151.Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem. 2004;279:48246–48254. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- 152.Huang R, Cao GJ, Guo H, Kordowska J, Albert Wang CL. Direct interaction between caldesmon and cortactin. Arch Biochem Biophys. 2006;456:175–182. doi: 10.1016/j.abb.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stork B, Engelke M, Frey J, Horejsi V, Hamm-Baarke A, Schraven B, et al. Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity. 2004;21:681–691. doi: 10.1016/j.immuni.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 157.Di Fulvio M, Frondorf K, Henkels KM, Lehman N, Gomez-Cambronero J. The Grb2/PLD2 interaction is essential for lipase activity, intracellular localization and signaling in response to EGF. J Mol Biol. 2007;367:814–824. doi: 10.1016/j.jmb.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abella JV, Parachoniak CA, Sangwan V, Park M. Dorsal ruffle microdomains potentiate Met receptor tyrosine kinase signaling and downregulation. J Biol Chem. 285:24956–24967. doi: 10.1074/jbc.M110.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hattan D, Nesti E, Cachero TG, Morielli AD. Tyrosine phosphorylation of Kv1.2 modulates its interaction with the actin-binding protein cortactin. J Biol Chem. 2002;277:38596–38606. doi: 10.1074/jbc.M205005200. [DOI] [PubMed] [Google Scholar]
- 160.Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 161.Stuible M, Dube N, Tremblay ML. PTP1B regulates cortactin tyrosine phosphorylation by targeting Tyr446. J Biol Chem. 2008;283:15740–15746. doi: 10.1074/jbc.M710534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Okamura H, Resh MD. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J Biol Chem. 1995;270:26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- 163.Oser M, Dovas A, Cox D, Condeelis J. Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur J Cell Biol. 2010 doi: 10.1016/j.ejcb.2010.08.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang X, Shrikhande U, Alicie BM, Zhou Q, Geahlen RL. Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol Cancer Res. 2009;7:634–644. doi: 10.1158/1541-7786.MCR-08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Linder S, Aepfelbacher M. Podosomes: adhesion hotspots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 167.Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, et al. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 168.Campbell DH, Sutherland RL, Daly RJ. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 1999;59:5376–5385. [PubMed] [Google Scholar]
- 169.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 170.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 171.Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 172.Stofega MR, Sanders LC, Gardiner EM, Bokoch GM. Constitutive p21-activated kinase (PAK) activation in breast cancer cells as a result of mislocalization of PAK to focal adhesions. Mol Biol Cell. 2004;15:2965–2977. doi: 10.1091/mbc.E03-08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu W, Mayer BJ. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene. 1999;18:797–806. doi: 10.1038/sj.onc.1202361. [DOI] [PubMed] [Google Scholar]
- 174.Shang X, Zhou YT, Low BC. Concerted regulation of cell dynamics by BNIP-2 and Cdc42GAP homology/Sec14p-like, proline-rich and GTPase-activating protein domains of a novel Rho GTPase-activating protein, BPGAP1. J Biol Chem. 2003;278:45903–45914. doi: 10.1074/jbc.M304514200. [DOI] [PubMed] [Google Scholar]
- 175.Lua BL, Low BC. BPGAP1 interacts with cortactin and facilitates its translocation to cell periphery for enhanced cell migration. Mol Biol Cell. 2004;15:2873–2883. doi: 10.1091/mbc.E04-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Le Clainche C, Pauly BS, Zhang CX, Engqvist-Goldstein AE, Cunningham K, Drubin DG. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 2007;26:1199–1210. doi: 10.1038/sj.emboj.7601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, et al. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J. 2006;20:2588–2590. doi: 10.1096/fj.06-6152fje. [DOI] [PubMed] [Google Scholar]
- 178.Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, et al. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, et al. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, et al. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol. 2008;10:85–92. doi: 10.1038/ncb1672. [DOI] [PubMed] [Google Scholar]
- 182.Taagepera S, McDonald D, Loeb JE, Whitaker LL, McElroy AK, Wang JY, et al. Nuclear-cytoplasmic shuttling of C-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr Biol. 2004;14:1222–1231. doi: 10.1016/j.cub.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 184.Matas OB, Martinez-Menarguez JA, Egea G. Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic. 2004;5:838–846. doi: 10.1111/j.1600-0854.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 185.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 186.Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jones SM, Howell KE, Henley JR, Cao H, McNiven MA. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 188.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Redecker P, Bockmann J, Bockers TM. Secretory granules of hypophyseal and pancreatic endocrine cells contain proteins of the neuronal postsynaptic density. Cell Tissue Res. 2007;328:49–55. doi: 10.1007/s00441-006-0309-y. [DOI] [PubMed] [Google Scholar]
- 191.Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 192.Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boeckers TM. The postsynaptic density. Cell Tissue Res. 2006;326:409–422. doi: 10.1007/s00441-006-0274-5. [DOI] [PubMed] [Google Scholar]
- 194.Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, et al. Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem. 2006;281:1461–1469. doi: 10.1074/jbc.M509786200. [DOI] [PubMed] [Google Scholar]
- 195.McWilliams RR, Gidey E, Fouassier L, Weed SA, Doctor RB. Characterization of an ankyrin repeat-containing Shank2 isoform (Shank2E) in liver epithelial cells. Biochem J. 2004;380:181–191. doi: 10.1042/BJ20031577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum Mol Genet. 2003;12:1981–1993. doi: 10.1093/hmg/ddg209. [DOI] [PubMed] [Google Scholar]
- 197.Estrada L, Caron E, Gorski JL. Fgd1, the Cdc42 guanine nucleotide exchange factor responsible for faciogenital dysplasia, is localized to the subcortical actin cytoskeleton and Golgi membrane. Hum Mol Genet. 2001;10:485–495. doi: 10.1093/hmg/10.5.485. [DOI] [PubMed] [Google Scholar]
- 198.Le Bras S, Massaad M, Koduru S, Kumar L, Oyoshi MK, Hartwig J, et al. WIP is critical for T cell responsiveness to IL-2. Proc Natl Acad Sci USA. 2009;106:7519–7524. doi: 10.1073/pnas.0806410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, et al. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat Cell Biol. 2001;3:484–491. doi: 10.1038/35074551. [DOI] [PubMed] [Google Scholar]
- 200.de la Fuente MA, Sasahara Y, Calamito M, Anton IM, Elkhal A, Gallego MD, et al. WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP) Proc Natl Acad Sci USA. 2007;104:926–931. doi: 10.1073/pnas.0610275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brown M, Adyshev D, Bindokas V, Moitra J, Garcia JG, Dudek SM. Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc Res. 80:75–88. doi: 10.1016/j.mvr.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 203.Lin J, Liu J, Wang Y, Zhu J, Zhou K, Smith N, et al. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005;24:2059–2066. doi: 10.1038/sj.onc.1208412. [DOI] [PubMed] [Google Scholar]
- 204.Gonzalez-Quevedo R, Shoffer M, Horng L, Oro AE. Receptor tyrosine phosphatase-dependent cytoskeletal remodeling by the hedgehog-responsive gene MIM/BEG4. J Cell Biol. 2005;168:453–463. doi: 10.1083/jcb.200409078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lynch DK, Winata SC, Lyons RJ, Hughes WE, Lehrbach GM, Wasinger V, et al. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J Biol Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 206.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 207.Brett TJ, Traub LM, Fremont DH. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure. 2002;10:797–809. doi: 10.1016/s0969-2126(02)00784-0. [DOI] [PubMed] [Google Scholar]
- 208.Welsch T, Endlich N, Gokce G, Doroshenko E, Simpson JC, Kriz W, et al. Association of CD2AP with dynamic actin on vesicles in podocytes. Am J Physiol Renal Physiol. 2005;289:1134–1143. doi: 10.1152/ajprenal.00178.2005. [DOI] [PubMed] [Google Scholar]
- 209.Monzo P, Mari M, Kaddai V, Gonzalez T, Le Marchand-Brustel Y, Cormont M. CD2AP, Rabip4 and Rabip4′: analysis of interaction with Rab4a and regulation of endosomes morphology. Methods Enzymol. 2005;403:107–118. doi: 10.1016/S0076-6879(05)03010-7. [DOI] [PubMed] [Google Scholar]
- 210.Cormont M, Meton I, Mari M, Monzo P, Keslair F, Gaskin C, et al. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4:97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]