Abstract
Objectives/Hypothesis
To evaluate antitumor efficacy of the generic mTOR inhibitor sirolimus in preclinical animal models of head and neck squamous cell carcinoma (HNSCC) and compare its effects with those of the patented analog Temsirolimus.
Study Design
In vivo study.
Methods
To develop xenograft established tumor model (ETM) of HNSCC, FaDu cells were injected subcutaneously into nude mice. When tumors reached 50–60mm3 mice were randomized into 5 groups and treated daily i.p. with sirolimus at various doses for 5 days a week for 3 weeks. Tumor volumes were measured. The results were compared with historical data on Temsirolimus effects. In the minimal residual disease (MRD) model surgical wounds were created and FaDu cells implanted. After 72h, animals were randomized into two groups and were injected i.p. with 0 or 5 mg/kg sirolimus for 5 days a week for one month.
Results
In the ETM, sirolimus significantly inhibited tumor growth (p<0.01) although there was no overall significant difference in tumor growth inhibition between sirolimus and Temsirolimus. In the MRD model, sirolimus significantly suppressed growth of tumors (p<0.001) and improved survival compared with controls (p<0.01). There was a significant decrease in pS6 expression, indicating mTOR inhibition.
Conclusion
In this study we demonstrate that the generic mTOR inhibitor sirolimus shows potent antitumor activity in HNSCC and produces comparable effects to the patent drug Temsirolimus. Sirolimus has the potential of serving as an economic and comparative targeted agent to Temsirolimus in the treatment of HNSCC.
Level of Evidence
1b Individual randomized controlled trial.
Keywords: sirolimus, Temsirolimus, head and neck squamous cell carcinoma
INTRODUCTION
Rapamycin is a mammalian target of rapamycin (mTOR) inhibitor that was initially isolated more than 30 years ago from Streptomyces hygroscopicus on Easter Island (also known as Rapa Nui) and used as an antifungal agent1. It was soon found to have immunosuppressive and antiproliferative effects on human tumor cells by the inhibition of mTOR2. The PI3K/Akt/mTOR kinase pathway is a major regulator of cell proliferation, metabolism, survival and angiogenesis3,4. In many cancers, including head and neck squamous cell carcinoma (HNSCC), this pathway is upregulated5. mTOR phosphorylates and inactivates the translation suppressor initiation factor 4E-binding protein 1 (4E-BP1) and activates ribosomal p 70 S6 kinase (S6K1)6. The mTOR pathway presents an attractive therapeutic target by acting downstream of broader function upstream proteins in the PI3K/Akt pathway7. Due to the promising effects of mTOR inhibitors, a number of rapamycin analogues have been developed.
Sirolimus was the first rapalogue introduced on the market. It was initially used for its immunosuppressive properties in organ transplant recipients8. Through these patients, sirolimus was also found to inhibit growth of smooth muscle cells which led to its use in drug-eluting stents9. In post-transplant patients, sirolimus was seen to cause regression of Kaposi’s sarcoma and renal angiomyolipomas in tuberous sclerosis10,11. Phase I studies have been performed using sirolimus in combination with chemotherapy to treat acute myelogenous leukemia and showed the possibility of combined therapy12. The role of sirolimus in combination with other chemotherapeutic to treat advanced colorectal cancer is also promising13. Currently, prospective randomized controlled studies are underway looking at the effects of sirolimus in preventing non-melanoma skin cancers and the recurrence of hepatocellular carcinoma in post-transplant patients14,15.
Since the patent for the anti-cancer application of rapamycin (sirolimus, Rapamune®) expired in 1993, other rapalogues have been introduced. Wyeth patented an intravenous form, Temsirolimus in 2007. Novartis recently revealed an oral rapalogue Everolimus. Merck and Ariad Pharmaceuticals most recently co-developed Deforolimus.
Early phase clinical trials have demonstrated these rapalogues to be effective in treating refractory mantle cell lymphoma, glioblastoma, breast cancer, endometrial cancer, non-Hodgkin lymphomas, and multiple myeloma16. Temsirolimus and Everolimus are currently FDA-approved for treatment of renal cell carcinoma17. In head and neck squamous cell carcinoma, we have shown that Temsirolimus can inhibit tumor growth and increase tumor free rates18. We have also demonstrated that Temsirolimus has radiosensitizing effects in an experimental model of HNSCC19. Rapalogues also demonstrate a relatively low side effect profile as it is used for extended periods in renal transplant patients and angioplasty patients who use rapalogues as immunosuppressant and anti-proliferative agents, respectively20.
With the increasing numbers of designer drugs on the market, and an increasing emphasis on biosimilars and generics, there has been a rising focus in determining the cost and comparative effectiveness of drugs. The main goals of this study are to evaluate antitumor efficacy of the cheaper generic mTOR inhibitor sirolimus in an in vivo HNSCC animal model and to compare its effectiveness with patented analog Temsirolimus.
MATERIALS AND METHODS
Cell line
The eIF4E-overexpressing HNSCC cell line FaDu, derived from a hypopharyngeal squamous cell carcinoma (obtained from American Type Culture Collection, Manassas, VA) was grown in RPMI 1640 (Life Technologies, Grand Island, NY), supplemented with 10% bovine calf serum and nonessential amino acids (Life Technologies). Cells were grown in monolayers and maintained in humidified 5% CO2 atmosphere at 37°C. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) except for sirolimus which was obtained from LC Laboratories (Woburn, MA).
Established Tumor Model
BALB/c nu/nu mice (Charles River Laboratories, Wilmington, MA) were injected with 1 × 106 FaDu cells subcutaneously. Animals were housed in a barrier facility and maintained on a normal diet ad libitum. All studies were conducted in compliance with the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee guidelines. Tumor volume was determined with digital caliper, (length × width2)/2, and established at a volume of 50–60 mm3. Animals were randomized into five groups of five mice each and treated with vehicle or sirolimus at a daily i.p dose of 5, 10, 15, and 20 mg/kg for 5 days per week for 3 weeks. Sirolimus was prepared in 4% ethanol, 5.2% Tween 80, and 5.2% polyethylene glycol 400 (Sigma-Aldrich). Tumor volumes were measured three times a week.
Minimal Residual Disease Model
Nude mice were anesthetized and incisions were made in the dorsal flanks and subcutaneous flaps were elevated with sharp dissection. Pipette dispensers were used to introduce tumor cells (1 × 106 FaDu cells) in 100 μL saline into the flap, which was then sutured. 72 hours following tumor seeding, the animals were randomized into two groups, 10 mice in the control group and 21 mice treated with sirolimus 5 mg/kg daily for 5 days per week till the end of the experiment. Tumor volume was determined with digital calipers with the formula (length × width2)/2. Mice were sacrificed due to significant tumor burden (>2,000 mm3), weight loss >15%, or at a maximum time point of 30 days after treatment initiation. As a surrogate marker of toxicity, body weight was measured weekly for the duration of the experiment at the time of tumor measurement without any differences observed between treated and control animals (Supplemental Figure 1).
Western blot analysis of 4E-BP1 and S6 ribosomal protein
Protein was extracted from tumor samples following treatment with sirolimus to confirm the observed effects of sirolimus from mTOR inhibition. Western blot analysis was performed according to previously published laboratory protocol21.
The following antibodies were used: rabbit polyclonal anti-4E-BP1 (1:500), rabbit anti-S6 ribosomal protein (1:100), rabbit anti-phospho-S6 ribosomal protein (Ser 235/236;1:100); all antibodies were obtained from Cell Signaling (Beverly, MA).
RESULTS
Sirolimus is as effective as Temsirolimus in inhibiting HNSCC tumor growth
The effect of sirolimus on tumor growth was evaluated in an established xenograft tumor model (Table 1). Using established tumor model we determined that all doses of sirolimus (5–20 mg/kg) significantly inhibited the growth of FaDu xenograft tumor compared to control (P<0.01; repeated measures ANOVA with the Bonferroni post-test). When the results of this study was compared with the results of our published study on the effects of Temsirolimus18 there were no significant differences in tumor volumes between sirolimus and Temsirolimus at 5, 15, and 20 mg/kg (Table 1). Interestingly, at 10 mg/kg, sirolimus performed significantly better than Temsirolimus in preventing tumor growth (p <0.01; the Wilcoxon rank-sum test). Therefore, sirolimus was at least as effective as Temsirolimus in inhibiting HNSCC tumor growth.
Table 1.
Comparisons between sirolimus-treated and control mice on average tumor volume in an established xenograft tumor model
| Day | Study groups (n=5) Mean±SD, 95% confidence range |
p-value vs. corresponding control | p-value vs. historical data on Temsirolimus18 |
|---|---|---|---|
| Control | |||
| Day 0 | 55.7±15.2, 38.2 – 76.9 | ||
| Day 7 | 176.8±85.6, 54.8 – 282.5 | ||
| Day 14 | 460.0±262.2, 105.3 – 832.3 | ||
| Day 21 | 684.0±439.0, 143.9 – 1349 | ||
| Rapamycin = 5 mg/kg | |||
| Day 0 | 56.4±19.2, 33.7 – 74.0 | 0.92 | 0.92 |
| Day 7 | 71.8±11.4, 55.0 – 84.5 | 0.12 | 0.60 |
| Day 14 | 96.4±25.1, 68.2 – 127.6 | 0.03* | 0.60 |
| Day 21 | 106.8±28.4, 72.3 – 139.9 | 0.009** | 0.60 |
| Rapamycin = 10 mg/kg | |||
| Day 0 | 56.0±9.3, 42.3 – 66.5 | 0.92 | 0.60 |
| Day 7 | 57.3±28.5, 32.5 – 94.5 | 0.03* | 0.009** |
| Day 14 | 78.1±19.5, 50.5 – 101.4 | 0.009** | 0.009** |
| Day 21 | 102.1±22.7, 78.4 – 126.2 | 0.009** | 0.009** |
| Rapamycin = 15 mg/kg | |||
| Day 0 | 55.1±12.6, 36.9 – 69.3 | 0.92 | 0.60 |
| Day 7 | 68.9±7.6, 60.1 – 81.1 | 0.12 | 0.12 |
| Day 14 | 106.3±10.1, 99.2 – 123.9 | 0.02* | 0.12 |
| Day 21 | 155.4±27.3, 112.3 – 187.5 | 0.04* | 0.07 |
| Rapamycin = 20 mg/kg | |||
| Day 0 | 55.9±9.1, 49.2 – 70.3 | 0.92 | 0.92 |
| Day 7 | 51.1±16.1, 22.8 – 63.3 | 0.04* | 0.12 |
| Day 14 | 78.6±29.2, 41.1 – 122.7 | 0.02* | 0.75 |
| Day 21 | 114.4±56.4, 65.4 – 109.8 | 0.02* | 0.60 |
Significant difference at 5% level (0.01<p-value<0.01
Significant difference at 1% level (p-value<0.01)
The efficacy of sirolimus in a MRD model
The effect of sirolimus was also tested in a MRD model. Tumor formation was measured up to day 30 after sirolimus treatment initiation (5 mg/kg) and compared to control mice (Fig. 1A). Similarly to its effects in ETM sirolimus significantly inhibited tumor growth compared to control in MRD model as well (P<0.001). On closer inspection of the tumor growth curve, it appears on the days of no treatment, the rate of tumor growth increased (Figure 1B). This observation may imply that continuous treatment of sirolimus may provide better results in inhibiting tumor growth than a cyclical treatment regimen. Sirolimus demonstrated a significant improvement in survival compared to control on the Kaplan-Meier survival curve, p<0.01 (Figure 2). Among the control mice, 60% have been sacrificed by the end of the study with median survival time of 27.5 days. All the mice treated with sirolimus survived till the end of the study.
Figure 1.
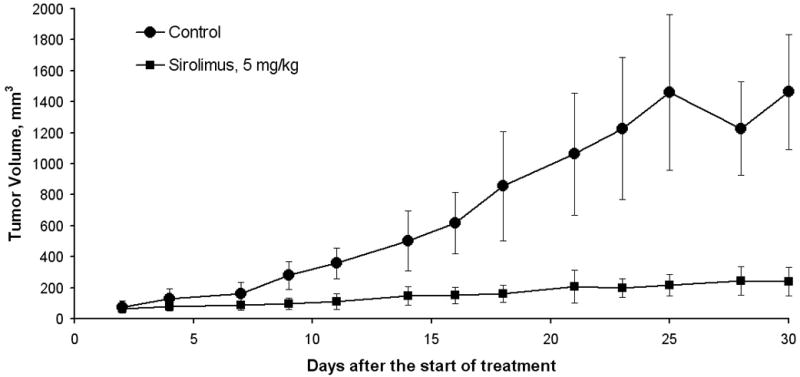
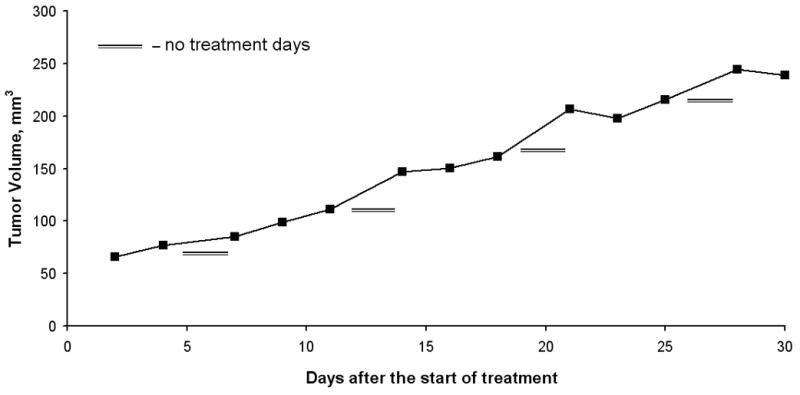
A, Tumor growth curve in the minimal residual disease model comparing sirolimus to control, revealing a significant difference in tumor growth inhibition in the sirolimus treated group. B, Tumor growth curve: volume growth rates (mm3 per mouse per day) increased on “no treatment” days, resulting in growth “spikes” in the sirolimus treatment group.
Figure 2.
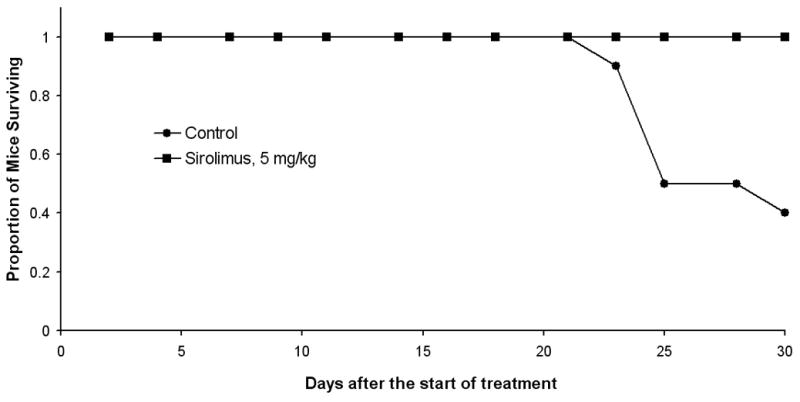
Kaplan-Meier curves of survival comparison between control and sirolimus-treated FaDu xenograft mice in MRD. Mice were sacrificed due to significant tumor burden (>2,000 mm3), weight loss >15%, or at maximum time point of 30 days after the treatment initiation. Sirolimus (5 mg/kg) significantly improved survival compared to control (p<0.01).
The effects of sirolimus on mTOR signaling
Inhibition of mTOR signaling by sirolimus in tumor tissues was assessed by western blot analysis. Treatment with sirolimus (5 mg/kg) led to an inhibition in the phosphorylation of 4E-BP1 with a decrease in the phosphorylated γ isoform and an increase of the β isoform and unphosphorylated α isoform (Figure 3). Sirolimus also decreased pS6 expression by 64.9% which was comparable to the effects of Temsirolimus treatment in our previous study20. These analyses confirmed the activity of sirolimus used in our study.
Figure 3.
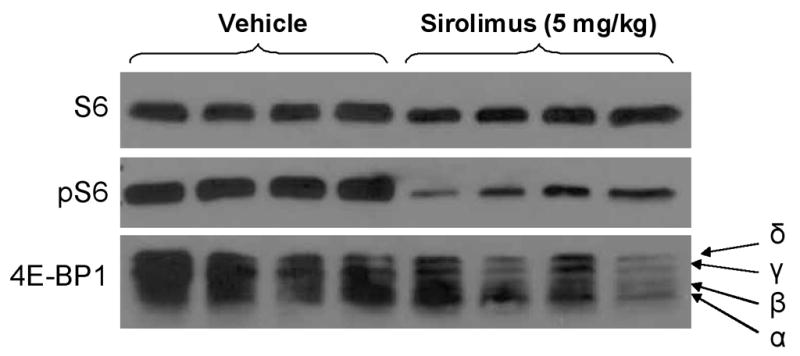
Western blot analysis showing a decrease in phospho-S6 and shift from the more phosphorylated ‘δ’ and ‘γ’ isoforms to the less phosphorylated ‘β’ and unphosphorylated ‘α’ isoforms of 4E-BP1 in the tumor tissue of the mice treated with sirolimus.
DISCUSSION
HNSCC accounts for 4% of all malignancies in the country. Locoregionally advanced head and neck cancer is treated with multimodality therapy consisting of either definitive concurrent chemoradiotherapy or resection followed by postoperative radiotherapy22. However, high risk HNSCC patients with locally advanced stage III and IV tumors, demonstrate a 50–60% recurrence rate. The addition of complementary drugs such as targeted agents to the concurrent chemoradiotherapy regimen has shown promise in optimizing treatment by potentially increasing survival rates and decreasing recurrence rates.
Molecular targeting agents have been used successfully in treatment of other cancers. Trastuzumab, a monoclonal antibody, that binds and inhibits HER2/neu receptors is now an integral part of breast cancer treatment for qualifying patients. Gleevec, a tyrosine kinase inhibitor, is used in the treatment of chronic myologenous leukemia. In HNSCC, cetuximab is a monoclonal antibody that inhibits epidermal growth factor receptors (EGFR) used in combination or alone to treat metastatic disease. Another emerging targeting agent is rapamycin, an mTOR inhibitor.
Rapamycin is an mTOR inhibitor that has an established effect on suppressing tumor growth in a number of solid tumors. It has demonstrated antiproliferative effects and apoptosis in HNSCC cell lines4. We previously demonstrated that Temsirolimus can inhibit tumor growth in a HNSCC animal model and increase the tumor free period in minimal residual disease18.
We have also shown that Temsirolimus radiosensitizes HNSCC in vivo19. A case report further supports potential radiosensitizing properties of the rapalogues in HNSCC. It describes a patient post liver transplantation that developed laryngeal cancer and was subsequently switched to sirolimus (2 mg daily) from Tacrolimus. The patient was then treated with radiation for his HNSCC and demonstrated an exceptional response to therapy with complete resolution of the tumor23.
As research and clinical evidence continue to confirm the effectiveness of mTOR inhibitors as an anti-cancer agent, the number of commercially available rapamycin analogues has increased. There are a handful of rapamycin-related mTOR inhibitors on the market, at varying costs. Sirolimus, the first introduced rapamycin analog, currently costs $20 – $37 a day for 4 mg as the generic. Temsirolimus, a rapamycin analog introduced in 2007 by Wyeth carries a cost of $1916 for one 25 mg IV treatment weekly. In 2009, Novartis revealed another oral version of rapamycin, pricing their drug Everolimus at $99 for 5 mg.
Furthermore, the cost effectiveness of drug treatment is becoming an increasing focus in today’s health care spending. Generic drugs have moderated drug expenditures, and health care reform initiatives are looking into biosimilars legislation24. These changes will most likely affect drug treatment plans, including the delivery of combined chemoradiotherapy regimens in the future.
As research begins to reveal that rapamycin analogues have the potential to serve as complementary agents in treatment of HNSCC, drug comparison and effectiveness studies will help determine the clinical applicability and cost-effective of future drug therapies. The purpose of this study was to evaluate the efficacy of the generic mTOR inhibitor sirolimus in HNSCC and compare its effectiveness to the patented analog Temsirolimus.
We showed, in our study, sirolimus significantly inhibits tumor growth at all dosages (5–20 mg/kg) compared to control (P<0.01; repeated measures ANOVA with the Bonferroni post-test). In the minimal residual disease model, sirolimus slowed the growth of tumors and increased survival, similar to previous studies of Temsirolimus18. Using weight as a marker of toxicity, we observed no significant difference in weights of treated mice, suggesting the toxicity of sirolimus is low. Finally, in the established tumor model, when we compared the results of the current study with the results of our published study on the effects of Temsirolimus18 there was no significant difference in antitumor activity between sirolimus and Temsirolimus. This information suggests that sirolimus has the potential to provide equally effective results in the clinical setting as a complementary agent in HNSCC as more expensive patented rapamycin analogues. A better approach would perhaps have been to directly compare sirolimus and Temsirolimus in a randomized mice study. However, our previous study with Temsirolimus was performed in a similar fashion, as to provide an adequate historical control for our current study with sirolimus. Future studies may be considered comparing a five day treatment regimen to a seven day regimen of both sirolimus and Temsirolims.
We found that final tumor volumes were relatively similar in the establised tumor model, despite increasing dosages of the drug at 5, 10, 15 and 20 mg/kg. This suggests that higher dosages of sirolimus may not provide any additional benefit in preventing HNSCC tumor growth. Guba et al. found a similar pattern in their studies of rapamycin on tumor growth in mice. They went on to claim that tumor volumes were most effectively controlled with lower dosages of rapamycin. The inhibitory effect of rapamycin on angiogenesis and growth was found to be optimal at lower ranges. They speculate that this may be due to increasing side effects and immunosuppression induced by higher concentrations of the drug. This may in turn provide conditions advantageous for cancer growth25. Our data also suggests that a 7 day/week treatment regimen may produce better outcomes than a 5 day/week regimen. These findings will be applied to a multi-institutional phase I clinical trial looking at treatment of advanced head and neck cancers with Everolimus.
This study confirmed that the generic mTOR inhibitor sirolimus demonstrates potent anti-tumorigenic effects on HNSCC xenografts. Furthermore there was no significant difference between sirolimus and patented analog Temsirolimus. Additional studies comparing rapamycin analogues should also be constructed to include other analogues such as, Everolimus and Deforolimus, currently on the market. Our studies also showed that sirolimus inhibited the mTOR pathway, as a decrease in pS6 and a shift in the phosphorylated isoforms of 4EBP1 were observed. Although other markers could have been evaluated, these particular markers have been used in our previous publications for mTOR inhibitors26. A decrease in pS6 is also a well accepted marker for mTOR activity27. Ultimately, our findings need to be confirmed in the clinical setting, comparing the effectiveness of rapamycin analogues in treatment of HNSCC patients.
CONCLUSION
This study demonstrated potent antitumor activity of sirolimus in HNSCC and showed that both sirolimus, a cheaper generic rapamycin, and Temsirolimus, a more expensive patented rapamycin analogue, have comparable effectiveness in inhibiting tumor growth in a HNSCC animal model. In the future, sirolimus could serve as a cost effective complementary agent to treatment of HNSCC.
Supplementary Material
Acknowledgments
Funding: The study was supported by grant # R01CA102363 from the National Cancer Institute to C.O. Nathan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of Interest: None
BIBLIOGRAPHY
- 1.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975;28:727–32. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 2.Huang S, Houghton PJ. Inhibitors of mammalian target of rapamycin as novel antitumor agents: from bench to clinic. Curr Opin Investig Drugs. 2002;3:295–304. [PubMed] [Google Scholar]
- 3.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signaling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 4.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 5.Amornphimoltham P, Patel V, Sodhi A, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 6.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer. 2004;91:1420–4. doi: 10.1038/sj.bjc.6602162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepkowski SM, Tian L, Wang ME, Qu X, Napoli K, Kahan BD. Sirolimus in transplantation. Arch Immunol Ther Exp (Warsz) 1997;45:383–90. [PubMed] [Google Scholar]
- 9.Abizaid A. Sirolimus-eluting coronary stents: a review. Vasc Health Risk Manag. 2007;3:191–201. doi: 10.2147/vhrm.2007.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolhe N, Mamode N, Van der Walt J, Pattison J. Regression of post-transplant Kaposi’s sarcoma. Int J Clin Pract. 2006;60:1509–12. doi: 10.1111/j.1742-1241.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 11.Krischock L, Beach R, Taylor J. Sirolimus and tuberous sclerosis-associated renal angiomyolipomas. Arch Dis Child. 2010;95:391–2. doi: 10.1136/adc.2009.159210. [DOI] [PubMed] [Google Scholar]
- 12.Perl AE, Kasner MT, Tsai DE, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;15:6732–9. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 13.Ghiringhelli F, Guiu B, Chauffert B, Ladoire S. Sirolimus, bevacizumab, 5-Fluorouracil and irinotecan for advanced colorectal cancer: a pilot study. World J Gastroenterol. 2009;15:4278–83. doi: 10.3748/wjg.15.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgo R, Gossmann J, Schöfer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385–93. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzbauer AA, Zuelke C, Graeb C, et al. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer. 2010;10:190. doi: 10.1186/1471-2407-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–46. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 17.Pal SK, Figlin RA. Treatment Options in Metastatic Renal Cell Carcinoma: Focus on mTOR Inhibitors. Clin Med Insights Oncol. 2010;4:43–53. doi: 10.4137/cmo.s1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan CO, Amirghahari N, Rong X, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67:2160–8. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 19.Ekshyyan O, Rong Y, Rong X, et al. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8:2255–65. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77:76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Nathan CA, Amirghahari N, Abreo F, et al. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10:5820–7. doi: 10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 22.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara ET, Maity A, Jha N, Lustig RA. Sirolimus as a potential radiosensitizer in squamous cell cancer of the head and neck. Head Neck. 2009;31:406–11. doi: 10.1002/hed.20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman JM, Doloresco F, Vermeulen LC, Shah ND, Matusiak L, Hunkler RJ, Schumock GT. Projecting future drug expenditures 2010. Am J Health Syst Pharm. 2010;67:919–28. doi: 10.2146/ajhp100068. [DOI] [PubMed] [Google Scholar]
- 25.Guba M, Koehl GE, Neppl E, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int. 2005 Jan;18(1):89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- 26.Clark C, Shah S, Herman-Ferdinandez L, Ekshyyan O, Abreo F, Rong X, McLarty J, Lurie A, Milligan EJ, Nathan CA. Teasing out the best molecular marker in the AKT/mTOR pathway in head and neck squamous cell cancer patients. Laryngoscope. 2010;120:1159–65. doi: 10.1002/lary.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–14. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


