Abstract
Evidence from cell, animal, and human studies demonstrates that α1-adrenergic receptors mediate adaptive and protective effects in the heart. These effects may be particularly important in chronic heart failure, when catecholamine levels are elevated and β-adrenergic receptors are down regulated and dysfunctional. This review summarizes these data and proposes that selectively activating α1-adrenergic receptors in the heart may represent a novel and effective way to treat heart failure.
Keywords: alpha-1-adrenergic receptors, cardiac myocytes, heart failure, drug development
Description of α1-ARs
The neurohormonal alterations of heart failure (HF) are characterized by marked elevations in sympathetic catecholamines, norepinephrine (NE) and epinephrine (EPI) [1]. NE and EPI activate two main classes of myocardial adrenergic receptors (ARs), alpha-1-ARs (α1-ARs) and beta-ARs (β-ARs). The most abundant cardiac AR is the β1-AR, though there are also smaller but functionally important populations of β2- and α1-ARs. All ARs are prototypical G-protein coupled receptors (GPCRs) with seven transmembrane domains, though they differentially activate Gα subunits: β-ARs couple predominantly to Gs, and α1-ARs to Gq, although β2- and α1-ARs can also couple to Gi.
Acute activation of β1-ARs increases heart rate and myocardial contractility. However excessive chronic stimulation of cardiac β1-ARs, as with elevated catecholamines in HF, mediates harmful processes, including cell death, fibrosis, and adverse remodeling [2–9]. Interestingly, recent investigations suggest that myocardial β2-ARs might mitigate the harm associated with chronic β1-AR activation (article by Talan et al in this issue, and [10, 11]). Nevertheless, drugs that block the activation of β-ARs (β-blockers) reduce HF morbidity and mortality and have become a cornerstone of HF therapy [12]. α1-ARs in the heart have been the subject of less intensive investigation, but multiple lines of evidence define adaptive and protective roles for cardiac α1-ARs (Tables 1–3) that contrast sharply with the toxic effects of excessive chronic β-AR activation [9].
Table 1.
α1-AR Gain of Function Models: Pharmacology
| IN VITRO MYOCYTES | ||
|---|---|---|
| System | Agonist | Findings & References |
| NMVMs | PE | ↓ Cell death with prolonged hypoxia [97] |
| NRVMs | NE, EPI, PE | ↑ Myocyte HT, fetal gene induction [79–87] |
| NRVMs | PE | ↓ Apoptosis [4, 99–101] |
| NRVMs | PE | ↓ Doxorubicin toxicity [102] |
| NRVMs & ARVMs | NE, methoxamine | ↓ Cell death with hypoxia-reoxygenation [95, 96] |
| ARVMs | NE, PE, EPI | ↑ Myocyte HT, protein synthesis & cell survival [89–91] |
| AMVMs | PE | ↓ Apoptosis & necrosis caused by β-AR agonism, H2O2 or doxorubicin; requires ERK signaling [27] |
| AMVMs | NE, EPI | ↑ ERK with β-blocker, via α1-ARs [135] |
| ACVMs | PE | ↑ Myocyte HT, fetal gene induction [88, 92, 93] |
| IN VITRO PERFUSED HEART | ||
| Species | Agonist | Findings & References |
| Rat | EPI | ↑ Glycolysis in heart [94] |
| Rat | EPI | ↑ Protein synthesis in heart [89] |
| Rat | PE | ↓ Ca++ injury in Ca++ depletion-repletion [98] |
| Rat | NE, PE | ↓ global I-R injury [21, 110, 114, 115, 120] |
| Rabbit | PE | ↓ regional I-R injury [113] |
| ANIMAL IN VIVO | ||
| Species | Agonist, Model | Findings & References |
| Mouse | PE, A6 infusion | Physiologic HT without ↑ BP [107, 127] |
| Mouse | PE in vivo & global ischemia in perfused heart | ↓ I-R injury [124] |
| Mouse | NE & LAD ligation | ↑ Right ventricular inotropy [73] |
| Mouse | PE, A6 infusion & Doxorubicin | Prevents CM, decreases apoptosis [102, 127] |
| Rat | NE & regional I-R | ↓ I-R injury and arrhythmia [125] |
| Rat | NE in vivo & global ischemia in perfused heart | ↓ I-R injury (delayed cardioprotection) [116, 117, 130] |
| Rabbit | PE in vivo & global ischemia in perfused heart | ↓ I-R apoptosis & necrosis [122, 123] |
| Rabbit | PE & hypoxic cardiac arrest | PE infusion preconditions donor hearts [119] |
| Rabbit | NE, Tyr & LAD ligation | ↓ I-R injury [112, 118, 128] |
| Cat | NE infusion | Physiologic HT without ↑ BP [39] |
| Dog | NE, Methoxamine & LAD ligation | ↓ I-R injury [109, 111, 121] |
| Dog | NE infusion | Physiologic HT without ↑ BP [103–105] [106] |
| HUMAN | ||
| System | Agonist, Model | Phenotype/Findings |
| Atrium in vitro | PE | ↑ Ischemic preconditioning [166–168] |
| Ventricle in vitro | PE | ↑ Ischemic preconditioning [165] |
| Ventricle in vitro | NE | α1-AR = β-AR inotropy in failing heart [66, 67] |
| In vivo | Methoxamine | Improved exercise performance in HF [169] |
| In vivo | Midodrine | ↓ Symptoms, ↑ EF, ↓ hospitalizations in HF [170] |
A6 = A61603 (α1A-selective agonist); ARVM = adult rat ventricular myocytes; ACVM = adult cat ventricular myocytes; AMVM = adult mouse ventricular myocytes; CM = cardiomyopathy; EPI = epinephrine; HT = hypertrophy; I-R = ischemia-reperfusion; LAD = Left Anterior Descending coronary artery; NE = norepinephrine; NMVM = neonatal mouse ventricular myocytes; NRVM = neonatal rat ventricular myocytes; PE = phenylephrine; Tyr = tyramine (releases NE)
Table 3.
α1-AR Loss of Function Models
| ANIMAL PHARMACOLOGY | ||
|---|---|---|
| Species | α1-Antagonist & Model |
Findings & References |
| Rat | Prazosin & hemorrhage | ↑ I-R injury with α1-blockade [154] |
| Rabbit | CEC & I-R | ↑ I-R injury with α1B-blockade [153] |
| Rabbit | Doxazosin & rapid pacing | ↓ Efficacy of β-blockade with α1-blockade [155] |
| Pig | Prazosin & I-R | ↑ I-R injury with α1-blockade (second window of preconditioning) [132] |
| KNOCKOUT MICE | ||
| α1 Subtype or Gene | Model | Findings & References |
| A | In vivo: basal & agonist infusion | Mixed genetic background: normal heart size & function, ↓ resting BP and pressor response to α1A agonist [50] |
| Congenic: normal heart size and BP [157] | ||
| B | In vivo: basal, agonist infusion, TAC | Mixed background: normal heart size, normal BP, ↓ pressor response [107, 158–160]; normal HT with TAC, but ↓ HT with subpressor PE [107] |
| Congenic: small heart, normal BP, sinus bradycardia [157] | ||
| D | In vivo: basal, agonist infusion, salt loading | Mixed background: normal heart size, ↓ resting BP, ↓ pressor response, ↓ hypertension with salt loading [160, 161]; ↓ coronary vasoconstriction with PE [37] |
| Congenic: normal heart size, ↓ resting BP (unpublished data) | ||
| A & B | In vivo: basal, exercise, TAC | Congenic: small heart & myocytes (males), normal BP, bradycardia, ↓ exercise, ↓ ERK [25]; ↓ myocardial contractility [162]; normal HT with TAC, but ↓ fetal gene induction, ↑ apoptosis and fibrosis, ↑ cardiomyopathy, ↑ HF, and ↑ death [26] |
| A & B | In vitro | ↓ ERK activation with PE, but not ET or PMA [25]; ↑ apoptosis [26]; α1A but not α1B subtype rescues ABKO myocyte survival via ERK [27] |
| B & D | In vivo: basal, agonist infusion | Mixed: normal heart, ↓ BP, ↓ pressor response [160] |
| Congenic: small heart, normal BP (unpublished data) | ||
| A & D | In vivo: basal | Congenic: normal heart size, normal BP (unpublished data) |
| A, B, & D | In vivo:basal | Congenic: small heart, normal BP (unpublished data) |
| TH, DBH | In vivo:basal | NE required for cardiac development in utero [198–200] |
| HUMAN RANDOMIZED CLINICAL TRIALS | ||
| Subtype | Test Drug | Findings & References |
| All ARs | moxonidine or bucindolol | Sympatholysis with ↓ NE (↓ α1 occupancy) increases HF [177–181] |
| A, B, D | prazosin | Non-selective α1-blocker trend toward ↑ mortality [173] |
| A, B, D | doxazosin | Non-selective α1-blocker ↑ incident HF [171, 172, 201] |
| A, B, D | phentolamine | Non-selective α-blocker ↓ preconditioning [175] |
↑ and ↓ = relative to WT mice or control treatment; BP = blood pressure; CEC = chloroethylclonidine; DBH = dopamine beta-hydroxylase; ET = endothelin; HT = hypertrophy; I-R = ischemia-reperfusion; NE = norepinephrine; PE = phenylephrine; PMA = phorbol myristate acetate; TH = tyrosine hydroxylase
α1-ARs exist as three distinct molecular subtypes, named α1A, α1B, and α1D (reviews in [13–19]). All three subtypes are activated by NE and EPI and blocked by the α1-antagonist prazosin. There are significant differences among subtypes in amino acid sequence, signaling, and tissue distribution. However, all α1-ARs couple to Gq to activate phospholipase Cβ1, with increases in diacylglycerol and activation of protein kinase C. In cardiac myocytes, increases in inositol trisphosphate and subsequent release of intracellular calcium are controversial. The α1B subtype might also couple to Gi [20–24]. The α1A subtype protects cardiac myocytes via ERK activation [25–27].
Further α1-AR intracellular signaling is diverse: over seventy molecules have been identified as downstream effectors of α1-AR-stimulated hypertrophy in cultured neonatal rat ventricular myocytes (NRVMs). Functionally, cardiac α1-ARs control numerous adaptive processes, including positive inotropy, gene transcription, protein synthesis, glucose metabolism, and inhibition of cell death (reviews in [16, 28–32]).
This review explores the cell, animal, and human data that reveal beneficial roles for α1-AR activation in the heart, and collectively encourage a reexamination of the currently prevailing paradigm wherein chronic catecholamine elevation is felt to be wholly maladaptive in HF [33, 34].
α1-AR expression and regulation in animal models and the human heart
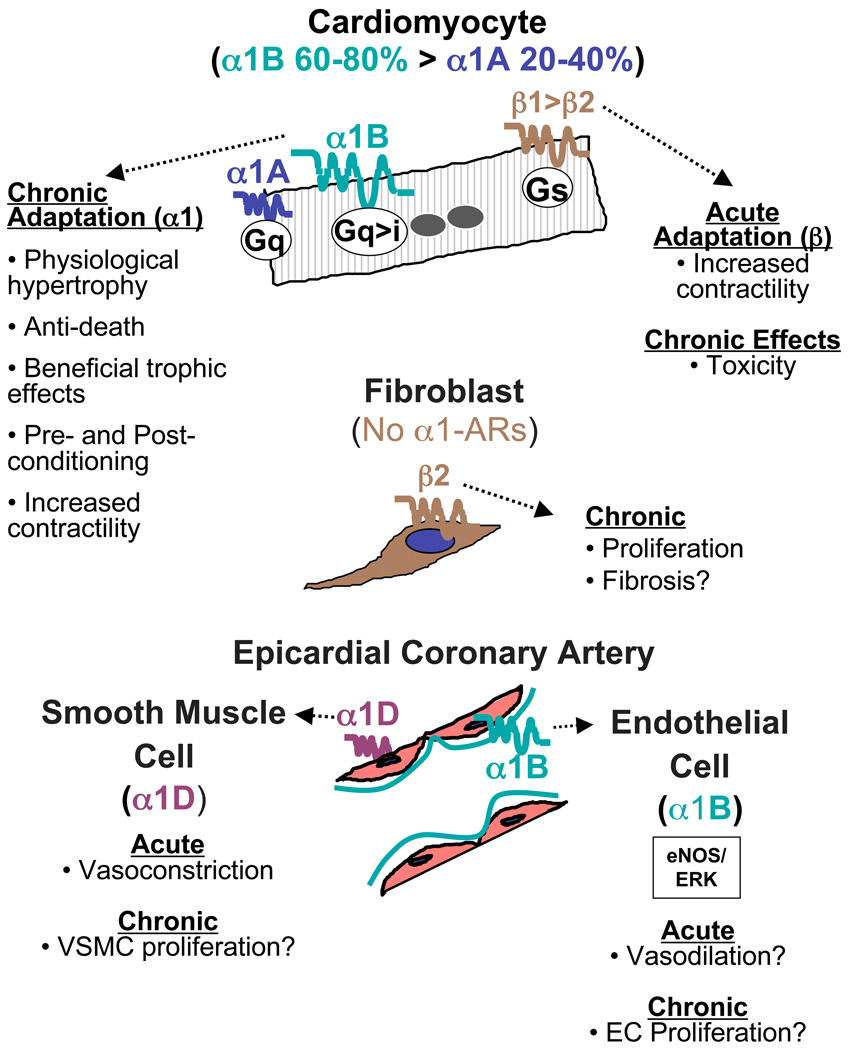
Figure 1 summarizes expression and function of α1-ARs and β-ARs in the main cells of the animal and human heart.
Figure 1.
Summary of α1-AR subtypes and functions in different cardiac cells.
α1-ARs IN HEART OF ANIMAL MODELS
α1-AR binding in the heart is similar among species, except for the rat, where binding is six-fold higher than either human or mouse [35]. In the rodent heart, cardiomyocytes express only the α1A and α1B subtypes [25], with α1B more abundant than α1A, whereas the α1D subtype is in coronary arteries [36, 37].
Rodent cardiac fibroblasts (FBs) do not express α1-ARs at all [38], and thus are uninvolved in the FB proliferation that characterizes maladaptive remodeling. Indeed, α1-agonist treatment does not cause fibrosis [39], in contrast with some β-AR agonists [40, 41], that stimulate cardiac FB proliferation through β2-ARs [42–45].
Numerous studies have identified functional α1-ARs in endothelial cells (ECs) of multiple systemic vascular beds in the rat [46, 47], but their presence and function in cardiac ECs of animal models remains unknown.
In vitro and in vivo studies suggest that the α1A and α1B subtypes in rat cardiomyocytes might be differentially regulated by chronic stimulation [48], but total cardiac α1-ARs are not desensitized or down-regulated in hypertrophy in vitro or HF in vivo [48, 49]. Strikingly, in myocardium and arteries where the α1A is expressed, it is present in only subpopulations of vascular or cardiac myocytes [50–52], unlike the α1D, which is present in most or all vascular myocytes [53]. Another recent, unexpected finding is that the α1A and α1B subtypes in cardiac myocytes are located primarily on the nuclear membrane, not the sarcolemma [54].
α1-ARs IN HUMAN HEART
The distribution of α1-AR subtypes in the human heart mirrors the rodent heart (summarized in [55]). The α1A and α1B are the most abundant subtypes in the myocardium [56], whereas the α1D is the predominant and functional subtype in epicardial coronary arteries and smooth muscle cells [55]. Human epicardial coronary artery ECs express α1B-ARs that activate ERK and eNOS, and increase DNA synthesis [57], and could play a role in coronary vasodilation and angiogenesis.
Numerous studies show that total α1-AR expression remains stable or increases in the failing human heart [56, 58–62], whereas β1-ARs reliably decrease [63, 64], so that the fraction of total ARs consisting of α1-ARs increases substantially. In non-failing human myocardium, α1-ARs constitute 2–23% of total AR binding (mean of 5 studies 11%), whereas that percentage increases to 9–41% (mean 25%) in failing myocardium [56, 58–62]. Levels of the α1A and α1B subtypes are undiminished in both the left ventricle (LV) and right ventricle (RV) of the failing human heart [56]. The decrease in β-ARs in HF is accompanied by an uncoupling of some beneficial pathways activated by β-ARs, including those that mediate positive inotropy [63, 65]. In contrast, as in animals, α1-ARs appear to maintain their function in HF, as evidenced by the finding that the degree of positive inotropy induced by α1-AR stimulation can be equal to that induced by β-AR stimulation in failing human heart muscle [66, 67].
Evidence from α1-AR gain of function in animal models
α1-AR GAIN OF FUNCTION USING PHARMACOLOGY (Table 1)
Early physiologic studies of the heart’s response to α1-AR activation focused on the coronary arteries, where NE infusion causes vasoconstriction of epicardial coronary arteries, primarily or only in the setting of atherosclerosis (reviewed in [55]). Multiple studies also identify a positive inotropic response to α1-AR activation in humans [66–69], and some animals [70–73], though results vary according to species [74, 75] and developmental stage [76], and are different in the normal mouse RV (negative inotropy) [76–78] and LV (positive inotropy) [73].
Subsequently, cell culture experiments using AR agonists identified a number of important functions of α1-ARs in cardiomyocytes, most notably the induction of hypertrophy and stimulation of transcription [79–87]. The initial experiments were conducted in NRVMs, though later work in cardiomyocytes from adult rat and cat confirmed the findings [88–93]. α1-AR stimulation, often with phenylephrine ("PE"), remains a standard model for assaying hypertrophic signaling, although it needs to be appreciated that PE can have substantial β-AR agonism. Further in vitro studies using AR agonists identified additional cardioprotective processes mediated by α1-AR activation, including energy production [94], preconditioning against hypoxia and calcium overload [95–98], and prevention of apoptosis and necrosis [4, 27, 99–102].
In vivo gain-of-function studies using pharmacology bolster the in vitro findings and demonstrate important biologic roles for cardiac α1-ARs. Chronic low-dose NE infusion in the mouse, cat, and dog stimulates adaptive hypertrophy, characterized by normal or enhanced cardiac function, without increasing blood pressure, promoting fibrosis, or accelerating cell death [39, 103–107].
α1-AR activation by NE or PE infusion in the isolated heart and in vivo also reliably ameliorates ischemia-reperfusion-induced apoptosis and necrosis in mouse, rats, dogs and rabbits [21, 108–125]. Interestingly, methoxamine was ineffective in some studies [113, 126], and effective in others [109, 111]. α1-Agonism also protects against doxorubicin cardiotoxicity [102, 127], and calcium overload [98]. Pleiotropic mechanisms implicated in these cardioprotective effects include ecto-5’-nucleotidase activation and increased adenosine release [96, 108, 111, 128]; activation of ERK [27, 129], K ATP channels [125], and protein kinase C [115, 130]; increased heat shock proteins [116], β1-integrins [131], and fetal genes [130]; induction and activation of inducible nitric oxide synthase (iNOS) [124, 132], superoxide dismutase (SOD) [95, 97], cyclooxygenase-2 [132], and GATA-4 [102]; phosphorylation and inactivation of Bad [31, 101]; and up-regulation of anti-apoptotic Bcl proteins [99, 102, 122].
A recent novel finding concerns α1-ARs in the RV. α1-ARs mediate a negative inotropic effect in the normal mouse RV, and a positive inotropic effect in the normal LV. However, in HF after myocardial infarction (MI), α1-AR stimulation causes positive inotropy in the RV [73]. This "switch" might be mediated partly by changes in coupling to myosin light chain kinase, though the details are under investigation. This finding might enhance the implications of α1-AR activation in chronic HF, as the development of RV failure in the setting of chronic left ventricular failure is known to be highly predictive of poor outcomes [133].
β-Blockers provide an unexpected example of α1-AR gain-of-function. NE and EPI signal predominantly through the β1-AR in the normal and failing heart, because β1-ARs are the most abundant cardiac AR, and have the highest affinity for NE and EPI [134]. In cultured adult mouse myocytes, NE or EPI inactivate ERK via β-ARs, whereas NE or EPI activate ERK via α1-ARs, in the presence of a neutral β-blocker, such as propranolol [135]. Since ERK activation by α1-ARs is cardioprotective [27], β-blockers might “work” in HF partly by unmasking beneficial α1-AR signaling, at the same time that they inhibit maladaptive β-AR pathways.
TRANSGENIC MOUSE α1-AR SUBTYPE GAIN OF FUNCTION (Table 2)
Table 2.
α1-AR Gain of Function Models: α1 Subtype Transgenics
| Subtype | α1 Receptor (fold level) with Promoter |
Findings & References |
|---|---|---|
| A | CAM rat A (2×) with mouse α1A | Protection from ischemia [136] |
| A | WT rat A (148–170×) with rat αMyHC | ↑ Contractility, no HT [137] |
| A | WT rat A (66×) with rat αMyHC | ↑ Protection against myocardial infarction and transverse aortic constriction [138, 139] |
| A | WT rat A (112–170×) with rat αMyHC | ↑ Fibrosis & death in aged mice [140] |
| B | CAM B hamster (3×) with mouse αMyHC/ | ↑ HT, normal BP [141, 142]; ↓ I-R arrhythmias [144]; no preconditioning [197]; ↑ cardiomyopathy after TAC [143] |
| B | CAM or WT hamster (2×) with mouse α1B | ↑ HT, ↓ BP, ↓ HR, autonomic dysfunction [145, 146]; no preconditioning [136]; ↓ inotropy [37, 147] |
| B | WT hamster B (40–70×) with mouse αMyHC | No HT, ↑ fetal genes, ↓ β-AR response, ↑ receptor coupling to Gi, ↑ GRK2, ↓ inotropy, maladaptive HT with PE, dilated cardiomyopathy in aged mice [22, 148–150] |
CAM = constitutively activated mutant receptor; HT = hypertrophy; WT = wild type receptor
The limited number of pharmacologic agents specific for the three α1-AR subtypes prompted the creation of transgenic mouse models to explore which of the subtypes regulated these beneficial effects. Different labs used receptor cDNAs from different species, with varying activating mutations, and with MyHC or native receptor promoters to create mice with very different receptor levels. It is perhaps not surprising that the phenotypes vary.
In general, however, α1A-transgenics show enhanced contractility and cardioprotection without hypertrophy, even at extraordinarily high over-expression levels. In contrast, α1B-transgenics have variable hypertrophy without hypertension, and are predominantly maladaptive.
A constitutively active mutant (CAM) α1A causes preconditioning, when 2- to 3-fold over-expressed in heart with the endogenous mouse α1A promoter [136]. The WT α1A expressed in myocytes with the α-myosin heavy chain (α-MyHC) promoter causes increased contractility and ANF levels without hypertrophy, with 148- to 170-fold over-expression [137], and cardioprotection after coronary ligation or pressure overload, with 66-fold over-expression [138, 139]. However, long-term α1A over-expression (112- to 170-fold) causes fibrosis and early death [140].
α1B transgenic mice have less consistent results. A CAM α1B made with the α-MyHC promoter causes hypertrophy with 2- to 3-fold myocyte-specific over-expression [141, 142], and worsens pathological hypertrophy after TAC [143], but reduces reperfusion arrhythmias [144]. A CAM α1B made with the endogenous mouse α1B promoter for systemic overexpression (2-fold cardiac) also causes hypertrophy, without increased blood pressure [145, 146], but with decreased contractility [37]. A WT α1B with the same endogenous promoter causes variable hypertrophy and negative inotropy [145, 147]. In contrast, a WT α1B 40- to 70-fold over-expressed in myocytes with the α-MyHC promoter shows no hypertrophy, but rather fetal gene induction, decreased inotropy, pathological response to PE, dilated cardiomyopathy, and early death [22, 148–150].
Normal expression of the α1D subtype in heart is limited to coronary arteries and smooth muscle cells [36, 37, 151], and there are no formal reports of a vascular transgenic mouse [152].
Evidence from α1-AR loss of function in animal models (Table 3)
α1-Antagonists have negative effects on adaptive cardiac processes in vitro and in animal models in vivo [132, 153–155] (Table 3), supporting the data from pharmacology gain of function (Table 1). However, the pharmacologic tools can have nonspecific effects, and are inadequate to distinguish α1-subtypes in vivo. The shortcomings of pharmacology and the inconsistencies of the transgenic mice prompted the creation of knockout (KO) mouse models for the three α1-AR subtypes (reviewed in [16]). Importantly, phenotypes vary markedly between mice that are on a mixed genetic background versus congenic. Furthermore, only a few studies analyze mice separately by sex, an essential precaution given sex differences in cardiovascular phenotypes [156].
Mice lacking the α1A on a mixed genetic background (FVB/N × 129SvJ) have normal heart size but low blood pressure (BP), and no vasopressor response to the α1A subtype agonist A61603 [50]. The pressor response to PE is normal [50]. In the congenic C57Bl/6J background, the α1A-KO has normal heart size and BP [157].
α1B-KOs created on a mixed background (C57Bl/6J × 129Sv) have normal heart size, and a decreased pressor response to α1-agonist infusion [107, 158–160], whereas α1B-KOs on a congenic C57BL/6J background have small hearts [157]. Regardless of genetic background, α1B-KO mice have a normal blood pressure. The α1B-KO heart enlarges normally with TAC. However, a subpressor dose of PE, which causes an adaptive hypertrophy in WT mice, has no effect in α1B-KO mice [107].
α1D-KO mice in a mixed genetic background have normal hearts, but decreased blood pressure and reduced coronary vasoconstriction in response to PE infusion [37, 160, 161].
Mice lacking both the α1B and α1D in a mixed genetic background have a normal heart, but decreased blood pressure and a decreased pressor response to agonist infusion [160].
The double α1AB-KO has been characterized in a congenic C57BL/6J background. The double KO eliminates all cardiac α1-AR binding. A key role for ERK in the phenotype is suggested by the facts that activated ERK in the KO myocardium is reduced to 30% of WT, as assayed by phosphorylation of Elk1 in vitro by ERK immunoprecipitated from intact hearts, and PE no longer activates ERK and downstream kinases (p90RSK, p70S6K) in KO myocytes [25].
α1AB-KO mice have normal blood pressure, but males have decreased heart and myocyte hypertrophy during post-weaning development. Other organs are normal [25]. Contractility is normal by echocardiography in awake mice, but cardiac output is decreased due to lower stroke volume and bradycardia; contractility of isolated myocardium is abnormal; β-ARs are desensitized; and exercise tolerance is impaired [25, 26, 162].
After pressure overload by transverse aortic constriction (TAC), the α1AB-KO mice have worse dilated cardiomyopathy, HF, and increased mortality [25, 26], confirming the importance of α1-ARs in cardioprotection. Mechanisms underlying this dilated cardiomyopathy include increased apoptosis, increased fibrosis, and failure to induce fetal and other genes [26]. Hypertrophy after TAC measured by heart and myocyte size is the same or greater in α1AB-KO mice as in WT mice, illustrating a dissociation between hypertrophy per se (unaffected) and fetal genes (not induced) [26].
Thus, the double α1AB-KO impairs the physiological hypertrophy of normal post-weaning development, and worsens pathological hypertrophy after TAC. Importantly, the double β-AR KO is opposite the double α1-AR KO. Double KO of the β1- and β2-ARs has no effect on developmental heart growth, but induces fetal genes in the basal state, and improves pathological hypertrophy after TAC [163, 164].
Experiments using cultured cardiomyocytes from α1AB-KO mice provide insight into the mechanisms underlying the in vivo findings, revealing increased myocyte death with toxic stimuli, including β-AR stimulation, H2O2 and doxorubicin [25, 27]. Adenoviral reconstitution of the α1A subtype in double KO myocytes rescues the phenotype, through a pathway that requires activation of ERK [27]. However, reintroduction of the α1B subtype does not rescue toxin-induced death of α1AB-KO myocytes [27]. Taken together, these data demonstrate that the α1A subtype is necessary and sufficient for myocyte protection, and that the mechanism is myocyte-autonomous and requires ERK activation.
We have made all combinations of α1-KOs congenic in C57Bl/6J, and find that heart size is smaller than WT in all genotypes lacking the B, whereas it is normal when the B is present, clearly implicating the α1B subtype in developmental hypertrophy ([157] and unpublished data).
Tentative summary of α1-AR subtype functions revealed in genetic mouse models
Although some results are conflicting, a general pattern emerges from genetically altered mouse models, wherein the α1A subtype mediates cardioprotection; the α1B stimulates developmental and α1-induced hypertrophy; and the α1D has a predominant role in vasoconstriction and maintaining blood pressure [16]. The α1A and α1B both mediate myocardial inotropic effects [78]. The α1A and α1B are not required for heart or myocyte enlargement after TAC, but are necessary for fetal gene induction.
Human α1-AR gain and loss of function
HUMAN α1-AR GAIN OF FUNCTION (Table 1)
Gain-of-function data in humans demonstrate adaptive and protective roles for cardiac α1-ARs, including positive inotropy and preconditioning. In non-failing hearts, β–ARs account for the vast majority of the catecholamine-induced increase in inotropy. However in failing hearts, α1-ARs can increase contractility equal to β̃-ARs [66, 67]. As predicted by animal and cell models, α1-ARs also cause preconditioning against ischemic injury both in vitro and in vivo [165–168], and can improve cardiac performance in HF patients [169, 170].
HUMAN α1-AR LOSS OF FUNCTION (Table 3)
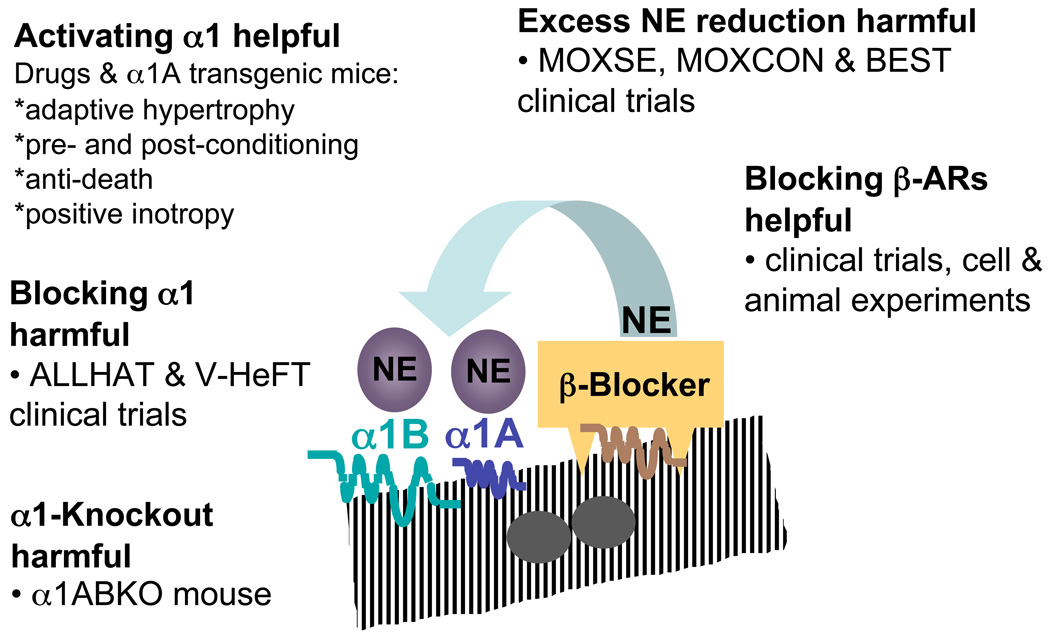
Two large clinical trials provide loss-of-function data that support the benefit of cardiac α1-AR activation. The ALLHAT (Antihypertensive and Lipid-Lowering treatment to prevent Heart ATtack) trial included an arm in which 24,000 hypertensive men and women received the non-selective α1-blocker doxazosin. The incidence of HF in the subjects who received the α1-blocker was twice as high as in those who received any of the other three antihypertensive agents, and the Data Safety Monitoring Board stopped the doxazosin arm of the trial prematurely [171]. Subsequent analysis confirmed that this excess harm persisted after adjustment for covariate risk factors, including blood pressure [172].
These results substantiated the findings of the earlier V-HeFT (Vasodilator-Heart Failure Trials), in which the non-selective α1-blocker prazosin was associated with a trend toward increased mortality, in contrast with the beneficial effects of other vasodilators [173]. Recently, a smaller retrospective study found evidence of increased HF hospitalizations in patients taking α1-blockers without concomitant β-blockers [174]. Phentolamine, a nonselective α-blocker, prevents ischemic preconditioning [175].
α1-Blockers might have off-target effects [176], but the maladaptive phenotype of the α1AB double KO mouse supports that the adverse results in the ALLHAT and V-HeFT trials were due to α1-AR inhibition itself, rather than some nonspecific drug effect.
Additional support for the concept that harm results from reducing α1-AR occupancy in HF arises from clinical trials evaluating the effect of sympatholysis [177]. The MOXSE and MOXCON trials (using moxonidine) [178–180] and BEST (using bucindolol) [181] all revealed harmful effects resulting from marked systemic reduction of NE levels. Given the beneficial effect of decreasing NE binding to β-ARs, these findings suggest that the observed harm might result from decreasing binding to α1-ARs below some critical threshold. Indeed, the α1AB double KO mouse indicates that the heart requires some degree of α1-AR activation by NE and/or EPI.
Translational potential of α1-AR agonists (Table 4)
Table 4.
Concerns & Answers About Potential α1-Agonist Therapy
| Concerns | Answers & References |
|---|---|
| Transgenics: α1B-AR over-expression can be maladaptive (Table 2). | Pharmacology and KOs are congruent on adaptive effects (Tables 1 & 3), and germline KOs are predictive of drug effects in humans [19, 202]; high-level over-expression is non-physiological; over-expressed or constitutively activated α1- and β-ARs can signal differently from endogenous receptors [203–205]; over-expressed receptors can inhibit other GPCRs by "stealing" G proteins [206, 207] |
| Hypertension: α1-receptors cause vasoconstriction, and α1-agonists will cause hypertension. | Cardiac trophic effects occur at low, cardioselective doses that do not increase blood pressure [39, 103–107, 127] |
| Angina: α1-receptors constrict coronary arteries, and an agonist will cause angina. | α1-Receptors do not constrict normal coronary arteries [208]; smooth muscle contraction occurs at higher doses than required for cardiac trophic effects [36, 127]; the α1D is the subtype present in coronary smooth muscle, and could be avoided with selective agonists [36, 37, 55]. |
| Prostatism: α1-receptors constrict prostate smooth muscle, and agonists will cause urinary retention or prostate symptoms. | Cardiac effects might occur at doses below those activating prostate smooth muscle, as with vascular; α1D antagonists are effective to treat prostate symptoms [209], and the α1D subtype could be avoided with α1A- and/or α1B-selective agonists. |
| Carvedilol: carvedilol blocks α1-receptors and is efficacious in heart failure. | Carvedilol in chronic therapy does not block α1-effects [187, 188], and might even enhance them [189]. |
| Hypertrophy: α1-receptors cause hypertrophy, which is bad. | α1-Receptor agonists stimulate an adaptive or "physiological" hypertrophy, with no fibrosis, and normal or improved cardiac function [39, 103–107, 127]. |
| Fetal genes: α1-receptors increase β-MyHC and other fetal genes in rodent models, and these are hallmarks of pathological hypertrophy. | It is arguable whether fetal gene induction is maladaptive, or causative in pathological hypertrophy [195, 196, 210, 211] |
| Gq: α1-receptors are coupled to Gq, and Gq over-expression in mice causes pathological hypertrophy. | Two-fold, life-long α-MyHC-driven Gq over-expression in mice has no phenotype [212, 213], and 5-fold adult myocyte over-expression does not cause pathology [214]; 2-fold increases in endogenous Gq are the maximum seen in heart failure [215, 216], and the Gq is shared among many cardiac cells and receptors in those cells. |
As summarized above, abundant evidence from cell, animal and human studies indicates that activating cardiac α1-ARs is beneficial. α1-ARs are highly "druggable", and recruit numerous downstream adaptive and protective signaling mechanisms. Thus, α1-AR agonists could represent a novel approach to the treatment of myocardial diseases and HF. α1-AR augmentation of adaptive hypertrophy, cardioprotection, and positive inotropy might have multiple clinical applications, including acute myocardial ischemia, cardiotoxicity with cancer therapy, and chronic systolic HF. As previously mentioned, multiple studies have shown that α1-AR levels are either unchanged or increased in human HF [56, 58–62]. Furthermore, myocardial α1-ARs are thought to be only 10% occupied by NE, even in HF [134], indicating the potential for additional activation by an exogenous agonist. The safety of α1-AR activation by an exogenous agonist is well established, as oral (midodrine) and intravenous (PE) agents are already in clinical use. In fact, a recent small clinical trial demonstrated a significant benefit associated with the use of midodrine in patients with advanced HF already receiving contemporary therapy [170].
Given the wealth of data in multiple models from many different labs over three-plus decades, it is important to consider reasons for possible resistance to the idea of α1-agonist therapy. Potential concerns and answers are summarized in Table 4.
First, α1B subtype over-expression in transgenic mice causes a maladaptive phenotype, or at least not adaptive, whereas the KO approach and pharmacology point to the α1A and α1B in adaptive effects. We believe that pharmacology and the KOs provide the more reliable evidence, for reasons noted in Table 4, but the role of the α1B requires more study.
Second, α1-ARs are irrefutably linked to smooth muscle contraction, for example, in the vascular and GU systems, raising concerns of hypertension, angina, or prostatism with α1-agonist therapy. Against these possibilities is the key observation, repeated in many labs, that adaptive cardiac effects of α1-agonists occur at doses that do not increase BP, or cause myocardial ischemia. Furthermore, the α1D subtype appears to have a key role in smooth muscle contraction, but is not involved in adaptive cardiac effects, and thus could be avoided with α1A and/or α1B agonists. As with any systemic therapy, other potential extra-cardiac effects of an α1-agonist still need to be determined. Some might be favorable. For example, in the brain, there is evidence that α1-ARs might be neuroprotective [182, 183]. KO of the α1B causes abnormal glucose metabolism and obesity [184], implying that an α1B agonist might have favorable metabolic effects, opposite to the view that α1-blockers have favorable metabolic profiles [185].
The proven efficacy of carvedilol in the treatment of HF [186] would also seem to argue against the therapeutic benefit of an α1-AR agonist, since carvedilol blocks both α1- and β-ARs. However, it is important to recognize that the α1-blocking properties of carvedilol extinguish shortly after initiation of therapy [187, 188]. In fact, chronic carvedilol use actually increases the blood pressure response to PE infusion in HF patients [189]. Thus the benefits associated with chronic carvedilol use are likely related to β-blockade, not α1-blockade, as well as to a number of salutary effects unrelated to ARs [155, 190–194].
Finally, α1-ARs are associated with "pathological" hypertrophy, because they are coupled to Gq, and induce fetal genes in rodent models. On the contrary, the studies reviewed here indicate clearly that α1-ARs stimulate adaptive and protective effects in heart, not pathological. For reasons outlined in Table 4, it is not appropriate to extrapolate from Gq over-expression to the conclusion that all cardiac Gq-coupled receptors mediate pathology. Likewise, induction of fetal genes, such as ANF, BNP, skeletal α-actin, and β-MyHC is considered a hallmark of pathological hypertrophy. However, it is not clear that induction of these genes is causal, or even maladaptive. For instance, one fetal gene, BNP is even used as therapy in HF (nesiritide, Natrecor). As another example, skeletal α-actin is increased by 5-fold in BALB/c mouse hearts, yet cardiac structure is normal and contractility is enhanced [195]. Finally, recent work suggests that the prototypical fetal gene, β-MyHC, is induced by pressure overload only in a minor population of myocytes, and that the cells with β-MyHC are smaller than those without β-MyHC, not larger [196]. The low fraction of myocytes expressing β-MyHC casts some doubt on contractile function significance, and the small cell size suggests that β-MyHC is not a marker for cell hypertrophy.
Future Directions
Given the valid concerns regarding the activation of non-cardiac α1-ARs with a putative agonist, ongoing studies will need to focus on assuring cardioselectivity. Cardioselective α1-AR activation with low doses of systemically delivered agonists appears to be feasible and beneficial, though careful investigation for previously undetected systemic effects is required.
An alternate approach to cardioselectivity would be the use of a subtype-selective agent for activation of myocardial α1A or α1B-ARs, thereby eliminating undesirable coronary vasoconstriction by activation of α1D-ARs. Indeed, our lab showed recently that a low, nonhypertensive dose of an α1A-selective agonist (A61603) prevents doxorubicin-induced cardiomyopathy and death in a mouse model of HF [127]. Future efforts should focus on further unraveling the roles of the α1A and α1B subtypes in the heart, to determine whether both should be targeted. Importantly, the distribution of the cardiac α1-AR subtypes appears to be identical in rodents and humans, suggesting that rodent models could offer accurate platforms for assessing the cardioselectivity and safety of novel therapies, as well as for the further elucidation of mechanism.
Figure 2.
Summary of α1-AR cell, animal, and clinical loss and gain of function studies.
ACKNOWLEDGEMENTS
We thank the NIH (BCJ, TDO, PCS), the Department of Veterans Affairs Research Service (PCS), the GlaxoSmithKline Research and Education Foundation for Cardiovascular Disease (BCJ), the University of California, San Francisco, Foundation for Cardiac Research (BCJ); the American Heart Association, Western States Affiliate (PCS); and the American Heart Association, Greater Midwest Affiliate (TDO)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
A patent application is submitted to use α1-agonist compounds as treatment.
Contributor Information
Brian C. Jensen, Email: brian_jensen@med.unc.edu.
Timothy D. O'Connell, Email: Tim.OConnell@sanfordhealth.org.
Paul C. Simpson, Email: paul.simpson@ucsf.edu.
REFERENCES
- 1.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984 Sep 27;311(13):819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL, Kent RL, Parsons B, Cooper Gt. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992 Feb;85(2):790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 3.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998 Sep 29;98(13):1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 4.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999 Jul 20;100(3):305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta(1)-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96(12):7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32(5):817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 7.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003 Nov 14;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 8.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003 Mar;111(5):617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009 Nov 3;54(19):1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Xydas S, Kherani AR, Chang JS, Klotz S, Hay I, Mutrie CJ, et al. beta(2)-Adrenergic stimulation attenuates left ventricular remodeling, decreases apoptosis, and improves calcium homeostasis in a rodent model of ischemic cardiomyopathy. J Pharmacol Exp Ther. 2006 May;317(2):553–561. doi: 10.1124/jpet.105.099432. [DOI] [PubMed] [Google Scholar]
- 11.Ahmet I, Morrell C, Lakatta EG, Talan MI. Therapeutic efficacy of a combination of a beta1-adrenoreceptor (AR) blocker and beta2-AR agonist in a rat model of postmyocardial infarction dilated heart failure exceeds that of a beta1-AR blocker plus angiotensin-converting enzyme inhibitor. J Pharmacol Exp Ther. 2009 Oct;331(1):178–185. doi: 10.1124/jpet.109.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009 Apr 14;119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 13.Graham RM, Perez DM, Hwa J, Piascik MT. alpha 1-adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ Res. 1996 May;78(5):737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- 14.Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999 Jun 30;375(1–3):261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]
- 15.Piascik MT, Perez DM. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001 Aug;298(2):403–410. [PubMed] [Google Scholar]
- 16.Simpson P. Lessons from knockouts: the alpha1-ARs. In: Perez DM, editor. The Adrenergic Receptors in the 21st Century. Totowa, New Jersey: Humana Press; 2006. pp. 207–240. [Google Scholar]
- 17.Hein P, Michel MC. Signal transduction and regulation: are all alpha1-adrenergic receptor subtypes created equal? Biochem Pharmacol. 2007 Apr 15;73(8):1097–1106. doi: 10.1016/j.bcp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Bylund DB, Bond RA, Eikenburg DC, Hieble JP, Hills R, Minneman KP, et al. Adrenoceptors: IUPHAR database (IUPHAR-DB), Last modified on 2010-07-21. 2010 http://wwwiuphar-dborg/DATABASE/FamilyMenuForward?familyId=4. [Google Scholar]
- 19.Zhu F, Han L, Zheng C, Xie B, Tammi MT, Yang S, et al. What are next generation innovative therapeutic targets? Clues from genetic, structural, physicochemical, and systems profiles of successful targets. J Pharmacol Exp Ther. 2009 Jul;330(1):304–315. doi: 10.1124/jpet.108.149955. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg SF, Drugge ED, Bilezikian JP, Robinson RB. Acquisition by innervated cardiac myocytes of a pertussis toxin-specific regulatory protein linked to the alpha 1-receptor. Science. 1985 Oct 11;230(4722):186–188. doi: 10.1126/science.2994230. [DOI] [PubMed] [Google Scholar]
- 21.Hu K, Nattel S. Mechanisms of ischemic preconditioning in rat hearts. Involvement of alpha 1B-adrenoceptors, pertussis toxin-sensitive G proteins, and protein kinase C. Circulation. 1995 Oct 15;92(8):2259–2265. doi: 10.1161/01.cir.92.8.2259. [DOI] [PubMed] [Google Scholar]
- 22.Akhter SA, Milano CA, Shotwell KF, Cho MC, Rockman HA, Lefkowitz RJ, et al. Transgenic mice with cardiac overexpression of a1B-adrenergic receptors. In vivo a1-adrenergic receptor-mediated regulation of b-adrenergic signaling. J Biol Chem. 1997;272(34):21253–21259. doi: 10.1074/jbc.272.34.21253. [DOI] [PubMed] [Google Scholar]
- 23.Melien O, Sandnes D, Johansen EJ, Christoffersen T. Effects of pertussis toxin on extracellular signal-regulated kinase activation in hepatocytes by hormones and receptor-independent agents: evidence suggesting a stimulatory role of G(i) proteins at a level distal to receptor coupling. J Cell Physiol. 2000 Jul;184(1):27–36. doi: 10.1002/(SICI)1097-4652(200007)184:1<27::AID-JCP3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Snabaitis AK, Muntendorf A, Wieland T, Avkiran M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of G(q/11), Gi and G(12/13) proteins in signalling by alpha1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell Signal. 2005 May;17(5):655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 25.O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, et al. The a1A/C- and a1B-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003 Jun;111(11):1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, et al. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006 Apr;116(4):1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, et al. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007 Feb 13;115(6):763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- 28.Benfey BG. Function of myocardial alpha-adrenoceptors. Life Sci. 1990;46(11):743–757. doi: 10.1016/0024-3205(90)90062-v. [DOI] [PubMed] [Google Scholar]
- 29.Li K, He H, Li C, Sirois P, Rouleau JL. Myocardial alpha1-adrenoceptor: inotropic effect and physiologic and pathologic implications. Life Sci. 1997;60(16):1305–1318. doi: 10.1016/s0024-3205(96)00650-9. [DOI] [PubMed] [Google Scholar]
- 30.Salvi S. Protecting the myocardium from ischemic injury: a critical role for alpha(1)-adrenoreceptors? Chest. 2001 Apr;119(4):1242–1249. doi: 10.1378/chest.119.4.1242. [DOI] [PubMed] [Google Scholar]
- 31.Mani K, Ashton AW, Kitsis RN. Taking the BAD out of adrenergic stimulation. J Mol Cell Cardiol. 2002 Jul;34(7):709–712. doi: 10.1006/jmcc.2002.2042. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock EA, Du XJ, Reichelt ME, Graham RM. Cardiac alpha 1-adrenergic drive in pathological remodelling. Cardiovasc Res. 2008 Feb 1;77(3):452–462. doi: 10.1093/cvr/cvm078. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb SS. The neurohormonal paradigm: have we gone too far? J Am Coll Cardiol. 2003 May 7;41(9):1458–1459. doi: 10.1016/s0735-1097(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 34.Mehra MR, Uber PA, Francis GS. Heart failure therapy at a crossroad: are there limits to the neurohormonal model? J Am Coll Cardiol. 2003 May 7;41(9):1606–1610. doi: 10.1016/s0735-1097(03)00245-6. [DOI] [PubMed] [Google Scholar]
- 35.Steinfath M, Chen YY, Lavicky J, Magnussen O, Nose M, Rosswag S, et al. Cardiac alpha 1-adrenoceptor densities in different mammalian species. Br J Pharmacol. 1992 Sep;107(1):185–188. doi: 10.1111/j.1476-5381.1992.tb14484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbull L, McCloskey DT, O'Connell TD, Simpson PC, Baker AJ. Alpha 1-adrenergic receptor responses in alpha 1AB-AR knockout mouse hearts suggest the presence of alpha 1D-AR. Am J Physiol Heart Circ Physiol. 2003 Apr;284(4):H1104–H1109. doi: 10.1152/ajpheart.00441.2002. [DOI] [PubMed] [Google Scholar]
- 37.Chalothorn D, McCune DF, Edelmann SE, Tobita K, Keller BB, Lasley RD, et al. Differential cardiovascular regulatory activities of the alpha 1B- and alpha 1D-adrenoceptor subtypes. J Pharmacol Exp Ther. 2003 Jun;305(3):1045–1053. doi: 10.1124/jpet.102.048553. [DOI] [PubMed] [Google Scholar]
- 38.Stewart AF, Rokosh DG, Bailey BA, Karns LR, Chang KC, Long CS, et al. Cloning of the rat alpha 1C-adrenergic receptor from cardiac myocytes. alpha 1C, alpha 1B, and alpha 1D mRNAs are present in cardiac myocytes but not in cardiac fibroblasts. Circ Res. 1994 Oct;75(4):796–802. doi: 10.1161/01.res.75.4.796. [DOI] [PubMed] [Google Scholar]
- 39.Marino TA, Cassidy M, Marino DR, Carson NL, Houser S. Norepinephrine-induced cardiac hypertrophy of the cat heart. Anat Rec. 1991 Apr;229(4):505–510. doi: 10.1002/ar.1092290411. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin IJ, Jalil JE, Tan LB, Cho K, Weber KT, Clark WA. Isoproterenol-induced myocardial fibrosis in relation to myocyte necrosis. Circ Res. 1989 Sep;65(3):657–670. doi: 10.1161/01.res.65.3.657. [DOI] [PubMed] [Google Scholar]
- 41.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, et al. Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol. 1997 Oct;29(10):2735–2746. doi: 10.1006/jmcc.1997.0508. [DOI] [PubMed] [Google Scholar]
- 42.Lau YH, Robinson RB, Rosen MR, Bilezikian JP. Subclassification of beta-adrenergic receptors in cultured rat cardiac myoblasts and fibroblasts. Circ Res. 1980 Jul;47(1):41–48. doi: 10.1161/01.res.47.1.41. [DOI] [PubMed] [Google Scholar]
- 43.Leicht M, Greipel N, Zimmer H. Comitogenic effect of catecholamines on rat cardiac fibroblasts in culture. Cardiovasc Res. 2000 Nov;48(2):274–284. doi: 10.1016/s0008-6363(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Eckhart AD, Eguchi S, Koch WJ. Beta-adrenergic receptor-mediated DNA synthesis in cardiac fibroblasts is dependent on transactivation of the epidermal growth factor receptor and subsequent activation of extracellular signal-regulated kinases. J Biol Chem. 2002 Aug 30;277(35):32116–32123. doi: 10.1074/jbc.M204895200. [DOI] [PubMed] [Google Scholar]
- 45.Turner NA, Porter KE, Smith WH, White HL, Ball SG, Balmforth AJ. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003 Mar;57(3):784–792. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 46.de Andrade CR, Fukada SY, Olivon VC, de Godoy MA, Haddad R, Eberlin MN, et al. Alpha1D-adrenoceptor-induced relaxation on rat carotid artery is impaired during the endothelial dysfunction evoked in the early stages of hyperhomocysteinemia. Eur J Pharmacol. 2006 Aug 14;543(1–3):83–91. doi: 10.1016/j.ejphar.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Filippi S, Parenti A, Donnini S, Granger HJ, Fazzini A, Ledda F. alpha(1D)-adrenoceptors cause endothelium-dependent vasodilatation in the rat mesenteric vascular bed. J Pharmacol Exp Ther. 2001 Mar;296(3):869–875. [PubMed] [Google Scholar]
- 48.Rokosh DG, Stewart AF, Chang KC, Bailey BA, Karliner JS, Camacho SA, et al. Alpha1-adrenergic receptor subtype mRNAs are differentially regulated by alpha1-adrenergic and other hypertrophic stimuli in cardiac myocytes in culture and in vivo. Repression of alpha1B and alpha1D but induction of alpha1C. J Biol Chem. 1996 Mar 8;271(10):5839–5843. doi: 10.1074/jbc.271.10.5839. [DOI] [PubMed] [Google Scholar]
- 49.Sjaastad I, Schiander I, Sjetnan A, Qvigstad E, Bokenes J, Sandnes D, et al. Increased contribution of alpha 1- vs. beta-adrenoceptor-mediated inotropic response in rats with congestive heart failure. Acta Physiol Scand. 2003 Apr;177(4):449–458. doi: 10.1046/j.1365-201X.2003.01063.x. [DOI] [PubMed] [Google Scholar]
- 50.Rokosh DG, Simpson PC. Knockout of the a1A/C-adrenergic receptor subtype: the a1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U S A. 2002;99(14):9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Methven L, McBride M, Wallace GA, McGrath JC. The alpha 1B/D-adrenoceptor knockout mouse permits isolation of the vascular alpha 1A-adrenoceptor and elucidates its relationship to the other subtypes. Br J Pharmacol. 2009 Sep;158(1):209–224. doi: 10.1111/j.1476-5381.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myagmar B-E, Rodrigo MC, Swigart PM, Simpson PC. The alpha-1A adrenergic receptor subtype Is expressed in only a sub-population of ventricular myocytes (abstract) Circulation. 2007;116 II-184. [Google Scholar]
- 53.Methven L, Simpson PC, McGrath JC. Alpha1A/B-knockout mice explain the native alpha1D-adrenoceptor's role in vasoconstriction and show that its location is independent of the other alpha1-subtypes. Br J Pharmacol. 2009 Dec;158(7):1663–1675. doi: 10.1111/j.1476-5381.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, et al. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008 Oct 24;103(9):992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen BC, Swigart PM, Laden ME, DeMarco T, Hoopes C, Simpson PC. The alpha-1D Is the predominant alpha-1-adrenergic receptor subtype in human epicardial coronary arteries. J Am Coll Cardiol. 2009 Sep 22;54(13):1137–1145. doi: 10.1016/j.jacc.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen BC, Swigart PM, De Marco T, Hoopes C, Simpson PC. {alpha}1-Adrenergic receptor subtypes in nonfailing and failing human myocardium. Circ Heart Fail. 2009 Nov;2(6):654–663. doi: 10.1161/CIRCHEARTFAILURE.108.846212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen BC, Swigart PM, Montgomery MD, Simpson PC. Functional alpha-1B adrenergic receptors on human epicardial coronary artery endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2010 doi: 10.1007/s00210-010-0558-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bristow MR, Minobe W, Rasmussen R, Hershberger RE, Hoffman BB. Alpha-1 adrenergic receptors in the nonfailing and failing human heart. J Pharmacol Exp Ther. 1988 Dec;247(3):1039–1045. [PubMed] [Google Scholar]
- 59.Bohm M, Diet F, Feiler G, Kemkes B, Erdmann E. Alpha-adrenoceptors and alpha-adrenoceptor-mediated positive inotropic effects in failing human myocardium. J Cardiovasc Pharmacol. 1988 Sep;12(3):357–364. doi: 10.1097/00005344-198809000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Vago T, Bevilacqua M, Norbiato G, Baldi G, Chebat E, Bertora P, et al. Identification of alpha 1-adrenergic receptors on sarcolemma from normal subjects and patients with idiopathic dilated cardiomyopathy: characteristics and linkage to GTP-binding protein. Circ Res. 1989 Mar;64(3):474–481. doi: 10.1161/01.res.64.3.474. [DOI] [PubMed] [Google Scholar]
- 61.Steinfath M, Danielsen W, von der Leyen H, Mende U, Meyer W, Neumann J, et al. Reduced alpha 1-and beta 2-adrenoceptor-mediated positive inotropic effects in human end-stage heart failure. Br J Pharmacol. 1992 Feb;105(2):463–469. doi: 10.1111/j.1476-5381.1992.tb14276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang KC, Gray CD, Sweet WE, Moravec CS, Im MJ. Alpha 1-adrenergic receptor coupling with Gh in the failing human heart. Circulation. 1996 Aug 15;94(4):718–726. doi: 10.1161/01.cir.94.4.718. [DOI] [PubMed] [Google Scholar]
- 63.Fowler MB, Laser JA, Hopkins GL, Minobe W, Bristow MR. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation. 1986 Dec;74(6):1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- 64.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986 Sep;59(3):297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 65.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 66.Skomedal T, Borthne K, Aass H, Geiran O, Osnes JB. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor- mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. J Pharmacol Exp Ther. 1997;280(2):721–729. [PubMed] [Google Scholar]
- 67.Scholz H, Eschenhagen T, Neumann J, Stein B. Receptor-mediated regulation of cardiac contractility: inotropic effects of alpha-adrenoceptor stimulation with phenylephrine and noradrenaline in failing human hearts. In: Endoh M, Morad M, Scholz H, Iijima T, editors. Molecular and cellular mechanisms of cardiovascular regulation. Tokyo: Springer-Verlag; 1996. pp. 317–325. [Google Scholar]
- 68.Skomedal T, Aass H, Osnes JB, Fjeld NB, Klingen G, Langslet A, et al. Demonstration of an alpha adrenoceptor-mediated inotropic effect of norepinephrine in human atria. J Pharmacol Exp Ther. 1985 May;233(2):441–446. [PubMed] [Google Scholar]
- 69.Aass H, Skomedal T, Osnes JB, Fjeld NB, Klingen G, Langslet A, et al. Noradrenaline evokes an alpha-adrenoceptor-mediated inotropic effect in human ventricular myocardium. Acta Pharmacol Toxicol (Copenh) 1986 Jan;58(1):88–90. doi: 10.1111/j.1600-0773.1986.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 70.Rabinowitz B, Chuck L, Kligerman M, Parmley WW. Positive inotropic effects of methoxamine: evidence for alpha-adrenergic receptors in ventricular myocardium. Am J Physiol. 1975 Sep;229(3):582–585. doi: 10.1152/ajplegacy.1975.229.3.582. [DOI] [PubMed] [Google Scholar]
- 71.Tohse N, Hattori Y, Nakaya H, Kanno M. Effects of alpha-adrenoceptor stimulation on electrophysiological properties and mechanics in rat papillary muscle. Gen Pharmacol. 1987;18(5):539–546. doi: 10.1016/0306-3623(87)90077-2. [DOI] [PubMed] [Google Scholar]
- 72.Williamson AP, Seifen E, Lindemann JP, Kennedy RH. The positive inotropic effect of alpha 1A-adrenoceptor stimulation is inhibited by 4-aminopyridine. Eur J Pharmacol. 1996 May 23;304(1–3):73–80. doi: 10.1016/0014-2999(96)00132-x. [DOI] [PubMed] [Google Scholar]
- 73.Wang GY, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the RV alpha1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol. 2010 Mar;298(3):H913–H920. doi: 10.1152/ajpheart.00259.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Endoh M, Shimizu T, Yanagisawa T. Characterization of adrenoceptors mediating positive inotropic responses in the ventricular myocardium of the dog. Br J Pharmacol. 1978 Sep;64(1):53–61. doi: 10.1111/j.1476-5381.1978.tb08640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hescheler J, Nawrath H, Tang M, Trautwein W. Adrenoceptor-mediated changes of excitation and contraction in ventricular heart muscle from guinea-pigs and rabbits. J Physiol. 1988 Mar;397:657–670. doi: 10.1113/jphysiol.1988.sp017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka H, Manita S, Matsuda T, Adachi M, Shigenobu K. Sustained negative inotropism mediated by alpha-adrenoceptors in adult mouse myocardia: developmental conversion from positive response in the neonate. Br J Pharmacol. 1995 Feb;114(3):673–677. doi: 10.1111/j.1476-5381.1995.tb17191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimaru K, Kobayashi M, Matsuda T, Tanaka Y, Tanaka H, Shigenobu K. alpha-Adrenoceptor stimulation-mediated negative inotropism and enhanced Na(+)/Ca(2+) exchange in mouse ventricle. Am J Physiol Heart Circ Physiol. 2001 Jan;280(1):H132–H141. doi: 10.1152/ajpheart.2001.280.1.H132. [DOI] [PubMed] [Google Scholar]
- 78.McCloskey DT, Rokosh DG, O'Connell TD, Keung EC, Simpson PC, Baker AJ. Alpha(1)-adrenoceptor subtypes mediate negative inotropy in myocardium from alpha(1A/C)-knockout and wild type mice. J Mol Cell Cardiol. 2002 Aug;34(8):1007–1017. doi: 10.1006/jmcc.2002.2049. [DOI] [PubMed] [Google Scholar]
- 79.Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- 80.Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha1-adrenergic response. J Clin Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- 82.Starksen NF, Simpson PC, Bishopric N, Coughlin SR, Lee WM, Escobedo JA, et al. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8348–8350. doi: 10.1073/pnas.83.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meidell RS, Sen A, Henderson SA, Slahetka MF, Chien KR. Alpha 1-adrenergic stimulation of rat myocardial cells increases protein synthesis. Am J Physiol. 1986 Nov;251(Pt 2) 5:H1076–H1084. doi: 10.1152/ajpheart.1986.251.5.H1076. [DOI] [PubMed] [Google Scholar]
- 84.Bishopric NH, Simpson PC, Ordahl CP. Induction of the skeletal alpha-actin gene in alpha 1-adrenoceptor-mediated hypertrophy of rat cardiac myocytes. J Clin Invest. 1987 Oct;80(4):1194–1199. doi: 10.1172/JCI113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee HR, Henderson SA, Reynolds R, Dunnmon P, Yuan D, Chien KR. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988 May 25;263(15):7352–7358. [PubMed] [Google Scholar]
- 86.Long CS, Ordahl CP, Simpson PC. Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. J Clin Invest. 1989 Mar;83(3):1078–1082. doi: 10.1172/JCI113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waspe LE, Ordahl CP, Simpson PC. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest. 1990 Apr;85(4):1206–1214. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simpson PC. Comments on "Load regulation of the properties of adult feline cardiocytes: The role of substrate adhesion" which appeared in Circ Res 58:692–705, 1986 (letter) Circ Res. 1988;62:864–866. doi: 10.1161/01.res.62.4.864. [DOI] [PubMed] [Google Scholar]
- 89.Fuller SJ, Gaitanaki CJ, Sugden PH. Effects of catecholamines on protein synthesis in cardiac myocytes and perfused hearts isolated from adult rats. Stimulation of translation is mediated through the alpha 1-adrenoceptor. Biochem J. 1990 Mar 15;266(3):727–736. doi: 10.1042/bj2660727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ikeda U, Tsuruya Y, Yaginuma T. Alpha 1-adrenergic stimulation is coupled to cardiac myocyte hypertrophy. Am J Physiol. 1991 Mar;260(Pt 2) 3:H953–H956. doi: 10.1152/ajpheart.1991.260.3.H953. [DOI] [PubMed] [Google Scholar]
- 91.Volz A, Piper HM, Siegmund B, Schwartz P. Longevity of adult ventricular rat heart muscle cells in serum-free primary culture. J Mol Cell Cardiol. 1991 Feb;23(2):161–173. doi: 10.1016/0022-2828(91)90103-s. [DOI] [PubMed] [Google Scholar]
- 92.Clark WA, Rudnick SJ, LaPres JJ, Andersen LC, LaPointe MC. Regulation of hypertrophy and atrophy in cultured adult heart cells. Circ Res. 1993 Dec;73(6):1163–1176. doi: 10.1161/01.res.73.6.1163. [DOI] [PubMed] [Google Scholar]
- 93.Clark WA, Rudnick SJ, Andersen LC, LaPres JJ. Myosin heavy chain synthesis is independently regulated in hypertrophy and atrophy of isolated adult cardiac myocytes. J Biol Chem. 1994 Oct 14;269(41):25562–25569. [PubMed] [Google Scholar]
- 94.Clark MG, Patten GS, Filsell OH. Evidence for an alpha-adrenergic receptor-mediated control of energy production in heart. J Mol Cell Cardiol. 1982 Jun;14(6):313–321. doi: 10.1016/0022-2828(82)90246-2. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita N, Nishida M, Hoshida S, Igarashi J, Hori M, Kuzuya T, et al. Alpha 1-adrenergic stimulation induces cardiac tolerance to hypoxia via induction and activation of Mn-SOD. Am J Physiol. 1996 Oct;271(Pt 2) 4:H1356–H1362. doi: 10.1152/ajpheart.1996.271.4.H1356. [DOI] [PubMed] [Google Scholar]
- 96.Kitakaze M, Minamino T, Node K, Komamura K, Inoue M, Hori M, et al. Activation of ecto-5'-nucleotidase by protein kinase C attenuates irreversible cellular injury due to hypoxia and reoxygenation in rat cardiomyocytes. J Mol Cell Cardiol. 1996 Sep;28(9):1945–1955. doi: 10.1006/jmcc.1996.0187. [DOI] [PubMed] [Google Scholar]
- 97.Karliner JS, Honbo N, Epstein CJ, Xian M, Lau YF, Gray MO. Neonatal mouse cardiac myocytes exhibit cardioprotection induced by hypoxic and pharmacologic preconditioning and by transgenic overexpression of human Cu/Zn superoxide dismutase. J Mol Cell Cardiol. 2000 Oct;32(10):1779–1786. doi: 10.1006/jmcc.2000.1212. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Ashraf M. Activation of alpha1-adrenergic receptor during Ca2+ pre-conditioning elicits strong protection against Ca2+ overload injury via protein kinase C signaling pathway. J Mol Cell Cardiol. 1998 Nov;30(11):2423–2435. doi: 10.1006/jmcc.1998.0802. [DOI] [PubMed] [Google Scholar]
- 99.Zhu H, McElwee-Witmer S, Perrone M, Clark KL, Zilberstein A. Phenylephrine protects neonatal rat cardiomyocytes from hypoxia and serum deprivation-induced apoptosis. Cell Death Differ. 2000 Sep;7(9):773–784. doi: 10.1038/sj.cdd.4400721. [DOI] [PubMed] [Google Scholar]
- 100.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, et al. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res. 2000 Feb 18;86(3):255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 101.Valks DM, Cook SA, Pham FH, Morrison PR, Clerk A, Sugden PH. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J Mol Cell Cardiol. 2002 Jul;34(7):749–763. doi: 10.1006/jmcc.2002.2014. [DOI] [PubMed] [Google Scholar]
- 102.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004 May 4;101(18):6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laks MM, Morady F, Swan HJ. Myocardial hypertrophy produced by chronic infusion of subhypertensive doses of norepinephrine in the dog. Chest. 1973 Jul;64(1):75–78. doi: 10.1378/chest.64.1.75. [DOI] [PubMed] [Google Scholar]
- 104.King BD, Sack D, Kichuk MR, Hintze TH. Absence of hypertension despite chronic marked elevations in plasma norepinephrine in conscious dogs. Hypertension. 1987 Jun;9(6):582–590. doi: 10.1161/01.hyp.9.6.582. [DOI] [PubMed] [Google Scholar]
- 105.Patel MB, Stewart JM, Loud AV, Anversa P, Wang J, Fiegel L, et al. Altered function and structure of the heart in dogs with chronic elevation in plasma norepinephrine. Circulation. 1991 Nov;84(5):2091–2100. doi: 10.1161/01.cir.84.5.2091. [DOI] [PubMed] [Google Scholar]
- 106.Stewart JM, Patel MB, Wang J, Ochoa M, Gewitz M, Loud AV, et al. Chronic elevation of norepinephrine in conscious dogs produces hypertrophy with no loss of LV reserve. Am J Physiol. 1992 Feb;262(Pt 2) 2:H331–H339. doi: 10.1152/ajpheart.1992.262.2.H331. [DOI] [PubMed] [Google Scholar]
- 107.Vecchione C, Fratta L, Rizzoni D, Notte A, Poulet R, Porteri E, et al. Cardiovascular influences of alpha1b-adrenergic receptor defect in mice. Circulation. 2002 Apr 9;105(14):1700–1707. doi: 10.1161/01.cir.0000012750.08480.55. [DOI] [PubMed] [Google Scholar]
- 108.Kitakaze M, Hori M, Tamai J, Iwakura K, Koretsune Y, Kagiya T, et al. Alpha 1-adrenoceptor activity regulates release of adenosine from the ischemic myocardium in dogs. Circ Res. 1987 May;60(5):631–639. doi: 10.1161/01.res.60.5.631. [DOI] [PubMed] [Google Scholar]
- 109.Kitakaze M, Hori M, Sato H, Iwakura K, Gotoh K, Inoue M, et al. Beneficial effects of alpha 1-adrenoceptor activity on myocardial stunning in dogs. Circ Res. 1991 May;68(5):1322–1339. doi: 10.1161/01.res.68.5.1322. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB, et al. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res. 1993 Oct;73(4):656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 111.Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, et al. Alpha 1-adrenoceptor activation mediates the infarct size-limiting effect of ischemic preconditioning through augmentation of 5'-nucleotidase activity. J Clin Invest. 1994 May;93(5):2197–2205. doi: 10.1172/JCI117216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bankwala Z, Hale SL, Kloner RA. Alpha-adrenoceptor stimulation with exogenous norepinephrine or release of endogenous catecholamines mimics ischemic preconditioning. Circulation. 1994 Aug;90(2):1023–1028. doi: 10.1161/01.cir.90.2.1023. [DOI] [PubMed] [Google Scholar]
- 113.Tsuchida A, Liu Y, Liu GS, Cohen MV, Downey JM. alpha 1-adrenergic agonists precondition rabbit ischemic myocardium independent of adenosine by direct activation of protein kinase C. Circ Res. 1994 Sep;75(3):576–585. doi: 10.1161/01.res.75.3.576. [DOI] [PubMed] [Google Scholar]
- 114.Tosaki A, Behjet NS, Engelman DT, Engelman RM, Das DK. Alpha-1 adrenergic receptor agonist-induced preconditioning in isolated working rat hearts. J Pharmacol Exp Ther. 1995 May;273(2):689–694. [PubMed] [Google Scholar]
- 115.Mitchell MB, Meng X, Ao L, Brown JM, Harken AH, Banerjee A. Preconditioning of isolated rat heart is mediated by protein kinase C. Circ Res. 1995 Jan;76(1):73–81. doi: 10.1161/01.res.76.1.73. [DOI] [PubMed] [Google Scholar]
- 116.Meng X, Brown JM, Ao L, Banerjee A, Harken AH. Norepinephrine induces cardiac heat shock protein 70 and delayed cardioprotection in the rat through alpha 1 adrenoceptors. Cardiovasc Res. 1996 Aug;32(2):374–383. doi: 10.1016/0008-6363(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 117.Meng X, Cleveland JC, Jr, Rowland RT, Mitchell MB, Brown JM, Banerjee A, et al. Norepinephrine-induced sustained myocardial adaptation to ischemia is dependent on alpha 1-adrenoceptors and protein synthesis. J Mol Cell Cardiol. 1996 Sep;28(9):2017–2025. doi: 10.1006/jmcc.1996.0194. [DOI] [PubMed] [Google Scholar]
- 118.Haessler R, Kuzume K, Wolff RA, Chien GL, Davis RF, Van Winkle DM. Adrenergic activation confers cardioprotection mediated by adenosine, but is not required for ischemic preconditioning. Coron Artery Dis. 1996 Apr;7(4):305–314. doi: 10.1097/00019501-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Cope JT, Mauney MC, Banks D, Binns OA, Moore CL, Rentz JJ, et al. Intravenous phenylephrine preconditioning of cardiac grafts from non-heart-beating donors. Ann Thorac Surg. 1997 Jun;63(6):1664–1668. doi: 10.1016/s0003-4975(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 120.Meldrum DR, Cleveland JC, Jr, Rowland RT, Banerjee A, Harken AH, Meng X. Early and delayed preconditioning: differential mechanisms and additive protection. Am J Physiol. 1997 Aug;273(Pt 2) 2:H725–H733. doi: 10.1152/ajpheart.1997.273.2.H725. [DOI] [PubMed] [Google Scholar]
- 121.Node K, Kitakaze M, Sato H, Minamino T, Komamura K, Shinozaki Y, et al. Role of intracellular Ca2+ in activation of protein kinase C during ischemic preconditioning. Circulation. 1997 Aug 19;96(4):1257–1265. doi: 10.1161/01.cir.96.4.1257. [DOI] [PubMed] [Google Scholar]
- 122.Baghelai K, Graham LJ, Wechsler AS, Jakoi ER. Delayed myocardial preconditioning by alpha1-adrenoceptors involves inhibition of apoptosis. J Thorac Cardiovasc Surg. 1999 May;117(5):980–986. doi: 10.1016/s0022-5223(99)70379-x. [DOI] [PubMed] [Google Scholar]
- 123.Baghelai K, Graham LJ, Wechsler AS, Jakoi ER. Phenylephrine induces delayed cardioprotection against necrosis without amelioration of stunning. Ann Thorac Surg. 1999 Oct;68(4):1219–1224. doi: 10.1016/s0003-4975(99)00979-0. [DOI] [PubMed] [Google Scholar]
- 124.Tejero-Taldo MI, Gursoy E, Zhao TC, Kukreja RC. Alpha-adrenergic receptor stimulation produces late preconditioning through inducible nitric oxide synthase in mouse heart. J Mol Cell Cardiol. 2002 Feb;34(2):185–195. doi: 10.1006/jmcc.2001.1500. [DOI] [PubMed] [Google Scholar]
- 125.Imani A, Faghihi M, Sadr SS, Keshavarz M, Niaraki SS. Noradrenaline reduces ischemia-induced arrhythmia in anesthetized rats: involvement of alpha1-adrenoceptors and mitochondrial K ATP channels. J Cardiovasc Electrophysiol. 2008 Mar;19(3):309–315. doi: 10.1111/j.1540-8167.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- 126.Sebbag L, Katsuragawa M, Verbinski S, Jennings RB, Reimer KA. Intracoronary administration of the alpha 1-receptor agonist, methoxamine, does not reproduce the infarct-limiting effect of ischemic preconditioning in dogs. Cardiovasc Res. 1996 Nov;32(5):830–838. [PubMed] [Google Scholar]
- 127.Chan T, Dash R, Simpson PC. An alpha-1A-adrenergic receptor subtype agonist prevents cardiomyopathy without increasing blood pressure (abstract) Circulation. 2008;118:S533. [Google Scholar]
- 128.Thornton JD, Daly JF, Cohen MV, Yang XM, Downey JM. Catecholamines can induce adenosine receptor-mediated protection of the myocardium but do not participate in ischemic preconditioning in the rabbit. Circ Res. 1993 Oct;73(4):649–655. doi: 10.1161/01.res.73.4.649. [DOI] [PubMed] [Google Scholar]
- 129.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004 Apr 27;109(16):1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 130.Meng X, Shames BD, Pulido EJ, Meldrum DR, Ao L, Joo KS, et al. Adrenergic induction of bimodal myocardial protection: signal transduction and cardiac gene reprogramming. Am J Physiol. 1999 May;276(Pt 2) 5:R1525–R1533. doi: 10.1152/ajpregu.1999.276.5.R1525. [DOI] [PubMed] [Google Scholar]
- 131.Communal C, Singh M, Menon B, Xie Z, Colucci WS, Singh K. beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J Cell Biochem. 2003 May 15;89(2):381–388. doi: 10.1002/jcb.10520. [DOI] [PubMed] [Google Scholar]
- 132.Kudej RK, Shen YT, Peppas AP, Huang CH, Chen W, Yan L, et al. Obligatory role of cardiac nerves and alpha1-adrenergic receptors for the second window of ischemic preconditioning in conscious pigs. Circ Res. 2006 Nov 24;99(11):1270–1276. doi: 10.1161/01.RES.0000251282.79411.44. [DOI] [PubMed] [Google Scholar]
- 133.Bleasdale RA, Frenneaux MP. Prognostic importance of right ventricular dysfunction. Heart. 2002 Oct;88(4):323–324. doi: 10.1136/heart.88.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bristow MR, Feldman AM, Adams KF, Jr, Goldstein S. Selective versus nonselective beta-blockade for heart failure therapy: are there lessons to be learned from the COMET trial? J Card Fail. 2003 Dec;9(6):444–453. doi: 10.1016/j.cardfail.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 135.Rodrigo MC, Swigart PM, Myagmar B-E, Cha J, Zhu B-Q, Yeh CC, et al. Beta-blockers switch catecholamine activation of MAPKs from maladaptive beta-adrenergic p38 to adaptive alpha-1-adrenergic ERK (abstract) Circulation. 2006;114(II):101. [Google Scholar]
- 136.Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, et al. alpha1A- but not alpha1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrinein-sensitive mechanism. Cardiovasc Res. 2005 Feb 1;65(2):436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 137.Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, et al. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001 Aug 17;89(4):343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- 138.Du XJ, Fang L, Gao XM, Kiriazis H, Feng X, Hotchkin E, et al. Genetic enhancement of ventricular contractility protects against pressure-overload-induced cardiac dysfunction. J Mol Cell Cardiol. 2004 Nov;37(5):979–987. doi: 10.1016/j.yjmcc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 139.Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, et al. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006 Sep 1;71(4):735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 140.Chaulet H, Lin F, Guo J, Owens WA, Michalicek J, Kesteven SH, et al. Sustained augmentation of cardiac alpha1A-adrenergic drive results in pathological remodeling with contractile dysfunction, progressive fibrosis and reactivation of matricellular protein genes. J Mol Cell Cardiol. 2006 Apr;40(4):540–552. doi: 10.1016/j.yjmcc.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 141.Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, et al. Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000 Jan 7–21;86(1):43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- 143.Wang BH, Du XJ, Autelitano DJ, Milano CA, Woodcock EA. Adverse effects of constitutively active alpha(1B)-adrenergic receptors after pressure overload in mouse hearts. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1079–H1086. doi: 10.1152/ajpheart.2000.279.3.H1079. [DOI] [PubMed] [Google Scholar]
- 144.Harrison SN, Autelitano DJ, Wang BH, Milano C, Du XJ, Woodcock EA. Reduced reperfusion-induced Ins(1,4,5)P3 generation and arrhythmias in hearts expressing constitutively active alpha1B-adrenergic receptors. Circ Res. 1998 Dec 14–28;83(12):1232–1240. doi: 10.1161/01.res.83.12.1232. [DOI] [PubMed] [Google Scholar]
- 145.Zuscik MJ, Chalothorn D, Hellard D, Deighan C, McGee A, Daly CJ, et al. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the alpha 1B-adrenergic receptor. J Biol Chem. 2001 Apr 27;276(17):13738–13743. doi: 10.1074/jbc.M008693200. [DOI] [PubMed] [Google Scholar]
- 146.Yun J, Zuscik MJ, Gonzalez-Cabrera P, McCune DF, Ross SA, Gaivin R, et al. Gene expression profiling of alpha(1b)-adrenergic receptor-induced cardiac hypertrophy by oligonucleotide arrays. Cardiovasc Res. 2003 Feb;57(2):443–455. doi: 10.1016/s0008-6363(02)00696-x. [DOI] [PubMed] [Google Scholar]
- 147.Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, et al. The alpha(1B)-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc Res. 2003 Dec 1;60(3):598–607. doi: 10.1016/j.cardiores.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 148.Grupp IL, Lorenz JN, Walsh RA, Boivin GP, Rindt H. Overexpression of alpha1B-adrenergic receptor induces left ventricular dysfunction in the absence of hypertrophy. Am J Physiol. 1998 Oct;275(Pt 2) 4:H1338–H1350. doi: 10.1152/ajpheart.1998.275.4.H1338. [DOI] [PubMed] [Google Scholar]
- 149.Lemire I, Ducharme A, Tardif JC, Poulin F, Jones LR, Allen BG, et al. Cardiac-directed overexpression of wild-type alpha1B-adrenergic receptor induces dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2001 Aug;281(2):H931–H938. doi: 10.1152/ajpheart.2001.281.2.H931. [DOI] [PubMed] [Google Scholar]
- 150.Iaccarino G, Keys JR, Rapacciuolo A, Shotwell KF, Lefkowitz RJ, Rockman HA, et al. Regulation of myocardial betaARK1 expression in catecholamine-induced cardiac hypertrophy in transgenic mice overexpressing alpha1B-adrenergic receptors. J Am Coll Cardiol. 2001 Aug;38(2):534–540. doi: 10.1016/s0735-1097(01)01396-1. [DOI] [PubMed] [Google Scholar]
- 151.Jensen B, Swigart P, Laden M-E, DeMarco T, Hoopes C, Simpson P. The alpha-1D is the predominant alpha-1-adrenergic receptor in human epicardial coronary arteries. JACC. 2009;54(13):1137–1145. doi: 10.1016/j.jacc.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanoue A, Koshimizu TA, Tsujimoto G. Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci. 2002 Sep 27;71(19):2207–2215. doi: 10.1016/s0024-3205(02)02012-x. [DOI] [PubMed] [Google Scholar]
- 153.Kariya T, Minatoguchi S, Ohno T, Yamashita K, Uno Y, Arai M, et al. Infarct size-reducing effect of ischemic preconditioning is related to alpha1b-adrenoceptors but not to alpha1a-adrenoceptors in rabbits. J Cardiovasc Pharmacol. 1997 Oct;30(4):437–445. doi: 10.1097/00005344-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 154.Meldrum DR, Cleveland JC, Jr, Sheridan BC, Rowland RT, Selzman CH, Banerjee A, et al. Alpha-adrenergic activation of myocardial NF kappa B during hemorrhage. J Surg Res. 1997 May;69(2):268–276. doi: 10.1006/jsre.1997.5023. [DOI] [PubMed] [Google Scholar]
- 155.Kawai K, Qin F, Shite J, Mao W, Fukuoka S, Liang CS. Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol. 2004 Sep;287(3):H1003–H1012. doi: 10.1152/ajpheart.00797.2003. [DOI] [PubMed] [Google Scholar]
- 156.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003 Aug;112(3):302–307. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodrigo MC, Joho S, Swigart PM, Foster E, Oɼonnell TD, Grossman W, et al. The alpha-1-B adrenergic receptor subtype is required for physiological cardiac hypertrophy in normal development (abstract) Circulation. 2005;112(II):123. [Google Scholar]
- 158.Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci U S A. 1997;94(21):11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9(2):85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- 160.Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005 Mar;67(3):912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- 161.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109(6):765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McCloskey DT, Turnbull L, Swigart P, O'Connell TD, Simpson PC, Baker AJ. Abnormal myocardial contraction in alpha(1A)- and alpha(1B)-adrenoceptor double-knockout mice. J Mol Cell Cardiol. 2003 Oct;35(10):1207–1216. doi: 10.1016/s0022-2828(03)00227-x. [DOI] [PubMed] [Google Scholar]
- 163.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem. 1999 Jun 11;274(24):16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- 164.Kiriazis H, Wang K, Xu Q, Gao XM, Ming Z, Su Y, et al. Knockout of beta(1)- and beta(2)-adrenoceptors attenuates pressure overload-induced cardiac hypertrophy and fibrosis. Br J Pharmacol. 2008 Feb;153(4):684–692. doi: 10.1038/sj.bjp.0707622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cleveland JC, Jr, Wollmering MM, Meldrum DR, Rowland RT, Rehring TF, Sheridan BC, et al. Ischemic preconditioning in human and rat ventricle. Am J Physiol. 1996 Nov;271(5 Pt 2):H1786–H1794. doi: 10.1152/ajpheart.1996.271.5.H1786. [DOI] [PubMed] [Google Scholar]
- 166.Cleveland JC, Jr, Meldrum DR, Rowland RT, Cain BS, Meng X, Gamboni-Robertson F, et al. Ischemic preconditioning of human myocardium: protein kinase C mediates a permissive role for alpha 1-adrenoceptors. Am J Physiol. 1997 Aug;273(2 Pt 2):H902–H908. doi: 10.1152/ajpheart.1997.273.2.H902. [DOI] [PubMed] [Google Scholar]
- 167.Loubani M, Galinanes M. alpha1-Adrenoceptors during simulated ischemia and reoxygenation of the human myocardium: effect of the dose and time of administration. J Thorac Cardiovasc Surg. 2001 Jul;122(1):103–112. doi: 10.1067/mtc.2001.114778. [DOI] [PubMed] [Google Scholar]
- 168.Loubani M, Galinanes M. Pharmacological and ischemic preconditioning of the human myocardium: mitoK(ATP) channels are upstream and p38MAPK is downstream of PKC. BMC Physiol. 2002 Jul 18;2:10. doi: 10.1186/1472-6793-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cabanes L, Costes F, Weber S, Regnard J, Benvenuti C, Castaigne A, et al. Improvement in exercise performance by inhalation of methoxamine in patients with impaired left ventricular function. N Engl J Med. 1992 Jun 18;326(25):1661–1665. doi: 10.1056/NEJM199206183262503. [DOI] [PubMed] [Google Scholar]
- 170.Zakir RM, Folefack A, Saric M, Berkowitz RL. The use of midodrine in patients with advanced heart failure. Congest Heart Fail. 2009 May–Jun;15(3):108–111. doi: 10.1111/j.1751-7133.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 171.ALLHAT CRG. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Jama. 2000;283(15):1967–1975. [see comments] [PubMed] [Google Scholar]
- 172.Davis BR, Cutler JA, Furberg CD, Wright JT, Farber MA, Felicetta JV, et al. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial. Ann Intern Med. 2002 Sep 3;137(5 Part 1):313–320. doi: 10.7326/0003-4819-137-5_part_1-200209030-00006. [DOI] [PubMed] [Google Scholar]
- 173.Cohn JN. The Vasodilator-Heart Failure Trials (V-HeFT). Mechanistic data from the VA Cooperative Studies Introduction. Circulation. 1993 Jun;87(6 Suppl):VI1–VI4. [PubMed] [Google Scholar]
- 174.Dhaliwal AS, Habib G, Deswal A, Verduzco M, Souchek J, Ramasubbu K, et al. Impact of alpha 1-adrenergic antagonist use for benign prostatic hypertrophy on outcomes in patients with heart failure. Am J Cardiol. 2009 Jul 15;104(2):270–275. doi: 10.1016/j.amjcard.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 175.Tomai F, Crea F, Gaspardone A, Versaci F, Ghini AS, De Paulis R, et al. Phentolamine prevents adaptation to ischemia during coronary angioplasty: role of alpha-adrenergic receptors in ischemic preconditioning. Circulation. 1997 Oct 7;96(7):2171–2177. doi: 10.1161/01.cir.96.7.2171. [DOI] [PubMed] [Google Scholar]
- 176.Gonzalez-Juanatey JR, Iglesias MJ, Alcaide C, Pineiro R, Lago F. Doxazosin induces apoptosis in cardiomyocytes cultured in vitro by a mechanism that is independent of alpha1-adrenergic blockade. Circulation. 2003 Jan 7;107(1):127–131. doi: 10.1161/01.cir.0000043803.20822.d1. [DOI] [PubMed] [Google Scholar]
- 177.Bristow M. Antiadrenergic therapy of chronic heart failure: surprises and new opportunities. Circulation. 2003 Mar 4;107(8):1100–1102. doi: 10.1161/01.cir.0000054530.87613.36. [DOI] [PubMed] [Google Scholar]
- 178.Coats AJ. Heart Failure 99 -- the MOXCON story. Int J Cardiol. 1999 Oct 31;71(2):109–111. doi: 10.1016/s0167-5273(99)00120-5. [DOI] [PubMed] [Google Scholar]
- 179.Cohn JN, Pfeffer MA, Rouleau J, Sharpe N, Swedberg K, Straub M, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON) Eur J Heart Fail. 2003 Oct;5(5):659–667. doi: 10.1016/s1388-9842(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 180.Swedberg K, Bristow MR, Cohn JN, Dargie H, Straub M, Wiltse C, et al. Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure. Circulation. 2002 Apr 16;105(15):1797–1803. doi: 10.1161/01.cir.0000014212.04920.62. [DOI] [PubMed] [Google Scholar]
- 181.Bristow MR, Krause-Steinrauf H, Nuzzo R, Liang CS, Lindenfeld J, Lowes BD, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation. 2004 Sep 14;110(11):1437–1442. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- 182.Dunn-Meynell AA, Yarlagadda Y, Levin BE. Alpha 1-adrenoceptor blockade increases behavioral deficits in traumatic brain injury. Journal of neurotrauma. 1997 Jan;14(1):43–52. doi: 10.1089/neu.1997.14.43. [DOI] [PubMed] [Google Scholar]
- 183.Hiramoto T, Ihara Y, Watanabe Y. alpha-1 adrenergic receptors stimulation induces the proliferation of neural progenitor cells in vitro. Neuroscience letters. 2006 Nov 6;408(1):25–28. doi: 10.1016/j.neulet.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 184.Burcelin R, Uldry M, Foretz M, Perrin C, Dacosta A, Nenniger-Tosato M, et al. Impaired glucose homeostasis in mice lacking the alpha 1b-adrenergic receptor subtype. J Biol Chem. 2003 Oct 27; doi: 10.1074/jbc.M307788200. [DOI] [PubMed] [Google Scholar]
- 185.Sever PS. Alpha 1-blockers in hypertension. Curr Med Res Opin. 1999;15(2):95–103. doi: 10.1185/03007999909113369. [DOI] [PubMed] [Google Scholar]
- 186.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003 Jul 5;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 187.Kubo T, Azevedo ER, Newton GE, Parker JD, Floras JS. Lack of evidence for peripheral alpha(1)-adrenoceptor blockade during long-term treatment of heart failure with carvedilol. J Am Coll Cardiol. 2001 Nov 1;38(5):1463–1469. doi: 10.1016/s0735-1097(01)01577-7. [DOI] [PubMed] [Google Scholar]
- 188.Hryniewicz K, Androne AS, Hudaihed A, Katz SD. Comparative effects of carvedilol and metoprolol on regional vascular responses to adrenergic stimuli in normal subjects and patients with chronic heart failure. Circulation. 2003 Aug 26;108(8):971–976. doi: 10.1161/01.CIR.0000085070.39873.B1. [DOI] [PubMed] [Google Scholar]
- 189.Van Tassell BW, Rondina MT, Huggins F, Gilbert EM, Munger MA. Carvedilol increases blood pressure response to phenylephrine infusion in heart failure subjects with systolic dysfunction: evidence of improved vascular alpha1-adrenoreceptor signal transduction. Am Heart J. 2008 Aug;156(2):315–321. doi: 10.1016/j.ahj.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Flesch M, Maack C, Cremers B, Baumer AT, Sudkamp M, Bohm M. Effect of beta-blockers on free radical-induced cardiac contractile dysfunction. Circulation. 1999 Jul 27;100(4):346–353. doi: 10.1161/01.cir.100.4.346. [DOI] [PubMed] [Google Scholar]
- 191.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001 May 22;103(20):2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 192.Karle CA, Kreye VA, Thomas D, Rockl K, Kathofer S, Zhang W, et al. Antiarrhythmic drug carvedilol inhibits HERG potassium channels. Cardiovasc Res. 2001 Feb 1;49(2):361–370. doi: 10.1016/s0008-6363(00)00265-0. [DOI] [PubMed] [Google Scholar]
- 193.Nakamura K, Kusano K, Nakamura Y, Kakishita M, Ohta K, Nagase S, et al. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002 Jun 18;105(24):2867–2871. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 194.Wang R, Miura T, Harada N, Kametani R, Shibuya M, Fukagawa Y, et al. Pleiotropic effects of the beta-adrenoceptor blocker carvedilol on calcium regulation during oxidative stress-induced apoptosis in cardiomyocytes. J Pharmacol Exp Ther. 2006 Jul;318(1):45–52. doi: 10.1124/jpet.105.099903. [DOI] [PubMed] [Google Scholar]
- 195.Hewett TE, Grupp IL, Grupp G, Robbins J. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res. 1994 Apr;74(4):740–746. doi: 10.1161/01.res.74.4.740. [DOI] [PubMed] [Google Scholar]
- 196.López JE, Myagmar B-E, Simpson PC. Beta-myosin heavy chain is induced by pressure overload only in a minor sub-population of smaller cardiac myocytes (abstract) Circulation. 2010 doi: 10.1161/CIRCRESAHA.111.243410. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gao XM, Wang BH, Woodcock E, Du XJ. Expression of active alpha(1B)-adrenergic receptors in the heart does not alleviate ischemic reperfusion injury. J Mol Cell Cardiol. 2000 Sep;32(9):1679–1686. doi: 10.1006/jmcc.2000.1201. [DOI] [PubMed] [Google Scholar]
- 198.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995 Apr 13;374(6523):643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 199.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, et al. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995 Nov 10;270(45):27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 200.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995 Apr 13;374(6523):640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 201.Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Jr, Williamson JD, et al. Validation of Heart Failure Events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants Assigned to Doxazosin and Chlorthalidone. Curr Control Trials Cardiovasc Med. 2002 Nov 14;3(1):10. doi: 10.1186/1468-6708-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003 Jan;2(1):38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 203.Perez DM, Hwa J, Gaivin R, Mathur M, Brown F, Graham RM. Constitutive activation of a single effector pathway: evidence for multiple activation states of a G protein-coupled receptor. Mol Pharmacol. 1996 Jan;49(1):112–122. [PubMed] [Google Scholar]
- 204.Zhou YY, Cheng H, Song LS, Wang D, Lakatta EG, Xiao RP. Spontaneous beta(2)-adrenergic signaling fails to modulate L-type Ca(2+) current in mouse ventricular myocytes. Mol Pharmacol. 1999 Sep;56(3):485–493. doi: 10.1124/mol.56.3.485. [DOI] [PubMed] [Google Scholar]
- 205.Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, et al. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999 Jan 8–22;84(1):43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 206.Tubio MR, Fernandez N, Fitzsimons CP, Copsel S, Santiago S, Shayo C, et al. Expression of a G protein-coupled receptor (GPCR) leads to attenuation of signaling by other GPCRs: experimental evidence for a spontaneous GPCR constitutive inactive form. J Biol Chem. 2010 May 14;285(20):14990–14998. doi: 10.1074/jbc.M109.099689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Birdsall NJ. Class A GPCR heterodimers: evidence from binding studies. Trends Pharmacol Sci. 2010 Sep 24; doi: 10.1016/j.tips.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 208.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, et al. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101(6):689–694. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]
- 209.Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006 Apr;97(4):747–751. doi: 10.1111/j.1464-410X.2006.06030.x. discussion 51. [DOI] [PubMed] [Google Scholar]
- 210.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004 Dec 21;44(12):2390–2397. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 211.Hoyer K, Krenz M, Robbins J, Ingwall JS. Shifts in the myosin heavy chain isozymes in the mouse heart result in increased energy efficiency. J Mol Cell Cardiol. 2007 Jan;42(1):214–221. doi: 10.1016/j.yjmcc.2006.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW. Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. (2nd.) 1998 Apr 21;97(15):1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 213.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, et al. Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Syed F, Odley A, Hahn HS, Brunskill EW, Lynch RA, Marreez Y, et al. Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circ Res. 2004 Dec 10;95(12):1200–1206. doi: 10.1161/01.RES.0000150366.08972.7f. [DOI] [PubMed] [Google Scholar]
- 215.Jalili T, Takeishi Y, Song G, Ball NA, Howles G, Walsh RA. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. Am J Physiol. 1999 Dec;277(6 Pt 2):H2298–H2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 216.Ponicke K, Vogelsang M, Heinroth M, Becker K, Zolk O, Bohm M, et al. Endothelin receptors in the failing and nonfailing human heart. Circulation. 1998 Mar 3;97(8):744–751. doi: 10.1161/01.cir.97.8.744. [DOI] [PubMed] [Google Scholar]