Abstract
The genetically unstable Mutator Strain of D. melanogaster is characterised by a high frequency of spontaneous mutations and their reversions. Three forked mutants were obtained independently and several reversions arose spontaneously with frequency of 10(-3)-10(-4). The sites of integration and excision of the gypsy retrotransposon were analysed by Southern blot analysis and sequencing of PCR fragments. In all cases gypsy had inserted at the end of the third exon of the major transcript of the forked gene, causing the duplication of TCCA target sequence. All the reversions resulted from precise excision of the gypsy. A double mutant containing ct6 and f1, caused by gypsy insertions into untranslated regions of the corresponding genes, was constructed. Two spontaneous ct6f+ revertants as well as one ct+f1 revertant were obtained from this line. Sequence analysis of gypsy integration and excision sites revealed that in all cases gypsy excision was also precise. These experiments constitute the first demonstration of precise excision of LTR-containing elements from their host genomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhipova I. R., Mazo A. M., Cherkasova V. A., Gorelova T. V., Schuppe N. G., Llyin Y. V. The steps of reverse transcription of Drosophila mobile dispersed genetic elements and U3-R-U5 structure of their LTRs. Cell. 1986 Feb 28;44(4):555–563. doi: 10.1016/0092-8674(86)90265-5. [DOI] [PubMed] [Google Scholar]
- Bayev A. A., Jr, Lyubomirskaya N. V., Dzhumagaliev E. B., Ananiev E. V., Amiantova I. G., Ilyin Y. V. Structural organization of transposable element mdg4 from Drosophila melanogaster and a nucleotide sequence of its long terminal repeats. Nucleic Acids Res. 1984 Apr 25;12(8):3707–3723. doi: 10.1093/nar/12.8.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonare B. D., Gehring W. J. Excision of copia element in a revertant of the white-apricot mutation of Drosophila melanogaster leaves behind one long-terminal repeat. Mol Gen Genet. 1985;199(1):1–6. doi: 10.1007/BF00327501. [DOI] [PubMed] [Google Scholar]
- Corces V. G., Geyer P. K. Interactions of retrotransposons with the host genome: the case of the gypsy element of Drosophila. Trends Genet. 1991 Mar;7(3):86–90. doi: 10.1016/0168-9525(91)90277-W. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Boeke J. D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988 Sep 23;54(7):955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Alphey L. S., Ross S. J., Leigh-Brown A. J. Complete reversions of a gypsy retrotransposon-induced cut locus mutation in Drosophila melanogaster involving jockey transposon insertions and flanking gypsy sequence deletions. Mol Gen Genet. 1990 Jan;220(2):181–185. doi: 10.1007/BF00260479. [DOI] [PubMed] [Google Scholar]
- Freund R., Meselson M. Long terminal repeat nucleotide sequence and specific insertion of the gypsy transposon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4462–4464. doi: 10.1073/pnas.81.14.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Reversion of a gypsy-induced mutation at the yellow (y) locus of Drosophila melanogaster is associated with the insertion of a newly defined transposable element. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3938–3942. doi: 10.1073/pnas.85.11.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
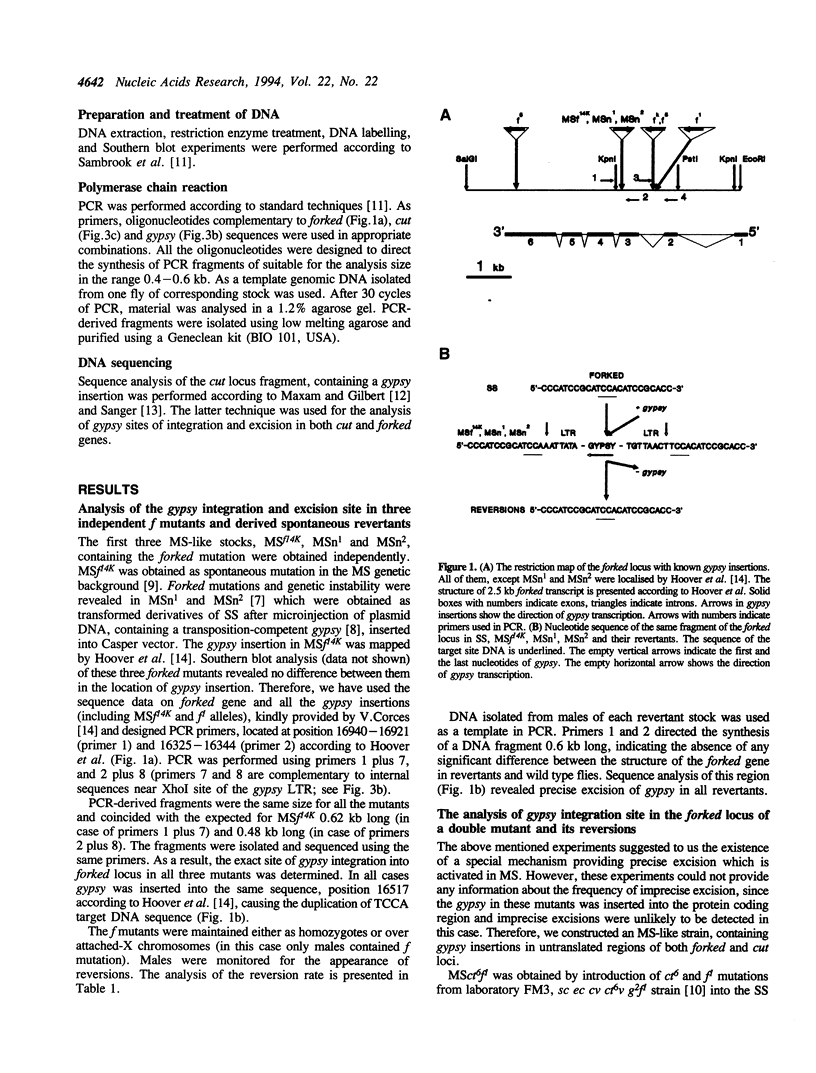
- Hoover K. K., Chien A. J., Corces V. G. Effects of transposable elements on the expression of the forked gene of Drosophila melanogaster. Genetics. 1993 Oct;135(2):507–526. doi: 10.1093/genetics/135.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. W. Molecular organization of the cut locus of Drosophila melanogaster. Cell. 1985 Oct;42(3):869–876. doi: 10.1016/0092-8674(85)90283-1. [DOI] [PubMed] [Google Scholar]
- Katzman M., Mack J. P., Skalka A. M., Leis J. A covalent complex between retroviral integrase and nicked substrate DNA. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4695–4699. doi: 10.1073/pnas.88.11.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. I., Beliaeva E. S., Larkina Z. G., Aslanian M. M. Geneticheskaia nestabil'nost' i transpozitsii mobil'nogo élement MDG4 v mutatornoi linii Drosophila melanogaster. Genetika. 1989 Oct;25(10):1747–1756. [PubMed] [Google Scholar]
- Kim A. I., Belyaeva E. S., Aslanian M. M. Autonomous transposition of gypsy mobile elements and genetic instability in Drosophila melanogaster. Mol Gen Genet. 1990 Nov;224(2):303–308. doi: 10.1007/BF00271566. [DOI] [PubMed] [Google Scholar]
- Kim A. I., Belyaeva E. S. Transposition of mobile elements gypsy (mdg4) and hobo in germ-line and somatic cells of a genetically unstable mutator strain of Drosophila melanogaster. Mol Gen Genet. 1991 Oct;229(3):437–444. doi: 10.1007/BF00267467. [DOI] [PubMed] [Google Scholar]
- Kim A. I., Lyubomirskaya N. V., Belyaeva E. S., Shostack N. G., Ilyin Y. V. The introduction of a transpositionally active copy of retrotransposon GYPSY into the Stable Strain of Drosophila melanogaster causes genetic instability. Mol Gen Genet. 1994 Feb;242(4):472–477. doi: 10.1007/BF00281799. [DOI] [PubMed] [Google Scholar]
- Lyubomirskaya N. V., Arkhipova I. R., Ilyin Y. V., Kim A. I. Molecular analysis of the gypsy (mdg4) retrotransposon in two Drosophila melanogaster strains differing by genetic instability. Mol Gen Genet. 1990 Sep;223(2):305–309. doi: 10.1007/BF00265067. [DOI] [PubMed] [Google Scholar]
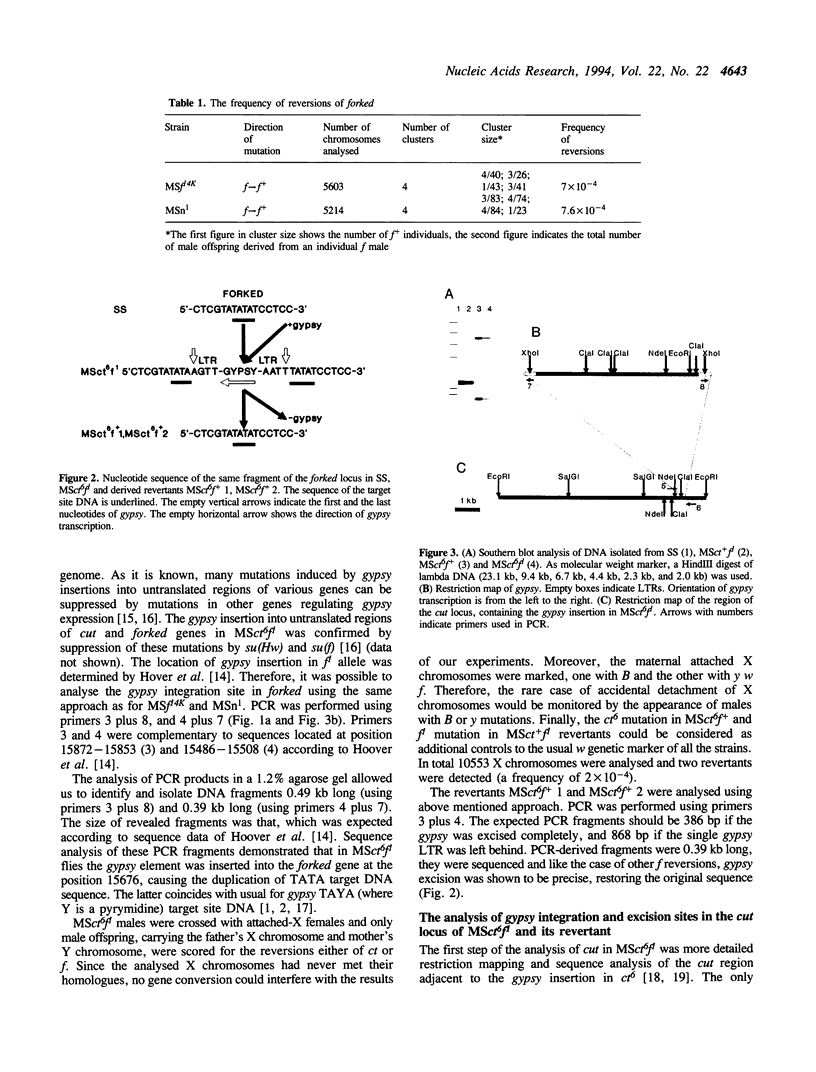
- Lyubomirskaya N. V., Avedisov S. N., Surkov S. A., Ilyin Y. V. Two Drosophila retrotransposon gypsy subfamilies differ in ability to produce new DNA copies via reverse transcription in Drosophila cultured cells. Nucleic Acids Res. 1993 Jul 11;21(14):3265–3268. doi: 10.1093/nar/21.14.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlor R. L., Parkhurst S. M., Corces V. G. The Drosophila melanogaster gypsy transposable element encodes putative gene products homologous to retroviral proteins. Mol Cell Biol. 1986 Apr;6(4):1129–1134. doi: 10.1128/mcb.6.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mizrokhi L. J., Obolenkova L. A., Priimägi A. F., Ilyin Y. V., Gerasimova T. I., Georgiev G. P. The nature of unstable insertion mutations and reversions in the locus cut of Drosophila melanogaster: molecular mechanism of transposition memory. EMBO J. 1985 Dec 30;4(13B):3781–3787. doi: 10.1002/j.1460-2075.1985.tb04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
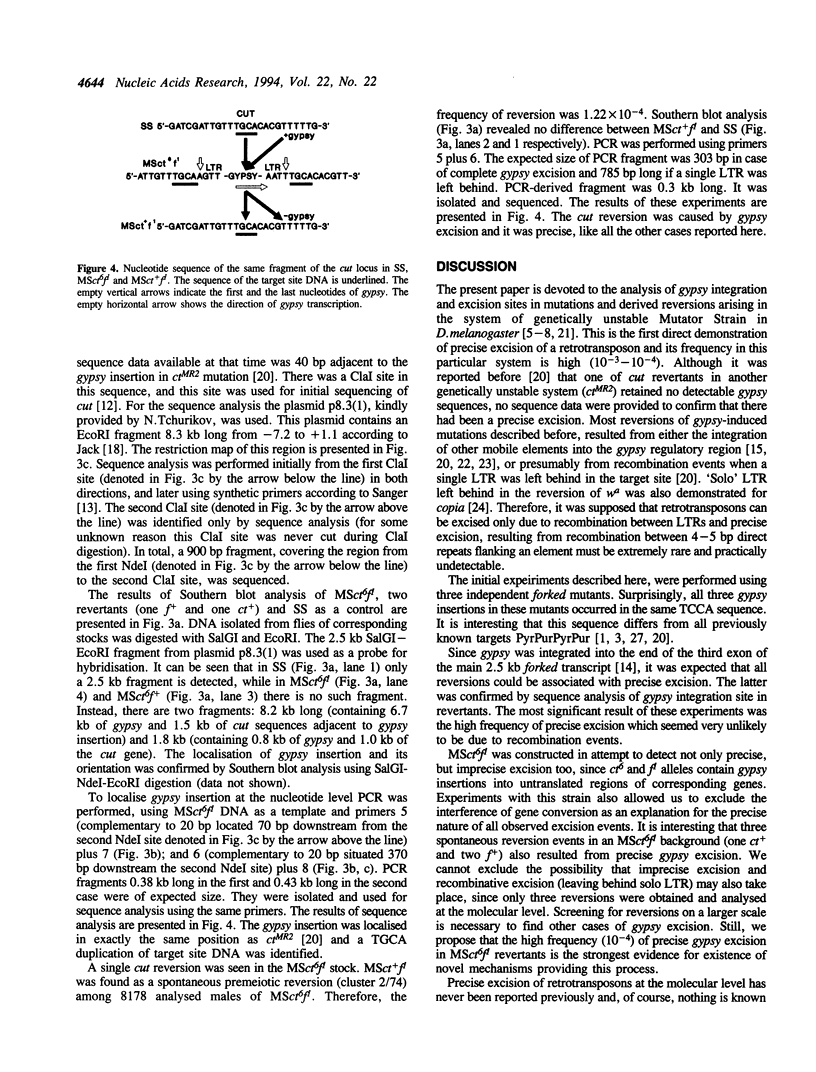
- Rutledge B. J., Mortin M. A., Schwarz E., Thierry-Mieg D., Meselson M. Genetic interactions of modifier genes and modifiable alleles in Drosophila melanogaster. Genetics. 1988 Jun;119(2):391–397. doi: 10.1093/genetics/119.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchurikov N. A., Gerasimova T. I., Johnson T. K., Barbakar N. I., Kenzior A. L., Georgiev G. P. Mobile elements and transposition events in the cut locus of Drosophila melanogaster. Mol Gen Genet. 1989 Oct;219(1-2):241–248. doi: 10.1007/BF00261183. [DOI] [PubMed] [Google Scholar]