Abstract
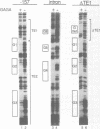
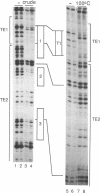
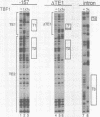
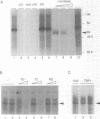
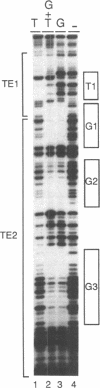
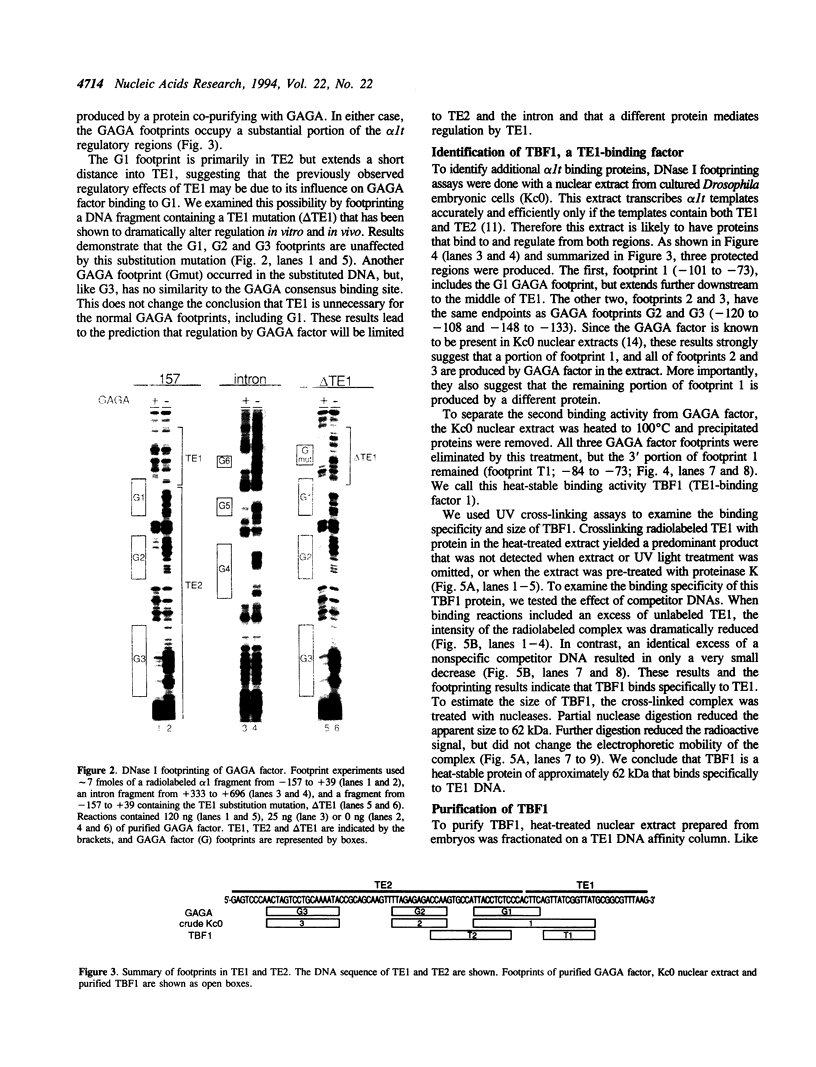
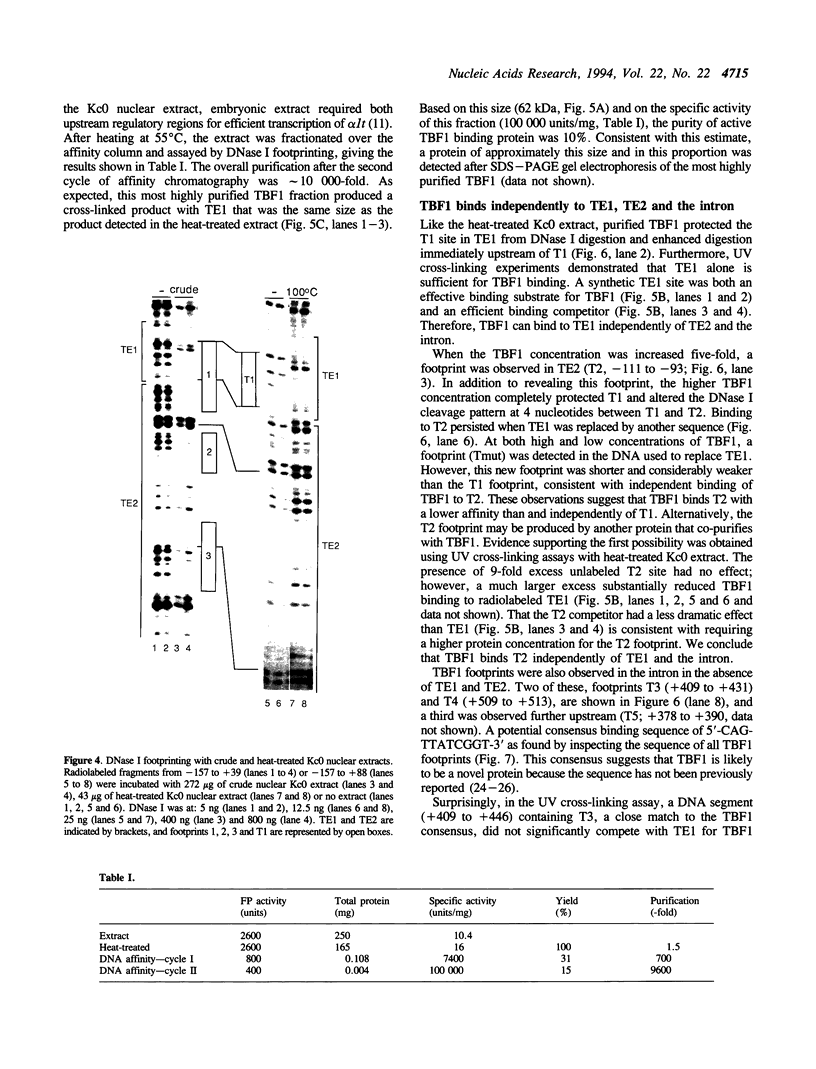
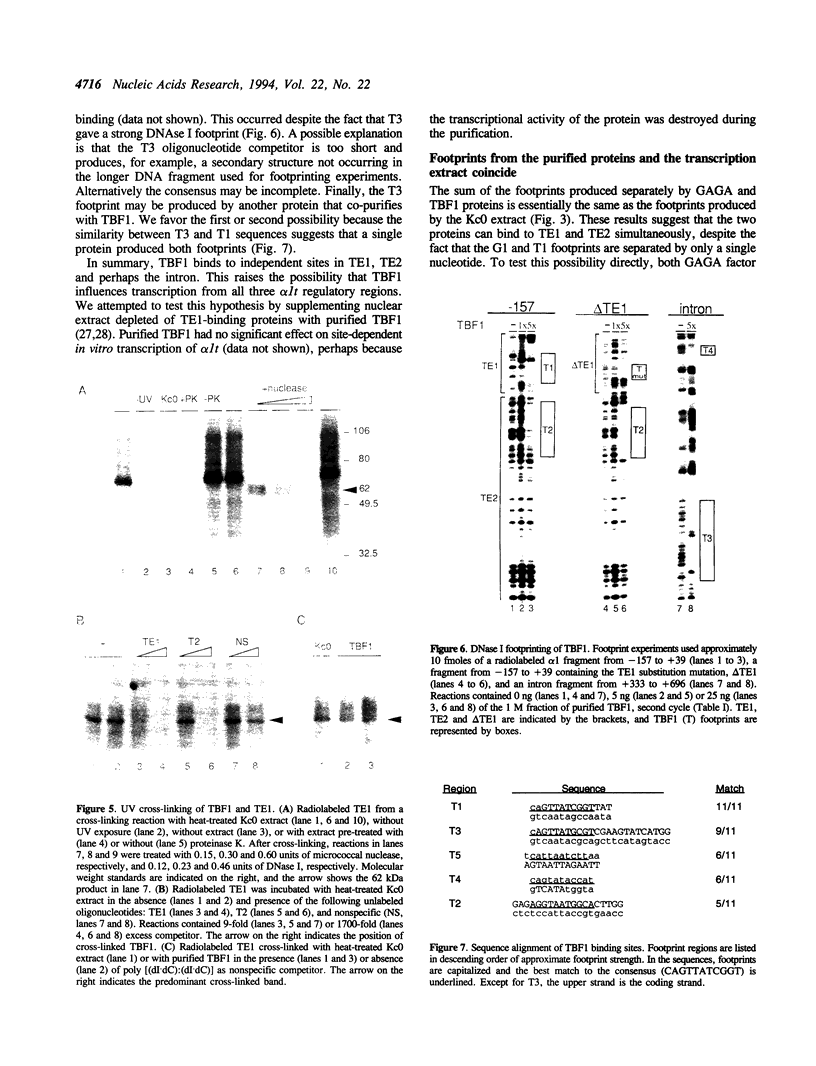
Three DNA regions (TE1, TE2 and the intron) regulate the ubiquitous expression of the alpha 1-tubulin gene of Drosophila melanogaster. In this report, we identify two proteins that bind these DNA regions. One is the previously characterized GAGA transcription factor and the other is a newly identified 62 kDa polypeptide, TBF1 (TE1-binding factor 1). Purified GAGA factor binds three sites in TE2 and at least three in the intron. TBF1 was purified from embryos and binds to both TE1 and TE2. Together, the two proteins produce the same DNase I footprints in TE1 and TE2 as does a nuclear extract that transcribes the gene accurately. These footprints cover most of the TE1 and TE2 DNA. Moreover, one binding site for each protein coincides with a site that activates transcription in vitro. The characteristics of the GAGA factor and the genes it regulates suggest roles these two proteins are likely to play in regulating ubiquitous expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Tjian R. Transcription factors and the control of Drosophila development. Trends Genet. 1989 Nov;5(11):377–383. doi: 10.1016/0168-9525(89)90173-x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Boyer T. G., Maquat L. E. Minimal sequence and factor requirements for the initiation of transcription from an atypical, TATATAA box-containing housekeeping promoter. J Biol Chem. 1990 Nov 25;265(33):20524–20532. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Coschigano K. T., Baker B. S., Wensink P. C. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991 Sep;10(9):2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M., Naik S., Johanning G. L., Ho L., Patel M. S. Multiple protein-binding domains and functional cis-elements in the 5'-flanking region of the human pyruvate dehydrogenase alpha-subunit gene. Biochemistry. 1993 Apr 27;32(16):4263–4269. doi: 10.1021/bi00067a014. [DOI] [PubMed] [Google Scholar]
- Chung Y. T., Keller E. B. Regulatory elements mediating transcription from the Drosophila melanogaster actin 5C proximal promoter. Mol Cell Biol. 1990 Jan;10(1):206–216. doi: 10.1128/mcb.10.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano K. T., Wensink P. C. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993 Jan;7(1):42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Dorn R., Szidonya J., Korge G., Sehnert M., Taubert H., Archoukieh E., Tschiersch B., Morawietz H., Wustmann G., Hoffmann G. P transposon-induced dominant enhancer mutations of position-effect variegation in Drosophila melanogaster. Genetics. 1993 Feb;133(2):279–290. doi: 10.1093/genetics/133.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. L., Thomas G. H., Siegfried E., Elgin S. C., Lis J. T. Optimal heat-induced expression of the Drosophila hsp26 gene requires a promoter sequence containing (CT)n.(GA)n repeats. J Mol Biol. 1990 Feb 20;211(4):751–761. doi: 10.1016/0022-2836(90)90075-W. [DOI] [PubMed] [Google Scholar]
- Heberlein U., England B., Tjian R. Characterization of Drosophila transcription factors that activate the tandem promoters of the alcohol dehydrogenase gene. Cell. 1985 Jul;41(3):965–977. doi: 10.1016/s0092-8674(85)80077-5. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Kerrigan L. A., Croston G. E., Lira L. M., Kadonaga J. T. Sequence-specific transcriptional antirepression of the Drosophila Krüppel gene by the GAGA factor. J Biol Chem. 1991 Jan 5;266(1):574–582. [PubMed] [Google Scholar]
- Laney J. D., Biggin M. D. zeste, a nonessential gene, potently activates Ultrabithorax transcription in the Drosophila embryo. Genes Dev. 1992 Aug;6(8):1531–1541. doi: 10.1101/gad.6.8.1531. [DOI] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science. 1992 Sep 18;257(5077):1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- Lee H., Kraus K. W., Wolfner M. F., Lis J. T. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 1992 Feb;6(2):284–295. doi: 10.1101/gad.6.2.284. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wallrath L. L., Allan B. D., Glaser R. L., Lis J. T., Elgin S. C. Promoter sequence containing (CT)n.(GA)n repeats is critical for the formation of the DNase I hypersensitive sites in the Drosophila hsp26 gene. J Mol Biol. 1992 Jun 20;225(4):985–998. doi: 10.1016/0022-2836(92)90099-6. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wallrath L. L., Granok H., Elgin S. C. (CT)n (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene. Mol Cell Biol. 1993 May;13(5):2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. C., Kim K. H. An enhancer element in the house-keeping promoter for acetyl-CoA carboxylase gene. Nucleic Acids Res. 1990 Jun 11;18(11):3249–3254. doi: 10.1093/nar/18.11.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F., Kaufman T. C. Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev Biol. 1989 Mar;132(1):45–61. doi: 10.1016/0012-1606(89)90203-0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Sutherland L. C., Adra C. N., Leclair B., Rudnicki M. A., Jardine K. The mouse Pgk-1 gene promoter contains an upstream activator sequence. Nucleic Acids Res. 1991 Oct 25;19(20):5755–5761. doi: 10.1093/nar/19.20.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. L., Farnham P. J. Transcription initiation from the dihydrofolate reductase promoter is positioned by HIP1 binding at the initiation site. Mol Cell Biol. 1990 Feb;10(2):653–661. doi: 10.1128/mcb.10.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzle J. E., McCarthy B. J. Regulation of Drosophila alpha- and beta-tubulin genes during development. Dev Biol. 1984 Jul;104(1):187–198. doi: 10.1016/0012-1606(84)90047-2. [DOI] [PubMed] [Google Scholar]
- O'Donnell K. H., Chen C. T., Wensink P. C. Insulating DNA directs ubiquitous transcription of the Drosophila melanogaster alpha 1-tubulin gene. Mol Cell Biol. 1994 Sep;14(9):6398–6408. doi: 10.1128/mcb.14.9.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988 Dec 20;7(13):4265–4273. doi: 10.1002/j.1460-2075.1988.tb03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. Postinitiation transcriptional control in Drosophila melanogaster. Mol Cell Biol. 1990 Nov;10(11):6041–6045. doi: 10.1128/mcb.10.11.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Oh C. E., Kornberg T. B. Isolation of cDNAs encoding the Drosophila GAGA transcription factor. Mol Cell Biol. 1993 Dec;13(12):7961–7970. doi: 10.1128/mcb.13.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Stapleton G., Somma M. P., Lavia P. Cell type-specific interactions of transcription factors with a housekeeping promoter in vivo. Nucleic Acids Res. 1993 May 25;21(10):2465–2471. doi: 10.1093/nar/21.10.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Becker P. B., Wu C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature. 1994 Feb 10;367(6463):525–532. doi: 10.1038/367525a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]