SUMMARY
Antigen presenting cell-associated four-domain MHC class-II molecules play a central role in activating autoreactive CD4+ T-cells involved in Multiple Sclerosis (MS) and Type 1 Diabetes (T1D). In contrast, two-domain MHC-II structures with the same covalently-attached self peptide (Recombinant T-cell receptor Ligands=RTLs) can regulate pathogenic CD4+ T-cells and reverse clinical signs of experimental autoimmune diseases. RTL1000, comprised of the β1α1 domains of HLA-DR2 linked to the encephalitogenic human MOG-35-55 peptide, was recently shown to be safe and well-tolerated in a Phase I clinical trial in MS. To evaluate the opposing biological effects of four- vs. two-domain class-II structures, we screened phage Fab antibodies (Abs) for neutralizing activity of RTL1000. . Five different TCR-like Abs were identified that could distinguish between the two- vs. four-domain MHC peptide complexes, while the cognate TCR was unable to make such a distinction. Moreover, Fab detection of native two-domain HLA-DR structures in human plasma implies that there are naturally-occurring regulatory MHC-peptide complexes. These results demonstrate for the first time distinct conformational determinants characteristic of activating vs. tolerogenic MHC-peptide complexes involved in human autoimmunity.
Keywords: Autoimmunity, Recombinant Antibodies, Immune tolerance, MHC class II
INTRODUCTION
A common basis for several autoimmune diseases, including Multiple Sclerosis (MS), Type 1 Diabetes (T1D) and Rheumatoid Arthritis (RA), is the strong linkage between HLA genotype and susceptibility to the disease [1–3]. While some alleles are tightly linked to certain diseases, others confer protection and are found extremely rarely in patients. This linkage is not surprising due to the involvement of T-cells in the progression of these diseases. Activation or disregulation of CD4+ T-cells directed to self organ-specific proteins, combined with yet-undefined events, may contribute to the pathogenesis of a variety of human autoimmune diseases.
Multiple sclerosis is an immune-mediated demyelinating and neurodegenerative disease of the central nervous system (CNS) [4]. Susceptibility to MS is associated with human leukocyte antigen (HLA) class II alleles, mostly the DR2 haplotype that includes the DRB1*1501, DRB5*0101, and DQB1*0602 genes [5]. DRB1*1501 is a well-studied risk factor of MS that occurs in about 60% of Caucasian MS patients vs. 25% of healthy controls. Contribution of these risk factors to disease process likely involves presentation of self antigens by disease-associated MHC expressed on antigen presenting cells (APC) that activate T-cell-mediated CNS inflammation. Suspected MS autoantigens include myelin proteins such as myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG). T-cells from MS patients were found to predominantly recognize MOG [6,7] as well as other myelin proteins, and the MOG-35-55 peptide was found to be highly encephalitogenic in rodents and monkeys [8,9] and induces severe chronic EAE in HLA-DRB1*1501-Tg mice [10].
Type 1 Diabetes involves progressive destruction of pancreatic beta-cells by autoreactive T-cells specific for antigens expressed in the pancreatic islets, including glutamic acid decarboxylase (GAD65) [11]. GAD65 is a suspected islet autoantigen in T1D, stimulating both humoral and cellular self reactivity in at-risk and diseased subjects. Antibodies to GAD65 in combination with antibodies directed at two additional islet autoantigens are predictive markers of T1D in at-risk subjects [12], and GAD-555-567 peptide has identical sequence in all GAD isoforms in human and mouse. This highly immunogenic determinant was found to be a naturally processed T-cell epitope both in disease-associated-HLA-DR4(*0401)-Tg-mice [13] and human T1D subjects [14,15].
Antigen-specific activation or regulation of CD4 T-cells is multistep process where co-ligation of the T-cell receptor (TCR) with complexes of MHC II/peptide on the surface of APC plays a central role. Full activation through the TCR of CD4+ T-cells requires co-stimulation of additional T-cell surface molecules such as CD4, CD28 and CD40, whereas absence of co-stimulation may lead to anergy, a state of unresponsiveness of the T-cells to their presented antigen [16,17]. Recombinant T-cell receptor Ligands (RTLs) are soluble two-domain MHC class II constructs with covalently attached antigenic peptides that can bind selectively to the TCR in the absence of co-stimulation [18] and induce specific immunological tolerance in pathogenic CD4+ inflammatory T-cells [19,20]. RTLs constructed with different combinations of MHC class II β1α1 domains and potentially pathogenic peptides can reverse clinical and histopathological signs of disease in animal models of multiple sclerosis [21,22], uveitis [23], arthritis [24] and stroke [25], and the RTL1000 construct (DR2/MOG-35-55) has been tested successfully in a Phase I clinical trial in MS.
We reported previously on the generation of a family of recombinant Fabs with peptide-specific, MHC class I allele-restricted specificity for a wide panel of tumor and viral derived T-cell epitopes [26–31]. These molecules, termed TCR-like (TCRL)-Fabs, were isolated by screening large Ab phage libraries. Here, we report the isolation and characterization of TCRL-Fabs directed at self MHC II/peptide complexes associated with autoimmunity. Surprisingly, a panel of Fabs selected to the DR2/MOG-35-55 specificity of RTL1000 distinguished RTL1000 from the native conformation of DR2/MOG-35-55 complexes presented by APC. In addition, Fabs directed at either two-domain RTLs or native four-domain DR4/GAD-555-567 complexes recognized the cognate structures but failed to react with the non-cognate complexes. These two novel groups of TCRL-Fabs confirm conformational differences between the two structures. Moreover, our TCRL-Fabs distinguished opposing functionalities of stimulatory four-domain vs. tolerogenic two-domain MHCII peptide complexes in autoimmune inflammation. Although our previous studies could not discern the mechanistic basis for altered T cell activation induced with two- versus four-domain MHC/peptide combinations, the current data describing distinct conformations of two- vs. four-domain forms of MHC class II represents a major conceptual advance in explaining these important functional differences.
By using a Fab specific for the two-domain conformation of HLA-DR, we were able to detect similar novel structures in human serum/plasma. We demonstrated the in vivo functionality of our TCRL-Fabs directed at the two-domain RTL structure by their ability to neutralize the RTL1000 treatment of EAE. Therefore, the TCRL-Fabs directed at the two-domain RTL structure represent a valuable tool to study Ag-specific therapeutic mechanisms and to study the appearance of the yet-uncharacterized partial MHC class II structures in human serum and plasma. Conversely, our TCRL-Fabs directed at native four-domain MHC class II/peptide complexes will enable the study of specific self-antigen presentation by MHC class II during autoimmunity.
RESULTS
Characterization of biotinylated RTLs
In our previous report, human RTLs were found to have a secondary structure composition similar to the TCR recognition/peptide-binding α1β1 domain of native human MHC class II [18,19]. In order to select for TCR-like Abs we generated biotinylated versions of HLA-DR2 derived RTLs, RTL1000 (DR2/MOG-35-55) and RTL340 (DR2/MBP-85-99). These constructs were produced by in vitro refolding of purified inclusion bodies and were found to be very pure, homogenous, and monomeric by SDS-PAGE and size exclusion chromatography analyses (Figure 1A). HLA-DR2 (DRA1*0101, DRB1*1501) contains a disulfide bond between conserved cysteines in the β1 domain (residues 15 and 79 of the DR-B chain) [32]. The formation of this native conserved disulfide bond within the RTL molecule was verified by gel-shift assay (Figure 1B). SDS-PAGE analyzes of reduced and non-reduced RTL1000 samples revealed that the non-reduced sample had a smaller apparent molecular weight, indicating the presence of an internal disulfide bond leading to a more compact structure. High biotinylation levels are essential for a successful screening of the desired Abs using our phage display screening strategy. The RTL constructs were found to have high biotinylation levels, identical to the compared 100% biotinylated MBP standard (Figure 1C).
Figure 1. RTL1000 is highly purified, monomeric, biotinylated and biologically active.
A) Purified RTL1000 was analyzed by Size Exclusion Chromatography and detected by absorbance at 280nm. * Indicates elution of known molecular weight proteins (43, 29, 13.7, 6.5kD)with increasing retention volumes. Insert: RTL1000 sample was analyzed by SDS-polyacrylamide gel electrophoresis (left lane) and compared to MW standards (right lane). B) Samples of RTLs with or without β-mercaptoethanol, analyzed by SDS-polyacrylamide gel. C) Biotinylated RTL1000 and 100% biotinylated MBP standard were separated by SDS-PAGE, blotted and stained with HRP-conjugated streptavidin. D) Inhibition of the MOG-35-55-specific response of H2-1 T-cell hybridoma by RTL1000. H2-1 cells were pre-incubated with RTL1000, RTL340 or medium alone before their Ag-specific activation with DR*1501 APC pulsed with MOG-35-55 peptide compared to medium alone. CTLL proliferation (triplicate mean of 3H-Tdy uptake) differences between the samples pre-incubated with RTL1000 and medium alone were evaluated using two-tailed t-test. Data in A–D are representative of at least three independent experiments.
In previous reports, RTLs were found to deliver peptide-specific rudimentary signals through the TCR of human Th1 cells [19] and a murine T-cell hybridoma [20]. We verified the interaction of biotinylated RTL1000 with the cognate TCR of H2-1 T-cell hybridoma specific for the DR2/MOG-35-55 complex. As shown in Figure 1D, MOG-35-55 specific activation of H2-1 hybridoma was inhibited by pre-incubation of H2-1 with RTL1000. Control RTL340 (DR2/MBP-85-99) did not inhibit this antigen-specific response, indicating selective RTL1000 ligation of the TCR leading to inhibitory signaling. We conclude that the RTL1000 construct mimics the minimal MHC II domains necessary for specific interaction with the TCR and therefore it was used as a soluble recombinant protein for the selection of Abs directed to the α1β1 DR2/MOG-35-55 T-cell epitope in a TCR-like fashion.
Isolation of recombinant Abs with TCR-like specificity toward RTL1000
For selection of TCRL Abs directed to MHC class II, we used a strategy of screening a large Ab phage library consisting of a repertoire of 3.7 × 1010 human recombinant Fab fragments [33]. RTL1000 was used as a minimal DR2/MOG-35-55 complex recognized by autoreactive T-cells. We applied the library to panning on soluble RTL1000. Seven hundred-fold enrichment in phage titer was observed following four |rounds of panning. The specificity of the selected phage Abs was determined by ELISA comparison of streptavidin-coated wells incubated with biotinylated RTL1000 (DR2/MOG-35-55) or RTL340 (DR2/MBP-85-99) (Figure 2A). Fab clones with peptide-dependent, MHC-restricted binding were picked for further characterization. DNA fingerprinting, by BstNI restriction reaction, revealed 23 different restriction patterns of MOG peptide-dependent DR2 specific Fabs, indicating the selection of several different Fabs with this unique specificity.
Figure 2. Specificity of recombinant Fab Ab phage clones selected on DR2/MOG-35-55 complexes (RTL1000).
A) Representative supernatant ELISA of Fab clones selected against RTL1000. The boxed areas and arrows indicate Fabs that specifically bind to the DR2/MOG-35-55 complex but not to a control DR2 complex containing the DR2-restricted MBP peptide (RTL340). These clones were picked for further characterization. B) ELISA binding of soluble purified Fabs to immobilized DR2/MOG-35-55 complex in the presence of various concentrations of the following competitors: DR2/MOG-35-55, DR2/MBP-85-99, MOG-35-55 peptide, or MBP-85-99 peptide. Data are representative of three independent experiments C) ELISA binding of the indicated soluble purified Fabs and anti-MHC II mAb TU39 (BD) to immobilized DR2/MOG-35-55, control complexes, and MOG-35-55 peptide. Data are representative of four independent experiments
Specificity and affinity of TCR-like Fabs reactive with RTL1000
We used E.coli cells to produce a soluble Fab form of a representative clone of each DNA restriction pattern. The specificity of the selected clones was characterized in a competition ELISA binding assay. Binding of the Fabs to the immobilized RTL1000 complex was competed with a soluble RTL1000, control RTL340 (DR2/MBP-85-99) or with free MOG-35-55 peptide alone. By this assay we were able to verify the binding of the Fabs to soluble DR/peptide complexes and to exclude a conformational distortion by direct binding to plastic. As shown in Figure 2B for two representative Fabs (2E4 and 2C3), neither RTL340 nor MOG-35-55 peptide alone could compete the Fab binding to immobilized RTL1000. By performing this assay we were able to discriminate between Fabs that bind soluble MOG-35-55 peptide (represented by 2B4) and those that bind a portion of this peptide when bound to two-domain DR2 molecules in a TCR-like fashion. Figure 2C presents five different Fabs that were found to have a DR2 restricted MOG-35-55 specific TCR-like reactivity to RTL1000. These Fabs were tested in an ELISA binding assay and were found to bind only RTL1000 and not the controls, RTL340, RTL302-5D (empty HLA-DR-derived RTL), or free MOG-35-55 peptide. Fab 1B11 was isolated and found to bind all HLA-DR-derived RTLs with no peptide-specificity and dependency. Commercially available TU39 anti-MHC class II mAb (BD Pharmingen) that binds a conserved determinant at the alpha1 domain was used to verify identical quantities of the different complexes that were compared. This DNA sequencing confirmed the selection of five different clones directed specifically to the RTL1000 complex (Table I). The affinities of the Fabs to RTL1000 were measured and analyzed by a Surface Plasmon Resonance (SPR) biosensor (ProteOn™ XPR36 ,Bio-Rad Laboratories) and found to be in the range of 30–150nM (Table I).
Table I.
CDR sequencing and affinities of the anti-RTL1000 TCRL Fab Abs.
| Variable L chain | Variable H chain | ||||||
|---|---|---|---|---|---|---|---|
| Name | Affinity (nM) |
CDR1 | CDR2 | CDR3 | CDR1 | CDR2 | CDR3 |
| 2E4 | 33 | RASQSVSSYLA | DASNRAT | QQRSNWPPSYT | GYTFTSYYMH | IINPSGGSTSYAQKFQ | EGDNYYGDAFDI |
| 1F11 | 60 | RASQSIINSHLA | GASSRAT | QQYGTSPLT | GVSISSRSGHWG | SISYSGSTYYNPSLKS | ESHPAAALVG |
| 3A3 | 58 | RASQVISSWLA | TASSLQS | QQANSFPLT | GFTFSSYSMN | SISSSSSYIYYADSVKG | VRGHRYYYDSSGYYSSDYYYYYGMDV |
| 3H5 | 129 | RSSQSLLHSNGNNYLD | LGSNRAS | MQALQTPLT | GGSISGYYWS | YIYYSGSTNYNPSLKS | DERDAYYYGMDV |
| 2C3 | 153 | RSSQSLLHSDGNNYLD | LGSNRAS | MQALHIPLT | GFTFSSYAMH | VISYDGSNKYYADSVKG | DRSFWSGYYIINYYYYGMDV |
Fine specificity of anti-two-domain DR2/MOG-35-55 TCRL Fabs
To analyze the fine specificity of our Fabs, we tested their recognition of RTL342m-two-domain DR2 complex with mouse MOG-35-55 peptide. Mouse (m)MOG-35-55 peptide carries a 42Pro->Ser substitution compared to human (h)MOG-35-55. This single amino-acid substitution altered the recognition of all the 5 anti-RTL1000 Fabs as detected by ELISA binding (Figure 3A). Fabs 2C3 and 3H5 completely lost their detected binding to the altered complex. Reduction in the binding of the Fabs to RTL342m compared to RTL1000 was obtained for 1F11 and 3A3 (5-fold) and 2E4 (2 fold). The dependence of reactivity of these selected Fabs on this 42Pro anchor residue implies a unique peptide conformation in the context of the HLA-DR2 α1β1 domains. In addition, none of the Fabs reacted with the mMOG-35-55 in the context of the murine allele I-Ab (RTL551) (Figure 3A), emphasizing the TCR-like requirement of the Fabs to the cognate peptide within the MHC allele.
Figure 3. Fine specificity of anti-RTL1000 TCRLs.
A) TCRLs selected to DR2/hMOG-35-55 complex (RTL1000) distinguish it from the DR2/mMOG-35-55 complex (RTL342m). ELISA binding assay of the indicated Fabs to RTL1000, RTL342m and RTL551. Data are representative of three independent experiments. B) ELISA binding assay comparing Fab binding to empty DR2-RTL alone or loaded with MOG-35-55 peptide. Data are representative of two independent experiments.
To exclude reactivity of the Fabs with the linker attaching the MOG-35-55 peptide to the RTL construct, we tested their binding to empty DR2 derived RTL (RTL302) loaded with free MOG-35-55 peptide. All the Fabs kept their peptide-specific, MHC restricted binding to the MOG-35-55 loaded empty RTL302 (Figure 3B), excluding any binding-dependence to non-native sequences of RTL1000. Additionally, we tested Fab binding to RTL1000 in different buffer conditions and found the Fabs to be conformationally sensitive, losing their ability to react with denatured RTL1000 (Supplementary Figure 1). Taken together, these data indicate selective Fab binding to the α1β1 DR2/MOG-35-55 native sequence of the folded RTL1000.
Conformational difference between RTL and full-length MHC II molecule
We next tested the ability of the anti-RTL1000 Fabs to bind the native full length four-domain form of MHC II complexes as expressed on APCs. L-cell DR*1501 transfectants (L466.1 cells) were loaded with MOG-35-55 or control peptide. The loaded cells were incubated with the purified Fabs following anti-Fab-FITC incubation. As shown in Figure 4A, no specific binding of Fabs was observed for MOG-35-55 loaded cells. MOG-35-55 and control peptide loaded cells produced the same fluorescence intensity as background. MHC expression on the APC surface was confirmed by anti-DR mAb (L243, BD). A portion of the loaded cells that were used for the FACS analysis was incubated with H2-1 T-cell hybridoma specific for the DR2/MOG-35-55 complex. Following 72h incubation, cell supernatants were transferred to IL-2-dependent CTLL cells for detection of IL-2 levels secreted from the H2-1 hybridoma (Figure 4B). H2-1 cells were activated only by the MOG-35-55 pulsed cells, secreting 8-fold higher levels of IL-2 compared to non-pulsed or control peptide-pulsed APCs. Peptide-specific H2-1 activation confirmed a successful loading of MOG-35-55 peptide to the native MHC on the APCs used for the FACS analysis. Despite the presence of a biologically active determinant in the form of DR2/MOG-35-55 molecules presented by the APCs, no staining of such complex was obtained by any of our anti RTL1000 Fabs. Considering the high affinity of the selected Fabs and the permissive conditions used for this experiment, we conclude that the Fabs do not bind the native DR2/MOG-35-55 complex presented by APCs. Further support for this finding came from blocking experiments which tested the Fabs ability to inhibit peptide-specific activation of the H2-1 hybridoma by DR2 APCs pulsed with MOG-35-55 peptide (Figure 4C). None of our selected Fabs were able to block this peptide-specific, MHC restricted activation, as compared to a control TCRL Fab (D2) specific for RTL2010 (DR4/GAD-555-567) that also failed to block H2-1 activation. In contrast, complete blocking was achieved by the control anti-MHC class II mAb (TU39, BD). The failure of the Fabs to interfere with MHC presentation to TCR implies an inability to bind native four domain DR2/MOG-35-55 complexes. This was indeed the case, as demonstrated by ELISA (Figure 4D). Altogether, these data |strongly suggest that there are conformational differences between two- vs. four-domain forms of HLA-DR2 loaded with the MOG-35-55 peptide.
Figure 4. Distinct conformations between two- vs. four-domain DR2/MOG-35-55 complexes revealed by anti-RTL1000 TCRLs.
DR2 APCs (L466.1 DR*1501 L cell transfectants) were pulsed with MOG-35-55 or MBP-85-99 peptides and tested for (A) the indicated Fab staining by FACS, or (B) activation of the H2-1 MOG-35-55 specific T-cell hybridoma. C) Ag-specific activation of H2-1 MOG-35-55 specific T-cell hybridoma was not affected by the anti-RTL1000 Fabs, as demonstrated by detection of IL-2 secreted (3H-Tdy uptake by CTLL cells incubated with cell supernatants) from the H2-1 T-cell hybridoma activated by DR2*1501 APC in the presence of anti-RTL1000 Fab Abs and control Fab (D2) compared to the inhibitory anti-MHC II Ab (TU39, BD). D) Binding of anti-RTL1000 Fabs and anti-MHC II (TU39, BD) to immobilized RTL1000 and full length recombinant DR2/MOG-35-55 complexes as detected by ELISA. Note lack of reactivity of Fabs to MOG peptide-loaded four-domain DR2 complexes. Data in A–D are representative of at least three independent experiments.
Reversal of RTL342m treatment of EAE in DR2 Tg mice
To further test the functional attributes of Fab specific for the two-domain RTL1000, we utilized an Fab specific for RTL1000 that was also cross-reactive with a similar antigenic determinant on RTL342m (α1β1 domains of DR2 linked to mouse (m)MOG-35-55 peptide). DR2 Tg mice were immunized with mMOG-35-55 peptide/CFA/Ptx to induce EAE and were treated with pre-formed complexes of 2E4 Fab:RTL342m, the control D2 Fab:RTL342m (specific for RTL2010 comprised of DR4/GAD-555-567 described below) or TRIS buffer (Figure 5).
Figure 5. Fab 2E4 specific for RTL342m (β1α1DR2/mMOG-35-55) neutralized RTL342m treatment of EAE in DR2*1501 mice in a dose dependent manner.
A) Fab 2E4 or control Fab D2 were incubated in vitro for 2 h at room temperature at 2:1 and 1:1 molar ratios with 20µg RTL342m and injected subcutaneously daily for 3 days (arrows) into DR2 mice with clinical signs of EAE (scores > 2.0) induced by mMOG-35-55 peptide/CFA/Ptx. EAE was measured over time. B) Differences between the Cumulative Disease Indices of the experimental groups in (A) over the 14 day observation period were evaluated using the Mann-Whitney test. *p<0.05; **p<0.01; ***p<0.001.
As is shown in Figure 5, mice receiving RTL342m plus TRIS buffer were effectively treated, whereas a 2:1 ratio of 2E4 Fab:RTL342m almost completely neutralized the RTL therapeutic effect on EAE. In contrast, a 1:1 ratio of Fab:RTL342m had less neutralizing activity as assessed by daily EAE scores (Fig 5A) and by the entire experimental effect on EAE for each group as assessed by the Cumulative Disease Index (CDI) (Fig 5B). Importantly, D2 Fab (also used at a 2:1 ratio) did not neutralize the therapeutic effect of RTL342m on EAE, indicating specificity of the 2E4 Fab for the two-domain RTL342m.
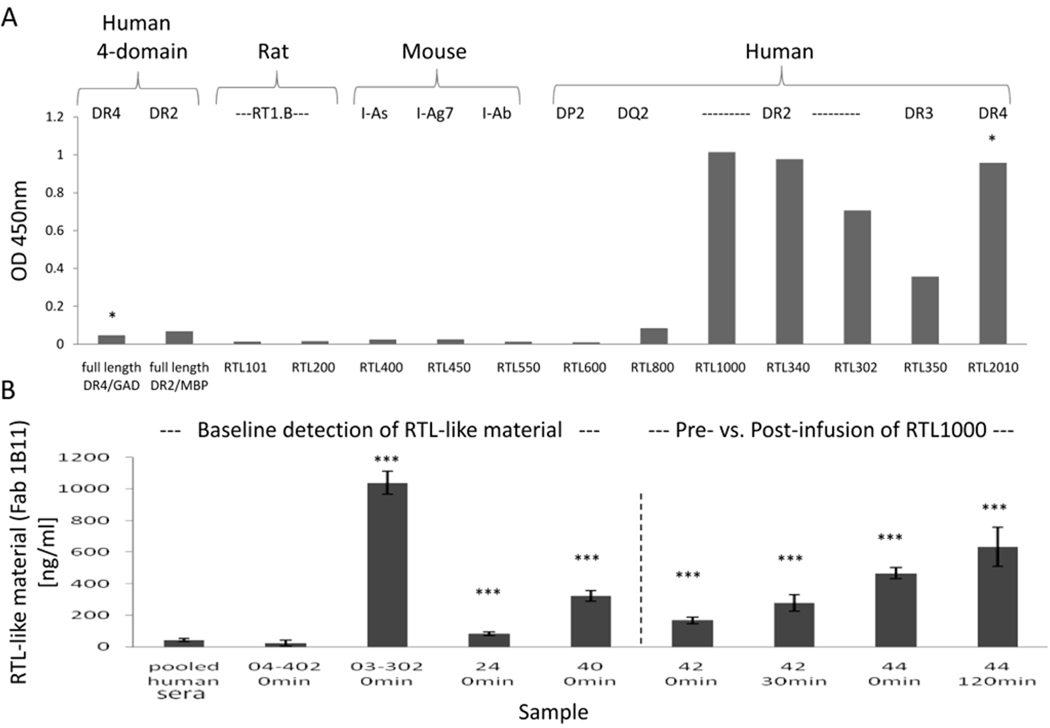
Detection of natural RTL-like two-domain MHC class II molecules in human plasma In a recent Phase I safety study in DR2+ MS subjects [34] to be treated with RTL1000 or placebo, we observed detectable baseline plasma levels of two-domain RTL-like structures in 4 of 13 donors (31%). This observation suggested the natural occurrence of two-domain structures that could be derived from four-domain intermediates possibly shed from class II expressing APC upon immunization. Using the power of our conformationally sensitive Fabs, we evaluated the appearance and persistence of naturally occurring two domain MHC class II structures in human MS subjects. Fab 1B11 is specific for two-domain HLA-DR-conformation. It was found to bind to all HLA-DR-derived RTLs (with no peptide specificity), but not to other human and murine allele-derived RTLs or four-domain HLA-DR molecules (Figure 6A). Serum or plasma samples were diluted 1:10 and adsorbed onto plastic wells pre-coated with the TU39 mAb (that detects all forms of MHC), washed and reacted with 1B11 Fab specific for HLA-DR-derived RTLs, followed by addition of enzyme-labeled anti-Fab and substrate for ELISA detection. As is shown in Figure 6B, the 1B11 Fab detected RTL-like material in serum or plasma from the healthy control pool as well as all six MS subjects tested at baseline, with detected levels of protein ranging from 13ng/ml to 1,100 ng/ml. These results indicate for the first time on the existence of soluble serum MHC class II structures with a distinct RTL-like conformation that differ from the classical membrane-bound MHC conformation. Increased signal for two-domain class II was also observed in Subject #42 after 30 minutes of infusion of 200mg RTL1000 and in Subject #44 after 2 hours of infusion of 100mg RTL1000, consistent with increased levels of injected RTL1000.
Figure 6. Detection of natural RTL-like two-domain MHC class II molecules in MS subject serum and plasma samples.
A) ELISA binding assay of purified 1B11 Fab to immobilized RTLs and four-domain recombinant MHC complexes. * indicates recombinant complexes that were compared only with RTL1000. Data are representative of three independent experiments B) Fab 1B11 detected RTL-like MHC class II material in serum and plasma samples from MS subjects and a pool of 3 healthy controls. Serum or plasma was collected prior to infusion of RTL1000 from MS Subjects #03–302, #04–402, #24, #40, #42 & #44 (0 time), from Subject #42 at 30min after initiating infusion of 200mg RTL1000; and #44 at 120min after initiating infusion of 100mg RTL1000. Differences between the samples and background were evaluated using two-tailed unpaired t-test. *p<0.05; **p<0.01; ***p<0.001.
Data are representative mean + SD of at least three independent experiments..
Detection of RTL1000 in plasma of treated MS subjects
In order to detect injected RTL1000 in serum and plasma samples of MS subjects treated with RTL1000 and to discriminate it from the native RTL-like structures obtained by Fab 1B11, we used Fab 2E4 which binds RTL1000 in a MOG-35-55 peptide-specific, DR2-restricted manner. As indicated in Figure 7A, 2E4 Fab successfully detected RTL1000 in plasma samples of MS subjects post-RTL1000 infusion (samples #42 at 30min and #44 at 120min) while the pre-infusion samples (#04–402, #03–302, #24, #40, #42, and #44 at 0min) and the pooled healthy human serum kept low background signal levels. The increase of the 1B11 Fab signal in the post- vs. pre-RTL1000 infusion samples is consistent with the detection of serum RTL1000 in the post-infusion samples by Fab 2E4. The combined Fab data strongly support the presence of other peptide-specificities of native two-domain structures in the serum/plasma samples and the high utility of our Fabs for such a sensitive and specific detection. Figure 7B demonstrates the utility of 2E4 Fab for pharmacokinetic (PK) studies of RTL1000 infusion. RTL1000 levels in plasma of DR2+ MS subject #42 were measured during 120min of RTL1000 infusion and during the following 60min. Results from this PK study verified a previously determined half-life of RTL1000 in plasma as ~5min [34].
Figure 7. Detection of RTL1000 in plasma of treated MS subjects.
A) Fab 2E4 detected RTL1000 in plasma samples from MS Subjects (#04–402, #03–302, #24, #40, # 42 and #44) before (0 min) and after infusion of the drug and circulating RTL1000 levels were discriminated from native RTL-like material by Fab 1B11. Differences between pre- and post-infusion samples of each subject were evaluated using two-tailed paired t-test. Right panel: Standard curves of various concentrations of RTL1000 and RTL340 that were used for calculation of RTL and RTL-like material concentrations in serum and plasma samples. The minimal thresholds for RTL detection were 12ng/ml for Fab2E4 and 0.1ng/ml for Fab1B11. B) Fab 2E4 detection of RTL1000 by ELISA in plasma of MS subject #42 collected during 120min of infusion of the drug and the following 60 min. Time from the beginning of RTL1000 infusion and the time after completion of the infusion are indicated by brackets. The completion of RTL1000 infusion is indicated by a dashed line. Differences between pre- and post-infusion samples of each subject were evaluated using two-tailed paired t-test. Data in A–B are representative mean + SD of at least three independent experiments. *p<0.05; **p<0.01; ***p<0.001.
Isolation of recombinant Abs with TCR-like specificity toward RTL and the native DR4/GAD-555-567 complex
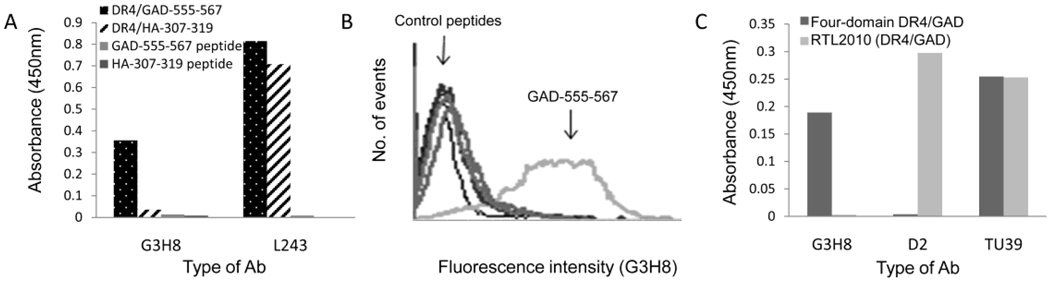
We expanded our TCRL repertoire toward the DR4/GAD-555-567 complex associated with autoimmune response during the course of type I diabetes. Similar to the isolation of anti-RTL1000 TCRLs described above, we constructed DR4/GAD RTL molecules and isolated a TCRL Fab, named D2, which is specific for the DR4/GAD RTL2010 in a GAD-peptide dependent, DR4-restricted manner. D2 failed to react with four-domain DR4/GAD-555-567 complexes, both as recombinant protein (Figure 8C) and as native complexes presented by APCs (Supplementary Figure 2). Thus, similar to anti-RTL1000 TCRLs, D2 identified a distinct conformational difference between the two domain RTL structure vs. the four domain native MHC/peptide.
Figure 8. Binding characterization of G3H8 and D2 Fabs.
A) ELISA of purified anti-G3H8 with immobilized DR4/GAD-555-567, control complex DR4/HA-307-319, GAD-555-567 peptide, and HA-307-319 peptide. Anti-DR mAb (L243) was used to determine the correct conformation and stability of the bound complexes during the binding assay. B) Flow cytometry analysis of Fab G3H8 binding to Preiss APCs pulsed with GAD-555-567 peptide or the control peptides: InsA-1-15, CII-261-273, and HA-307-319. C) ELISA binding of anti-RTL2010 (DR4/GAD-555-567) TCRL (D2), anti-full-length DR4/GAD-555-567 TCRL (G3H8) and anti-MHC II (TU39, BD) to immobilized RTL2010 and full length DR4/GAD-555-567 complexes. Data in A–C are representative of at least three independent experiments.
For the isolation of TCRLs directed to the native MHC/peptide complexes, we applied our phage display strategy directed to recombinant full-length DR4/GAD-555-567 peptide. Four different TCRL Fab Abs were isolated and found to bind solely to recombinant full length DR4/GAD-555-567 complexes and not to DR4 complexes with control peptides, or to the GAD-555-567 peptide alone (Figure 8A, for representative G3H8 Fab). Additionally, these TCRLs successfully detected native DR4/GAD-555-567 complexes presented by EBV transformed DR4+ B cells (Figure 8B for representative G3H8 Fab) and a variety of APC populations in PBMCs from a DR4+ donor (Manuscript in preparation). Of importance, G3H8 Fab did not recognize the DR4/GAD-555-567 derived RTL2010 in an ELISA binding assay (Figure 8C). By using these two novel distinct TCRL Fab groups, we have thus detected unique conformational differences between the two- and four-domain MHC versions of the DR4/GAD complexes. These findings are consistent with the conformational data demonstrated for the DR2 MHC II complexes and support our uniform novel concept of opposing functionalities derived from distinct conformations of MHC II/peptide structures.
DISCUSSION
In this study we have shown the ability to select, from a large non-immune repertoire of human Fab fragments, a panel of recombinant antibodies with TCR-like specificity directed to auto-reactive T-cell epitopes in the form of self peptide presented by MHC class II. Abs directed to MHC II/peptide complexes have been generated before, using epitope-specific immunization as the initial step for further conventional hybridoma technology or construction of a phage display library [35–39]. We report here, for the first time, the generation of MHC II/peptide TCRL Fabs from a naïve human Ab library. Moreover, due to the large size of our phage display library, we were able to isolate several different Fabs directed to each targeted MHC II peptide complex. Based on our successful experience in the generation of MHC I/peptide TCRLs and the current data, we believe that the described method can be duplicated for a relative rapid generation of TCRL Fabs directed to other MHC II/peptide complexes.
We isolated five different TCRL Fab clones directed to the minimal two-domain DR2/MOG-35-55 (RTL1000) complex . Characterization of these Fabs indicated a requirement for both DR2 and MOG-35-55 peptide for recognition. The Fabs could further discern conformational differences in the P42S variant of DR2-bound MOG-35-55 peptide present in RTL342m, demonstrating individual variation in binding to specific contact residues within the DR2/MOG-35-55 complex. Moreover, cross-recognition of RTL342m by the 2E4 Fab allowed neutralization of RTL treatment of mMOG-35-55 induced EAE, illustrating the functional activity of this highly characterized Fab in vivo. These Abs therefore mimic the fine specificity of TCRs with the advantages of high-affinity and stable characteristics of the recombinant Fab fragment.
Our TCRLs exhibited high structural sensitivity while firmly distinguishing two- vs. four-domain MHC II/peptide complexes. None of the anti-RTL1000 TCRL Fabs were able to recognize four-domain DR2/MOG-35-55 presented by APC or in a recombinant form. Similarly, two panels of TCRL Fabs directed to two- or four-domain DR4/GAD-555-567 complexes clearly distinguished these two conformational MHC II peptide determinants.
While our previous bio-physical and biochemical data suggest a similar secondary structure content for the RTL constructs and the peptide binding domains of native MHC, our novel TCRL Fabs have identified distinct conformational differences between MHC II/peptide and RTL/peptide complexes. This novel finding suggests that autoreactive four vs. two domain MHC class II TCR ligands have distinct conformational shapes that can be distinguished by human Fab molecules and that apparently confer opposing immunological functions (peptide-specific T cell activation vs. tolerance). These data establish a new conformational-based concept that explains the opposing regulatory function of the two-domain MHC II structures compared to their cognate four-domain MHC class II complexes. This concept is of fundamental importance for understanding immunological tolerance, since it implies that the distinct shape of class II complexes formed by truncated two-domain structures may provide a natural tolerogen for regulating inflammatory T cells selected originally on four-domain structures.
We have characterized specific interactions of both RTL1000 and four-domain DR2/MOG-35-55 with the cognate TCR present on the H2-1 T-cell hybridoma. The ability of defined TCR to bind these two TCRLs-distinguished conformational MHC II complexes highlights the permissive nature of the TCR as compared to our TCRL Fabs. The basis for the distinct specificity can be explained by major feature differences between cell surface TCRs and soluble Abs. Monomeric TCR affinities (in the range of 1–50uM [40]) are in orders of magnitude lower than our isolated TCRLs. However, in the cellular context, TCR functional avidity is defined by multiple factors such as receptor and co-receptor densities and affinities. Replacement of TCR with high-affinity TCRL-Ab results in loss of specificity of the engineered T lymphocytes (Oren, R et al, manuscript in preparation), supporting the theory of maximal TCR affinity threshold for improved T-cell function [41] and emphasizing the limitations of TCR mimics in an Ab form. Alternative explanation for these distinct specificities is that TCRL-Fab recognition of RTLs may require a structural motif that is exposed to the solvent only when the Ig-fold domains of the four-domain MHC molecule are removed. In this scenario, TCRs originally selected on four-domain MHC complexes are not educated to recognize this unexposed motif in the four-domain molecule.
Unlike TCRs, B-cell-secreted Abs potentially can discern two- vs. four-domain MHC II/peptide complexes similar to our phage-display Abs. We detected serum non-neutralizing Abs to RTLs in RTL immunized mice [22] and in MS patients (Arthur Vandenbark, personal communication). We predict that such Abs will not cross-react with native four-domain MHC II complexes due to self tolerance mechanisms and their diverse conformation. The naïve human phage display library origin of our isolated TCRL Fabs implies their possible existence in the native Ab sequence repertoire. However, to the best of our knowledge there is no evidence for B-cell expression of TCRL-Abs. The need to break self-tolerance and the predicted immunomodulation effect of circulating Abs specific for self MHC/peptide complexes are possible explanations for our prediction that such antibodies are not produced. While the genetic information for such antibodies exists in the germ line they are not produced or are negatively selected. The fact that such antibodies can be isolated from naïve antibody phage libraries may be the outcome of VH and VL gene recombination that does not naturally exist or that is being eliminated by negative selection.
It is conceivable that our RTL constructs are representative of naturally occurring soluble two-domain MHC class II structures that may function as inhibitors of T-cell responses. In our recent Phase I safety study of RTL1000 in DR2+ MS subjects discussed above, we observed detectable pre-infusion plasma levels of two-domain RTL-like structure in 4 of 13 donors (31%). To verify these intriguing results, we re-evaluated pre- and post-infusion serum or plasma samples from 6 MS subjects from our trial and serum from a pool of 3 healthy donors using the 1B11 Fab specific for two-domain MHC class II structures (with no specificity for bound peptide). Diverse quantities of such structures (ranged from 13ng/ml to 1038 ng/ml) were found in all evaluated subjects. These novel results suggest the natural occurrence of two-domain structures that could be derived from four-domain intermediates possibly shed from class II expressing APC upon immunization [42]. The conformational sensitivity of Fab 1B11 for the distinct RTLs shape implies that such native MHC class II-derived structures carry an RTL-like conformation and therefore may act as natural analogues of RTL constructs and induce similar regulatory effects on T-cell responses. Most importantly, the appearance of natural two-domain class II molecules in human plasma would provide support for the biological relevance of our RTL constructs. Our Abs directed to the two-domain MHC conformation are valuable tool for isolation and identification of such native structures. The comparison between the signal levels detected by Fab 1B11 (pan DR two-domain structures) and Fab 2E4 (DR2/MOG-35-55 two-domain structure of RTL1000) in the plasma of subjects after infusion of RTL1000 demonstrate the high sensitivity of our Fabs. We are currently in the process of increasing the avidity of 1B11 Fab by expressing it as whole IgG, which will allow us to immunoprecipitate and further study such novel serum structures.
In PK studies of our clinical trial discussed above we observed a short half-life (~5min) of circulating RTL1000 post infusion [34]. For the detection of RTL1000 in plasma and serum samples of the subjects, we used polyclonal Abs in sera from mice immunized with RTL1000. The high specificity of Fab 2E4 to RTL1000 in a peptide-restricted manner enabled its sensitive detection of circulating RTL1000 in plasma samples with no background of native MHC and other-peptide specificities of RTL-like structures. Using Fab 2E4 we developed a new assay for PK studies and measurement of RTL1000 levels in serum. This assay was found to have greater sensitivity (~two-fold) compared to the use of poly-clonal serum Abs in the original assay and therefore allows more accurate PK studies (manuscript in preparation).
The therapeutic effects of RTLs on T-cell mediated autoimmunity may involve several complementary pathways. In addition to direct TCR ligation, RTL regulatory effects on inflammatory CD4+ T-cells might work through manipulation of APCs. Our recent studies demonstrated high avidity binding of RTLs to macrophages, dendritic cells and B cells, and such RTL “armed” myeloid cells (but not B cells) could tolerize T-cells specific for the RTL-bound peptide [43]. The current study clearly demonstrates that two-domain MHC II complexes embodied by RTLs are distinct from the corresponding four-domain complexes, and these two-domain structures deliver tolerogenic rather than activating signals through the cognate TCR. We believe that the RTL-armed APCs are tolerogenic through two possible mechanisms: 1) that the RTLs present on the APC surface can still ligate the TCR of cognate T-cells suboptimally as partial agonists; and 2) the RTLs induce inhibitory cell surface co-inhibitory molecules (eg. PD-1 or PD-L1/2) and/or secreted inhibitory cytokines (eg. IL-10) that inhibit T cell activation in concert with RTL ligation of the TCR, with or without prior processing and re-presentation of RTL-derived antigenic peptide and MHC determinants. Our TCRL Fabs will be used to further elucidate the in-vivo therapeutic pathways of RTL1000 in the humanized DR2-Tg EAE model. RTL342m idiotype-specific TCRLs can be used to both inhibit RTL binding to APC and block RTL association with the TCR, as would be predicted for Fab 2E4. A similar approach can shed light on the functionality of the novel native two-domain structures and address whether they constitute Ag-specific tolerogens which resemble RTLs regulatory pathways. By using our conformational-sensitive Fabs we will test our hypothesis that natural RTL-like structures are degradation products of soluble 4-domain MHC class II molecules that have undergone partial enzymatic cleavage. In addition, we are in the process of isolating TCRL Fabs specific for the native DR2/MOG-35-55 complex. Such Fabs will enable us to monitor possible processing and re-presentation of RTL peptides by APCs.
In recent years, with the advantage of fluorochrome-labeled MHC class II multimers, there is increased knowledge about specific CD4+ T-cells in various inflammatory autoimmune conditions [14, 44–47]. T1D patients and at-risk subjects were found to have a significantly higher prevalence of GAD-555-567 specific CD4 T-cells than control subjects [48]. Our novel TCRL to four vs. two-domain MHC II peptide complexes have the potential to selectively recognize APCs presenting disease-inducing or regulatory determinants, respectively, to islet cell-responsive CD4+ T-cells during T1D. Similarly, Fabs to four vs. two domain DR2/MOG-35-55 determinantsmay be invaluable in localizing and quantifying encephalitogenic vs. tolerogenic APC in subjects with MS.
MATERIALS AND METHODS
Production of biotinylated RTLs
RTL1000 and RTL340 constructs were modified for a biotinylated version. In these constructs, a Bir-A tag for biotinylation was introduced to the N-terminus using a 20-aa flexible linker. DNA constructs encoding the RTLs on the pRB98 plasmid were transformed into BL21(DE3)pBirA-competent cells for protein expression. These cells carry an additional plasmid with exogenous BirA ligase under the lac promoter. Bacteria were grown in 1-liter cultures to mid-logarithmic phase (OD600 0.6–0.8) in Luria-Bertani broth containing ampicillin (100µg/ml) at 37°C. Recombinant protein production was induced by addition of 1mM isopropyl-β-D-thiogalactoside and incubated overnight at 30°C. Biotinlated inclusion bodies containing RTLs were produced and purified using the principles described previously for rat [18] and human RTLs [49].
Production of DR4 molecules in S2 cells
DES TOPO DR-A1*0101/DR-B1*0401(HA-307-319) plasmids for inducible expression in Schneider S2 cells, a gift from Dr. Lars Fugger, were used for cloning of the DR-B1*0401(GAD-555-567) construct, transfection and expression of recombinant four-domain MHC class II as previously reported [45]. The correct folding of the recombinant complexes was verified by recognition of anti-HLA-DR conformational sensitive mAb (clone L243) in an ELISA binding assay.
Selection of phage Abs on biotinylated complexes
Selection of phage Abs on biotinylated complexes was performed according to principles described before [50]. Briefly, a large human Fab library containing 3.7 × 1010 different Fab clones was used for the selection. Phages were first preincubated with streptavidin-coated paramagnetic beads (200 µl; Dynal) to deplete the streptavidin binders. The remaining phages were subsequently used for panning with decreasing amounts of biotinylated MHC-peptide complexes. The streptavidin-depleted library was incubated in solution with soluble biotinylated RTLs or four-domain DR4/GAD (500nM for the first round, and 100nM for the following rounds) for 30 min at room temperature. Streptavidin-coated magnetic beads (200µl for the first round of selection and 100µl for the following rounds) were added to the mixture and incubated for 10–15 min at room temperature. The beads were washed extensively 12 times with PBS/0.1% Tween 20 and an additional two washes were with PBS. Bound phages were eluted with triethylamine (100mM, 5 min at room temperature), followed by neutralization with Tris-HCl (1M, pH 7.4), and used to infect E. coli TG1 cells (OD = 0.5) for 30 min at 37°C. The diversity of the selected Abs was determined by DNA fingerprinting using a restriction endonuclease (BstNI), which is a frequent cutter of Ab V gene sequences. Selected Fab Ab clones were expressed and purified as described before [50].
ELISA with phage clone supernatants and purified Fab antibodies
Binding specificity of individual phage clone supernatants and soluble Fab fragments were determined by ELISA using biotinylated two and four-domain MHC/peptide complexes. ELISA plates (Falcon) were coated overnight with BSA-biotin (1µg/well). After being washed, the plates were incubated (1 h at room temperature) with streptavidin (10µg/ml), washed extensively and further incubated (1 h at room temperature) with 5µg/ml of MHC/peptide complexes. The plates were blocked for 30 min at room temperature with PBS/2% skim milk and subsequently were incubated for 1 h at room temperature with phage clone supernatants (induced at OD600 = 0.8–1.0 for overnight expression at 30°C) or 5µg/ml soluble purified Fab. After washing, plates were incubated with horseradish peroxidase-conjugated/anti-human-Fab antibody. Detection was performed using TMB reagent (Sigma). For binding of peptide-loaded RTLs, ELISA plates were coated 2h at 37°C with purified Fab, washed extensively and blocked for 30min with PBS/2% skim milk. Loaded complexes were incubated for 1 hour followed by 1 hour incubation with anti-MHC class II mAb (TU39, BD). After washing, plates were incubated with horseradish peroxidase-conjugated/anti-mouse-IgG antibody and detection was performed as described above.
Competition binding assays
ELISA plates were coated with BSA-biotin and MHC-peptide complexes were immobilized as described above. Binding of soluble purified Fabs was performed by competitive binding analysis, which examined the ability of varied concentrations of soluble recombinant MHC-peptide complexes to inhibit the binding of the purified Fab to the specific immobilized MHC-peptide complex. Detection of Fabs binding to the immobilized MHC-peptide complexes was performed as described above.
Flow cytometry
Cells were incubated for 4h with medium containing 70µM MOG-35-55 (MEVGWYRPPFSRVVHLYRNGK) or MBP-85-99 (ENPVVHFFKNIVTPR) for L-cell DR*1501 transfectants and with GAD-555-567 (NFFRMVISNPAAT) or control peptide: HA-307-319 (PKYVKQNTLKLAT), InsA-1-15 (GIVEQCCTSICSLYQ), and CII-261-273 (AGFKGEQGPKGEP)- for DR4-EBV-transformed B lymphoblast Preiss cells. Cells (106) were washed and incubated with 1–2µg of specific Fab for 1 h at 4°C, followed by incubation with FITC-labeled anti-human Ab for 45 min at 4°C. Cells were finally washed and analyzed by a FACSCalibur flow cytometer (BD Biosciences).
IL-2 bioassay for the H2-1 T-cell hybridoma
H2-1 T-cell hybridoma cells [51] (2×105/well in a 96-well plate) in 100µl of 10% FBS-containing medium were combined with 2×105 irradiated (4,500 rad) HLA-DRB1*1501-transfected L cells in 100µl alone or in the presence of 10µg/ml individual peptides and incubated at 37°C and 7% CO2 for 72hr. Supernatants were collected from the top of the culture, followed by centrifugation for 1 min at 1,000 rpm. Hybridoma supernatants were added in triplicate into wells containing 5,000 CTLL-2 cells in 100µl of 10% FBS culture medium. After 24 hr of culture, the cells were pulsed with 0.5µCi [3H]thymidine for an additional 5 hr and the net cpm (mean +/− SD) were calculated.
RTL in vitro potency assay using H2-1 T-cell hybridomas
Human MOG-35–55 peptide-specific H2-1 T-cell hybridoma cells (2×105/well) were co-cultured in triplicate with 2mM Tris-containing medium alone, 8µM RTL1000, or 8µM RTL340 in 2mM Tris-containing medium for 72hr. Aliquotted hybridoma cell cultures were thoroughly washed with RPMI and further stimulated with and without 10µg/ml hMOG-35–55 peptide presented by irradiated (4,500 rad) DRB1*1501-transfected cell lines at a 1:1 ratio in triplicate for 48 hr. Half of the supernatant was collected from the top of each well and transferred into corresponding wells of another culture plate in which 100µl of 10% FBS-containing medium with 5,000 CTLL cells per well had been seeded. After 24 hr of culture, the CTLL cells were pulsed with [3H]thymidine for an additional 4 h and the net cpm (mean + SD) were calculated.
RTL treatment of EAE in DR2-Tg mice
HLA-DR2 mice between 8 and 12 weeks of age were immunized s.c. at four sites on the flanks with 0.2ml of an emulsion of 200µg mouse MOG-35-55 peptide and complete Freund’s adjuvant containing 400µg of Mycobacterium tuberculosis H37RA (Difco, Detroit, MI). In addition, mice were given pertussis toxin (Ptx) from List Biological Laboratories (Campbell, CA) on days 0 and 2 post-immunization (75ng and 200ng per mouse, respectively). HLA-DR2 mice were treated with vehicle, RTL342m alone, or RTL342m pre-incubated with one of the FAbs beginning on the first day that the combined clinical EAE score for each individual mouse reached 2 or higher. Once-daily treatments were administered to each mouse subcutaneously in the interscapular region for three days. RTL342m and RTL342m + FAb were prepared in 100µl of 20mM Tris-HCl pH 8.0 with 5% w/v D-glucose (Sigma-Aldrich, St. Louis, MO). Vehicle treatments consisted of only Tris-HCl pH 8.0 with 5% w/v D-glucose. Mean EAE scores and standard deviations for mice grouped according to initiation of RTL or vehicle treatment were calculated for each day. The Cumulative Disease Index (CDI) was determined for each mouse by summing the daily EAE scores. Group CDI scores were calculated by determining the mean + SD of the individual mice in the group.
The IACUC Protocol #2108, Vandenbark AA PI, was in place and is currently approved for the animal experiments reported in the manuscript.
Serum ELISA with Fabs
Detection of RTL-like material in human serum or plasma was determined by ELISA using Fab 1B11. ELISA plates (Falcon) were coated for 2 h with anti-MHC mAb TU39 (10µg/well). The plates were blocked for 30 min at room temperature with PBS/2% skim milk and subsequently were incubated for 2 h at room temperature with serial dilutions of RTL1000 (for standard curve) and 1:10 serum dilutions. After being washed, the plates were incubated (1 h at room temperature) with 1B11 Fab (10µg/ml), washed extensively and further incubated (1 h at room temperature) with anti-myc-biotin Ab (9E10 clone, Covance). The plates were washed and incubated for 30 min with horseradish peroxidase-conjugated streptavidin. Further amplification steps were performed using the ELAST ELISA amplification system (PerkinElmer), according to the manufacturer’s protocol. Detection was performed using TMB reagent (Sigma). Detection of RTL1000 in human serum or plasma was determined by ELISA using biotinylated Fab 2E4. ELISA plates (Falcon) were coated overnight with BSA-biotin (1µg/well). After being washed, the plates were incubated (1 h at room temperature) with streptavidin (10µg/ml), washed extensively and further incubated (1 h at room temperature) with 5µg/ml of biotinylated Fab 2E4. The plates were blocked for 30 min at room temperature with PBS/2% skim milk and subsequently were incubated for 2 h at room temperature with serial dilutions of RTL1000 and RTL340 (for standard curve) and 1:10 serum dilutions. After washing, plates were incubated with anti-DR/DP/DQ mAb (Tu39 clone, BD) followed by horseradish peroxidase-conjugated/anti-mouse antibody. Detection was performed using TMB reagent (Sigma).
Surface Plasmon Resonance
Kinetic studies for measures of Fabs affinities to RTLs were performed on a ProteOn XPR36 Protein Interaction Array System (Bop Rad Laboratories, Hercules, CA, USA) as described before [52].
Statistics
All experiments performed under this study are presented as independent assays which are representative of three to nine independent experiments. IL-2 bioassays were performed in triplicates with SD bars indicated. For neutralization of RTL treatment of DR2-mice by Fabs, a two- tailed Mann-Whitney test for nonparametric comparisons was used to gauge the significance of difference between the mean daily and CDI scores of vehicle vs. RTL treatment groups. A one sided Fisher’s exact test was used to gauge the significance of the number of “treated” mice between groups. A Kruskal-Wallis nonparametric analysis of variance test was also performed with a Dunn’s multiple-comparison post-test to confirm significance between all groups. A two-tailed unpaired t-test was used to confirm significance of signal in 1B11 serum ELISA, while two-tailed paired t-test was used to gauge the significance between pre-vs. post-infusion samples. All statistical tests were computed using GraphPad Prism 4 (GraphPad software, Inc.).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the US-Israel Educational Foundation which supported this study and enabled collaborative visit to the United States under the auspices of the Fulbright Program. This work was supported by NIH grants NS47661 (AAV), AI43960 (GGB), DK068881 (GGB) and the Biomedical Laboratory R&D Service, Department of Veterans Affairs, USA.
Abbreviations
- MS
Multiple Sclerosis
- RTL
Recombinant T-cell receptor Ligands
- TCRL
T-cell receptor-like
- T1D
Type 1 Diabetes
Footnotes
CONFLICT OF INTEREST
Dr. Burrows, Dr. Offner, Dr. Vandenbark, and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees. Dr. Ferro has a financial interest in Artielle ImmunoTherapeutics.
References
- 1.Nepom GT, Erlich H. MHC Class-II Molecules and Autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- 2.Sawcer S, Ban M, Maranian M, Yeo TW, Compston A, Kirby A, Daly MJ, et al. International Multiple Sclerosis Genetics Consortium. A High-Density Screen for Linkage in Multiple Sclerosis. Am J Hum Genet. 2005;77:454–467. doi: 10.1086/444547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDaniel DO, Barger BO, Acton RT, Koopman WJ, Alarcn GS. Molecular analysis of HLA-D region genes in seropositive rheumatoid arthritis. Tissue Antigens. 1989;34:299–308. doi: 10.1111/j.1399-0039.1989.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 4.Trapp BD, Nave KA. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 5.Olerup O, Hilert J. HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens. 1991;38:1–15. doi: 10.1111/j.1399-0039.1991.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 6.Kerlero de Rosbo N, Ben-Nun A. T-cell responses to myelin antigens in multiple sclerosis: relevance of the predominant autoimmune reactivity to myelin oligodendrocyte glycoprotein. J Autoimmun. 1998;11:287–295. doi: 10.1006/jaut.1998.0202. [DOI] [PubMed] [Google Scholar]
- 7.Kerlero de Rosbo N, Milo R, Lees MB, Burger D, Bernard CC, Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis: peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92:2602–2608. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2 mice: Fine specificity and T -cell receptor Vbeta expression of encephalitogenic T-cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 9.Johns TG, Kerlero de Rosbo N, Menon KK, Abo S, Gonzales MF, Bernard CC. Myelin oligodendrocyte glycoprotein induces a demyelinating encephalomyelitis resembling multiple sclerosis. J Immunol. 1995;154:5536–5541. [PubMed] [Google Scholar]
- 10.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, et al. Myelin oligodendrocyte glycoprotein-35-55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol. 2004;34:1251–1261. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 11.Karlsen AE, Hagopian WA, Grubin CE, Dube S, Disteche DA, Adler H, Bärmeier S, et al. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci U S A. 1991;88:8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 13.Patel SD, Cope AP, Congia M, Chen TT, Kim E, Fugger L, Wherrett D, Sonderstrup-McDevitt G. Identification of immunodominant T-cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR (alpha1*0101,beta1*0401) transgenic mice. Proc Natl Acad Sci U S A. 1997;94:8082–8087. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu A, Kwok W. Detection of GAD65-Specific T-Cells by Major Histocompatibility Complex Class II Tetramers in Type 1 Diabetic Patients and At-Risk Subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 15.Nepom GT, Lippolis JD, White FM, Masewicz S, Marto JA, Herman A, Luckey CJ, Falk B, et al. Identification and modulation of a naturally processed T-cell epitope from the diabetes-associated autoantigen human glutamic acid decarboxylase 65 (hGAD65) Proc Natl Acad Sci U S A. 2001;98:1763–1768. doi: 10.1073/pnas.98.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz RH. Models of T-cell anergy: is there a common molecular mechanism? J Exp Med. 1996;1:19–29. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quill H, Schwartz RH. Stimulation of normal inducer T-cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 18.Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T- cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 19.Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, et al. Rudimentary TCR Signaling Triggers Default IL-10 Secretion by Human Th1 Cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR Ligand Induces Early TCR Signaling and a Unique Pattern of Downstream Activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 21.Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H. Cytokine Switch and Bystander Suppression of Autoimmune Responses to Multiple Antigens in Experimental Autoimmune Encephalomyelitis by a Single Recombinant T-Cell Receptor Ligand. J. Neurosci. 2009;29:3816–3823. doi: 10.1523/JNEUROSCI.5812-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link JM, Rich CM, Korat M, Burrows GG, Offner H, Vandenbark AA. Monomeric DR2/MOG-35-55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clin Immunol. 2007;123:95–104. doi: 10.1016/j.clim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of Autoimmune Anterior Uveitis with Recombinant TCR Ligands. Invest. Ophthalmol. Vis. Sci. 2006;47:2555–2561. doi: 10.1167/iovs.05-1242. [DOI] [PubMed] [Google Scholar]
- 24.Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, Rosloniec EF, et al. MHC Class II Derived Recombinant T-Cell Receptor Ligands Protect DBA/1LacJ Mice from Collagen-Induced Arthritis. J Immunol. 2008;180:1249–1257. doi: 10.4049/jimmunol.180.2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian S, Zhang b, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T-Cell Receptor Ligand Treats Experimental Stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P, Hoogenboom HR, Reiter Y. Isolation and Characterization of Human Recombinant Antibodies Endowed with the Antigen-specific, Major Histocompatibility Complex-restricted Specificity of T-Cells Directed toward the Widely Expressed Tumor T-cell Epitopes of the Telomerase Catalytic Subunit. Cancer Res. 2002;62:3184–3194. [PubMed] [Google Scholar]
- 27.Denkberg G, Cohen CJ, Lev A, Chames P, Hoogenboom HR, Reiter Y. Direct visualization of distinct T -cell epitopes derived from a melanoma tumor-associated antigen by using human recombinant antibodies with MHC- restricted T-cell receptor-like specificity. Proc Natl Acad Sci U S A. 2002;99:9421–9426. doi: 10.1073/pnas.132285699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen CJ, Hoffmann N, Farago M, Hoogenboom HR, Eisenbach L, Reiter Y. Direct Detection and Quantitation of a Distinct T-Cell Epitope Derived from Tumor-specific Epithelial Cell-associated Mucin Using Human Recombinant Antibodies Endowed with the Antigen-specific, Major Histocompatibility Complex-restricted Specificity of T-Cells. Cancer Res. 2002;62:5835–5844. [PubMed] [Google Scholar]
- 29.Denkberg G, Lev A, Eisenbach L, Benhar I, Reiter Y. Selective Targeting of Melanoma and APCs Using a Recombinant Antibody with TCR-Like Specificity Directed Toward a Melanoma Differentiation Antigen. J Immunol. 2003;171:2197–2207. doi: 10.4049/jimmunol.171.5.2197. [DOI] [PubMed] [Google Scholar]
- 30.Epel M, Carmi I, Soueid-Baumgarten S, Oh SK, Bera T, Pastan I, Berzofsky J, Reiter Y. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T-cell receptor-like human recombinant antibodies. Eur J Immunol. 2008;38:1706–1720. doi: 10.1002/eji.200737524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaeli Y, Denkberg G, Sinik K, Lantzy L, Chih-Sheng C, Beauverd C, Ziv T, et al. Expression Hierarchy of T-Cell Epitopes from Melanoma Differentiation Antigens: Unexpected High Level Presentation of Tyrosinase-HLA-A2 Complexes Revealed by Peptide-Specific, MHC-Restricted, TCR-Like Antibodies. J Immunol. 2009;182:6328–6341. doi: 10.4049/jimmunol.0801898. [DOI] [PubMed] [Google Scholar]
- 32.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal Structure of HLA-DR2 (DRA*0101, DRB1*1501) Complexed with a Peptide from Human Myelin Basic Protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Brune AP, et al. A Large Non-immunized Human Fab Fragment Phage Library That Permits Rapid Isolation and Kinetic Analysis of High Affinity Antibodies. J Biol Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 34.Yadav RV, Bourdette D, Bowen JD, Lynch SG, Mattson D, Preinigerova J, Rose C, et al. Recombinant T-Cell Receptor Ligand (RTL) for the Treatment of Multiple Sclerosis: Report of a Phase I Clinical Trial. Neurology. 2010;74(S2):A293–A294. [Google Scholar]
- 35.Stang E, Guerra CB, Amaya M, Paterson Y, Bakke O, Mellins ED. DR/CLIP (Class II-Associated Invariant Chain Peptides) and DR/Peptide Complexes Colocalize in Prelysosomes in Human B Lymphoblastoid Cells. J Immunol. 1998;160:4696–4707. [PubMed] [Google Scholar]
- 36.Rudensky AY, Maric M, Eastman S, Shoemaker L, DeRoos PC, Blum JS. Intracellular assembly and transport of endogenous peptide-MHC class II complexes. Immunity. 1994;1:585–594. doi: 10.1016/1074-7613(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 37.Krogsgaard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J, Ditzel H, et al. Visualization of Myelin Basic Protein (Mbp) T-Cell Epitopes in Multiple Sclerosis Lesions Using a Monoclonal Antibody Specific for the Human Histocompatibility Leukocyte Antigen (Hla)-Dr2–Mbp 85–99 Complex. J Exp Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong G, Reis e Sousa C, Germain RN. Production, specificity, and functionality of monoclonal antibodies to specific peptide–major histocompatibility complex class II complexes formed by processing ofexogenousprotein. Proc Natl Acad Sci U S A. 1997;94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eastman S, Deftos M, DeRoos PC, Hsu DH, Teyton L, Braunstein NS, Hackett CJ, Rudensky A. A study of complexes of class II invariant chain peptide: major histocompatibility complex class II molecules using a new complex-specific monoclonal antibody. Eur J Immunol. 1996;26:385–393. doi: 10.1002/eji.1830260218. [DOI] [PubMed] [Google Scholar]
- 40.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 41.Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC, Gomez-Eerland R, et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol. 2010;184:4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 42.MacKay PA, Leibundgut-Landmann S, Koch N, Dunn AC, Reith W, Jack RW, McLellan AD. Circulating, soluble forms of major histocompatability complex antigens are not exosome-associated. Eur J Immunol. 36:2875–2884. doi: 10.1002/eji.200636041. [DOI] [PubMed] [Google Scholar]
- 43.Sinha S, Miller L, Subramanian S, McCarty O, Proctor T, Meza-Romero R, Burrows GG, et al. Binding of recombinant T-cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T-cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;25:52–61. doi: 10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, Kwok WW, et al. GAD65-Specific CD4+ T-Cells with High Antigen Avidity Are Prevalent in Peripheral Blood of Patients With Type 1 Diabetes. Diabetes. 2004;53:1987–1994. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 45.Svendsen P, Andersen CB, Willcox N, Coyle AJ, Holmdahl R, Kamradt T, Fugger L. Tracking of proinflammatory collagen-specific T cells in early and late collagen-induced arthritis in humanized mice. J Immunol. 2004;1:7037–7045. doi: 10.4049/jimmunol.173.11.7037. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, et al. Myelin-specific regulatory T-cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macaubas C, Wahlstrom J, Galvao da Silva AP, Forsthuber TG, Sonderstrup G, Kwok WW, DeKruyff RH, Umetsu DT. Allergen-Specific MHC Class II Tetramer+ Cells Are Detectable in Allergic, but Not in Nonallergic, Individuals. J Immunol. 2006;176:5069–5077. doi: 10.4049/jimmunol.176.8.5069. [DOI] [PubMed] [Google Scholar]
- 48.Oling V, Marttila J, Ilonen J, Kwok WW, Nepom G, Knip M, Simell O, Reijonen H. GAD65- and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J Autoimmun. 2005;25:235–243. doi: 10.1016/j.jaut.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan L, Fugger L, et al. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J. Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 50.Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y. Direct Phenotypic Analysis of Human MHC Class I Antigen Presentation: Visualization, Quantitation, and In Situ Detection of Human Viral Epitopes Using Peptide-Specific, MHC-Restricted Human Recombinant Antibodies. J Immunol. 2003;170:4349–4361. doi: 10.4049/jimmunol.170.8.4349. [DOI] [PubMed] [Google Scholar]
- 51.Chou YK, Culbertson N, Rich C, LaTocha D, Buenafe AC, Huan J, Link J, et al. T-cell hybridoma specific for myelin oligodendrocyte glycoprotein-35-55 peptide produced from HLA-DRB1*1501-transgenic mice. J Neurosci Res. 2004;77:670–680. doi: 10.1002/jnr.20201. [DOI] [PubMed] [Google Scholar]
- 52.Bronner V, Denkberg G, Peled M, Elbaz Y, Zahavi E, Kasoto H, Reiter Y, et al. Therapeutic antibodies: Discovery and development using the ProteOn XPR36 biosensor interaction array system. Anal Biochem. 2010;406:147–156. doi: 10.1016/j.ab.2010.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.