Abstract
The D2/D3 receptor agonist pramipexole has clinical efficacy as an antidepressant, but its neural mechanisms are unknown. We used 18FDG-PET to investigate the cerebral metabolic effects of pramipexole augmentation of mood stabilizers in bipolar II depression. Fifteen bipolar II depressed patients on mood stabilizers were imaged at baseline and following 6 wk of pramipexole (n=7) or placebo (n=8) augmentation. Relative to placebo, pramipexole treatment was associated with reductions in normalized metabolism in bilateral orbitofrontal cortex, left ventrolateral prefrontal cortex (PFC), and right anteromedial PFC. Voxel-wise analyses additionally showed decreased normalized metabolism in the left inferior parietal cortex and medial frontopolar cortical (BA 10P) area of the anteromedial PFC following pramipexole treatment. These pramipexole-induced effects on regional metabolism suggest a mechanism of antidepressant action distinct from that previously reported under serotonin reuptake inhibitor treatment and appear compatible with evidence that the central dopaminergic system plays a role in the pathophysiology of bipolar depression.
Keywords: Bipolar disorder, glucose metabolism, major depression, orbitofrontal cortex, positron emission tomography (PET)
Introduction
Pramipexole, a dopamine receptor agonist with high selectivity for the D2 dopamine receptor family (D2,D3, D4 receptor subtypes) and preferential affinity for the D3 receptor subtype, has shown antidepressant efficacy as an augmentation strategy for treatment-resistant unipolar and bipolar depression (with effect sizes relative to placebo ranging from 0.6 to 1.1; Aiken, 2007; Zarate et al. 2004). However, the neural mechanisms underlying pramipexole's antidepressant effects are unknown. In the current study, 18F-fluorodeoxyglucose-positron emission tomography (18FDG-PET) imaging was used to assess the cerebral metabolic effects of pramipexole in depressed subjects with type-II bipolar disorder (BD-II). We previously demonstrated, in BD-II depression, abnormally elevated limbic-cortical-striatal activity (Mah et al. 2007), which has been hypothesized to partly reflect the effects of deficient mesostriatal dopaminergic input on striatal outflow (Drevets et al. 1992; Hasler et al. 2008; Mah et al. 2007; Swerdlow et al. 1987). We therefore expected that the post-synaptic D2/D3 receptor agonist effects of pramipexole in BD-II depression would result in reduction of normalized limbic-cortical-striatal metabolic activity (regional/global tissue radioactivity).
Methods
Participants
Fifteen subjects (12 females, mean age=43±11 yr) with BD-II in a current major depressive episode (according to DSM-IV criteria) underwent PET imaging at baseline and after 6 wk of either pramipexole (target range 1.0–3.0 mg/d; maximum dose 4.5 mg/d) or placebo administration in combination with a mood stabilizer. Subjects were a subset of the treatment-refractory BD-II sample studied in a larger clinical trial of pramipexole augmentation of mood stabilizers (Zarate et al. 2004). Patients were treated with lithium or divalproex sodium (VPA) for o4 wk with at least two weekly blood levels within therapeutic range (lithium, 0.6–1.2 meq./l; VPA, 50–125 mg/ml) prior to the baseline scan and subsequent randomization to pramipexole (n=7; three lithium, four VPA) or placebo (n=8; four lithium, four VPA). Patients were maintained on mood stabilizers to minimize the risk of development of hypomania or mania. No other psychotropic medications were permitted within the 2 wk (5 wk for fluoxetine) preceding the baseline scan. Subjects scored ≥20 (moderate to severe level of depression) on the clinician-rated Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979) at screening and baseline evaluations. Subjects were excluded if they had a major medical or neurological disorder, substance abuse within 3 months or dependence within 12 months, rapid cycling, psychosis, serious suicidal risk, current pregnancy, or were breastfeeding. Subjects provided written informed consent as approved by the National Institute of Mental Health Institutional Review Board.
Image acquisition and processing
PET images were acquired using a GE Advance PET scanner in 3D mode (GE Medical Systems, USA; 35 slices 4.25-mm thick; axial resolution=5.3 mm full-width half-maximum). Subjects received 4.5 mCi of 18FDG following a fasting period of at least 6 h. Dynamic PET imaging of the heart in 2D mode for a total of 35 min followed, with concurrent serial venous blood sampling beginning 15-min post-tracer injection. A 10-min static emission scan was acquired 45-min post-tracer injection, followed by an 8-min transmission scan to attenuation-correct the emission scan (Carson et al. 1988). MRI scans were acquired to provide an anatomical framework for PET image analysis (3.0 T GE Signa Scanner; MP-RAGE sequence : TE=2.98 ms, TR=7.5 ms, inversion time= 725 ms, voxel size=0.85×0.85×1.2 mm).
The cerebral metabolic rate for glucose (CMRGlu) was quantitatively measured using a non-invasive method that combined left cardiac ventricular chamber time-activity imaging with venous blood sampling to generate the input function, a method previously validated against the more invasive approach of sampling arterial blood (Moore et al. 2003). This approach was well-tolerated by all subjects. However, because of the technical difficulties involved in performing serial venous blood sampling through an intravenous cannula during PET scanning, the input function was incomplete for some subjects' pre- or post-treatment studies. Consequently, a complete set of pre- and post-treatment quantitative CMRGlu data was available for only a subset of patients. Since the sample size of this subset was insufficient to generate meaningful statistical analyses, the available quantitative CMRGlu values are provided as Supplementary material (Supplementary Table S1, available online), and only the normalized metabolic results (regional/ global tissue radioactivity) – which were available for all scan sessions – are reported here.
Clinical data analysis
The number of pramipexole responders [defined, according to convention, as individuals whose baseline MADRS score decreased ≥50% by study end (Zarate et al. 2004)] was compared to the number of placebo responders using a x2 test. Between-group differences in the change in MADRS ratings pre- vs. post-treatment were assessed using an independent t test.
Region-of-interest (ROI) analysis
Each subject's PET and MRI scans were co-registered using MEDx (Medical Numerics Inc., USA). The whole-brain tissue radioactivity was measured within an MRI-based template to permit global normalization of the regional data. Regional tissue radioactivity was extracted from ROIs defined a priori on an MRI template image and then positioned individually on each subject's MRI scan, as described in Neumeister et al. (2004) (see also Supplementary material). The ROIs were defined in regions reported to have abnormal metabolism in depression (Brody et al. 2001; Drevets, 1999; Drevets et al. 1992; Mah et al. 2007; Mayberg et al. 1999): the orbitofrontal cortex (OFC), dorsolateral pre-frontal cortex (dlPFC), perigenual anterior cingulate cortex (pgACC), ventrolateral prefrontal cortex (vlPFC), anteromedial PFC (amPFC), amygdala, ventral striatum, and anterior insula (anatomical definitions for these ROI appear in Cannon et al. 2006; Drevets et al. 2002b; Neumeister et al. 2004; and also in the online Supplementary material). effect sizes were calculated using Cohen's d (Cohen, 1988) to assess the magnitude of the effects of pramipexole, relative to placebo within ROIs. effect sizes of ≥0.8 were considered large, independent of sample size. Between-group differences in metabolic change within ROIs following pramipexole or placebo treatment were assessed using independent t tests. Associations between clinical improvement on the MADRS, baseline metabolism, and treatment-associated metabolic changes were assessed post-hoc using Spearman's rho only in predefined ROIs with significant post-treatment change in metabolism. Fisher's Z transformation was used to assess differences in correlation coefficients between treatment groups (Rosenthal, 1991).
Voxel-wise analysis
Exploratory voxel-wise analyses were conducted post-hoc using SPM2 (Wellcome Department of Imaging Neuroscience, UK) to reduce Type-II error by identifying metabolic changes located outside the predefined ROIs and to more specifically localize the metabolic changes situated within the predefined ROIs. The co-registered PET and MRI images were spatially normalized, and smoothed using a 12-mm Gaussian kernel. Changes in metabolism following treatment were analysed in SPM2 using a randomeffects model and paired t tests with proportional scaling global normalization. Between-group changes were analysed using a multi-group conditions and covariates model in SPM2, with proportional scaling global normalization. The threshold for statistical significance was set at uncorrected p<0.001. Coordinates were converted to the stereotaxic array of Talairach & Tournoux (1988).
Results
At baseline the mean MADRS score did not differ between subjects randomized to pramipexole (34±5.8) vs. those randomized to placebo (31±5.3; t13=1.1, p=0.31). Consistent with results from the larger patient sample enrolled in the clinical trial (Zarate et al. 2004), a greater number of BD subjects responded to pramipexole (5/7) than to placebo (1/8; χ2=5.4, p= 0.041). The mean change on the MADRS was greater following pramipexole than placebo (pramipexole: 19.0±11.4; placebo: 6.9±7.3; t13=−2.38, p=0.03).
The pre-treatment normalized metabolism did not differ between groups in any ROI. Following pramipexole treatment, normalized metabolism decreased significantly relative to placebo in the left OFC [Effect size (ES)=−1.22; t13=2.37, p=0.03; Table 1] and showed non-significant trends towards decreasing in the right OFC (ES=−1.04; t13=2.01, p=0.07), right amPFC (ES=−1.1; t13=2.13, p=0.053), and left vlPFC (ES=−0.91, p=0.10).
Table 1.
Changes in normalized cerebral glucose metabolism (mean, standard deviation) in subjects randomized to receive either pramipexole or placebo and effect sizes of pramipexole in regions-of-interest defined a priori
| Region of interest | Effect size (Pram/placebo) | Pramipexole |
Placebo |
||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Orbitofrontal cortex | |||||
| Lefta | −1.22 | 1.14 (0.036) | 1.10 (0.058)c | 1.15 (0.031) | 1.15 (0.039) |
| Rightb | −1.04 | 1.12 (0.042) | 1.08 (0.049)c | 1.13 (0.037) | 1.12 (0.033) |
| Ventrolateral PFC | |||||
| Leftb | −0.91 | 1.14 (0.035) | 1.11 (0.042)d | 1.18 (0.040) | 1.19 (0.060) |
| Right | −0.39 | 1.13 (0.033) | 1.10(0.034) | 1.17 (0.053) | 1.16 (0.059) |
| Anteromedial PFC | |||||
| Left | −0.59 | 1.04 (0.035) | 1.01 (0.049) | 1.08 (0.077) | 1.09 (0.068) |
| Rightb | −1.10 | 1.09 (0.043) | 1.04 (0.046)c | 1.09 (0.072) | 1.09 (0.080) |
| Perigenual ACC | |||||
| Left | 0.78 | 1.11 (0.053) | 1.12 (0.062) | 1.15 (0.065) | 1.12 (0.051)d |
| Right | −0.07 | 1.10 (0.075) | 1.09 (0.058) | 1.10 (0.068) | 1.10 (0.068) |
| Dorsolateral PFC | |||||
| Left | −0.48 | 1.20 (0.036) | 1.18 (0.043) | 1.22 (0.047) | 1.21 (0.063) |
| Right | −0.28 | 1.19 (0.064) | 1.18 (0.053) | 1.20 (0.058) | 1.21 (0.060) |
| Amygdala | |||||
| Left | 0.27 | 0.94 (0.097) | 0.94 (0.075) | 0.86 (0.111) | 0.84 (0.107) |
| Right | 0.14 | 0.92 (0.062) | 0.93 (0.055) | 0.86 (0.063) | 0.86 (0.060) |
| Ventral striatum | |||||
| Left | −0.01 | 1.25 (0.100) | 1.27 (0.086) | 1.24 (0.099) | 1.26 (0.052) |
| Right | 0.37 | 1.31 (0.100) | 1.31 (0.139) | 1.26 (0.067) | 1.24 (0.087) |
| Anterior insula | |||||
| Left | −0.62 | 1.22 (0.051) | 1.18 (0.066) | 1.24 (0.058) | 1.24 (0.093) |
| Right | 0.49 | 1.20 (0.070) | 1.20 (0.062) | 1.25 (0.070) | 1.21 (0.110) |
ACC, Anterior cingulate cortex; PFC, prefrontal cortex.
Effect sizes (ES) of pramipexole relative to placebo in regions-of-interest calculated as the difference between mean pramipexole- and placebo-mediated metabolic change divided by the pooled standard deviation for the two means (Cohen, 1988).
ES: 0.2 = small, 0.5 = medium, 0.8 = large. A larger ES indicates greater percentage of non-overlap in the distribution of scores for the active treatment group with placebo group; e.g. an ES of 1.0 indicates a non-overlap of 55.4% of the two distributions.
Metabolic change during treatment differed between groups (p<0.05).
Metabolic change during treatment showed non-significant trend towards differing between groups at 0.05<p≤0.10.
Post-treatment metabolism changed from baseline at p<0.05.
Post-treatment metabolism showed non-significant trend towards differing from baseline at 0.05<p<0.10.
Lower pretreatment normalized metabolism in the left OFC predicted superior response to pramipexole (ρ=−0.87, p=0.01; Supplementary Fig. S2). This correlation coefficient differed significantly (Fisher's Z=−2.67, p=0.008) from the corresponding association observed in the placebo group (ρ=0.43). However, the change in depression ratings was not associated significantly with the change in normalized metabolism in the same region.
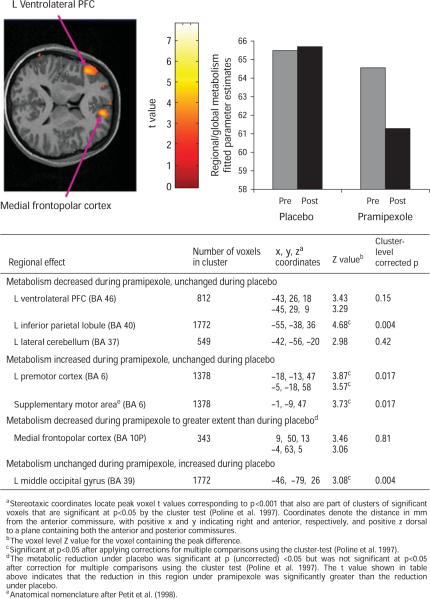
The post-hoc voxel-wise analysis identified areas where, under pramipexole, the normalized metabolism decreased significantly in the medial frontopolar [Brodmann area (BA) 10P] cortex of the amPFC, left inferior parietal cortex, and left vlPFC, and increased significantly in the right posterior cingulate, posterior hippocampus, left motor and premotor cortices, and accumbens (Supplementary Table S2). The reductions in metabolism under pramipexole differed significantly from the metabolic changes under placebo in the left vlPFC, medial frontopolar cortex, and left inferior parietal cortex (Fig. 1). Metabolism increased in the left premotor cortex and supplementary motor area to a greater extent under pramipexole than under placebo, and increased in the left middle occipital gyrus to a greater extent under placebo than under pramipexole (Fig. 1).
Fig. 1.
Regions of interest identified by voxel-wise analysis where changes in normalized metabolism associated with pramipexole treatment differed from those associated with placebo administration, as shown by (top left) horizontal section from the statistical parametric map of voxel t values computed using SPM2, p<0.001; (top right) fitted parameter estimates for regional/global metabolism (in which the mean value for individual subject is set to 50) in left ventrolateral prefrontal cortex; (bottom) table of stereotaxic coordinates of regions where changes in normalized metabolism associated with pramipexole treatment differed from those associated with placebo administration. PFC, Prefrontal cortex; L, left.
We also used the voxel-wise analysis to more specifically localize the area within the OFC where metabolic activity changed most significantly under pramipexole, since our predefined ROI in this region encompassed both medial and lateral orbital gyri. Although no regional change within the OFC reached our pre-specified significance threshold of p<0.001, we observed two clusters within the OFC where changes in metabolism following pramipexole treatment reached the more liberal threshold of p<0.01. These clusters were located in the medial OFC (with the peak voxel t value situated at x=−14, y=34, z=−21; Z=2.50, p=0.006, cluster size=108 voxels) and the lateral OFC (x=38, y=36, z=−4; Z=2.41; p=0.008, cluster size=126 voxels; coordinates interpreted as in Supplementary Fig. S2).
Discussion
Clinical improvement with pramipexole augmentation in BD-II depression was associated with a reduction in normalized regional metabolism in the OFC, amPFC, and vlPFC, regions where cerebral metabolic activity is reportedly elevated in the depressed state of unipolar or bipolar mood disorders (Drevets, 1999; Drevets et al. 1992; Mah et al. 2007). Post-hoc voxel-wise analyses suggest that the reduction in metabolism found in the predefined ROI in the OFC was driven by reductions in both left medial orbital and right lateral orbital cortex. However, given our inability to exclude the possibility of shifts in global metabolism due to the limited sample of quantitative cerebral metabolic data, we were unable to establish whether the absolute CMRGlu also changed in the OFC, amPFC, and vlPFC. Nevertheless, it is noteworthy that our findings of a reduction in relative metabolism (i.e. regional/global) in these areas following pramipexole treatment resemble the direction of metabolic changes reported in unipolar depressives following treatment with antidepressant medications or deep-brain stimulation (Drevets, 2007; Drevets et al. 2002a; Lozano et al. 2008). Further, the metabolic changes we found in depressed patients under chronic pramipexole administration appear compatible with PET data obtained in non-human primates which showed that blood flow decreased in the OFC, frontal operculum (vlPFC), insula, and cingulate cortex following acute pramipexole administration (Black et al. 2002).
In contrast to pramipexole's large effect sizes on normalized metabolic activity within the OFC, amPFC, and vlPFC, we observed small, non-significant effects in some other regions affected by selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), such as the amygdala and pgACC (Drevets et al. 2002a; Fu et al. 2004; Kennedy et al. 2001). However, negative findings are difficult to interpret in this small sample, e.g. the post-hoc voxel-wise analyses showed a cluster in the left amygdala where metabolism decreased under pramipexole treatment, but where the peak voxel t value corresponded to p>0.001 (x=−20, y=−1, z=−17; Z=1.88, p= 0.03, k=4), raising the possibility of Type II error.
The association between lower pretreatment metabolism in the OFC and superior response to pramipexole is noteworthy in light of previous reports of inverse relationships between OFC activity and depression severity in major depressive disorder (Drevets, 2007; Drevets et al. 1992) and BD (Mah et al. 2007), reduced OFC activity in treatment-resistant depression (Mayberg et al. 2000), and elevated risk for development of depression with reduced OFC volume (Lai et al. 2000). These data, taken together with pre-clinical evidence of modulatory effects of OFC over emotional expression, are consistent with hypotheses that increased OFC activity reflects a compensatory response in depression (Drevets, 2007). The inverse relationship between OFC metabolism and response to pramipexole suggests that those least capable of mounting this compensatory response are most likely to benefit from pramipexole.
A limitation to interpreting the specificity of our findings was that the sample size was too small to establish whether pramipexole treatment significantly altered global CMRGlu, particularly since technical problems precluded analysis of quantitative CMRGlu for some subjects (see Methods section). Black et al. (2002) reported significant dose-related decreases in whole-brain cerebral blood flow (CBF) following acute pramipexole administration in healthy baboons (n=7, p<0.05), with a maximal change of −23 % at the intermediate dose tested of 50 μg/kg i.v. It is unclear whether such an effect would be expected under the experimental conditions of our study, in which pramipexole was administered orally on a chronic basis to human subjects with BD. Moreover, while CMRGlu and CBF are coupled under resting conditions, dopamine agonists may exert non-specific vascular effects that could alter CBF without affecting CMRGlu. Nevertheless, if chronic pramipexole administration similarly reduced global CMRGlu, then the reductions in normalized metabolism we observed in the OFC, amPFC and vlPFC under pramipexole would remain interpretable, since they would become more pronounced without global normalization. In contrast, under this scenario the findings of increased normalized metabolism in motor and premotor cortices, supplementary motor area, and accumbens area (Supplementary Table S2) would be considered non-specific since they may have been driven by a reduction in CMRGlu in other regions.
In summary, the present study suggests that the antidepressant efficacy of pramipexole augmentation for bipolar depression may have neural mechanisms that are partly similar to, and partly distinct from, those associated with other somatic antidepressant therapies. Further, the pramipexole-induced effects on regional metabolism provide additional support for a role of the central dopaminergic system in the pathophysiology of bipolar depression.
Supplementary Material
Acknowledgments
We thank Dr Yu-Fei Duan and the staff of the NIH PET Department for their assistance in data collection, and Dr Malcolm Binns for guidance regarding statistical analyses.
The study was supported by the Intramural Research Program at the National Institute of Mental Health, the National Institutes of Health, and the Department of Health & Human Services.
Statement of Interest Dr Mah receives research support from the Geoffrey H. Wood Foundation, the Scottish Rite Charitable Foundation, and the University of Toronto. Dr Manji and Dr Singh are currently at Johnson & Johnson Pharmaceutical Research and Development L. L. C. Dr Drevets served as a consultant to Pfizer on imaging biomarkers in 2009.
Footnotes
Clinical Trials Identifier : NCT00025792 Clinical Trial of Pramipexole in Bipolar Depression (http://clinicaltrials.gov).
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/pnp).
References
- Aiken CB. Pramipexole in psychiatry : a systematic review of the literature. Journal of Clinical Psychiatry. 2007;68:1230–1236. [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, et al. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proceedings of the National Academy of Sciences USA. 2002;99:17113–17118. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, et al. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biological Psychiatry. 2001;50:171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Fromm SJ, Nugent AC, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biological Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Carson RE, Daube-Witherspoon ME, Green MV. A method for postinjection PET transmission measurements with a rotating source. Journal of Nuclear Medicine. 1988;29:1558–1567. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology. 2002a;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, et al. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology Biochemistry and Behavior. 2002b;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, et al. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment : a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Archives of General Psychiatry. 2008;65:521–531. doi: 10.1001/archpsyc.65.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biological Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Jr., Singh J, Duan YF, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biological Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, et al. Regional metabolic effects of fluoxetine in major depression : serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, et al. Reciprocal limbic-cortical function and negative mood : converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moore DF, Altarescu G, Barker WC, Patronas NJ, et al. White matter lesions in Fabry disease occur in `prior' selectively hypometabolic and hyperperfused brain regions. Brain Research Bulletin. 2003;62:231–240. doi: 10.1016/j.brainresbull.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Archives of General Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-Analytic Procedures for Social Research. revised edn. Sage Publications; Newbury Park, CA: 1991. [Google Scholar]
- Swerdlow NR, Amalric M, Koob GF. Nucleus accumbens opiate-dopamine interactions and locomotor activation in the rat : evidence for a pre-synaptic locus. Pharmacology Biochemistry and Behavior. 1987;26:765–769. doi: 10.1016/0091-3057(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Publishing Group; New York: 1988. [Google Scholar]
- Zarate CA, Jr., Payne JL, Singh J, Quiroz JA, et al. Pramipexole for bipolar II depression : a placebo-controlled proof of concept study. Biological Psychiatry. 2004;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.