Abstract
Background
Atrasentan is a potent, oral, selective endothelin-A (ETA) receptor antagonist with clinical activity in patients with hormone-refractory prostate cancer (HRPC). This reports the results of a Phase 3, randomized, double-blind, placebo-controlled trial of atrasentan in patients with nonmetastatic HRPC.
Methods
Of 941 patients with adequate androgen suppression, no radiographic evidence of metastases but rising prostate-specific antigen (PSA) levels 467 received atrasentan 10 mg daily and 474 received placebo daily. The primary endpoint was time to disease progression (TTP) defined as the onset of metastases. Secondary endpoints were time to PSA progression, change in bone alkaline phosphatase (BALP), PSA doubling time, and overall survival.
Results
Atrasentan delayed median TTP by 93 days but the difference from placebo was not statistically significant (P = .288). Large geographical differences in median TTP were noted: in the United States (US), the difference was 81 days longer with placebo, whereas in non-US sites, the difference was 180 days longer with atrasentan. Atrasentan lengthened PSA doubling time (P = .031) and slowed the increase in BALP (P < .001). Median survival was 1477 days with atrasentan and 1403 days with placebo. The most common adverse events associated with atrasentan were peripheral edema, nasal congestion, and headache, consistent with the vasodilatory properties of ETA receptor antagonists.
Conclusions
While the primary endpoint was not achieved, large regional differences in TTP were noted, suggesting that trial conduct might have influenced the results. The biological activity is consistent with findings from other clinical trials of atrasentan in HRPC.
INTRODUCTION
Worldwide, over 200,000 men die annually from metastatic hormone-refractory prostate cancer (HRPC), including more than 80,000 in Europe and an estimated 27,050 in the US.1,2 Widespread use of medical castration has resulted in large numbers of men undergoing androgen-deprivation therapy (ADT) prior to developing objective metastases. This practice has created a disease state defined by rising prostate-specific antigen (PSA) as the first and only sign of HRPC. In these otherwise asymptomatic men, delaying the emergence of objective metastatic disease is a worthwhile goal, particularly if the intervention is also well tolerated. Exploiting the generally slow progression of nonmetastatic HRPC, an effective therapy could convert this into a chronic illness where overall mortality is no longer defined primarily by HRPC-specific death.
Since the profound response of prostate cancer to ADT was first described, one fact remains unchanged: the initial effectiveness is, ultimately, lost. Eventually, approximately 85% of men with HRPC develop metastatic disease, predominantly in bone,3 and once these lesions develop, prognosis is dire. Quality of life declines rapidly, primarily reflecting the morbidity associated with bone metastases,4 and median survival for patients with progressive, castrate, metastatic HRPC treated with chemotherapy is only 16 to 18 months.5,6
Endothelin-1 (ET-1) and the ETA receptor are implicated in prostate cancer progression.7–9ET -1 is produced by normal prostatic epithelium and by primary and metastatic prostate cancer. Plasma ET-1 concentrations are higher in men with HRPC than in men with localized disease or in healthy volunteers. The predominant endothelin receptor on normal prostatic epithelium, ETB, commonly loses expression in prostate cancer, while expression of ETA is increased and ultimately predominates as a consequence of transformation. Indeed, ETA receptor expression in prostate cancer cells increases with worsening histological grade and stage of disease.10,11 ET-1 binding to the ETA receptor acts as a survival factor in a host of benign and malignant cells, including prostate cancer. For example, ET-1 reduces paclitaxel-induced apoptosis in prostate cancer in vitro, an effect prevented by ETA receptor blockade with the selective ETA receptor antagonist atrasentan; similarly, the combination of paclitaxel and atrasentan decreased prostate tumor growth in vivo, significantly more than either agent alone.12
More importantly in the setting of bone-metastatic HRPC, prostate cancer–derived ET-1 facilitates interactions between ET-1–secreting tumor cells and the bone microenvironment, where it acts as a mitogen for osteoblasts, which express ETA receptors at high density, and inhibits osteoclast bone resorptive activity and motility, resulting in new bone growth.13,14ET -1 acting through the ETA receptor is causal in the development of osteoblastic metastases in animal models. As a potent ETA selective receptor antagonist, atrasentan significantly inhibits development of osteoblastic response to cancer in bone in a variety of model systems.
Atrasentan has demonstrated clinical activity in patients with HRPC.15–17In placebo-controlled, Phase2 and 3 clinical trials in patients with asymptomatic or symptomatic metastatic HRPC, atrasentan delayed disease progression and PSA progression, improving progression-free survival (PFS), and attenuating the rise in bone alkaline phosphatase (BALP). While treatment differences for some efficacy endpoints did not attain statistical significance for the intent-to-treat (ITT) population, they were consistently significant for the evaluable population in each trial. Coupling this clinical activity with preclinical evidence for the role of ET-1 and ETA in prostate cancer progression, the current trial was designed to test the hypothesis that atrasentan would delay time to disease progression (TTP) in men with nonmetastatic HRPC.
PATIENTS AND METHODS
Patient Selection
Eligible patients had histologically confirmed nonmetastatic HRPC, had undergone surgical or pharmacological (with maintenance androgen-suppression therapy) castration at least 3months before randomization, and had castrate testosterone levels at screening. Before randomization, PSA levels were at least 20ng/mL within 12 months, or had increased by 50% within 6 months (minimum of 1.0 ng/mL at screening), or were rising (two sequential increases with a confirmatory third increase) within 12months (minimum of 1.0 ng/mL at screening) with no radiographic evidence of metastases. Other inclusion criteria included a documented, minimum withdrawal period from anti-androgen therapy of 4 to 6 weeks before randomization; a Karnofsky Performance Score of at least 70; no other malignancies except non-melanoma skin cancer within the previous 5 years; and adequate hematologic, hepatic, and renal function.
Patients were ineligible if they were candidates for local salvage therapy or had received any of the following therapies for prostate cancer or associated pain: cytotoxic chemotherapy or radionuclides; external beam radiotherapy, brachytherapy, or cryotherapy to the prostatic bed within 6 months before randomization; radiation therapy to a lesion outside the prostatic bed more than 6 months after either castration or initiation of hormonal therapy; steroids within 6 months before randomization; or opioid analgesic therapy within 6months before randomization. Prohibited treatments included hormonally active therapies (other than gonadotropin-releasing hormone agonists), intravenous or oral bisphosphonates, any investigational product within 4 weeks before randomization, or prior or current treatment with an endothelin antagonist. Patients with current cardiovascular disability (New York Heart Association Class 2 or greater); significant pulmonary disease requiring chronic or pulse steroid therapy within the preceding 3months; or any clinically significant, unstable, uncontrolled disease were ineligible.
The institutional review boards or independent ethics committees of all participating investigational sites approved the protocol. Each patient or his legal representative signed and dated an approved informed consent form.
Treatment
Eligible patients were randomly assigned in a 1:1 ratio to double-blinded, daily oral administration of atrasentan 10 mg or matching placebo on an outpatient basis. Study treatment continued until patients experienced confirmed disease progression or unacceptable adverse effects, decided to discontinue taking the study treatment or to discontinue participation in the study, or until the study was completed. Patients with confirmed disease progression and those that were active on study at the time the double-blind period ended (31 May2006) were eligible to enroll in an open -label extension study in which all participants received atrasentan 10 mg daily. All patients were followed for survival at 3-month intervals after their final study visit until 31 January 2007.
If a patient experienced a treatment-related grade 3 or 4 toxicity according to National Cancer Institute Common Toxicity Criteria (NCI CTC), version 2.0, study drug was interrupted until the toxicity resolved to within one grade level of baseline, but not exceeding grade 2. If the toxicity recurred, study drug was interrupted again, and reinstated upon resolution as described for the initial occurrence. A second recurrence required discontinuation of study drug. If resolution was not achieved within 2 weeks of study drug interruption, study drug was to be discontinued.
Evaluations
Evaluations to ensure that patients met eligibility criteria and to establish baseline values were performed during a 35-day screening period prior to randomization. The absence of bone and soft tissue metastases was evaluated by bone scans and chest/abdominal/pelvic computed tomography (CT) or magnetic resonance imaging (MRI) scans, respectively, and confirmed by centralized, independent review.
Eligible patients were enrolled and randomly assigned study drug on day1. Subsequent clinic visits, during which patients were assessed for safety and clinical evidence of disease progression, were conducted at day14, weeks 4, 8, and 12, and every 6 weeks thereafter. Bone scans were performed at week12 and thereafter at 12 -week intervals and underwent centralized, independent review for determination of disease progression. PSA and biochemical bone markers were evaluated at weeks 4, 8, and 12 and every subsequent 12 weeks.
Any of the following events was indicative of disease progression: one or more new metastatic skeletal lesions seen on bone scan; one or more new metastatic extraskeletal lesions, atleast 1.5 cm in longest diameter, shown on CT or MRI scan; or an event attributed to metastatic prostate cancer (eg, upper urinary tract obstruction, spinal cord compression, pain) supported by radiographic, surgical, or pathologic evidence of disease. Anincrease in PSA was not considered disease progression. An independent radiologist(s) read all radiographs and independent oncologist(s) confirmed all disease progression events: independent reviewers were blinded to randomization status.
Safety was assessed by review of treatment-emergent adverse events, laboratory test results, and vital sign measurements.
Statistical Methods
TTP and PFS were the primary efficacy endpoints of the study for regulatory agencies in the US and Europe, respectively. TTP was defined as the time from randomization to onset of the earliest confirmed event of disease progression. PFS was defined as the time from randomization to the earliest onset of a confirmed event of disease progression, grade3 or 4 hypercalcemia within 7 days of the last dose of study drug, or death within 42 days of the last available evaluation. All events of disease progression were confirmed by centralized, independent review. For TTP, data were censored at the date of the last available evaluation in the absence of a confirmed event of disease progression. For PFS, data were censored as described for TTP with the addition of a confirmed event of hypercalcemia or death.
Secondary efficacy endpoints were time to onset of PSA progression (TTPSA), mean change from baseline to the final BALP value, PSA doubling time, and survival (measured without censoring data for subsequent treatments). TTPSA was defined as the time from randomization to the first of two or more consecutive postbaseline PSA measurements, obtained 14 or more days apart, that were at least 50% greater (minimum of 5ng/mL increase) than the patient’s PSA nadir. A patient’s PSA nadir was defined as the smallest PSA value among his baseline and postbaseline PSA values measured through study drug dosing day105. Data for patients without PSA progression were censored at the date of the last post-baseline on-study or off-study PSA measurement obtained no more than 7 days after the last study drug dose. PSA doubling time was calculated using the formula (natural logarithm of2)/slope of the linear regression fit to natural logarithm of PSA vs. time (years) relative to the first dose of study drug. PSA doubling times were categorized into the following intervals (in years): > 0 to 0.25, > 0.25 to 0.5, > 0.5 to 0.75, > 0.75 to 1.0, > 1.0 to 1.5, > 1.5 to 2.0, and > 2.0.
Tertiary efficacy endpoints included time to the first skeletal metastasis. All efficacy analyses were performed on all randomized patients.
The distributions of all time-to-event endpoints were estimated for each treatment group using Kaplan-Meier methodology, and comparisons between treatment groups were performed using a weighted log-rank G1,1 statistic, 18,19 stratified by region (US sites vs. non-US sites) and the Cox proportional hazards model. For the primary and secondary time-to-event endpoints, the stratified G1,1 was the primary statistic used for comparisons between treatment groups. Mean change from baseline to final value in biomarkers was calculated for each treatment group and compared using an analysis of covariance with treatment group as the factor and baseline value as the covariate. The Cochran-Mantel-Haenszel mean score test was used to compare the rate of PSA rise, as determined by PSA doubling time categories, between treatment groups.
Safety was assessed for the following variables: study drug exposure; incidence of treatment-emergent adverse events, including deaths and other serious adverse events; laboratory data, and vital sign measurements. The Fisher exact test was used to compare the incidence of adverse events between treatment groups. Safety analyses included all patients who received at least one dose of study drug.
Data from an earlier study in patients with metastatic HRPC were the basis of simulations indicating the need to enroll 900 to 1000 patients to realize 650events of disease progression and attain 90% power to detect a 25% difference in TTP. Since the patient population in the present study had earlier-stage disease, it was determined that 500 events of disease progression would provide 80% power to detect a treatment difference using the log-rank test if the hazard ratio in favor of atrasentan was 0.77. It was anticipated that 500 events of disease progression would be achieved by 31 May2006, the primary cutoff date for statistical analyses. The cutoff date for data used in survival analyses was 31 January2007.
Safety and efficacy data, summarized by treatment group, were reviewed by an independent data monitoring committee during the course of the study. The committee met on six occasions to assess safety only and performed two formal interim analyses of safety and efficacy. Interim efficacy analyses were governed by a one-sided, formal group sequential stopping rule20 that was subject to constraints on the maximum allowed boundary.21 Because of these protocol -specified interim analyses, statistical significance for the primary efficacy endpoint was determined by a two-sided adjusted P value ≤ 0.05.
RESULTS
Patient Characteristics
Between July 2001 and April 2003, 941 patients were randomized at 75 investigational sites in the US and 108 investigational sites outside of the US. Demographic and baseline characteristics were generally balanced between groups (Table 1). Figure 1 shows the allocation of patients to the placebo (N = 474) and atrasentan (N = 467) groups and patient disposition.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Placebo (N= 474) | Atrasentan (N= 467) |
|---|---|---|
| Age, years | ||
| Mean | 73.4 | 73.9 |
| SD | 7.79 | 7.81 |
| Median | 74.0 | 75.0 |
| Range | 48.0–93.0 | 47.0–92.0 |
|
| ||
| Race, number of patients (%) | ||
| White | 447 (94.3) | 420 (89.9) |
| Black | 23 (4.9) | 27 (5.8) |
| Other | 4 (0.8) | 20 (4.2) |
|
| ||
| Karnofsky Performance Score, number of patients (%) | ||
| 100 | 331 (69.8) | 326 (69.8) |
| 90 | 115 (24.3) | 109 (23.3) |
| 80 | 24 (5.1) | 23 (4.9) |
| ≤70 | 4 (0.8) | 8 (1.7) |
| Missing | 0 (0) | 1 (0.2) |
|
| ||
| Enrollment by regions, number of patients (%) | ||
| US | 189 (39.9) | 191 (40.9) |
| Non-US | 285 (60.1) | 276 (59.1) |
|
| ||
| Time since initial diagnosis, years | (N = 473) | (N = 467) |
| Mean | 7.2 | 7.5 |
| SD | 3.86 | 4.03 |
| Median | 6.9 | 7.1 |
| Range | 0.9–23.4 | 0.9–22.9 |
|
| ||
| PSA, ng/mL | (N = 474) | (N = 467) |
| Mean | 29.8 | 28.9 |
| SD | 60.16 | 54.64 |
| Median | 13.1 | 13.1 |
| Range | 0.8–672.2 | 1.2–732.9 |
|
| ||
| BALP, ng/mL | (N = 455) | (N = 446) |
| Mean | 14.3 | 14.4 |
| SD | 8.02 | 7.73 |
| Median | 13.2 | 14.1 |
| Range | 2.1–79.0 | 2.4–58.4 |
|
| ||
| Total Gleason score | (N = 401) | (N = 390) |
| Mean | 7.1 | 6.9 |
| SD | 1.43 | 1.62 |
| Median | 7.0 | 7.0 |
| Range | 2.0–10.0 | 2.0–10.0 |
Fig. 1.
Study Flow Chart
Efficacy
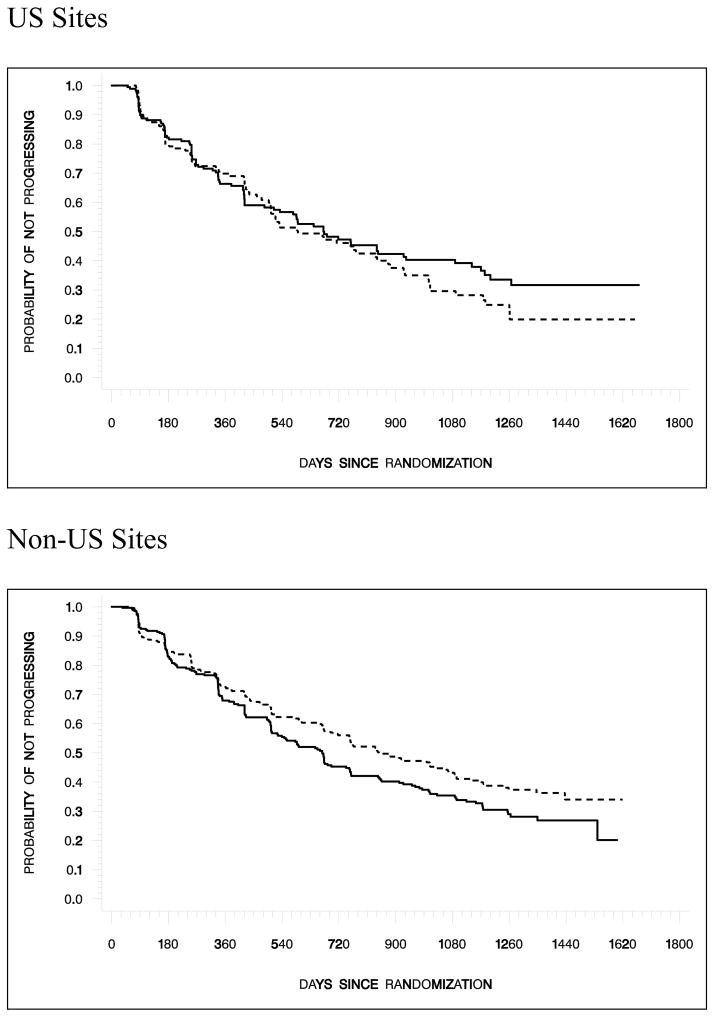
TTP and PFS endpoints were included to satisfy regulatory authorities in the US and Europe, respectively. Since the results for both endpoints were similar, only those for TTP are presented here (Table 2). Treatment with atrasentan delayed median TTP (764 days) compared with placebo (671 days); however, this 93-day delay was not statistically significant (P = .288) (Fig. 2a). When the data were analyzed by region (US vs. non -US), it became apparent that patients randomized to treatment with atrasentan at sites in the US had a shorter median TTP (590 days) than those at non-US sites (847 days), while median TTP was similar for placebo patients across regions(US sites, 671 days; non-US sites, 667 days) (Table 2 and Fig. 2b).
Table 2.
Analysis of Efficacy
| Endpoint | Placebo (N= 474) | Atrasentan (N= 467) | P Value |
|---|---|---|---|
| TTP (All sites) | |||
| Median, days | 671 | 764 | |
|
| |||
| TTP (US sites) | (N = 189) | (N = 191) | |
| Median, days | 671 | 590 | |
| TTP (Non-US sites) | (N = 285) | (N = 276) | |
| Median, days | 667 | 847 | |
| Hazard ratio for TTP (95% confidence interval) | .915 (.766, 1.092) | ||
| Stratified G1,1 P value | .288 | ||
|
| |||
| Overall survival (All sites) | |||
| Median survival, days | 1403 | 1477 | |
| Hazard ratio for overall survival (95% confidence interval) | .919 (.769, 1.098) | ||
| Stratified G1,1P value | .219 | ||
|
| |||
| PSA progression | (N = 467) | (N = 441) | |
| Median time to PSA progression, days | 253 | 254 | |
| Hazard ratio for PSA progression (95% confidence interval) | .919 (.784, 1.077) | ||
| Stratified G1,1 P value | .240 | ||
|
| |||
| PSA doubling time, years, % of patients | (N = 460) | (N = 414) | |
| >0 to 0.25 | 10.4 | 11.6 | |
| >0.25 to 0.5 | 25.4 | 18.6 | |
| >0.5 to 0.75 | 18.0 | 16.2 | |
| >0.75 to 1.0 | 9.8 | 9.7 | |
| >1.0 to 1.5 | 10.9 | 12.8 | |
| >1.5 to 2.0 | 5.4 | 5.8 | |
| >2.0 | 20.0 | 25.4 | |
| P value using Cochran -Mantel-Haenszel mean score test | .031 | ||
|
| |||
| Change from baseline in BALP, ng/mL | (N = 448) | (N = 425) | |
| Mean | 2.21 | −1.51 | |
| SE | .475 | .487 | |
| P value using analysis of covariance † | < .001 | ||
|
| |||
| Median time to first new skeletal lesion, days | 757 | 1008 | |
| Hazard ratio for time to first new skeletal lesion (95% confidence interval) | .880 (.722, 1.071) | ||
| Stratified G1,1 P value | .103 | ||
P value was determined using analysis of covariance with treatment group as the factor and baseline value as the covariate.
Fig. 2.
Fig. 2a. Kaplan -Meier Estimates of Time to Disease Progression
Median time to progression was 764 days with atrasentan and 671 days with placebo, corresponding to a hazard ratio (95% CI) of .915 (.766, 1.092) in the ITT population (P= .288).
Fig. 2b. Kaplan -Meier Estimates of Time to Disease Progression (US and Non-US)
Median time to progression was 590 days with atrasentan and 671 days with placebo at sites in the US and 847 days with atrasentan and 667 days with placebo at sites outside of the US. Hazard ratios (95% CI) and P values (G 1,1) were 1.154 (.862, 1.545), P= .177 for US sites and .800 (.639, 1.000), P = .021 for non -US sites.
Overall, a significantly higher percentage of atrasentan patients discontinued prematurely (atrasentan, 33.2% vs. placebo, 25.9%; P = .015). Premature discontinuations were significantly more frequent at US sites (40.8%) than non-US sites (21.9%) (P < .001). Respective cumulative discontinuation rates for atrasentan and placebo patients at US sites were 45.5% and 36.0%, while at non-US sites they were 24.6% and 19.3%. Adverse events were the most frequent primary explanation by investigators for premature discontinuations, and the percentage of atrasentan patients discontinuing for this reason was more than twice that of placebo patients overall (atrasentan, 15.8% vs. placebo, 7.4%) and by region (US: atrasentan, 20.9% vs. placebo, 9.5%; non-US: atrasentan, 12.3% vs. placebo, 6.0%). Although an increase in PSA was not considered disease progression per protocol, differences between US sites and non-US sites were noted in mean PSA increase from the penultimate to the last visit and the mean PSA values at the last visit for patients in both treatment groups (Table 3). In all cases, the mean increase in PSA and mean PSA values were lower at US than non-US sites.
Table 3.
PSA Levels among Patients Discontinuing the Study
| Region | Treatment Group | Mean PSA (ng/mL)
|
|||
|---|---|---|---|---|---|
| N | Penultimate Visit | Last Visit | Increase | ||
|
| |||||
| US | Atrasentan | 75 | 29.2 | 41.7 | 12.5 |
| Placebo | 61 | 36.8 | 53.2 | 16.4 | |
|
| |||||
| Non-US | Atrasentan | 61 | 65.9 | 86.1 | 20.3 |
| Placebo | 50 | 79.6 | 98.2 | 18.6 | |
A greater percentage of patients in the placebo group (56.3%) than in the atrasentan group (48.6%) had confirmed disease progression. New extraskeletal lesions and metastatic prostate cancer events were cited as the primary manifestation of disease progression by similar percentages of patients in the placebo (8.0% and 4.0%, respectively) and atrasentan (8.8% and 3.6%, respectively) groups. However, new skeletal lesions were cited as the primary reason for disease progression by a greater percentage of placebo patients (44.3%) than atrasentan patients (36.2%). Treatment with atrasentan delayed the median time to initial presentation with skeletal metastases by approximately 250 days (1008 days, atrasentan vs. 757 days, placebo; P = .103).
Median TTPSA progression was similar for the two groups (254 days for atrasentan and 253 days for placebo; P = .240). The rate of PSA rise as measured by doubling time distribution was significantly slower for atrasentan than for placebo (P = .031). Atrasentan significantly attenuated the rise in BALP from baseline to final assessment (mean change of −1.51 ng/mL, atrasentan vs. +2.21 ng/mL, placebo; P = .001).
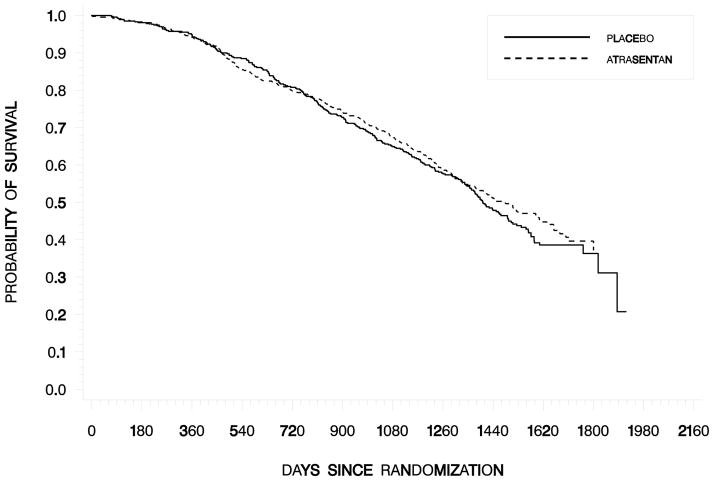
Median survival was 1477 days with atrasentan and 1403 days with placebo. Interpretation about the possible effect of atrasentan on overall survival is difficult since the survival data were not censored for subsequent treatment and 54% of placebo patients subsequently enrolled in the open-label study and received atrasentan.
Safety
Common treatment-emergent adverse events reported significantly more often with atrasentan included peripheral edema, nasal congestion, headache, dyspnea, and anemia (P ≤ .029). With placebo, these included constipation and hypertension (P ≤ .006) (Table 4). Most of these events reported with atrasentan were grade1 or 2 and resolved during the treatment period with or without the use of ancillary medication. Generally, the median time to onset of these events was within the initial 30days; the median time to onset of anemia was 225 days after starting treatment. Most important, there was no evidence of cumulative toxicity. All of these events have been observed in patients receiving atrasentan in earlier clinical trials. Peripheral edema, nasal congestion, dyspnea, and headache are consistent with the vasodilatory effects of ETA receptor blockade, while anemia reflects an underlying mechanism of plasma volume expansion resulting in hemodilution.
Table 4.
Treatment-Emergent Adverse Events
| Event | Placebo % of Pts (N= 470) | Atrasentan % of Pts (N= 462) | P value † |
|---|---|---|---|
| Any AE | 96.6 | 99.4 | .004 |
|
| |||
| Any AE, grade 3 or 4 | 44.7 | 46.5 | |
|
| |||
| Any SAE | 39.6 | 43.9 | |
|
| |||
| Any SAE resulting in death | 4.7 | 3.5 | |
|
| |||
| Deaths within 30 days of last dose of study drug | |||
| While still receiving study drug | 0.9 | 0.6 | |
| While no longer receiving study drug | 3.8 | 2.6 | |
|
| |||
| AEs resulting in discontinuation | 16.4 | 26.4 | < .001 |
|
| |||
| AEs all grades; incidence of ≥ 15% in either group‡ Preferred term | |||
| Peripheral edema | 21.1 | 49.4 | < .001 |
| Nasal congestion | 11.3 | 39.4 | < .001 |
| Fatigue | 15.1 | 17.7 | |
| Headache | 11.3 | 17.7 | .005 |
| Pain | 18.1 | 16.0 | |
| Back pain | 17.4 | 15.6 | |
| Dyspnea | 9.6 | 15.6 | .007 |
| Hematuria | 16.2 | 14.9 | |
| Constipation | 21.5 | 14.5 | .006 |
| Urinary tract infection | 15.1 | 13.2 | |
| Diarrhea | 15.5 | 12.3 | |
|
| |||
| AEs, grade3 or 4; incidence of ≥ 2% in either group Preferred term | |||
| Obstructive uropathy | 7.2 | 8.2 | |
| Urinary retention | 4.0 | 3.7 | |
| Hematuria | 2.8 | 3.5 | |
| Peripheral edema | 0 | 3.0 | < .001 |
| Anemia | 2.8 | 2.6 | |
| Bladder obstruction | 2.8 | 2.4 | |
| Bone pain | 1.9 | 2.2 | |
| Hydronephrosis | 2.1 | 1.9 | |
| Fracture | 2.8 | 1.5 | |
| Pain | 2.3 | 1.5 | |
| Myocardial infarction | 2.1 | 0.9 | |
| Fall | 2.1 | 0.2 | .011 |
Treatment-emergent adverse events were coded using the Medical Dictionary for Regulatory Activities, version 9.0.
P values for pairwise comparisons were determined using the Fisher exact test. If no P value is shown, the difference was not statistically significant.
Anemia and hypertension are not included in the table because the incidence was < 15% in each group (anemia: 7.9%, placebo vs. 12.3%, atrasentan; P = .029, and hypertension: 10.4%, placebo vs. 3.7%, atrasentan; P < .001).
A greater percentage of patients in the atrasentan group (26.4%) than in the placebo group (16.4%) experienced adverse events that resulted in discontinuation of study drug. Serious adverse events occurred at similar frequencies in both treatment groups (Table 4). The incidence of deaths resulting from serious adverse events also was similar between the atrasentan (3.5%) and placebo (4.7%) groups. As a class, endothelin receptor antagonists have demonstrated an increased incidence of cardiopulmonary events, which is likely related to their underlying vasoactive mechanism. Heart failure was the only cardiopulmonary event that occurred significantly more frequently with atrasentan (6.7%, atrasentan vs. 3.0%, placebo; P = .009) (Table 5). The median time to onset of heart failure was 47 days (range: 7days to 506 days). The overall incidence of cardiopulmonary events was low, and atrasentan-treated patients with heart failure tended to be older and have histories of significant cardiac disease compared with atrasentan-treated patients who did not experience heart failure (P ≤ .05). No atrasentan-treated patient died from heart failure or myocardial infarction. Aside from an increased incidence of anemia in the atrasentan group, there were no statistically or clinically significant differences between treatment groups in hematology and chemistry laboratory data.
Table 5.
Treatment-Emergent Cardiopulmonary Events of Interest
| Event | Placebo % of Pts (N = 470) | Atrasentan % of Pts (N= 462) | P value† |
|---|---|---|---|
| Arrhythmia (pooled) ‡ | 8.9 | 9.5 | |
| Heart failure (pooled) § | 3.0 | 6.7 | .009 |
| Hypotension | 3.4 | 4.1 | |
| Pneumonia | 3.2 | 3.9 | |
| Angina pectoris | 2.6 | 1.9 | |
| Coronary artery disease | 1.5 | 1.7 | |
| Myocardial infarction | 2.6 | 1.5 | |
| Cerebrovascular accident | 1.7 | 1.1 | |
| Transient ischemic attack | 0.4 | 1.1 | |
| Tachycardia | 1.1 | 0.6 | |
| Deep vein thrombosis | 1.1 | 0.4 |
P values for pairwise comparisons were determined using the Fisher exact test. The only statistically significant treatment group difference was for heart failure (pooled adverse events).
Arrhythmia includes pooled terms of arrhythmia, atrial fibrillation, atrial flutter, atrial tachycardia, bradycardia, cardiac flutter, extrasystoles, heart rate increased, heart rate irregular, palpitations, sinus arrhythmia, supraventricular tachycardia, tachycardia, and ventricular extrasystoles.
Heart failure includes pooled terms of cardiac failure, cardiac failure congestive, cardiogenic shock, left ventricular failure, pulmonary edema, and right ventricular failure.
DISCUSSION
Despite a clear demonstration of atrasentan’s biological activity in this randomized, placebo-controlled, multinational trial in patients with nonmetastatic HRPC, confirming earlier observations of its effects on PSA and biomarkers of bone disease, we were not able to show a significant treatment difference in TTP or survival. Treatment with atrasentan resulted in a significantly slower rate of PSA rise, a significant attenuation of BALP rise, and a trend toward a delay in the time to initial presentation with skeletal metastases. Although more atrasentan than placebo patients discontinued the study because of adverse events, the safety profile of atrasentan was consistent with that seen in earlier trials, primarily reflecting the vasodilatory effects of ETA receptor blockade, without evidence of cumulative toxicity or hematologic toxicity.
The obvious question is why didn’t the effects of atrasentan on PSA, biomarkers of bone lesions, and the incidence of bone lesions translate to a significant effect on the disease course and a delay in TTP. Atrasentan did show a favorable prolongation of TTP among patients at sites outside the US—representing the majority of patients enrolled in the study—whereas it did not delay TTP among US patients. Discontinuation for reasons other than disease progression was notably high in this trial. Almost 30% of all patients discontinued prematurely, and the percentage of atrasentan patients that discontinued for reasons other than disease progression was significantly greater than that of placebo patients. A high premature discontinuation rate has been reported previously in a multinational trial enrolling patients with metastatic HRPC.22In this trial, two -thirds or more of patients in each of three treatment groups (zoledronic acid 4 mg, zoledronic acid 8 mg reduced to 4mg, and placebo) discontinued prematurely and did not complete the 15-month treatment regimen. The most frequently cited reasons for premature discontinuation included consent withdrawal, adverse events, and death.
In the current trial, premature discontinuations were almost twice as frequent at US sites than at non-US sites. Cumulative discontinuation rates were higher for both treatment groups at US sites compared with non-US sites. Discontinuations because of adverse events were also more frequent at US sites and more than twice as frequent among atrasentan patients in both regions.
Are these observations indicative of regional differences in patient populations or physician practice patterns? The regional differences for placebo patients in both TTP and discontinuation rates were minimal, suggesting that differences in patient populations in the two regions were not a major factor. In contrast, a shorter median duration of treatment was seen for both treatment groups at US sites compared with non-US sites. Regional differences in physician practice patterns may have contributed to observed differences in treatment duration. At US sites, a shorter median duration of treatment was seen for both treatment groups compared with non-US sites. Another regional difference was in PSA levels at the time of premature discontinuation. Interestingly, while the percentage of patients that discontinued prematurely because of PSA progression was similar for both treatment groups overall, the mean increase in PSA from the penultimate to last visit and the mean PSA value at the last visit were lower at US sites than at non-US sites (Table 3). These data suggest that physicians and/or patients were more inclined toward premature discontinuation at US than non-US sites. Alternative treatment options may have been more available at US sites, but whether this contributed to differences in treatment duration and incidence of premature discontinuation is merely speculative.
The safety profile seen for atrasentan in this trial was consistent with observations in previous trials and its mechanism of action as a selective ETA receptor antagonist. The overall incidence of cardiopulmonary events was low, and the only cardiopulmonary event significantly more common in atrasentan than placebo patients was heart failure. Among atrasentan-treated patients, those with heart failure tended to be older and have histories of significant cardiac disease. The low incidence of hematologic toxicity seen with atrasentan treatment supports its use in combination with chemotherapy agents that affect hematologic parameters, such as docetaxel.
Atrasentan treatment did demonstrate biological activity, as evidenced by effects on biomarkers of disease progression. Significant differences from placebo in PSA doubling time and changes in BALP levels were seen with atrasentan treatment. Effects on bone lesions also were seen, with a median delay of approximately 250 days in initial presentation of skeletal metastases with atrasentan. Among patients with confirmed disease progression, fewer were treated with atrasentan (48.6%) than placebo (56.3%), and new skeletal metastases were cited as the primary disease progression event in a smaller percentage of atrasentan (36.2%) than placebo (44.3%) patients. These observations of the effects of atrasentan on bone and bone markers are consistent with preclinical findings and those seen in Phase2 and 3 clinical trials. 15–17,23The favorable effects of atrasentan on PSA are also consistent with observations from other clinical trials.
The evidence of biological activity, especially relevant to bone metastases, and the acceptable safety profile of atrasentan, particularly the lack of hematologic toxicity, warrant further evaluation of atrasentan in combination with chemotherapy agents in prospective, randomized, controlled trials. Once such trial, sponsored by the Southwest Oncology Group (SWOG-0421), is ongoing in HRPC patients with bone metastases in order to evaluate possible synergy between atrasentan and docetaxel.
Fig. 3. Kaplan-Meier Estimates of Overall Survival.
Median duration of survival was 1477 days with atrasentan and 1403 days with placebo, corresponding to a hazard ratio (95% CI) of .919 (.769, 1.098) in the ITT population (P= .219).
Acknowledgments
Grant support: Supported by a grant from Abbott Laboratories, Abbott Park, Illinois
We thank Rachelle Weiss, PhD, and Sarah Duban, ELS, for their excellent assistance in preparing this manuscript.
Footnotes
Previously Presented: American Society of Clinical Oncology 43rd Annual Meeting, 2007
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Disani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Chung LWK. Bone metastases: improving the therapeutic index. Semin Oncol. 1994;12:630–656. [PubMed] [Google Scholar]
- 4.Cella D, Petrylak DP, Fishman M, Tergland C, Young J, Mulani P. Role of quality of life in men with metastatic hormone-refractory prostate cancer: How does atrasentan influence quality of life? Eur Urol. 2006;49:781–789. doi: 10.1016/j.eururo.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JB, Chan-Tack K, Hedican SP, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 9.Nelson JB, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Cancer Rev. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 10.Gohji K, Kitazawa S, Tamada H, et al. Expression of endothelin receptor A associated with prostate cancer progression. J Urol. 2001;165:1033–1036. [PubMed] [Google Scholar]
- 11.Godara G, Pecher S, Jukic DM, et al. Distinct patterns of endothelin axis expression in primary prostate cancer. Urology. 2007;70:209–215. doi: 10.1016/j.urology.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Akhavan A, McHugh KH, Guruli G, et al. Endothelin receptor A blockade enhances taxane effects in prostate cancer. Neoplasia. 2006;8:725–732. doi: 10.1593/neo.06388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JJ, Mohammad KS, Kakonen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. [accessed Aug 3, 2007];J Transl Med [serial online] 2004 2:16. doi: 10.1186/1479-5876-2-16. Available from URL: http://www.translational-medicine.com/content/2/1/16. [DOI] [PMC free article] [PubMed]
- 15.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JB, Nabulsi AA, Vogelzang NJ, et al. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003;169:1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 17.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 18.Fleming TR, Harrington DP. Weighted logrank statistics, Counting processes and survival analysis. New York: John Wiley & Sons; 1991. pp. 255–271. [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- 20.Kittelson JM, Emerson SS. A unifying family of group sequential test designs. Biometrics. 1999;55:874–872. doi: 10.1111/j.0006-341x.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 21.Emerson SS, Bruce AG, Baldwin K. S + SeqTrial User’s Manual. Seattle, WA: Mathsoft, Inc; 2000. [Google Scholar]
- 22.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 23.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97(3 Suppl):779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]