Abstract
Female sexual behavior in rodents, typified by the lordosis posture, is hormone-dependent and sex-specific. Ovarian hormones control this behavior via receptors in the hypothalamic ventromedial nucleus (VMH). This review considers the sex differences in the morphology, neurochemistry and neural circuitry of the VMH to gain insights into the mechanisms that control lordosis. The VMH is larger in males compared with females, due to more synaptic connections. Another sex difference is the responsiveness to estradiol, with males exhibiting muted, and in some cases reverse, effects compared with females. The lack of lordosis in males may be explained by differences in synaptic organization or estrogen responsiveness, or both, in the VMH. However, given that damage to other brain regions unmasks lordosis behavior in males, a male-typical VMH is unlikely the main factor that prevents lordosis. In females, key questions remain regarding the mechanisms whereby ovarian hormones modulate VMH function to promote lordosis.
Keywords: estradiol, estrogen receptor, hypothalamus, lordosis, reproductive behavior, sexual differentiation, ventromedial nucleus
I. INTRODUCTION
The basic pattern of sex differences in mating behavior has been conserved by evolution. As a case in point, in the well-studied insect model system, drosophila melanogaster, females display a receptive posture while approached from behind for insemination by males [1]. This invertebrate model system has proven useful for the dissection of the genetic controls of these behaviors. Such sex-specific mating behaviors also are seen in mammals. The laboratory rat is a widely used, tractable model system for the study of the neural controls that underlie mammalian mating behavior [2]. Rather than direct effects of sex chromosomes on the patterning of brain sex differences, as occurs in insects, abundant evidence suggests that in rodents the presence or absence of gonadal steroids during development exerts epigenetic effects in the brain to specify the adult mating behavior [3]. In particular, the absence of hormone action during development is associated with lordosis behavior in adult female rats.
During the hours just before ovulation, a female rat becomes sexually receptive. At this time, courting males approach the female and attempt to mount her to elicit the receptive stance in females, termed the lordosis reflex. This reflex is gated by the sequential actions of the ovarian hormones estradiol and progesterone. Ovariectomy prevents the display of the lordosis reflex, and this effect is reversed by estradiol and progesterone replacement [4]. The lordosis response is not exhibited by intact males or even by adult castrated males treated with ovarian hormones [5], although there are some illuminating exceptions to this general rule, to be discussed below. Therefore, this behavior has provided a platform to examine the neurological mechanisms underlying a sex-specific mammalian behavior. The goal of this review is to consider what has been learned about sex differences in the neural circuitry that underlies female rat mating behavior.
The hypothalamic ventromedial nucleus (VMH) is a critical site of ovarian hormone action to permit the lordosis response, as detailed below. Axonal projections from the VMH to the periaqueductal gray enable the lordosis reflex by modulating posture-control relays to the reticular formation [6; 7]. A few sex differences have been observed in the periaqueductal gray itself [8]. Given that considerably more attention has been focused on sex differences in the VMH than its downstream relays, the present review will attend to the ontogeny of the VMH and the various sex differences that have been observed. I also will consider mechanistic studies that have begun to elucidate the manner in which sex differences are established in the VMH. Lastly, I will discuss how the pattern of sex differences in the VMH may elucidate the neural components that contribute to VMH function.
II. THE BEHAVIORAL SIGNIFICANCE OF THE VMH
Numerous lines of evidence support the important role of the VMH in female rat sexual behavior. First, the VMH can retain estradiol and progesterone [9; 10], based on its ability to express estrogen and progestin receptors [11; 12; 13]. The local application of ovarian hormones to the VMH is sufficient for the behavioral effectiveness of these hormones [14; 15; 16], which further implicates steroid receptors in the VMH as a major target in the control of lordosis. Moreover, VMH lesions disrupt sexual behavior in female rats [17; 18], as do transections of the axonal projections that arise from the VMH and descend to the midbrain [19]. Conversely, electrical stimulation of the VMH enhances lordosis responses in the VMH [20]. Finally, female sexual behavior induces immediate early gene expression in the rat VMH, indicative of neuronal activation [21; 22; 23; 24; 25; 26]. These results are consistent with electrophysiological studies that showed that VMH neurons are responsive to flank and VCS stimulation [27]. Thus, the fundamental role of the VMH in the control of reproductive behavior in female rats is well established.
Beyond laboratory rats, the importance of the VMH for female sexual behavior has been documented in a range of other mammals. For example, the VMH contributes to the lordosis response in guinea pigs [28], hamsters [29; 30; 31], cats [32], and sheep [33]. Studies in reptiles, such as whiptail lizards [34; 35], have indicated that this neural mechanism is phylogenetically old. Furthermore, electrophysiological recordings have supported an active role of the VMH in female sexual behavior in non-human primates [36]. Although mice have not been the mainstay for the behavioral neuroscience of female reproductive behavior, transgenic mice have been tremendously informative regarding the role of developmental transcription factors and ovarian hormone receptors in VMH structure and function [37; 38; 39; 40; 41]. The similarities and differences between mice and rats make the case for both species providing valuable complementary information [42]. Thus, it remains true that the elucidation of the neural mechanisms within the VMH that mediate female mating behavior in laboratory rats may pertain to diverse vertebrate species.
With regard to the human VMH, neuroanatomical studies suggest a similar organization compared with other species, including its cytoarchitecture, source of afferents, and the peptide content of its afferents [43; 44; 45; 46; 47]. In addition, the human VMH expresses estrogen, androgen and oxytocin receptors [48; 49], as seen in other species, and a variety of sex differences have been reported in the human VMH [50]. A functional imaging study suggested that a sex-specific activation of the ventromedial hypothalamic area occurs in humans after exposure to putative pheromones [51]. These findings suggest that many aspects of the human VMH have been conserved, and that it is not only sexually dimorphic, but it may processes sexually relevant information.
Interestingly, the VMH has been implicated in both male and female mating behavior [24; 52; 53], although the role of the VMH in male mating behavior is in need of further study. In this regard, it is important to remember that while females experience cyclic variations in gonadal hormones, the levels in males remain steady across days. A better understanding of VMH neural circuitry will provide a better explanation the neural basis of sex-specific, hormone-gated behaviors. Next, I will consider the ontological events that establish the VMH as a first step towards explaining the sex differences in the structure and function of the VMH.
III. GENERAL FEATURES OF THE VMH
A. DEVELOPMENT OF THE VMH
The VMH is defined as an elliptical condensation of neurons surrounded by a cell-poor fiber-rich zone in the caudal, mediobasal hypothalamus. This basic structure is apparent before birth in rats. As with other hypothalamic cell groups, VMH development begins with neurogenesis in the proliferative zone of the third ventricle. In the case of the VMH, neurons are born around embryological day 10 (E10; as reviewed by [54]). Terminally mitotic cells migrate ventrolaterally along the processes of radial glia and then differentiate into neurons. A discernable cytoarchitectonic VMH is visible between E18 and E19 in rats [55]. Axonal projections then are established and synaptic inputs are arranged. The surrounding neuropil that makes the VMH so conspicuous histologically includes dendrites extending from VMH neurons, and these dendrites provide a receptive zone for axons arising from other brain regions [56].
An array of transcription factors contributes to the ontogeny of hypothalamic neurons during brain development. For example, Islet-1 is a marker for the developing hypothalamus [57]. Nkx2.1 has a narrower role, establishing the medial basal hypothalamus [58]. More restricted still, the normal development of the VMH depends on the expression of a transcription factor known as SF-1, officially designated NR5A1, encoded by the FTZ-F1 gene, with a rat homolog identified as Ad4BP [59; 60; 61]. This protein is a member of the orphan nuclear receptor superfamily, and within the brain it is uniquely expressed in the VMH [61]. When the expression of SF-1 is disrupted, a striking malformation of the VMH occurs, with various cell types inappropriately positioned within or outside the nucleus [62; 63; 64]. When SF-1 is selectively disrupted in the brain, the resulting malformation of the VMH is associated with impaired lordosis behavior [65].
In sum, the VMH develops as a typical hypothalamic nucleus, although it is unique in its expression and developmental regulation by SF-1, which contributes to the spatial organization of VMH neurons. With the importance of the developmental positioning of VMH neurons in mind, it becomes clear that another key feature of VMH function would be the chemical phenotypes of its neurons. As discussed below, several important phenotypic markers have emerged for VMH neurons, although our understanding is not yet complete.
B. CYTOARCHITECTURE OF THE VMH
The VMH has been parceled into two hemi-ovals, the dorsomedial (DM-VMH) and the ventrolateral (VL-VMH), with a narrow cell-poor central region between them [56; 66; 67; 68; 69]. The DM-VMH and the VL-VMH differ in their patterns of gene expression, as summarized in Table 1. Soma size in the VL-VMH is larger than soma size in the DM-VMH and the central region [70]. Analyses of the subdivision-specific afferents and projection targets also indicate unique patterns of connectivity for these subdivisions [67]. The surrounding shell, also referred to as the fiber plexus, the neuropil, or the lateral rim, contains axonal processes from other brain regions containing neurotransmitters and modulators, including norepinephrine, serotonin, gonadotropin releasing hormone, and oxytocin [71; 72; 73]. There also are sparse neurons found in the shell [74].
Table 1.
Phenotypic markers that are specifically localized to the ventrolateral (VL-VMH) versus the dorsomedial (DM-VMH).
| VL, not DM | DM, not VL | |
|---|---|---|
| Receptors | ER-α, OTR | GHRH-R |
| Peptides, Growth Factors, signaling |
Enk, SST, SubP, CCK, nNOS | NPY, BDNF |
| Transcription Factors | Islet-1, Nkx2.1 | SF-1 |
Abbreviations: Brain-derived neural growth factor, BDNF; Cholecystokinin, CCK; Enkephalin, Enk; Estrogen receptor-α; ERα; Growth hormone releasing hormone receptor, GHRH-R; Neuronal nitrous oxide synthase, nNOS; Neuropeptide Y, NPY; Oxytocin receptor, OTR; Somatostatin, SST; Substance P, SubP. References: [9; 57; 63; 105; 106; 174; 175; 176; 177; 178; 179; 180; 181; 182].
VMH neurons maintain a simple dendritic tree, with the following characteristics: a single very long primary dendrite, a few much shorter primary dendrites, and a few secondary dendrites [75]. The long primary dendrite may be uniquely positioned to contact afferents terminating in the fiber plexus surrounding the VMH. The length of these long primary dendrites are regulated by a variety of physiological conditions [76; 77; 78; 79], thus potentially titrating VMH sensitivity to extranuclear inputs. The short primary dendrites, in contrast, may be situated to receive input from local interneurons. In this way, individual VMH neurons can integrate local computations with afferent inputs arriving from other brain regions.
At the ultrastructural level, both excitatory and inhibitory synaptic contacts exist in the VMH, with approximately half of these remaining after deafferentation of the VMH [80]. About one third of the synapses are axospinous and appear to be exclusively excitatory based on their ultrastructure. A relatively small portion, approximately 10 to 15 percent, of the axodendritic synapses are inhibitory, again based on ultrastructure. Thus, the VMH is rife with intranuclear connectivity, including excitatory inputs to dendritic spines.
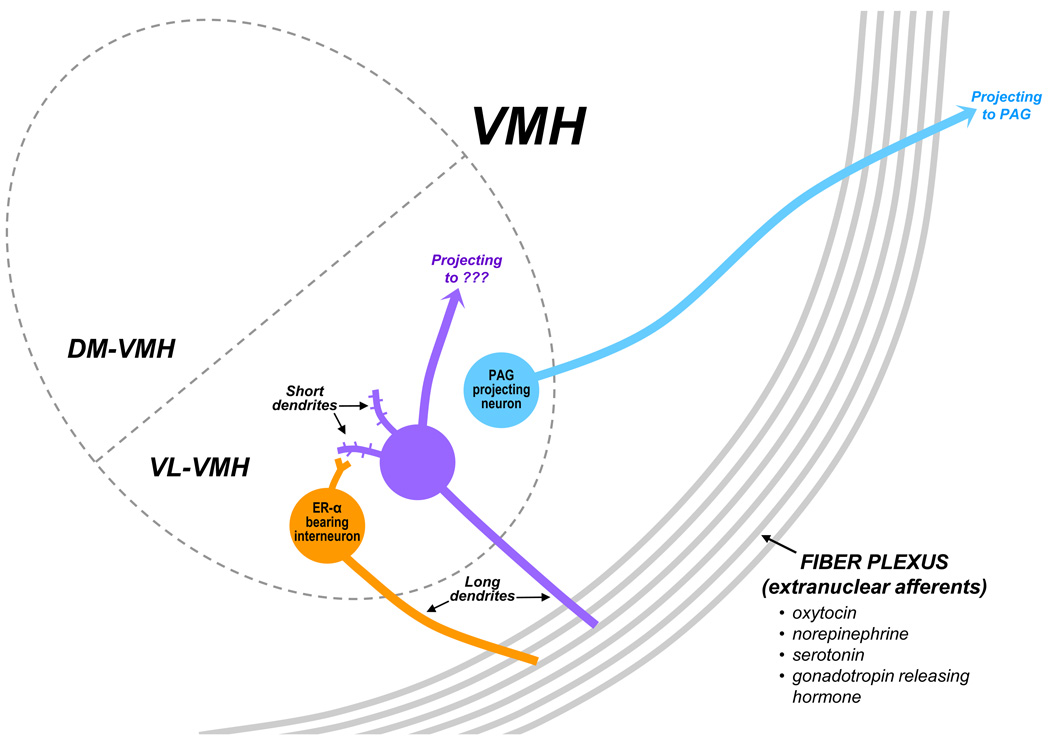
Several lines of evidence suggest that the VMH is not homogenous, but instead is comprised of several cell types (summarized in Figures 1 and 2). For example, several markers are co-localized with the alpha subtype of the estrogen receptor (ER-α), whilst others are not, as detailed below. It should be noted that many studies have used nuclear labeling to identify the ER-α-expressing cells. Given that membrane-associated ER-α may be more difficult to visualize with immunohistochemistry, it remains unclear whether these same neurons also mediate the membrane-based effects of estradiol discussed below.
FIGURE 1.
Working model of the cell types in the VL-VMH, including their dendritic arbors, axonal targets and possible connections between them. A) The ER-containing neurons (gold) are known to release glutamate as their neurotransmitter and may serve as local interneurons. Other neurons (purple) exhibit estradiol-induced increases in dendritic spines. A third cell type (blue) sends descending projections to the periaqueductal gray (PAG) to affect behavior. All three cell types extend long dendrites into the lateral fiber field, which includes axonal fibers containing a variety of peptides and transmitters.
FIGURE 2.
Summary of our current understanding of the neuron types in the VMH. Several lines of evidence indicate that the ERα-containing neurons are largely separate from the PAG-projecting neurons. Various markers have been co-localized with the ERα-containing neurons. The available evidence suggests that there is a third cell type, that is, non-ERα-containing neurons and non-PAG-projecting neurons. Although several features may be attributed to these neurons, it is not yet known if this represents a single cell type. Abbreviations: Enkephalin, Enk; Estrogen receptor-α; ERα; Neuronal nitrous oxide synthase, nNOS; Oxytocin receptor, OTR; Vesicular glutamate transporter 2, vGlut2. References: [75; 81; 82; 83; 84; 85; 94; 102; 103; 104; 109].
As a second cell type, my laboratory has observed that the ER-α-containing neurons are largely a separate population from those neurons with axonal connections to the periaqueductal gray [81; 82; 83], with approximately 15 to 25% overlap. Somewhat surprisingly, mating-induced Fos expression occurs mainly in neurons that lack both ER-α and axonal projections to the midbrain [82], thus indicating a third cell type. Likewise, other groups have observed that mating cues activate Fos expression in many VMH neurons that lack immunostaining for nuclear receptors for ovarian hormones [84; 85]. In particular, these papers quantified that less than half of the mating cue-induced Fos labeling was found in neurons with estrogen or progestin receptors. Although the neurochemistry of these mating-activated neurons remains undetermined, previous work from our laboratory suggests that such neurons alter their synaptic connectivity in response to estradiol treatment [75]. Thus, elements of the VMH circuit that participate in lordosis behavior includes ER-α-containing neurons, midbrain-projecting neurons, and neurons that neither express a key estrogen receptor nor project to the critical downstream relay, but are activated during mating. Further studies are needed to clarify the extent of involvement of these neurons that neither express a key estrogen receptor nor project to the critical downstream relay in mediating lordosis behavior.
Taken together, a key structural feature of the VMH is its functionally distinct domains, with the VL-VML likely to be especially important for mating behavior. VMH neurons have a signature dendritic tree that allows for the integration of intrinsic and extrinsic activity and exhibits plasticity. Finally, at least three cell types within the VMH contribute to the control of female sexual behavior, although the connectivity between these cell types and key aspects of their neurochemistry has not been determined.
C. NEUROCHEMISTRY OF THE VMH
Several receptors, neuropeptides, and transcription factors intrinsic to the VMH are common across VMH subdivisions, whereas others are found in one region and not the other, as listed in Table 1. Many of the markers specifically localized within the VL-VMH have been implicated in the control of female sexual behavior, most notably estrogen receptor subtype α (ER-α) [86]. The aggregation of these lordosis-modulating factors within the VL-VMH has contributed to the notion that the control of female sexual behavior is housed within the VL-VMH rather than the VMH more broadly.
An important neurotransmitter in the VMH is gamma-aminobutyric acid (GABA). GABA is present in the VMH, based on immunoelectron microscopy [87], as well as light-level immunohistochemistry [88], which revealed terminals labeled for the GABA biosynthetic enzyme glutamic acid decarboxylase-65 (GAD-65). Electrophysiological recordings of dissociated VMH neurons found spontaneous GABAergic inhibitory postsynaptic currents [89], which suggested that the GABA neurons may be local interneurons. Numerous studies have indicated that GABA activity in the VMH is regulated by estradiol levels and modulates the lordosis response (as reviewed by [90]); [91]. Activity in these GABA neurons may facilitate reproductive behavior, as infusion of a GABAA receptor agonist into the VMH promotes sexual behavior [92], whereas a local infusion of a GABAA receptor antagonist has an inhibitory effect [91]. Thus, the presence of GABA in the VMH is behaviorally relevant. However, it is important note that GAD65 and 67 are not synthesized by VMH neurons [93]. Therefore, the GABAergic influences on VMH neurons arise from cell bodies that reside elsewhere.
Excitatory neurotransmission in the VMH also has been implicated in lordosis. The critical ER-α-containing neurons express the vesicular glutamate transporter, indicating that their main neurotransmitter is glutamate [94]. Another receptor abundant in the VL-VMH is the oxytocin receptor (OTR), which also promotes female sexual behavior [72; 95; 96; 97]. Likewise, neuronal nitric oxide synthase (nNOS), enkephalin and substance P have been shown to promote lordosis [98; 99; 100; 101]. Each of these VL-VMH-specific factors is co-localized with estrogen receptors in the VMH [102; 103; 104], and estradiol treatment up-regulates the levels of these factors in the adult female VMH [105; 106; 107; 108]. Thus, estradiol acts within glutamatergic neurons, in which the enhanced production and release of enkephalin, substance P and nitric oxide promotes sexual behavior. Oxytocin also acts on these neurons to promote lordosis. Thus, several neurochemical properties of ER-α-containing neurons have been revealed, although the specific role of each of these signals in the context of the local circuit is not yet clear.
In contrast, cholecystokinin is found in VMH neurons that do not co-express estrogen receptors [109]. When administered into the VMH, cholecystokinin has an inhibitory effect on female sexual behavior [110]. Thus, cholecystokinin-expressing neurons must play a distinct role in the VMH local circuitry compared with the ER-expressing neurons.
In sum, investigations of phenotypic markers in the VMH are in accordance with the aforementioned tracing studies and Fos analysis studies in that both sets of literature suggest that the VMH consists of several cell types. Although several neurochemical features of the ER-α-containing neurons have emerged (summarized in Figure 2), it remains unclear how the GABA-ergic input and cholecystokinin-labeled neurons correspond to the Fos-activated neurons and to the projection neurons. It also seems likely that there are subpopulations amongst the ER-α-containing neurons.
IV. SEX DIFFERENCES IN THE VMH
A. NEUROCHEMISTRY
A fundamental difference between the male and female VMH is its responsiveness to steroid hormones in adulthood. In particular, males exhibit higher levels of both the androgen receptor and aromatase, the enzyme that converts testosterone to estradiol, than females in the VL-VMH [111; 112]. Conversely, the female manifests more receptors for estradiol, including both ER-α and ER-β subtypes [113; 114; 115], and for progesterone [116; 117; 118] than males. Thus, it may not be surprising that a number of estradiol-induced changes in the VMH are more robust in females than males, as detailed below. Nevertheless, there are several cases in which estradiol-induced changes persist in males or occur in the reverse direction.
In addition to the levels of steroid receptors, many of the gene products uniquely expressed in the VL-VMH, with known contributions to lordosis behavior, also exhibit sex differences in their expression. As summarized in Figure 2, these VMH neurochemical signaling molecules can be organized into three categories. The first category includes receptors for estradiol and progesterone, but also includes GABA and enkaphalin. Each of these is found in greater amounts in females, is increased when estradiol levels are elevated, and promotes lordosis behavior in females [119; 120; 121; 122]. Thus, these signals may activate a lordosis-specific network within the VMH.
A second category includes signals that are elevated in males compared with females, and are known to inhibit lordosis. This category includes cholecystokinin, glutamate, and serotonin [107; 123]. For cholecystokinin, the inhibitory effect on behavior in females depends on the normal lack of exposure to gonadal steroids during development in females [124]. Thus, developmental effect of hormones on sex differences in the VMH (discussed in more detail below) includes determining the responsiveness of the adult VMH to some of these modulators of lordosis. There is a sex difference in glutamate receptors, specifically the dl-α-amino-3-hydroxy-5-methyl-4-isoxazone-propionate (AMPA) receptor subunit 2/3 with males exhibiting a diminished level of estradiol-induced up-regulation compared with females [125]. This is reminiscent of the aforementioned blunted effect of estradiol on enkephalin in males, and may be secondary to lower levels of ER-α. Further investigation is needed on glutamate’s behavioral effects and the sex differences and hormonal regulation of its receptors. With regard to serotonin, projections arrive from the dorsal raphe nucleus [126], and serotonin suppresses lordosis when released in the VMH [127]. A recent study elegantly demonstrated that there are substantially more serotonin-labeled fibers in the male VMH compared with females [88]. Although the total number of neurons in the VMH is the same in males versus females, more neurons receive serotonin inputs in males. This increase in serotonin innervation is specific for non-ER-a-containing neurons, as the percentage of ER-a-containing neurons that are innervated by serotonin is approximately 70 percent in both males and females. Thus, the pronounced sex difference in the serotonin innervation of the VMH consists of a doubling of the input to the non-ER-α-containing cells in males.
In a third category, some neuromodulators, are more abundant in males, yet are known to increase lordosis behavior. For the oxytocin receptor, which promotes lordosis behavior in females, males have a substantially higher level of receptor binding activity and mRNA expression compared with females [128; 129]. Likewise, males express higher levels of nNOS than females [130]. One explanation for this pattern of results is that oxytocin receptors and nNOS may generically promote reproductive behavior, whether mounting or lordosis, in both males and females, respectively. In fact, central injections of oxytocin promote sexual behavior in males [131]. Thus, these neuromodulators may not influence a sex-specific posture, but may activate a circuit within the VMH that promotes mating in a non-sex specific way.
In closing this section, a complex picture has emerged regarding sex differences in the neurochemistry of the VMH. Overall, males and females seem to express the same signaling molecules in the VMH, with differing mechanisms in the regulation of these signals. The fact that some signals that promote lordosis in female are present in higher amounts in males suggests that some elements of VMH activity are not sex-specific with regard to facilitating reproductive behavior. On the other hand, some sex differences in classical neurotransmitters do correlate with the ability to express lordosis behavior. A critical difference is the lower level of ER-α in males, which may explain the blunted estradiol-mediated changes in the expression of several genes in males compared with females. In this way, developmental exposure to steroids, to be discussed shortly, may epigenetically blunt the effect of estradiol on a number of genes that are estrogen-responsive and promote lordosis in females. This may include downstream effects on neural plasticity, as discussed next.
B. SYNAPTIC ORGANIZATION
The volume of the adult VMH is approximately 25% larger in males compared with females, based on Nissl stain analysis [70; 132; 133; 134], and this sex difference is most pronounced in the VL-VMH [134]. This volume difference is secondary to males having a larger neuronal soma size than females, rather than males having a greater total number of neurons [70; 134]. In addition, the space occupied by neuropil in the VMH is greater in males than females [70]. At the ultrastructural level, several laboratories have reported a higher density of spine and shaft synapses in males compared with females [135; 136; 137], although this is not always reported [70; 138]. As mentioned above, serotonin innervation contributes to the greater levels of synaptic input in the male VMH [88]. Given that dendritic spines are induced and maintained by neuronal activity, the higher level of spine density in the male VMH implies intrinsic differences in basal synaptic activity. Overall, the sex difference in the volume of the VMH is based on males exhibiting the same number of neurons, albeit of greater size and with more synaptic inputs. Thus, to understand the sex-specific function of the VMH, it is necessary to delve into its synaptic organization.
Connectivity within the VMH is regulated quite differently in males versus females. Estradiol treatment decreases dendritic spine density in adult male rats [139; 140], whereas estradiol generally increases spine density and axospinous synapses in females [75; 141; 142; 143] (but see [81]. Estradiol is a physiological regulator of spine density in male rats based on the evidence that estradiol treatment reverses the castration-induced increase in dendritic spine density in the male VMH [139; 140]. The spine density on short primary dendrites, but not other dendrite types, is markedly increased by estradiol treatment in females, possibly via afferent input from nearby ER-a-containing interneurons [75]. It remains unclear whether the effect of estradiol on dendritic spines in males is confined to the same dendrite types that are affected in the opposite direction in females. A mechanistic accounting for this opposite effect of estradiol in males versus females has not been provided, and the known effects of estradiol on local neuromodulators, as discussed above, do not explain it. It seems likely that the sex difference in the density of synapses in the VMH is not merely quantitative, but that it represents a fundamentally different kind of synaptic organization that allows estradiol to have such radically different re-wiring effects.
We recently conducted a comparison of the VMH dendritic arbor in males versus females to test the hypothesis that a sex difference might be localized to a specific dendrite type [77]. VMH neurons were visualized with Golgi impregnation. Male rats displayed significantly longer dendrites than females in both the DM-VMH and the VL-VMH. Longer dendrites were observed in males for all dendrite types, regardless of which direction the dendrites extended. The elongated male VMH dendrites may provide additional sites to process input from both local interneurons and extranuclear afferents. In addition, the longer dendrites may be needed to traverse a greater VMH area to reach the surrounding fiber plexus. This sex difference occurred regardless of the hormonal status of the females, and is thus a true sex difference.
To date, relatively few studies have investigated possible sex differences in the connectivity between the VMH and other brain regions. One such study observed a sex difference in the innervation of the VL-VMH from the fornix, with the male VMH receiving approximately 25 percent more terminals than the female VMH [136]. It seems likely that similar differences occur with other afferents to the VMH, contributing to the sex difference in the volume of neuropil and the density of synapses. Likewise, sex differences in the outflow from the VMH to the periaqueductal gray, either in terms of the density of the projection, or the subdivisions being targeted, may be critical for the behavioral specificity of the VMH.
In summary, the synaptic organization of the VMH differs by virtue of males having more dendritic surface, including more synaptic contacts. At least some of this additional connectivity in males arises from a higher density of input coming from other brain regions. Another fundamental sex difference in the synaptic organization of the VMH is the direction of changes in wiring wrought by estradiol. Future studies should address how that additional information processing in the male VMH yields distinct neurobehavioral functions.
C. MECHANISMS OF HORMONE-INDUCED SEX DIFFERENCES
Neurological sex differences can arise from two very different mechanisms: direct effects of genes expressed by sex chromosomes and epigenetic effects mediated by the developmental activation sex steroid receptors [144]. In the case of the VMH, neonatal exposure to gonadal hormones can explain many of the sex differences that have been documented; in fact neonatal manipulation of exposure to testosterone and estradiol can reverse the sex differences in the VMH studied to date. Thus, neonatally castrated males have a female-like VMH, and, conversely, females treated neonatally with testosterone or estradiol have a male-like VMH, according to studies assessing a variety of parameters. For example, the perinatal testicular secretion in males contributes to the masculinization of the general volume of the VMH [133], the number of synapses [136; 137], including the serotonergic innervation [88], the pattern of gene expression [115], and the adult pattern of electrophysiological activity [145]. Therefore, although neuronal effects of sex chromosomes may mediate some sex differences in the brain, dichotomies in the VMH seem to be largely explained by the developmental, organizing surge of testosterone.
The developmental surge in testicular secretions occurs between the ages of E18 and E19 in male rats [146]. Several biochemical sex differences in the VMH emerge in close succession to this event. For example, on E22 the male VMH contains half the level of the serine/threonine kinase Raf-1 compared with females [147]. On the day of birth, the male VMH contains twice the number of cells labeled with phosphorylated CREB compared with females [148]. Another sex difference, in this case GABA-A receptor activity, emerges shortly after birth, with a slower decay of the GABA-A-mediated current in males compared with females [149]. Finally, between PN5 and PN14, males express less ER-α and ER-β than females [150; 151]. This may reflect auto-down-regulation based on the aromatization of testosterone and subsequent estradiol action in males. Thus, sexual dichotomies in VMH biochemistry and electrophysiology are temporally linked with an organizational surge in testosterone.
A detailed study of the sex difference in dendritic spines in the VMH has provided insights into the mechanisms of estradiol-induced sexual differentiation in the brain [152]. As mentioned above, males exhibit higher levels of dendritic spines in the VMH compared with females. Likewise, the level of the spine-specific protein spinophilin is approximately twice as high in males compared with females in the hypothalamus. The effect of perinatal estradiol to increase spinophilin levels is mediated by glutamate. Specifically, blockade of glutamate receptors prevented an estradiol-dependent increase in spinophilin levels. More specifically, estradiol treatment increased the depolarization-induced release of glutamate. The timing of this presynaptic effect of estradiol on glutamate release occurs within three hours after estradiol treatment. This time frame challenges the traditional view that estradiol masculinizes the brain mainly through a transcriptional mechanism. In fact, the translation inhibitor cyclohexamide did not interfere with estradiol-induced glutamate release. Instead, an inhibitor of phosphatidylinositol 3 (PI3) kinase blocked the effects of estradiol on both glutamate release and spinophillin levels. Indeed, estradiol treatment increased levels of phosphorylated Akt, a substrate of PI3 kinase. Subsequent to estradiol-induced glutamate release, there is a postsynaptic increase in the levels of phosphorylated mitogen-activated protein kinase (MAPK). The inhibition of MAPK phosphorylation blocked the effect of glutamate on spinophilin levels, and presumably spine formation. Taken together, masculinization of the VMH involves a cascade of presynaptic PI3 kinase activity, which phosphorylates Akt, which then promotes glutamate release. Consequently, glutamate binds to its receptors post-synaptically, which in turn leads to MAPK activation, which then promotes spinophilin expression and spine formation. Thus, control of glutamate activity in this early time window is a critical feature of the sexual differentiation of synaptic organization in the VMH. The receptor mechanisms mediating this action of estradiol is unclear.
While the aforementioned study implicates a novel mechanism of estradiol action in mediating sex differences in the VMH, at least for the masculinization of dendritic spines, there are parallel roles for the classic sex steroid receptors. Recent work has established the involvement of both ER-α and ER-β in VMH sexual differentiation [150; 151; 153]. In addition to these critical organizational mechanisms in the VMH involving estradiol, androgen receptors also contribute to the sex differences in the size of the VMH, as shown in rats with mutant androgen receptors [134]. It is possible that androgen receptor activation is important for the control of the expression of the aromatase enzyme [111], which would suggest that estrogen receptors are involved afterall. Androgen-based mechanisms also extend to sex differences in neurochemistry. For example, males express higher levels of nNOS than females. This male pattern of nNOS expression is blocked by a loss of effective androgen receptors [130]. It should be noted that androgen receptors and aromatase are expressed in the VMH in modest amounts compared to other brain regions, but limbic regions with high expression of androgen receptors and aromatase provide afferents to the VMH [11] . As for the outer limits during which gonadal steroids can masculinize the VMH, a postnatal time course analysis indicated that the adult pattern of sex-specific connectivity is established by PN45, but not at PN20 [137]. Thus, the secretion of gonad hormones during puberty may normally contribute to the final pattern of male and female VMH circuitry in adults.
In addition to permanent effects on the VMH morphology and circuitry, developmental exposure to steroids affects later responsiveness to estradiol, as mentioned above. In particular, a number of estradiol-mediated effects are weaker in adult males compared with females. For example, estradiol does not induce progestin receptor expression in males, despite this being a classic effect of estradiol in the female VMH [117]. As mentioned above, estradiol treatment enhances enkephalin expression in females but not males [119]. One possibility is that this reduced estrogen responsiveness in males merely represents a quantitative difference in estrogen receptors between the sexes. Given that males express lower levels of estradiol receptors than females, the blunted capacity to respond to estradiol may be a consequence of fewer receptors. The lack of estradiol-induced progestin receptors in the male VMH would certainly explain the lack of effect of progesterone on oxytocin receptor binding in males than has been observed in females [154]. Alternatively, the sex difference in estrogen responsivity may reflect epigenetic modifications on the accessibility of estrogen-regulated genes. This would be manifested as qualitative differences in hormone-induced genotropic effects later in life, compared with females. Another possible source of quantitative sex differences in the responses to estradiol treatment may occur if the effects depend on transynaptic actions. Given that the organization of sex differences in the VMH includes robust differences in connectivity, and given that some effects of estradiol are secondary to neurotransmission, the effect of estradiol to indirectly influence the pattern of gene expression may be sex-specific. Given that these mechanisms are not mutually exclusive, sex differences in estradiol responsiveness could be both quantitative and qualitative.
In addition to distinct responses in adult males versus females to the genotropic actions of estradiol, it remains unclear whether the sex differences in the synaptic organization of the VMH are quantitative or qualitative. As mentioned in the previous section, male rats may require additional dendritic surface to process more inputs, with the source of those inputs being identical to females. Conversely, sex differences in synaptic connectivity may represent categorical differences in the afferents to the VMH and/or the targets of its axonal projection [24]. In this way, the greater number of synapses in male VMH may reflect a categorical difference in computational processing, rather than simply stronger excitatory or inhibitory influences. To date, there has not been a quantitative comparison of the density and source of VMH afferents in males versus females.
In summary, the extant studies have established that during development, gonadal hormones produce long-term changes in the wiring of the VMH as well as its responsiveness to estradiol in adulthood. These mechanisms are summarized in Figure 3. Although in some cases cellular mechanisms have been further elaborated, many questions remain regarding the functional consequences of the dichotomies in VMH connectivity and transcriptional potential.
FIGURE 3.
Working model of the mechanisms of sexual differentiation in the VMH. Testosterone acts on androgen receptors, which in turn upregulate the enzyme aromatase. Aromatase allows the conversion of testosterone to estradiol, and many masculinizing effects then are mediated by estrogen receptors. ER-α is certainly a critical receptor, but other estrogen receptors are likely to participate as well. Membrane-based actions may mediate the sex differences in dendrite morphology, which lead to permanent sex differences in synaptic organization. In parallel, developmental effects of estrogen receptors may modify the chromatin to permanently alter the responsiveness to sex hormones in adult hood.
V. STRUCTURE-FUNCTION RELATION OF THE VMH AND LORDOSIS
As noted thus far, certain attributes of the VMH are not sex-dependent. For example, both males and female exhibit an ovoid structure with neurochemically distinct dorsomedial and ventrolateral regions, each comprised of equivalent numbers of neurons with simple dendritic arbors. However, within this overall structure, specific dimensions are dichotomous for the male versus female VMH, including the levels of certain signaling molecules, the pattern of synaptic organization, and the responses to adult hormone exposure. Each of these specific differences may contribute to the sex-specific behavioral functions of the VMH.
An obstacle to explaining how the sex differences observed in the VMH provide a biological substrate for sex-specific mating behaviors lies in the fact that the neurochemistry and neural plasticity that allow the female VMH to promote lordosis remain only partly understood. As mentioned above, the normal fluctuations in estradiol and progesterone across the estrous cycle are critical for controlling the timing of lordosis behavior in intact females based on their actions in the VMH. A number of neurochemical changes take place in the VMH when estradiol levels become elevated, as already described. Ovarian hormones also reorganize neuronal connections within the VMH. Golgi impregnation studies showed that the number of dendritic spines in the VMH correlates with reproductive behavior across the estrous cycle. In particular, the density of dendritic spines in VMH neurons is highest at the time when ovarian hormones are elevated [142]. In ovariectomized rats, estradiol treatment increases the number of spines on the dendrites of VMH neurons two-fold [142]. Similar effects of estradiol to increase synaptic connectivity in the VMH have been reported with a variety of methods [70; 141; 142; 143]. This finding also was replicated and extended using the cell filling technique [75]. Keeping in mind that the dendritic tree of VMH neurons includes a single long primary dendrite that extends toward the lateral afferent fiber field, as well as several short primary dendrites [56; 75], my laboratory tested the hypothesis that these dendrite categories represent different roles in neuronal computation. In support of this notion, we found that the estradiol treatment induced a robust increase in dendritic spine number specifically on the short primary dendrites. Thus, the estradiol-induced spines in the VMH may signify additional local connectivity within the VMH induced by estrogen to mediate reproductive behavior. We also demonstrated that these changes occur on VMH neurons that do not express nuclear ER-α nor send axonal projections to the periaqueductal gray [75; 81].
At the same time that ovarian hormones promote the formation of dendritic spines on short primary dendrites, they dynamically regulate the length of the long primary dendrites. In particular, estradiol treatment causes a marked shortening of these dendrites, a processes that is reversed with subsequent progesterone treatment. This regulation of dendrite extension was first observed with Golgi analysis, which allowed for straightforward measurement of dendrite length [76]. A subsequent electron microscopy analysis of the lateral fiber plexus verified that estradiol treatment caused an attrition of dendritic profiles in that region [155]. This pattern is consistent with findings reported from intact cycling female rats [70]. Thus, estradiol acts to shorten the long primary dendrites, removing them from the lateral fiber plexus at the same time that the short primary dendrites have heightened excitatory input. Intriguingly, a neurochemical marker for the dendrites that remain after estradiol treatment is oxytocin labeling within these dendrites [155]. Thus, ovarian hormones re-wire VMH dendrites with notable spatial and neurochemical specificity.
Putting these findings together with our knowledge of VMH neurons, the following working model emerges. As the estrous cycle proceeds and estradiol levels rise, ER-α neurons respond with changes in the transcription of a host of signaling and effector proteins. Given that these are glutamate neurons, the release of glutamate is likely to increase. As suggested in the developing brain, these neurons may also have a rapid, non-genotopic response to increase the release of glutamate [152]. We propose that a second cell type then responds to the glutamate released by the ER-α-containing neurons by developing dendritic spines at these sites of contact on their short primary dendrites (Figure 1). The emergence of these dendritic spines may enhance the neurotransmission between the ER-containing neurons and this second cell type. The identity of this second cell type may be the mating-activated neurons and/or the cholecystokinin-expressing neurons. It is also possible that estradiol initially acts on extranuclear afferents to transynaptically increase spines [80]. Based on this change in excitability after the marked increase in dendritic spines, these neurons may impinge on the projection neurons, which send their axons to the periaqueductal gray, to exert effects on behavior.
In this model, estradiol treatment stimulates the expression of gene products that promote lordosis in the ER-α-containing neurons, and enhances the excitability of a second set of neurons. As mentioned above, the long primary dendrites of some neurons disengage from extranuclear afferents in the lateral fiber plexus until progesterone comes along. Thus, the behavioral effects of progesterone may derive from the re-establishment of sensory or arousal cues arriving from other brain regions and impinging on the long primary dendrites.
With these inferences about the effects of lordosis-promoting hormones on the dendritic tree of VMH neurons, one can consider the dendrite morphology in males. As mentioned above, VMH neurons in males have the same dendrite components, although the dendrites are longer and they receive more synaptic input. Thus, male VMH neurons do not simply resemble those of a female without ovarian hormones, which would entail a low number of dendritic spines. Just as the neurochemistry of the male VMH does not mirror that of a female without ovarian hormone exposure, as described below, the synaptic organization of the male VMH is not simply that of a non-estrous female. Thus, the masculinization of the VMH is more than imposing the synaptic arrangement seen in a non-estrus female.
The VMH expresses the oxytocin receptor, nNOS, enkephalin and substance P, all of which promote lordosis in females [72; 95; 96; 97; 98; 99; 100; 101]. These factors are also present in males, but in some cases to a lesser degree. Thus, the lack of lordosis behavior in males may result, in part, from a relative dearth of lordosis-promoting factors in the VMH. Likewise, males exhibit higher levels of cholecystokinin and serotonin in the VMH than females [88; 107]. Given that cholecystokinin and serotonin have inhibitory effects on female sexual behavior in the VMH [110; 156], the sex difference in cholecystokinin and serotonin in the VMH may contribute to the lack of lordosis behavior in males. Thus, a combination of low amounts of lordosis-promoting signals and high levels of lordosis-inhibiting signals may be responsible for the lack of female sexual behavior in males (summarized in Table 2). An exception to this scheme is glutamate levels, as discussed above.
Table 2.
Proposed differential functions of neurochemical markers in the VL-VMH based on their pattern of sex differences and their effects on lordosis behavior.
| Proposed function |
Signaling molecule |
Sex with higher levels |
Effect on lordosis in females |
|---|---|---|---|
| Promotes lordosis (female sex behavior) |
ER-α | Females | increase |
| ER-β | Females | increase | |
| Progesterone receptor |
Females | increase | |
| Enkephalin | Females | increase | |
| GABA | Females | increase | |
| Inhibits lordosis in both sexes |
Cholecystokinin | Males | decrease |
| Glutamate (+/−) | Males | decrease | |
| GluR2/3 | Males | decrease | |
| Promotes mating in both sexes |
Oxytocin receptors |
Males | increase |
| nNOS | Males | increase |
The notion that sex-specific behavior is controlled by the ratio of lordosis promoting and inhibiting substances in the VMH is challenged by reports that under certain circumstances, lordosis behavior can be observed in males when primed with estradiol and progesterone. One example is the transection of dorsal afferents to the anterior hypothalamus, implicating the lateral septum as a lordosis-inhibiting system [157]. The unmasking of lordosis in males with this denervation persisted when a concomitant VMH lesion was performed. Thus, circuits outside the VMH are sufficient to support lordosis behavior in males. Another situation that reveals a latent lordosis circuitry in adult males is the loss of serotonergic innervation to the hypothalamus [156; 158]. Furthermore, given that the extent of the lesions were not verified, the change in behavior may have reflected a loss of serotonin input elsewhere, such as to the lateral septum. The lateral septum was already implicated in inhibiting lordosis [159; 160]. It is unknown whether or not these interventions cause a sex reversal in the levels of lordosis-promoting and lordosis-inhibiting factors in the VMH. Given that the unmasking of lordosis persists after the VMH has been lesioned, any feminization that may occur in VMH neurochemistry or neural circuitry does not seem to mediate the sex reversal of reproductive behavior in these conditions. This VMH-independent expression of lordosis in males questions the importance of sex differences in the VMH as being responsible for the lack of lordosis in males. Furthermore, a developmental treatment that masculinizes the serotonin innervation to the VMH does not concomitantly abolish lordosis behavior [88]. Thus, despite the host of sex differences in the VMH and the abundant evidence that the VMH controls lordosis behavior in females, there has not been a clear demonstration that masculinization of the VMH is the key factor that prevents males from exhibiting lordosis behavior. A transgenic experiment that allowed a VMH-specific, developmentally controlled manipulation of estradiol and androgen action could test the hypothesis that masculinization of the VMH is necessary and sufficient for the sex-specificity of lordosis. Although such an experiment would not be trivial to conduct, it is need to demonstrate that masculinization of the VMH and only the VMH is critical for the lack of lordosis in males.
It may be useful to note that there are several other examples of sexually dimorphic brain structures that are associated with known dichotomous effects on behavior or physiology that exhibit much greater sex differences in size that the VMH. For example, in songbirds, areas that control the male-typical behavior of singing, such as the high vocal center, are three times larger in males than females [161]. An example in the mammalian brain is the pathway that controls the female-typical positive feedback effect of estradiol to trigger gonadotropin secretion and ovulation. This circuit, which is twenty times larger in males versus females, includes a projection from the principal nucleus of the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus [162]. The modest sexual dimorphism of VMH size, which is 20% larger in males in its most dimorphic subregion, the VL-VMH [134], may suggest that masculinization of VMH, or the lack thereof, does not dictate the sex-specificity of lordosis.
If it is true that the masculinization of the VMH is not the key to preventing lordosis in males, the functional significance of sex differences in the VMH must be addressed. A clue to this question may be the many documented sex differences in the VMH pertain to the response to estradiol. In addition to the control of female mating behavior, estradiol also contributes to the regulation of body weight [163; 164; 165; 166]. This particular role of estradiol may also be mediated in the VMH [167]. It has been noted that the effect of estradiol on body weight is opposite for males and females [168]. Thus, the relatively weak sex difference in VMH size may be a reflection of the relevant function not being completely dichotomous between males and females, such as body weight regulation.
IV. SUMMARY AND FUTURE DIRECTIONS
Previous investigations on the mechanisms in the VMH that control female mating behavior have provided a valuable heuristic for understanding the organizational and behavioral effects of steroid hormones at the cellular and molecular levels [152; 169]. Nevertheless, this neurobehavioral system still has secrets to reveal about how its circuitry and neurochemistry are configured and regulated to control reproductive behavior. To provide a full account of the neurological mechanisms that operate in the VMH to mediate female sexual behavior, the cell types in the VMH and their connectivity must be further elucidated. Given that ovarian hormones cause specific changes in connectivity, an important goal for my laboratory is to reveal the behavioral significance of ovarian hormone-induced re-wiring of the synaptic organization of the VMH. Although studies of the female VMH can help explain the neural underpinnings of lordosis behavior, comparisons with male sexual behavior are urgently needed to provide unique insights regarding structure-function relationships.
With regard to sex differences in the VMH, there are well-documented differences in a number of neurochemical markers. The role that this shift in the chemical environment of the VMH plays in the sex-specificity of mating behavior is not clear. Likewise, there are sex differences in the synaptic organization of the VMH, but whether or not this represents a dichotomy in the functional output of the VMH remains unclear. Another fascinating aspect of the sex differences in the VMH is the sex-specific estrogen responsiveness. A number of mechanisms are possible, but the mechanisms of chromatin modifications have not been systematically explored. Finally, it remains to be proven that sex differences in the VMH actually explain the lack of lordosis behavior in normal males. This may be addressed with transgenic experiments that anatomically and developmentally restrict the disruption of estrogen receptors. If the absence of lordosis in males is not controlled by the VMH, the functional significance of the VMH sex differences will require an explanation. Answering questions about the male VMH would represent a major advancement in our understanding of the numerous sex differences in neurochemical and circuitry.
Aside from a general interest in normal biological systems, there are practical reasons to elucidate the mechanisms of sexual differentiation in the brain. In recent years, evidence has accumulated to show that various human-made compounds in our environment, such as bisphenol A (BPA), can have endocrine disrupting effects, including during development. In particular, neonatal exposure to BPA caused a down-regulation of ER-α in the female VMH. These females also exhibited lower levels of mating behavior [170]. BPA is a widely used compound [171], including in our food containers, and recent epidemiological studies have found that a high percentage of the human population with readily detectable levels in their bodies [172; 173]. Animal models, including further studies of the VMH, may provide a useful screen for endocrine disruptors, as well as possible antidotes. Basic research on normal VMH responsiveness to hormones will afford a tremendous foundation for future studies to protect neuroendocrine health.
ACKNOWLEDGEMENTS
L.M.F.-C. is supported by R01HL091314. Dr. Daniel K. Yee contributed to the design of the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 2.Pfaff DW. Estrogens and Brain Function. New York: Springer-Verlag; 1980. [Google Scholar]
- 3.McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology. 1939;25:359–364. [Google Scholar]
- 5.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 6.Cottingham SL, Femano PA, Pfaff DW. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp. Neurol. 1987;97:703–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma Y, Pfaff DW. Facilitation of female reproductive behavior from mesencephalic central gray in the rat. Am. J. Physiol. 1979;237:R278–R284. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- 8.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J. Comp. Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaff DW, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 10.MacLusky NJ, McEwen BS. Progestin receptor in rat brain: distribution and properties of cytoplasmic progestin-binding sites. Endocrinology. 1980;106:192–202. doi: 10.1210/endo-106-1-192. [DOI] [PubMed] [Google Scholar]
- 11.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 12.DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J. Comp. Neurol. 1991;305:591–612. doi: 10.1002/cne.903050406. [DOI] [PubMed] [Google Scholar]
- 13.Blaustein J, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res. 1988;474:1–15. doi: 10.1016/0006-8993(88)90664-6. [DOI] [PubMed] [Google Scholar]
- 14.Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: An autoradiographical analysis. Endocrinol. 1982;111:1581–1586. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- 15.Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrin. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- 16.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 17.Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol. Behav. 1977;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- 18.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeifle JK, Shivers M, Edwards DA. Parasaggital hypothalamic knifecuts and sexual receptivity in the female rat. Physiol. Behav. 1980;24:145–150. doi: 10.1016/0031-9384(80)90026-8. [DOI] [PubMed] [Google Scholar]
- 20.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 21.Flanagan LM, Pfaus JG, Pfaff DW, McEwen BS. Induction of FOS immunoreactivity in oxytocin neurons after sexual activity in female rats. Neuroendocrinology. 1993;58:352–358. doi: 10.1159/000126562. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan-Cato LM, McEwen BS. Pattern of Fos and Jun expression in the female rat forebrain after sexual behavior. Brain Research. 1995;673:53–60. doi: 10.1016/0006-8993(94)01395-x. [DOI] [PubMed] [Google Scholar]
- 23.Pfaus JG, Kleopoulos SP, Mobbs CV, Gibbs RB, Pfaff DW. Sexual stimulation activates c-fos within estrogen-concentrating regions of the female rat forebrain. Brain Res. 1993;624:253–267. doi: 10.1016/0006-8993(93)90085-2. [DOI] [PubMed] [Google Scholar]
- 24.Wersinger SR, Baum MJ, Erskine MS. Mating -induced FOS-like immunoreactivity in the rat forebrain: a sex comparison and a dimorphic effect of pelvic nerve transection. J. Neuroendocrinology. 1993;5:557–568. doi: 10.1111/j.1365-2826.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Rowe DW, Erskine MS. c-Fos proto-oncogene activity induced by mating in the preoptic area, hypothalamus and amygdala in the female rat: role of afferent input via the pelvic nerve. Brain Res. 1993;621:25–34. doi: 10.1016/0006-8993(93)90294-w. [DOI] [PubMed] [Google Scholar]
- 26.Polston EK, Erskine MS. Patterns of induction of the immediate early genes c-fos and egr-1 in the female rat brain following differential amounts of mating stimulation. Neuroendocrinology. 1995;62:370–384. doi: 10.1159/000127027. [DOI] [PubMed] [Google Scholar]
- 27.Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- 28.Goy RW, Phoenix CH. Hypothalamic regulation of female sexual behavior: establishment of behavioral oestrus in spayed guinea-pigs following hypothalamic lesions. J. Reprod. Fertil. 1963;5:23–40. doi: 10.1530/jrf.0.0050023. [DOI] [PubMed] [Google Scholar]
- 29.Malsbury CW, Kow L-M, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol. Behav. 1977;19:223–237. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- 30.Sterner MR, Meisel RL, Diekman MA. Forebrain sites of estradiol-17 beta action on sexual behavior and aggression in female Syrian hamsters. Behav. Neurosci. 1992;106:162–171. doi: 10.1037//0735-7044.106.1.162. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi LK, Lisk RD. Estrogen action in anterior and ventromedial hypothalamus and the modulation of heterosexual behavior in female golden hamsters. Physiol. Behav. 1985;34:233–239. doi: 10.1016/0031-9384(85)90111-8. [DOI] [PubMed] [Google Scholar]
- 32.Leedy MG, Hart BL. Female and male sexual responses in female cats with ventromedial hypothalamic lesions. Behav. Neurosci. 1985;99:936–941. doi: 10.1037//0735-7044.99.5.936. [DOI] [PubMed] [Google Scholar]
- 33.Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity, and luteinizing hormone surge of the ewe. Brain Res. 1991;546:241–249. doi: 10.1016/0006-8993(91)91488-m. [DOI] [PubMed] [Google Scholar]
- 34.Kendrick AM, Rand MS, Crews D. Electrolytic lesions of the ventromedial hypothalamus abolish sexual receptivity in female whiptail lizards, Cnemidophorus uniparens. Brain Res. 1995;680:226–228. doi: 10.1016/0006-8993(95)00191-r. [DOI] [PubMed] [Google Scholar]
- 35.Rand MS, Crews D. The bisexual brain: sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res. 1994;663:163–167. doi: 10.1016/0006-8993(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 36.Aou S, Oomura Y, Yoshimatsu H. Neuron activity of the ventromedial hypothalamus and the medial preoptic area of the female monkey during sexual behavior. Brain Res. 1988;455:65–71. doi: 10.1016/0006-8993(88)90115-1. [DOI] [PubMed] [Google Scholar]
- 37.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Molecular & Cellular Endocrinology. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budefeld T, Grgurevic N, Tobet SA, Majdic G. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackshaw S, Scholpp S, Placzek M, Ingraham H, Simerly R, Shimogori T. Molecular pathways controlling development of thalamus and hypothalamus: from neural specification to circuit formation. Journal of Neuroscience. 2010;30:14925–14930. doi: 10.1523/JNEUROSCI.4499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. Journal of Neuroendocrinology. 2009;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonthuis PJC, Searcy KH, Kumar BT, Tobet P, Rissman S, EF Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Frontiers in Neuroendocrinology. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mufson EJ, Benoit R, Mesulam MM. Immunohistochemical evidence for a possible somatostatin-containing amygdalostriatal pathway in normal and Alzheimer's disease brain. Brain Research. 1988;453:117–128. doi: 10.1016/0006-8993(88)90149-7. [DOI] [PubMed] [Google Scholar]
- 44.Fliers E, Noppen NWAM, Wiersinga WM, Visser TJ, Swaab DF. Distribution of thyrotropin releasing hormone (TRH)-containing cells and fibers in human hypothalamus. J. Comp. Neurol. 1994;348:1–13. doi: 10.1002/cne.903500213. [DOI] [PubMed] [Google Scholar]
- 45.Stopa EG, Koh ET, Svendsen CN, Rogers WT, Schwaber JS, King JC. Computer-assisted mapping of immunoreactive mammalian gonadotropin-releasing hormone in adult human basal forebrain and amygdala. Endocrinology. 1991;128:3199–3207. doi: 10.1210/endo-128-6-3199. [DOI] [PubMed] [Google Scholar]
- 46.Mai JK, Stephens PH, Hopf A, Cuello AC. Substance P in the human brain. Neuroscience. 1986;17:709–739. doi: 10.1016/0306-4522(86)90041-2. [DOI] [PubMed] [Google Scholar]
- 47.Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., III Opioid precursor gene expression in the human hypothalamus. J. Comp. Neurol. 1995;353:604–622. doi: 10.1002/cne.903530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael RP, Clancy AN, Zumpe D. Distribution of androgen receptor-like immunoreactivity in the brains of cynomolgus monkeys. J. Neuroendocrinology. 1995;7:713–719. doi: 10.1111/j.1365-2826.1995.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Guasti A, Kruijver FPM, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J. Comp. Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 50.Ishunina TA, Unmehopa UA, van Heerikhuize JJ, Pool CW, Swaab DF. Metabolic activity of the human ventromedial nucleus neurons in relation to sex and aging. Brain Research. 2001;893:70–76. doi: 10.1016/s0006-8993(00)03289-3. [DOI] [PubMed] [Google Scholar]
- 51.Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in human brain. Neuron. 2001;31:661–668. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 52.Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiology & Behavior. 2004;81:671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behavioral Neuroscience. 2005;119:1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- 54.McClellen KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Frontiers in Neuroendocrinology. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Tobet SA, Henderson RG, Whiting PJ, Sieghart W. Special relationship of g-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. J. Comp. Neurol. 1999;405:88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 56.Millhouse OE. The organization of the ventromedial hyothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- 57.Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor a in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- 58.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 59.Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J. Biol. Chem. 1993;268:7494–7502. [PubMed] [Google Scholar]
- 60.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinology. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 61.Roselli CE, Jorgensen EZ, Doyle MW, Ronnekleiv OK. Expression of the orphan receptor steroidogenic factor-1 mRNA in the rat medial basal hypothalamus. Mol. Brain Res. 1997;44:66–72. doi: 10.1016/s0169-328x(96)00187-8. [DOI] [PubMed] [Google Scholar]
- 62.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor-1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinology. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 63.Luo X, Ikeda Y, Lala DS, Baity LA, Meade JC, Parker KL. A cell-specific nuclear receptor plays essential roels in adrenal and gonadal development. Endocrine Res. 1995;21:517–524. doi: 10.3109/07435809509030469. [DOI] [PubMed] [Google Scholar]
- 64.Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki J, Osawa Y, Ninomiya Y, Niwa O, Morohashi K-I, Li E. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dynamics. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 65.Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JD, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Molecular Endocrinology. 2010;24:1240–1250. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cajal S, Ramon Y. Histologie du systeme nerveux de l'homme et des vertebres. Paris: Maloine; 1911. [Google Scholar]
- 67.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 68.Fahrbach SE, Morrell JI, Pfaff DW. Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. Exp. Brain Res. 1989;77:221–233. doi: 10.1007/BF00274980. [DOI] [PubMed] [Google Scholar]
- 69.Van Houten M, Brawer JR. Cytology of neurons of the hypothalamic ventromedial nucleus in the adult male rat. J. Comp. Neurol. 1978;178:89–116. doi: 10.1002/cne.901780106. [DOI] [PubMed] [Google Scholar]
- 70.Madiera MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J. Comp. Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 71.Merchenthaler I, Gorcs T, Setalo G, Petrusz P, Flerko B. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell. Tissue Res. 1984;237:15–29. doi: 10.1007/BF00229195. [DOI] [PubMed] [Google Scholar]
- 72.Schumacher M, Coirini H, Frankfurt M, McEwen BS. Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA. 1989;86:6798–6801. doi: 10.1073/pnas.86.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine beta hydroxylase as a marker. J. Comp. Neurol. 1975;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 74.Millhouse OE. Certain ventromedial hypothalamic afferents. Brain Res. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- 75.Calizo LH, Flanagan-Cato LM. Estrogen selectively induces dendritic spines within the dendritic arbor of rat ventromedial hypothalamic neurons. J. Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) J. Comp. Neurol. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffin GD, Flanagan-Cato LM. Sex differences in the dendritic arbors of neurons in the hypothalamic ventromedial nucleus. Physiology and Behavior under review. 2009 doi: 10.1016/j.physbeh.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flanagan-Cato LM, Fluharty SJ, Weinreb EB, LaBelle DR. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2008;149:93–99. doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LaBelle DR, Cox JM, Dunn-Meynell AA, Levin BE, Flanagan-Cato LM. Genetic and Dietary Effects on Dendrites in the Hypothalamic Ventromedial Nucleus. Physiol. Behav. 2009;98:511–516. doi: 10.1016/j.physbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishizuka M, Pfaff DW. Intrinsic synapses in the ventromedial nucleus of the hypothalamus: An ultrastructural study. J. Comp. Neurol. 1989;286:260–268. doi: 10.1002/cne.902860210. [DOI] [PubMed] [Google Scholar]
- 81.Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J. Comp. Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- 82.Calizo LH, Flanagan-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-a, retrograde tract tracing from the periaqueductal gray, and mating-induced fos expression. Endocrinology. 2003;144:5430–5440. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- 83.Daniels D, Flanagan-Cato LM. Functionally-defined compartments of the lordosis neural circuit in the ventromedial hypothalamus in female rats. J. Neurobiol. 2000;45:1–13. [PubMed] [Google Scholar]
- 84.Auger AP, Moffatt CA, Blaustein JD. Reproductively relevant stimuli induce fos immunoreactivity within progestin receptor-containing neurons in localized regions of female forebrain. J. Neuroendocrinol. 1996;8:831–838. doi: 10.1046/j.1365-2826.1996.02684.x. [DOI] [PubMed] [Google Scholar]
- 85.Tetel MJ, Celentano DC, Blaustein JD. Intraneuronal convergence of tactile an hormonal stimuli associated with female reproduction in rats. J. Neuroendocrinol. 1994;6:211–216. doi: 10.1111/j.1365-2826.1994.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 86.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-a gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 87.Commons KG, Kow L-M, Milner TA, Pfaff DW. In the ventromedial nucleus of the rat hypothalamus, GABA-immunolabeled neurons are abundant and are innervated by both enkephalin- and GABA immunolabeled axon terminals. Brain Research. 1999;816:58–67. doi: 10.1016/s0006-8993(98)01084-1. [DOI] [PubMed] [Google Scholar]
- 88.Patisaul HB, Fortino AE, Polston EK. Sex differences in serotonergic but not gamma-aminobutyric acidergic (GABA) projections to therat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:397–408. doi: 10.1210/en.2007-0666. [DOI] [PubMed] [Google Scholar]
- 89.Jang I-S, Rhee JS, Watanabe T, Akaike N, Akaike N. Histaminergic modulation of GABAergic transmission in rat ventromedial hypothalamic neurones. J. Physiol. 2001;534:791–803. doi: 10.1111/j.1469-7793.2001.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarthy MM. Functional significance of steroid modulation of GABAergic neurotransmission: analysis at the behavioral, cellular and molecular levels. Horm. Behav. 1995;29:131–140. doi: 10.1006/hbeh.1995.1010. [DOI] [PubMed] [Google Scholar]
- 91.Luine VN, Wu V, Hoffman CS, Renner KJ. GABAergic regulation of lordosis: influence of gonadal hormones on turnover of GABA and interaction of GABA with 5-HT. Neuroendocrinology. 1998;69:438–445. doi: 10.1159/000054447. [DOI] [PubMed] [Google Scholar]
- 92.McCarthy MM, Malik KF, Feder HH. Increased GABAergic transmission in medial hypothalamus facilitates lordosis but has opposite effects in the preoptic area/anterior hypothalamus. Brain Res. 1990;507:40–44. doi: 10.1016/0006-8993(90)90519-h. [DOI] [PubMed] [Google Scholar]
- 93.Okamura H, Abitbol M, Julien J, Dumas S, Bérod A, Geffard M, Kitahama K, Bobillie rP, Mallet J, Wiklund L. Neurons containing messenger RNA encoding glutamate decarboxylase in rat hypothalamus demonstrated by in situ hybridization, with special emphasis on cell groups in medial preoptic area, anterior hypothalamic area and dorsomedial hypothalamic nucleus. Neuroscience. 1990;39:675–699. doi: 10.1016/0306-4522(90)90252-y. [DOI] [PubMed] [Google Scholar]
- 94.Eyigor O, Lin W, Jennes L. Identification of neurones in the female rat hypothalamus that express oestrogen receptor-alpha and vesicular glutamate transporter-2. J. Neuroendocrinology. 2004;16:26–31. doi: 10.1111/j.1365-2826.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 95.McCarthy MM, Kleopoulos SP, Mobbs CV, Pfaff DW. Infusion of antisense oligonucleotides to the oxytocin receptor in the ventromedial hypothalamus reduces estrogen-induced sexual receptivity and oxytocin receptor binding in the female rat. Neuroendocrinology. 1994;59:432–440. doi: 10.1159/000126689. [DOI] [PubMed] [Google Scholar]
- 96.Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6:247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- 97.Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- 98.Chu HP, Etgen AM. Ovarian hormone dependence of alpha(1)-adrenoceptor activation of the nitric oxide-cGMP pathway: relevance for hormonal facilitation of lordosis behavior. J. Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicot A, Ogawa S, Berman Y, Carr KD, Pfaff DW. Effects of an intrahypothalamic injection of antisense oligonucleotides for preproenkephalin mRNA in female rats: evidence for opioid involvement in lordosis reflex. Brain Res. 1997;777:60–68. doi: 10.1016/s0006-8993(97)00967-0. [DOI] [PubMed] [Google Scholar]
- 100.Dornan WA, Malsbury CW, Penney RB. Facilitation of lordosis by injection of substance P into the midbrain central gray. Neuroendocrinol. 1987;45:498–506. doi: 10.1159/000124781. [DOI] [PubMed] [Google Scholar]
- 101.Dornan WA, Akesson TR, Micevych PE. A substance P projection from the VMH to the dorsal midbrain central gray: implications for lordosis. Brain Res. Bull. 1990;25:791–796. doi: 10.1016/0361-9230(90)90061-4. [DOI] [PubMed] [Google Scholar]
- 102.Turcotte JC, Blaustein JD. Convergence of substance P and estrogen receptor immunoreactivity in the midbrain central gray of female guinea pigs. Neuroendocrin. 1997;66:28–37. doi: 10.1159/000127216. [DOI] [PubMed] [Google Scholar]
- 103.Olster DH, Blaustein JD. Immunocytochemical colocalization of progestin receptors and beta-endorphin or enkephalin in the hypothalamus of female guinea pigs. J. Neurobiology. 1990;21:768–780. doi: 10.1002/neu.480210510. [DOI] [PubMed] [Google Scholar]
- 104.Devidze N, Mong JA, Jasnow AM, Kow L-M, Pfaff DW. Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc. Natl. Acad. Sci. USA. 2005;102:14446–14451. doi: 10.1073/pnas.0507144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Kloet ER, Voorhuis DAM, Boschma Y, Elands J. Estradiol modulates density of putative 'oxytocin receptors' in discrete rat brain regions. Neuroendocrinology. 1986;44:415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 106.Romano GJ, Harlan RE, Shivers BD, Howells RD, Pfaff DW. Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol. Endocrinol. 1988;2:1320–1328. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 107.Micevych PE, Matt DW, Go VL. Concentrations of cholecystokinin, substance P, and bombesin in discrete regions of male and female rat brain: sex differences and estrogen effects. Experimental Neurology. 1988;100:416–425. doi: 10.1016/0014-4886(88)90119-7. [DOI] [PubMed] [Google Scholar]
- 108.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Regulation of neuronal nitric oxide synthase mRNA in lordosis-relevant neurons of the ventromedial hypothalamus following short-term estrogen treatment. Molecular Brain Research. 1998;59:105–108. doi: 10.1016/s0169-328x(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 109.Akesson TR, Micevych PE. Evidence for an absence of estrogen-concentration by CCK-immunoreactive neurons in the hypothalamus of the female rat. J Neurobiol. 1988;19:3–16. doi: 10.1002/neu.480190103. [DOI] [PubMed] [Google Scholar]
- 110.Babcock AM, Bloch GJ, Micevych PE. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol. Behav. 1988;43:195–199. doi: 10.1016/0031-9384(88)90237-5. [DOI] [PubMed] [Google Scholar]
- 111.Roselli CE, Klosterman SA. Sexual differentiation of aromatase activity in the rat brain: effects of perinatal steroid exposure. Endocrinology. 1998;139:3193–3201. doi: 10.1210/endo.139.7.6101. [DOI] [PubMed] [Google Scholar]
- 112.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 113.Scott CJ, Tilbrook AJ, Simmons DM, Rawson JA, Chu S, Fuller PJ, Ing NH, Clarke IJ. The distribution of cells containing estrogen receptor-a (ERa) and ERb messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinol. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- 114.Brown TJ, MacLuskey NJ, Toran-Allerand CD, Zielinski JE, Hochberg RB. Characterization of 11b-methoxy-16a-[125I]iodoestradiol binding: neuronal localization of estrogen binding sites in the developing brain. Endocrinology. 1989;124:2074–2088. doi: 10.1210/endo-124-5-2074. [DOI] [PubMed] [Google Scholar]
- 115.Orikasa C, Sakuma Y. Sex and region-specific regulation of oestrogen receptor β in the rat hypothalamus. Journal of Neuroendocrinology. 2004;16:964–969. doi: 10.1111/j.1365-2826.2004.01254.x. [DOI] [PubMed] [Google Scholar]
- 116.Bogic L, Gerlach JL, McEwen BS. The ontogeny of sex differences in estrogen-induced progesterone receptors in rat brain. Endocrinol. 1988;122:2735–2741. doi: 10.1210/endo-122-6-2735. [DOI] [PubMed] [Google Scholar]
- 117.Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology. 1991;53:608–613. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- 118.Rainbow TC, Parsons B, McEwen BS. Sex differences in rat brain oestrogen and progestin receptors. Nature. 1982;300:648–649. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- 119.Romano GJ, Mobbs CV, Lauber A, Howells RD, Pfaff DW. Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: implications for the molecular basis of a sexually differentiated behavior. Brain Research. 1990;536:63–68. doi: 10.1016/0006-8993(90)90009-z. [DOI] [PubMed] [Google Scholar]
- 120.Gratten DR, Selmanoff M. Sex differences in the activity of g-aminobutyeric acidergic neurons in the rat hypothalamus. Brain Research. 1997;775:244–249. doi: 10.1016/s0006-8993(97)01069-x. [DOI] [PubMed] [Google Scholar]
- 121.Frankfurt M, Fuchs E, Wuttke W. Sex differences in g-aminobutyric acid and glutamate concentrations in discreet rat brain nuclei. Neurosci. Lett. 1984;50:245–250. doi: 10.1016/0304-3940(84)90493-2. [DOI] [PubMed] [Google Scholar]
- 122.Luine VN, Grattan DR, Selmanoff M. Gonadal hormones alter hypothalamic GABA and glutamate levels. Brain Res. 1997;747:165–168. doi: 10.1016/s0006-8993(96)01255-3. [DOI] [PubMed] [Google Scholar]
- 123.Frankfurt M, Siegel RA, Sim I, Wuttke W. Cholecystokinin and substance P concentrations in discrete areas of the rat brain: sex differences. Brain Research. 1985;358:53–58. doi: 10.1016/0006-8993(85)90947-3. [DOI] [PubMed] [Google Scholar]
- 124.Ulibarri C, Popper P, Micevych PE. Role of postnatal androgens in sexual differentiation of the lordosis-inhibiting effect of central injections of cholecystokinin. Journal of Neurobiology. 1990;21:796–807. doi: 10.1002/neu.480210513. [DOI] [PubMed] [Google Scholar]
- 125.Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- 126.Willoughby WO, Blessing WW. Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Res. 1987;418:170–173. doi: 10.1016/0006-8993(87)90975-9. [DOI] [PubMed] [Google Scholar]
- 127.Uphouse L. Female gonadal hormones , serotonin, and sexual receptivity. Brain Research Reviews. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 128.Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135:147–154. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995a;136:27–32. doi: 10.1210/endo.136.1.7828541. [DOI] [PubMed] [Google Scholar]
- 130.Martini M, Di Sante G, Collado P, Pinos H, Guillamon A, Panzica GC. Androgen receptors are required for full masculinization of nitric oxide synthase system in rat limbic-hypothalamic region. Hormones & Behavior. 2008;54:557–564. doi: 10.1016/j.yhbeh.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 131.Arletti R, Bazzani C, Castelli M, Bertonlini A. Oxytocin improves male copulatory behavior in rats. Horm. Behav. 1986;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- 132.Dorner G, Staudt J. Structural changes in the hypothalamic ventromedial nucleus of the male rat, following neonatal castration and androgen treatment. Neuroendocrinology. 1969;4:278–281. doi: 10.1159/000121758. [DOI] [PubMed] [Google Scholar]
- 133.Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol. Japon. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- 134.Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Hormones and behavior. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsumoto A, Arai Y. Male-female differences in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 136.Larriva-Sahd J, Rondan-Zarate A, Ramirez-Degollado M. Sexually dimorphic contribution from the fornix to the ventromedial hypothalamic nucleus: a quantitative electron microscopic study. Neurosci. Lett. 1995;200:147–150. doi: 10.1016/0304-3940(95)12129-r. [DOI] [PubMed] [Google Scholar]
- 137.Pozzo Miller LD, Aoki A. Stereological analysis of the hypothalamic ventromedial nucleus II. Hormone-induced changes in the synaptigenic pattern. Dev. Brain Research. 1991;61:189–196. doi: 10.1016/0165-3806(91)90131-2. [DOI] [PubMed] [Google Scholar]
- 138.Sa SI, Madiera MD. Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J. Comp. Neurol. 2005;484:68–79. doi: 10.1002/cne.20451. [DOI] [PubMed] [Google Scholar]
- 139.Frankfurt M, McEwen BS. 5,7-Dihydroxytryptamine and gonadal steroid manipulation alter spine density in ventromedial hypothalamic neurons. Neuroendocrinology. 1991;54:653–657. doi: 10.1159/000125975. [DOI] [PubMed] [Google Scholar]
- 140.Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult GDX. Dev. Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- 141.Carrer HF, Aoki A. Ultrastructural changes in the hypothalamic ventromedial nucleus of ovariectomized rats after estrogen treatment. Brain Research. 1982;240:221–233. doi: 10.1016/0006-8993(82)90218-9. [DOI] [PubMed] [Google Scholar]
- 142.Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: A golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- 143.Frankfurt M, McEwen BS. Estrogen increases axodendritic synapses in the VMN of rats after ovariectomy. NeuroReport. 1991;2:380–382. doi: 10.1097/00001756-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 144.Arnold AP. Sex chromosomes and brain gender. Nature Reviews Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 145.Sakuma Y. Influences of neonatal gonadectomy or androgen exposure on the sexual differentiation of the rat ventromedial hypothalamus. J. Physiol. (Lond.) 1984;349:273–286. doi: 10.1113/jphysiol.1984.sp015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 147.Whorf RC, Tobet SA. Expression of the Raf-1 protein in rat brain during development and its hormone regulation in hypothalamus. J. Neurobiology. 1992;23:103–119. doi: 10.1002/neu.480230202. [DOI] [PubMed] [Google Scholar]
- 148.Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Research. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- 149.Smith ST, Brennan C, Clark AS, Henderson LP. GABAA receptor-mediated responses in the ventromedial nucleus of the hypothalamus of female and male neonatal rats. Neuroendocrinology. 1996;64:103–113. doi: 10.1159/000127105. [DOI] [PubMed] [Google Scholar]
- 150.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. Journal of Comparative Neurology. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 151.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Sexually dimorphic and estrogen-dependent expression of estrogen receptor beta in the ventromedial hypothalamus during rat postnatal development. Endocrinology. 2003;144:5098–5104. doi: 10.1210/en.2003-0267. [DOI] [PubMed] [Google Scholar]
- 152.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) beta modulates ERalpha responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Coirini H, Johnson AE, Schumacher M, McEwen BS. Sex differences in the regulation of oxytocin receptors by ovarian steroids in the ventromedial hypothalamus in rat. Neuroendocrinology. 1992;55:269–275. doi: 10.1159/000126125. [DOI] [PubMed] [Google Scholar]
- 155.Griffin GD, Ferri-Kolwicz SL, Reyes BAS, VanBockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J. Comp. Neurol. 2010;518:4531–4545. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moreines J, Kelton M, Luine VN, Pfaff DW, McEwen BS. Hypothalamic serotonin lesions unmask hormone responsiveness of lordosis behavior in adult male rats. Neuroendocrinology. 1988;47:453–458. doi: 10.1159/000124949. [DOI] [PubMed] [Google Scholar]
- 157.Yamanouchi K, Arai Y. Presence of a neural mechanism for the expression of female sexual behaviors in the male rat brain. Neuroendocrinology. 1985;40:393–397. doi: 10.1159/000124104. [DOI] [PubMed] [Google Scholar]
- 158.Kakeyama M, Yamanouchi K. Lordosis in male rats: The facilitatory effect of mesencephalic dorsal raphe nucleus lesion. Physiology & Behavior. 1992;51:181–184. doi: 10.1016/0031-9384(92)90221-m. [DOI] [PubMed] [Google Scholar]
- 159.Gordon JH, Nance DM, Wallis CJ, Gorski RA. Effects of septal lesions and chronic estrogen treatment on dopamine, GABA and lordosis behavior in male rats. Brain Res. Bull. 1979;4:85–89. doi: 10.1016/0361-9230(79)90062-5. [DOI] [PubMed] [Google Scholar]
- 160.Nance DM, Shryne J, Gorski RA. Septal lesions: effects on lordosis behavior and pattern of gonadotropin release. Horm. Behav. 1974;5:73–81. doi: 10.1016/0018-506x(74)90008-7. [DOI] [PubMed] [Google Scholar]
- 161.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 162.Hutton LA, Gu G, Simerly RB. Development of a sexually dimorphic projection from the bed nuclei of the stria terminalis to the anteroventral periventricular nucleus in the rat. Journal of Neuroscience. 1998;18:3003–3013. doi: 10.1523/JNEUROSCI.18-08-03003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J. Comp. Physiol. Psychol. 1970;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 164.Butera PC, Beikirch RJ. Central implants of diluted estradiol: Independent effects on ingestion and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 165.King J, Cox VC. The effects of estrogens on food intake and body weight following ventromedial hypothalamic lesions. Physiol. Psychol. 1973;1:261–269. [Google Scholar]
- 166.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-a null mice. Endocrinol. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 167.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Nat. Acad. Sci. (USA) 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beatty WW, Powley TL, Keesey RE. Effects of neonatal testosterone injection and hormone replacement in adulthood on body weight and body fat in female rats. Physiology & Behavior. 1970;5:1093–1098. doi: 10.1016/0031-9384(70)90194-0. [DOI] [PubMed] [Google Scholar]
- 169.Mani SK, Blaustein JD, Allen JMC, Law SW, O'Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 170.Monje L, Varayoud J, Munoz de Toro M, Luque EH, Ramos JG. Neonatal exposure to bisphenol A alters estrogen dependent mechanisms governing sexual behavior in the adult female rat. Reproductive Toxicology. 2009;28:435–442. doi: 10.1016/j.reprotox.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 171.Burridge E. Bisphenol A: product profile. Eur Chem News. 2003;17:14–20. [Google Scholar]
- 172.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VWV, Mackenbach JP, Steegers EAP, Tiemeier H, MP L. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environmental Research. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernandez M, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reproductive Toxicology. 2007;24:259–264. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 174.De Vente J, Hopkins DA, Markerink-Van Ittersum M, Emson PC, Schmidt HHHW, Steinbusch HWM. Distribution of nitric oxide synthase and nitric oxide receptive, cyclic GMP-producing structures in rat brain. Neuroscience. 1998;87:207–241. doi: 10.1016/s0306-4522(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 175.Nakamura K, Kimura S, Yamazaki M, Kawaguchi A, Inoue K, Sakai T. Immunohistochemical analysis of thyroid-specific enhancer-binding protein in the fetal and adult rat hypothalami and pituitary glands. Dev. Brain Res. 2001;130:159–166. doi: 10.1016/s0165-3806(01)00226-7. [DOI] [PubMed] [Google Scholar]
- 176.Akesson TR, Mantyh PW, Mantyh CR, Matt DW, Micevych PE. Estrous cyclicity of 125I-cholecystokinin octapeptide binding in the ventromedial hypothalamic nucleus. Neuroendocrinol. 1987;45:257–262. doi: 10.1159/000124737. [DOI] [PubMed] [Google Scholar]
- 177.Nielsen Ricciardi KH, Blaustein JD. Projections from ventrolateral hypothalamic neurons containing progestin receptor- and substance P-immunoreactivity to specific forebrain and midbrain areas in female guinea pigs. J. Neuroendocrinol. 1994;6:135–144. doi: 10.1111/j.1365-2826.1994.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 178.Akesson TR, Micevych PE. Estrogen concentration by substance P immunoreactive neurons in the medial basal hypothalamus of the female rat. J. Neurosci. Res. 1988;19:412–419. doi: 10.1002/jnr.490190405. [DOI] [PubMed] [Google Scholar]
- 179.Herbison AE. Somoatostatin-immunoreactive neurones in the hypothalamic ventromedial nucleus possess oestrogen receptors in the male and female rat. J. Neuroendocrinology. 1994;6:323–328. doi: 10.1111/j.1365-2826.1994.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 180.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, DSirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol. Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 181.Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker KL, Tobet SA. Disruption of gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J. Comp. Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 182.Sugiyama N, Kanba S, Arita J. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Mol. Brain Res. 2003;115:69–77. doi: 10.1016/s0169-328x(03)00184-0. [DOI] [PubMed] [Google Scholar]