Abstract
The Syrian golden hamster (Mesocricetus auratus) is a valuable but under-utilized animal model for studies of human viral pathogens such as bunyaviruses, arenaviruses, flaviviruses, henipaviruses, and SARS-coronavirus. A lack of suitable reagents and specific assays for monitoring host responses has limited the use of this animal model to clinical observations, pathology and humoral immune responses. The objective of this study was to establish and validate assays to monitor host immune responses in the hamster including important pro-inflammatory, anti-inflammatory and innate immune responses, as well as markers of apoptosis, cell proliferation, cell junction integrity and coagulation. Commercially available mouse and rat ELISA and luminex panels were screened for potential cross-reactivity, but were found to be of limited value for studying host responses in hamsters. Subsequently, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assays for the detection of 51 immune-related and four internal reference genes were developed. To validate the immune-related assays, hamsters were infected with vesicular stomatitis virus (VSV), Indiana species, or treated with lipopolysaccharide (LPS) and host immune responses were monitored in selected organs. Ribosomal protein L18 was identified as the most stable internal reference gene. In conclusion, these new assays will greatly improve the use of the hamster as an important small animal model in infectious disease research.
Keywords: Syrian Golden hamster, qRT-PCR, Host immune response, Reference genes
Abbreviations: β2M, Beta-2-microglobulin; BBQ, Blackberry quencher; HPRT, Hypoxanthine phosphoribosyltransferase; LPS, Lipopolysaccharide; RPL18, Ribosomal protein L18; VSV, vesicular stomatitis Indiana virus; Yak, Yakima yellow
1. Introduction
Syrian golden hamsters (Mesocricetus auratus; hereafter referred to as hamster) have been used in numerous studies as infection and disease models (Table 1 ) due to their ease of handling and disease development, which often mimics the natural course of human diseases. In particular, hamsters have been recognized as valuable animal models for studying emerging and high consequence acute human viral diseases caused by bunyaviruses (Niklasson et al., 1984, Hooper et al., 2001, Milazzo et al., 2002, Fisher et al., 2003) arenaviruses (Smee et al., 1993, Sbrana et al., 2006), henipaviruses (Wong et al., 2003), flaviviruses (Tesh et al., 2001, Xiao et al., 2001, Siirin et al., 2007) and SARS-coronavirus (Roberts et al., 2005). For some of these pathogens, such as Andes virus, a New World hantavirus and a causative agent of hantavirus pulmonary syndrome, hamsters are the only lethal disease model (Hooper et al., 2001). However, due to a lack of available reagents and specific assays to monitor host responses in hamsters, including disease-decisive acute and early innate immune responses, investigators are currently limited to studies on disease progression (clinical symptoms), humoral immune responses (antibodies) and pathology. For other disease models using rodents, nonhuman primates and ferrets, expression microarray proteome and luminex technology-based quantification assays have been developed and utilized (Kayo et al., 2001, Datson et al., 2007, Bruder et al., 2010, Ierna et al., 2010). However, for Syrian (Golden) hamsters similar specific assays are not available due to the lack of a complete genome sequence.
Table 1.
Selected biological agent disease models in Syrian Golden hamsters.
| Agent | Disease modeled | Reference |
|---|---|---|
| Amebic liver abscess | Entamoeba histolytica | Ghadirian et al., 1980 |
| Andes virus | Hantavirus pulmonary syndrome | Hooper et al., 2001 |
| Babesia | Babesiosis | Braga et al., 2006 |
| Eastern equine encephalitis virus | Eastern equine encephalitis | Paessler et al., 2004 |
| Gabek Forest virus | Rift Valley fever-likea | Fisher et al., 2003 |
| Japanese encephalitis virus | Japanese encephalitis | Larson et al., 1980 |
| Leishmania | Visceral leishmania | Melby et al., 2001 |
| Leptospira | Leptospirosis | Haake, 2006 |
| Maporal virus | Hantavirus pulmonary syndrome | Milazzo et al., 2002 |
| Nipah virus | Nipah virus encephalitis | Wong et al., 2003 |
| Oncolytic adenoviruses | Pancreatic carcinoma | Spencer et al., 2009 |
| Pichinde virus | Lassa fever-like | Smee et al., 1993 |
| Pirital virus | Lassa fever-like | Sbrana et al., 2006 |
| Prions | Scrapie, Creutzfeldt–Jakob disease | Beekes et al., 1996, Chabry et al., 1999 |
| Punta Tora virus | Rift Valley fever-like | Fisher et al., 2003 |
| Rift Valley virus | Rift Valley fever | Niklasson et al., 1984 |
| St. Louis encephalitis virus | Chronic St. Louis encephalitisa | Siirin et al., 2007 |
| SARS coronavirus | Severe acute respiratory syndromea | Roberts et al., 2005 |
| Venezuelan equine encephalitis virus | Venezuelan equine encephalitis** | Jackson et al., 1991 |
| West Nile virus | West Nile neurological syndrome | Xiao et al., 2001 |
| Western equine encephalitis virus | Western equine encephalitis | Zlotnik et al., 1972 |
| Yellow fever virusb | Yellow fever | Tesh et al., 2001 |
Infection model, not disease model.
Adapted viruses used in model.
Here, we describe the testing of a large panel of ELISA and luminex based assays for cross-reactivity against hamster proteins and the development of an extended panel of real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assays which were based on hamster mRNA sequences currently registered in Genbank. Due to limited cross-reactivity in ELISA and luminex based assays, the development of qRT-PCR assays seemed the most practical and useful strategy to quantify specific host responses in the hamster model. A few qRT-PCR assays for the quantification of a limited number of immunological responses have been previously reported (Melby et al., 1998, Muller et al., 2001, Vernel-Pauillac and Merien, 2006). We developed a unique extended panel of TaqMan® primer/probe assays for 51 different genes targeting specific markers in the pro-inflammatory, anti-inflammatory, innate immunity, apoptosis, cell junction and coagulation responses of the hamster. With ribosomal protein L18 we identified a more suitable and stable housekeeping gene. The assays were validated by monitoring host responses to treatment with lipopolysaccharide (LPS) and infection with vesicular stomatitis Indiana virus (VSV), both of which are known to strongly activate host responses in the hamster (Fultz et al., 1981b, Muller et al., 2001). The new assays will allow for a broader monitoring of host responses against infection and an advanced utilization of the hamster model.
2. Materials and methods
2.1. Inoculations and sample preparation
Recombinant VSV was rescued from cloned cDNA and infectivity was titered by conventional plaque forming assay, as described previously (Lawson et al., 1995, Garbutt et al., 2004). Three groups of six hamsters (female, 4–6 weeks old Harlan Laboratories Inc, Indianapolis, IN, USA) were injected intraperitoneally (ip) with either 50 μg LPS (Sigma, St. Louis, MO USA), 106 plaque forming units (pfu) of VSV (both diluted in Dulbecco's modified Eagle's medium (DMEM)), or DMEM only (control) in a total volume of 400 μL. Three of the controls and VSV infected, and six of the LPS treated hamsters were euthanized by exsanguination while under deep anesthesia (inhalational isoflurane) one day post inoculation. The remaining control and VSV inoculated hamsters were euthanized at 3 days post inoculation. Whole blood was collected by cardiac puncture into EDTA vacutainers, after which plasma was separated by centrifugation and frozen at − 80 °C. Lung, liver and spleen samples were harvested from euthanized hamsters, cut into approximately 50–100 mg pieces and submerged in 1 mL of RNAlater (Qiagen, Valencia, CA, USA) overnight at 4 °C. The following day RNAlater was removed and the tissues were frozen at − 80 °C. Approximately 30 mg of the tissue samples were mechanically homogenized in RLT buffer and the RNA was extracted from the homogenate using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. The concentration of the extracted RNA was determined using a NanoDrop 8000 instrument (Thermo Scientific, Waltham, MA, USA) and adjusted to a final concentration of 10 ng/μL. All experiments were conducted under BSL2 conditions, and approval for animal experiments was obtained from the Institutional Animal Care and Use Committee. Animal work was performed by certified staff in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
2.2. ELISA and Milliplex panel preparation
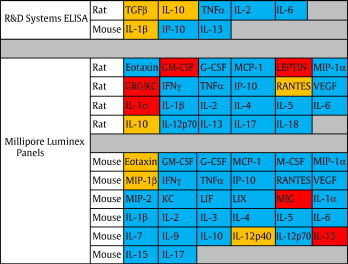
Rat TNFα, IL-2, IL-6 and IL-10, mouse CXCL10/IP-10, IL-13 and IL-1β/IL-1F2, and mouse/rat/porcine/canine TGFβ1 ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA) and tested according to manufacturer's instructions with individual hamster plasma (Table 2 ). Individual control and VSV infected and pooled LPS hamster plasma were sent to Millipore (Billerica, MA, USA) for analysis by Milliplex® MAP Mouse (32 plex) and Rat (23 plex) Cytokine panels (Table 2).
Table 2.
List of commercially available, rodent specific, ELISA and Luminex based assays examined for cross-reactivity with hamster proteins. Red indicates acceptable cross-reactivity (within the range of supplied standards), orange indicates marginal cross-reactivity (low or suspiciously high concentrations detected) and blue indicates no cross-reactivity.
2.3. Selection of target genes for development qRT-PCR assay
Over 800 hamster mRNA sequences have been registered in Genbank. Of those, 51 target genes related to host immunological responses elicited by pathogen infections were selected. These included pro-inflammatory, anti-inflammatory, innate immune (primarily Type I IFN-relating genes), and T-cell-mediated responses, as well as apoptosis, cell junctions and coagulation markers (Table 3 ) (Adler, 2008).
Table 3.
Detailed sequence information of the primers and probes utilized in these studies. Primers and probes for the indicated genes were designed based on Syrian golden hamster specific sequences available on GenBank (accession number provided in brackets). The melting temperature of all these primers was ~ 55 °C and the melting point of the probes was ~ 65 °C.
| β-2-Microglobulin (X17002) | B2M F | GGCTCACAGGGAGTTTGTAC | Fibrinogen A α- chain (D43757) | FAAC F | GCACAAGCACGACACGT |
| B2M R | TGGGCTCCTTCAGAGTTATG | FAAC R | TGGGTCATGCCTAAGTCTCC | ||
| B2M TM | YAK-CTGCGACTGATAAATACGCCTGCA-BBQ | FAAC TM | 6FAM-CGATGGTCACCGAGAAGTGGTCA-BBQ | ||
| β-actin (AJ312092) | bactin F | ACTGCCGCATCCTCTTCCT | Forkhead box P3 (FJ664148) | FbP3 F | AAGCAGATCACCTCCTGGAT |
| bactin R | TCGTTGCCAATGGTGATGAC | FbP3 R | AGCTGCTGCTCCAGAGAC | ||
| bactin TM | 6FAM-CCTGGAGAAGAGCTATGAGCTGCCTGATG-BBQ | FbP3 TM | 6FAM-CACCACTTCTCTCTGGAGGAGGCAC-BBQ | ||
| B-cell lymphoma 2 protein (bcl-2) (AJ582074) | Bcl2 F | CTTCGCAGAGATGTCCAGTC | Hypoxanthine phosphoribosyltransferase (AF047041) | HPRT F | TGCGGATGATATCTCAACTTTAACTG |
| Bcl2 R | CATCTCCCTGTTGACGCTC | HPRT R | AAAGGAAAGCAAAGTTTGTATTGTCA | ||
| Bcl2 TM | 6FAM-TGACGCCCTTCACCGCGA-BBQ | HPRT TM | 6FAM-AAAGAATGTCTTGATTGTTGAAGGTAAAACTGACATTGG-BBQ | ||
| Bcl-2 associated protein (AJ582075) | Bax F | GGCAACTTCAACTGGGG | Inducible nitric oxide synthase-2 (AY297461) | iNOS F | TGGCAGGATGGGAAACTGA |
| Bax R | CCACCCTGGTCTTGGATC | iNOS R | GCACCGCTTTCACCAAGACT | ||
| Bax TM | 6FAM-CCAGCCCATGATGGTTCTGATTAGC-BBQ | iNOS TM | 6FAM-CCCAGGAGGAGAGAGATCCGGCTC-BBQ | ||
| CD83 protein DQ094177 | CD83 F | AACCTGGTACGGAACAAGCT | Intracellular adhesion molecule-1 (DQ093373) | ICAM1 F | TGCAGCCGGAGAACAGATG |
| CD83 R | CAAAGGAAGGTTGCCGTC | ICAM1 R | ATCTCCCGTGTGACAGTCTTCA | ||
| CD83 TM | 6FAM-TCCAGGCAGCATTCAGGTACACTGA-BBQ | ICAM1 TM | 6FAM-AGCCCTGCTGCCCATCGGG-BBQ | ||
| Chemokine (C-X-C motif) ligand 10 (IP-10) (AY007988) | IP-10F | GCCATTCATCCACAGTTGACA | Interferon-α inducible protein (p27-h) (AF212039) | p27 F | TCGTTGCTGCTCCCGTAGTC |
| IP-10 R | CATGGTGCTGACAGTGGAGTCT | p27 R | ATGGATCCCGCTGCAATTC | ||
| IP-10 TM | 6FAM-CGTCCCGAGCCAGCCAACGA-BBQ | p27 TM | 6FAM-TGGGTGCTGTGGGCTTCACTGG-BBQ | ||
| Chemokine CCL20/MIP-3 α (AY924377) | CCL20 F | AGTCAGTCAGAAGCAAGCAACT | Interferon-γ (AF034482) | IFNg F | GGCCATCCAGAGGAGCATAG |
| CCL20 R | TGAAGCGGTGCATGATCC | IFNg R | TTTCTCCATGCTGCTGTTGAA | ||
| CCL20 TM | 6FAM-CACAAGGAGCACTATCCCACCCAGA-BBQ | IFNg TM | 6FAM-CACCATCAAGGCAGACCTGTTTGCTAACTT-BBQ | ||
| Chemokine ligand 17 (FJ664143) | CL17 F | CGAGTGCTGCCTGGAGATC | Interferon regulatory factor-1 (DQ092344) | IRF1 F | GGCATACAACATGTCTTCACG |
| CL17 R | TGATGGCCTTCTTCACATGC | IRF1 R | GCTATGCTTTGCCATGTCAA | ||
| CL17 TM | 6FAM-TGGACCTGCCCTGGACAGTCACA-BBQ | IRF1 TM | 6FAM-CACAATGACGCCAGACCTTGCTCA-BBQ | ||
| Chemokine ligand 22 (FJ664144) | CL22 F | CGCGTAGTGAAGGAGTTCTTC | Interferon regulatory factor-2 (AY714581) | IRF2 F | AATGCCTTCAGAGTGTACCG |
| CL22 R | TCTTCACCAGGCCAGCTTA | IRF2 R | TGTTCACCGTACTATCCACTTCAT | ||
| CL22 TM | 6FAM-ACCTCAAAGTCCTGCCGCAAGCC-BBQ | IRF2 TM | 6FAM-CTGAAGTCAGGACCGCATACTCAGGA-BBQ | ||
| Claudulin-1 (EU856105) | ham cld1 F2 | GCCACAGCATGGTATGGAA | Interleukin-1β (AB028497) | IL-1b F | GGCTGATGCTCCCATTCG |
| ham cld1 R1 | GCAAGAAAGTAGGGCACCTC | IL-1b R | CACGAGGCATTTCTGTTGTTCA | ||
| ham cld1 TM | 6FAM- CCCGTCAATGCCAGGTATGAATT-BBQ | IL-1b TM | 6FAM-CAGCTGCACTGCAGGCTCCGAG-BBQ | ||
| Complement C3 (complement C3d region) (AB024425) | CC3d F | GGAGCCTTACCTCAGCAAGT | Interleukin-2 (EU729351) | IL-2F | GTGCACCCACTTCAAGCTCTAA |
| CC3d R | TAGCCGCCTCCGTAGTATCT | IL-2 R | AAGCTCCTGTAAGTCCAGCAGTAAC | ||
| CC3d TM | 6FAM-CAGAAGCTCTACAATGTGGAGGCCA-BBQ | IL-2 TM | 6FAM-AGGAAACCCAGCAGCACCTCGAGC-BBQ | ||
| Complement component 5 (DQ369042) | CC5 F | GTAGTTCCCGATGCTGAAGTG | Interleukin-4 (AF046213) | IL-4F | CCACGGAGAAAGACCTCATCTG |
| CC5 R | TGATTAACTCCATTGACCAACG | IL-4 R | GGGTCACCTCATGTTGGAAATAAA | ||
| CC5 TM | 6FAM-TGTGACTTGCATCGCTTTCGGC-BBQ | IL-4 TM | 6FAM-CAGGGCTTCCCAGGTGCTTCGCAAGT-BBQ | ||
| Complement protein C1qBP (DQ367730) | CP1qBP F | CAGAGGATGAGGTTGGACAA | Interleukin-6 (AB028635) | IL-6F | CCTGAAAGCACTTGAAGAATTCC |
| CP1qBP R | CCATTAGGTGGTCATACAAGGC | IL-6 R | GGTATGCTAAGGCACAGCACACT | ||
| CP1qBP TM | 6FAM- TCCATTCAGAGTCACCAGTGGTCTGGA-BBQ | IL-6 TM | 6FAM-AGAAGTCACCATGAGGTCTACTCGGCAAAA-BBQ | ||
| E-cadherin (DQ237892) | Ecad F | GTTAAGGTTCTGGAGATGAGATTGG | Interleukin-10 (AF046210) | IL-10F | GTTGCCAAACCTTATCAGAAATGA |
| Ecad R | CATCTTTCCCCTCCGAGACA | IL-10 R | TTCTGGCCCGTGGTTCTCT | ||
| Ecad TM | 6FAM-TTATGTAGATGACCATGACTTTAATGACAA-BBQ | IL-10 TM | 6FAM-CAGTTTTACCTGGTAGAAGTGATGCCCCAGG-BBQ | ||
| Epithelial mucin (Muc1) (L41545) | Muc1 F | CGGAAGAACTATGGGCAGCT | Interleukin-12 p35 subunit (AB085791) | IL-12p35 F | GGCCTTCCCTGGCAGAA |
| Muc 1 R | GCCACTACTGGGTTGGTGTAAG | IL-12p35 R | ATGCTGAAAGCCTGCAGTAGAAT | ||
| Muc 1 TM | 6FAM-TGCCTGCCGAGACCTCCTCGTA-BBQ | IL-12p35 TM | 6FAM-CGGATCCCTACAAAGTGAAAATGAAGCTCTG-BBQ | ||
| Interleukin-12 p40 subunit (AB085792) | IL12p40 F | TGGTTACCTCCTTAGCAGTCC | Signal transducer and activator of transcription-1 β (AB177397) | STAT1b F | AGGTCCGTCAGCAGCTTAA |
| IL12p40 R | TCAGCCTGATGATGAACCTGA | STAT1b R | GCCGTTCCACCACAAAT | ||
| IL12p40 TM | 6FAM-TCCAGAGTGCCATAATAGCCACACAAA-BBQ | STAT1b TM | 6FAM-TCTGAATGAGCTGCTGGAAGAGGACA-BBQ | ||
| Interleukin-21 (FJ664142) | IL21 F | TCAACTGATGTGAAAGGAGC | Signal transducer and activator of transcription-2 (AB177399) | STAT2 F | AATGCCTTCAGAGTGTACCG |
| IL21 R | ATCTTGTGGAGCTGGCAG | STAT2 R | TGTTCACCGTACTATCCACTTCAT | ||
| IL21 TM | 6FAM-TCAGGGTCCTAGCCAAAAGAGAATC-BBQ | STAT2 TM | 6FAM-CTGAAGTCAGGACCGCATACTCAGGA-BBQ | ||
| Interleukin-2 receptor-α (DQ093372) | IL2Ra F | AAAGCAAGCTACACCTAACCC | Tight junction protein 2 (EU856099) | ham tjp2 F1 | CTACACTGACAATGAGCTGGA |
| IL2Ra R | GCCTTGTATCCTTGAATGCG | ham tjp2 R1 | CTCTGGGCTGGATTTCCTTA | ||
| IL2Ra TM | 6FAM-CAGAAATCAGCACAGTCTGTGCACCA-BBQ | ham tjp2 TM | 6FAM-TCATGCTGCACCGGCTCCGA-BBQ | ||
| Interleukin-6 signal transducer (EF442778) | IL6ST F | TGAAGATACAGCATCTTCCCG | Tissue inhibitor of matrix metalloproteinase-2 (AF260255) | TIMM2 F | AGAGCCTGAACCACAGGT |
| IL6ST R | TGAAGATACAGCATCTTCCCG | TIMM2 R | CGGGTCCTCGATGTCAA | ||
| IL6ST TM | 6FAM-TCACTCCAGTAGCCTTTGCCATCCT-BBQ | TIMM2 TM | 6FAM-CGAGTGCAAGATCACACGCTGCC-BBQ | ||
| Junction adhesion molecule (EU856104) | ham jam F1 | CGTCCAAGTTCCCGAGAGTA | Transforming growth factor-β1 (AF046214) | TGFb F | TGTGTGCGGCAGCTGTACA |
| ham jam R1 | CGTGATCTGGCTGTTATAGCA | TGFb R | TGGGCTCGTGAATCCACTTC | ||
| ham jam TM | 6FAM-TAGTGCCACCCTGGACGAACTTC-BBQ | TGFb TM | 6FAM-CGACTTTCGCAAGGACCTGGGCT-BBQ | ||
| Matrix metalloproteinase-2 (AF260254) | MM2 F | GATGCTGCCTTTAACTGGAGT | Transforming growth factor 2 (AY007214) | TGFb2 F | TGCTGCCCTCCTACAGACT |
| MM2 R | GAGCTTAGGGAAACCAGGAT | TGFb2 R | GCACAGAAGTTGGCATTATACC | ||
| MM2 TM | 6FAM-CATACATCTTCGCTGGAGACAAGTTC-BBQ | TGFb2 TM | 6FAM-CACAACAGTCCAATCGGCGGA-BBQ | ||
| MHC class II antigen alpha chain (DQ092501) | MHCAAC F1 | CAGGGAGGACTGCAAGCTATA | Transforming growth factor-β 3 (AF298188) | TGFb3 F | CAAGCTCAGGCTCACCAGT |
| MHCAAC R | TGTCCACGAAGCAGATGAG | TGFb3 R | CCGACTCTGTGTTCTCCTGAG | ||
| MHCAAC TM | 6FAM-TGCAGCAAAGCAGAACTTGGACATC-BBQ | TGFb3 TM | 6FAM-AGCCATCGGTGATGACCCACGT-BBQ | ||
| Myxovirus resistance protein-2 (EU616539) | Mx2 F | CCAGTAATGTGGACATTGCC | Transforming growth factor-β type I receptor (AF298187) | TGFbTIR F | ATCAAACTTGCTCTGTCTACGG |
| Mx2 R | CATCAACGACCTTGTCTTCAGTA | TGFbTIR R | TGTCTGTGGCAGAATCATGC | ||
| Mx2 TM | 6FAM-TGTCCACCAGATCAGGCTTGGTCA-BBQ | TGFbTIR TM | 6FAM-ACAGCCAGTCCCAAGTCTGCAATAC-BBQ | ||
| Nitric oxide synthase-2 (DQ355357) | NOS2 F | TGCCTTGCATCCTCATTGG | Tumor Necrosis Factor-α (AF315292) | TNFa F | GGAGTGGCTGAGCCATCGT |
| NOS2 R | GTCGCTGTTGCCAGAAACTG | TNFa R | AGCTGGTTGTCTTTGAGAGACATG | ||
| NOS2 TM | 6FAM-CCTGGCACGGGCATCGCTC-BBQ | TNFa TM | 6FAM-CCAATGCCCTCCTGGCCAACG-BBQ | ||
| Occuldin (EU856106) | ham occ F1 | CTATTCTGGGCATCCTGGT | Vascular endothelial growth factor (AF297627) | VEGF F | CAGGAGTACCCCGATGAGATAGA |
| ham occ R1 | TTGCACATGGCATAGATCTG | VEGF R | CCCCCACACCGCATCA | ||
| ham occ TM | 6FAM- AGTCAACCCAACTGCCCAGGCT-BBQ | VEGF TM | 6FAM-TCTTCAAGCCGTCCTGTGTGCCC-BBQ | ||
| p75 tumor necrosis factor membrane receptor (AF315291) | p75 F | CCCCAGGCCACAGTCAC | |||
| p75 R | GCCGTGGGAGGAATCTGAA | ||||
| p75 TM | 6FAM-CTGCACAGGCCTCCTGAGACCCT-BBQ | ||||
| Platelet endothelial adhesion molecule (AF508040) | PECAMF | CAGGATCAGAACTTCAGCAAGAT | |||
| PECAMR | GCAGCTGATGGTTATAGCATGT | ||||
| PECAM TM | 6FAM-TGTACCGCAGGCATCGGCAGA-BBQ | ||||
| Protein kinase R (DQ645944) | Eif2ak2 F | ACGGACCTAAGAGATGGCAT | |||
| Eif2ak2 R | AGGTAACTAAAGCGGAGTGC | ||||
| Eif2ak2 TM | 6FAM-CCACGGATCGACCTAGTGCTTCTGA-BBQ | ||||
| Ribosomal Protein L 18 (DQ403027) | RPL18 F | GTTTATGAGTCGCACTAACCG | |||
| RPL18 R | TGTTCTCTCGGCCAGGAA | ||||
| RPL18 TM | YAK-TCTGTCCCTGTCCCGGATGATC-BBQ | ||||
| Signal transducer and activator of transcription-1 (DQ092343) | STAT1 F | GCCAACGATGATTCCTTTGC | |||
| STAT1 R | GCTATATTGGTCATCCAGCTGAGA | ||||
| STAT1 TM | 6FAM-ACCATCCGTTTCCATGACCTCC-BBQ | ||||
2.4. Internal control selection
Of the available hamster sequences on Genbank, the following four “housekeeping” genes were selected as internal reference genes: Hypoxanthine phosphoribosyltransferase (HPRT), β-actin, β-2-microglobulin (β2M) and ribosomal protein L18 (RPL18). β-actin and HPRT were chosen as these are seen as classical housekeeping genes and have been previously utilized by other researchers performing quantitative RT-PCR on hamster tissues (Melby et al., 1998). β2M and RPL18 were selected based on the work of Dheda et al. (2004). All 4 genes were tested in qRT-PCR experiments to determine which had the most stable expression in the tissues of infected/treated and control hamsters.
2.5. Primers and probes
qRT-PCR primers and probes were either designed with the aid of Primer Express® Software v3.0 (Applied Biosystems, Foster City, CA, USA), or were directly designed and synthesized by TIB MOLBIOL (Adelphia, NJ, USA) based on the sequence information of target transcripts. Sequence information of primers and probes are shown in Table 3. Prior to use in qRT-PCR assays all the primer sets were tested to ensure the primers can amplify target transcripts by conventional reverse transcriptase PCR; amplicons were analyzed by agarose gel electrophoresis. Briefly, using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and random hexamers, cDNA pools were generated according to manufacturer's instructions from RNA derived from hamster spleen, lung or liver. The cDNA was used as template for conventional PCR. All PCR reactions were carried out using Taq polymerase (Qiagen Cat. No. 201203) at a denaturation temperature of 95 °C for 30 s, annealing temperature of 50 °C for 30 s, and an extension temperature of 72 °C with an extension time of 30 s for 40 cycles. If a band was evident at the correct size on an agarose gel, the primers were used for subsequent qRT-PCR runs. In total, 51 different primer/probe sets were validated on lung, liver and spleen tissues (Table 3). All experimental gene probes were labeled with 5′ 6FAM dye and quenched by 3′ BlackBerry Quencher (BBQ), and all internal control gene probes were labeled with Yakima Yellow (Yak) and quenched by BBQ (TIB MOLBIOL).
2.6. Primer and probe efficiency and limit of detection
The limit of detection and efficiency of cDNA amplification was determined by selecting a few representative cloned genes. The clones were altered to contain a T7 promoter directly upstream to the qRT-PCR forward primer binding site. Following amplification the PCR fragments were gel purified and in vitro transcribed by Riboprobe Combination System T3/T7 RNA Polymerase (Promega, Madison, WI, USA). Transcripts were DNase I digested and the RNA purified using TRIzol reagent (Invitrogen) according to manufacturer's instructions. The purified RNA was diluted from 5 × 101 to 5 × 10− 8 ng RNA and used as a template for qRT-PCR reactions. To determine the minimum amount of total hamster tissue RNA required for the experiments, representative genes were selected and run in a 2-fold dilution series (from 200 ng to 50 ng total RNA per reaction).
To test whether the efficiency of the primer/probe sets was suitable for direct comparison between reactions, approximately one third of the available genes were selected and cloned into pCR2.1-TOPO (Invitrogen) according to the manufacturer's instructions. The inserts were verified by sequencing and entire plasmids were diluted in series of log10 from 5 × 101 to 5 × 10− 9 ng of total DNA. Quantitative PCR and qRT-PCR were carried out on the plasmid and RNA dilution series, respectively, and the crossing threshold (CT) values plotted and efficiency determined by measuring the slope of the best fit line through the CT values. If the efficiencies were within 10% of each other the primer/probe sets were considered as being comparable (Ling et al., 2008).
2.7. qRT-PCR methods
All qRT-PCR experiments were performed on the Rotor-Gene 6000 thermal cycler (Corbett, San Francisco, CA, USA) using Rotor-Gene Probe RT-PCR kits (Qiagen) according to manufacturer's instructions. Each reaction was multiplexed with RPL18 as the internal reference, using 50 ng of template RNA. The final concentration of each test primer and probe set was 0.4 μM and 0.1 μM, respectively. The final concentration of the internal control primer and probe set was 0.2 μM and 0.05 μM, respectively. Cycling conditions were as follows: 10 min at 50 °C (initial reverse transcription), 5 min at 95 °C (denaturation and HotStarTaq activation), and 40 cycles of 5 s at 95 °C (denaturation), followed by 10 s at 60 °C per cycle (annealing/amplification). Data acquisition was carried out at the end of the annealing/amplification step in the green (510 nm) and yellow (555 nm) channels.
2.8. Data analysis
The data were analyzed using the ΔΔCT method (Livak and Schmittgen, 2001). Briefly, the CT of each test gene in a treated hamster was first normalized to the RPL18 CT (ΔCT) and then compared to the same normalized gene in a mock-treated (calibrator) hamster to determine the ΔΔCT. The final value is displayed as the relative fold increase between the infected/treated and mock treated hamsters.
3. Results and discussion
3.1. Examination of cross-reactivities of ELISA and luminex based cytokine/chemokine detection assays
Many important genes are highly conserved between species; therefore cross-reactivity of antibodies isolated against other rodents may detect hamster proteins. First, we compared amino acid sequence homologies of representative cytokines and chemokines between hamsters, rats, and mice. We selected target proteins showing > 90% homology at the amino acid level (data not shown) and tested cross-reactivities of ELISA kits for their detection. In total 63 antibody-based (55 luminex, 8 ELISA) assays were tested for cross-reactivity with hamster proteins and only 14 were determined to display cross-reactivity (Table 2). The mouse and rat ELISA kits tested in this study had limited cross-reactivity with hamster proteins. Of the eight ELISA kits tested, only three (mouse IL-1β, rat IL-10 and rat/mouse/porcine/canine TGFβ1) displayed cross-reactivity against the hamster homologues (Fig. 1A, Table 2 top panel). Additional problems with the ELISA results are the unusually high (roughly 1000-fold higher than to be expected) levels of TGFβ1 detected in comparison to mice. Therefore, we concluded that the tested ELISA kits are unreliable and of limited use for monitoring proteins involved in immune responses of hamsters. Similarly, the luminex rat and mouse panel data suggests that of the 55 different proteins tested only mouse IL-13 and MIG, and rat GM-CSF, IL-1α, Leptin and GRO/KC were detected with confidence in hamster plasma (Fig. 1B, Table 2 bottom panel). In addition, mouse Eotaxin, MIP-1β and IL-12 p40 as well as rat RANTES and IL-10 showed weak cross-reactivity for hamster proteins (Fig. 1C). All other cytokine analyses failed to show values above the limit of detection. These data suggest that, as with the ELISA kits, the tested luminex panels for rat and mouse cytokines/chemokines, with the exception of the six mentioned previously, are unreliable for the detection of immune responses in hamsters.
Fig. 1.
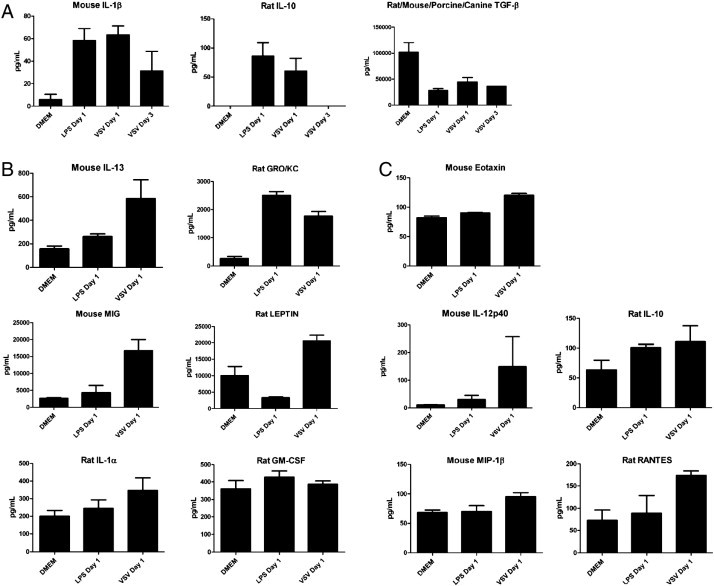
Evaluation of commercially available, rodent specific, ELISA and Luminex based assays for the detection of hamster homologues. (A) ELISA based cross-reactivity data for IL-1β, IL-10 and TGFβ. Luminex based cross-reactivity data displaying (B) acceptable and (C) marginal cross reactivity between mouse and rat homologues. Error bars indicate standard error of the mean (n = 6 of LPS, n = 3 of VSV day 1 and n = 2 of VSV day 3).
3.2. Gene data mining and selection
Given the limited cross-reactivity (Table 2) and high costs of mouse and/or rat ELISA and luminex based assays, qRT-PCR assays were considered and selected as the most practical detection system of choice to analyze hamster immune responses until further genome sequences become available. For this we analyzed specific hamster sequences registered on Genbank. Of the ~ 800 sequences available, 51 hamster specific genes involved in pro-inflammatory, anti-inflammatory, innate immunity, apoptosis, cell junction and coagulation responses, and four constitutively expressed reference genes were selected and TaqMan based qRT-PCR assays developed (Table 3).
3.3. Evaluation of internal reference genes
The identification of a suitable reference gene was of paramount importance for the assay development. Studies focusing on human tissues previously concluded that commonly used reference genes such as HPRT and β-actin may not be suitable as internal references due to large variations in expression between tissues and in responses to stresses (Dheda et al., 2004). Hamsters are outbred animals and therefore variability is inherent between individual animals. This makes selection of an appropriate internal reference critical (Dheda et al., 2004). β-actin, β2M, HPRT, and RPL18 were selected as potential housekeeping genes and individually evaluated for their expression levels in lung, liver and spleen tissues of treated/infected and control hamsters (n = 17 per tissue, n = 51 total). Fig. 2 shows the distribution of the fluorescence curves within representative tissues of infected, treated and untreated hamster tissues. β2M and RPL18 vary less than HPRT and β-actin (β2M standard deviation (SD) ≤ 1 cycle within tissues, 1.29 cycles between all samples; RPL18 SD ≤ 0.5 cycles within tissues, 0.87 cycles between all samples; HPRT SD ≤ 4.6 cycles within tissues, 3.4 cycles between all samples; β-actin SD ≤ 7.2 cycles within tissues, 7.5 cycles between all samples). Though previous work in hamsters has used HPRT and β-actin as reference genes (Melby et al., 1998, Muller et al., 2001, Vernel-Pauillac and Merien, 2006), in this study these internal reference genes showed some variation between infected/treated and untreated hamsters and in comparison of different tissues (Fig. 2). The variation of β2M and, especially, RPL18 was much less in all instances. Therefore we chose to use RPL18 as the most reliable internal reference gene and propose that it should be strongly considered for future work.
Fig. 2.

Distribution of CT values of the internal control genes in treated and untreated hamster tissues. Ribosomal protein L18 (RPL18) has the lowest variation between treated and untreated hamsters, followed by β-2-microglobulin (β2M), Hypoxanthine–guanine phosphoribosyltransferase (HPRT) and β-actin (βAct). The boxes indicate the 25th percentile to 75th percentile values, the middle line is the median value and the whiskers the total range of the values (n = 17 per tissue, n = 51 combined).
3.4. Primer/probe efficiency and limit of detection
The average efficiency or E value of the primers was determined to be 3.112 (range 2.904–3.321, n = 15,) (Fig. 3A), i.e. 3.112 cycles per log10 dilution, when used on DNA template and 3.178 (range 2.727–3.414, n = 12) (Fig. 3B), i.e. 3.178 cycles per log10 dilution, when used on RNA template. The average r2 values of the slopes were determined to be 0.9969 (range 0.9911–0.9996) from the DNA template (Fig. 3A) and 0.9616 (range 0.8148–0.9995) (Fig. 3B) from the RNA template. The signal generated per target template between the control and reference primer/probe sets was considered to be comparable as the efficiencies of the primer/probe sets were within 10% of the average (Ling et al., 2008). The limit of detection for the assayed primer/probe sets was determined to be between 10− 6 to 10− 8 ng gene-specific RNA which corresponds to 9–900 copies of the target RNA. The lowest amount of total RNA which could be used in these sets of experiments was determined to be 50 ng/25 μL reaction. The advantage of the approach is the sheer number of available assays and the diverse aspects of the immune system that can be analyzed, as well as the low amount of RNA necessary to perform each of the assays. Together, this allows for detailed analysis of several important genes even with lower RNA isolation yields.
Fig. 3.
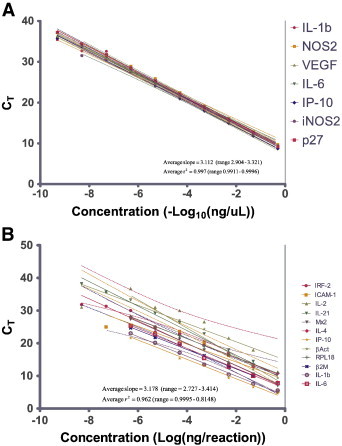
Representative standard curve slope results for hamster qRT-PCR assays. Standard curves for selected assays were generated using (A) sequence specific plasmids as template (n = 15) and (B) sequence specific in vitro transcribed RNA as template (n = 12). Solid lines are the best fit curve and dashed lines represent the 95% confidence interval of the linear regression analysis. IL = Interleukin, IP-10 = Chemokine (C-X-C motif) ligand 10, NOS = Nitric Oxide Synthase, iNOS = inducible NOS, VEGF = Vascular Endothelial Growth Factor, p27 = Interferon α inducible protein p27, IRF = Interferon Regulatory Factor, ICAM = Intercellular Adhesion Molecule, Mx = myxovirus resistance protein, RPL18 = ribosomal protein L18, β2M = β-2-microglobulin, and βAct = β actin.
3.5. Target RNA levels following LPS treatment
Previous studies using hamsters as a sepsis model reported complete fatality within three days post inoculation of E. coli (Steinwald et al., 1999). Hamsters used in our study developed severe “sepsis-like” symptoms (lethargy, ruffled fur, hunched posture and labored breathing) within one day following LPS treatment (50 μg) and were therefore euthanized and their tissues harvested. Hamster immune modulation was studied in selected tissues on day 1 post treatment (Fig. 4 ). The expression of pro-inflammatory genes was up-regulated with high transcript levels detected in the spleens and livers of the treated animals. Up-regulation of pro-inflammatory gene expression was less pronounced in lung tissue. Anti-inflammatory and immunoregulatory genes were up-regulated in all three tissues examined with liver being the strongest. The cell regulatory response was enhanced in spleen and liver tissues, but the anti-apoptotic gene expression was unaltered in all three tissues. Cell junction and integrity responses appeared to be minimally altered in the spleen, increased in the lung and nearly unaffected in the liver in response to LPS treatment and the expression level of vascular endothelial growth factor (VEGF) was strongly decreased in the three tissues examined, suggesting a lack of endothelial cell proliferation (Ferrara and Davis-Smyth, 1997). A previous study focusing on LPS induced sepsis in the hamster model noted that liver and spleen showed higher levels of gene expression than lung and other tissues (Muller et al., 2001). In our study here the responses to LPS inoculation were also more pronounced in liver and spleen than in lung tissue, suggesting a much greater impact on these organs.
Fig. 4.
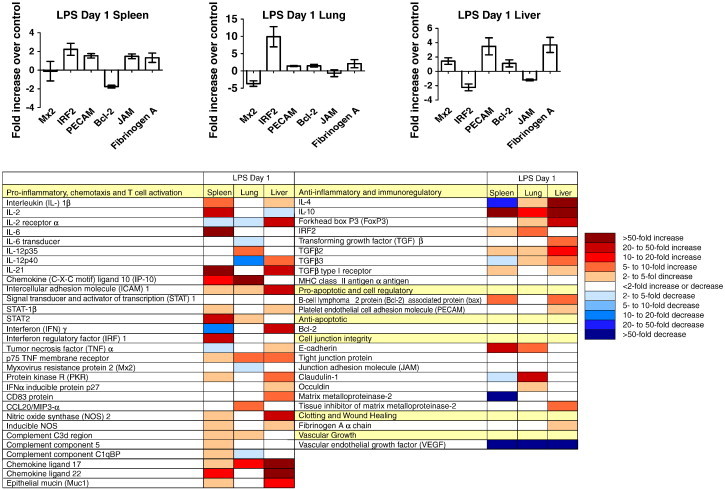
Immune responses to LPS treatment in hamsters. Hamster immune gene expression profiles, with representative graphs (randomly selected), in spleen, lung and liver samples collected 1 day post-inoculation with 50 μg of LPS. Data is presented as fold increase over uninfected controls. Error bars indicate standard error of the mean (n = 6). For abbreviations see table in bottom part.
3.6. Target RNA levels following VSV infection
Hamsters are especially susceptible to VSV infection. Previous studies report that, in hamsters, as few as 10 pfu of VSV inoculated i.p. will result in uniform lethality within 72 h (Fultz et al., 1981a, Fultz et al., 1981b). Upon infection with VSV (106 pfu) pro-inflammatory, innate and anti-inflammatory responses were largely up-regulated in all three organs examined with some being down-regulated (Fig. 5 ). Down- and up-regulation were especially pronounced in liver and spleen tissue (Fig. 5A and C). Pro-apoptotic genes were minimally up-regulated in expression in all tissues, but anti-apoptotic gene expression was increased only in the livers of infected animals. Expression of genes encoding for cell junction proteins was generally unaffected by VSV infection in liver, increased in the lungs, and reduced in the spleen of infected hamsters. Expression of fibrinogen was increased initially followed by a substantial decrease in expression in spleen and liver in final stages of the disease. This suggests that after infection with VSV there is a deregulation of cytokine and chemokine expression, which may precede or induce coagulation abnormalities in spleen and liver tissues of hamsters. Interestingly, the opposite was found for lung tissue (Fig. 5B). VEGF expression was strongly reduced in all tissues monitored during both early and terminal stages of infection. Overall the data imply that hamsters mount more robust immune responses in the liver and spleen than in the lung during VSV infection. Previous work identified the spleen and liver as the organs displaying pathological changes during VSV infection without notable involvement of the lungs, kidney, brain or intestinal tract (Fultz et al., 1981b). The similarities between our results and previous studies demonstrate the usefulness of our assays for more in depth evaluation of host responses to infection in an important animal model for which tools are largely lacking.
Fig. 5.


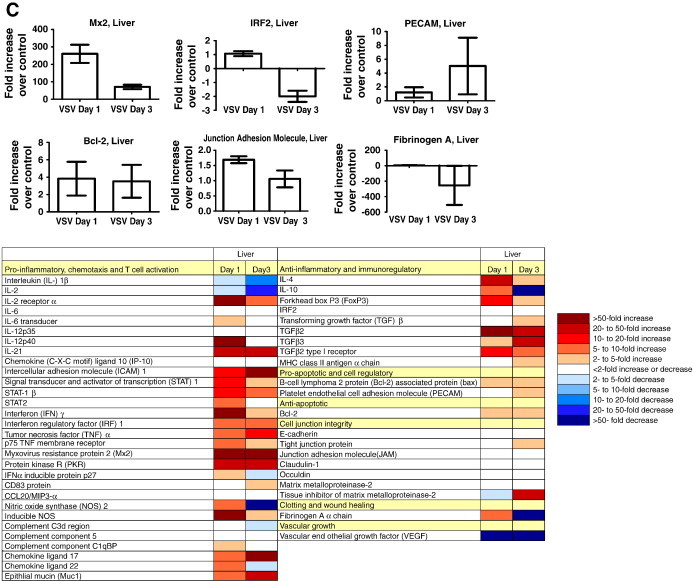
Immune responses to VSV infection in hamsters. Hamster immune gene expression profiles, with representative graphs (randomly selected), in (A) spleen, (B) lung and (C) liver collected at 1 and 3 days post infection with 106 pfu VSV. Data is presented as fold increase over uninfected controls. Error bars indicate standard error of the mean (n = 3 VSV day 1, n = 2 VSV day 3). For abbreviations see table in bottom part.
4. Conclusions
Mouse and rat ELISA and luminex panels tested in these studies were found to be of limited value for detecting hamster host responses. Instead, qRT-PCR assays for selected host response genes were established and validated on LPS-treated and VSV-infected hamsters. The assays allow for broad range monitoring of hamster immune and other cellular activities in host response to stress factors and the identification of RPL18 as an internal reference gene allows for accurate standardization. Thus, the developed assays will be instrumental in future applications of the hamster as a disease model until the full genome sequence is available allowing for the development of large scale RNA microarrays or deep sequencing.
Acknowledgements
The authors would like to thank Allen Grolla, Public Health Agency of Canada, Winnipeg, Canada, for help with the initial primer design and PCR; Katharine N. Bossart, Boston University, Boston, USA, for her fruitful discussion concerning realtime PCR; Gary Hettrick, Division of Intramural Research (DIR), National Institute of Allergy and Infectious Disease (NIAID), NIH, Hamilton, USA, for his help with visual graphics; and Joseph Prescott, DIR, NIAID, NIH, for his helpful suggestions regarding the manuscript. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Disease, National Institutes of Health.
Contributor Information
Heinz Feldmann, Email: feldmannh@niaid.nih.gov.
Hideki Ebihara, Email: ebiharah@niaid.nih.gov.
References
- Adler R. Vol. 45. 2008. Janeway's immunobiology; p. 1793. (Choice: Current Reviews for Academic Libraries). [Google Scholar]
- Beekes M., Baldauf E., Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 1996;77(Pt 8):1925. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- Braga W., Venasco J., Willard L., Moro M.H. Ultrastructure of Babesia WA1 (Apicomplexa: Piroplasma) during infection of erythrocytes in a hamster model. J. Parasitol. 2006;92:1104. doi: 10.1645/GE-712R.1. [DOI] [PubMed] [Google Scholar]
- Bruder C.E., Yao S., Larson F., Camp J.V., Tapp R., McBrayer A., Powers N., Granda W.V., Jonsson C.B. Transcriptome sequencing and development of an expression microarray platform for the domestic ferret. BMC Genomics. 2010;11:251. doi: 10.1186/1471-2164-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabry J., Priola S.A., Wehrly K., Nishio J., Hope J., Chesebro B. Species-independent inhibition of abnormal prion protein (PrP) formation by a peptide containing a conserved PrP sequence. J. Virol. 1999;73:6245. doi: 10.1128/jvi.73.8.6245-6250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson N.A., Morsink M.C., Atanasova S., Armstrong V.W., Zischler H., Schlumbohm C., Dutilh B.E., Huynen M.A., Waegele B., Ruepp A., de Kloet E.R., Fuchs E. Development of the first marmoset-specific DNA microarray (EUMAMA): a new genetic tool for large-scale expression profiling in a non-human primate. BMC Genomics. 2007;8:190. doi: 10.1186/1471-2164-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheda K., Huggett J.F., Bustin S.A., Johnson M.A., Rook G., Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Fisher A.F., Tesh R.B., Tonry J., Guzman H., Liu D., Xiao S.Y. Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus. Am. J. Trop. Med. Hyg. 2003;69:269. [PubMed] [Google Scholar]
- Fultz P.N., Shadduck J.A., Kang C.Y., Streilein J.W. Genetic analysis of resistance to lethal infections of vesicular stomatitis virus in Syrian hamsters. Infect. Immun. 1981;32:1007. doi: 10.1128/iai.32.3.1007-1013.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P.N., Shadduck J.A., Kang C.Y., Streilein J.W. Involvement of cells of hematopoietic origin in genetically determined resistance of Syrian hamsters to vesicular stomatitis virus. Infect. Immun. 1981;34:540. doi: 10.1128/iai.34.2.540-549.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt M., Liebscher R., Wahl-Jensen V., Jones S., Moller P., Wagner R., Volchkov V., Klenk H.D., Feldmann H., Stroher U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 2004;78:5458. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E., Hartmann D.P. Protection against amebic liver abscess in hamsters by means of immunization with amebic antigen and some of its fractions. Am. J. Trop. Med. Hyg. 1980;29:779. doi: 10.4269/ajtmh.1980.29.779. [DOI] [PubMed] [Google Scholar]
- Haake D.A. Hamster Model of Leptospirosis. Curr. Protoc. Microbiol. 2006 doi: 10.1002/9780471729259.mc12e02s02. Chapter 12, Unit 12E 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J.W., Larsen T., Custer D.M., Schmaljohn C.S. A lethal disease model for hantavirus pulmonary syndrome. Virology. 2001;289:6. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- Ierna M., Kerr A., Scales H., Berge K., Griinari M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010;11:136. doi: 10.1186/1471-2474-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.C., SenGupta S.K., Smith J.F. Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet. Pathol. 1991;28:410. doi: 10.1177/030098589102800509. [DOI] [PubMed] [Google Scholar]
- Kayo T., Allison D.B., Weindruch R., Prolla T.A. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc. Natl Acad. Sci. USA. 2001;98:5093. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E.W., Dominik J.W., Slone T.W. Aerosol stability and respiratory infectivity of japanese B encephalitis virus. Infect. Immun. 1980;30:397. doi: 10.1128/iai.30.2.397-401.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D., Stillman E.A., Whitt M.A., Rose J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl Acad. Sci. USA. 1995;92:4477. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K.H., Hewitt C.A., Kinkel S.A., Smyth G.K., Scott H.S. High-throughput and complex gene expression validation using the Universal ProbeLibrary and the LightCycler® 480 System. Biochemical. 2008;2:23. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(− Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Melby P.C., Tryon V.V., Chandrasekar B., Freeman G.L. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. Infect. Immun. 1998;66:2135. doi: 10.1128/iai.66.5.2135-2142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby P.C., Chandrasekar B., Zhao W., Coe J.E. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 2001;166:1912. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- Milazzo M.L., Eyzaguirre E.J., Molina C.P., Fulhorst C.F. Maporal viral infection in the Syrian golden hamster: a model of hantavirus pulmonary syndrome. J. Infect. Dis. 2002;186:1390. doi: 10.1086/344735. [DOI] [PubMed] [Google Scholar]
- Muller B., White J.C., Nylen E.S., Snider R.H., Becker K.L., Habener J.F. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001;86:396. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- Niklasson B.S., Meadors G.F., Peters C.J. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathol. Microbiol. Immunol. Scand. C. 1984;92:197. doi: 10.1111/j.1699-0463.1984.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Paessler S., Aguilar P., Anishchenko M., Wang H.Q., Aronson J., Campbell G., Cararra A.S., Weaver S.C. The hamster as an animal model for eastern equine encephalitis — and its use in studies of virus entrance into the brain. J. Infect. Dis. 2004;189:2072. doi: 10.1086/383246. [DOI] [PubMed] [Google Scholar]
- Roberts A., Vogel L., Guarner J., Hayes N., Murphy B., Zaki S., Subbarao K. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J. Virol. 2005;79:503. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrana E., Mateo R.I., Xiao S.Y., Popov V.L., Newman P.C., Tesh R.B. Clinical laboratory, virologic, and pathologic changes in hamsters experimentally infected with Pirital virus (Arenaviridae): a rodent model of Lassa fever. Am. J. Trop. Med. Hyg. 2006;74:1096. [PubMed] [Google Scholar]
- Siirin M.T., Duan T., Lei H., Guzman H., da Rosa A.P., Watts D.M., Xiao S.Y., Tesh R.B. Chronic St. Louis encephalitis virus infection in the golden hamster (Mesocricetus auratus) Am. J. Trop. Med. Hyg. 2007;76:299. [PubMed] [Google Scholar]
- Smee D.F., Gilbert J., Leonhardt J.A., Barnett B.B., Huggins J.H., Sidwell R.W. Treatment of lethal Pichinde virus infections in weanling LVG/Lak hamsters with ribavirin, ribamidine, selenazofurin, and ampligen. Antivir. Res. 1993;20:57. doi: 10.1016/0166-3542(93)90059-r. [DOI] [PubMed] [Google Scholar]
- Spencer J.F., Sagartz J.E., Wold W.S., Toth K. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Ther. 2009;16:912. doi: 10.1038/cgt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwald P.M., Whang K.T., Becker K.L., Snider R.H., Nylen E.S., White J.C. Elevated calcitonin precursor levels are related to mortality in an animal model of sepsis. Crit. Care. 1999;3:11. doi: 10.1186/cc300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R.B., Guzman H., da Rosa A.P., Vasconcelos P.F., Dias L.B., Bunnell J.E., Zhang H., Xiao S.Y. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 2001;183:1431. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Vernel-Pauillac F., Merien F. Proinflammatory and immunomodulatory cytokine mRNA time course profiles in hamsters infected with a virulent variant of Leptospira interrogans. Infect. Immun. 2006;74:4172. doi: 10.1128/IAI.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.T., Grosjean I., Brisson C., Blanquier B., Fevre-Montange M., Bernard A., Loth P., Georges-Courbot M.C., Chevallier M., Akaoka H., Marianneau P., Lam S.K., Wild T.F., Deubel V. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol. 2003;163:2127. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.Y., Guzman H., Zhang H., Travassos da Rosa A.P., Tesh R.B. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 2001;7:714. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Peacock S., Grant D.P., Batter-Hatton D. The pathogenesis of western equine encephalitis virus (W.E.E.) in adult hamsters with special reference to the long and short term effects on the C.N.S. of the attenuated clone 15 variant. Br. J. Exp. Pathol. 1972;53:59. [PMC free article] [PubMed] [Google Scholar]


