Introduction
There have been occasional lively debates about the actual number of sexes that can be defined [42]. However, there is little debate that mammalian sexual differentiation starts from the perspective of two primary sexes that correspond to differential sex chromosomes (X versus Y) that lead to individuals with sex typical characteristics, ranging from breast development and function, facial hair, different reproductive organs in humans, to larger body sizes in many mammals, presence or absence of antlers in ungulates, and differential plumage in birds [10]. Beside obvious external differences, sex differences exist in many other organs or organ systems such as liver, immune system and brain [20, 26, 27, 51, 132]. While genes on sex chromosomes are usually credited as the key trigger for generating sex differences, most sex differences (at least in mammals) are thought to arise due to differential exposure to sex steroid hormones secreted by the gonads during development. In male mammals, the formation of the testis is triggered by the expression of the Sry gene on the Y chromosome. Sry induces a genetic cascade that leads gonadal primordia to develop into testes [34]. Subsequently, testes secrete different hormones, key among them being the steroid hormone testosterone and the peptide anti-mullerian hormone, which are responsible for development of the male phenotype [50]. While dogma states that ovaries develop in the absence of Sry, newer data indicate a critical genetic cascade for ovarian development [34].
More than 50 years ago a study of guinea pigs exposed to exogenous testosterone during pregnancy [104] led to a large number of studies showing that sex steroids play important roles for the sexual differentiation of brain and behavior [14]. Sex steroids, in particular testosterone and its aromatized metabolite estradiol [73], influence brain development and plasticity throughout the lifespan. Sex differences in morphology, physiology or behavior can be achieved through processes that occur early in life with long-lasting consequences (often termed organizational effects) and/or through processes that occur by direct action of sex steroids immediately prior to changes that are observed (often termed activational effects). Interestingly, in rodents, the metabolite estradiol tends to be more responsible for masculinization of the developing brain (an organizational influence), while in primates (including humans) the precursor testosterone likely plays the more important role for masculinization [46, 123]. Regardless of species, testosterone is converted with the help of the enzyme aromatase into estradiol locally in specific brain regions [9, 110]. This renders the active local concentration of estradiol different than that in the peripheral circulation. To add to the anatomical specificity of potential responses, androgen and estrogen receptors are also present in discrete brain areas throughout life [72, 86, 125, 134].
Although sex steroid hormones account for most aspects of brain sexual differentiation, a growing literature has raised important questions about the direct role of genes on sex chromosomes separate from sex steroid actions [3, 10]. Sex chromosomes obviously differ by sex, but it has been controversial as to what extent the genes on these chromosomes might affect brain development directly and differentially to cause differences in the brain between males and females. One straight-forward answer to this question has come from studies in zebra finches. Birds utilize similar mechanisms for sexual differentiation as mammals, although in birds, females are the heterogametic sex with Z and W chromosomes while males are the homogametic sex with two Z chromosomes. Through the 80's and 90's there were a number of studies showing that exogenous steroid hormones could cause masculinization of zebra finch brains in development [57, 90], but also several studies that indicated that it was not that simple [81, 113]. In the mid-90's a now classic experiment showed that creating testes in female birds was insufficient to drive brain masculinization [137]. This launched extended efforts in birds and subsequently mammals to determine factors other than secretions from the gonads that could drive brain sexual differentiation. Perhaps the exclamation point in the bird story derived from the fortuitous finding of a gynandromorphic zebra finch [1]. As songbirds are usually sexually dimorphic in plumage as well as brain, the gynandromorphic finch was one that was uniquely defined with characteristics of both sexes, separated in the midline of the body with one half of the body male (i.e., colorful plumage) and the other side with typical female characteristics. This midline division was also evident in the brain, where in situ hybridization for W and Z chromosomal markers showed that the W chromosome markers were only present on the female side. Most importantly, at least one brain nucleus important for sexually dimorphic singing behavior was asymmetric in size indicating a sex chromosomal gene contribution to sexual differentiation. This nucleus was still larger in the gynandromorph than in normal females suggesting that there was also a sex steroid hormone component, but there was now a striking visible indication that it was no longer the only story. However, birds are different from mammals in many aspects and this review will focus on the literature suggesting that hormone independent sex differences in the brain exist also in mammals and that sex chromosomal genes and hormonal influences synergize to result in brain sexual differentiation.
1. Models to study hormone independent brain sex differentiation in mammals
1.1 Four core genotype mice
Four core genotype (FCG) mice were produced originally by manipulating the Sry gene [32]. As mentioned earlier and reviewed elsewhere [34, 116], the Sry gene bears primary responsibility for the development of testes in mammals. Mutation of the Sry gene causes male to female sex reversal in the presence of a Y chromosome, while translocation of the Sry gene to either the X chromosome or one of the autosomes is sufficient to cause testis development and consequently many male specific sex characteristics including male external genitalia in genetically female (XX) mice [12, 70]. Although the testes form and testosterone is produced, they do not provide for spermatogenesis as other necessary regulatory genes are on the Y chromosome. Such sex reversed male mice are infertile, and it is likely that the brains are also not completely sex reversed. All of these factors go into the creation of FCG mice where Sry gene was either deleted from the Y chromosome, or translocated to the autosomes, creating four different genotype/phenotype combinations [3, 32] (Figure 1):
XY male mice that have intact Sry gene on an autosome (XY gonadal male)
XX female mice without Sry gene (XX gonadal female)
XY female mice with deleted Sry gene from the Y chromosome (XY gonadal female)
XX male mice carrying Sry gene on one of the autosomes (XX gonadal male)
Figure 1.
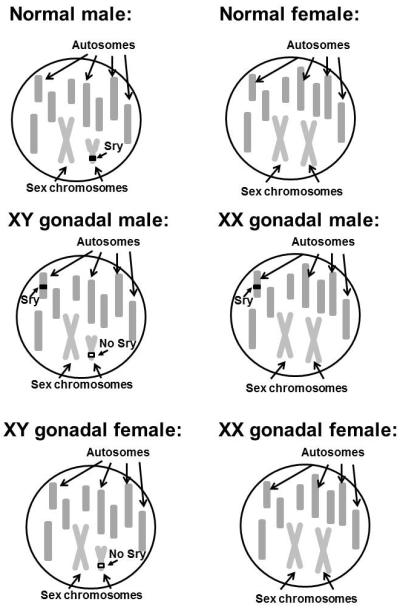
Schematic representation of a genotype in WT male and female mice and in all four groups of FCG mice. The number and shapes of chromosomes are purely schematic and do not represent real metaphase chromosomal shapes.
These mice are an important model for studying brain sexual differentiation since genetic sex can be dissociated from phenotypic sex and hormonal status (i.e., XX males and XY females). Therefore, mice with testes can have two X chromosomes and mice with ovaries can have one X and a Y chromosome. However, results from studies with FCG mice must be carefully analyzed and interpreted since these mice are still exposed to gonadal hormones, even when their gonadal sex does not match their chromosomal sex [3].
1.2. Steroidogenic factor 1 knockout mice
Steroidogenic factor 1 knockout mice (SF-1 KO) were developed independently at approximately the same time by Keith Parker in the United States and Kenichiro Morohashi in Japan, who were interested in the regulation of adrenal steroidogenesis. Creation of SF-1 KO mice revealed much wider roles for the SF-1 gene than expected based simply on a role in steroidogenesis. Both groups surprisingly found that SF-1 KO mice are born without gonads and adrenal glands, have dysfunctional pituitary gonadotropes and a disorganized brain region containing the ventromedial nucleus of the hypothalamus (VMH) [77, 117]. In SF-1 KO mice, genital ridges initially form but the gonads regress soon after their formation, before steroidogenesis starts in the fetal testis [77]. Consequently, these mice are not exposed to endogenous gonadal hormones. It is likely, however, that KO fetuses are exposed in utero to sex steroid hormones deriving from their mothers, and possibly from neighboring male fetuses [112, 131]. This type of exposure would not be expected to differ between KO males and females. Long-term studies with SF-1 KO mice are hampered by neonatal mortality due to adrenal insufficiency. This can be overcome by using a protocol that calls for glucocorticoid replacement and adrenal transplantation that enables raising SF-1 KO mice into adulthood [18, 54, 78]. SF-1 KO mice represent another useful model for studying sex differences in the brain in the absence of endogenous sex steroid hormones, however, like the FCG mice one also has to be careful with interpretation of the data obtained from them. In particular, SF-1 KO mice also have a disruption of brain organization in a region (VMH) that is important for regulating a number of key aspects of physiology and behavior. This by itself may lead to some behavioral deficits/discrepancies in comparison to WT mice that are not necessarily due to the absence of gonadal hormones. A majority of the review of sex chromosomal genes and endocrine influences for sexual differentiation below will drawn from studies utilizing FCG or SF-1 KO mice. Examples from other mammalian species, including humans are difficult to find, although Turner Syndrome patients that have one X chromosome may provide some information relevant to hormone and sex chromosomal gene interactions [88, 108]. Some information about this interaction might also be drawn from clinical examinations of patients with either estrogen receptor or cyp19 mutations [63]; or patients with testicular feminization (TFM) syndrome [92]. However, in all these cases, hormone deficiencies are incomplete and provide only partial answers about the contributions of sex chromosomal genes to brain development in humans.
1.3 Other genetic models of hormone influences on sexual differentiation in mice
There are several other molecular genetic murine models, developing in the partial absence of sex steroid hormones or increased exposure to sex steroid hormones. Mice lacking steroid hormone receptors such as estrogen receptor-α knockout mice (ERαKO/ERKO mice), estrogen receptor-β knockout mice (ERβKO/BERKO mice), naturally occurring TFM (testicular feminization) mice with mutated androgen receptor (as well as the deliberately created androgen receptor knockout mouse) and hypogonadal (HPG) mice with mutated GnRH gene, or altered enzymes for producing steroid hormones like aromatase knockout mice (ArKO mice) are all useful in studies of brain sexual differentiation. These models provide complementary answers in regard to which sex steroids or which pathways are responsible for differentiation of certain brain characteristic/behavior. Perhaps the most well-studied models in these respects are ERKO and ArKO mice, which show several behavioral deficits such as reduced aggression in adult male mice, reduced male and female sexual behavior, and interestingly, increased female aggressive behavior, confirming the importance of estrogens for development of certain sex specific behaviors [6-8, 84, 93-95]. Studies with ERβKO mice did not find major effects on typical sexually differentiated behaviors such as sexual or aggressive behavior, although some differences between WT and ERβKO mice were found in different cognitive/learning behaviors [71, 109]. In TFM mice lacking fully functional androgen receptors, feminine behavior was observed in several tests of odor preference, although there were no differences between WT and Tfm male mice in aggressive or masculine sexual behaviors, suggesting that these two behaviors are not regulated by developmental exposure to androgens, but rather dependent on estradiol (intermale aggressive behavior) or not sexually dimorphic at all (male sexual behavior)[15, 115]. One final interesting model to consider is mice in which the alpha-fetoprotein gene is disrupted [4]. Alpha-fetoprotein is believed to protect the female rodent brain from the action of estradiol that may be in the fetal circulation. A key test of this hypothesis was provided by studies showing that alpha-fetoprotein KO mice were partially defeminized as evident by reduced lordosis behavior and masculinized based on enhanced male sexual behavior [4]. There were no changes in olfactory preferences [5], suggesting that this particular behavior is not regulated by prenatal estrogen exposure.
2 Sex differences in adulthood
2.1. Morphology
2.1.1. Sexually dimorphic nucleus in the medial preoptic area
Sex steroids have important organizational roles during development that result in morphological differences between male and female brains. Although studies of morphological differences are often difficult and hampered by the allomorphic appearance of such differences (in contrast to dimorphic) there are nevertheless some consistent morphological differences in mammals that are detected in different species. Perhaps the best known is the so called sexually dimorphic nucleus (SDN), first described in rats in the late 1970's [37, 52, 53] that is larger in males than females. Subsequent studies have shown sex differences in nuclear grouping in this region in many different species including rat, ferret, sheep, monkeys and also humans [132]. One of the best molecular signatures of this cell grouping in rats is the calcium binding protein calbindin [118]. Interestingly, in mice, nuclear grouping is not morphologically distinguishable with classic Nissl stains (e.g., Thionin; [17], but can be visualized by examining immunoreactive calbindin [39]. The exact function of this cell grouping is yet to be clarified in any species. Although it's location suggests a role in male sexual behavior, some experimental evidence argues directly against this [2]. Early studies in rats using hormone antagonists had indicated the likely hormone-dependence of the differentiation of the rat SDN [38]. Formation of the calbindin immunoreactive nucleus in male mice is completely hormone dependent, as it is only becomes a cell group in gonadally intact males, and is absent in females, and SF-1 KO males [18]. The use of the SF-1 KO model therefore confirms that gonadal steroids during development are needed for the normal development of this nucleus.
2.1.2. Anteroventral periventricular area
Another well studied brain region that exhibits strong sexual dimorphism in different mammalian species is the anteroventral periventricular nucleus (AVPV), a rostral part of preoptic area, thought to be involved in the regulation of the ovulatory surge of luteinizing hormone in females [61]. In this nucleus in rodents, there are more cells present in females than males (leading to larger nuclear volume [45, 119]). There are even more striking differences in the number of cells expressing different neurochemical signatures such as the rate limiting enzyme for dopamine production, tyrosine hydroxylase, as well as estrogen receptor alpha, and GABA/glutamate neurons [24, 100, 120]. Kisspeptin, a peptide involved in the regulation of GnRH release and onset of puberty is found in cells of AVPV in rats and in the region that extends more caudally in mice (rostral periventricular area of the third ventricle – RP3V; [25, 61, 65]). Several studies have shown that sexually dimorphic development of this area is also hormone dependent as sex differences in both tyrosine hydroxylase and kisspeptin containing cells are not evident in gonadectomized animals. In addition, immunoreactive kisspeptin is absent from the RP3V in the absence of sex steroid hormone exposure in SF-1 KO mice [19]. Analysis of mice from the FCG model further indicated that there was no influence of sex chromosomal gene background on the number of neurons in AVPV containing immunoreactive tyrosine hydroxylase [32]. FCG mice with testes during development always developed with fewer immunoreactive neurons. This may not apply to all dopaminergic neurons, as there is strong evidence for a genotype effect independent of hormone action in mesencephalic dopaminergic neurons [21]. Finally, not all sex differences in the AVPV region are likely due to sex steroid hormones as studies of immunoreactive neuronal nitric oxide synthase (nNOS) in this same area revealed a sex difference in wild-type mice that was maintained between SF-1 KO males and females, suggesting that some aspects of sexual dimorphism of this area develop in a hormone-independent manner [18].
2.1.3. Lateral septum
The projection of vasopressin immunoreactive fibers to the lateral septum is one of the more robust sex differences in the vertebrate kingdom in adult animals (review by [29]). Although the main role of vasopressin in the mammalian body is regulation of fluid homeostasis, vasopressin in the lateral septum is believed to be involved in the regulation of social behaviors like social recognition, pair bonding and others [13, 28, 59]. Sex differences in fibers containing immunoreactive vasopressin in the lateral septum were first described in the early 80's by De Vries and colleagues [31]. These fibers arise from cells bodies in the bed nucleus of the stria terminalis (BNST) and amygdala and theoretically release their contents to target cells in the lateral septum [30, 135, 140]. For maximal content of immunoreactive vasopressin in the lateral septum, testosterone is needed both organizationally and activationally, since in females and in gonadectomized male rats (either neonatally or in adulthood at three months of age) vasopressin expression is barely detectable [140]. Important for this review, De Vries and colleagues have shown in FCG mice that the sexual dimorphic portrait of immunoreactive vasopressin in the lateral septum is also dependent on sex chromosome complement. In FCG mice, XY females exhibited greater immunoreactive vasopressin than XX females and likewise, levels of immunoreactive vasopressin were higher in XY males in comparison to XX males suggesting that some aspect of Y chromosome function, or absence of a second X chromosome, may also contribute to this particular sex difference [32]. By contrast, no differences in immunoreactive vasopressin between male and female SF-1 KO mice were detected in mice treated with testosterone propionate for 3 weeks (Budefeld and Majdic, unpublished results), suggesting that regulation of sexually dimorphic vasopressin fibers in the lateral septum may be complicated by some aspect of gonadal function across the lifespan. The difference between FCG and SF-1 KO mice may be due to the complete absence of sex steroid hormone exposure during development for the SF-1 KO mice. An organizational influence of sex steroids for vasopressin fibers in the lateral septum was an early component of the discovery [30], and this idea is further supported by the observation that even after 3 weeks exposure to testosterone, vasopressin expression in SF-1 KO mice was lower in comparison to gonadally intact male mice (Budefeld and Majdic, unpublished results).
2.1.4. Ventromedial hypothalamus
Another area that has been noted to be sexually dimorphic is the VMH, which is disrupted in SF-1 KO mice. Several studies have shown different sex differences such as in the volume of this nucleus [82], in dendritic tree patterns [56, 83], serotonergic innervations [101] and expression of different genes such as estrogen (alpha and beta) and progestin receptors [98, 139, 145]. Many of these characteristics have been connected with the regulation of female sexual behaviors, particularly measures of receptivity [80, 103].
The VMH is an interesting nucleus to consider from the perspective of sex differences in SF-1 KO mice because it is a region that is directly affected by loss of the SF-1 gene independent of any influence of the gonads. This was demonstrated using mice in which the loss of SF-1 function was targeted selectively to brain sites [67, 147]. In the brain selective SF-1 KO, the region of the VMH is disorganized similar to the global KO. Nevertheless, the VMH in SF-1 KO mice is a site that provides a window to observe steroid hormones influences on the rearrangement of cells in development that are also impacted by the more direct loss of SF-1. Calbindin and nNOS provide molecular signatures of interest in this regard as cells containing either of these immunoreactive proteins are sexually dimorphic in wildtype adult mice. As expected for the reorganization of the region of the VMH in the KO mice, cells that were immunopositive for calbindin or nNOS were located in more dorsomedial positions in comparison to WT mice. Interestingly, for calbindin the significant sex difference in number of calbindin immunopositive cells was maintained in SF-1 KO mice [18]. Therefore, even though the cells were present in different positions, a sex difference was maintained in mice that were not exposed to endogenous gonadal steroids – suggesting a strong genetic influence on the sex difference in the number of calbindin positive cells in the context of their altered final locations. The situation, by contrast, was notably different for nNOS. The sex difference in immunoreactive nNOS containing cells in the ventrolateral VMH of wildtype mice was lost with the dorsomedial shift in cell positions in the SF-1 KO mice. The hormone receptor sensitivity of cells containing calbindin or nNOS is not yet clear for the ventrolateral VMH. The region is well known for its content of estrogen receptors and estrogen-induced progestin receptors [138, 145]. Early studies showed clear shifts in the locations of estrogen receptor alpha containing cells to medial positions in development [35, 78]. Recent experiments using double-label immunofluorescence indicate that in WT mice some of calbindin cells also express estrogen receptor alpha (Budefeld and Majdic, unpublished observations). It is more difficult to determine where the progestin receptor containing cells might end up in the SF-1 KO as progestin receptor immunoreactivity is greatly reduced ([67]). At least a small subset of these cells remains in the normal position in the ventrolateral part of the VMH (Budefeld and Majdic, unpublished observations). There were no sex differences in the number of progestin receptor positive cells in the VMH in these same mice, suggesting that remaining progestin receptor positive cells are not responsible for driving sex differences in female sex behavior.
2.2. Sexual differentiation of behaviors related to hypothalamic function
Behaviors are complex phenotypic traits that develop under the influence of many different factors. The relationship between genes and behavior is complex and frequently misunderstood [128]. Many animal behaviors are connected with basic survival functions (e.g., searching for food, avoidance of predators, territorial aggression to ensure access to resources) and continuation of the species (e.g., male and female sexual behavior, parental behavior, female aggressive behavior for protection of offspring, male aggressive behavior in fights for mating privileges). Many of these behaviors are sex dependent. Rodent females show typical female like behaviors such as lordosis when in estrus under the influence of estrogenic hormones and in the presence of stimulus males. Males under the influence of testosterone show mounting, intromission and ejaculatory behaviors with receptive females [89]. Sex differences in behaviors have been studied in many species. In virtually all cases, it is critical to define the contributions of sex steroid hormones that are essential during critical periods early in development (organizational) versus those that are necessary at the time of testing (activational). In both of these cases it is important to define factors that are important for the development of behavioral capacity and behavioral performance – and these must be defined in terms of hormone-dependent versus hormone-independent. These issues have been studied using both FCG and SF-1 KO mice.
2.2.1. Female sex behavior
Female sex behavior refers to the typical female response to the presence of a male that is actively trying to mate with a female in proestrus – an estrous cycle stage that is conducive to reproduction because of circulating estradiol and progesterone. In experimental studies, this is usually achieved by hormone priming, adding estradiol (48h) and progesterone (4h) to female mice or rats before testing [16, 40]. The VMH is an important brain region regulating lordosis since disruption of this nucleus abolishes lordotic responses [103] and direct implantation of estradiol alone or estradiol and progesterone induces lordosis [111]. Another region involved in lordotic response is preoptic area [64, 127], which is thought to act as a restraint, providing fine tuning of this behavior together with the VMH.
Since female sex behavior is clearly regulated by hormones, it is not surprising that there is strong sex difference in the expression of such behavior, not only in native rodents but also in gonadectomized, hormone primed rats and mice of both sexes (reviewed in [33]. Lordotic responses are much stronger in females, suggesting that not only activational, but also organizational effects of sex steroids are needed for the proper development of the capacity to display female sexual behavior [36, 96, 124]. Interestingly, a recent study using SF-1 KO mice suggests that perhaps in addition to sex steroids, there is also a hormone-independent, sex chromosomal gene dependent contribution to the development of this behavior. Sex differences were seen in both lordosis quotient and in the number of intromissions received that was higher in tested females than in tested males whether they were wild-type or SF-1 KO, suggesting that this behavior might be partially influenced by hormone-independent developmental processes [55]. In addition, it was somewhat surprising to see SF-1 KO mice showing any lordosis behavior as their VMH is disrupted and this alone resulted in reduced fertility in brain-selective SF-1 KO (Kim et al., 2010). However, preliminary observations as noted above indicated that a reduced number of immunoreactive progestin receptors were present in their normal locations in the ventrolateral hypothalamus. It is possible that even a small number of PR-immunoreactive cells in the VMH may be sufficient to drive a minimal amount of lordotic behavior.
2.2.2. Male aggressive behavior
Aggression is a complex social behavior that can be triggered by a number of different causes. In laboratory rodents, different types of aggression could be distinguished such as maternal aggression with the aim to protect offspring, and intermale aggression thought to develop as evolutionary response to protect resources (territorial aggression) and ensure transfer of genes into a next generation [89]. Since intermale aggressive behavior is generally thought to be the most relevant for understanding human aggressive behavior, the current discussion will be restricted to this behavior.
Rodent males (both mouse and rat) under the influence of testosterone are capable of showing strong aggressive behavior that can result in the death of the opponents [49]. Intermale aggressive behavior is believed to be primarily influenced by serotoninergic systems. Serotonergic systems usually dampen aggression in both animals and humans, and reduced serotonin levels or turnover are associated with increased aggression [11, 22, 74]. This behavior is also strongly sex dependent due in large part to sex differences in the modulation of serotonergic systems by sex steroid hormones. Interestingly, there may be strain differences with regard to which sex steroid hormone is important for the regulation of aggressive behavior in mice. Namely, the CF-1 strain is sensitive to both estrogens and androgens, the CFW strain only to estrogens, while aggression in C57BL/6J mice seems to be influenced only by testosterone and not by its aromatized metabolites in adult life [121, 122]. Nevertheless, estrogens are likely to be important for organizational development of the capacity to display aggressive behavior in C57BL/6J mice. This is suggested by data from ERKO mice that display reduced aggression [93, 94]. This organizational influence of sex steroid hormones on aggressive behavior probably arises from the modulation of serotonergic systems during the neonatal period [43, 126, 146].
Adult activational effects of testosterone are needed for the normal expression of aggressive behavior since castrated males usually do not show strong aggressive responses to male intruders [87, 121, 122], and several studies have shown that organizational effects of testosterone during neonatal development are also needed for development of capacity to exhibit aggressive behavior [48, 121]. In a study of SF-1 KO mice raised to adulthood, only wild type males gonadectomized prior to puberty showed aggressive responses to intruder males when treated with testosterone prior to testing [54]. Despite much evidence confirming the importance of sex steroid hormones in the regulation of intermale aggressive behavior (either androgens or estrogens), a study using FCG mice [47] found differences in aggressive behavior that were in part related to sex chromosomes of origin. In this study, XX gonadal female mice showed less aggression than any other group of mice (XY gonadal female, XY gonadal male, XX gonadal male), suggesting that an interaction between hormonal exposure and sex chromosomal genes is important for development of the capacity to express aggressive behavior. It is important to note that the FCG and SF-1 KO models provide for hormonal exposure in different ways. Although SF-1 KO mice are sometimes discussed as a null hormone environment, this is an oversimplification. SF-1 KO mice are exposed to maternal steroids, any sources of steroids outside of the endogenous gonads or adrenals (e.g., such as brain neurosteroids [85], and adrenal steroids following transplant. Most of these sources should not produce sex differences in hormone exposure, but it remains to be determined whether subtle sex differences in neurosteroids play key roles in early development [69]. FCG mice are exposed to additional sex steroid hormones during development, since they maintain gonads and adrenals capable of steroidogenesis in addition to the common mechanisms. In the aforementioned FCG study [48], mice were not gonadectomized until puberty. For both animal models (FCG or SF-1 WT controls) the time of gonadectomy may impact the expression of particular characteristics that are examined. Some of these hormonal differences in addition to the genetic differences could account for alternate results between experiments using FCG and SF-1 KO mice.
2.2.3. Parental behavior
Parental behavior is needed to ensure survival of the offspring. While in birds both males and females often exhibit parental behavior, in mammals it is usually mothers that are responsible for parental care. Nevertheless, male parental behavior is also present in some species of carnivores, rodents and primates, including humans [89]. In rats and mice, both parents usually exhibit parental behavior and often, males take care of newborns [76, 144]. Parents respond to ultrasonic vocalizations and smells/pheromones secreted by offspring with typical behaviors such as crouching, nest building, retrieval, and protection from intruders [75, 107]. Although parental behavior is induced after delivery by hormonal changes in the female, both rats and mice that are not parents are also capable of showing parental behavior. Juvenile or gonadectomized mice and rats will thus usually show some patterns of parental behavior, although, especially in males, this is not always the case and some males will also exhibit infanticide when presented with unfamiliar pups [89].
Parental behavior is hormonally regulated by several hormones like prolactin, estrogens, oxytocin and others [23, 41, 79, 91, 102]. In addition, some parental behavior must be hormone independent as parental behavior is also observed in gonadectomized mice without hormone replacement. Still, parental behavior is sexually differentiated in juvenile or gonadectomized mice [76]. A recent study has also shown that prenatal exposure to estradiol is probably involved in defeminization of parental behavior. Female alpha-fetoprotein knockout mice that are not protected from maternal estrogens as normal females are, exhibit reduced levels of parental behavior, similar to the parental behavior observed in WT males [66]. Important for the current review, studies with FCG mice have shown that XX gonadal females exhibit better parental behavior [47]. In this study, latency to retrieve pups as well as number of retrieved pups was better in XX gonadal females than in any other group (XY gonadal females, XX gonadal males, XY gonadal males) suggesting that both genetic and hormonal influences must synergize for the development of this behavior. Namely, XX and XY females are exposed to similar levels of sex steroid hormones during development; yet, XX gonadal females show better parental behavior. This would suggest the influence of genes on sex chromosomes, perhaps reduced levels of some X chromosome genes (that escape X inactivation) in XY gonadal females. However, it is also clear that hormones also contribute to this behavior as XX gonadal males performed similarly as XY gonadal males and XY gonadal females, suggesting that a combination of long term hormonal and direct sex chromosomal gene influences lead to fully developed parental behavior.
3. Sex differences in development
All of the preceding discussion presupposes the sex-dependent characteristics develop across the lifespan, with likely emphasis on early developmental processes. These processes fundamentally include cell birth, migration, specification, death, and connectivity [129, 133]. Accumulating evidence suggests that both hormone dependent and independent mechanisms are active in the developing preoptic area and anterior hypothalamus of rodents. Two studies indicate hormone independence for the retention of cells born at early ages to be sex dependent when examined later. This was originally seen for cells in the SDN of rats using tritiated-thymidine autoradiography [62] and more recently for the same region of mice using bromodeoxyuridine (BrdU) immunohistochemistry [68]. In both cases, incorporation of a labeled nucleotide during DNA synthesis resulted in more cells being observed in females when the labeled nucleotide was given at the earliest stages of neurogenesis in this region. Interestingly, the more frequently cited data are that more labeled cells were observed in male rats when the label was given at the end of the neurogenic period [62]. In either case, this could indicate differences in cell proliferation, or a role for cell survival. If the sex difference is generated based on differences in proliferation early in the neurogenic period, then it may be more likely hormone independent (e.g., the gonads are not sufficiently formed to produce gonadal steroids). If the sex difference is generated later by a selective action on the survival of cells that are born early, then it may be a synergistic interaction of hormone-independent and dependent mechanisms. Hormonal mechanisms of cell death in nervous system development are well studied and reviewed elsewhere [44]. However, the presence of sex differences in agonadal SF-1 mice injected with BrdU at the early age, suggests that the sex difference in labeling was not dependent upon gonadal steroids at the older age [68]. This renders the argument for hormone-dependent cell survival at later ages less viable for this particular cohort of cells born at the earliest ages. Still, it is entirely possible that the early age of birth results in cells arriving at a maturational state during later development when they are most sensitive to sex-dependent (hormone dependent or not) events that influence viability.
The sex and hormone dependence for cell movements have been directly visualized by video microscopy and indirectly visualized by examining the positions of cells with defined molecular phenotypes as a function of sex and age. Organotypic slices have been used in vitro from mice harvested at E13 to E15 during the earliest period of gonad development at a particularly important time in hypothalamic development [60, 68]. Although cells from slices started at E13 and 14 were capable of responding to estradiol (activational hormone influence), only cells in slices started on E14 or E15 showed sex differences in cell movements in the absence of hormone treatment. There are two probable sources of ‘programming’ for basal sex differences seen in cells from E14 or E15 slices. First, a hormone-independent mechanism similar to one that led to more early-born BrdU immunoreactive cells in females than males may alter the movement behavior of cells. Secondarily, hormone exposure prior to slices being prepared on E14 [105, 106, 136] may alter the subsequent behavior of cells examined in the absence of gonadal steroids (organizational hormone influence). In general, the data suggest that a key focal point for sexual programming of brain structure occurs early in brain development and prior to the commonly considered prenatal surge in testicular hormones [141].
If there are hormone-dependent and independent influences on cell positions, then there should be instances of sexual dimorphism where the sex difference is in the position of particular cell populations as much as in the number of cells. This has been found in several instances. In embryonic mice, there are sex differences in the position of cells containing both immunoreactive estrogen receptor beta and the R1 subunit of the GABAB receptor [142]. Similar differences in cell positions have been observed in the preoptic area of adult mice for cells containing immunoreactive nNOS [115] and in the anteroventral periventricular preoptic area of adult rats for estrogen receptor beta containing cells [97]. For these 3 molecular signatures, there are good data suggesting the hormone-dependence of the position of cells containing immunoreactive estrogen receptor beta. This is based on the use of embryonic SF-1 KO mice in one case [142] and neonatal hormone treatments in the other [97].
Cells containing immunoreactive calbindin (and to some extent nNOS) provoke an interesting question. Data in adults clearly indicates that the positions of these cells differ in male and female mice [18, 39, 58, 99]. However, in development there is a sex difference that is not in the positions of cells, but rather in the number of cells. A key component of the developmental process for these cell populations is that the number of immunoreactive cells decreases with age (opposite from the rat pattern). There is clearly the potential of cell death and neuronal re-specification (e.g., turning off calbindin expression) as terms in a mechanistic equation.
However, a critical twist in such an equation must be the role of location. Either cells reorganize in some sort of movement-dependent process or the loss of immunoreactive cells must be more likely in some locations than others. There is an interesting precedent for this among cells that contain immunoreactive gonadotropin-releasing hormone (GnRH) early in development. There are almost twice as many GnRH neurons in early development as in adulthood [143]. One subset that ‘disappears’ (death or loss of expression) are those that are found in greater abundance in development in places where they are no longer found in adulthood such as in the cerebral cortex (e.g., mice; [114] or ferrets [130].
Conclusions
Sex differences in brain structure and function are found in many regions. There is a large body of accumulated evidence that shows the importance of gonadal steroids during development for the genesis of sexual dimorphism. In recent years, however, there is a growing realization that there are hormone-independent processes that also contribute. Gonadal steroids do not write on a clean molecular white board (blackboard perhaps to older readers). There may be writing already in place that in some cases influences the hormonal outcome. For example, hormone independent influences on cell movements or positions may set the stage for later hormone dependent influences [68]. Conversely, excessive experimental hormonal treatments or disorders of hormone metabolism might overwrite underlying genetic instructions. There may be some writing in place that can be read in the final version regardless of hormonal overwriting. For example, calbindin expression in the adult VMH is sexually dimorphic in wildtype and SF-1 KO mice [18]. Alternatively, there may be some writing that is uncovered when hormonal signals are not present and some writing that is only visible when hormones have been present to amplify. For example, differences between FCG and SF-1 KO mice might be due to the cooperation of sex chromosome and hormonal mechanisms that operate in the FCG mice (e.g., vasopressin) that have not been observed in the SF-1 KO mice. In the final analysis, we are left with an ongoing need to account for the influence of hormones in the context of the underlying genetic condition and null hormone condition.
Acknowledgement
Supported by NIH R01-MH61376 (SAT, GM) and ARRS grant P4-0053 (GM). We would like to thank the anonymous referees for helpful comments in the preparation of a final version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 5.Bakker J, De Mees C, Szpirer J, Szpirer C, Balthazart J. Exposure to oestrogen prenatally does not interfere with the normal female-typical development of odour preferences. J Neuroendocrinol. 2007;19:329–334. doi: 10.1111/j.1365-2826.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 6.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann N Y Acad Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 8.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 10.Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young E. Sex differences in the brain: From Genes to Behavior. Oxford university press; Oxford New York: 2007. [Google Scholar]
- 11.Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci Biobehav Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 12.Berkovitz GD, Seeherunvong T. Abnormalities of gonadal differentiation. Baillieres Clin Endocrinol Metab. 1998;12:133–142. doi: 10.1016/s0950-351x(98)80512-0. [DOI] [PubMed] [Google Scholar]
- 13.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Blaustein JD, McCarthy MM. Phoenix, Goy, Gerall, and Young, Endocrinology, 1959: 50 years young and going strong. Endocrinology. 2009;150:2501. doi: 10.1210/en.2009-0414. [DOI] [PubMed] [Google Scholar]
- 15.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown AE, Mani S, Tobet SA. The preoptic area/anterior hypothalamus of different strains of mice: sex differences and development. Brain Res Dev Brain Res. 1999;115:171–182. doi: 10.1016/s0165-3806(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 18.Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budefeld T, Tobet SA, Majdic G. Expression of Kiss1 in the hypothalamus of agonadal steroidogenic factor-1 knockout (SF-1 KO) mice; 14th Congress of European neuroendocrine association; Liege, Belgium. 2010. [Google Scholar]
- 20.Carrer HF, Cambiasso MJ. Sexual differentiation of the brain: genes, estrogen, and neurotrophic factors. Cell Mol Neurobiol. 2002;22:479–500. doi: 10.1023/A:1021825317546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 22.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catala S, Deis RP. Effect of oestrogen upon parturition, maternal behaviour and lactation in ovariectomized pregnant rats. J Endocrinol. 1973;56:219–225. doi: 10.1677/joe.0.0560219. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor {alpha} in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 2005;230:49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- 25.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 27.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 28.Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–414. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- 29.de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 30.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 31.de Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 32.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeFalco T, Capel B. Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol. 2009;25:457–482. doi: 10.1146/annurev.cellbio.042308.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Diaz DR, Fleming DE, Rhees RW. The hormone-sensitive early postnatal periods for sexual differentiation of feminine behavior and luteinizing hormone secretion in male and female rats. Brain Res Dev Brain Res. 1995;86:227–232. doi: 10.1016/0165-3806(95)00029-d. [DOI] [PubMed] [Google Scholar]
- 37.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- 38.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Sickmoller PM, Jarzab B, Gorski RA. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Neuroendocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- 39.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 41.Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- 42.Fausto-Sterling A. Sciences. Vol. 40. New York: 2000. The five sexes, revisited; pp. 18–23. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari PF, Lowther S, Tidbury H, Greengrass P, Wilson CA, Horton RW. The influence of gender and age on neonatal rat hypothalamic 5-HT1A and 5-HT2A receptors. Cell Mol Neurobiol. 1999;19:775–784. doi: 10.1023/A:1006909207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. Hormones. Vol. 7. Athens: 2008. Androgen insensitivity syndrome: clinical features and molecular defects; pp. 217–229. [DOI] [PubMed] [Google Scholar]
- 47.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giammanco M, Tabacchi G, Giammanco S, Di Majo D, La Guardia M. Testosterone and aggressiveness. Med Sci Monit. 2005;11:RA136–145. [PubMed] [Google Scholar]
- 49.Giammanco S, La Guardia M. The influence of sex, of castration in new-born males and of androgen treatment in new-born females on the mouse-killing behaviour of the rat. Arch Int Physiol Biochim. 1979;87:943–947. doi: 10.3109/13813457909070542. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert SF. Developmental Biology. Sinauer Associates, Inc.; Subderland, Massachusetts: 1994. [Google Scholar]
- 51.Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149:5747–5757. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- 52.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 53.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 54.Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development. Behav Neurosci. 2008;122:876–884. doi: 10.1037/0735-7044.122.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grgurevic N, Budefeld T, Rissman EF, Tobet SA, Majdic G. Differentiation of sexual behavior potential in agonadal Steroidogenic factor 1 knockout mice 12th annual meeting of the society for behavioral neuroendocrinology. Groningen, Netherlands: 2008. [Google Scholar]
- 56.Griffin GD, Flanagan-Cato LM. Sex differences in the dendritic arbor of hypothalamic ventromedial nucleus neurons. Physiol Behav. 2009;97:151–156. doi: 10.1016/j.physbeh.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- 58.Hamada T, Sakuma Y. Estrogen receptor {alpha} gene promoter 0/B usage in the rat sexually dimorphic nucleus of the preoptic area. Endocrinology. 2010;151:1923–1928. doi: 10.1210/en.2009-1022. [DOI] [PubMed] [Google Scholar]
- 59.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson RG, Brown AE, Tobet SA. Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol. 1999;41:252–266. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 61.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- 63.Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Kato A, Sakuma Y. Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Res. 2000;862:90–102. doi: 10.1016/s0006-8993(00)02076-x. [DOI] [PubMed] [Google Scholar]
- 65.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 66.Keller M, Pawluski JL, Brock O, Douhard Q, Bakker J. The alpha-fetoprotein knock-out mouse model suggests that parental behavior is sexually differentiated under the influence of prenatal estradiol. Horm Behav. 2010;57:434–440. doi: 10.1016/j.yhbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JK, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol Endocrinol. 2010;24:1240–1250. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knoll JG, Wolfe CA, Tobet SA. Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus. Eur J Neurosci. 2007;26:1091–1099. doi: 10.1111/j.1460-9568.2007.05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koopman P, Gubbay J, Vivian N, Goodfellow PN, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 71.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lauber AH, Romano GJ, Pfaff DW. Gene expression for estrogen and progesterone receptor mRNAs in rat brain and possible relations to sexually dimorphic functions. J Steroid Biochem Mol Biol. 1991;40:53–62. doi: 10.1016/0960-0760(91)90167-4. [DOI] [PubMed] [Google Scholar]
- 73.Lenz KM, McCarthy MM. Organized for sex - steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32:2096–2104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 75.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 76.Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 77.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 78.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 79.Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 80.Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol Behav. 1977;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- 81.Mathews GA, Brenowitz EA, Arnold AP. Paradoxical hypermasculinization of the zebra finch song system by an antiestrogen. Horm Behav. 1988;22:540–551. doi: 10.1016/0018-506x(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 82.Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 84.Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- 85.Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 87.Motelica-Heino I, Edwards DA, Roffi J. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol Behav. 1993;53:1017–1019. doi: 10.1016/0031-9384(93)90284-m. [DOI] [PubMed] [Google Scholar]
- 88.Mullaney R, Murphy D. Turner syndrome: neuroimaging findings: structural and functional. Dev Disabil Res Rev. 2009;15:279–283. doi: 10.1002/ddrr.87. [DOI] [PubMed] [Google Scholar]
- 89.Nelson RN. An Introduction to Behavioral Endocrinology Sinauer Associates Inc. Sunderland, MA: 2005. [Google Scholar]
- 90.Nordeen KW, Nordeen EJ, Arnold AP. Estrogen establishes sex differences in androgen accumulation in zebra finch brain. J Neurosci. 1986;6:734–738. doi: 10.1523/JNEUROSCI.06-03-00734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Numan M, Leon M, Moltz H. Interference with prolactin release and the maternal behavior of female rats. Horm Behav. 1972;3:29–38. doi: 10.1016/0018-506x(72)90004-9. [DOI] [PubMed] [Google Scholar]
- 92.Oakes MB, Eyvazzadeh AD, Quint E, Smith YR. Complete androgen insensitivity syndrome- -a review. J Pediatr Adolesc Gynecol. 2008;21:305–310. doi: 10.1016/j.jpag.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 94.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 96.Olster DH, Blaustein JD. Progesterone facilitation of lordosis in male and female Sprague-Dawley rats following priming with estradiol pulses. Horm Behav. 1988;22:294–304. doi: 10.1016/0018-506x(88)90002-5. [DOI] [PubMed] [Google Scholar]
- 97.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci U S A. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orikasa C, Sakuma Y. Sex and region-specific regulation of oestrogen receptor beta in the rat hypothalamus. J Neuroendocrinol. 2004;16:964–969. doi: 10.1111/j.1365-2826.2004.01254.x. [DOI] [PubMed] [Google Scholar]
- 99.Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J Comp Neurol. 2010;518:3618–3629. doi: 10.1002/cne.22419. [DOI] [PubMed] [Google Scholar]
- 100.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patisaul HB, Fortino AE, Polston EK. Sex differences in serotonergic but not gamma-aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:397–408. doi: 10.1210/en.2007-0666. [DOI] [PubMed] [Google Scholar]
- 102.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 103.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 104.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 105.Pointis G, Latreille MT, Cedard L. Gonado-pituitary relationships in the fetal mouse at various times during sexual differentiation. J Endocrinol. 1980;86:483–488. doi: 10.1677/joe.0.0860483. [DOI] [PubMed] [Google Scholar]
- 106.Pointis G, Latreille MT, Mignot TM, Janssens Y, Cedard L. Regulation of testosterone synthesis in the fetal mouse testis. J Steroid Biochem. 1979;11:1609–1612. doi: 10.1016/0022-4731(79)90357-1. [DOI] [PubMed] [Google Scholar]
- 107.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 108.Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, Murphy DD. Cortical anatomy in human X monosomy. Neuroimage. 2010;49:2915–2923. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- 111.Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- 112.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 113.Schlinger BA, Arnold AP. Plasma sex steroids and tissue aromatization in hatchling zebra finches: implications for the sexual differentiation of singing behavior. Endocrinology. 1992;130:289–299. doi: 10.1210/endo.130.1.1727704. [DOI] [PubMed] [Google Scholar]
- 114.Schwarting GA, Kostek C, Bless EP, Ahmad N, Tobet SA. Deleted in colorectal cancer (DCC) regulates the migration of luteinizing hormone-releasing hormone neurons to the basal forebrain. J Neurosci. 2001;21:911–919. doi: 10.1523/JNEUROSCI.21-03-00911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 116.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 117.Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 118.Sickel MJ, McCarthy MM. Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- 119.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 120.Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 121.Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev. 1998;23:325–336. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 122.Simon NG, Masters DB. Activation of male-typical aggression by testosterone but not its metabolites in C57BL/6J female mice. Physiol Behav. 1987;41:405–407. doi: 10.1016/0031-9384(87)90073-4. [DOI] [PubMed] [Google Scholar]
- 123.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 124.Sodersten P. Lordosis behaviour in male, female and androgenized female rats. J Endocrinol. 1976;70:409–420. doi: 10.1677/joe.0.0700409. [DOI] [PubMed] [Google Scholar]
- 125.Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–1170. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- 126.Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998;59:205–214. doi: 10.1016/s0169-328x(98)00148-x. [DOI] [PubMed] [Google Scholar]
- 127.Takeo T, Chiba Y, Sakuma Y. Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiol Behav. 1993;53:831–838. doi: 10.1016/0031-9384(93)90258-h. [DOI] [PubMed] [Google Scholar]
- 128.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 129.Tobet SA. Genes controlling hypothalamic development and sexual differentiation. Eur J Neurosci. 2002;16:373–376. doi: 10.1046/j.1460-9568.2002.02105.x. [DOI] [PubMed] [Google Scholar]
- 130.Tobet SA, Basham ME, Baum MJ. Estrogen receptor immunoreactive neurons in the fetal ferret forebrain. Brain Res Dev Brain Res. 1993;72:167–180. doi: 10.1016/0165-3806(93)90182-a. [DOI] [PubMed] [Google Scholar]
- 131.Tobet SA, Dunlap JL, Gerall AA. Influence of fetal position on neonatal androgen-induced sterility and sexual behavior in female rats. Horm Behav. 1982;16:251–258. doi: 10.1016/0018-506x(82)90025-3. [DOI] [PubMed] [Google Scholar]
- 132.Tobet SA, Fox TO. Sex differences in neural morphology influenced hormonally throughout life. In: Gerall AA, Moltz H, Ward IL, editors. Sexual differentiation: A Lifespan Approach. Plenum Press; New York: 1992. pp. 41–83. [Google Scholar]
- 133.Tobet SA, Hanna IK. Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cell Mol Neurobiol. 1997;17:565–601. doi: 10.1023/A:1022529918810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 135.van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- 136.vom Saal FS, Bronson FH. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science. 1980;208:597–599. doi: 10.1126/science.7367881. [DOI] [PubMed] [Google Scholar]
- 137.Wade J, Springer ML, Wingfield JC, Arnold AP. Neither testicular androgens nor embryonic aromatase activity alters morphology of the neural song system in zebra finches. Biol Reprod. 1996;55:1126–1132. doi: 10.1095/biolreprod55.5.1126. [DOI] [PubMed] [Google Scholar]
- 138.Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 139.Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ. Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor alpha expression. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- 140.Wang Z, Bullock NA, De Vries GJ. Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology. 1993;132:2299–2306. doi: 10.1210/endo.132.6.8504734. [DOI] [PubMed] [Google Scholar]
- 141.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 142.Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 143.Wu TJ, Gibson MJ, Rogers MC, Silverman AJ. New observations on the development of the gonadotropin-releasing hormone system in the mouse. J Neurobiol. 1997;33:983–998. doi: 10.1002/(sici)1097-4695(199712)33:7<983::aid-neu9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 144.Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 145.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 146.Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 147.Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22:1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]


