Abstract
The extracellular fluid space is the site of intercellular communication and represents an important source of mediators that can shed light on the parenchymal environment. Sampling of this compartment using continuous microdialysis allows assessment of the temporal changes in extracellular mediators involved in tissue homeostasis and disease processes. However, novel biomarker identification is limited by the current need to utilize specific, targeted molecular assays. The aim of our study was to explore the use of qualitative and quantitative proteomic approaches to define the protein content of dermal dialysate. Timed dermal dialysate samples were collected from healthy human volunteers for 5 h following probe insertion, using a 3,000-kDa MWCO membrane perfused at a rate of 3 μl/min. Dialysate proteins were identified using GeLC–MS/MS and iTRAQ approaches and functions assigned according to the Gene Ontology classification system. More than 80 proteins (size range 11–516 kDa) originating from both extracellular and intracellular fluid space were identified using the qualitative approach of GeLC–MS/MS. Quantitative iTRAQ data were obtained for 27 proteins with relative change ratios between consecutive timed samples showing changes of >1.5-fold. Interstitial proteins can be identified and measured using shotgun proteomic techniques and changes detected during the acute inflammatory response. Our findings provide a platform from which to explore novel protein biomarkers and their modulation in health and disease.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-011-9269-6) contains supplementary material, which is available to authorized users.
KEY WORDS: injury, interstitial fluid, microdialysis, proteomics, skin
INTRODUCTION
Microdialysis sampling is a well-established and widely accepted method for the in vivo collection of solutes from a number of complex matrices but principally from the extracellular fluid space. It involves implantation and perfusion of a small porous hollow fibre dialysis membrane into the tissue which allows the exchange of fluid and dissolved solutes between the tissue space and the perfusion fluid across the membrane, via diffusion and ultrafiltration. This provides a relatively analytically clean sample that requires little to no further treatment before analysis. For this reason, microdialysis sampling has become a standard technique in many laboratories to explore the mediator mechanisms of physiological and pathological tissue events.
Microdialysis has been used to recover a wide range of molecules from both animal and human tissues, including the skin (1), brain (2,3), liver (4) and eye (5). In the skin, it is frequently used to study the mechanisms driving inflammation, coupled with cytokine arrays or ELISAs to quantify the recovery of specific targets (6). With the increase in availability of proteomics technologies, it has now become possible to measure multiple proteins that have not been specifically targeted, e.g. for biomarker discovery and to reveal proteins involved in specific processes. Proteomic analysis of dialysate from human brain has revealed a small number of proteins, the majority of which were acute phase proteins, with ten specific to the brain as compared with cerebral spinal fluid (3). In skin, proteomic analysis of tissue fluid extracted from biopsy samples or suction blister fluid (7–9) has predominantly identified proteins that are abundant in plasma, including members of the albumin and apolipoprotein families and other proteins involved associated with the acute phase of inflammation, as well as structural proteins such as keratins and collagen. To date, there have been no proteomic analyses of human dermal dialysate samples. The primary aim of this study was to apply qualitative proteomic profiling using GeLC–MS/MS (10,11) and quantitative (iTRAQ) protein identification (12) to define the principal components in dermal dialysate and to explore their temporal changes during the acute inflammatory response in skin. In the former method, proteins are separated by conventional 1-D gel electrophoresis prior to in situ trypsin treatment. The resulting tryptic peptides are recovered, separated by nano-scale liquid chromatography (nano-LC) and identified by tandem MS and database searching. In the second approach, tryptic peptides are labelled at their primary amines with specific isobaric tags. The labelled peptides are then combined, fractionated and identified by tandem MS. Because the tags release reporter ions into a region of the mass spectrum that is chemically silent with respect to amino acids and peptides, it is also possible to obtain quantitative information on the peptides and hence the proteins from which they originate.
One concern with determining the proteome of dermal dialysate is the expected dynamic range of protein expression. In particular, it is possible that highly abundant plasma proteins, e.g. immunoglobulins and serum albumin, may interfere with the detection of less abundant proteins. It is unknown whether this is likely to be a problem in profiling the proteome of dialysate from skin. However, given that serum albumin is estimated to comprise approximately 70% of the total protein in cerebral (3) and dermal (13) dialysate, this is likely. Thus, a second aim of our study is to explore approaches by which less abundant proteins in dermal dialysate may best be quantified by mass spectrometry. The combination of these methodologies should provide a novel and informative approach to the understanding of the healthy skin proteome and give a baseline to which studies of skin pathologies can be compared and molecular mechanisms explored.
MATERIALS AND METHODS
Study Participants
Healthy male volunteers were recruited from the staff and students of the University of Southampton (n = 38, age range 18–40 years). The study was conducted using healthy volunteers and was approved by the Southampton and South West Hampshire Research Ethics Committee (REC 346/03/t and 06/Q1702/7) and registered with Southampton University Hospitals NHS Trust R&D Department (RHM HOS0157) in accordance with the uniform guidelines from the World Medical Association (www.wma.net/e/policy/b3.htm). All volunteers gave informed written consent.
Microdialysis
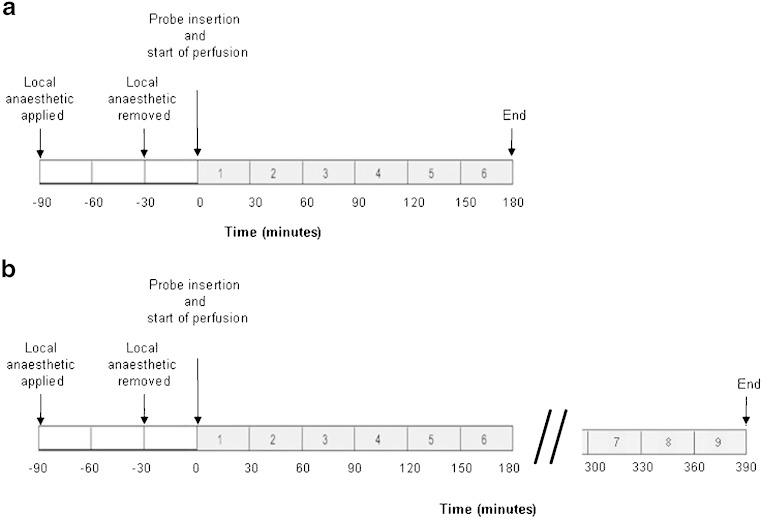
Up to six linear microdialysis probes (3,000 kDa molecular weight cut-off, polyethersulphone membrane, Asahi Medical) were inserted intra-dermally using a guide cannula into the anaesthetized skin (EMLA cream, 2.5% prilocaine, 2.5% lidocaine, Astra Pharmaceuticals, Kings Langley, UK) of the non-dominant volar forearm. Each membrane ran for a length of 20 mm, at a depth of approximately 0.7 mm and was perfused with physiological saline (Ringer’s Saline, 8.60 g l−1 NaCl, 0.30 g l−1 KCL and 0.33 g l−1 CaCl2.2H2O, Baxter, UK) at a rate of 3 μl min−1. Dialysate was collected into weighed plastic vials snap frozen in liquid nitrogen and then stored at −80°C prior to analysis. Figure 1 shows the protocols used to obtain dialysate for (a) assessment of total protein recovery and for qualitative proteomic profiling using GeLC–MS/MS and (b) quantitative (iTRAQ) protein identification.
Fig. 1.
Dialysate collection protocols. Protocol a was used to determine the time course of protein recovery during continuous perfusion (timed 30 min samples) and for qualitative analysis using GeLC–MS/MS (pooled samples collected between 30 and 150 min). Protocol b was used to collect dialysate from an early (60–150 min) and a late (300–390 min) time point for temporal analysis of protein content using iTRAQ. Periods over which dialysate was collected are represented by grey bars
In Vitro Assessment of Relative Recovery
The relative recovery of the microdialysis probe membrane was assessed in vitro in a 5 ml bath containing 10 mg ml−1 human serum albumin (66 kDa) in Ringer’s saline. Probes were perfused at 3 μl min−1 with Ringer’s saline and 3 × 30-min samples collected. Protein concentration in these samples and bath samples was estimated using the Bio-Rad DC assay (Bio-Rad, Hertfordshire, UK), based on the Lowry method. Relative recovery was calculated as  and was 10.5 ± 3.1% (mean ± SEM, n = 11).
and was 10.5 ± 3.1% (mean ± SEM, n = 11).
Estimation of Protein Concentration for Mass Spectroscopy
The protein concentration of the dialysate samples was determined using a bicinchoninic acid (BCA) method (14,15), using a kit from Sigma–Aldrich (Catalogue number: BCA-1).
Qualitative Assessment of Dialysate Protein Content Using Gelc–MS/MS
1-D SDS–PAGE
Two hundred fifty micrograms of protein from dialysate (30–150-min collection period) was denatured for 10 min at 70°C in final sample buffer containing 10 mM dithiothreitol. The denatured samples were resolved in a NuPage 4–12% Bis–Tris Gel (Invitrogen) at 200 V for approximately 45 min. The gels were stained with Colloidal Coomassie Blue (Bio-Rad) for 1 h at room temperature and developed overnight in deionised water.
Sample preparation for GeLC–MS/MS studies
Following SDS–PAGE of dialysate proteins and staining with Colloidal Coomassie Blue, gel tracks (7 cm length) were excised, cut into 25 equal-sized pieces and subjected to in situ trypsin digestion overnight at 37°C (16) (see supplementary methods for further details). The resulting peptides were extracted and separated by nanocapillary liquid chromatography (LC) prior to tandem mass spectrometry, performed as described previously (11,17).
Immuno-depletion to remove highly abundant proteins
The six most abundant proteins found in plasma are albumin, IgG, IgA, transferrin, haptoglobin and alpha-1-antitrypsin and account for 90–95% of the total protein in dialysate. These were removed by immunodepletion chromatography (Multiple Affinity Removal Column HU-6, 4.6-mm inner diameter (ID) × 100 mm; Agilent, Wilmington, DE). The immunodepletion column was equilibrated in Buffer A for 10 min at a flow rate of 200 μl min−1. Next, 150 μl of dialysate was made up to 1 ml in Buffer A and then injected onto the immunodepletion column. The flow-through fractions containing the low abundant proteins were collected in a microtitre plate, pooled and then stored at −80°C until analysis. Bound high-abundant proteins were eluted from the column in buffer B at a flow rate of 200 μl min−1, after which the column was re-equilibrated in buffer A.
Quantitative Assessment of Protein Content Using iTRAQ
Labelling of proteins with iTRAQ reagents
iTRAQ™ labelling of samples was performed according to the manufacturer’s instructions (Applied Biosystems iTRAQ™ Reagents—Amine-Modifying Labelling Reagents for Multiplexed Relative and Absolute Protein Quantification. Chemistry Reference Guide. Part Number 4351918 Rev. A. 05/2004).
Dialysate samples collected between 60–150 min (early) and 300–390 min (late) after insertion were labelled in separate duplex iTRAQ™ experiments. To each sample containing ∼100 μg of protein per sample in ≤34 μl of 0.5 M triethylammonium bicarbonate, 1 μl of 2% SDS and 2 μl of the reducing agent, 50 mM (tris-(2-carboxyethyl)) phosphine was added and mixed. Samples were incubated at 60°C for 1 h. Methyl methane-thiosulfonate in isopropanol (1 μl of 200 mM) was then added and incubated for a further 10 min at room temperature. Proteins were digested by adding 10 μl of 1 mg ml−1 trypsin in 80 mM CaCl2. Samples were incubated for 16 h at 37°C. Labeling of tryptic peptides with iTRAQ™ tags was achieved by mixing the contents of the appropriate reagent vial with the relevant sample and incubation at room temperature for 1 h. Samples taken from the early time-point were labelled with isobaric tag 114, and those from the late time-point were labelled with isobaric tag 116; they were then combined and lyophilized in vacuo.
iTRAQ™ Sample preparation prior to MS
The combined iTRAQ™ peptide mixtures were separated by reverse phase chromatography using a Dionex Ultimate LC system and Jupiter C18 RP column (2.1 mm ID × 250 mm, 4 μm; Phenomenex). Samples were solubilised in 500 μl of solvent (5% (v/v) acetonitrile, 0.1% formic acid) and loaded onto the column using a 500-μl loop. The loaded sample was injected and washed with the solvent for 20 min at 200 μl min−1 to remove excess reagent. Peptides were eluted with a linear gradient of 0–80% using a second solvent (95% acetonitrile, 0.1% formic acid) at 200 μl min−1 with fractions collected at 1-min intervals. Peptide elution was monitored at 214, 235 and 280 nm. Fractions were lyophilised and each re-suspended in 10 μl of 10% acetonitrile containing 0.1% formic acid prior to analysis by nano-LC–MS/MS.
Mass Spectrometry Data Analysis and Protein Annotation
MASCOT Searches
Peak lists were submitted to the MASCOT search engine version 2.2.04 (MatrixScience) and the data searched against the human NCBInr protein sequence database containing 40,877 protein sequences (accessed December 2007). A maximum of one missed cleavage was allowed for tryptic digestion while the allowed fixed and variable modifications were carboxy-amidomethylation of cysteine and the oxidation of methionine, respectively.
Annotation of the Identified Proteins
UniProt identifiers were assigned to identified proteins using the open-access Protein Identifier Cross Reference service available from EBI at http://www.ebi.ac.uk/Tools/picr/, the search was limited to human proteins and was mapped to the Swiss-Prot and TrEMBL databases. Each UniProt identifier was then searched manually against the AmiGO database using the GO Term Mapper tool (http://go.princeton.edu/cgi-bin/GOTermMapper), searching the list of UniProt IDs against the goa_human (GOA GO Slim) database for biological process terms.
iTRAQ Data Analysis
Peak lists obtained from the MS analysis of skin microdialysate were submitted to the MASCOT search engine version 2.2.04 (MatrixScience) and the data searched against a SwissProt human protein sequence database for peptide identification (accessed May 2008, 17659 sequence entries). The corresponding quantitative information using the iTRAQ™ reporter ions was obtained via MASCOT version 2.2.04. Search criteria included allowing a maximum of one missed cleavage for tryptic digestion and allowed fixed modifications of methyl methane-thiosulfonation of cysteine and modification of the N terminus and lysine side chains using the iTRAQ™ 4-plex reagent (Applied Biosystems). Variable modification for the oxidation of methionine and iTRAQ™ modification of tyrosine were also allowed. Precursor ion and sequence ion mass tolerances were set at 100 ppm and 0.2 Da, respectively. The proteomic data generated during the course of these studies is freely available from the authors and is currently being submitted to the PRIDE repository (www.ebi.ac.uk/pride).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (version 5, GraphPad Software Inc). One-way ANOVA with Bonferroni multiple comparisons post-test was used to compare the total protein in the 30 min in vivo dialysate collections and to determine whether there was a significant change in protein recovery over the 3-h period studied. The combined list of protein IDs identified from GeLC–MS/MS whole and depleted samples was compared to the Homo sapiens background provided in the Database for Annotation, Visualization and Integrated Discovery (DAVID) database for enrichment analysis. Functional clustering was performed using DAVID 6.7 (18,19) with the medium classification settings and functional annotation categories limited to GO ontology terms, biological process, molecular function and Cellular component and KEGG pathways (20). Multiple testing correction was performed using the Bonferroni correction implemented in the software. The mean, standard error and coefficient of variance (CV) were calculated for the ratio between the late and early samples for each protein and mean values converted into fold-change (see supplementary methods for further details).
RESULTS
Time Course of Continuous Total Protein Recovery
The total protein recovery in timed 30-min collections during continuous microdialysis is shown in Fig. 2. The protein concentration was highest in the first 30-min sample (p < 0.0001, repeat measures ANOVA). The average protein concentration (0.83 ± 0.3 mg/ml; mean ± SD, n = 5 volunteers) was similar to that described previously (13,21,22)
Fig. 2.
Changes in protein recovery over time during continuous perfusion of the microdialysis probes at 3 μl min−1. Protein concentration in 30-min dialysate collections, as described in Fig. 1a, was estimated by BCA assay. Dotted line represents mean protein concentration (30–150 min) of 0.83 ± −.3 mg ml−1 used for GeLC–MS/MS. Values are mean ± SE, n = 5 volunteers
Protein Content of Dermal Dialysate is Dominated by Serum Albumin
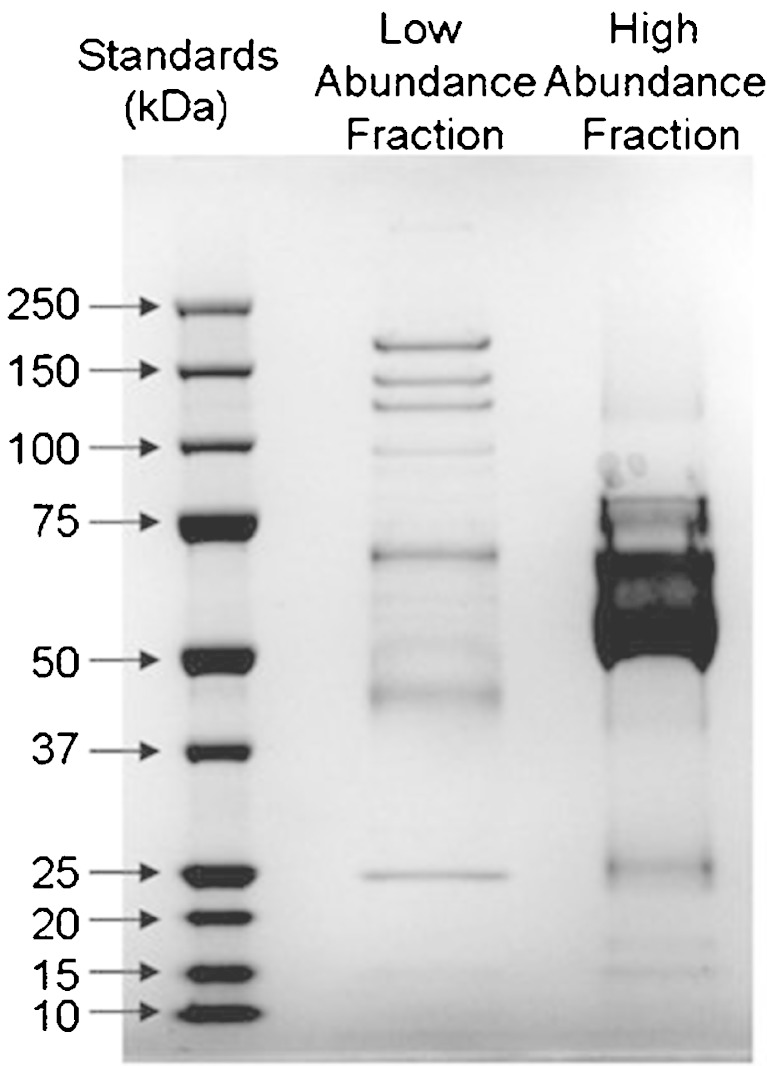
Qualitative proteomic analysis was performed on pooled dialysate collected over a period of 120 min from four probes from two volunteers. The total amount of protein in the pooled dialysate sample was 1.8 ± 0.1 mg. 1-D SDS–PAGE showed several distinct bands, including a large band at 66 kDa corresponding to the molecular weight of human serum albumin (Fig. 3). To confirm their identities, gel slices were excised and digested in situ with trypsin. The resulting tryptic peptides were recovered, separated by nano-scale liquid chromatography (nano-LC) and identified by tandem MS and database searching using MASCOT and the NCBInr database. This GeLC–MS/MS analysis identified six proteins: serum albumin, alpha-2-macroglobulin, ceruloplasmin, complement component 3, hemopexin and transferrin (see supplementary data, Table 1).
Fig. 3.
One-dimensional SDS–PAGE of dialysate before and after depletion. Gel image showing the profile obtained from dialysate before depletion and the two fractions obtained following depletion; the high-abundance fraction containing the proteins removed and the low-abundance, depleted fraction
Immuno-Depletion of Highly Abundant Components Revealed a Further 25 Proteins
To reveal less abundantly expressed proteins, a second pooled dialysate sample was depleted of the six most abundant proteins (albumin, anti-trypsin, haptoglobin, IgA, IgG and transferrin). Removal of these proteins reduced the protein content of the sample by 85% but revealed several distinct bands on the gel, previously obscured by serum albumin (Fig. 3). A further 25 proteins were identified in this depleted dialysate sample (Table I and Supplementary data, Table 1), a 4.8-fold increase over that identified in the non depleted pooled sample. The sizes of the identified proteins ranged from 15.9 to 188.5 kDa, indicating that dermal dialysates contain proteins across most of the molecular weight spectrum. Analysis of the proteins’ cellular compartment of origin indicated that proteins were derived from both intra- and extracellular sources, although it cannot be shown whether the intracellular proteins were released actively or passively due to damage or apoptosis.
Table I.
Proteins Identified in Depleted Dermal Dialysate Using GeLC–MS/MS
| GI | Uniprot ID | Protein Name | Mass (Da) | pI | Coverage (%) | Peptides |
|---|---|---|---|---|---|---|
| 21071030 | Q68CK0 | α-1B-Glycoprotein | 54790 | 5.56 | 29.5 | 9 |
| 4502067 | P02760 | α-1-Microglobulin | 39888 | 5.95 | 15.9 | 4 |
| 66932947 | P01023 | α-2-Macroglobulin | 164614 | 6.00 | 44.4 | 44 |
| 4557287 | P01019 | Angiotensinogen | 53406 | 5.87 | 13.4 | 5 |
| 4757756 | P07355 | Annexin A2 | 38808 | 7.57 | 10.9 | 3 |
| 4557327 | P02647 | Apolipoprotein A-1 | 30759 | 5.56 | 47.9 | 17 |
| 4557321 | P02749 | Apolipoprotein H | 39598 | 8.34 | 17.1 | 4 |
| 4504349 | P68871 | βglobin | 16102 | 6.75 | 68.7 | 8 |
| 4502517 | P00915 | Carbonic Anhydrase 1 | 28909 | 6.59 | 23 | 4 |
| 4557485 | P00450 | Ceruloplasmin | 122983 | 5.44 | 30 | 23 |
| 73858568 | P05155 | Component 1 inhibitor | 55347 | 6.09 | 14.4 | 6 |
| 4557385 | P01024 | Complement Component 3 | 188585 | 6.02 | 19.1 | 7 |
| 67782358 | P00751 | Complement Factor B | 86847 | 6.67 | 24.3 | 12 |
| 11321561 | P02790 | Hemopexin | 52385 | 6.55 | 40.3 | 15 |
| 4504579 | P05156 | I Factor (complement) | 68120 | 7.72 | 3.3 | 2 |
| 17318569 | P04264 | Keratin 1 | 66198 | 8.16 | 33.1 | 15 |
| 47132620 | P35908 | Keratin 2a | 65678 | 8.07 | 13.5 | 8 |
| 17318574 | P19013 | Keratin 4 | 57649 | 6.25 | 4.3 | 2 |
| 4504919 | P05787 | Keratin 8 | 53671 | 5.52 | 5 | 2 |
| 55956899 | P35527 | Keratin 9 | 62255 | 5.14 | 21.8 | 11 |
| 9257232 | P02763 | Orosomucoid 1 | 23725 | 4.93 | 40.8 | 7 |
| 4505529 | P19652 | Orosomucoid 2 | 23873 | 5.03 | 32.8 | 7 |
| 4505881 | P00747 | Plasminogen | 93247 | 7.04 | 10 | 5 |
| 4506355 | P20742 | Pregnancy zone protein | 165215 | 5.97 | 7.4 | 7 |
| 50659080 | P01011 | SERPIN A3 | 47792 | 5.33 | 17.3 | 5 |
| 4502261 | P01008 | SERPIN C1 | 53025 | 6.32 | 27.6 | 12 |
| 39725934 | P36955 | SERPIN F1 | 46454 | 5.97 | 14.8 | 6 |
| 4507725 | P02766 | Transthyretin | 15991 | 5.52 | 49 | 5 |
| 32483410 | P02774 | Vitamin D-binding protein | 54480 | 5.32 | 38 | 15 |
None of the proteins specifically removed by depletion were identified in the treated sample, showing the depletion protocol to be very efficient. The proteins identified in the depleted sample were predominantly those found in high levels in plasma, including complement proteins and protease inhibitors.
Functional Classifications Suggests Many of the Proteins are Associated with the Response to Injury
An enrichment analysis performed using a modified Fishers Exact Test implemented in the DAVID software (ver 6.7) (18,19) was used to determine significant functional terms from the identified proteins. The terms identified from the enrichment analysis are shown in Supplementary Table 2 as functionally clustered groups based on the commonality of their shared proteins as defined by DAVID 6.7. The most significantly enriched biological processes included acute inflammatory response (Bonferroni corrected P value 5 × 10−10) and regulation of response to external stimuli (Bonferroni corrected P value 4.2 × 10−8) terms. In keeping with the dialysate sample, the most significantly enriched cellular compartment term reported was associated with the extracellular space. Furthermore, the same analysis performed on the KEGG pathway database showed a significant enrichment (43-fold enrichment, Bonferroni corrected P value 1.5 × 10−8) for the Complement and Coagulation Cascade pathway in keeping with a response to injury (Fig. 4).
Fig. 4.
KEGG Pathway Map (20). Complement and Coagulation Cascade identified as significantly enriched using DAVID software. Proteins identified in dialysate material highlighted in bold; A2M alpha-2-macroglobulin, PLG plasminogen, BF complement factor B, IF complement factor I, C3 complement component C3, SerpinC1 (antithrombin) and SerpinG1 (complement component 1 inhibitor)
Quantitative Analysis Shows Changes in the Expression of Several Proteins
To avoid the significant loss of protein from the dialysate sample caused by depletion, iTRAQ labelling was performed on non-depleted samples. A total of 274,533 mass spectra from 23 volunteers led to the identification of 7,791 peptides that were assigned to 89 proteins. The proteins range in mass from 11–516 kDa, and the pIs range from 4.5–9.5 (raw iTRAQ data for each sample, along with associated statistics are available in supplementary data, Table 3). Table II shows the mean fold change of 34 proteins between the early and late samples, where quantitative data were available from ≥3 participants (n = 23 volunteers). Two proteins (apolipoprotein A-II and Zinc-alpha-2-glycoprotein precursor) showed an up-regulation of more than 1.5-fold, while three proteins showed more than 1.5-fold down-regulation (vitamin-D binding protein, alpha-1-antichymotrypsin and ceruloplasmin).
Table II.
Mean Fold-Change in Protein Concentration Between Early and Late Samples, as Determined by iTRAQ Analysis
| Protein name | Mean (ratio) | SE | CV | Mean fold change |
|---|---|---|---|---|
| Proteins up-regulated in the late sample | ||||
| Apolipoprotein A-II precursor | 1.23 | 0.82 | 1.34 | 2.34 |
| Zinc-alpha-2-glycoprotein precursor | 0.62 | 0.71 | 1.99 | 1.54 |
| Hemoglobin subunit alpha | 0.55 | 0.24 | 1.72 | 1.46 |
| Hemoglobin subunit delta | 0.43 | 0.40 | 2.50 | 1.34 |
| Ig kappa chain V-I region CAR | 0.34 | 0.32 | 1.61 | 1.27 |
| Antithrombin-III precursor | 0.29 | 0.33 | 1.97 | 1.22 |
| Hemopexin precursor | 0.29 | 0.39 | 4.11 | 1.22 |
| Alpha-1-acid glycoprotein 1 precursor | 0.20 | 0.26 | 3.70 | 1.14 |
| Hemoglobin subunit beta | 0.18 | 0.40 | 9.11 | 1.13 |
| Complement C3 precursor | 0.08 | 0.18 | 7.97 | 1.06 |
| Beta-2-glycoprotein 1 precursor | 0.05 | 0.22 | 7.50 | 1.04 |
| Ig gamma-2 chain C region | 0.03 | 0.15 | 22.25 | 1.02 |
| Ig gamma-1 chain C region | 0.02 | 0.11 | 24.74 | 1.01 |
| Trypsin-1 precursor | 0.01 | 0.40 | 104.16 | 1.01 |
| Ig gamma-3 chain C region | 0.01 | 0.20 | 138.30 | 1.00 |
| Proteins down-regulated in the late sample | ||||
| Vitamin D-binding protein precursor | −1.48 | 0.94 | −1.10 | 2.79 |
| Alpha-1-antichymotrypsin precursor | −0.83 | 0.75 | −1.56 | 1.78 |
| Ceruloplasmin precursor | −0.61 | 0.40 | −1.14 | 1.53 |
| Ig mu chain C region | −0.60 | 0.53 | −2.18 | 1.51 |
| Haptoglobin precursor | −0.52 | 0.21 | −1.20 | 1.43 |
| Fibrinogen alpha chain precursor | −0.50 | 0.62 | −2.12 | 1.42 |
| Alpha-2-HS-glycoprotein precursor | −0.42 | 0.57 | −2.71 | 1.34 |
| Alpha-2-macroglobulin precursor | −0.37 | 0.16 | −1.66 | 1.29 |
| Ig alpha-1 chain C region | −0.36 | 0.17 | −1.34 | 1.29 |
| Serotransferrin precursor | −0.28 | 0.12 | −1.88 | 1.21 |
| Ig kappa chain C region | −0.20 | 0.24 | −4.70 | 1.15 |
| Apolipoprotein A-I precursor | −0.19 | 0.45 | −6.26 | 1.14 |
| Alpha-1-antitrypsin precursor | −0.18 | 0.21 | −4.50 | 1.13 |
| Ig kappa chain V-I region DEE | −0.16 | 0.20 | −2.22 | 1.12 |
| Ig kappa chain V-III region B6 | −0.16 | 0.47 | −6.02 | 1.11 |
| Ig gamma-4 chain C region | −0.11 | 0.21 | −5.33 | 1.08 |
| Ig lambda chain C regions | −0.09 | 0.24 | −10.97 | 1.07 |
| Alpha-1B-glycoprotein precursor | −0.08 | 0.40 | −10.71 | 1.05 |
| Serum albumin precursor | −0.04 | 0.19 | −20.28 | 1.03 |
DISCUSSION
This study gives the first report of the proteome of healthy human dermal dialysate and shows that highly abundant plasma proteins comprise approximately 85% of this fluid. Once these were removed, a further 25 proteins were revealed by GeLC–MS/MS, many with functions associated with the response to injury. A total of 89 proteins were assigned in the iTRAQ analysis, although many were variants of those identified in the GeLC–MS/MS experiments. Analysis of the changes in protein ratios between the early and late samples, 60–150 and 300–390 min after probe insertion respectively, showed changes in the recovery of several proteins. Further, our study provides evidence that such proteomic approaches can be used to investigate the protein content of dermal dialysate with a view to identifying novel biomarkers of the response to injury.
The amount of protein recovered from the interstitial space was low compared to other more commonly studied biological fluids such as plasma. It was similar to that recovered previously using membranes of similar molecular weight cut-off (21,22). The reduced microdialystae total protein concentration is in part due not only to the relatively lower concentration of the abundant proteins in the interstitium but also to the extraction efficiency of the microdialysis membrane (13). The use of a 3,000-kDa molecular weight cut-off membrane at a perfusion rate of 3 μl min−1 represents the best compromise between sample concentration and volume, giving the optimal protein recovery of 10.5 ± 3.1% (23). It should also be noted that using a shotgun proteomic approach, we were only able to detect proteins in the nanograms per millilitre and greater concentration range. We were unable to detect important signalling molecules such as the cytokines and wound healing peptides found at lower concentrations that we and others have shown to be present immediately after wounding using other analytical approaches (1,3,6,24). It is likely that a number of components will have been removed during depletion, especially given the carrier functions of the highly abundant proteins. At this time, depletion remains the ‘least worst’ option, as without it, the identification capacity of the instrumentation is taken up analyzing fragments of the most abundant proteins, as demonstrated by our initial analysis of non-depleted dialysate. Our experiments show that it is possible to obtain sufficient protein from dialysate to enable one–off and temporal analyses of tissue status, and this situation will improve as the sensitivity of proteome analyses develops.
The use of shotgun proteomics technology has enabled an assessment of molecular changes that occur following the insertion of a microdialysis probe and the measurement of components that may not have otherwise been considered. Proteomics provides a step further on from gene and mRNA analysis, allowing study of the ultimate effectors of essentially all cellular processes. This is of advantage, because of the reported disparity between mRNA and proteins levels (25,26) and also allows analysis of biological fluids with multiple sources from which there is no directly associated genome.
Reports of the proteome of dermal interstitial fluid, as sampled using suction blister techniques, give some indication of the different classes of proteins that can be found within the intercellular space (8). While this is a more protein-rich fluid, it is derived from a single injury event that can only be sampled once and thus cannot provide a description of changes within the same wound over time. Unfortunately, the full proteome of suction blister fluid has not yet been published, making a direct comparison between the two fluids impossible at this time.
The high proportion of plasma proteins identified suggests a considerable extravasation of protein during probe insertion and injury. However, the profile of dialysate is different to plasma (27), with several of the most abundant plasma proteins, and key systemic inflammatory markers (e.g. serum amyloid, C-reactive protein) not detected. The absence of such proteins may be explained by their large size (C-reactive protein forms a pentamer of 105 kDa) which may cause them to be retained by the dialysis membrane. However, it is surprising that no low molecular weight fragments were detected (28). This suggests that dialysate is an independent type of sample that offers a representative view of the tissue fluid.
Comparison of the protein profiles of dialysate obtained from the skin and from the brain also showed differences. Eight of the proteins found in cerebral microdialysate (2) were not present in dermal dialysate, while 15 proteins were found in dermal dialysate by GeLC–MS/MS but were not reported in cerebral dialysate. This may be a reflection of the difference in composition of the dialysate from the two locations, analysis protocols and instrumentation, or could be a result of a differing response to the probe insertion trauma.
Several recent publications have reported on the trauma that is caused following the insertion of microdialysis probes (1,6,24,29); and that this is long-lasting (6,24,30). All arise from the specific, targeted analyses of components that are expected to be involved, rather than exploring unknowns that may enhance the current understanding of the processes involved. In the current study, the functional vocabulary associated with each protein shows that an injury response occurs and gives a useful first insight into the processes that may be occurring in response to the trauma caused by probe insertion. Proteins involved in response to stimulus, cellular and molecular transport, and responses to other organisms were proportionally over-represented, compared to the frequency with which these terms occur within the database of annotated proteins. This suggests a bias towards proteins relevant to the injury process within dialysate. Examples of such proteins include the serine anti-proteases, which may be involved in regulating the activity of proteases released either to facilitate removal of wound debris, or as a result of cellular damage.
At the very early stage in the response to an injury under investigation (60–150 min after wounding), the proteome may be dominated by acute phase reactants and anti-microbial peptides that act to prevent an infection (31). Quantitative analysis to assess the changes in dialysate protein content between the early and late samples uncovered five proteins that exhibited a >1.5-fold change: apolipoprotein A-II (apo A-II), alpha-1-antichymotrypsin, ceruloplasmin, vitamin-D binding protein and Zinc-alpha-2-glycoprotein precursor. The increase in recovery of apo A-II is of particular interest, as it has been hypothesised to augment the monocyte response to bacterial lipopolysaccharide and thus acts as an anti-microbial agent (32). Equally, the reduction in alpha-1-antichymotrypsin is of relevance, as it is an inhibitor of proteases including chymotrypsin and MMP-9. These are known to be involved in wound debridement and extracellular matrix degradation necessary for the influx of inflammatory cells and later, tissue re-modelling (33).
Vitamin-D binding protein was the only component to be reduced in all volunteers from whom it was recovered. Vitamin D is known to enhance the innate inflammatory response through up-regulating the anti-microbial peptide cathelecidin, so it may be expected that it would be up-regulated following injury. Furthermore, this protein is also involved in actin clearance from necrotic cells, preventing unwanted disseminated intravascular coagulation and, following specific deglycosylation, becomes a macrophage activating factor, reviewed by (34). However, it was reduced in the second dialysate sample collected 300–390 min (late) after wounding and is known to be reduced during inflammation, perhaps converse to expectation. The review by Meier et al. (34) offers an interesting explanation for this observed reduction; the half-life of actin-bound vitamin-D binding protein in vivo is approximately 30 min, compared to 24 h in the free form. With such a quick turnover and 90-min collection periods, it is entirely possible that the protein binds actin and is removed faster that it can be sampled, thus appearing to be reduced in dialysate.
CONCLUSIONS
In summary, the combination of microdialysis and proteomics has the potential to identify relevant, novel markers of injury and inflammatory. However, the low concentration of protein within this biological fluid makes identification of less abundant components difficult. Despite this challenge, both qualitative and quantitative data were obtained which show changes consistent with an injury response can be measured using these technologies. Furthermore, it has been possible to study tissue status dynamically, which cannot be achieved with sequential biopsies and other discontinuous sampling methods.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 65 kb)
(DOC 571 kb)
(DOC 103 kb)
(DOC 365 kb)
ACKNOWLEDGEMENTS
The authors extend their thanks to many colleagues at Unilever and Southampton, and in particular Therese Nestor and Shanon Pead (CPR) for their technical support. Instrumentation in the Centre for Proteomic Research is supported by a grant from the Science Research Infrastructure Fund. CAG was supported by a Gerald Kerkut Charitable Trust studentship and additional funding for this work including support for EP was provided as part of Unilever’s ongoing support in developing novel ways of delivering consumer safety.
ABBREVIATIONS
- BCA
Bicinchoninic Acid
- GeLC
In situ trypsin digestion of proteins in SDS–PAGE gel slices and separation of resulting peptides by nano-scale liquid chromatography
- GO
Gene ontology
- iTRAQ
Isobaric tagging for relative and absolute quantification
- MS/MS
Tandem mass spectrometry
- TEAB
Triethylammonium bicarbonate
- UV
Ultraviolet
Footnotes
Carolyn Gill and Erika Parkinson he contributed equally to this work.
REFERENCES
- 1.Clough GF, Jackson CL, Lee JJP, Jamal SC, Church MK. What can microdialysis tell us about the temporal and spatial generation of cytokines in allergen-induced responses in human skin in vivo. J Invest Dermatol. 2007;127:2799–2806. doi: 10.1038/sj.jid.5700930. [DOI] [PubMed] [Google Scholar]
- 2.Maurer MH, Berger C, Wolf M, Futterer CD, Feldmann RE, Jr, Schwab S, et al. The proteome of human brain microdialysate. Proteome Sci. 2003;14:7–15. doi: 10.1186/1477-5956-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1β, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Meth. 2002;119:45–50. doi: 10.1016/S0165-0270(02)00153-X. [DOI] [PubMed] [Google Scholar]
- 4.Yang CS, Tsai PJ, Chen WY, Liu L, Kuo JS. Determination of extracellular glutathione in livers of anaesthetized rats by microdialysis with on-line high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1995;667:41–48. doi: 10.1016/0378-4347(94)00590-2. [DOI] [PubMed] [Google Scholar]
- 5.Wei G, Ding PT, Zheng JM, Lu WY. Pharmacokinetics of timolol in aqueous humor sampled by microdialysis after topical administration of thermosetting gels. Biomed Chromatogr. 2006;20:67–71. doi: 10.1002/bmc.529. [DOI] [PubMed] [Google Scholar]
- 6.Sjögren F, Anderson CD. Are cutaneous microdialysis cytokine findings supported by end point biopsy imminohistochemistry findings? AAPS J. 2010;12:741–749. doi: 10.1208/s12248-010-9235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aden N, Shiwen X, Aden D, Black C, Nuttal A, Denton CP, et al. Proteomic analysis of scleroderma lesional skin reveals activated wound healing phenotype of epidermal cell layer. Rheumatol Oxf. 2008;47(12):1754–1760. doi: 10.1093/rheumatology/ken370. [DOI] [PubMed] [Google Scholar]
- 8.Kool J, Reubsaet L, Wesseldijk F, Maravilha RT, Pinkse MW, D’Santos CS, et al. Suction blister fluid as potential body fluid for biomarker proteins. Proteomics. 2007;7:3638–3650. doi: 10.1002/pmic.200600938. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald N, Cumberbatch M, Singh M, Moggs JG, Orphanides G, Dearman RJ, et al. Proteomic analysis of suction blister fluid isolated from human skin. Clin Exp Dermatol. 2006;31(3):445–448. doi: 10.1111/j.1365-2230.2006.02078.x. [DOI] [PubMed] [Google Scholar]
- 10.Schirle M, Heurtier MA, Kuster B. Profiling core proteomes of human cell lines by one-dimensional PAGE and liquid chromatography-tandem mass spectrometry. Mol Cell Proteomics. 2003;2:1297–1305. doi: 10.1074/mcp.M300087-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Skipp P, Robinson J, O’Connor CD, Clarke IN. Shotgun proteomic analysis of Chlamydia trachomatis. Proteomics. 2005;5:1558–1573. doi: 10.1002/pmic.200401044. [DOI] [PubMed] [Google Scholar]
- 12.Ross PL, Huang YN, Marchese JN, Williamson B, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Clough GF. Microdialysis of large molecules. AAPS J. 2005;7(3):E686–E692. doi: 10.1208/aapsj070369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1987;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 15.Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem. 1988;175:231–237. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- 16.Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two-dimensional gels. Proc Natl Acad Sci USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholas B, Skipp P, Mould R, Rennard S, Davies DE, O’Connor CD, et al. Shotgun proteomic analysis of human-induced sputum. Proteomics. 2006;6:4390–4401. doi: 10.1002/pmic.200600011. [DOI] [PubMed] [Google Scholar]
- 18.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 20.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. NeuroSci Let. 1997;230:117–120. doi: 10.1016/S0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- 22.Klede M, Clough GF, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-Nitro-L-Arginine-Methyl Ester on neuropeptide-induced vasodilatation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- 23.Chaurasia CS. In vivo microdialysis sampling: theory and applications. Biomed Chromatogr. 1999;13:317–332. doi: 10.1002/(SICI)1099-0801(199908)13:5<317::AID-BMC891>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Clausen TS, Kaastrup P, Stallknecht B. Proinflammatory tissue response and recovery of adipokines during 4 days of subcutaneous large-pore microdialysis. J Pharmacol Toxicol Meth. 2009;60(3):281–287. doi: 10.1016/j.vascn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Glanemann C, Loos A, Gorret N, Willis LB, O’Brien XM, Lessard P, et al. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: implications for DNA microarray analysis. Appl Microbiol Biotechnol. 2003;61:61–68. doi: 10.1007/s00253-002-1191-5. [DOI] [PubMed] [Google Scholar]
- 26.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3(4):311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Kiernan UA, Tubbs KA, Nedelkov D, Niederkofler EE, Nelson RW. Detection of novel truncated forms of human serum amyloid A protein in human plasma. FEBS Lett. 2003;537:166–170. doi: 10.1016/S0014-5793(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 29.Stenken JA, Church MK, Gill CA, Clough GF. How minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studies. AAPS J. 2010;12:73–78. doi: 10.1208/s12248-009-9163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Averbeck M, Beilharz S, Bauer M, Gebhardt C, Hartmann A, Hochleitner K, et al. In situ profiling and quantification of cytokines released during ultraviolet B-induced inflammation by combining dermal microdialysis and protein microarrays. Exp Dermatol. 2006;15(6):447–454. doi: 10.1111/j.0906-6705.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 31.Roupé KM, Nybo M, Sjöbring U, Alberius P, Schmidtchen A, Sørensen OE. Injury is a major inducer of epidermal innate immune responses during wound healing. J Invest Dermatol. 2010;130:1167–1177. doi: 10.1038/jid.2009.284. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PA, Berbée JF, Rensen PC, Kitchens RL. Apolipoprotein A-II augments monocyte responses to LPS by suppressing the inhibitory activity of LPS-binding protein. Innate Immun. 2008;14(6):365–374. doi: 10.1177/1753425908099171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han YP, Yan C, Garner WL. Proteolytic activation of matrix metalloproteinase-9 in skin wound healing is inhibited by alpha-1-antichymotrypsin. J Invest Dermatol. 2008;128(9):2334–2342. doi: 10.1038/jid.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulin: roles in response to injury. Clin Chem. 2006;52:1247–1253. doi: 10.1373/clinchem.2005.065680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 65 kb)
(DOC 571 kb)
(DOC 103 kb)
(DOC 365 kb)