Abstract
Over fifty years of rigorous empirical attention to the study of sexual differentiation of the brain has produced sufficient data to reveal fundamental guiding principles, but has also required the generation of new hypotheses to explain non-conforming observations. An early emphasis on the powerful impact and essential role of gonadal steroids is now complemented by an appreciation for genetic contributions to sex differences in the brain. The organizing effects of early steroid hormones on reproductively relevant brain regions and endpoints are largely dependent upon neuronal aromatization of androgens to estrogens. The effect of estradiol is mediated via estrogen receptors (ER). The presence or absence of ER can restrict hormone action to select cells and either prevent or invoke cell death. Alternatively, ER activation can initiate signaling cascades that induce cell-to-cell communication and thereby transduce organizational steroid effects to large numbers of cells. However, the specific details by which cell death and cell-to-cell communication are achieved appear to be locally, even cellularly, unique and specific to that particular subpopulation. As the field moves forward the increasingly specific and detailed elucidation of mechanism challenges us to generate new guiding principles in order to gain a holistic understanding of how the brain develops in males and females.
Introduction
The progression of scientific discovery occurs in phases. First, a phenomenon is described. Second, speculations about the general origins and significance of the observed phenomenon lead to formulation of specific testable hypothesis. Once the initial hypothesis is tested and approved, there is usually a series of new observations that either confirm, challenge or modify the initial observation and hypothesis. Ultimately the questions move towards elucidating the mechanism and a detailed characterization of the cellular and molecular events that underlie and determine the initially observed phenomenon. Frequently the elaboration of the underlying mechanism(s) opens new avenues of discovery and refines interpretation of the initial observation. This sequence of discovery has been realized several times in the neurosciences. It is evident in the arena of hippocampal control of spatial learning, in determining the sensitive period of development of the visual system and in the neural control of circadian rhythms. Detailed understanding of the cellular and molecular events underlying learning and memory, visual perception and daily hormonal and activity rhythms confirm at the most fundamental level the originally observed phenomenon while simultaneously opening up new avenues of exploration (Figure 1). The question here is, has the field of sexual differentiation of the brain also reached this level of maturity? The description phase, consisting of observations that the sexual behavior and reproductive endocrinology of males and females are fundamentally different, has long since past and can be considered essentially self-evident. The onset of the second phase began with the iconic Phoenix, Goy, Gerall and Young paper of 1959 [1] elucidating and then testing the Organizational/Activational Hypothesis of hormonally-mediated sexual differentiation of reproductive behavior. The original specific hypothesis has since been adapted to broadly codify the phenomenon of steroid-mediated sexual differentiation of the brain, a process in which copious steroid production by the testis of the late gestation male fetus gains access to the brain and initiates a series of organizational changes to the neuroarchitecture which will subsequently be re-activated in adulthood to mediate sex-specific physiology and behavior. The confirmation of the hypothesis came in its replication in other species (originally guinea pigs), particularly rats and mice, and expansion to other phenomenon such as sexual differentiation of hormonal control of gonadotropin secretion [2]. Challenges have also been numerous and range from questioning the foundations of the described phenomenon to its applicability to other sexually differentiated species, such as song birds [3] or primates [4; 5]. These challenges, among others, have produced important and substantial refinements to the hypothesis, perhaps best exemplified in the now widely accepted caveat that genetics also contributes to sex differences in the brain; its not just about hormones anymore [6].
Figure 1. Phases in scientific discovery.
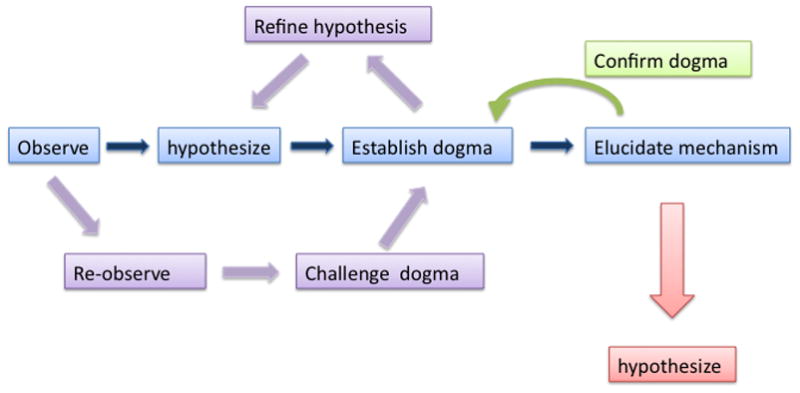
Most scientific discoveries begin with the observation and description of a fundamental phenomenon, thereby forming the basis for an initial hypothesis. Once accepted as true, the hypothesis becomes a tenet which over time becomes a dogma. This stimulates challenges in the form of new observations which result in a refining and/or expanding of the initial hypothesis and associated dogma. Eventually, the focus of the field turns to mechanism, which mostly reconfirms the initial dogma but also provides a new set of observations upon which new hypotheses can be based. The field of sexual differentiation of the brain is currently in this period of scientific discovery as we make great inroads into determining the mechanism of steroid hormone action while also forming new hypotheses regarding the importance of genetic and epigenetic variables.
Simultaneous to the challenges and refinements of the organizational/activational hypothesis there have been major advances on the mechanistic front. As with other fields, these discoveries have also opened new avenues and provided additional insights into both the origins and significance of sexual differentiation of the brain. But perhaps unlike other fields, the discoveries are being made on multiple simultaneous and largely independent fronts. Thus, rather than building upon each other in an inexorable march towards the ultimate truth, the study of the mechanisms establishing sex differences in the brain are disparate and isolated, with little attempt to create a coherent whole [7]. Perhaps this is because there is no coherent whole to create, or, perhaps we are suffering from an inability to see the forest because we are too busy looking at the trees. In other words, when it comes to understanding the significance of the mechanisms of sexual differentiation is it more productive to be a lumper or a splitter? Should we look for similarities and patterns presented by multiple independent observations, or is it better to take each observation on its own as a representation of that isolated phenomenon and nothing more? There is value in both approaches, and therefore there is value in exploring whether either or both interpretations help to advance our understanding of the phenomenon of sexual differentiation of the brain (Figure 2).
Figure 2. A Lumpers versus Splitters approach to sexual differentiation of the brain.
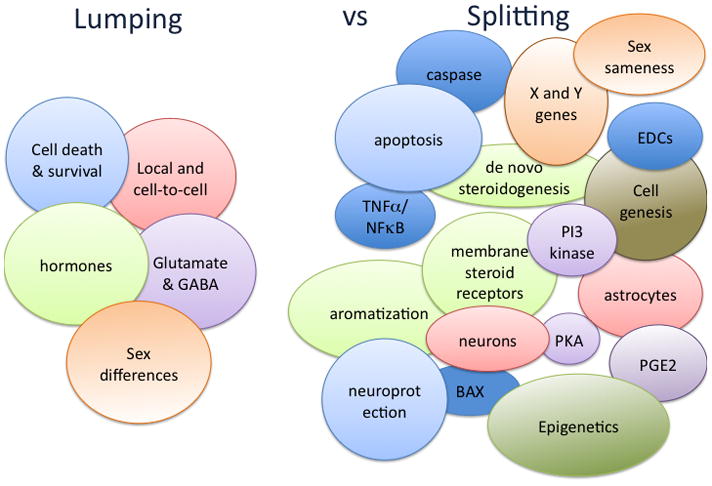
Studies on the sexual differentiation of the brain have been going on for 50+ years, providing a rich and complex collection of findings. Several fundamental principles arise from the dogma of early organizational effects of steroids determining adult reproductive physiology and sexual behavior. These can be lumped into broad categories. But elucidation of mechanism reveals highly unique region specific cellular pathways that underlie the organizational actions of steroids. Moreover, examination of sex differences outside of the context of reproduction reveals many new and possibly fundamental principles that both induce and reduce sex differences in brain and behavior. The relative lumping and splitting of various components is fluid and likely to change with additional new discoveries and reinterpretation of old ones. The colors used to designate each major category under Lumping is then used to designate the related set of individual findings under Splitting. New colors under Splitting indicates areas that are so recent as to not yet be associated with any fundamental concepts.
Common mechanisms – The lumpers approach to sexual differentiation
Estradiol is the principle mediator of masculinization of the rodent brain
The Aromatization Hypothesis reconciled conflicting evidence on the precise nature of hormonally mediated sexual differentiation of the brain. There was no doubt that the male gonad was the source of the testosterone that masculinizes the male brain, but the observation that treating neonatal females with exogenous estradiol was more masculinizing than exogenous testosterone was confusing until the elucidation of the elegant solution of sequestering all maternal estrogens in the fetal bloodstream by binding it to alpha-fetoprotein while allowing testicular androgens into neurons where it was locally converted to estrogens by aromatase [8; 9]. This strategy not only prevented the masculinization of females from their mothers estrogens, but also provided for highly localized estradiol production within the male brain, meaning estradiol is only made in substantial amounts where the aromatase enzyme is found, and this is largely in the reproductively relevant regions of the brain, the preoptic area and hypothalamus [10; 11]. Confirmation of the dominance of estradiol as the masculinizing hormone of the rodent brain comes from many and divergent quarters. This includes the predicted effect of absent or compromised masculinization in multiple varieties of mutant mice either lacking estrogen receptors or the ability to make estradiol [12; 13; 14; 15; 16], with relatively little but specific effects in mice lacking androgen receptors [17; 18; 19]. Moreover, mice lacking the ability to synthesize alpha-fetoprotein confirm the risk imposed by maternal estrogens as the female offspring are indeed masculinized [20]. Thus these new (mechanistic?) observations confirm the long-held dogma (Figure 1), and additional behavioral observations prompted a re-evaluation of an old hypothesis regarding a delayed sensitive period for feminization of the brain from estradiol of ovarian origin [21], opening new avenues for understanding the origins of sex differences in the brain.
The dominance of estradiol is also confirmed in elucidation of the detailed cellular mechanisms by which many sex differences are established. This is best exemplified when estradiol is used to treat cultured neurons and astrocytes in a particular brain region and found to mimic the effects observed in vivo. The value of observing analogous effects of steroid treatment in cultured neurons versus in the brain is the ability to rule out indirect steroid effects emanating from other brain areas, or effects occurring due to further metabolism of the steroid. For instance, estradiol stimulates the production of prostaglandin E2 (PGE2) by inducing expression of the rate-limiting synthetic enzymes COX1&2, and through a cascade of cellular events induces the formation of dendritic spine synapses on preoptic area neurons, resulting in a masculinized synaptic pattern [22; 23; 24]. The cascade of cellular events includes activation of protein kinase A (PKA), specifically PKA that is associated with the actin matrix found in dendritic spines and anchored there by AKAPs, protein kinase A anchoring proteins [24]. One function of PKA associated with dendritic spines is phosphorylation of specific amino acid residues of the GluR1 subunit of the AMPA glutamate receptor, and the subsequent trafficking of the receptor to the post-synaptic membrane. Thus increased PGE2 ultimately results in increased AMPA receptor insertion at the membrane and the formation and stabilization of dendritic spine synapses. Amazingly, the entire sequence of events can be recapitulated in the dish by treating cultured POA neurons and astrocytes with PGE2 and monitoring the movement of fluorescently labeled GluR1 to the membrane (Figure 3). Taken together these results confirm that estradiol is the masculinizing hormone for POA dendritic spine synapses and that the cellular effects are local to the POA. We have observed the same level of concordance in dendritic spine synapse development and dendritic branching in the mediobasal hypothalamus, both are increased by estradiol whether the neurons are in the brain or growing under artificial culture conditions [25; 26]. These findings suggest highly local mechanisms are relatively common in estradiol-induced sexual differentiation of neuronal morphology.
Figure 3. Complex cellular cascades mediate organizational effects of steroids.
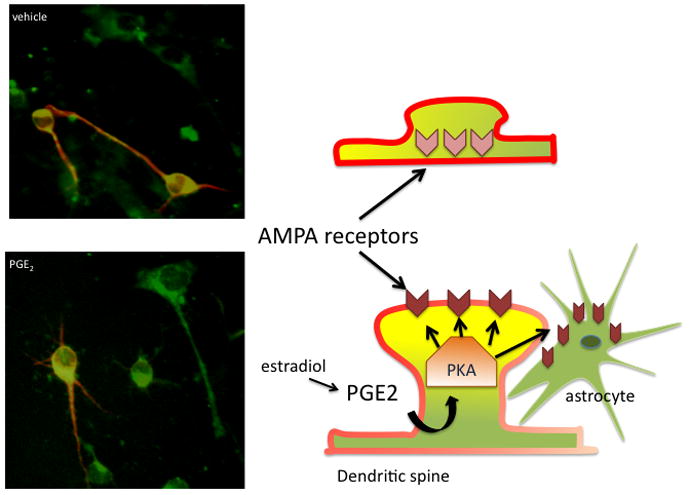
In the preoptic area, estradiol up regulates the synthesis of prostaglandin E2 (PGE2), which binds to EP2&4 receptors found on neurons and astrocytes. The EP2&4 receptors are positively linked to adenylate cyclase and the production of cAMP, thereby activating protein kinase A (PKA), which is associated with the actin scaffolding in the head and neck of dendritic spines. Activated PKA phosphorylates the GluR1 subunit of the AMPA receptor, which causes it to move into the cellular membrane and promotes the development and stabilization of the post-synaptic density of the dendritic spine. In the absence of phosphorylated GluR1, the AMPA receptor is not maintained in the membrane and dendritic spine synapses collapse or fail to form. In the photomicrographs on the left, POA neurons grown in vitro are visualized by red staining for the neuron specific microtubule associated protein (MAP-2) and phosphorylated GluR1 is visualized as green. The combination of MAP-2 and phosphorylated GluR1 is visualized as yellow. Cells that appear entirely green are presumptive astrocytes. Cells in the top panel were treated with vehicle while those in the bottom panel were treated with PGE2 two hours prior. The intensity and amount of colocalization of phosphorylated GluR1 and MAP-2 is increased and the movement of GluR1 to the membranes of astrocytes is readily apparent (photo courtesy of Katherine Lenz). This combined effect of PGE2 on neurons and glial is speculated to be the result of cell-to-cell communication and to be the basis for organizationally determined sex differences in dendritic spine density and astroglial morphology.
Estradiol effects are local but not cell autonomous
The effects of estradiol on POA neuron dendritic and astrocyte morphology confirm two things for that brain region; 1) there is no need for external input to the POA and 2) estradiol effects are not isolated to those cells expressing estrogen receptor (ER) but instead involve cell-to-cell, and most likely in this case neuron-to-astrocyte communication. A similar scenario is found in the immediately adjacent mediobasal hypothalamus where estradiol also induces the formation of dendritic spine synapses, also by co-opting glutamate, and the effects are also reproducible in the dish, meaning they are local to the hypothalamus [25]. And, there is again a requirement for cell-to-cell communication as the changes in dendritic spine number occur in the post-synaptic neuron while the induction of glutamate release involves estradiol binding to ER in the presynaptic neuron. The arcuate nucleus presents yet another example of the importance of cell-to-cell communication, with estradiol initiating events in neurons by up regulating the synthesis of GABA via increased production of the rate limiting enzyme, GAD, but the changes are manifested on astrocytes which express GABA receptors but do not make GABA [27; 28]. In this instance there is a de facto requirement for cell-to-cell communication as only neurons can make GABA and it is GABA that changes the morphology of the astrocytes, so again there is a critical role for neuron-to-astrocyte communication [29; 30].
Cell death is a critical mechanism establishing volumetric sex differences
Sex differences in the brain are established during the process of steroid-mediated sexual differentiation. A frequently observed type of sex difference is volumetric, meaning a region is larger in volume in one sex versus the other. The first such sex difference noted was in the size of the song control nuclei of birds [31], which was quickly followed by the discovery of the sexually dimorphic nucleus (SDN) of rats [32; 33]. Subsequently there was a protracted period of cataloging and characterizing neuroanatomical sex differences throughout the brain. These include many other volumetric sex differences, where the overall size of a brain region is larger in one sex, which is usually but not always male. But regardless of which sex ultimately retains a larger size nucleus, the mechanism by which it occurs is fundamentally the same, differential cell death, meaning the two sexes start with the same number of neurons and in response to differences in the steroid hormone milieu, neurons will selectively die in either males or females. This is true for the spinal nucleus of the bulbocavernosus (SNB) of the spinal cord, where in males androgens rescue the motor neurons innervating muscles at the base of the penis. The same neurons wither and die in the female as she has little to no androgen and no penis, and therefore no need for these particular neurons [34; 35; 36]. This is also true for the song control nuclei, the RA and HVC of the song bird brain where estrogens rescue the neurons in the male brain so that he can produce courtship song in adulthood, a behavior not in the repertoire of females and therefore there is much less need for these neurons [37; 38]. The volume of song control nuclei change seasonally, however, which belies a simple organizationally determined sex difference [39; 40]. Hormones also mediate the sex difference in the size of the anteroperiventricular nucleus (AVPV), a subnucleus in the preoptic area that is a critical node in the neural circuitry controlling GnRH neurons [41]. Here estrogens play the opposite role and actually induce cell death, resulting in a smaller nucleus in males. The selective elimination of these neurons in females is presumably required for the appropriate construction of a neural circuit capable of producing an ovulation-inducing LH surge from the anterior pituitary at reproductive maturity. A second critical node in this circuit is the bed nucleus of the stria terminalis, in particular the principal subdivision, the pBNST, and here gonadal steroids protect cells from dying, just as they do in the nearby SDN. In both the pBNST and the SDN the end result is more neurons in males, and in both cases these seem to be mostly GABAergic neurons and/or neurons that express GABA-A receptors [42; 43]. Thus there is a great deal of commonality in the strategy for generating a brain region that is larger in one sex versus the other; start with the same number of neurons and then either selectively rescue them in males with the inherently higher steroid levels of the developing male brain, or selectively kill them in males by the same approach [44; 45; 46]. But this gratifyingly simple solution to the creation of size differences in brain regions of males and females begs the next question, what is regulating the cell death? Again we get some degree of commonality, a lumping of mechanisms in that the classic apoptotic mediators, BAX and Bcl-2 are intimately involved in each instance [47; 48]. But there are also some components that remain unexplained. Recent studies highlight unique and specific mechanisms regulating sex differences in cell death, i.e. splitting, and these are reviewed further below.
In summary, there are least three commonalities in the one phenomenon of sexual differentiation of the rodent brain; 1) the dominance of estradiol as the masculinizing hormone, 2) the narrowly restricted regional specificity of the effects combined with cell-to-cell communication and a critical role for GABA and glutamate, and 3) the importance of cell death as a means to alter the size of a particular subnucleus. Multiple sources of convergent evidence support each of these conclusions, fully justifying a “lumpers” approach to sexual differentiation of the brain. The appeal of lumping is the ability to discern patterns and thereby simplify the rules governing a complex process in order to guide and inform future studies. But the great risk of lumping is missing other important variables, ignoring other contributing influences or just plain over simplifying a complex situation. This is particularly important to avoid in the realm of sex differences in the brain as there is the added burden of potentially fostering false prejudices about the relative cognitive and emotional abilities of men versus women.
Multiple mechanisms – The splitters approach to sexual differentiation
Multiple hormones of diverse origin influence sexual differentiation of the rodent brain
There was pushback from the very first suggestion of a preeminent role for estradiol in rodent brain masculinization, surely there must be a role for that most masculine of hormones, testosterone. And surely there is, most specifically in the spinal cord and the survival of the motoneurons of the SNB [49]. But there has always been some lingering evidence of an androgen contribution to hypothalamic and preoptic area sexual differentiation. A role for androgens in adult sex differences in the amygdala [50] is often interpreted as evidence for androgen induced differentiation but sex differences and sexual differentiation are not the same thing. Studies of rodents with naturally occurring mutations of androgen receptor (AR) function clearly indicate a role for this steroid in behaviors associated with anxiety, stress responding and emotionality [18] and these effects may be the product of being deprived of the organizational actions of androgens, the lack of androgen responsiveness in the adult or a combination of both. What has been largely missing, however, is the cellular mechanisms by which androgens work, providing the final confirmation of the importance of androgens to sexually differentiated processes. Some may argue the development of transgenic mice with selective changes to aromatase or the AR further supports the importance of androgen [51], but in the absence of knowing what the androgens are actually doing, and how, the story remains incomplete.
A basic tenet of the Aromatization Hypothesis is that estradiol synthesized in the brain is exclusively the result of conversion of testicularly synthesized androgens gaining access to the brain by circumventing the sequestering effect of alpha-fetoprotein. In addition to the crucial role of the testis, emerging evidence indicates an additional and equally important source of estradiol, the brain itself. This has been most firmly and enduringly established for the bird brain [52; 53; 54], and is becoming increasingly generalized to the rodent brain [55; 56; 57]. Not only is estradiol synthesized entirely from locally derived precursor, not just testicular androgens, but it can also be synthesized rapidly, on demand and possibly even at the synapse, functioning in many ways analogous to a neurotransmitter [58]. There has been speculation, but no definitive evidence that locally de novo synthesized steroids contribute to the establishment of brain sex differences and this seems an important area for future development.
Nuclear steroid receptors also reside in cell membranes
Empirical evidence for so-called rapid membrane-mediated effects of steroids was first reported over 30 years ago by Clara Szego [59] and was met with skepticism for many decades. We now collectively accept that there are indeed biologically important signaling cascades initiated by steroid receptors residing in the membrane, especially ER which is often denoted as mER to distinguish it from the nuclear receptor even though they may be the same protein [60]. The majority if not all of the definitive work establishing a membrane receptor for estrogens has been conducted in adult brains and so applicability to the developing brain is not always clear. In fact, in the realm of rapid estradiol effects initiated at the membrane it was not unreasonable to assume that this expressly would NOT occur in the developing brain as the long-term consequences of short-term ER activation during a critical period could be profound and perhaps inappropriate. But the study of mechanism proved otherwise. In the neonatal mediobasal hypothalamus, estradiol activates ERα which in turn rapidly activates PI3 kinase, within as little as one hour, and via additional steps not yet elucidated the activated PI3 kinase increases the probability of glutamate release from presynaptic terminals [25; 61]. Post-synaptic glutamate receptors of the AMPA and NMDA subtype initiate a further cellular cascade involving activation of MAP kinase, increases in spinophilin protein and ultimately the production of dendritic spine synapses. The end result is a masculinized pattern of dendritic spine synapses on mediobasal neurons which correlates with the defeminization of sexual behavior. Thus while the mechanism here supports the dogma of the hegemony of estradiol, it also refines our view of how estradiol acts to induce enduring changes in neuronal morphology of the developing brain.
Its not just hormones anymore, genes matter too
It was not so much mechanism as it was observational data that defied conformity to the notion of a straight-forward estradiol mediated masculinization of the bird brain, both for song and for sex, that ultimately led Art Arnold of UCLA to explore the role of genetics and sex differences in the brain [6]. The sex chromosomes, X and Y in mammals and W and Z in birds, contribute genetic variability to other parts of the body, so why not the brain? Indeed, a substantial portion of genes on the X chromosome are associated with brain function [62; 63; 64]. The challenge of separating gonadal from chromosomal effects was greatly advanced by the development of the 4-core-genotype line of mice in which the Sry gene, which is critical for the differentiation of the testis, has been translocated to an autosome and deleted from the Y chromosome. Through carefully conducted crosses, animals can be generated with an XX genotype and a testis phenotype or an XY genotype with an ovarian phenotype [63; 65]. By comparing them to animals with the normal sexual genotype and phenotype combination, the relative role of gonadal hormones versus genes can be assessed on various endpoints. For the most part those endpoints that are principle to reproduction, i.e. fertility, sex behavior and associated brain regions, the critical role of hormones remains apparent. But for several endpoints outside the realm of reproduction, such as aggression, habit formation, pain perception and alcohol preference, there is a clear influence of genetics [66; 67; 68; 69]. Identifying which genes are involved and precisely how they contribute to the behavioral phenotypes, in other words determining the mechanism, will confirm the new dogma that genes on the X or Y chromosome contribute to the establishment and maintenance of sex differences in brain and behavior.
There are many ways to die – mechanisms of cell death
The commonality of cell death as a mechanism for generating sex differences in the size of particular subnuclei is appealing both for its simplicity and its confirmation in multiple brain regions across multiple species. Given the robustness and reliability of the phenomenon there seemed no real reason to consider regionally specific differences, with the exception of those instances where estradiol actually promotes cell death as opposed to prevents it. But again, studies of mechanism reveal an unexpectedly complex control of cell death in the service of constructing sex differences in brain size. One of the more startling discoveries is that the early period of sex differences in cell death is subsequently replaced by sex differences in cell birth. Thus in the SDN and BNST, where more cells die in females due to a lack of estradiol during the perinatal sensitive period, there is a later onset of greater cell genesis in males than females in these same nuclei, thereby retaining the larger regional volume in males. Conversely, in the AVPV where there is more cell death in the neonatal male, there is also less cell genesis during the peripubertal period compared to females which show high rates of cell genesis [70]. The time period of sex differences in cell death is sufficiently close to the period of sex differences in steroid hormones that it is not implausible that the steroids are directly influencing cell survival. But the sex difference in cell genesis is long past the sensitive period for sexual differentiation, and while it may be a product of hormonal changes associated with puberty, there is also clearly some type of cellular memory formed by the earlier differentiating events. That this is possible was confirmed in studies exploring a potential epigenetic contribution to hormonally mediated cell death in the BNST, which these authors felt occurred sufficiently long after hormone exposure to not be a direct effect of the steroids [71]. By preventing epigenetic changes to the chromatin induced by steroid exposure, the steroid-mediated sex difference in cell death was also prevented. The role of epigenetics in the establishment and maintenance of sex differences in the brain is an emerging area and certainly one to watch for exciting new developments that will both confirm existing dogma and open new avenues for exploration [72; 73].
The AVPV is a sexually dimorphic nucleus of considerable interest due both to its apparently critical role in the control of GnRH neurons and the fact that it is larger in females than males, making it notably different from the majority of male-biased volumetric sex differences. The smaller AVPV of males is a direct consequence of estradiol action, meaning estradiol is killing cells instead of protecting them. Even more striking is that the cell death is targeted to selective cellular phenotypes via apparently cell specific pathways. Both GABAergic and dopaminergic neurons are reduced in number in the male AVPV. The death of GABAergic cells involves the suppression of TNFα, a proinflammatory cytokine that activates NFkB receptors and promotes cell survival. In GABAergic neurons of the female AVPV, the TNFα-NFkB survival pathway is constitutively active. The suppression in males is the result of higher expression of an associated protein called TRIP (TNF receptor associated-associated factor 2-inhibiting protein) which inhibits both the TNFα-NFkB survival pathway and the anti-apoptotic protein, bcl-2, resulting in higher levels of the proapoptotic signaling molecules, bax and bad [74]. Whether the up regulation of TRIP in males is induced by estradiol has not been established, but seems highly likely. The selective killing of GABA neurons is complemented by a parallel effect of estradiol-induced activation of caspase-dependent cell death in the dopaminergic neurons of the AVPV, reducing the number of this class of neurons in males as well [75]. Thus, one hormone, estradiol, has one effect, the induction of apoptosis in the AVPV, but this is achieved via two distinct mechanisms that will selectively reduce GABAergic and dopaminergic neuron number (Table 1).
Table 1. Cell Death versus Cell Birth.
Sex differences in the size of multiple brain regions have been attributed to sex differences in the rate of cell death, with steroid hormones either stimulating or repressing apoptotic processes. Recent evidence indicates that precise cellular mechanisms mediated cell death are specific for each sub-nuclei, and additional mechanisms likely remain to be discovered. In the rat brain, estrogens are a dominant mediator of sex differences in cell death, with the exception of the SNB of the spinal cord which is regulated by androgens. In contrast to these reproductively relevant nuclei, the hippocampus and amygdala are characterized by sex differences in cell birth, with more new neurons born in male hippocampus compared to female but more new astrocytes born in the female amygdala compared to the developing male.
| Brain Region | Cell fate | Sex difference | Mechanism | References |
|---|---|---|---|---|
| SNB | death | M>F | CNTF, androgen | 34, 35, 36 |
| SDN-POA | death | M>F | unknown | 32 |
| AVPV | death | F>M | TNFα, caspase | 74, 75 |
| BNST | death | M>F | epigenetics | 71 |
| Hippocampus | birth | M>F | unknown | 77, 78 |
| Amygdala | birth | F>M | endocannabiniods | 89 |
These findings suggest that both cell death and cell birth are important contributors to sex differences in the brain and highlight how much we still have to learn regarding mechanisms that establish the cellular profiles of the male and female brain. SNB – spinal nucleus bulbocavernosus, SDN-POA – sexually dimorphic nucleus of the preoptic area, AVPV = anteroventral periventricular nucleus, BNST = bed nucleus of the stria terminalis, M = male, F = female.
Cell birth – a new source of sex differences in the brain
Hippocampus
The dogma that sex differences in the size of specific subnuclei of the preoptic area, hypothalamus and spinal cord are the result of sex differences in cell death is classic, confirmed by mechanism and expanded upon by new observations (Figure 1). But challenges to the dogma emerge from new observations of brain areas outside of the traditional regions closely associated with reproductive endpoints. In the adult there are two locations where neurogenesis is reliably documented to continue and is subject to physiological regulation, including hormones. These are the subventricular zone (SVZ) and the proliferative zone of the subgranular layer of the dentate gyrus, a component of the hippocampal complex [76]. The early postnatal period is also a time of ongoing neurogenesis, with levels of proliferation being much higher than in the adult but less than in the developing embryo. We have discovered that there is a considerable level of cell genesis occurring in the neonatal dentate gyrus, as would be expected, but also in the CA1 and CA3 regions of Ammon’s horn. Moreover, when littermates are compared there is almost twice the rate of cell proliferation in males compared to females [77; 78]. When the fate of these cells are tracked over the long term, meaning three to four weeks later, some 70–80% have differentiated into neurons. Treatment of females with estradiol during the neonatal proliferative period increases the number of new neurons to that of males, and antagonizing estrogen action at the receptor or inhibiting its synthesis via inhibitors of aromatase, reduces neurogenesis in males to even below that of females. Notably, the level of endogenous estradiol and aromatase activity in the hippocampus at this developmental time point is exceedingly low relative to the hypothalamus or preoptic area, but it is detectable [79]. Thus estradiol-induced neurogenesis in the hippocampus may be mediated by locally synthesized estradiol, or the effect of estradiol may begin outside the hippocampus or even the brain itself. In the adult much of estradiol’s effects on hippocampal neural plasticity involves changes in afferent input, originating in the cholinergic neurons in the septal region outside of the hippocampus [80]. It is possible the effects on the developing hippocampus are also initiated in the septal or other extra-hippocampal regions. Estradiol might also be acting outside the brain by inducing changes in vascular blood flow and thereby affecting the delivery of oxygen, nutrients and growth factors to the brain. Among the variables known to increase adult neurogenesis is exercise, which may also act in part by increasing blood flow to the brain [76; 81; 82; 83]. Until the mechanism of estradiol-induced neurogenesis in the developing hippocampus is established, the brain or body region regulating estradiol effects will remain unknown.
Beyond mechanism there remains an additional substantial gap in our understanding of the observed sex difference in neurogenesis in the developing hippocampus; what is it for? A natural assumption is that if twice as many new neurons are being born in the male hippocampus at any given time, then presumably the male has twice as big a hippocampus. But this is decidedly not the case, the male hippocampus is at best 10–12% larger in the female [84; 85]. Moreover, it appears the period of differential cell birth in male versus female hippocampus may be relatively brief, occurring only during the first few postnatal days, a time when the life of a rodent consists largely of sleeping, suckling and pooping, not unlike the life of a human baby during the first few days. But we do know something is happening to the brain during this semi-vegetative state of development, how the dam treats her offspring, meaning how much attention she provides in terms of licking and grooming, can have life long consequences [86; 87], and there is a degree of olfactory learning that occurs during this time as well, with the neonatal pups learning the smell of the dam, as well as possibly the natal nest [88]. One potential function of the neurogenesis during this period may be to code this information for latter recall as an adult, and there must be something about it that requires more neurons in males than females, but what that is remains to be determined.
Amygdala
There is also a sex difference in cell birth in the developing medial amygdala, but in contrast to the hippocampus here there are more new cells born in the developing female brain compared to the male (Table 1). In further contrast to the hippocampus, while the majority of the cells being born during this period will become neurons, those cells that account for the sex difference, which is in the range of 20–50% more new cells in females, are destined to become astrocytes [89]. Astrocytes are intimately associated with synapses and actively participate in both the construction and maintenance of synaptic connections [90]. Thus it is plausible that the greater number of astrocytes in the developing female amygdala is associated with a change in the synaptic profile. There is a rather surprising cellular mediator of the observed sex difference in astrocyte proliferation, endocannabinoids, lipid-derived signaling molecules that are ubiquitous throughout the brain. There are two main endocannabiniods, anandamide and 2-AG, and they bind to two principle receptors, CB1 and CB2, which are G-protein coupled membrane receptors. In the brain CB1 is heavily and ubiquitously expressed and is frequently found on presynaptic nerve terminals. Activation of CB1 results in reduced neurotransmitter release, usually GABA or glutamate, and thereby serves as a critical component of retrograde signaling from the postsynaptic neuron [91]. CB2, on the other hand, is more heavily expressed in the periphery and is strongly associated with the immune system. But there are CB2 receptors in the brain, and they have been found on proliferating cells [92]. We found that administration of the promiscuous ligand, WIN 55,212-2, to neonatal males and females, significantly reduced cell genesis in females to that of males, but had no impact on cell genesis in males across a wide range of doses. We further found that antagonizing the CB1 receptor simultaneous to administration of WIN had no effect on cell proliferation, but administrating a CB2 antagonist completely prevented the effect of WIN, leading to the conclusion that the CB2 receptor mediates the effect of WIN on cell proliferation [89].
One plausible explanation for the observed sex difference in response to endocannabinods in the developing amygdala would be a difference in the amount or functionality of the CB2 receptor. There are multiple examples of sex differences in the amount of a particular receptor expressed in a specific brain region, both developmentally and in adulthood in response to circulating steroids. However in our studies of the cellular mechanisms of steroid mediated sexual differentiation of the brain we have found it is rarely the receptors that are different in males versus females. Instead, it is almost always the ligand, either in its synthesis or release. This is now also true for the endocannabiniods. We found no evidence of a sex difference in the amount of CB1 or CB2 receptors in the developing amygdala, but there was a substantial difference in the content of anandamide and 2-AG, with females having significantly less than males. This was paralleled by an opposite sex difference in the amount of the associated degradation enzymes, FAAH, and MAGL, which is a dominant mechanism controlling the level of endocannabinoids. Thus on balance, females have a lower overall tone of endocannabinoid activation. That this mediates the increased level of cell genesis in females was confirmed by inhibiting the degradation enzymes, FAAH and MAGL, which increased endocannabinoid content and subsequently decreased the number of new cells being born to that of males (Figure 4). A further logical prediction is that antagonizing the endocannabinoid system in males, to reduce tone, should increase cell genesis to the level seen in females. However we found this not to be the case, no manipulation we could find altered the level of cell genesis in males, either up or down. Note that we did not increase cell genesis in females either. This may reflect that the cellular events necessary to promote cell division, i.e. increase proliferation rates, are multiple and complex. By contrast stopping proliferation may be a much simpler process. Alternatively, there could be a ceiling of cell proliferation rate that has been reached in females, therefore they can only go down, and males may simply be unresponsive to this signaling cascade. Regardless, this is an intriguing and unusual sex difference, and whether hormones mediate the sex difference in MAGL and FAAH levels is at the moment an untested prediction.
Figure 4. Cell genesis may become a new fundamental principle of sexual differentiation of the brain.
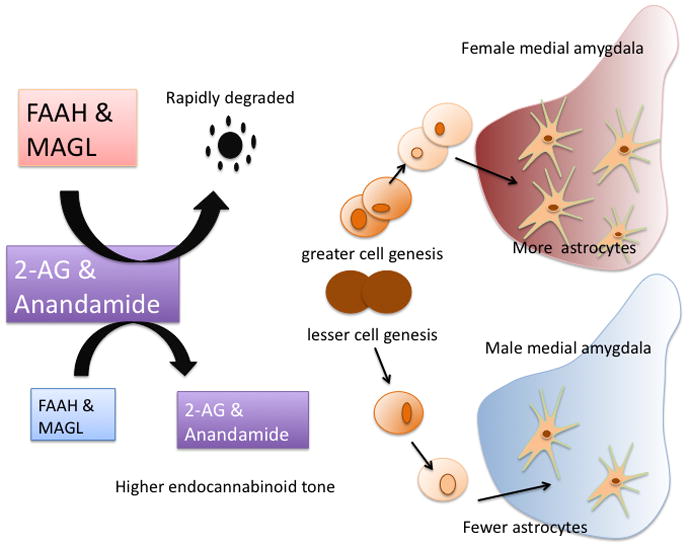
There are almost twice as many future neurons being born in the developing hippocampus of male rat pups compared to their female littermates. Treating females with estradiol increases the rate of neurogenesis to that of males, while treating males with either an estrogen receptor antagonist or aromatase inhibitor decreases the rate to that of females [97]. This sex difference is notably different than the well established fundamental principle of sex differences in cell death in select nuclei during the perinatal period. A second example of a sex difference in cell genesis is illustrated here and is unusual in several ways. First is that a sex difference in the overall tone of endocannabinoids mediates the sex difference. Females have higher levels of the degradation enzymes, FAAH and MAGL, and as a result have lower resting levels of the endocannabinoids, 2-AG and anandamide. There is also a higher rate of cell proliferation in the medial amygdala of females during the early neonatal period. Raising the endocannabinoid tone by either inhibiting FAAH and MAGL or supplying exogenous endocannabinoids, reduces the rate of proliferation in females to that of males, but has no effect on males. Lastly, most of the new cells being born during the perinatal period will differentiate into neurons, but a small population that accounts for the observed sex difference will become astrocytes, and as a result females have more astrocytes in the medial amygdala compared to males [89].
There is one last component of this set of observations that is of interest, its functional significance. The amygdala is an important brain region for a variety of emotional and social behaviors, being an essential node in the learning component of fear conditioning, a gateway for olfactory information for social interactions, including sexual behavior, and the primary brain region implicated in the sexual differentiation of juvenile play behavior. Social play, sometimes called rough-and-tumble play, exhibits a robust and consistent sex difference. On average, males engage in more frequent and more intense physical interactions, be they puppies, colts, monkeys, mice, rats or boys. The sex difference is established early in development by gonadal steroid hormones, just as with sex behavior, and both androgens and more recently estrogens have been implicated as the principle mediators of the sex difference yet the expression of the behavior occurs during a life phase during which there is little to no steroid hormone exposure, i.e. prior to puberty. Thus rough and tumble play is an example of an organized sex difference that does not require hormonal activation in order to be apparent. The cellular mechanisms by which play is differentiated are largely unknown [93]. We found that treatment of neonatal females with a regime of WIN that reduces glialgenesis resulted in juvenile females with masculinized play, meaning they played more frequently and more intensely than their untreated female littermates. To our knowledge, this is the first demonstration of masculinization of play by females without exogenous hormone manipulation. Establishing the impact of reduced glialgenesis on the synaptic profile of the amygdala will provide further insight into how this complex social behavior is regulated and why it is sometimes dysregulated in individuals with autism or related disorders.
Summary and conclusions
It was not the goal of this review to provide a comprehensive state-of-the-art understanding of sexual differentiation of the brain, as this has been admirably achieved by others on numerous and recent occasions [44; 94]([45; 46; 95]. The objective here was to take already established information and reformulate it in a way that hierarchically organizes our understanding along the lines of fundamentals versus exceptions. It may not have escaped the readers notice that most of what has been proposed as fundamental, and therefore “lumped” because it applies broadly across a wide range of species, brain areas and behaviors, tends to be information that is older and supported by a larger body of literature. It also tends to be concepts that are relatively simple so as to be generalizable. This is in contrast to those ideas or findings placed under the rubric “splitters”, which are mostly newer and heavily restricted to a specific brain region or endpoint. But this isn’t always the case, the importance of cell-to-cell communication has only become apparent with recent mechanistic studies for instance. Nonetheless, with time, many items now listed under the splitters category may move to the lumpers side, in fact this will most assuredly be the case regarding genetic and epigenetic contributions to sex differences in the brain. The current presentation is meant only to present the study of sex difference in a new way, not to fundamentally alter our interpretation.
The subject of sexual differentiation of the brain continues to garner wide attention and will likely continue to do so for some time to come. The combination of social and political implications, major significance to human health and heuristic value inherent in comparing males and females provides for a rich and multilayered topic of investigation. As a discipline, behavioral endocrinology predates neuroscience and as a result may have been slow to embrace many of the advances being made on other fronts in the quest to understand how the brain works. But advances are being made and by lumping related observations we can now state fundamental principles that apply broadly to many aspects of sexual differentiation. Simultaneously, it is apparent that there are multiple unique and unanticipated components of sexual differentiation, and these can be split into isolated observations.
As it stands now the fundamentals are the overwhelming importance of hormones acting during a perinatal sensitive period to orchestrate sex differences in reproductively relevant endpoints by inducing permanent structural changes in brain regions associated with those endpoints. The hormonally induced changes are manifest by either inducing or inhibiting cell death and changing synaptic profiles. The latter effect involves cell-to-cell communication and frequently utilizes GABA or glutamate. Conversely, brain regions more associated with cognitive or emotional endpoints, such as the hippocampus and amygdala, are subject to a different set of rules governing sexual differentiation which may or may not involve hormones and an important component of which is differences in cell proliferation as opposed to cell death. Among the many unique observations are the role of TNFα in cell death in the AVPV, the regulation of prostaglandin production in the preoptic area, rapid membrane mediated effects of estradiol in the mediobasal hypothalamus (reviewed in [96]) and regulation of glialgenesis by endocannabinoids in the developing amygdala. Only time will tell which of these will remain unique and which will form the basis of the next fundamental concept, but discovering and elucidating mechanism is the key step in making that happen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MM. How it’s made: organisational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22:736–42. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wade J, Swender DA, McElhinny TL. Sexual differentiation of the zebra finch song system parallels genetic, not gonadal, sex. Horm Behav. 1999;36:141–152. doi: 10.1006/hbeh.1999.1537. [DOI] [PubMed] [Google Scholar]
- 4.Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: Comparative aspects of steroid hormone action. In: Pfaff D, editor. Hormones Brain and Behavior. Academic Press; London, UK: 2002. pp. 385–424. [Google Scholar]
- 6.Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–8. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: Important for sexual differentiation of the neonatal rat brain. Hormones and Behavior. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 9.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 10.Roselli CE, Klosterman SA. Sexual differentiation of aromatase activity in the rat brain: effects of perinatal steroid exposure. Endocrinology. 1998;139:3193–201. doi: 10.1210/endo.139.7.6101. [DOI] [PubMed] [Google Scholar]
- 11.Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach K, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Chan J, Chester AE, Gustaffsson J-A, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–81. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rissman E, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor α. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 16.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–43. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 17.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–72. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008;54:758–66. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–26. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 21.Bakker J, Brock O. Early oestrogens in shaping reproductive networks: evidence for a potential organisational role of oestradiol in female brain development. J Neuroendocrinol. 2010;22:728–35. doi: 10.1111/j.1365-2826.2010.02016.x. [DOI] [PubMed] [Google Scholar]
- 22.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–50. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 24.Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain, behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–82. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: A mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- 27.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendo. 2002;14:1–16. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 29.Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Hormones and Behavior. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- 30.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 31.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 32.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 33.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 34.Forger NG, Wagner CK, Contois M, Bengston L, MacLennan AJ. Ciliary neurotrophic factor receptor alpha in spinal motoneurons is regulated by gonadal hormones. J Neurosci. 1998;18:8720–9. doi: 10.1523/JNEUROSCI.18-21-08720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forger NG, Hodges LL, Roberts SL, Breedlove SM. Regulation of motoneuron death in the spinal nucleus of the bulbocavernosus. J Neurobiol. 1992;23:1192–203. doi: 10.1002/neu.480230910. [DOI] [PubMed] [Google Scholar]
- 36.Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: role of early androgen. Proc Natl Acad Sci U S A. 1986;83:7527–31. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball GF, Absil P, Balthazart J. Assessment of volumetric sex differences in the song control nuclei HVC and RA in zebra finches by immunocytochemistry for methionine enkephalin and vasoactive intestinal polypeptide. Brain Res. 1995;699:83–96. doi: 10.1016/0006-8993(95)00875-q. [DOI] [PubMed] [Google Scholar]
- 38.Gahr M. Delineation of a brain nucleus: comparisons of cytochemical, hodological, and cytoarchitectural views of the song control nucleus HVc of the adult canary. J Comp Neurol. 1990;294:30–6. doi: 10.1002/cne.902940104. [DOI] [PubMed] [Google Scholar]
- 39.Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1999;409:224–36. [PubMed] [Google Scholar]
- 40.Smith GT, Brenowitz EA, Wingfield JC. Seasonal changes in the size of the avian song control nucleus HVC defined by multiple histological markers. J Comp Neurol. 1997;381:253–61. doi: 10.1002/(sici)1096-9861(19970512)381:3<253::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40:501–10. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 42.Herbison AE, Fenelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J Neurosci. 1995;15:2328–37. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sagrillo CA, Selmanoff M. Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the rostral hypothalamus. Journal of Neuroendocrinology. 1997;9:699–706. doi: 10.1046/j.1365-2826.1997.00630.x. [DOI] [PubMed] [Google Scholar]
- 44.De Vries GJ, Simerly RB. Anatomy, development and funtion of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 137–192. [Google Scholar]
- 45.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 47.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–9. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: firsts in androgen-dependent neural sex differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke BM, Breedlove SM, Jordan CL. A brain sexual dimorphism controlled by adult circulating androgens. PNAS. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviors. Horm Behav. 2008;53:627–37. doi: 10.1016/j.yhbeh.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–5. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- 53.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–34. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci. 1992;89:7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/Autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- 57.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 58.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 60.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–56. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav. 2008;54:662–668. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci. 2009;29:768–76. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- 65.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–4. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–30. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–7. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–23. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: Implications for sexual differentiation. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci U S A. 2009;106:16692–7. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–8. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 77.Zhang JM, Tonelli L, Regenold WT, McCarthy MM. Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. Neuroscience. 2010;169:544–54. doi: 10.1016/j.neuroscience.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–35. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leranth C, Shanabrough M. Supramammillary area mediates subcortical estrogenic action on hippocampal synaptic plasticity. Exp Neurol. 2001;167:445–50. doi: 10.1006/exnr.2000.7585. [DOI] [PubMed] [Google Scholar]
- 81.Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 82.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 84.Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- 85.Nunez JL, Alt J, McCarthy MM. A new model for prenatal brain damage: I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–56. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- 87.Champagne FA. Epigenetic mechanisms and the transgernational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schapiro S, Salas M. Behavioral response of infant rats to maternal odor. Physiol Behav. 1970;5:815–7. doi: 10.1016/0031-9384(70)90285-4. [DOI] [PubMed] [Google Scholar]
- 89.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci U S A. 2010;107:20535–40. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ullian E, Chistophersen K, Barres B. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 91.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 92.Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–7. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- 93.Auger AP, Olesen KM. Brain sex differences and the organisation of juvenile social play behaviour. J Neuroendocrinol. 2009;21:519–25. doi: 10.1111/j.1365-2826.2009.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Vries GJ, Sodersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–96. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 96.Wright CL, Schwarz JS, Dean SL, McCarthy MM. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab. 2010;21:553–61. doi: 10.1016/j.tem.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: A novel source of sex dimorphism? Eur, J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


