Abstract
Few studies have shown the correlation between metabolic syndrome and bone mineral density (BMD). The main pathogenic mechanisms of metabolic syndrome rely on chronic low-level inflammatory status and oxidative stress. There are few studies that examine the gender-specific effects of inflammation and antioxidants on BMD. In this study, we evaluated the relative contribution of these factors in patients with metabolic syndrome. We conducted a cross-sectional study of 67 men and 46 postmenopausal women with metabolic syndrome; metabolic syndrome was defined as having three or more metabolic syndrome risk factors. BMD, body fat mass, and lean body mass were evaluated. We also examined the levels of high sensitive C-reactive protein (hs-CRP), interleukin-6 (IL-6), adiponectin, vitamin E, and C in serum. Log-transformed hs-CRP levels were significantly higher in lumbar spine osteoporotic subjects than in normal subjects for women but not for men. There was no significant difference between the normal group and the osteoporotic group in other inflammatory markers. Stepwise regression analyses for BMD of the lumbar spine showed that lean body mass and vitamin E were significant determinants in men. Lean body mass and log-transformed hs-CRP were significant determinants in women Analysis for BMD of the femoral neck showed that lean body mass was a significant determinant for both men and women. There was no significant factor among the inflammatory markers or antioxidant vitamins affecting the femoral neck BMD for either gender. In conclusion, while hs-CRP is an independent predictor of the BMD of the lumbar spine in women, vitamin E showed profound effects on BMD in men but not women with metabolic syndrome.
Keywords: BMD, inflammation, antioxidant, metabolic syndrome, gender
Introduction
Metabolic syndrome is associated with various health risk factors, such as hypertension, abnormal blood sugar and lipid profiles, and abdominal obesity. In the United States, approximately 25% of the total population has been reported to have metabolic syndrome, 45% of that in people age 50 or older [1-3]. According to the Korean National Health and Nutrition Examination Survey conducted in 2005, 32.9% of Korean men and 31.8% of Korean women have metabolic syndrome [4]. Patients with metabolic syndrome tend to have a higher risk of developing diabetes mellitus and cardiovascular diseases (CVD) [5]. Therefore, according to the National Cholesterol Education Program Adult Treatment Panel III (NECP-ATP III), the active prevention of metabolic syndrome is recommended to prevent or delay the onset of CVD [3].
The main pathogenic mechanism of metabolic syndrome relies on insulin resistance, low-level inflammation, and oxidative stress [6]. In the category of inflammation, it has been shown that high sensitive C-reactive protein (hs-CRP), IL-6, and adiponectin are independent factors for CVD [7]. In metabolic syndrome, excessive fat accumulation induces free radical generation and fosters oxidative damage in tissue [8]. In addition, low lycopene, β-carotene [9], vitamin C, and α-tocopherole consumption rates enhance systemic oxidative stress in metabolic syndrome patients [10]. This may explain the correlation between the complications associated with metabolic syndrome, oxidative stress, and inflammation.
Recently, risk factors associated with metabolic syndrome, such as hypertriglyceridemia, low high-density lipoprotein (HDL), cholesterolemia, and abdominal obesity, were shown to be associated with decreased bone mineral density (BMD) [11-12]. In one study, BMD was significantly lower in male patients with metabolic syndrome compared to a control group [13]. In a study of the Korean population, the odds ratio of the prevalence of metabolic syndrome was 3.07 times higher in male patients with osteodyspenia [14], and the BMD of the femoral neck was significantly lower in patients with metabolic syndrome compared to healthy people [15]. Although this mechanism has not been clarified, the origin of adipose cells and osteoblasts from the same mesenchymal stem cells has been postulated as the key factor contributing to the correlation between metabolic syndrome and BMD [16]. In addition, peroxisome proliferator-activated receptor-γ (PPAR-γ) and many types of cytokines have been reported to regulate the differentiation and proliferation of these two cells [17-18]. Adiponectin, which is involved in bone formation and inflammation, functions in maintaining energy homeostasis, anti-inflammatory effects, and anti-atherogenic effects. It also protects body fat during osteolysis [19], and inhibits bone formation by interfering with the stimulation of the receptor activator of nuclear factor-kappaB ligand (RANKL) and the formation of Osteoprotegerin (OPG) [20]. An acute inflammatory molecule, C-reactive protein (CRP), which is formed by IL-6 and tumor necrosis factor-α (TNF-α) in the liver, is a sensitive marker that predicts the risk of developing CVD [21-23]. In association with bone metabolism, hs-CRP reportedly has a negative correlation with BMD [24] but shows a positive correlation with bone turnover rate [25]. A recent study has shown that there is a positive correlation between hs-CRP and biochemical bone markers, which implies a higher possibility of an asymptomatic inflammatory response to mediate the increased bone turnover [26].
To date, a limited number of studies have been conducted to examine the relationship between BMD, various types of inflammation biomarkers, and antioxidant vitamins in patients with metabolic syndrome. In addition, there are few studies of gender-specific principal risk factors that affect bone formation. Therefore, we examined the gender-specific relationship of inflammatory markers, antioxidants, and BMD in patients with metabolic syndrome.
Subjects and Methods
Subjects
The subjects of this study were a subset of all patients who visited the Health Promotion Center for a regular medical check-up at the University Hospital in Kyounggi province between 21 September 2009 and 5 February 2010. Clinical diagnosis of metabolic syndrome was based on the criteria outlined in a joint interim statement from the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute [27]. Metabolic syndrome was diagnosed in cases with: serum triglyceride > 150 mg/dl, serum HDL-cholesterol < 40 mg/dl in men and < 50 mg/dl in women, fasting blood sugar > 100 mg/dl or taking anti-diabetic drugs, systolic blood pressure > 130 mmHg or diastolic pressure > 85 mmHg or taking anti-hypertensive drugs and an abdominal circumference > 90 cm in men and > 80 cm in women. Patients who exhibited more than three of the five factors associated with metabolic syndrome and whose BMD was measured were selected as subjects for the study. Patients with diabetes complications, cardiovascular or cerebrovascular disease, or chronic liver disease were excluded. The study was approved by the Institutional Review Board (IRB) of the Bundang Seoul National University Hospital. A total of 67 men and 46 postmenopausal women were enrolled in the study and submitted written informed consent.
Physical examination
The height and weight of the subjects were measured using an automatic recorder, DS-102 (Jenix Co, Korea). Body mass index (BMI) was calculated as weight divided by height squared. Lean body mass and body fat percentage were measured with a bioelectrical impedance analysis, X-SCAN Plus II (Jawon medical, Korea); subjects wore minimal clothing, no shoes, socks, or metal substances.
Abdominal circumference was measured at the mid-point between the iliac crest and the inferior border of the ribs in a standing position. Blood pressure was measured using an automatic blood pressure monitor, Sysmex XE-2100(Sysmex, Japan) from a sitting position.
Measurement of BMD
BMD was assessed with a Dual Energy X-ray Absorptiometry, measured at the lumbar spine from L1 to L4. BMD of the femoral neck of the femur was measured with Lunar Prodigy (General Electric system, USA). BMD was expressed in g/cm2. At each site, patients with a t-score ≧ -1 were put into the normal group, and those with a t-score < -1 were put into the osteopenia group, as defined by the World Health Organization [28].
Biochemical assay
Venous blood was collected from test subjects after an overnight fast, and blood was centrifuged at 3,000rpm for 15 minutes. The isolated serum was used to analyze glucose, triglycerides, HDL-cholesterol, LDL-cholesterol, total cholesterol, and gamma glutamyl transferase using BS-220 (Mindray, China). Hs-CRP was analyzed using a Hitachi 7600-110 (Hitachi, Japan), based on latex agglutination immunoassay. Serum Adiponectin was measured using an Enzyme Immuno Assay (EIA) with an Adiponectin ELISA kit (Adipogen, Korea). Serum IL-6 concentrations were also measured using an EIA with an IL-6 kit (Bender Medsystems, Austria). A vitamin E assay was performed based on the Bieri methods [29], for which High Performance Liquid Chromatography (HPLC) was used. In the serum two internal standards and n-hexane were extracted twice for a total volume of 400 µl. Centrifugation was done again at 1,500 rpm for ten minutes, and the supernatant was taken. The isolated supernatant was filtered through a 0.45 µm syringe filter, dried with nitrogen gas, and then dissolved with 50 µl methanol. The column was analyzed with Nova-Pak C18 (Waters, USA), and the mobile phase was analyzed with methanol/H2O (95%/5%). At a UV wavelength of 292 nm, a quantitative analysis was performed at a flow rate of 1.5 ml/min using HPLC (Shimadzu, Japan). An analysis of serum vitamin C was comissioned to Samkwang Medical Labora fories and was performed with HPLC [30]. For this, 200 µl of serum was mixed with 5% metaphosphoric acid and centrifuged to remove the precipitated proteins. A 20 µl aliquot of the centrifuged extract was injected into HPLC.
Statistical analysis
All of the statistical analyses were performed using Statistical Package for Social Science 12.0, and statistical significance was considered to be P < 0.05. Mean differences in antioxidant vitamins and other continuous variables between men and women were analyzed with a t-test. The gender difference distribution of metabolic syndrome indicators and BMD were determined with a chi-square test. To identify the correlation between inflammation biomarkers, BMD, and antioxidant vitamins, partial correlation coefficients were obtained after an age and weight adjustment. An evaluation of normality was performed with a Shapiro-Wilk test, and logarithmic transformations were performed for serum hs-CRP, adiponectin, and IL-6 concentrations due to a positively skewed distribution. In both male and female patients, after adjustments for age and BMI, the means and 95% confidence intervals of log-transformed hs-CRP concentrations were compared by analysis of covariance (ANCOVA) among the subjects' BMD status (normal vs. osteopenia + osteoporosis).
To identify the factors affecting BMD, a multiple regression analysis was performed after adjustment for age. Body compositions (percentage of body fat and lean body mass), antioxidant vitamins (vitamin E and C), and inflammatory markers (hs-CRP, IL-6, adiponectin) were examined using a stepwise analysis, retaining only those variables that were statistically significant.
Results
General characteristics and biochemical assessment
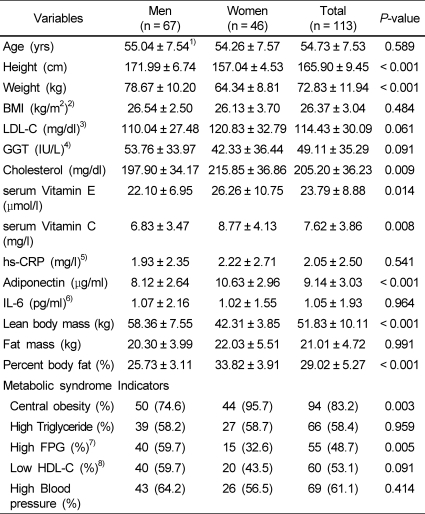
The baseline characteristics of the subjects are summarized in Table 1. There was no significant difference in the distribution of triglycerides, blood pressure, or HDL-cholesterol between men and women. There was no significant difference in the level of serum hs-CRP or IL-6 between men and women. The mean value of adiponectin was significantly higher in women than in men (P < 0.001). Antioxidant vitamin levels, including vitamin E (P < 0.05) and C (P < 0.01), were also higher in women than in men. Lean body mass was significantly higher in men than in women (P < 0.001), and percentage of body fat was significantly higher in women than in men (P < 0.001).
Table 1.
Characteristics of study subjects
1)Mean ± SD
2)BMI: body mass index
3)LDL-C: low-density lipoprotein cholesterol
4)GGT: gamma-glutamyl transferase
5)hs-CRP: high-sensitivity C-reactive protein
6)IL-6: interleukin-6
7)FPG: fasting plasma glucose
8)HDL-C: high-density lipoprotein cholesterol
BMD analysis
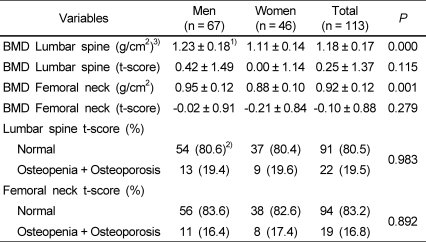
The results for BMD are presented in Table 2. The BMD of the lumbar spine (P < 0.001) and femoral neck (P < 0.001) was higher in men than in women.
Table 2.
Bone mineral density status
1)Mean ± SD
2)N (%)
3)BMD: bone mineral density
Based on the t-score of the lumbar spine and femoral neck, subjects were classified into two groups: the normal BMD group (t-score ≧-1) and the osteopenia group (t-score < -1). Based on the BMD of the lumbar spine, 54 male subjects (80.6%) were included in the normal BMD group, and 13 male subjects (19.4%) were included in the osteopenia group. When considering the BMD of the femoral neck, these numbers were 56 (83.6%) and 11 male subjects (16.4%), respectively. Based on the BMD of the lumbar spine, there were 37 female subjects (80.4%) in the normal group and 9 female subjects (19.6%) in the osteopenia group. When considering the BMD of the femoral neck, these numbers were 38 (82.6%) and 8 female subjects (17.4%), respectively.
Correlation between inflammatory markers, antioxidant vitamins, and BMD
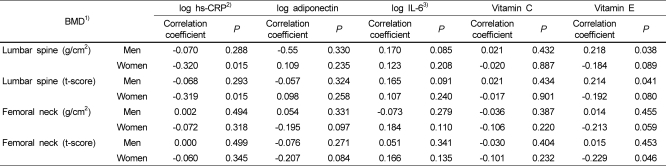
The correlations between BMD and hs-CRP, adiponectin, IL-6, and antioxidant vitamins (vitamins E and C) are presented in Table 3. An analysis following adjustment for age and weight revealed that male subjects had a significantly positive correlation between BMD and serum vitamin E (r = 0.218, P = 0.038) and between the t-score of the lumbar spine and serum vitamin E (r = 0.214, P = 0.041). In women, there was a significantly negative correlation between log-transformed hs-CRP and the BMD of the lumbar spine (r = -0.320, P = 0.015) and between log-transformed hs-CRP and the t-score of the lumbar spine (r = -0.319, P = 0.015). There was a significantly negative correlation between the t-score of the femoral neck and serum vitamin E in women (r = -0.229, P = 0.046).
Table 3.
Correlation of bone mineral density with inflammatory markers and antioxidant vitamins
1)BMD: bone mineral density
2)hs-CRP: high-sensitivity C-reactive protein
3)IL-6: interleukin-6
Comparison of hs-CRP concentrations according to BMD status
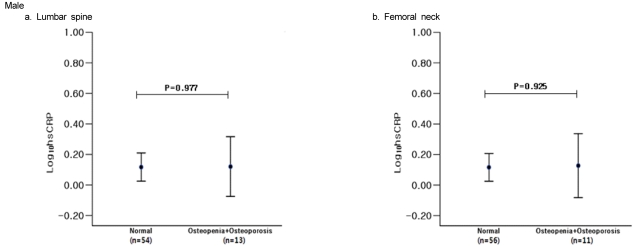
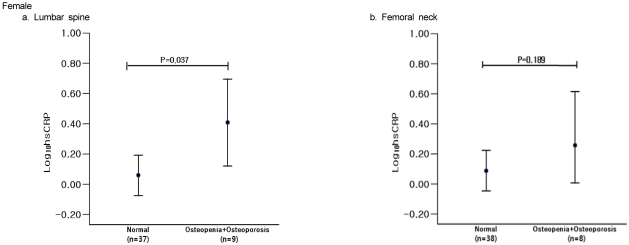
We compared log-transformed serum hs-CRP concentrations with BMD status. There was no significant difference in hs-CRP concentrations related to BMD status in men (Fig. 1). In women, however, hs-CRP levels were significantly higher in the lumbar spine osteoporotic group than in the normal group (F = 4.447, P = 0.037) (Fig. 2).
Fig. 1.
Serum high-sensitivity C-reactive protein (hs-CRP) concentrations among normal and osteopenic male subjects. Serum hs-CRP concentrations were given as estimated mean ± 95% confidence intervals.
Fig. 2.
Serum high-sensitivity C-reactive protein (hs-CRP) concentrations among normal and osteopenic female subjects. Serum hs-CRP concentrations were given as estimated mean ± 95% confidence intervals.
Analysis of factors related to BMD
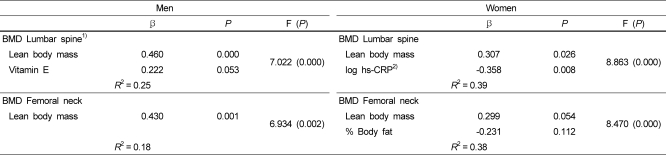
Table 4 shows the effects of factors associated with BMD in patients with metabolic syndrome. Following stepwise regression analyses for BMD of lumbar spine, lean body mass, and vitamin E were independent variables in men, and lean body mass and log-transformed hs-CRP were significant variables in women. Lean body mass was a significant variables in men, and lean body mass and percentage of body fat were significant variables in BMD of women in femoral neck.
Table 4.
Multiple regression analysis for gender-specific bone mineral density
1)BMD: bone mineral density
2)hs-CRP: high-sensitivity C-reactive protein
Discussion
Many studies have reported a close link between metabolic syndrome and CVD [1-3]. Also, recent studies have shown that there is a correlation between metabolic syndrome and BMD [13-15]. Understanding the main factors that affect BMD in patients with metabolic syndrome would contribute to identifying the common risk factors that link CVD and osteoporosis. Gender has been identified as a risk factor associated with CVD [31], so we extrapolated that there might be a gender difference affecting bone formation in metabolic syndrome patients. Results of this study show that hs-CRP level was a negatively associated significant variable predicting lumbar spine BMD in women. There was no significant relationship between BMD and hs-CRP level in men, and IL-6 and adiponectin levels were not associated with BMD in either men or women. Pro-inflammatory cytokines, such as interleukin IL-6 and CRP, have been shown to regulate bone metabolism in healthy women. According to Bae et al. [25], as hs-CRP serum concentration levels increase BMD decreases and bone turnover rate increases. In another population-based study, women with osteopenia had higher serum hs-CRP levels than those with normal BMD. Therefore, it is suggested that subclinical systemic inflammation may be associated with bone turnover rate and bone mass in healthy women [32]. The relationship between pro-inflammatory cytokines and BMD in men is inconclusive. In men with metabolic syndrome, a trend toward reduced BMD with higher CRP levels was identified [33]. According to an epidemiologic study, increased systemic inflammation was a risk factor for lower BMD among premenopausal women, but not for men [34]. IL-6 has been known to promote osteoclast differentiation and is associated with femoral bone loss in healthy postmenopausal women [35]. The relationship between serum adiponectin levels and BMD is controversial [36-37]. In this study, we found that change in serum hs-CRP levels might be a more sensitive marker for predicting lumbar spine BMD change than other inflammatory markers, especially in women patients. The association of hs-CRP levels and BMD at the lumbar spine was higher than that at the femoral neck. The reason for this is not entirely clear; it may be that higher surface-to-volume ratio in the spine than in the femoral neck causes more metabolic activity. Because there not enough definite results about inflammatory markers and the mechanisms associated with BMD, further studies should be conducted.
For femoral neck BMD, percentage of body fat was a significant variable in women. Although the mechanism underlying this relationship is not clear, this finding suggests that body fat may have harmful effects on bone. It has been widely understood that visceral fat is not only used for the storage and mobilization of lipids but is also a remarkable endocrine organ that releases adipokines, which play an important role in bone formation and the pathogenesis of osteoporosis [38-39]. It is feasible that low-grade inflammation derived from visceral adiposity may be associated with bone loss in postmenopausal patients with metabolic syndrome.
This study revealed an opposite relationship between vitamin E levels and BMD in men and women. In a multiple linear regression analysis, vitamin E was a predictor for BMD in men only, which suggests that vitamin E may exert a positive influence on bone mass in patients with metabolic syndrome. Recent evidence suggests that vitamin E, in addition to its antioxidant properties, has a protective effect against bone deformation from inflammatory cytokine synthesis. Vitamin E consumption has been associated with a lowered risk of coronary heart disease and reduced low-density lipoprotein oxidation [9]. Palmieri et al. [10] reported that serum vitamin E concentration was lower in patients with metabolic syndrome than in controls, showing unbalanced serum redox with decreased lipid antioxidant capacity. However, the positive association seen between increased hs-CRP levels and increased vitamin E levels in women may be due the pro-oxidant effects of vitamin E [40-41]. Further research is required to confirm the gender difference in the potential of vitamin E and to understand bone and oxidative stress in patients with metabolic syndrome.
There were several limitations to this study. First, the experimental group consisted of a small, heterogeneous sample of metabolic syndrome patients who visited a health promotion center, which may have resulted in a biased selection. We would like to confirm our findings in a larger number of subjects. Second, this was cross-sectional study that did not clarify whether there was a causal relationship between inflammatory markers, antioxidant vitamins, and BMD. Further studies would be necessary to clarify if there is a causal relationship among these factors. We have shown that incremental serum hs-CRP concentrations are associated with lower BMD, and that hs-CRP is an independent factor of BMD in women but not men with metabolic syndrome. These finding suggest that reducing subclinical systematic inflammation may be an important factor in conserving bone mass, especially in women with metabolic syndrome.
Footnotes
This work was supported in part by a grant from National Research Foundation of Korea (2010-0015498).
References
- 1.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Ministry of Health and Welfare (MOHW) Korea National Health and Nutrition Examination Survey Report (KNHANES III)-Health Examination. 2005. [Google Scholar]
- 5.Kim JH, Choi SR, Lee JR, Shin JH, Lee SJ, Han MA, Park J, Bae HY, Kim SY. Association of hemoglobin A1c with cardiovascular disease risk factors and metabolic syndrome in nondiabetic adults. Korean Diabetes J. 2008;32:435–444. [Google Scholar]
- 6.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa S, Fujita T, shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations. Diabetes. 2003;52:2346–2352. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri VO, Grattagliano I, Portincasa P, Palasciano G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006;136:3022–3326. doi: 10.1093/jn/136.12.3022. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 12.Cui LH, Shin MH, Chung EK, Lee YH, Kweon SS, Park KS, Choi JS. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16:1975–1981. doi: 10.1007/s00198-005-1977-2. [DOI] [PubMed] [Google Scholar]
- 13.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 14.Kang YH, Kam S. Association of bone mineral density with the metabolic syndrome. J Radiol Sci Technol. 2008;31:259–266. [Google Scholar]
- 15.Kim HY, Choe JW, Kim HK, Bae SJ, Kim BJ, Lee SH, Koh JM, Han KO, Park HM, Kim GS. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif Tissue Int. 2010;86:350–358. doi: 10.1007/s00223-010-9347-2. [DOI] [PubMed] [Google Scholar]
- 16.Oh KW. Diabetes and osteoporosis. Korean J Bone Metab. 2008;15:91–98. [Google Scholar]
- 17.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorocéanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol. 2004;183:203–216. doi: 10.1677/joe.1.05723. [DOI] [PubMed] [Google Scholar]
- 19.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi N, Kukita T, Li YJ, Kamio N, Fukumoto S, Nonaka K, Ninomiya Y, Hanazawa S, Yamashita Y. Adiponectin inhibits induction of TNF-alpha/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett. 2008;582:451–456. doi: 10.1016/j.febslet.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41:97–101. doi: 10.2337/diab.41.2.s97. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 24.Whitcomb BW, Bruder JM, Bauer RL, Mahaney MC, Tracy RP, Kammerer CM, Mitchell BD. C-reactive protein levels are associated with decreased bone mineral density in Mexican Americans. J Bone Miner Res. 2004;19:S289. [Google Scholar]
- 25.Bae SJ, Son HY, Pyun DK, Nah SS, Koh JM, Kim GS. Higher circulating hs-CRP levels are associated with lower bone mineral density in healthy premenopausal and postmenopausal women: evidence for link between systemic inflammation and osteoporosis. Korean J Bone Metab. 2004;11:147–157. [Google Scholar]
- 26.Kim BJ, Kim WG, Jung CH, Byun SW, Koh JM, Kim GS. Relationship between bone turnover rate and a systemic inflammatory marker in Korean women. Korean J Bone Metab. 2006;13:129–138. [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994. [PubMed] [Google Scholar]
- 29.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of α-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 30.Kutnink MA, Hawkes WC, Schaus EE, Omaye ST. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987;166:424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- 31.Nishida M, Moriyama T, Ishii K, Takashima S, Yoshizaki K, Sugita Y, Yamauchi-Takihara K. Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta. 2007;384:99–104. doi: 10.1016/j.cca.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16:1263–1271. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 33.Kinjo M, Setoguchi S, Solomon DH. Bone Mineral Density in adults with the Metabolic Syndrome: analysis in a population-based U.S. Sample. J Clin Endocrinol Metab. 2007;92:4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- 34.McLean RR, Zhang X, Benjamin EJ, Cupples LA, Kiel DP, Hannan MT. No link between C-Reactive Protein (CRP) and Bone Mineral Density (BMD) in men, but menopause status modifies the relation in women: The Framingham Osteoporosis Study. Arthritis Rheum. 2009;60(Suppl 10):1131. [Google Scholar]
- 35.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86:2032–2042. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 36.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 37.Zoico E, Zamboni M, Di Francesco V, Mazzali G, Fantin F, De Pergola G, Zivelonghi A, Adami S, Bosello O. Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest. 2008;31:297–302. doi: 10.1007/BF03346361. [DOI] [PubMed] [Google Scholar]
- 38.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim CJ, Rhee EJ, Kim HM, Kim HS, Lee EA, Kim YS, Choi JH, Jo SK, Jung CH, Won JC, Park CY, Lee WY, Oh KW, Park SW, Kim SW. Relationship between body composition and metabolic bone disease in Korean male adults. Korean J Bone Metab. 2008;15:25–32. [Google Scholar]
- 40.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 41.Lim Y, Traber MG. Alpha-tocopherol transfer protein: insights from alpha-tocopherol transfer protein knockout mice. Nutr Res Pract. 2007;1:247–253. doi: 10.4162/nrp.2007.1.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]