Abstract
Degenerative lung diseases such as chronic obstructive pulmonary disease (COPD) are common with huge worldwide morbidity. Anti-inflammatory drug development strategies have proved disappointing and current treatment is aimed at symptomatic relief. Only lung transplantation with all its attendant difficulties offers hope of cure and the outlook for affected patients is bleak. Lung regeneration therapies aim to reverse the structural and functional deficits in COPD either by delivery of exogenous lung cells to replace lost tissue, delivery of exogenous stem cells to induce a local paracrine effect probably through an anti-inflammatory action or by the administration of small molecules to stimulate the endogenous regenerative ability of lung cells. In animal models of emphysema and disrupted alveolar development each of these strategies has shown some success but there are potential tumour-inducing dangers with a cellular approach. Small molecules such as all-trans retinoic acid have been successful in animal models although the mechanism is not completely understood. There are currently two Pharma-sponsored trials in progress concerning patients with COPD, one of a specific retinoic acid receptor gamma agonist and another using mesenchymal stem cells.
LINKED ARTICLES
This article is part of a themed issue on Respiratory Pharmacology. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-1
Keywords: COPD, emphysema, lung regeneration, retinoic acid, novel therapies, MSCs, ES cells
COPD – what is it?
Chronic obstructive pulmonary disease (COPD) is a common syndrome comprising the related diseases of airways (chronic bronchitis) and lung parenchyma (emphysema) often related to cigarette smoking. The worldwide burden of COPD is increasing (Murray and Lopez, 1996) with the market for COPD drugs estimated to be valued at over $9.3 billion in 2007 in just six developed countries (Yasothan and Kar, 2008). Remarkably, despite huge investment from big Pharma, treatment modalities have changed little since the middle of the last century with only lung transplantation offering a hope of cure. Excellent treatments for reversible airway diseases such as the use of corticosteroids in asthma have limited effects in clinical trials in COPD patients suggesting that despite phenotypic similarities (breathlessness and obstructive lung function), COPD is not asthma.
Patients with COPD describe breathlessness: symptoms are related to changes in lung structure including variable contributions from reduction in gas-exchanging surface area, small airway obstruction caused in part by loss of alveolar attachments (Saetta et al., 1985; Hogg, 2004), chronic bronchitis including goblet cell hyperplasia and pulmonary hypertension. These pathologic changes lead to unfavourable lung mechanics, reduced alveolar ventilation and repeated cycles of infection or exacerbation (Hurst et al., 2010). COPD is therefore a structural lung disease.
COPD is largely a disease of older people. Classic epidemiological studies demonstrate a progressive decline in lung function over time, hastened in patients with COPD (Fletcher and Peto, 1977). It is hypothesized that COPD represents accelerated ageing of the lung with progressive decline in measures of lung function such as FEV1 and FVC driven by an increased burden of oxidative stress, failure of DNA repair and telomere shortening (reviewed in Ito and Barnes, 2009). There are compelling similarities between the histopathology of COPD and the ageing lung with loss of matrix proteins, reduction in alveolar number enlargement of alveoli and reduction in total gas-exchanging surface area (Janssens et al., 1999).
Global prevalence studies looking at risk factors for the development of COPD suggest that that while smoking and age are major contributors to the development of other diseases, as yet unknown factors appear to be important (Buist et al., 2007). Genetic susceptibility clearly plays a role and candidate genes in addition to the well-known α-1 anti-trypsin gene (Laurell and Eriksson, 1963) are actively being sought (Ito et al., 2005; Demeo et al., 2006). One further factor might be the adult number of alveoli, which in part determines the gas-exchanging surface area of the lung. There is wide variation in the total number of alveoli corrected for lung volume and height in the normal adult lung (Thurlbeck, 1967). As alveoli are formed largely during a developmentally regulated period in mammals (Massaro and Massaro, 2002) and lost during ageing (Thurlbeck, 1967; Janssens et al., 1999), differences in alveolar gas-exchanging surface area in the adult might therefore be a critical determinant, not only on which patients are likely to develop COPD but at what age they may develop symptoms. This hypothesis highlights the potential importance of early developmental events in the later emergence of respiratory disease (reviewed in Massaro and Massaro, 2004a).
Destruction versus repair in COPD
It has generally been assumed there is little alveolar turnover in the adult lung; once alveoli are destroyed or have failed to develop normally there is little spontaneous effective alveolar repair or regeneration. The factors that drive tissue damage include oxidative stress, matrix metalloproteases (MMPs) and inflammation. In the normal lung these factors are finely balanced against protective proteins such as α-1 anti-trypsin (α-1AT) and tissue inhibitors of metalloprotease (TIMPs). The disturbance of this delicate equilibrium forms the basis of the elastase/anti-elastase hypothesis of the pathogenesis of emphysema whereby destructive airspace enlargement is caused by an overproduction of elastase and MMPs from neutrophils and macrophages (Laurell and Eriksson, 1963; reviewed in Shapiro, 1995). However, little is known about endogenous repair programs that must surely exist to maintain the thin and fragile alveolar epithelium, which is constantly exposed to air and under constant attack both from within and outside. It can therefore be argued that the emphysema component of COPD is not caused solely by injury but rather an imbalance of injury and ongoing alveolar repair.
The existence of rapid alveolar repair mechanisms has been clearly shown in rodents. When adult mice are ovariectomized their lungs have larger alveoli and less alveolar surface area than controls and three weeks of oestrogen treatment resulted in alveolar regeneration (Massaro and Massaro, 2004b). Similarly calorie restricted mice show alveolar loss within 72 h preceded by molecular changes associated with apoptosis in 2 h followed by alveolar regeneration again within 72 h after ad libitum refeeding (Massaro et al., 2004). These data suggest that the distal lung in rodents and possibly in humans (Coxson et al., 2004) has a previously unforeseen capacity for plasticity and structural remodelling under the correct regenerative cues.
How might COPD be treated by regenerative therapy?
Regenerative medicine aims to repair or replace functional tissue lost during damage, ageing or following disrupted development. Airspace destruction distal to the terminal bronchiole defines the emphysema component of COPD (Snider et al., 1985). Therefore restoration of functional alveoli and alveolar ducts is a central goal for regenerative therapies in COPD (Massaro and Massaro, 2006). As the distal lung has an apparently remarkable capacity for remodelling as described earlier, discovering these regenerative cues must be a central feature of COPD therapies. In addition the recent exciting advances in embryonic stem (ES) cells and adult derived stem cells and the ability to differentiate them into chosen cell types has raised the possibility that such cells can be used to replace damaged or missing lung tissue. These are the two broad strategies that are being used to reverse structural disease: the use of extrinsic cell therapy (the following sections Exogenous stem cells, Paracrine effects of stem cells and Ex vivo tissue engineering) and the use of small molecules to induce lung regeneration through actions on intrinsic populations of cells – an intrinsic cell therapy.
Extrinsic cell therapy
Exogenous stem cells
Stem cells have characteristics of self-renewal and the ability to generate a variety of specialized differentiated daughter cells. ES cells taken from the inner cell mass of the blastocyst can generate all three germ layers of the embryo namely endoderm (the gut lining and associated structures including lung) ectoderm (skin and nervous system) and mesoderm (skeletal muscle, blood and cardiovascular system). Once isolated, ES cells can be maintained indefinitely in an undifferentiated pluripotent state and potentially differentiated into any desired cell type. Both mouse and human ES cells have been shown to be capable of differentiating into type II pneumocytes, which express surfactant proteins and ultrastructurally generate lamellar bodies (Denham et al., 2006; Samadikuchaksaraei et al., 2006; van Vranken et al., 2007; Van Haute et al., 2009). To use these cells in transplantation would require the generation of essentially pure populations, and this has now been achieved using either combinations of the embryonic signalling molecules Wnt3a, activin and FGF2 (Roszell et al., 2009), or transfection and selection methods (Wang et al., 2007).
The primary reason for producing pure populations of differentiated cells for regeneration therapy of lung tissue is that if pluripotent ES cells are used with the intention of allowing them to differentiate in situ using local signals then there is a high risk of generating teratomas. The same applies if the differentiated population is not pure and there are contaminating undifferentiated ES cells. Nevertheless the proof of principle that these purified type II cells can engraft into the lung has been shown following their intratracheal instillation into embryonic day 18 embryos (Roszell et al., 2009), and remarkably, when derived from human ES cells they can engraft into the adult mouse lung and greatly reduce the extent of injury after bleomycin treatment and recover lung tidal volumes (Wang et al., 2010). The utility of this approach as a way of repairing the damaged lung was established in adult rats by intratracheal instillation of type II cells taken from the lung (not from ES cells) whereupon they engrafted and repaired bleomycin induced fibrosis (Serrano-Mollar et al., 2007).
Despite this major progress there are two remaining hurdles to the transition of this technology to the clinic. The first is an ethical one concerning the supply of human embryos from which to obtain the stem cells, an especially controversial topic in the United States and the second one is rejection problems as the differentiated type II cells would not be autologous.
Both of these issues can be overcome by the use of autologous adult stem cells. Mesenchymal stem cells (MSCs) are an adult bone marrow-derived stem cell population. They have a potential therapeutic advantage in that they can be harvested from a patient's own bone-marrow circumventing immunological rejection. MSCs express low levels of HLA class I and do not express HLA class II or co-stimulatory antigens and are therefore thought to be non-immunogenic allowing autologous or allogenic transplantation without immunosuppression. When MSCs are injected intravenously they appear to home to areas of damaged tissue in increased numbers and have been postulated to have a role in tissue repair in numerous organs including the lung (Kotton et al., 2001; Krause et al., 2001; Theise et al., 2002; Ortiz et al., 2003). The damaged lung produces factors that cause MSCs to proliferate and migrate to the sites of injury where they can differentiate into many of the cell types of the lung including fibroblasts endothelia type I and type II cells and myofibroblasts and repair bleomycin or elastase injury (Ishizawa et al., 2004; Rojas et al., 2005; Aliotta et al., 2006). Another technique using surgically joined parabiotic mice demonstrated that fibroblasts and type I cells appeared to derive from circulating stem/progenitor cells and contributed to lung repair following radiation/elastase injury (Abe et al., 2004). Similar results have been obtained by injecting a novel source of stem cells amniotic fluid stem cells into the tail vein and observing their differentiation into distal type II pneumocytes or proximal Clara cells after injury (Carraro et al., 2008). In an attempt to understand the mechanism of action of these effects it became apparent that the rates of engraftment using exogenous MSCs are too low (1–2%) to provide cellular replacement for damaged tissue and that even this low estimate may not be due to engraftment of the MSCs but instead due to fusion of the injected cells with endogenous lung cells (Chang et al., 2005; Kotton et al., 2005). A paracrine effect of MSCs is now suggested as the mechanism of action (see section Paracrine effects of exogenous stem cells), but whatever the case may be two cautionary notes are important. Firstly, if MSCs can become myofibroblasts, rather than alleviate conditions such as fibrosis, they can actually contribute to its worsening (Epperly et al., 2003), and secondly, sarcomas have been shown to develop within the lung after MSC administration (Tolar et al., 2006; Aguilar et al., 2007).
It therefore seems that direct administration of type II cells to the lung rather than intravenous administration of MSCs will be of greater therapeutic potential. If so then how can the immune rejection problem be overcome? Remarkably stem cells can now be generated from adult somatic cells allowing the possibility of taking the patients own skin fibroblasts returning them to pluripotency and turning them into lung cells. These induced pluripotent (iPS) cells are generated by retroviral transfection of just four factors Oct3/4 Sox-2 c-Myc and Klf-4 are functionally equivalent to ES cells and can be taken from a patient's own somatic cells (Takahashi and Yamanaka, 2006). Modifications of iPS technology using synthetic modified mRNA rather than DNA retroviruses to deliver the factors (Warren et al., 2010) or using small molecule cocktails including HDAC inhibitors (Li et al., 2011) may overcome potential problems associated with integrating DNA viruses.
Paracrine effects of exogenous stem cells
Recently interest has shifted to exploring an immunomodulatory role for MSCs in an effort to explain why positive effects of MSCs are seen when there is such as low rate of cell engraftment. MSCs release a variety of mediators in response to the specific microenvironment and can have inhibitory effects on specific immune cells and inflammatory cytokines (Nauta and Fibbe, 2007; Sueblinvong and Weiss, 2010) thus suggesting a non-engraftment mechanism of action. Indeed MSCs administered directly to the lungs induced the down-regulation of pro-inflammatory cytokines and up-regulated the anti-inflammatory cytokine IL-10, an effect unrelated to cell engraftment (Gupta et al., 2007). In elastase treated rabbits MSCs administered to the lungs improved lung function and rescued alveolar dimensions (Yuhgetsu et al., 2006). The homing properties of MSCs have also been exploited in experiments where MSCs are used as vectors to deliver gene therapy. Haematopoetic stem cells transduced with a lentiviral vector encoding the α1 anti-trypsin gene were transplanted into irradiated mice whilst the authors successfully demonstrated a sustained increase in AAT for 31 weeks in vivo; however, this did not alter disease progression (Wilson et al., 2008).
Clinical reports and trials of MSCs have been described in immune-mediated diseases such as Crohn's disease and graft versus host disease (Uccelli et al., 2008; Newman et al., 2009). Safety data from a clinical trial of allogeneic MSCs in patients with acute myocardial infarction demonstrated a surprising improvement in FEV1 (Hare et al., 2009). Early data from these studies suggest the use of MSCs is feasible and safe prompting a clinical trial in patients with COPD (Osiris – see Human trials section in the following). Advocates of cell therapy point to decades of clinical experience in bone marrow transplantation, the positive position of both funding agencies and regulatory authorities and the fact the respiratory medicine has lagged behind specialties such as cardiology and haematology in trials of cell therapy. Critics of this approach highlight the lack of data demonstrating that MSCs can reverse lung disease.
Ex vivo tissue engineering
A major hurdle for utilization of exogenous cells toward lung regeneration is the lack of scaffold on to which cells might be seeded for implantation. Tissue engineering and bioprocessing success in other organs such as skin has not been matched in the lung, which in part probably reflects the architectural complexity of the organ. The recent report of a localized airway defect treated using a bioengineered airway segment generated with recipient epithelial cells and MSC-derived chondrocytes to seed denuded donor trachea demonstrates the potential of this approach (Macchiarini et al., 2008). Longer term follow-up data of this case will be eagerly awaited. More dramatic experiments in rats using stem cell populations to seed denuded explanted lung transplanted into a recipient demonstrates the engineered lung is capable of limited gas exchange (Petersen et al., 2010).
Intrinsic cell therapy
Endogenous stem cells
An alternative approach to the complications of providing extrinsic cells namely low engraftment levels or rejection is to harness the regenerative potential of existing lung cells. Tissue specific stem cell populations have been reported in many organs including the lung. Within the lung the presence of several niches containing stem cell populations that have a broader regenerative potential have been described associated with discrete regions including basal cells of the trachea and proximal airways submucosal gland ducts neuroepithelial bodies of the bronchiolar epithelium and cells at the bronchioalveolar duct junction [(BADJs or bronchioalveolar stem cells (BASCs)] (Reynolds et al., 2000; Giangreco et al., 2002; Kim et al., 2005) (Figure 1). Each of these stem cell populations can respond to both acute and chronic damage and regenerate their own niches and damaged airway epithelium – the submucosal duct cells; a relatively undifferentiated Clara cell known as a Clara variant, which seem to congregate adjacent to the neuroepithelial bodies; the basal cells in the proximal airways; and the BASCs in the bronchioles (review Rawlins and Hogan, 2006).
Figure 1.
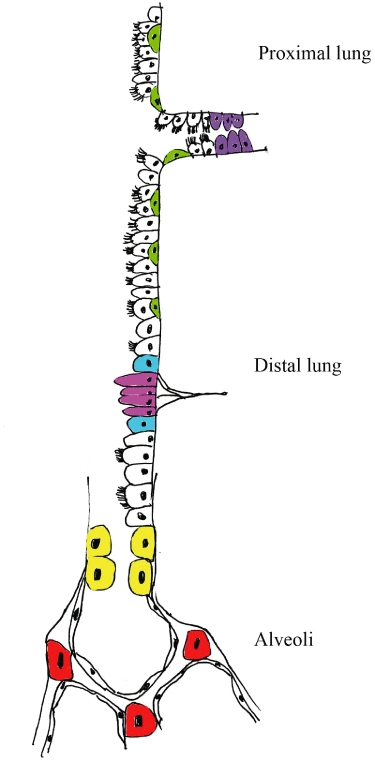
Varying forms of endogenous cells found in the lung, which can proliferate after lung injury including the more classical stem cells (redrawn from Rawlins and Hogan, 2006). Throughout the proximal lung are K-14 +ve basal cells (green), which give rise to Clara cells and ciliated cells after injury. Also in the proximal lung are cells in the ducts of the submucosal glands (purple), which can take up BrdU. In the distal lung there are neuroendocrine cells (pink), which can proliferate but do not give rise to other cell types. Adjacent to neuroendocrine bodies are variant Clara cells (blue), which can give rise to more Clara cells and ciliated cells after injury. At the bronchioalveolar junction are bronchioalveolar stem cell (BASCs) (yellow), which can give rise to ciliated cells, Clara cells and type II cells. In the alveoli are type II cells (red), which give rise to type I cells. Type I cells in the alveoli, and ciliated cells and Clara cells in the proximal and distal lung are uncoloured.
In the alveoli, however, these more proximal stem cell populations play no part in alveolar repair (Rawlins et al., 2009) and it is the replacement of alveoli, which is the critical requirement for COPD therapies. Type II cells are thought to be the progenitor cell population whose proliferation leads to daughter type II cells and differentiation into type I cells (Figure 1) although lineage tracing studies of this important concept have not yet been performed. Assuming this is the case then to develop regenerative treatments for COPD it will be crucial to concentrate on understanding the endogenous signals that control cell proliferation differentiation and replacement of these type II cells (see next section).
Perhaps it will be possible to induce tissue regeneration without utilizing specific stem cell populations. A striking example of cellular reprogramming in an adult foregut-derived organ is illustrated in the pancreas. In adult mice the use of key developmental molecules Ngn3 Pdx1 and Mafa has been used to turn exocrine pancreas into endocrine pancreas, that is, cells have been transformed from one cell type into another in a predictable and controlled manner without reversion to a pluripotent stem cell state (Zhou et al., 2008).
Endogenous signalling molecules to induce lung regeneration
This approach has roots in developmental biology using factors important in lung development and maintenance and is exemplified by investigation into the role of vitamin A derivatives (retinoids). Retinoids including the biologically active molecule all-trans-RA (atRA) are essential for correct development of a number of organs including the lung. atRA is generated from vitamin A (retinol) through a series of reactions dependent on retinaldehyde dehydrogenase (RALDH1-3) enzymes. The expression of these enzymes correlates with atRA activity in vivo. atRA acts via nuclear retinoic acid receptors (RARs), which are members of the glucocorticoid/thyroid hormone receptor superfamily. There are three RARs (αβ and γ) each of which has multiple isoforms. RARs form heterodimers with retinoid X receptors (RXRs) that bind atRA to form a ligand-activated transcription factor complex that regulates downstream gene transcription. atRA and retinol are bound within the cell complexed to cellular retinol binding proteins (CRBP1 and 2) and cellular retinoic acid binding proteins (CRABP1 and 2). Active atRA is oxidized to polar metabolites such as 4-oxo-RA through the CYP26 class of cytochrome P450 enzymes. Precise intracellular levels of atRA are regulated by a balance between synthesizing and degrading enzymes (Duester, 1999). The lung is second only to the liver as the largest store of retinoids in the body and retinoids are stored as retinyl esters in lipid-laden fibroblasts (Okabe et al., 1984) that are abundant in the alveolar wall often in close approximation with type II pneumocytes. Lipid-laden fibroblasts generate biologically active atRA that can regulate gene transcription in pulmonary microvascular endothelial cells (Dirami et al., 2004) and atRA regulates elastin, an essential structural component of lung matrix in perinatal fibroblasts (McGowan et al., 1997). Levels of retinoid synthesizing enzymes RARs and retinoid-binding proteins demonstrate dynamic patterns of regulation in whole lung during alveologenesis in the in rat (McGowan et al., 1995) and mouse (Hind et al., 2002a,b;). In mice mutant for RAR genes alveolar formation is disrupted: RARγ functions as a positive regulator of alveologenesis (McGowan et al., 2000) whereas RARβ is a negative regulator of alveologenesis (Massaro et al., 2000). atRA supplementation during alveolar septation increases the number of alveoli but not total surface area in normal rats and prevents the reduction in both number of alveoli and low surface area corrected for body mass in rats treated with dexamethasone during septation (Massaro and Massaro, 1996). These data provided the first experimental evidence to suggest that pharmacological regenerative therapy might be a potential approach for human diseases characterized by too few alveoli and reduced surface area such as emphysema.
Do retinoids affect lung development in man? It appears that the answer is yes. Mutations in the cell surface receptor for retinol STRA6 have been identified in a screen of children born with anophthalmia (Golzio et al., 2007; Pasutto et al., 2007). Interestingly these infants also had structural lung defects and a failure of normal alveologenesis. Remarkably, vitamin A supplementation during pregnancy in women in areas of endemic dietary retinoid deficiency increases lung function in their offspring (Checkley et al., 2010). This suggests conservation of retinoid signalling between mouse and human lung development and demonstrates gas exchanging surface area can be manipulated by retinoids in man.
It is therefore conceivable that the re-awakening of the retinoid signalling pathway used in alveolar development would induce regeneration of alveoli in the damaged lung. This is indeed the case as the administration of atRA to elastase-induced emphysema in adult rats restored alveoli and reversed the pathologic features of the disease (Massaro and Massaro, 1997). This phenomenon also occurs in several other models of airspace enlargement, for example the dexamethasone treated mouse, where mean chord length and lung surface area were recovered after atRA administration (Hind and Maden, 2004; Stinchcombe and Maden, 2008). Using retinoic acid receptor agonists it was shown that the effect of atRA can be replicated by either a RARα or a RARγ agonist (Maden, 2006) paving the way for the current clinical trial of one of these compounds (see the following). These positive effects of atRA have now been replicated several times in elastase or dexamethasone models of lung damage (Belloni et al., 2000; Massaro and Massaro, 2000; Tepper et al., 2000; Ishizawa et al., 2004; Garber et al., 2006; Perl & Gale, 2009); in the tight skin mouse mutant (Massaro and Massaro, 2000); in pneumonectomized lungs (Kaza et al., 2001) and it protects from 02 induced damage (Veness-Meehan et al., 2000, 2002; Ozer et al., 2005).
Oestrogens have also been demonstrated to have a regulatory role in alveolar formation. Ovariectomy in immature female rats reduces alveolar formation and this can be prevented by exogenous oestrogens. In adult mice ovariectomy induces loss of total number of alveoli and surface area within 3 weeks. This loss of alveoli can be reversed by oestrogen replacement (Massaro and Massaro, 2004b). These data suggest that oestrogens have a role in the alveolar maintenance program in adult mice and can induce regeneration of alveoli in female mice. In humans there is accelerated loss of lung function together with other features of ageing in women in both never smokers and smokers following the menopause. Hormone replacement using oestradiol slows the rate of decline in FEV1 in females with COPD (Carlson et al., 2001).
Adrenomedulin (AM) is a vasoactive regulatory peptide originally isolated from human phaeochromocytoma. It is found in many tissues including the lung and is synthesized by endothelial and smooth muscle cells of the systemic and pulmonary circulation. AM acts through calcitonin receptor-like receptor (CRLR). AM promotes vascular regeneration in vivo in models such as hindlimb ischaemia in rabbits (Tokunaga et al., 2004). AM has been located by in situ hybridization in airway basal cells and type II cells of the lung (Martinez et al., 1997). AM and CRLR mRNA peak during alveologenesis in mice; AM antagonists arrest lung vascular and alveolar development and exogenous AM attenuates developmental arrest in rats with oxygen-induced BPD (Vadivel et al., 2010). AM improves morphometry and lung function in adult rats with elastase-induced emphysema (Murakami et al., 2005) and has been used clinically for the treatment of pulmonary hypertension (Nagaya et al., 2000; reviewed in O'Callaghan and Gaine, 2007).
Hepatocyte growth factor (HGF) is a pleotropic growth factor that acts through its receptor c-Met. Initially isolated as a mitogen from hepatocyes in vitro (Nakamura et al., 1984) it has been shown to be a potent mitogen for type II cells and endothelial cells both in vitro and in vivo (Mason et al., 1994; Panos et al., 1996). HGF increases the Sca-1+/ Flk-1+ population of cells in mice and when transfected into rats with elastase-induced emphysema, induces proliferation of bone marrow (BM)-derived and resident endothelial-derived cells and an increase in radial alveolar counts suggestive of alveolar regeneration (Shigemura et al., 2005). Intranasal HGF has been demonstrated to reverse elastase-induced emphysema in mice (Hegab et al., 2008). There is no published data for the use of exogenous HGF in patients with COPD.
Granulocyte colony-stimulating factor (GCSF) induces angiogenesis in cardiac brain and limb ischaemia models by mobilization of BM-derived stem cell populations. GCSF has been reported to improve morphometric parameters of emphysema in a mouse model the same degree as that achieved with atRA alone. However, when used together an additive effect was described. These data raise the possibility that BM-derived cells might be important in the atRA response a factor that might be limited in an ageing population (Ishizawa et al., 2004). However GCSF had no effect in the Dex model of alveolar insufficiency (Maden, 2006).
Fibroblast growth factor-7 (FGF-7 or KGF) is an important signalling molecule in alveologenesis and has been investigated in animal models of emphysema. In elastase-treated rats FGF7 prevented elastase-induced emphysema but did not reverse morphometric parameters in established disease (Plantier et al., 2007).
The 3-hydroxy-3 methyl glutaryl coenzyme A reductase inhibitor simvastatin has been demonstrated to reverse emphysema in an adult elastase-induced emphysema in the mouse with reduction in Lm and increase in proliferation of lung cells (Takahashi et al., 2008). Statins have also been reported to have roles in the protection of vascular injury (Indolfi et al., 2000).
Human trials to induce lung regeneration
BM-MSCs
As discussed earlier potential use of bone marrow derived-MSCs is under investigation as a potential therapy for COPD. Despite uncertainty regarding potential mechanisms of action, a double blinded placebo controlled trial of Prochymal™, an allogenic adult bone marrow derived MSC preparation is underway in patients with moderate-severe COPD. The 6 month interim safety data report on 62 patients, suggests that four doses of the stem cell infusion were safe and had some effect on systemic inflammation, but as yet no effect on pulmonary function (Osiris Therapeutics Reports interim data for COPD stem cell study 2009, http://osir.client.shareholder.com/releasedetail.cfm?ReleaseID=391580). The trial has a 2 year observation period and results are awaited.
RA and RAR specific agonists
The first retinoid study in patients with emphysema was a 3 month placebo controlled crosover study of 50 mg·m−2 oral atRA in 20 patients with severe emphysema (Mao et al., 2002). The patients in this pilot study were largely men (16 vs. 4). This dose of atRA was well tolerated with only minor side effects of rash, transaminitis, transient headache and dyslipidaemia. Outcome measures including computed tomography (CT) imaging lung function and quality of life scores were unchanged; plasma levels of atRA decreased significantly over time in 35% of patients. MMP-9 levels in patients receiving atRA declined by 45% with no effect on TIMP-1 levels. The MMP-9/TIMP-1 molar ratio declined suggesting that atRA alters the protease/antiprotease balance in patients with emphysema (Mao et al., 2003). A larger randomized multi-centre feasibility study of atRA was undertaken in patients with moderately-severe emphysema (Roth et al., 2006). In this trial, again a crossover designed study, patients received either atRA low dose (1 mg·kg−1) atRA high dose (2 mg·kg−1) 13-cis RA (1 mg·kg−1) or placebo for a six month period followed by a 3 month crossover period. Side effects were common but generally mild and self-limiting. There were no changes in the outcome measures of CT densitometry, imaging or quality of life questionnaires at 6 months. However, the investigators found a delayed improvement in gas-transfer (DLCO) measurements that correlated with plasma drug levels in those patients receiving the higher dose of atRA (2 mg·kg−1). In addition, five of the 25 patients in the higher dose atRA group had delayed improvements in CT densitometry scores that correlated with plasma drug levels.
These two studies suggest that oral retinoids are tolerated in patients with emphysema but mild, self-limiting side effects are common. Of the four doses investigated only atRA at 50 mg·m−2 and atRA at 2 mg·kg−1 appeared to have biological effect in patients with emphysema. Importantly oral atRA dosing results in significant enzyme induction and changes in drug levels. The crossover design of the studies limits longer term follow-up of these patients. A report of a single patient receiving an off-license liposomal atRA preparation delivered through a nebulizer at a dose of 3 mg has been reported (Frankenberger et al., 2009). The preparation was tolerated with no immediate side effects. A reduction in urinary desmosine a marker of elastin breakdown was demonstrated suggesting possible biological effects on ongoing matrix destruction.
The development of an RAR specific agonist R667 or Palovoratene (Roche Holding AG) is under investigation in patients with α-1AT deficiency and cigarette smoke-related emphysema as an oral preparation in 1 and 2 year studies (Stolk et al., 2010). Specific RAR agonists do not induce their own metabolism in the same way as atRA and pharmacokinetic studies suggest drug levels are dose-proportional. Incomplete data has been published in abstract only and results of clinical trials are awaited (reviewed in Hind and Stinchcombe, 2009).
Conclusions
The emphysema component of COPD results in destruction of alveoli distal to the terminal bronchiole and so any regenerative therapies must result in the development of new alveoli. While this hope may sound a difficult and perhaps an unattainable goal it is important to bear in mind that the adult alveolus is much more dynamic structure than what is commonly envisaged – in rodents and perhaps in humans too, alveoli can be lost and readily reformed under certain conditions. The discovery of regenerative therapies is concentrated on two broad strategies – one, adding differentiated stem cells to replace the missing tissue, and two, inducing the endogenous stem cells to proliferate and differentiate into the missing tissue in situ. In the former strategy, ES cells differentiated into type II pneumocytes or using type II cells themselves have been shown to engraft after intratracheal administration and to some degree, repair fibrosis in animal studies. But this approach may have clinical problems either with rejection, the appearance of teratomas from undifferentiated ES cells or ethical issues related to the derivation of the ES cells. Adult MSCs from bone marrow would circumvent all of these problems but they seem to engraft only in very low numbers when applied intravenously, although in animal models lung recovery from injury has been seen. Their positive effect may not be related to actual cellular engraftment but the fact that they can have inhibitory influences on immune cells and inflammatory cytokines. However, problems with this approach include the appearance of sarcomas in the lung and the induction of fibrosis, which may worsen any disease. In the future it may be possible to generate a patient's own IPS cells from skin fibroblasts and differentiate them into type II cells for intratracheal transplantation and perhaps seed these onto engineered scaffolds to improve the structural basis for engraftment, which is an often ignored aspect of tissue regeneration in the lung. One current human trial using this strategy uses bone marrow derived MSCs for patients with COPD.
The second strategy focuses on understanding the signals involved in regulating endogenous stem cells, of which there are several types in the lung. The type II cell is the one involved in alveolar regeneration and several molecules have been shown to induce some degree of regeneration in animal studies including retinoids, oestrogen, adrenomedullin, HGF and statins. The only other current human trial for patients with COPD concerns the use of one of these compounds, a retinoic acid receptor gamma agonist, and the result of this and the MSC trial are eagerly awaited.
Glossary
Abbreviations
- α-1AT
alpha-one antitrypsin
- AM
adrenomedulin
- atRA
all-trans-retinoic acid
- BADJ
bronchioalveolar duct junction
- BASC
bronchioalveolar stem cell
- COPD
chronic obstructive pulmonary disease
- CRABP
cellular retinoic acid binding protein
- CRBP
cellular retinol binding protein
- CRLR
calcitonin receptor like receptor
- ES
embryonic stem (cell)
- FEV1
forced expiratory volume in 1 second
- FGF7
fibroblast growth factor 7
- FVC
forced vital capacity
- GCSF
granulocyte colony stimulating factor
- HGF
hepatocyte growth factor
- MMP
matrix metalloprotease
- MSC
mesenchymal stem cell
- RALDH
retinaldehyde dehydrogenase enzyme
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- Stra6
stimulated by retinoic acid 6
- TIMP
tissue inhibitor of metalloprotease
Conflict of interest
None of the authors have a financial interest in work from this paper.
Supporting Information
Teaching Materials; Fig 1 as PowerPoint slide.
References
- Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, et al. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, et al. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- Aliotta JM, Keaney P, Passero M, Dooner MS, Greer D, Demers D, et al. Bone marrow production of lung cells: the impact of G-CSF cardiotoxin graded doses of irradiation and subpopulation phenotype. Exp Hematol. 2006;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 2000;117:6. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Cushman M, Enright PL, Cauley JA, Newman AB. Hormone replacement therapy is associated with higher FEV1 in elderly women. Am J Respir Crit Care Med. 2001;163:423–428. doi: 10.1164/ajrccm.163.2.2003040. [DOI] [PubMed] [Google Scholar]
- Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, West KP, Wise RA, Baldwin MR, Wu L, LeClerq SC. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362:1784–1794. doi: 10.1056/NEJMoa0907441. [DOI] [PubMed] [Google Scholar]
- Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med. 2004;170:748–752. doi: 10.1164/rccm.200405-651OC. [DOI] [PubMed] [Google Scholar]
- Demeo DL, Mariani TJ, Lange C, Srisuma S, Litonjua AA, Celedon JC, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet. 2006;78:253–264. doi: 10.1086/499828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham MT, Cole J, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1210–L1215. doi: 10.1152/ajplung.00427.2005. [DOI] [PubMed] [Google Scholar]
- Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D, et al. Lung retinol storing cells synthesize and secrete retinoic acid an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L249–L256. doi: 10.1152/ajplung.00140.2003. [DOI] [PubMed] [Google Scholar]
- Duester G. Function of alcohol dehydrogenase and aldehyde dehydrogenase gene families in retinoid signaling. Adv Exp Med Biol. 1999;463:311–319. doi: 10.1007/978-1-4615-4735-8_38. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberger M, Heimbeck I, Möller W, Mamidi S, Kassner G, Pukelsheim K, et al. Inhaled all-trans retinoic acid in an individual with severe emphysema. Eur Respir J. 2009;34:1487–1489. doi: 10.1183/09031936.00105309. [DOI] [PubMed] [Google Scholar]
- Garber S, Zhang JH, Foley JP, Zhao H, Butler SJ, Godinez RI, et al. Hormonal regulation of alveolarization: structure-function correlation. Respir Res. 2006;7:47. doi: 10.1186/1465-9921-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, et al. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized double-blind placebo-controlled dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab AE, Kubo H, Yamaya M, Asada M, He M, Fujino N, et al. Intranasal HGF administration ameliorates the physiologic and morphologic changes in lung emphysema. Mol Ther. 2008;16:1417–1426. doi: 10.1038/mt.2008.137. [DOI] [PubMed] [Google Scholar]
- Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J. 2004;23:20–27. doi: 10.1183/09031936.03.00119103. [DOI] [PubMed] [Google Scholar]
- Hind M, Stinchcombe S. Palovarotene a novel retinoic acid receptor gamma agonist for the treatment of emphysema. Curr Opin Investig Drugs. 2009;10:1243–1250. [PubMed] [Google Scholar]
- Hind M, Corcoran J, Maden M. Alveolar proliferation retinoid synthesizing enzymes and endogenous retinoids in the postnatal mouse lung. Different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol. 2002a;26:67–73. doi: 10.1165/ajrcmb.26.1.4575. [DOI] [PubMed] [Google Scholar]
- Hind M, Corcoran J, Maden M. Temporal/spatial expression of retinoid binding proteins and RAR isoforms in the postnatal lung. Am J Physiol Lung Cell Mol Physiol. 2002b;282:L468–L476. doi: 10.1152/ajplung.00196.2001. [DOI] [PubMed] [Google Scholar]
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A, et al. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol. 2000;35:214–221. doi: 10.1016/s0735-1097(99)00526-4. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- Kaza AK, Kron IL, Kern JA, Long SM, Fiser SM, Nguyen RP, et al. Retinoic acid enhances lung growth after pneumonectomy. Ann Thorac Surg. 2001;71:1645–1650. doi: 10.1016/s0003-4975(01)02478-x. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer ES, Williams MC, et al. Bone marrow derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Mulit-organ multi-lineage engraftment by a single bone marrow derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Laurell CB, Eriksson SE. Electrophoretic alpha globulin pattern os serum in alpha-1 antitrypsin deficiency. Scand J Clin Invest. 1963;15:132–140. [Google Scholar]
- Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, et al. Generation of iPSCs from mouse fibroblasts with a single gene Oct4 and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol. 1997;273(2 Pt 1):L410–L416. doi: 10.1152/ajplung.1997.273.2.L410. [DOI] [PubMed] [Google Scholar]
- McGowan SE, Harvey CS, Jackson SK. Retinoids retinoic acid receptors and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Physiol. 1995;269(4 Pt 1):L463–L472. doi: 10.1152/ajplung.1995.269.4.L463. [DOI] [PubMed] [Google Scholar]
- McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoids have differing efficacies on alveolar regeneration in a dexamethasone-treated mouse. Am J Respir Cell Mol Biol. 2006;35:260–267. doi: 10.1165/rcmb.2006-0029OC. [DOI] [PubMed] [Google Scholar]
- Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- Mao JT, Tashkin DP, Belloni PN, Baileyhealy I, Baratelli F, Roth MD. All-trans retinoic acid modulates the balance of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with emphysema. Chest. 2003;124:1724–1732. doi: 10.1378/chest.124.5.1724. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Miller J, Unsworth EJ, Siegfried JM, Cuttitta F. Adrenomedullin receptor expression in human lung and in pulmonary tumors. J Histochem Cytochem. 1997;45:159–164. doi: 10.1177/002215549704500202. [DOI] [PubMed] [Google Scholar]
- Mason RJ, McCormick-Shannon K, Rubin JS, Nakamura T, Leslie CC. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol. 1994;11:561–567. doi: 10.1165/ajrcmb.11.5.7524567. [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. Invited Review: pulmonary alveoli: formation the call for oxygen and other regulators. Am J Physiol Lung Cell Mol Physiol. 2002;282:L345–L358. doi: 10.1152/ajplung.00374.2001. [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. Critical period for alveologenesis and early determinants of adult pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2004a;287:L715–L717. doi: 10.1152/ajplung.00166.2004. [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation loss and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004b;287:L1154–L1159. doi: 10.1152/ajplung.00228.2004. [DOI] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. Toward therapeutic pulmonary alveolar regeneration in humans. Proc Am Thorac Soc. 2006;3:709–712. doi: 10.1513/pats.200605-127SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro D, Massaro GD, Baras A, Hoffman EP, Clerch LB. Calorie-related rapid onset of alveolar loss regeneration and changes in mouse lung gene expression. Am J Physiol Lung Cell Mol Physiol. 2004;286:L896–L906. doi: 10.1152/ajplung.00333.2003. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270(2 Pt 1):L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, et al. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics. 2000;4:51–57. doi: 10.1152/physiolgenomics.2000.4.1.51. [DOI] [PubMed] [Google Scholar]
- Murakami S, Nagaya N, Itoh T, Iwase T, Fujisato T, Nishioka K, et al. Adrenomedullin regenerates alveoli and vasculature in elastase-induced pulmonary emphysema in mice. Am J Respir Crit Care Med. 2005;172:581–589. doi: 10.1164/rccm.200409-1280OC. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The Global Burden of Disease. A Comprehensive Assesment of Mortality and Disablilty from Diseases Injuries and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: World Health Organisation; 1996. [Google Scholar]
- Nagaya N, Nishikimi T, Uematsu M, Satoh T, Oya H, Kyotani S, et al. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart. 2000;84:653–658. doi: 10.1136/heart.84.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- O'Callaghan DS, Gaine SP. Combination therapy and new types of agents for pulmonary arterial hypertension. Clin Chest Med. 2007;28:169–185. doi: 10.1016/j.ccm.2006.11.011. ix. [DOI] [PubMed] [Google Scholar]
- Okabe T, Yorifuji H, Yamada E, Takaku F. Isolation and characterization of vitamin-A-storing lung cells. Exp Cell Res. 1984;154:10. doi: 10.1016/0014-4827(84)90673-6. [DOI] [PubMed] [Google Scholar]
- Ortiz L, Gambelli AF, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E, Kumral AA, Ozer E, Duman N, Yilmaz O, Ozkal S, et al. Effect of retinoic acid on oxygen-induced lung injury in the newborn rat. Pediatr Pulmonol. 2005;39:35–40. doi: 10.1002/ppul.20131. [DOI] [PubMed] [Google Scholar]
- Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol. 1996;15:574–581. doi: 10.1165/ajrcmb.15.5.8918364. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia congenital heart defects diaphragmatic hernia alveolar capillary dysplasia lung hypoplasia and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol. 2009;297:L299–L308. doi: 10.1152/ajplung.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantier L, Marchand-Adam S, Antico VG, Boyer L, De Coster C, Marchal J, et al. Keratinocyte growth factor protects against elastase-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1230–L1239. doi: 10.1152/ajplung.00460.2006. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszell BM, Mondrinos J, Seaton A, Simons DM, Koutzaki SH, Fong GH, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Connett JE, D'Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, et al. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- Saetta M, Ghezzo H, Kim WD, King M, Angus GE, Wang NS, et al. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis. 1985;132:894–900. doi: 10.1164/arrd.1985.132.4.894. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen SK, Rippon HJ, Polak JM, Bielby RC, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. The pathogenesis of emphysema: the elastase:antielastase hypothesis 30 years later. Proc Assoc Am Physicians. 1995;107:346–352. [PubMed] [Google Scholar]
- Shigemura N, Sawa Y, Mizuno S, Ono M, Ohta M, Nakamura T, et al. Amelioration of pulmonary emphysema by in vivo gene transfection with hepatocyte growth factor in rats. Circulation. 2005;111:1407–1414. doi: 10.1161/01.CIR.0000158433.89103.85. [DOI] [PubMed] [Google Scholar]
- Snider G, Kleinerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema. Report of a National Heart Lung and Blood Institute Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- Stinchcombe SV, Maden M. Retinoic acid-induced alveolar regeneration: critical differences in strain sensitivity. Am J Respir Cell Mol Biol. 2008;38:185–191. doi: 10.1165/rcmb.2007-0252OC. [DOI] [PubMed] [Google Scholar]
- Stolk J, Cooper BG, Stoel B, Rames A, Rutman O, Soliman S, et al. Retinoid treatment of emphysema in patients on the alpha-1 international registry. The REPAIR study: study design methodology and quality control of study assessments. Ther Adv Respir Dis. 2010;4:319–332. doi: 10.1177/1753465810379617. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Trans Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura H, Seki M, Shiraishi Y, Yamamoto M, Furuuchi M, Nakajima T, et al. Reversal of elastase-induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L882–L890. doi: 10.1152/ajplung.00238.2007. [DOI] [PubMed] [Google Scholar]
- Tepper J, Pfeiffer J, Aldrich M, Tumas D, Kern J, Hoffman E, et al. Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest. 2000;117(Suppl.):2. doi: 10.1378/chest.117.5_suppl_1.242s. [DOI] [PubMed] [Google Scholar]
- Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, et al. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis. 1967;95:765–773. doi: 10.1164/arrd.1967.95.5.765. [DOI] [PubMed] [Google Scholar]
- Tokunaga N, Nagaya N, Shirai M, Tanaka E, Ishibashi-Ueda H, Harada-Shiba M, et al. Adrenomedullin gene transfer induces therapeutic angiogenesis in a rabbit model of chronic hind limb ischemia: benefits of a novel nonviral vector gelatin. Circulation. 2004;109:526–531. doi: 10.1161/01.CIR.0000109700.81266.32. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2006;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Vadivel A, Abozaid S, van Haaften T, Sawicka M, Eaton F, Chen M, et al. Adrenomedullin promotes lung angiogenesis alveolar development and repair. Am J Respir Cell Mol Biol. 2010;43:152–160. doi: 10.1165/rcmb.2009-0004OC. [DOI] [PubMed] [Google Scholar]
- Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vranken BE, Rippon HJ, Samadikuchaksaraei A, Trounson AO, Bishop AE. The differentiation of distal lung epithelium from embryonic stem cells. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01g01s2. Chapter 1: Unit 1G 1. [DOI] [PubMed] [Google Scholar]
- Veness-Meehan KA, Bottone FG, Stiles AD. Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr Res. 2000;48:434–444. doi: 10.1203/00006450-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L971–L980. doi: 10.1152/ajplung.00266.2001. [DOI] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren LP, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Kwok LW, Hovav AH, Ohle SJ, Little FF, Fine A, et al. Sustained expression of alpha1-antitrypsin after transplantation of manipulated hematopoietic stem cells. Am J Respir Cell Mol Biol. 2008;39:133–141. doi: 10.1165/rcmb.2007-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothan U, Kar S. Therapies for COPD. Nat Rev Drug Dis. 2008;7:285–286. doi: 10.1038/nrd2620. [DOI] [PubMed] [Google Scholar]
- Yuhgetsu H, Ohno Y, Funaguchi N, Asai T, Sawada M, Takemura G. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp Lung Res. 2006;32:413–426. doi: 10.1080/01902140601047633. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


