Abstract
Cough is among the most common complaints for which patients worldwide seek medical attention. Thus, the evaluation and treatment of cough result in tremendous financial expenditure and consumption of health care resources. Yet, despite the clinical significance of cough, research efforts aimed at improving diagnostic capabilities and developing more effective therapeutic agents have been, to date, disappointing in their limited scope and outcomes. Acute cough due to the common cold represents the most common type of cough. Currently, available medications for the symptomatic management of acute cough are inadequate due to lack of proven efficacy and/or their association with undesirable or intolerable side effects at anti-tussive doses. Subacute cough, often representing a prolonged post-viral response, is typically refractory to standard anti-tussive therapy. Few clinical trials have evaluated therapeutic options for subacute cough. Diagnostic challenges facing the clinician in the management of chronic cough include the determination of whether symptoms of upper airway cough syndrome (formerly, postnasal drip syndrome) or gastro-oesophageal reflux disease are indeed the underlying cause of cough. Chronic, refractory unexplained (formerly, idiopathic) cough must be distinguished from cough that has not been fully evaluated and treated according to current guideline recommendations. Eagerly awaited are new safe and effective anti-tussive agents for use when cough suppression is desired, regardless of underlying aetiology of cough, as well as practical, validated ambulatory cough counters to aid clinical assessment and future research in the field of cough.
LINKED ARTICLES
This article is part of a themed issue on Respiratory Pharmacology. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-1
Keywords: cough, anti-tussive, rhinosinusitis, common cold, bronchitis, asthma, gastro-oesophageal reflux disease (GERD), ACE inhibitors
Introduction
Cough is among the most common symptoms for which patients seek medical attention (Cherry et al., 2008). Hence, the assessment and treatment of cough necessitates enormous financial expenditure. For example, annual amounts spent solely on non-prescription, over-the-counter (OTC) remedies for the self-treatment of cough and cold symptoms are estimated at $3.6 billion in the United States (The Nielsen Company, 2007) and over £100 million in the United Kingdom (Proprietary Association of Great Britain, 2005). The perceived importance of cough as a clinical problem worldwide is evidenced by the fact that recently, no less than five major pulmonary societies have published guidelines on the diagnosis and management of cough: the European Respiratory Society (Morice et al., 2004), the American College of Chest Physicians (Irwin et al., 2006), the British Thoracic Society (Morice et al., 2006), the Japanese Respiratory Society (Kohno et al., 2006) and the German Respiratory Society (Kardos et al., 2010). Yet, despite the availability of numerous published guideline documents, physicians, especially subspecialists, remain challenged with a significant percentage of patients whose cough remains unimproved, or only partially treated, despite adherence to recommended protocols (Pavord and Chung, 2008). Among the shortcomings of all published cough management guidelines is the dearth of evidence from adequately performed, randomized, controlled trials to support that proposed therapeutic interventions will indeed address the underlying aetiology of a patient's cough and thus ameliorate or eliminate the symptom. Thus, in the setting of cough refractory to standard therapy, a physician is left uncertain as to whether: the correct aetiology of cough has not yet been discerned; a correct diagnosis has been established and recommended treatment has been administered, yet the patient's cough persists; or, the patient has unexplained cough, a term proposed in the American College of Chest Physicians cough guidelines (Irwin et al., 2006) to describe a cough that persists despite a thorough diagnostic evaluation, specific and appropriate therapeutic trials aimed at potential causes of cough, and exclusion of uncommon causes.
The goal of the present review is to identify and discuss current diagnostic and therapeutic challenges facing the clinician treating patients with cough. The discussion will consider cough in terms of its duration (acute cough <3 weeks; subacute cough 3–8 weeks; chronic cough >8 weeks) as is generally accepted and proposed in current cough management guidelines (Morice et al., 2004; Irwin et al., 2006). Focus on the duration of cough is appropriate, as it aids in generating a differential diagnosis (Table 1).
Table 1.
Major causes of cough*
| Acute |
| Acute viral upper respiratory tract infection (common cold) |
| Subacute |
| Post-viral |
| Bordetella pertussis (whooping cough) |
| Chronic cough not yet of 8-weeks duration |
| Chronic |
| Upper airway cough syndrome (postnasal drip syndrome) |
| Eosinophilic airway inflammation |
| Asthma |
| Non-asthmatic eosinophilic bronchitis |
| Gastro-oesophageal reflux diseases |
| Laryngopharyngeal reflux |
| Angiotensin converting-enzyme inhibitors |
| Occupational/Environmental |
| Unexplained |
In adult non-smokers without history of chronic lung disease.
Acute cough
Acute cough is an extremely common condition, most often due to acute viral upper respiratory tract infection (URI; common cold). Cough due to URI is usually transient and self-limited. However, if cough is bothersome and disruptive to the patient, symptomatic treatment is appropriate. Unfortunately, therapeutic options for cough suppression are extremely limited. In the United States, only three non-prescription (over-the-counter; OTC) agents are approved as anti-tussives: (i) chlophedianol, a medication available for purchase only through the Internet from a limited number of suppliers, and about which no research has been published since the early 1960s; (ii) diphenhydramine, a first-generation sedating antihistamine; and (iii) dextromethorphan (FDA, 1987, 1994). Animal studies in various species (Eddy et al., 1969; Bolser et al., 1993; Kotzer et al., 2000; McLeod et al., 2010) and studies of induced cough in humans (Bickerman et al., 1957; Karttunen et al., 1987; Grattan et al., 1995; Ramsay et al., 2008) have clearly demonstrated the anti-tussive effect of dextromethorphan. Recently, controversy has arisen because of the dearth of adequately performed clinical trials demonstrating the efficacy of dextromethorphan in acute cough due to URI (Bolser, 2006; Dicpinigaitis et al., 2009). Similarly, a paucity of data exists for the efficacy of prescription cough products. The widely used narcotic anti-tussive, codeine, has not been shown to be efficacious against acute cough due to the common cold in prospective, blinded, controlled trials (Eccles et al., 1992; Freestone and Eccles, 1997). When narcotics do provide symptomatic relief at recommended doses, they often do so at the expense of undesirable side effects such as sedation and gastrointestinal discomfort. Recently, the anti-cholinergic agent tiotropium has been shown to inhibit induced cough in subjects with acute URI (Dicpinigaitis et al., 2008), but the clinical significance of this observation awaits elucidation in prospective clinical trials.
Thus, a significant need exists for safe, effective anti-tussives that can suppress acute cough without undesirable or intolerable side effects. Unfortunately, it will be difficult to demonstrate the efficacy of a potential new agent for acute cough due to URI, for the same reasons that data on currently available medications are scant: (i) because cough associated with the common cold is typically transient and self-limited, very large and hence, expensive trials would be necessary to provide adequate power to detect a clinically relevant effect; (ii) the large placebo effect observed in most trials of anti-tussives (Eccles, 2010); and (iii) the lack of well-validated, commercially available technology for objective cough counting (Smith and Woodcock, 2008).
Subacute cough
Subacute cough refers to a cough of 3–8-week duration (Morice et al., 2004; Irwin et al., 2006). Most cases of subacute cough likely represent a post-viral cough that has extended beyond 3 weeks. Post-viral cough may be particularly refractory to treatment. Based on limited trial data and anecdotal experience/expert opinion, recommended therapies include inhaled ipratropium, inhaled corticosteroids, short courses of oral corticosteroids (for severe paroxysms), codeine and dextromethorphan (Braman, 2006). Prospective, randomized controlled trials of therapeutic agents aimed at post-viral cough are lacking.
Cough due to Bordetella pertussis (whooping cough) typically lasts for 4–6 weeks but can persist for months. Usually, by the time severe cough occurs, the effective treatment window for the underlying pathogen has passed. Symptomatic therapy with inhaled long-acting β-agonists, antihistamines, corticosteroids and pertussis Ig have not been shown to be helpful for the paroxysmal cough (Braman, 2006). Symptoms classically associated with pertussis, i.e. post-tussive emesis and an inspiratory whooping sound, are not strong predictors of the presence of B pertussis infection (Cornia et al., 2010). Recent evidence from animal experiments suggests that the severe, paroxysmal cough of pertussis may be mediated by the inflammatory peptide bradykinin (Hewitt and Canning, 2010).
Some patients presenting with subacute cough will progress to a diagnosis of chronic cough once the duration of cough reaches 8 weeks. The differential diagnosis of chronic cough is discussed below.
Chronic cough
Upper airway cough syndrome (UACS; post-nasal drip syndrome)
The American College of Chest Physicians 2006 guidelines adopted the term upper airway cough syndrome or UACS to replace the term post-nasal drip syndrome, as it was felt that the new terminology more effectively addressed the possibility that cough could be due, not only to the post-nasal drip itself, but to irritation or inflammation of upper airway structures that directly stimulate cough receptors independent of, or in addition to, post-nasal drip (Irwin et al., 2006). More recently, the concept of a ‘unified airway’ has been proposed based on epidemiological and physiological data suggesting that the upper and lower airways may be linked through local inflammatory processes. The model suggests that systemic propagation of inflammation occurs through trafficking of inflammatory mediators, thus promoting a system-wide response in the respiratory mucosa through which pathology in one portion of this system can stimulate and influence pathophysiological changes at a site distal to the initial site of inflammation (Krouse and Altman, 2010). Consistent with this hypothesis, recent studies incorporating induced cough in humans have demonstrated enhancement of cough reflex sensitivity in the presence of rhinosinusitis (Tatar et al., 2009).
UACS can result from a multiplicity of rhinosinus conditions (Table 2), and has been considered the most common cause of chronic cough in adults in the United States (Pratter, 2006a). Treatment for UACS-induced cough should be targeted at the underlying aetiology, and is often effective. However, when UACS is due to acute viral upper respiratory tract infection, or, is being treated empirically as the first step in a diagnostic-therapeutic algorithm for chronic cough of unclear aetiology, the combination of a first-generation antihistamine and decongestant is recommended (Irwin et al., 2006). A complicating factor in interpreting a response to this form of therapy, and thus assigning a diagnosis to the patient, is the possibility that symptomatic relief is achieved not through amelioration of post-nasal drip, but by a primary anti-tussive effect of the antihistamine. Indeed, diphenhydramine has been demonstrated to inhibit citric acid-induced cough in healthy volunteers (Packman et al., 1991), while more recently, dexbrompheniramine has been shown to inhibit activation of the transient receptor potential vanilloid-1 (TRPV1) ion channel in human TRPV1-expressing human embryonic kidney (HEK) cells and rat dorsal root ganglia neuron preparations (Sadofsky et al., 2008). TRPV1 has received significant attention recently as a particularly relevant receptor in human cough, and thus represents a compelling target for the development of potential new anti-tussive agents (Adcock, 2009).
Table 2.
Potential causes of upper airway cough syndrome*
| Allergic rhinitis |
| Perennial non-allergic rhinitis |
| Vasomotor rhinitis |
| Nonallergic rhinitis with eosinophilia (NARES) |
| Post-infectious rhinitis |
| Following upper respiratory tract infection |
| Bacterial sinusitis |
| Allergic fungal sinusitis |
| Rhinitis due to anatomic abnormalities |
| Rhinitis due to physical or chemical irritants |
| Occupational rhinitis |
| Rhinitis medicamentosa |
| Rhinitis of pregnancy |
Adapted from Pratter (2006a).
Eosinophilic airway inflammation (asthma and non-asthmatic eosinophilic bronchitis)
Multiple prospective studies have shown that asthma is among the most common aetiologies of chronic cough (24–29%) in adult non-smokers (Irwin et al., 1990; Pratter et al., 1993; McGarvey et al., 1998). Usually, cough is accompanied by the typical features of dyspnoea and wheezing, but may represent the sole or predominant symptom in the setting of an unremarkable physical examination and normal pulmonary function studies. Such a presentation describes a subgroup of asthmatics termed to have cough-variant asthma, or CVA (Dicpinigaitis, 2006a).
Asthmatic cough typically responds to standard asthma therapy of inhaled bronchodilators and inhaled corticosteroids. The leukotriene receptor antagonists (LTRA) zafirlukast (Dicpinigaitis et al., 2002) and montelukast (Spector and Tan, 2004; Kita et al., 2010) have been shown to be particularly effective in CVA, and should be added to a regimen of inhaled corticosteroids before escalation to therapy with systemic corticosteroids (Dicpinigaitis, 2006a). Given a paucity of data on the natural history of CVA, it remains unclear whether monotherapy with LTRAs is sufficient to prevent the sequelae of chronic airway inflammation as are seen in the typical form of asthma.
Non-asthmatic eosinophilic bronchitis distinguishes itself from asthma by the absence of reversible airway obstruction and bronchial hyperresponsiveness, but the associated cough, as in asthma, is usually quite responsive to inhaled corticosteroid therapy (Brightling, 2010). Clinical response has been correlated with decreases in sputum eosinophil count and cough reflex sensitivity, thus supporting a causal relationship between cough and eosinophilic airway inflammation (Brightling, 2010). Only rarely is systemic treatment with oral corticosteroids required. The efficacy of LTRAs in chronic cough due to non-asthmatic eosinophilic bronchitis has not been investigated. Furthermore, the natural history of non-asthmatic eosinophilic bronchitis is yet to be elucidated fully. Preliminary long-term (>1 year) follow-up data from 32 patients suggest that although a minority (16%) of patients develop fixed airflow obstruction, the condition is rarely self-limiting; 66% of patients had persistent symptoms and/or ongoing airway inflammation (Berry et al., 2005).
Recently, exhaled nitric oxide has been proposed as a useful biomarker to confirm the presence of eosinophilic airway inflammation and thus, predict a favourable response to inhaled corticosteroid therapy in both asthmatic cough as well as cough due to non-asthmatic eosinophilic bronchitis (Lim, 2010). Absence of a response to systemic corticosteroids should prompt evaluation for other aetiologies of chronic cough.
Gastro-oesophageal reflux disease (GERD)
Perhaps the greatest challenges to the clinician evaluating a patient with chronic cough are the confirmation of the presence of gastro-oesophageal reflux and, more importantly, the determination that reflux, if present, is responsible for the cough (Table 3). Indeed, the diagnosis of GERD, including the use of empiric therapeutic trials with proton pump inhibitor medications (PPIs), remains problematic, even in the setting of typical reflux symptoms (Lacy et al., 2010). The response rate to PPI therapy in patients in whom cough is the sole or predominant symptom is even less (Chang et al., 2006; Pauwels et al., 2009).
Table 3.
Challenges in the management of cough due to gastro-oesophageal refluxdisease (GERD)
| Diagnosis |
| Poor sensitivity and specificity of empiric therapeutic trials with proton pump inhibitor medications (PPIs) |
| Standard diagnostic studies for acid reflux, such as 24-hour oesophageal pH measurement, may fall within normal range of number of reflux events, even when reflux is the cause of chronic cough. |
| Treatment |
| Aggressive acid suppression therapy may be inadequate due to the presence of non-acid or weakly-acid refluxate as the cause of cough. |
| When aggressive acid suppression therapy fails, and reflux remains the suspected aetiology of chronic cough, addition of a prokinetic agent is appropriate. However, the available therapeutic options are severely limited. |
| In the setting of failure of maximal medical therapy, surgical intervention (laparoscopic Nissen fundoplication) may be considered. However, data on the outcome of surgical intervention for chronic cough due to GERD are limited, and do not demonstrate a uniformly positive outcome. |
Potential mechanisms proposed to explain GERD-induced cough include: (i) refluxate within the oesophagus triggering a distal oesophageal-tracheobronchial reflex; (ii) refluxate extending beyond the oesophagus into the larynx (laryngopharyngeal reflux; LPR) where sensory afferent cough receptors reside; and (iii) microaspiration (Figure 1). Furthermore, it has recently been appreciated that cough may be induced by non-acidic or weakly acidic refluxate, thus explaining why some patients whose cough was refractory to aggressive acid suppression experienced resolution of cough subsequent to anti-reflux surgery (laparoscopic Nissen fundoplication) (Irwin et al., 2002).
Figure 1.
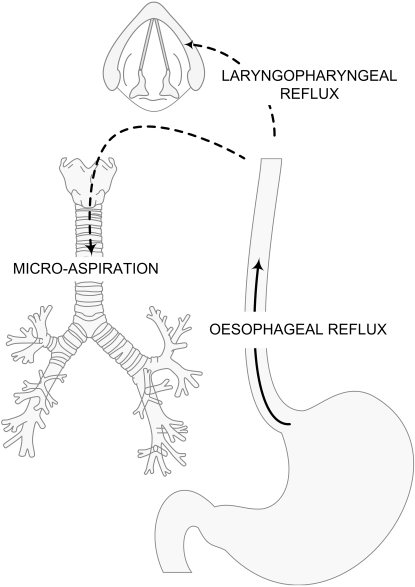
Potential mechanisms linking gastro-oesophageal reflux events and cough (reprinted with permission from Smith et al., 2010a).
Recently, the diagnostic evaluation of the patient with unexplained cough has been improved by the increased availability of combined oesophageal pH-impedance monitoring that allows detection of both acid (pH > 4) and non-acid reflux events (Tokayer, 2008). Initial studies demonstrate that this modality significantly enhances the diagnostic yield for detecting gastro-oesophageal reflux in patients with atypical GERD symptoms, and has been proposed as the new diagnostic gold standard (Bajbouj et al., 2007; Kahrilas, 2010; Lee et al., 2010). Interestingly, multiple studies incorporating this technology to evaluate chronic cough sufferers have not demonstrated an increased number of reflux events compared with data from healthy volunteers, but proposed a causal relationship based on observed temporal associations between reflux events and cough (Smith et al., 2010a). Though most recent studies incorporating these newest diagnostic methods have demonstrated intra-oesophageal reflux as being most relevant (Smith et al., 2010a), other investigators have suggested a prominent role for laryngopharyngeal reflux in patients with chronic cough (Patterson et al., 2009).
A recent study combining simultaneous acoustic cough recording with pH-impedance monitoring, in addition to cough reflex sensitivity measurement, in a group of unselected patients with chronic cough has shed further light on the association of reflux and cough (Smith et al., 2010b). Subjects in this study demonstrated a symptom association probability (SAP) of cough preceded by reflux, as well as reflux preceded by cough. This observation supports the concept that not only may reflux induce cough, but cough may cause or exacerbate reflux, through mechanisms including increased intra-thoracic pressure, thus generating a self-perpetuating process of cough stimulation. Notably, SAP-positive subjects did not have a greater degree of oesophageal acid exposure or degree of erosive disease compared with SAP-negative subjects, but they did have enhanced cough reflex sensitivity, thus suggesting a mechanism of sensitization at the level of sensory afferents in the oesophagus and/or central sensitization at the level of the brainstem.
For patients with chronic cough in whom GERD is confirmed or suspected, acid suppressive therapy is indicated, and can be escalated to an aggressive regimen of a twice-daily PPI, taken one-half hour before breakfast and dinner, with or without a histamine-2 antagonist (i.e. ranitidine 150 mg) at bedtime. Recently, liquid alginate suspensions have been evaluated as alternate or complementary therapy for symptoms of LPR. The alginate is believed to form a physical barrier floating above the gastric contents, thus inhibiting backflow of refluxate into the oesophagus (McGlashan et al., 2009). In addition to pharmacological therapy, anti-reflux lifestyle measures should be stressed: sleep with head elevated, do not eat within 2 h of bedtime, and avoid reflux-promoting foods and beverages including alcohol, caffeine, chocolate, peppermint, and spicy or greasy foods (Irwin, 2006).
If cough persists despite strict adherence to the above-described regimen for 2–3 months, and reflux remains the presumed aetiology, then empiric addition of a prokinetic agent is appropriate. Unfortunately, the clinician's options in this regard are extremely limited (Merati, 2010). One prospective study demonstrated the ability of a prokinetic agent (cisapride or metoclopramide) to reduce or eliminate cough in subjects whose cough did not respond to PPI therapy alone (Poe and Kallay, 2003). Cisapride was removed from the United States market in 2000 due to its association with QT interval prolongation and ventricular arrhythmias (Layton et al., 2003). Other available prokinetic agents include bethanechol, a muscarinic agonist that must be avoided in asthmatics, and erythromycin, the use of which raises concern of numerous drug interactions, cardiac arrhythmias and emergence of resistant bacteria (Berthet et al., 2010). Furthermore, neither bethanechol nor erythromycin has ever been specifically evaluated in cough associated with GERD.
Hence, physicians in the United States and elsewhere are essentially left with metoclopramide to treat reflux-induced cough refractory to PPI therapy, but this agent is often associated with sedation and other undesirable effects and has been implicated as a cause of tardive dyskinesia after prolonged use (Rao and Camilleri, 2010). Domperidone, a dopaminergic antagonist like metoclopramide, has been used as a prokinetic outside the United States. Published data for the efficacy of this drug in reflux-induced cough are also lacking. Furthermore, domperidone has been associated with serious ventricular arrhythmias and sudden cardiac death (Johannes et al., 2010). Thus, the lack of safe and effective prokinetic agents for physicians to use in the setting of presumed reflux-induced cough refractory to acid suppression therapy represents a significant unmet clinical need.
An area of current investigation that may yield therapeutic options beyond that of acid suppression involves the γ-aminobutyric acid type B (GABA-B) receptor agonists. Transient lower oesophageal sphincter relaxation (TLESR) promotes the occurrence of gastro-oesophageal reflux. The GABA-B agonist baclofen has been shown to inhibit TLESR, and reduces the number of reflux episodes in subjects with GERD, reduces GERD symptoms in one study of chronic administration and increases basal lower oesophageal sphincter pressure in healthy volunteers and subjects with GERD (Kuo and Holloway, 2010). In addition, baclofen has been shown to be an anti-tussive agent independent of its effect on reflux by inhibiting capsaicin-induced cough in healthy volunteers (Dicpinigaitis and Dobkin, 1997). Despite such compelling evidence, baclofen has not gained widespread use because of undesirable side effects including sedation, dizziness, headache and confusion. Hence, newer GABA-B agonists with more specific peripheral activity and less central effects are currently the subject of active research (Kuo and Holloway, 2010).
When maximal medical therapy has failed to alleviate reflux-induced cough, surgical intervention may be considered. Data on outcomes of surgical fundoplication are still limited, and results to date confirm that a satisfactory outcome is not guaranteed. In one retrospective study of long-term (median 53 months) outcomes of laparoscopic anti-reflux surgery, atypical GERD symptoms including cough were reported to have improved in 65–75% of 128 patients. Of note, 33% of these post-operative patients were receiving daily antacid therapy at the time of interview (Kaufman et al., 2006). Another retrospective study evaluated 51 patients, out of a total of 240 who underwent surgical fundoplication, with predominantly extra-oesophageal symptoms of GERD. The 40 patients available for analysis (at a mean of 53.3 months post-surgery) were asked to grade their overall quality of life (QOL); 25% reported QOL as excellent, 32.% as good, 32.5% as satisfactory and 10% reported overall QOL as bad (Iqbal et al., 2009). One prospective study describing a community hospital experience with laparoscopic Nissen fundoplication found, as in other studies, greater improvement in typical rather than atypical GERD symptoms after long-term follow up (mean 24.6 months) of 84 patients. Comparison of pre- and post-operative symptom scores demonstrated a 58% improvement in cough. In the study group as a whole, 74% reported significant atypical GERD symptoms preoperatively; only 7% reported exclusively atypical symptoms (Ranson et al., 2007). Thus, further guidance is awaited from prospective trials of anti-reflux surgery in patients with refractory, GERD-induced chronic cough to assist the physician and patient in the difficult decision of whether to pursue a surgical option. Of particular significance from future studies would be the identification of specific characteristics predictive of a successful outcome after surgery.
Angiotensin-converting enzyme (ACE) inhibitors
Chronic cough is a well-described class effect of the ACE inhibitor medications. The incidence of ACE inhibitor-induced cough has been reported to be in the range of 5–35%, and occurs more often in women, non-smokers and persons of Chinese origin (Dicpinigaitis, 2006b). Cough occurs more commonly in patients receiving ACE inhibitor therapy for congestive heart failure than in those receiving these drugs for treatment of hypertension (Bangalore et al., 2010). Cough may occur within hours of the first dose of medication, or its onset may be delayed for weeks to months after the initiation of therapy. Although numerous small studies have shown various drugs to be partially effective, the only uniformly successful intervention for ACE inhibitor-induced cough is cessation of the offending agent. Cough usually resolves within 1 week of discontinuation of the ACE inhibitor. However, in a subgroup of patients, a month or more may be required for resolution of cough. In a patient with chronic cough of unknown aetiology, ACE inhibitor therapy must be discontinued regardless of the temporal relation between the onset of cough and the initiation of the ACE inhibitor. If cough resolves after cessation of therapy with an ACE inhibitor, and a compelling reason remains for treatment with this drug class, a repeat trial of ACE inhibitor therapy may be attempted. Studies have shown that the occurrence of cough with angiotensin-receptor blockers is similar to that of the non-ACE-inhibitor drugs against which they were compared (Dicpinigaitis, 2006b).
Occupational/environmental
In the evaluation of a patient with chronic cough, most physicians will appropriately seek to eliminate potential exogenous causative agents such as primary inhaled cigarette smoke and ACE inhibitor medications. In addition, a thorough history must be performed to identify other potentially relevant factors in the environment or workplace that could be causing or enhancing chronic cough. Epidemiological studies have demonstrated that environmental exposure to particulate matter, irritant gases, second-hand tobacco smoke, mixed pollutants and moulds is associated with increased cough (Joad et al., 2007). Furthermore, chronic cough is among the most prevalent work-related airway disorders, as documented in, for example, coal miners, hard-rock miners, tunnel workers and concrete manufacturing workers (Groneberg et al., 2006). In addition, some individuals appear to experience irritation and cough after exposure to non-toxic amounts of common substances such as perfumes, pesticides, paint and automobile exhaust, among many others (Brooks, 2010). This group presents a particular challenge in terms of discerning the underlying cause(s) of chronic cough.
Unexplained cough
In their 2006 cough management guidelines, the American College of Chest Physicians adopted the term unexplained cough to replace the previously used term idiopathic cough, as it was felt that the term unexplained cough better describes a condition that is likely due to multiple underlying aetiologies rather than a single entity (Pratter, 2006b). The challenge facing the clinician is to ascertain that a complete and thorough evaluation has been performed, including diagnostic-therapeutic trials with appropriate medications at adequate doses and for sufficient duration, before a diagnosis of unexplained cough is conferred. Nevertheless, there appears to exist a group of patients with genuine unexplained cough, and recent investigations have allowed the description of a particular phenotype of such individuals. Patients with apparent true unexplained chronic cough are often middle-aged (perimenopausal) women with prolonged dry cough and demonstrable cough reflex hypersensitivity (McGarvey, 2008). Additional studies have shown that such patients tend to have airway inflammation characterized by increased numbers of mast cells (McGarvey et al., 1999) and lymphocytes (Birring et al., 2003), and an increased incidence of organ-specific autoimmune disorders (Birring et al., 2004). Interestingly, the recently proposed entity of post-viral vagal neuropathy, in which chronic cough associated with laryngeal symptoms such as throat clearing follow an URI, appears to affect predominantly women in their fifth decade of life (Rees et al., 2009; Greene and Simpson, 2010).
Recently, the Cough Hypersensitivity Syndrome has been proposed to explain why a subgroup of individuals with common conditions such as rhinitis, asthma and GERD develop chronic, refractory cough, whereas the majority of the population with the same underlying conditions does not (Morice, 2010). At present, satisfactory therapeutic agents to manage chronic, unexplained cough are lacking. New safe and effective anti-tussive medications, as well as pharmacological agents aimed at the hypersensitised cough reflex likely underlying this condition, are eagerly awaited.
Non-pharmacological therapy of cough
The clinician must be cognizant of the fact that non-pharmacological therapeutic strategies may be vital to the successful treatment of cough. As discussed above, elimination of exogenous factors potentially causing or exacerbating cough is essential. These include cigarette smoking, ACE inhibitors, and potential environmental as well as occupational triggers. For cough due to GERD, anti-reflux lifestyle measures are an essential component of a multifaceted treatment approach. Recent evidence supports an important role for speech language pathology management in the treatment of chronic cough refractory to medical therapy (Gibson and Vertigan, 2009; Ryan et al., 2010).
Acknowledgments
There are no acknowledgements nor was there any financial support of this review paper.
Glossary
Abbreviations
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- CVA
cough-variant asthma
- FeNO
exhaled nitric oxide
- GERD
gastro-oesophageal reflux disease
- LPR
laryngopharyngeal reflux
- LTRA
leukotriene receptor antagonist
- OTC
over the counter
- PNDS
postnasal drip syndrome
- QOL
quality of life
- SAP
symptom association probability
- TLESR
transient lower oesophageal sphincter relaxation
- TRPV1
transient receptor potential vanilloid-1
- UACS
upper airway cough syndrome
- URI
acute viral upper respiratory tract infection
Conflict of interest
I have no conflicts of interest related to this manuscript.
Supporting Information
Teaching Materials; Fig 1 as PowerPoint slide.
References
- Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther. 2009;22:65–70. doi: 10.1016/j.pupt.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Bajbouj M, Becker V, Neuber M, Schmid RM, Meining A. Combined ph-metry/impedance monitoring increases the diagnostic yield in patients with atypical gastroesophageal reflux symptoms. Digestion. 2007;76:223–228. doi: 10.1159/000112728. [DOI] [PubMed] [Google Scholar]
- Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians' Desk Reference. Am J Med. 2010;123:1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Berry MA, Hargadon B, McKenna S, Shaw D, Green RH, Brightling CE, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598–601. doi: 10.1111/j.1365-2222.2005.02222.x. [DOI] [PubMed] [Google Scholar]
- Berthet S, Charpiat B, Mabrut JY. Erythromycin as a prokinetic agent: risk factors. J Visc Surg. 2010;147:e13–e18. doi: 10.1016/j.jviscsurg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bickerman HA, German E, Cohen BM, Itkin SE. The cough response of healthy human subjects stimulated by citric acid aerosol. Am J Med Sci. 1957;234:191–205. doi: 10.1097/00000441-195708000-00010. [DOI] [PubMed] [Google Scholar]
- Birring SS, Brightling CE, Symon FA, Barlow SG, Wardlaw AJ, Pavord ID. Idiopathic chronic cough: association with organ specific autoimmune disease and bronchoalveolar lymphocytosis. Thorax. 2003;58:1066–1070. doi: 10.1136/thorax.58.12.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birring SS, Murphy AC, Scullion JE, Brightling CE, Browning M, Pavord ID. Idiopathic chronic cough and organ-specific autoimmune diseases: a case-control study. Respir Med. 2004;98:242–246. doi: 10.1016/j.rmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Bolser DC. Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):238S–249S. doi: 10.1378/chest.129.1_suppl.238S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Aziz SM, DeGennaro FC, Kreutner W, Egan RW, Siegel MI, et al. Antitussive effects of GABA-B agonists in the cat and guinea-pig. Br J Pharmacol. 1993;110:491–495. doi: 10.1111/j.1476-5381.1993.tb13837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):138S–146S. doi: 10.1378/chest.129.1_suppl.138S. [DOI] [PubMed] [Google Scholar]
- Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188(Suppl 1):S13–S17. doi: 10.1007/s00408-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Brooks SM. Occupational, environmental, and irritant-induced cough. Otolaryngol Clin North Am. 2010;43:85–96. doi: 10.1016/j.otc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ. 2006;332:11–17. doi: 10.1136/bmj.38677.559005.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Report. 2008;3:1–39. [PubMed] [Google Scholar]
- Cornia PB, Hersh AL, Lipsky BA, Newman TB, Gonzales R. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890–896. doi: 10.1001/jama.2010.1181. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest. 2006a;129(Suppl 1):75S–79S. doi: 10.1378/chest.129.1_suppl.75S. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006b;129(Suppl 1):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB. Antitussive effect of the GABA-agonist baclofen. Chest. 1997;111:996–999. doi: 10.1378/chest.111.4.996. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma. 2002;39:291–297. doi: 10.1081/jas-120002285. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Spinner L, Santhyadka G, Negassa A. Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung. 2008;186:369–374. doi: 10.1007/s00408-008-9114-6. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Colice GL, Goolsby MJ, Rogg GI, Spector SL, Winther B. Acute cough: a diagnostic and therapeutic challenge. Cough. 2009;5:11. doi: 10.1186/1745-9974-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. Importance of placebo effect in cough clinical trials. Lung. 2010;188(Suppl 1):S53–S61. doi: 10.1007/s00408-009-9173-3. [DOI] [PubMed] [Google Scholar]
- Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther. 1992;17:175–180. doi: 10.1111/j.1365-2710.1992.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Friebel H, Hahn K-J HH. Codeine and its alternates for pain and cough relief. Bull World Health Organ. 1969;40:639–719. [PMC free article] [PubMed] [Google Scholar]
- FDA. Cold, cough, allergy, bronchodilator and antiasthmatic drug products for over-the-counter human use; final monograph for OTC antitussive drug products. U.S. Department of Health and Human Services. Fed Regist. 1987;52:30042–30057. [Google Scholar]
- FDA. Cold, cough, allergy, bronchodilator and antiasthmatic drug products for over-the-counter human use; amendment of final monograph for OTC antitussive drug products. U.S. Department of Health and Human Services. Fed Regist. 1994;59:29172–29174. [Google Scholar]
- Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Vertigan AE. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Ther. 2009;22:159–162. doi: 10.1016/j.pupt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Grattan TJ, Marshall AE, Higgins KS, Morice AH. The effect of inhaled and oral dextromethorphan on citric acid induced cough in man. Br J Clin Pharmacol. 1995;39:261–263. doi: 10.1111/j.1365-2125.1995.tb04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SM, Simpson CB. Evidence for sensory neuropathy and pharmacologic management. Otolaryngol Clin North Am. 2010;43:67–72. doi: 10.1016/j.otc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Nowak D, Wussow A, Fischer A. Chronic cough due to occupational factors. J Occup Med Toxicol. 2006;1:3. doi: 10.1186/1745-6673-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt M, Canning BJ. Coughing precipitated by Bordetella pertussis infection. Lung. 2010;188(Suppl 1):S73–S79. doi: 10.1007/s00408-009-9196-9. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Batch AJ, Moorthy K, Cooper BT, Spychal RT. Outcome of surgical fundoplication for extra-oesophageal symptoms of reflux. Surg Endosc. 2009;23:557–561. doi: 10.1007/s00464-008-9861-8. [DOI] [PubMed] [Google Scholar]
- Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):80S–94S. doi: 10.1378/chest.129.1_suppl.80S. [DOI] [PubMed] [Google Scholar]
- Irwin RS, Curley FJ, French CL. Chronic cough: the spectrum and frequency of causes, key components of the diagnostic evaluation, and outline of specific therapy. Am Rev Respir Dis. 1990;141:640–647. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- Irwin RS, Zawacki JK, Wilson MM, French CT, Callery MP. Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near-total elimination of esophageal acid. Chest. 2002;121:1132–1140. doi: 10.1378/chest.121.4.1132. [DOI] [PubMed] [Google Scholar]
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joad JP, Sekizawa S, Chen CY, Bonham AC. Air pollutants and cough. Pulm Pharmacol Ther. 2007;20:347–354. doi: 10.1016/j.pupt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Johannes CB, Varas-Lorenzo C, McQuay LJ, Midkiff KD, Fife D. Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: a nested case-control study. Pharmacoepidemiol Drug Saf. 2010;19:881–888. doi: 10.1002/pds.2016. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ. Chronic cough and gastroesophageal reflux disease: new twists to the riddle (ed) Gastroenterology. 2010;139:716–718. doi: 10.1053/j.gastro.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos P, Berck H, Fuchs K-H, Gillissen A, Klimek L, Morr H, et al. Guidelines of the German Respiratory Society for diagnosis and treatment of adults suffering from acute or chronic cough. Pneumologie. 2010;64:336–373. doi: 10.1055/s-0030-1255526. [DOI] [PubMed] [Google Scholar]
- Karttunen P, Tukiainen H, Silvasti M, Kolonen S. Antitussive effect of dextromethorphan and dextromethorphan-salbutamol combination in healthy volunteers with artificially induced cough. Respiration. 1987;52:49–53. doi: 10.1159/000195303. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Houghland JE, Quiroga E, Cahill M, Pellegrini CA, Oelschlager BK. Long-term outcomes of laparoscopic antireflux surgery for gastroesophageal reflux disease (GERD)-related airway disorder. Surg Endosc. 2006;20:1824–1830. doi: 10.1007/s00464-005-0329-9. [DOI] [PubMed] [Google Scholar]
- Kita T, Fujimura M, Ogawa H, Nakatsumi Y, Nomura S, Ishiura Y, et al. Antitussive effects of the leukotriene receptor antagonist montelukast in patients with cough variant asthma and atopic cough. Allergol Int. 2010;59:185–192. doi: 10.2332/allergolint.09-OA-0112. [DOI] [PubMed] [Google Scholar]
- Kohno S, Ishida T, Uchida Y, Kishimoto H, Sasaki H, Shioya T, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology. 2006;11(Suppl 4):S135–S186. doi: 10.1111/j.1440-1843.2006.00920_1.x. [DOI] [PubMed] [Google Scholar]
- Kotzer CJ, Hay DWP, Dondio G, Giardina G, Petrillo P, Underwood DC. The antitussive activity of δ-opioid receptor stimulation in guinea pigs. J Pharmacol Exp Ther. 2000;292:803–809. [PubMed] [Google Scholar]
- Krouse JH, Altman KW. Rhinogenic laryngitis, cough, and the unified airway. Otolaryngol Clin North Am. 2010;43:111–121. doi: 10.1016/j.otc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Kuo P, Holloway RH. Beyond acid suppression: new pharmacologic approaches for treatment of GERD. Curr Gastroenterol Rep. 2010;12:175–180. doi: 10.1007/s11894-010-0102-7. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Weiser K, Chertoff J, Fass R, Pandolfino JE, Richter JE, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123:583–592. doi: 10.1016/j.amjmed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Layton D, Key C, Shakir SA. Prolongation of the QT interval and cardiac arrythmias associated with cisapride: limitations of the pharmacoepidemiological studies conducted and proposals for the future. Pharmacoepidemiol Drug Saf. 2003;12:31–40. doi: 10.1002/pds.781. [DOI] [PubMed] [Google Scholar]
- Lee BE, Kim GH, Ryu DY, Kim DU, Cheong GH, Lee DG, et al. Combined dual channel impedance pH-metry in patients with suspected laryngopharyngeal reflux. J Neurogastroenterol Motil. 2010;16:157–165. doi: 10.5056/jnm.2010.16.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG. Nitric oxide measurement in chronic cough. Lung. 2010;188(Suppl 1):S19–S22. doi: 10.1007/s00408-009-9187-x. [DOI] [PubMed] [Google Scholar]
- McGarvey LP. Does idiopathic cough exist? Lung. 2008;186(Suppl 1):S78–S81. doi: 10.1007/s00408-007-9048-4. [DOI] [PubMed] [Google Scholar]
- McGarvey LP, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53:738–743. doi: 10.1136/thx.53.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey LP, Forsythe P, Heaney LG, MacMahon J, Ennis M. Bronchoalveolar lavage findings in patients with chronic nonproductive cough. Eur Respir J. 1999;13:59–65. doi: 10.1183/09031936.99.13105999. [DOI] [PubMed] [Google Scholar]
- McGlashan JA, Johnstone LM, Sykes J, Strugala V, Dettmar PW. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2009;266:243–251. doi: 10.1007/s00405-008-0708-7. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Tulshian DB, Bolser DC, Varty GB, Baptista M, Fernandez X, et al. Pharmacological profile of the NOP agonist and cough suppressing agent SCH 486757 (8-[bis(2-chlorophenyl)methyl]-3-(2-pyrimidinyl)-8-azabicyclo[3,2,1]octan-3-ol) in preclinical models. Eur J Pharmacol. 2010;630:112–120. doi: 10.1016/j.ejphar.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merati AL. Reflux and cough. Otolaryngol Clin North Am. 2010;43:97–110. doi: 10.1016/j.otc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Morice AH. The Cough Hypersensitivity Syndrome: a novel paradigm for understanding cough. Lung. 2010;188(Suppl 1):S87–S90. doi: 10.1007/s00408-009-9185-z. [DOI] [PubMed] [Google Scholar]
- Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004;24:481–492. doi: 10.1183/09031936.04.00027804. [DOI] [PubMed] [Google Scholar]
- Morice AH, McGarvey L, Pavord I, British Thoracic Society Cough Guideline Group Recommendations for the management of cough in adults. Thorax. 2006;61(Suppl 1):i1–24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packman EW, Ciccone PE, Wilson J, Masurat T. Antitussive effects of diphenhydramine on the citric acid aerosol-induced cough response in humans. Int J Clin Pharmacol Ther Toxicol. 1991;29:218–222. [PubMed] [Google Scholar]
- Patterson N, Mainie I, Rafferty G, McGarvey L, Heaney L, Tutuian R, et al. Nonacid reflux episodes reaching the pharynx are important factors associated with cough. J Clin Gastroenterol. 2009;43:414–419. doi: 10.1097/MCG.0b013e31818859a3. [DOI] [PubMed] [Google Scholar]
- Pauwels A, Blondeau K, Dupont L, Sifrim D. Cough and gastroesophageal reflux: from the gastroenterologist end. Pulm Pharmacol Ther. 2009;22:135–138. doi: 10.1016/j.pupt.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Chung KF. Chronic cough 2: management of chronic cough. Lancet. 2008;371:1375–1384. doi: 10.1016/S0140-6736(08)60596-6. [DOI] [PubMed] [Google Scholar]
- Poe RH, Kallay MC. Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment. Chest. 2003;123:679–684. doi: 10.1378/chest.123.3.679. [DOI] [PubMed] [Google Scholar]
- Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest. 2006a;129(Suppl 1):63S–71S. doi: 10.1378/chest.129.1_suppl.63S. [DOI] [PubMed] [Google Scholar]
- Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest. 2006b;129(Suppl 1):220S–221S. doi: 10.1378/chest.129.1_suppl.220S. [DOI] [PubMed] [Google Scholar]
- Pratter MR, Bartter T, Akers S, DuBois J. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977–983. doi: 10.7326/0003-4819-119-10-199311150-00003. [DOI] [PubMed] [Google Scholar]
- Proprietary Association of Great Britain. IRI 2005 OTC market size statistics. 2005. Available at: http://www.pagb.co.uk.
- Ramsay J, Wright C, Thompson R, Hull D, Morice AH. Assessment of antitussive efficacy of dextromethorphan in smoking related cough:objective vs. subjective measures. Br J Clin Pharmacol. 2008;65:737–741. doi: 10.1111/j.1365-2125.2008.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson ME, Danielson A, Maxwell JG, Harris JA. Prospective study of laparoscopic Nissen fundoplication in a community hospital and its effect on typical, atypical, and nonspecific gastrointestinal symptoms. JSLS. 2007;11:66–71. [PMC free article] [PubMed] [Google Scholar]
- Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11–19. doi: 10.1111/j.1365-2036.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- Rees CJ, Henderson AH, Belafsky PC. Postviral vagal neuropathy. Ann Otol Rhinol Laryngol. 2009;118:247–252. doi: 10.1177/000348940911800402. [DOI] [PubMed] [Google Scholar]
- Ryan NM, Vertigan AE, Bone S, Gibson PG. Cough reflex sensitivity improves with speech language pathology management of refractory chronic cough. Cough. 2010;6:5. doi: 10.1186/1745-9974-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky LR, Campi B, Trevisani M, Compton SJ, Morice AK. Transient receptor potential vanilloid-1-mediated calcium responses are inhibited by the alkylamine antihistamines dexbrompheniramine and chlorpheniramine. Exp Lung Res. 2008;34:681–693. doi: 10.1080/01902140802339623. [DOI] [PubMed] [Google Scholar]
- Smith J, Woodcock A. New developments in the objective assessment of cough. Lung. 2008;186(Suppl 1):S48–S54. doi: 10.1007/s00408-007-9059-1. [DOI] [PubMed] [Google Scholar]
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung. 2010a;188(Suppl 1):S81–S86. doi: 10.1007/s00408-009-9210-2. [DOI] [PubMed] [Google Scholar]
- Smith J, Decalmer S, Kelsall A, McGuiness K, Jones H, Galloway S, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010b;139:754–762. doi: 10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough-variant asthma. Ann Allergy Asthma Immunol. 2004;93:232–236. doi: 10.1016/S1081-1206(10)61493-7. [DOI] [PubMed] [Google Scholar]
- Tatar M, Plevkova J, Brozmanova M, Pecova R, Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm Pharmacol Ther. 2009;22:121–126. doi: 10.1016/j.pupt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- The Nielsen Company. ACNielsen Strategic Planner:1. 2007.
- Tokayer AZ. Gastroesophageal reflux disease and chronic cough. Lung. 2008;186(Suppl 1):S29–S34. doi: 10.1007/s00408-007-9057-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


