Abstract
Cortical development depends on the active integration of cell autonomous and extrinsic cues, but the coordination of these processes is poorly understood. Here, we show that the apical complex protein Pals1 and Pten have opposing roles in localizing the Igf1R to the apical, ventricular domain of cerebral cortical progenitor cells. We found that the cerebrospinal fluid (CSF), which contacts this apical domain, has an age-dependent effect on proliferation, much of which is attributable to Igf2, but that CSF contains other signaling activities as well. CSF samples from patients with glioblastoma multiforme show elevated Igf2 and stimulate stem cell proliferation in an Igf2-dependent manner. Together, our findings demonstrate that the apical complex couples intrinsic and extrinsic signaling, enabling progenitors to sense and respond appropriately to diffusible CSF-borne signals distributed widely throughout the brain. The temporal control of CSF composition may have critical relevance to normal development and neuropathological conditions.
Introduction
Neural development involves a dynamic interplay between cell autonomous and diffusible extracellular signals that regulate symmetric and asymmetric division of progenitor cells (Johansson et al., 2010). In mammalian neural progenitors, homologues of C. elegans and Drosophila polarity proteins, including Par3 (Partitioning Defective Protein 3) and Pals1 (Protein Associated with Lin 7), assemble as apical complexes that play essential roles in regulating self-renewal and cell fate (Margolis and Borg, 2005). The unequal distribution of apical surface components during mitosis is a key determinant of daughter cell fate in C. elegans and Drosophila (Fishell and Kriegstein, 2003; Kemphues, 2000; Wodarz, 2005). Recently, mammalian Par3 was shown to promote asymmetric cell division by specifying differential Notch signaling in radial glial daughter cells (Bultje et al., 2009), suggesting that the inheritance of the apical complex guides progenitor responses to proliferative signals as well.
Secreted signals can act at a distance to guide decisions governing progenitor proliferation and cell fate (Johansson et al., 2010), but little is known of how secreted signals interact with cell-autonomous ones. Insulin-like growth factor 1 (Igf1) promotes progenitor proliferation (Hodge et al., 2004; Popken et al., 2004). Insulin/Igf1 signaling is regulated by E-catenin in keratinocytes (Vasioukhin et al., 2001) and β-catenin in oligodendrocyte progenitors (Ye et al., 2010), suggesting that cell polarity proteins govern cellular responses to extrinsic cues.
Direct interactions between Par3 and Pten (Phosphatase and Tensin homolog) (Feng et al., 2008; Pinal et al., 2006; von Stein et al., 2005; Wu et al., 2007) suggest that the apical complex interacts with growth factor signaling pathways. Indeed, disrupting the apical complex via Pals1 leads to attenuated pS6 signaling, premature cell cycle exit, and rapid cell death, resulting in the absence of nearly the entire cerebral cortex (Kim et al., 2010). In turn, Pals1-deficiency can be partially rescued by concomitant activation of mTOR (Mammalian Target of Rapamycin) (Kim et al., 2010), a downstream effector of growth factor signaling. Growth factor signaling, in particular via the type 1 Igf receptor (Igf1R), mediates powerful, age-dependent effects on the development and maintenance of many organ systems including the brain through the regulation of progenitor cell division (Baker et al., 1993; Hodge et al., 2004; Liu et al., 2009; Popken et al., 2004; Randhawa and Cohen, 2005). Nevertheless, the mechanisms coordinating the availability of Igf ligands to cortical progenitor cells have remained unclear.
Though vascular sources of secreted proliferative signals are well characterized (Palmer et al., 2000; Shen et al., 2004; Shen et al., 2008; Tavazoie et al., 2008), the apical surfaces of early cortical precursors and their primary cilia do not approximate blood vessels but instead directly contact the cerebrospinal fluid (CSF) (Fuchs and Schwark, 2004; Kim et al., 2010), suggesting that secreted factors may interact with progenitor cells at this interface. The CSF proteome shows a complex and dynamic pattern of protein expression (Dziegielewska et al., 1981; Parada et al., 2005; Zappaterra et al., 2007), suggesting important roles beyond provision of a fluid cushion for the central nervous system and maintenance of extracellular ionic balance. The CSF has recently been implicated in carrying secreted proteins in several contexts, including Fgf2 to midbrain progenitors (Martin et al., 2006), Sonic hedgehog to cerebellar progenitors (Huang et al., 2010), and Slit guidance of neuroblasts in adult brain (Sawamoto et al., 2006). Regulation of cerebral cortical progenitor cells by growth factors distributed in the lateral ventricular CSF would provide potentially global control over cerebral cortical neurogenesis, but this hypothesis has not been examined.
Here we show that the apical complex couples autonomous regulation of progenitor proliferation to CSF-borne signals in the developing cerebral cortex. Pals1 and Pten interact genetically to regulate cerebral cortical size and progenitor proliferation, and have opposing roles in localizing the Igf1R to the apical domain of cortical progenitors. Apically localized Igf1Rs respond to CSF-borne Igf ligands, particularly Igf2, and CSF regulates cortical progenitor proliferation and neural stem cell survival in an Igf2-dependent fashion. Finally, CSF Igf2 concentration is elevated in patients with malignant glioblastoma, suggesting that CSF proteins may regulate CNS tumorigenesis. Our findings suggest that the apical complex couples autonomous and extrinsic signaling in cerebral cortical progenitors, enabling these cells to respond appropriately to diffusible CSF-borne signals that regulate cortical neural stem cells during development and disease.
Results
Genetic interactions of Pals1 and Pten at the apical surface region
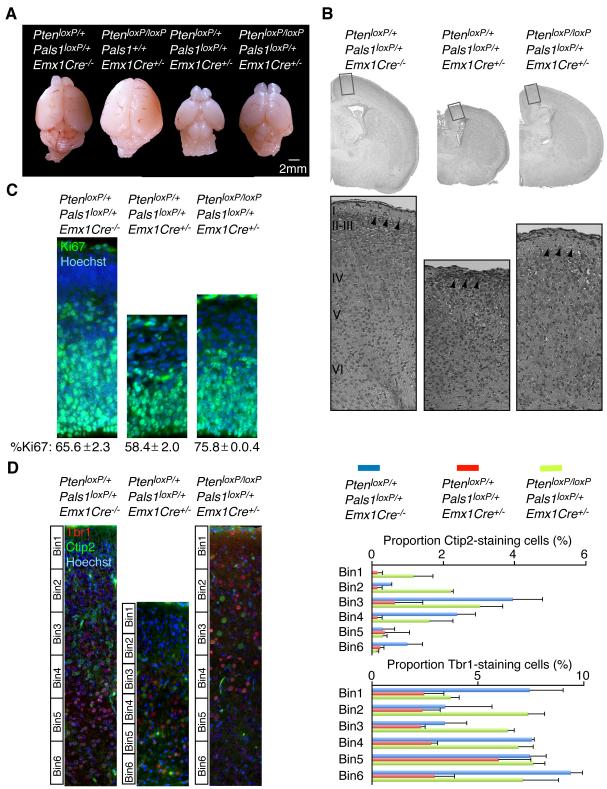
Since Pals1 loss disrupts growth factor signaling and cortical development (Kim et al., 2010), we looked for potential interactions of Pals1 with other regulators of growth factor signaling and found genetic interactions between Pals1 and Pten (Groszer et al., 2001). Cerebral cortex-specific deletion of Pals1 was achieved by crossing mice with a conditional Pals1 allele (Pals1loxP/loxP) (Kim et al., 2010) with mice carrying Emx1-promoter-driven Cre recombinase (Emx1Cre+/−) (Gorski et al., 2002). Pals1loxP/loxP/Emx1Cre+/− mice lacked nearly the entire cortical structure due to premature cell cycle exit and cell death (Kim et al., 2010), with heterozygotes having an intermediate phenotype (Figure 1A). In contrast, Pten deficiency, obtained by crossing PtenloxP/loxP mice (Groszer et al., 2001) with either Emx1Cre+/− or NestinCre+/− mice, resulted in cortical hyperplasia arising from excessive and extended proliferation of apical progenitors (Figure 1A, Figure S1A-S1E) (Groszer et al., 2001). While the broadest groupings of cells were preserved in Pten mutants, the cortical plate was disorganized across its entire radial extent (Figure S1A-S1C). No phenotypic abnormalities were observed in either heterozygous PtenloxP/+/NestinCre+/− mice or in PtenloxP/loxP/NestinCre−/− littermate controls (Figure S1A and data not shown). Conditional deletion of Pten in the Pals1loxP/+/Emx1Cre+/− mice resulted in an almost normal cortical size (Figure 1A). Histological analyses of Pals1loxP/+/Emx1Cre+/− mice, or PtenloxP/+/Pals1loxP/+/Emx1Cre+/− mice, revealed a severely disrupted laminar organization of the dorso-medial cortex (Figure 1B) (Kim et al., 2010). Double mutants showed a relatively normal organization of the marginal zone (Figure 1B), consistent with a genetic interaction between the apical complex and Pten. The expression of apical complex components, especially Cdc42, were abnormal in Pten cortex (Figure S1F and data not shown). The proportion of proliferative progenitor cells marked by Ki67-positive staining cells was greater in the double mutant cortex compared to conditional Pals1 heterozygotes (Figure 1C) and brain size was also more normal by embryonic day (E) 14.5 (Figure S1G and S1H). Proportions of early-born neurons marked by Tbr1 and Ctip2 were also more normal in the PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− mice than in either Pals1 or Pten mutants alone (Figure 1D and data not shown). However, cells in the double mutant brain appeared irregular in size and lamination (Figure 1D), a finding consistent with roles for Pten in the regulation of cell size and polarity (Figure S1C) (Chalhoub et al., 2009; Groszer et al., 2001), and with a role for Pten downstream of the apical complex.
Figure 1. The apical complex and Pten modulate brain size.
(A) Conditional Pten deletion (PtenloxP/loxP/Emx1Cre+/−) resulted in hyperplagia and an enlarged cerebral cortex. Ablation of Pten in PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− mice largely restored the small brain phenotype of Pals1loxP/+/Emx1Cre+/− neonates. (B) H&E staining of PtenloxP/+/Pals1loxP/+/Emx1Cre−/−, PtenloxP/+/Pals1loxP/+/Emx1Cre+/−, and PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− neonates. Arrowheads point to marginal zone. (C) The proportion of Ki67-positive staining progenitors was restored in the E14.5 PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− cortex compared to PtenloxP/+/Pals1loxP/+/Emx1Cre+/− (Percent Ki67-positive staining cells ± S.E.M.; PtenloxP/+/Pals1loxP/+/Emx1Cre−/−: 65.6 ± 2.3; PtenloxP/+/Pals1loxP/+/Emx1Cre+/−: 58.4 ± 2.0; PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/−: 75.8 ± 0.4; ANOVA, p<0.01, n=3). (D) Left panels: Representative images of Ctip2-positive and Tbr1-positive staining neurons analyzed in PtenloxP/+/Pals1loxP/+/Emx1Cre−/−, PtenloxP/+/Pals1loxP/+/Emx1Cre+/−, and PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− neonates. Right panels: The cortical plate was subdivided into six equal bins and Ctip2 and Tbr1 positive cells quantified per bin are expressed as percent of total cells per bin. Pten deletion in the PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− mice restored the proportions of early-born cells marked by Tbr1 and Ctip2 (Percent positive staining cells/total: PtenloxP/+/Pals1loxP/+/Emx1Cre−/− Ctip2 = 8.13 ± 2.0, Tbr1 = 38.7 ± 2.4; PtenloxP/+/Pals1loxP/+/Emx1Cre+/− Ctip2 = 1.6 ± 1.2, Tbr1 = 18.8 ± 3.1; PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− Ctip2 = 8.5 ± 1.6, Tbr1 = 39.1 ± 2.6; ANOVA, p<0.05, n = 3). See also Figure S1.
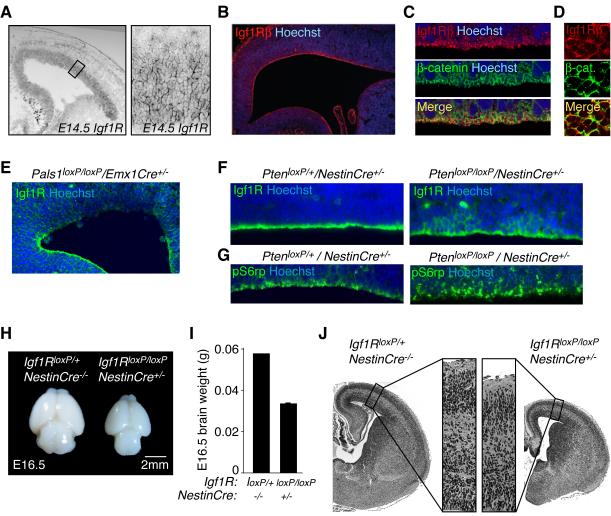
The genetic interaction between Pals1 and Pten, and the decreased proliferation of progenitors and prominent cell death in Pals1 mutants (Kim et al., 2010), prompted us to test whether the apical complex interacts with Igf signaling, since Igfs play a prominent role in cell cycle kinetics of cortical progenitors, cell survival, and brain size (Hodge et al., 2004; Liu et al., 2009; Popken et al., 2004; Schubert et al., 2003). The Igf1R, which binds both Igf1 and Igf2, mediates the proliferative response to Igf signaling (Weber et al., 1992). Surprisingly, Igf1R was enriched in cortical progenitors at the apical, ventricular surface, interdigitating with β-catenin (Figure 2A-2D), suggesting the apical region as the likely site for binding of Igf1R ligand. Apical Igf1R expression was strikingly decreased in Pals1loxP/loxP/Emx1Cre+/− mice (Figure 2E). By contrast in the absence of Pten, Igf1R immunoreactivity demonstrated a considerable basolateral spread in clusters of radial glia (Figure 2F and data not shown). Analyses of downstream signaling events, using a specific antibody against the phosphorylated form of Rsk substrate S6 ribosomal protein (phospho-S6rp), revealed an apical pattern of activity within control brains (Figure 2G). In contrast in Pten mutants, phospho-S6rp showed a broad distribution across the cortical tissue, with many robust phospho-S6rp positive cells extending basally away from the lateral ventricle (Figure 2G). While the majority of cells positive for Igf1R were clearly apical progenitors, some upregulation of Igf1R in basal progenitors is possible. Though we cannot rule out that Pals1 and Pten could function independently to regulate Igf signaling and cortical growth, we interpret our data to suggest that within the cortical ventricular zone, Pals1 and Pten spatially restrict IgfR expression and Igf signaling to the apical membrane domain.
Figure 2. Igf1R expression in cortical progenitor cells.
(A) Left panel: Igf1R in situ hybridization at E14.5 mouse. Right panel: High magnification image of area denoted in left panel. (B) Igf1R enriched along the ventricular surface of E17 rat cortex. (C) Confocal images of Igf1Rβ and β-catenin immunostaining in rat E17 ventricular zone. (D) En face view of the mouse E16.5 ventricular zone immunostained with Igf1Rβ and β-catenin. (E) Ventricular Igf1R expression was disrupted in E12.5 Pals1loxP/loxP/Emx1Cre+/− cortex. (F) Left panel: Igf1R expression was enriched along the apical, ventricular zone of E14.5 PtenloxP/+/NestinCre+/− controls. Right panel: Igf1R expression expanded basolaterally in PtenloxP/loxP/NestinCre+/− radial glia. (G) Left panel: pS6rp activity along the ventricular progenitors of E14.5 PtenloxP/+/NestinCre+/− controls. Right panel: pS6rp localization extended basolaterally in PtenloxP/loxP/NestinCre+/− radial glia. See also Figure S1. (H) Igf1R deficiency in NestinCre expressing cells diminished brain size at E16.5. (I) Brain weights of Igf1RloxP/loxP/NestinCre+/− and controls at E16.5 (brain weight (g) ± S.E.M.: Igf1RloxP/loxP/NestinCre+/−: 0.06; Igf1RloxP/loxP/NestinCre+/−: 0.03 ± 0.001; n = 2 [+/+], n = 3 [−/−]). (J) H&E staining of brains shown in panel (I).
Loss and gain of Igf signaling in mutant mice produced phenotypes similar to those seen when apical complex signaling is disrupted. Mice with Igf1R deficiency limited to neural precursors (Igf1RloxP/loxP/NestinCre+/−) were microcephalic (Figure 2H-J) (Kappeler et al., 2008; Liu et al., 2009), and had a reduced frequency of phospho-Histone H3 (PH3, a marker of cell division) proliferative progenitors in the ventricular zone (PH3-positive cells/100μm VZ ± S.E.M. at E16.5: Control: 2.9 ± 0.3; Igf1RloxP/loxP/NestinCre+/−: 1.7 ± 0.1; unpaired t-test, p<0.01; n=4 and n=3, respectively). We did not observe differences in progenitor cell survival at the ventricular zone in these mice as assessed by cleaved caspase 3 (CC3) immunoreactivity (data not shown). Conversely, mice with increased Igf activity (Igf1 expressed from the human GFAP promoter) were macrocephalic (data not shown) (Ye et al., 2004), and had increased proliferative progenitors at the ventricular surface (PH3-positive cells/100μm VZ ± S.E.M. at E18.5: Control: 0.9 ± 0.08; Igf1_Tg: 1.2 ± 0.07; unpaired t-test, p<0.05, n=3 and n=4, respectively). Together with published work demonstrating that Insulin receptor substrate 2 (Irs2) deletion leads to microcephaly (Schubert et al., 2003), these data suggest that Igf signaling in cortical progenitors, facilitated at the apical surface via Pals1 and an intact apical complex, regulates cortical development.
CSF-borne Igf signaling
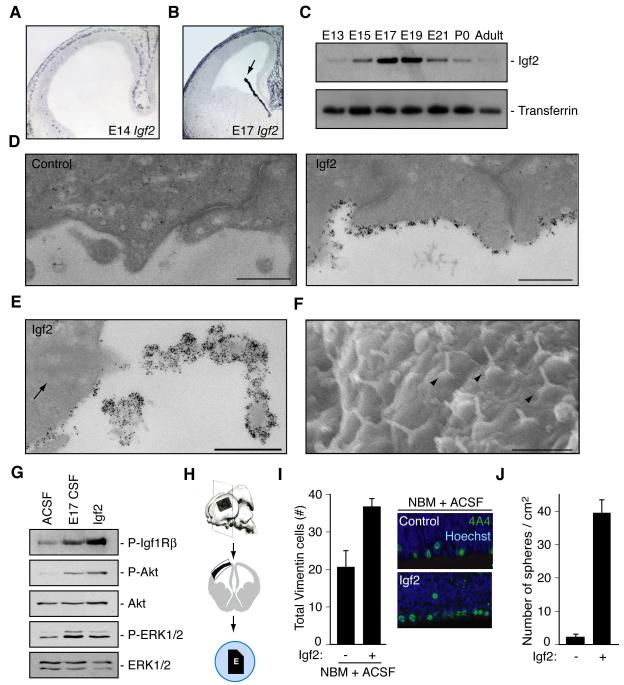
The normal apical localization of the Igf1R, and the fact that we did not observe Igf1 or Igf2 mRNA in neural progenitor cells by in situ hybridization (Figure 3A, B, and data not shown) (Ayer-le Lievre et al., 1991), suggested that progenitor cells may be exposed to Igfs derived from the lateral ventricle CSF. We confirmed the presence of Igf2 in an unbiased tandem mass spectrometry (LC-MS/MS) analysis of CSF (Table S1) (Binoux et al., 1986), and detected Igf1 in CSF by ELISA (E14 CSF [Igf1]: 72.2ng/ml, n=2; E17 CSF [Igf1]: 69.6ng/ml; adult CSF [Igf1]: 68.8ng/ml, n=3). Igf1 expression in the CSF remained stable across the ages sampled (see above). In contrast, expression of Igf2 in rat CSF was temporally dynamic; it peaked during periods of neurogenesis and declined in adulthood (Figure 3C). High levels of Igf2 mRNA expression by the choroid plexus suggested this as a source of CSF Igf2 (Figure 3B), and quantitative PCR revealed that rat choroid plexus expressed 10.7-fold more Igf2 than its cortical counterpart at E17 (data not shown). We confirmed that Igf2 mRNA was also expressed in vascular endothelial cells, and leptomeninges in the rat embryo at E14 and E17 as well as pericytes at E17 (Figure 3A, B and data not shown) (Bondy et al., 1992; Dugas et al., 2008; Stylianopoulou et al., 1988), suggesting that extra-choroidal sources of Igf2 may contribute to CSF-Igf2 content as well. Immunogold labeling revealed Igf2 binding to progenitors along the apical, ventricular surface (Figure 3D). Moreover, Igf2 binding to progenitors was highly enriched along primary cilia (Figure 3E), which extend directly into the ventricular space (Figure 3F) (Cohen et al., 1988). We did not observe enriched Igf2 binding beyond the apical surface of ventricular zone progenitor cells (data not shown). Thus, the robust expression of Igf2 by the choroid plexus and the apical binding of Igf2 to progenitors along the ventricular zone strongly suggest that the CSF distributes choroid plexus secreted Igf2 to cortical progenitor cells.
Figure 3. Igf2 is expressed in cerebrospinal fluid and stimulates progenitor proliferation.
(A, B) Igf2 in situ hybridization of rat E14 and E17 cortex. Arrow points to choroid plexus. (C) Transient Igf2 expression in rat CSF. (D) Immunogold labeling of endogenous Igf2 in E17 rat brain. Left panel: no primary control. Right panel: Igf2 binding to ventricular surface of cortical progenitors. Scale bar represents 500nm. (E) Igf2 binding to primary cilium of cortical progenitor cell. Arrow points to ciliary basal body. Scale bar represents 500nm. (F) Scanning EM of mouse ventricular surface at E12.5. Arrowheads point to primary cilia projecting into the ventricular space. Scale bar represents 2μm. (G) Lysates of cortical cells deprived of growth factors for 6 hours and treated with ACSF, E17 CSF, or Igf2 for 5 minutes were immunoblotted with antibodies to P-Igf1R, P-Akt, Akt, P-ERK1/2, and ERK1/2. (H) Schematic of cortical explant dissections: explant placed on membrane with ventricular side down contacting CSF and notch making medial-caudal side. (I) Left panels: E16 explants cultured with NBM plus ACSF (control) or with supplemental Igf2 immunostained with anti-Vimentin 4A4 and Hoechst represented as mean ± SEM (Igf2 mean: 36.7 ± 2.1; control mean: 20.4 ± 4.46; n = 8; Mann-Whitney; p<0.005). Vimentin 4A4-positive cells increased in explants cultured with Igf2 compared to control. Right panels: Representative images of explants quantified in left panels. (J) Single cells dissociated from primary neurospheres cultured in control media or control media containing Igf2 (20ng/ml). Igf2 stimulated secondary sphere formation after 10 DIV (Igf2 mean: 39.3 ± 4.1; control mean: 2.2 ± 0.75; n = 3; t-test; p<0.005).
Purified rat E17 CSF directly stimulated Igf1R mediated signaling activity, reflected by Igf1Rβ phosphorylation as well as phosphorylation of Akt and MAPK (Figure 3G), two downstream targets of Igf signaling as well as other growth factors that may be present in CSF. Igf2 treatment by itself induced Igf signaling similar to embryonic CSF (Figure 3G). Igf2 binding to progenitors, the localization of the Igf1R, its phosphorylation, as well as the phosphorylation of its downstream targets Akt and MAPK in response to CSF, strongly suggest that the CSF is a primary source of Igf ligands for cerebral cortical neuroepithelial cells, although additional sources cannot be completely excluded.
We next tested whether Igf2 supports progenitor proliferation in a cerebral cortical explant system. In this system, rat embryonic cortex dissected from the lateral pallium is placed on polycarbonate membranes and floated on defined media (Figure 3H). We found that Igf2 added to Neurobasal Medium (NBM) with 20% artificial CSF (ACSF) stimulated the proliferation of progenitor cells marked by phospho-Vimentin 4A4 in rat cortical explants (Figure 3I) (Noctor et al., 2002). In addition, Igf2 treatment alone maintained GLAST-positive neurospheres, an in vitro model of neural stem cells, even in the absence of Fgf2 (Fibroblast growth factor 2) and Egf (Epidermal growth factor) (Figure 3J) (Vescovi et al., 1993). Finally, pharmacologic activation of the signaling pathway with insulin demonstrated that activation of Igf signaling by ligands other than Igf2 is sufficient to stimulate proliferation (PH3-positive cells/100μm VZ ± S.E.M. in E16 rat explant: control mean: 5.6 ± 0.7; insulin (10ug/ml) mean: 11.2 ± 0.4; Mann-Whitney, p<0.05; n=6). Therefore, Igf signaling modulates proliferation of isolated cortical precursors or those maintained in their pallial environment in vitro.
CSF promotes proliferation of progenitor cells in an age-dependent manner
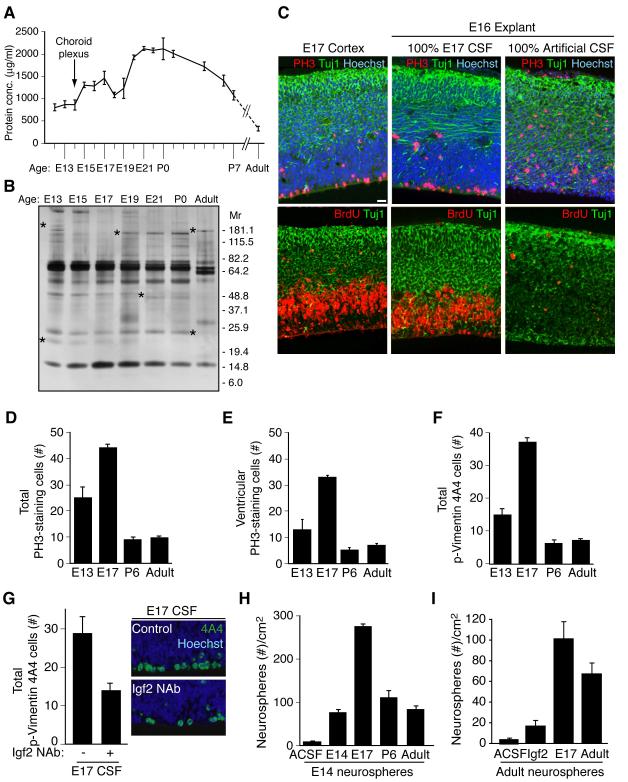
Since the CSF is a complex fluid containing many factors including Igf binding proteins that may modulate Igf2 bioavailability and signaling (Table S1) (Clemmons, 1997; Zappaterra et al., 2007), we tested whether native CSF alone could support cortical tissue growth. We used a heterochronic “mix-and-match” approach for exposing cortical tissue to CSF collected at different ages. E16 rat cortical explants with intact meninges and vasculature cultured with 100% E17 rat CSF for 24 hours, without any additional exogenous media or factors, retained remarkable tissue architecture, cell viability and proliferation, approximating in vivo E17 rat cortex (Figure 4C). In contrast, E16 explants cultured with 100% artificial CSF failed to thrive, had decreased mitotic activity, disorganized neuronal morphology, and increased cell death (Figure 4C, Figure S2A and S2B). Filtration analysis of E17 CSF showed that the sizes of CSF factors that support stem cells likely range from 10kDa – 100kDa, suggesting that they are proteins (Table S2 and data not shown). Thus, the embryonic CSF proteome provides essential growth and survival factors for the developing cortex.
Figure 4. Embryonic CSF supports cortical explant viability and stimulates proliferation of neural progenitor cells.
(A) Total CSF protein concentration over rat development. (B) Silver stain of embryonic rat CSF revealed a dynamic fluid with numerous changes in protein composition over time. Asterisks indicate proteins with varying CSF expression during development. (C) E17 rat cortex and E16 explants grown for 24 hours in 100% embryonic E17 CSF or 100% artificial CSF respectively. Upper panels: anti-PH3 (red), and anti-Tuj1 (green), Hoechst (blue) immunostaining. Lower panels: anti-BrdU (red), and anti-Tuj1 (green) immunostaining. Explants cultured in 100% E17 CSF in vitro maintained tissue histology similar to embryo in vivo. Survival and proliferation of explants cultured with E17 CSF indicated by immunoreactivity for PH3 along the ventricular surface, BrdU incorporation in the ventricular zone, and Tuj1-positive-staining neurons in the developing cortical plate. (D) E16 explants cultured in 100% E13, E17, P6, or adult CSF for 24 hours were immunostained with anti-PH3 (red) and Hoechst (blue)(see Figure S2C). Quantification of total PH3-positive-staining cells per 400μm explant showed that proliferating cells increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF. Immuno-positive cells are represented as mean ± SEM (E17 mean: 44.1 ± 1.43; E13 mean: 25 ± 4.2; P6 mean: 9.2 ± 0.8; adult mean: 9.6 ± 0.9, n = 4; Kruskal-Wallis; p<0.005). (E) Quantification of ventricular PH3-staining cells in explants (panel D). PH3-positive cells along the ventricle were significantly increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF (E17 mean: 32.3 ± 0.79; E13 mean: 12.8 ± 3.9; P6 mean: 4.9 ± 1.0; adult mean: 6.9 ± 0.73; n = 4; Kruskal-Wallis; p<0.01). (F) E16 explants (panel D) immunostained with anti-Vimentin 4A4 (green)(see Figure S2C) were quantified. Vimentin 4A4-positive cells were significantly increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF (E17 mean: 37.1 ± 1.4; E13 mean: 14.9 ± 1.9; P6 mean: 6.1 ± 1.05; adult mean: 7.3 ± 0.6; n = 4; Kruskal-Wallis; p<0.005). (G) Left panels: E16 explants cultured in control E17 CSF or E17 CSF with Igf2 neutralizing antibody (Igf2 NAb), immunostained with anti-Vimentin 4A4 and Hoechst (E17 control mean: 28.8 ± 4.3; E17 IGF2 NAb mean: 13.9 ± 2.0; n = 4; Mann-Whitney; p<0.05). Vimentin 4A4-positive cells decreased in explants cultured with E17 CSF plus Igf2 NAb compared to control. Right panels: Representative images of explants quantified in left panels. (H) Primary neurospheres derived from E14.5 cortex were grown in 20% E13/E14, E17, P6, or adult CSF for 10 days in vitro (DIV). E17 CSF generated the most spheres/cm2 (E17 mean: 274 ± 8.0; E13 mean: 77 ± 7.0; P6 mean: 110 ± 17.5; adult mean: 81 ± 8.8; n = 3; ANOVA; p<0.005). See also Figure S2. (I) Neurospheres derived from adult rat SVZ were cultured in artificial (A)CSF, Igf2 (20ng/ml), E17 CSF, or adult rat CSF for 10DIV. Igf2, E17 CSF, and adult CSF supported the growth and maintenance of adult neurospheres (ACSF: 4.76 ± 0.67; Igf2: 17.3 ± 3.2; E17 CSF: 101.7 ± 15.8; Adult CSF: 67.8 ± 12.6; Kruskal-Wallis: Igf2 vs. E17 CSF, p<0.05; E17 CSF vs. Adult CSF, N.S.; n=3). See also Figure S2.
By comparing rat CSF from several ages, we determined that the effects of CSF on survival and proliferation are strikingly age-dependent, and mimicked the temporal profile of CSF-Igf2 expression (Figure 3C). E17 CSF (near the middle of neurogenesis) maintained the healthiest explants and produced the maximal increase in the frequency of PH3 labeled proliferating cells in E16 cortical explants compared to explants cultured with E13 (early in neurogenesis), P6, or adult CSF (Figure 4D, E, Figure S2C, and data not shown). Many mitotic cells were identified as proliferating neuroepithelial progenitor cells by their immunoreactivity for phospho-Vimentin (4A4, Figure 4F and Figure S2C). In contrast, no differences were seen in Tbr2-positive basal progenitors, which do not contact the CSF directly (data not shown). Together, these data suggest that age-dependent differences in CSF signals are both supportive and instructive for neuroepithelial precursor proliferation in the developing cortex. The CSF effects may be specific to neuroepithelial progenitors, which contact the ventricle through the apical complex, without affecting the intermediate progenitors of the SVZ.
We tested directly whether CSF-borne Igf2 was necessary to explain the effects of age-specific CSF on rat cortical explants. The frequency of proliferating cells declined in explants grown in E17 CSF in the presence of Igf2 neutralizing antibodies (Igf2 Nab; Figure 4G). Igf2 neutralization with Igf2 NAb did not interfere with Igf1 levels in CSF compared to control as assayed by ELISA (data not shown). While Igf signaling is known to promote neuronal survival (Popken et al., 2004), we did not observe differences in ventricular progenitor cell survival in these explant experiments (data not shown), suggesting that Igf actions on neural cell survival likely depends on the cell type, developmental stage, and microenvironment. These data confirm the important role for CSF borne Igf2 in regulating cerebral cortical progenitor cells, but do not rule out roles of other CSF borne factors as well.
CSF influence on isolated neural stem cells requires Igf signaling
Neural stem cells cultured as neurospheres confirmed the age-dependent capacity of CSF to maintain neural stem cells (Reynolds and Weiss, 1996), and provided additional evidence suggesting that Igf2 mediated signaling is an essential determinant of CSF activity on neural stem cells. CSF from any age supported the proliferation and maintenance of isolated cortical stem cells cultured as primary or secondary neurospheres (Figure 4H and data not shown) (Vescovi et al., 1993). However, E17 CSF was maximally effective in generating increased numbers of neurospheres, larger neurospheres, and maintained neurospheres even in long-term cultures for up to 44 days in vitro (Figures 4H, S2D-S2G, and data not shown). Neurospheres grown in CSF retained responsiveness to Fgf2 and Egf, indicating that the CSF maintains stem cells in an uncommitted fate (Figure S2H). CSF generated neurospheres from adult SVZ precursors as well (Figure 4I). Consistent with these observations and our explant studies, the Igf1R inhibitor picropodophyllin blocked the formation of spheres in the presence of E17 CSF (data not shown). Our data suggest that the choroid plexus is the most prominent source of Igf2 in CSF (Figures 3 and S3A). Accordingly, media conditioned with E17 choroid plexus provided enhanced support for neurosphere formation compared to media conditioned with embryonic cortex, adult choroid plexus, or adult brain (Table S3), demonstrating that one or more factors actively secreted from the embryonic choroid plexus, including potentially Igf2, is sufficient for stem cell growth and maintenance. Thus, distinct factors secreted by the choroid plexus into the embryonic CSF, including Igf2, confer E17 CSF with an age-associated advantage to stimulate and maintain neural stem cell proliferation, and Igf signaling is likely one pathway that promotes this process.
Genetic inactivation of Igf signaling impairs brain development
Mouse explant experiments confirmed a requirement for Igf signaling in the proliferation of progenitor cells. Mouse embryonic CSF supported the survival and proliferation of mouse cortical progenitors (C57BL/6 explants: ACSF mean: 7.4 ± 0.2; E16.5 mean: 14.1 ± 1.4; Mann-Whitney; p<0.01; n=3), and purified Igf2 in ACSF stimulated cortical progenitor proliferation (Figure 5A). When the Igf1R was genetically inactivated in cortical progenitors (Igf1RloxP/loxP/NestinCre+/−) (Liu et al., 2009), wild type CSF no longer stimulated cortical progenitor proliferation (ACSF: 17.6 ± 2.9; E16.5 CSF: 16.4 ± 3.0; Mann-Whitney; N.S.; n=3). Importantly, CSF obtained from Igf2−/− mice failed to stimulate progenitor proliferation in wild type explants compared to control (Figure 5B), suggesting that Igf2 in its native CSF environment stimulates proliferation of progenitor cells during cerebral cortical development.
Figure 5. CSF Igf2 regulates progenitor proliferation and brain size.
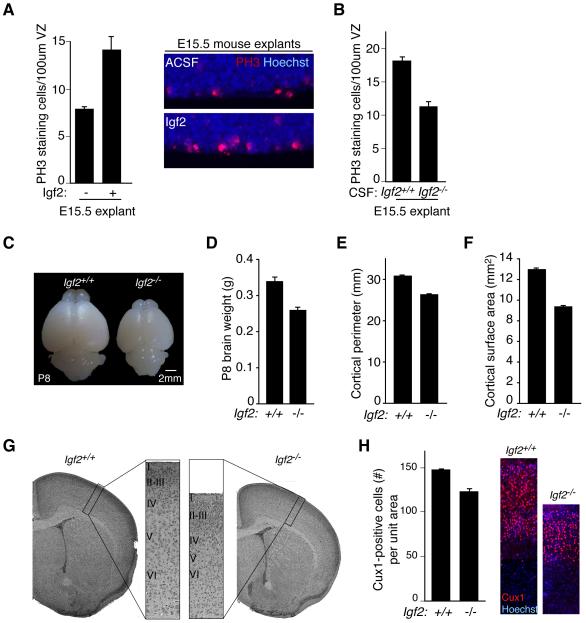
(A) Left panels: E15.5 C57BL/6 explants cultured in NBM supplemented with 20% ACSF or ACSF/Igf2. Igf2 stimulated the proliferation of PH3-positive cortical progenitor cells (C57BL/6 explants: ACSF mean: 7.4 ± 0.2; E16.5 mean: 14.1 ± 1.4; Igf2 mean: 11.2 ± 0.3; Kruskal-Wallis; E16.5 vs. ACSF, p<0.01; Igf2 vs. ACSF, p<0.05; E16.5 vs. Igf2, N.S.; n=3). Right panels: Representative images of explants quantified in left panels. (B) E15.5 C57BL/6 explants cultured in NBM supplemented with 20% ACSF or E16.5 Igf2−/− CSF. Igf2-deficient CSF failed to stimulate progenitor cell proliferation compared to control (ACSF: 17.9 ± 0.8; Igf2−/− CSF: 11.4 ± 1.0; Mann-Whitney; p<0.06; n=3 and n=4, respectively). (C) Representative images of P8 Igf2−/− and control brains. (D) Igf2-deficiency reduced P8 brain weight (Igf2+/+: 0.34g ± 0.008; Igf2−/−: 0.26g ± 0.004; Mann-Whitney, p<0.0001, n=11). (E) Igf2-deficiency reduced P8 cortical perimeter (Igf2+/+: 30.9mm ± 0.01; Igf2−/−: 26.4mm ± 0.1; Mann-Whitney, p<0.0001, n=11). (F) Igf2-deficiency reduced P8 cortical surface area (Igf2+/+: 13.0mm2 ± 0.1; Igf2−/−: 9.4mm2 ± 0.1; Mann-Whitney, p<0.0001, n=11). (G) H&E staining of Igf2−/− and control brains at P8. (H) Left panels: Igf2−/− brains have reduced numbers of upper layer neurons marked by Cux1 (Total Cux1-positive staining cells in equally sized cortical columns expressed as mean ± S.E.M.: Igf2+/+: 157 ± 1.5; Igf2−/−: 131.3 ± 3.3; t-test, p<0.005, n=3). Right panels: Representative images of Igf2−/− and control brains quantified in left panels. See also Figure S3.
As expected for the roles we have shown for Igf2 in regulating proliferation, we found that Igf2-deficiency reduced brain size. Igf2−/− brain weight decreased by 24% at P8 compared to controls (Figures 5C, D). Accordingly, the overall cortical perimeter and surface area were reduced in Igf2−/− brains compared to controls as well (Figures 5E-G). Profound defects in somatic size couple to brain size (Purves, 1988). As previously reported (DeChiara et al., 1991; Baker et al., 1993), Igf2−/− body weight was reduced compared to control (mean body weight (g) at P8: Igf2+/+: 5.6 ± 0.01; Igf2−/−: 2.8 ± 0.1; Mann-Whitney; p<0.0001; n=11), suggesting that Igf2 may be a secreted factor that scales brain size to body size. Consistent with the mouse CSF Igf2 expression pattern that is significantly increased during later embryonic development (Figure S3B), blunting Igf2 expression markedly reduced the proliferating progenitor cells at E16.5 compared to controls (PH3-positive cells/100μm VZ ± S.E.M. at E16.5: Igf2+/+: 2.5 ± 0.3; Igf2−/−: 1.7 ± 0.1; Mann-Whitney; p<0.05; n=5) (DeChiara et al., 1990). NeuN- and late-born Cux1-staining neurons were reduced in Igf2−/− mice (Figure 5H and data not shown), confirming that Igf2 contributes to cortical progenitor proliferation and to late stages of neurogenesis. Taken together, our genetic experiments support a model in which the apical complex localizes Igf signaling in progenitors by ensuring the apical, ventricular localization of the Igf1R. In this manner, the apical complex couples cell autonomous and extracellular signals to the regulation of cortical development.
Glioblastoma CSF expresses high Igf2
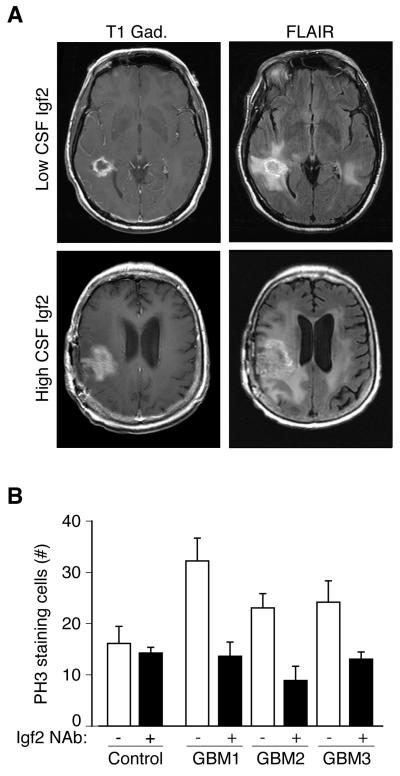
Our data, together with recent findings implicating Igf signaling in the maintenance of adult neural stem cells (Llorens-Martin et al., 2010), raised the possibility that abnormalities of the CSF may be relevant to conditions showing abnormal proliferation, including in glioblastoma multiforme (GBM), a malignant astrocytic brain tumor. Igf-PI3K-Akt signaling has been implicated as a key regulator of gliomagenesis (Louis, 2006; Soroceanu et al., 2007), and mutations in PTEN are commonly found in patients with GBM (Louis, 2006). We analyzed Igf2 concentration in a panel of 56 human GBM patient CSF samples collected from 21 individuals representing the full-range of disease progression, and 8 disease-free controls, and found that CSF from GBM patients contained significantly more Igf2 than CSF from disease-free controls (Igf2 concentration expressed as mean ± S.E.M. for GBM patients: 340.4 ± 12.9ng/ml; n=56; disease-free controls: 222.9 ± 41.5ng/ml; n=8; Mann-Whitney, p<0.01). Three GBM samples containing the highest Igf2 concentrations (605.8ng/ml, 502.8ng/ml, and 468.7ng/ml) came from patients with advanced disease (Figure 6A and Table 1). By contrast, the three patients with the lowest levels of Igf2 (142.1ng/ml, 145.4ng/ml, and 148.2ng/ml) all had early or stable glioma (Figure 6A and Table 1). Similar to rodent ventricular CSF, human lumbar CSF stimulated cortical progenitor cell proliferation in our explant assay, with CSF from GBM patients causing greater proliferation than CSF from disease-free controls (Figure 6B). Moreover, human GBM patient CSF neutralized with Igf2 antibodies failed to stimulate the proliferation of progenitor cells (Figure 6B; Igf2 concentration following NAb absorption: GBM1(PBS): 605.8ng/ml; GBM1(NAb): 45.6ng/ml; GBM2(PBS): 502.8ng/ml; GBM2(NAb): 218.3ng/ml; GBM3(PBS): 468.7ng/ml; GBM3(NAb): 248.8ng/ml). Taken together, these data suggest that beyond embryonic brain development, CSF-Igf2, in particular, is a potential mediator of GBM pathology, and that the CSF mechanisms that normally regulate neural stem cells are misregulated in GBM.
Figure 6. Glioblastoma CSF Igf2 supports progenitor proliferation.
(A) MRI scans from subjects with low and high CSF Igf2 levels. Gadolinium-enhanced T1-weighted (T1-Gad) MRI sequence delineated the contrast-enhanced portion of the tumor where tumor angiogenesis developed. Fluid attenuation inversion recovery (FLAIR) images included area of non-vascularized and invasive tumor (Macdonald et al., 1990). (B) 20% human GBM CSF in NBM stimulated PH3-positive proliferating cells compared to an average of 3 disease-free control CSFs in E16 rat explants (control = 16.0 ± 4.1 (n=3); GBM1 = 32.3 ± 4.3 (n=4); GBM2 = 23.0 ± 2.8 (n=5); GBM3 = 23.4 ± 3.8 (n=4); Mann-Whitney, p<0.05). Igf2(NAb) inhibited GBM CSF-stimulated progenitor proliferation (GBM1 = 13.5 ± 2.9 (n=4); GBM2 = 9.0 ± 2.7 (n=4); GBM3 = 13.0 ± 1.5 (n=3); Mann-Whitney, p<0.05). CSF Igf2 concentration before and after Igf2 NAb absorption: GBM1(PBS) = 605.8ng/ml; GBM1(NAb) = 45.6ng/ml; GBM2(PBS) = 502.8ng/ml; GBM2(NAb) = 218.3ng/ml; GBM3(PBS) = 468.7ng/ml; GBM3(NAb) = 248.8ng/ml).
Table 1. Clinical presentation of GBM patients with lowest and highest CSF Igf2 concentrations.
Patients with the lowest CSF Igf2 concentrations (L1-L3) had early or stable GBM disease state, while patients with the highest CSF Igf2 concentrations (H1-H3) had advanced disease and aggressive tumor progression at time of CSF collection. Tumor size was determined by Macdonald’s criteria, where T1-Gad MRI sequence delineated the contrast-enhanced portion of tumor, and FLAIR images include areas of non-vascularized and invasive tumor (Macdonald et al., 1990). High CSF Igf2 patients had larger T1-Gad tumor sizes compared to low CSF Igf2 patients (Mann-Whitney; p < 0.05; n=3).
| Patient | [Igf2] ng/ml |
Clinical Presentation | |||
|---|---|---|---|---|---|
| Tumor size: T1-Gad (cm2) | Tumor size: FLAIR (cm2) | Lifespan | |||
| Low CSF Igf2 |
L1 | 142.1 | 7.14 | 6.46 | Stable disease at follow-up; 3 weeks post CSF collection |
| L2 | 145.4 | 8.50 | 54.12 | Stable disease at follow-up; 3 weeks post CSF collection |
|
| L3 | 153.9 | 5.94 | 20.5 | Stable disease at follow-up; 5 weeks post CSF collection |
|
| High CSF Igf2 |
H1 | 605.8 | 47.31 | 102.83 | Deceased at 1 week post CSF collection |
| H2 | 502.8 | 13.69 | 53.90 | Deceased at 52 weeks post CSF collection |
|
| H3 | 468.7 | 23.94 | 36.48 | Deceased at 30 weeks post CSF collection |
|
CSF-mediated long-range distribution of additional secreted factors
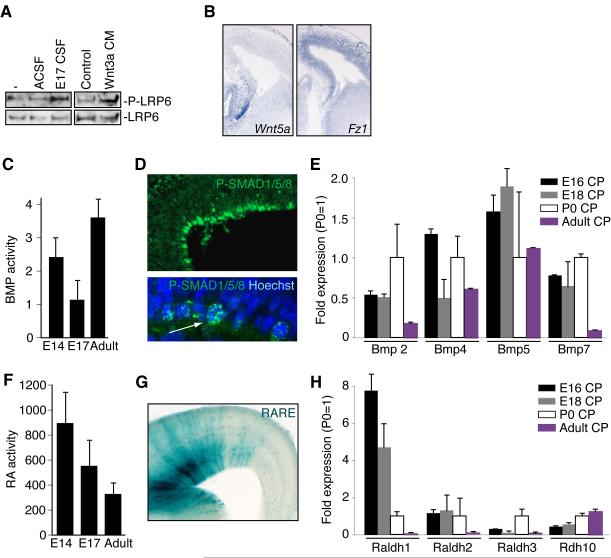
Whereas our studies suggest an important role for Igf2 in controlling proliferation in late stages of neurogenesis and potentially postnatally, they do not rule out the presence of other secreted factors that may act at long ranges via the CSF, and so we performed functional screening tests for several other families of factors. The CSF contained Wnt signaling activity (Zhou et al., 2006), based upon phosphorylation of LRP6, a Wnt co-receptor in response to CSF exposure (Figure 7A). Several Wnt ligands were expressed along the ventricular surface and in the choroid plexus (Figure 7B and data not shown) (Grove et al., 1998). Frizzled (Fz) receptors, which bind LRP6 to transduce Wnt signals, showed enhanced expression in ventricular progenitors (Figure 7B and data not shown) (Zhou et al., 2006), suggesting that CSF may distribute Wnts to precursors throughout the ventricular surface. Additional signaling activities that influence cortical development were also found in the CSF, with responsive cells seen broadly in the ventricular zone. There were dynamic levels of Bone morphogenetic protein (Bmp) activity in the CSF during different stages of cortical development (Figure 7C). Using a luciferase-based assay in which overall Bmp activity can be quantified between 0.1-100ng/ml (data not shown), we found that Bmp activity in the CSF decreased during embryogenesis and peaked in adulthood (Figure 7C). CSF-borne Bmp activity may be responsible for stimulating progenitors widely throughout the cortical ventricular zone in vivo, based on widespread labeling for nuclear phospho-SMAD1/5/8 (Figure 7D) in the absence of any known Bmp ligands localizing to the ventricular zone (Shimogori et al., 2004), whereas Bmps 2, 4, 5, and 7 are expressed in embryonic and adult choroid plexus (Figure 7E) (Hebert et al., 2002; Shimogori et al., 2004). Moreover, Growth and Differentiation Factors 3 and 8 (GDF3 and GDF8), both members of the TGF-β superfamily of proteins that can influence Bmp signaling (Levine and Brivanlou, 2006) were found in our MS analyses of CSF (data not shown), though we do not consider our MS analysis to have recovered all potential smaller ligands in the CSF. Retinoic acid (RA) (Haskell and LaMantia, 2005; Siegenthaler et al., 2009) activity in CSF also varied over the course of cortical development (Figure 7F). A luciferase-based assay that quantifies RA activity ranging between 10−9-10−6M (data not shown) revealed that RA activity in CSF peaked early and decreased in adulthood (Figure 7F). In parallel, RA responsive cortical progenitors localized to the developing ventricular zone (Figure 7G). Similar to Wnts and Bmps, RA is most likely released into CSF since RA synthetic and catabolic enzymes were expressed in the choroid plexus (Figure 7H) and meninges (data not shown). Thus, CSF shows bioavailability of a wide range of activities known to regulate neurogenesis, patterning, and neuronal survival in the cerebral cortex and throughout the CNS.
Figure 7. The CSF proteome coordinates multiple signaling pathways that regulate brain development.
(A) Lysates of cortical cells were left untreated or treated with 20% ACSF or E17 CSF and 10% Wnt3a conditioned medium or its control medium for 2 hours and subjected to immunoblotting with the P-LRP6 or LRP antibodies. (B) In situ hybridization for Wnt5a and Fz1 in mouse E14.5 cortex. (C) Bmp activity was measured in E14, E17, and adult rat CSF as luciferase signal in a clonally derived Bmp-sensitive cell line. Responses were compared to linear responses generated in the same cell line by pure ligand (Bmp4; data not shown). Bmp activity levels varied with age, and were statistically significant between E17 and adult (ANOVA, p<0.001; n=4). (D) Top panel: Expression and nuclear localization of phospho-Smad (P-SMAD) 1/5/8 in E14 rat cortical ventricular cells. Bottom panel: Arrow points to expression and nuclear localization of P-SMAD1/5/8 in E16.5 mouse cortical ventricular cells. (E) qPCR measurement of Bmps 2,4,5, and 7 in the E16, E18, P0, and adult rat choroid plexus (CP). (F) Quantification of RA activity in E14, E17, and adult rat CSF. RA activity declined, based on comparison of CSF activation of an RA responsive, clonally derived cell line with response to RA at known concentrations, from mid-gestation through adulthood (ANOVA, p=0.07; n=4). (G) RA responsive progenitor cells at the cortical ventricular zone from an E16.5 DR5-RARE transgenic mouse (LaMantia et al., 1993). (H) qPCR of Raldh1, 2, 3, and Adh10, in rat CP.
Discussion
We show that the CSF plays an essential, active role in distributing signals in the central nervous system. The key findings of our study are: (1) the apical complex is essential for the apical localization of Igf1R; (2) Pten deficiency in the Pals1 background results in an almost normally sized brain; (3) CSF Igf2 binds to the apical domain of cortical progenitor cells, stimulating their proliferation in an age-dependent manner; (4) Igf2 is upregulated in GBM patient CSF, contributing to the range of proliferative activities of GBM patient CSF; and (5) the CSF provides an adaptive library of secreted factors throughout life. The dynamic regulation of several potent modulators of neural stem cells reinforces the central relationship between local signaling at the apical surface via ligands delivered by the CSF during cortical neurogenesis.
Asymmetric growth factor-based signaling
It has been suggested that asymmetry of signaling at the apical versus basolateral aspect of cortical progenitors regulates progenitor progress through the cell cycle (Bultje et al., 2009; Sun et al., 2005). The basolateral expansion of the Igf1R signaling domain we report in Pten mutants suggests potential links between asymmetric growth factor signaling and proliferation. Although asymmetric localization of the EgfR in cortical progenitors has previously been reported (Sun et al., 2005), the ventricular enrichment of the Igf1R was not known and raises the possibility that the apical enrichment of the Igf1R along with other apical proteins confers a differential responsiveness to mitogenic signals, akin to Notch signaling (Bultje et al., 2009). Since Igfs are potent mitogens for cortical progenitors (Hodge et al., 2004; Popken et al., 2004), one model might suggest that inheritance of the apical complex promotes progenitor fate by differentially concentrating Igf1R and its downstream signaling proteins into cells that retain their perikarya or at least a process (likely a cilium) in the ventricular zone, causing these cells to remain in the cycling pool. The presence of proliferation-inducing factors in the CSF suggests that withdrawal of the progenitor’s apical ventricular process may be an important step in neuronal differentiation (Cappello et al., 2006), by insulating progenitor cells from proliferative signals in CSF, with vascular niches potentially supplying sources of secreted factors for stem cells at other stages (Palmer et al., 2000; Shen et al., 2004; Shen et al., 2008; Tavazoie et al., 2008).
Our data provides a new perspective on the production and provision of Igf ligands, which are known to regulate stem cell populations in the brain and other proliferative epithelia (Bendall et al., 2007; Hodge et al., 2004; Liu et al., 2009; Popken et al., 2004; Ye et al., 2004; Zhang and Lodish, 2004). In the E17 rat brain, the choroid plexus was the strongest source of Igf2, though we cannot discount a contribution by the vasculature or other cellular sources of Igf2 that may percolate into the CSF. Indeed, both pericytes and endothelial cells express Igf2 (Dugas et al., 2008), and Igfs from vascular tissue may have local effects beyond apically-mediated Igf1R signaling shown here. Thus, locally-derived Igf2 may play distinct roles at different developmental time points and in different cellular contexts, and Igf signaling may also be influenced by CSF Igf1 and insulin. Although Igf2 availability decreased in adult CSF (Figures 3C and S3B), Igf2 continued to be expressed in adult choroid plexus (data not shown) and maintained adult neurospheres (Figure 4I), suggesting that low levels of CSF Igf2 contribute to the maintenance of adult neural stem cells. The aberrant increase in Igf2 in advanced GBM patients reinforces the hypothesis that Igf signaling has an influence on proliferation of cortical precursors. Our identification of Igf2 regulation of neurogenesis and brain size complements a literature in which Igf signaling is well known to influence body and brain size (Baker et al., Cell 1993; DeChiara et al., 1991; Purves, 1988), raising the intriguing possibility that Igf2 represents a secreted factor that may scale brain size to body size.
Fluid-based signaling in the CNS and beyond
The activity of growth promoting factors in the CSF and their action on progenitors across the apical surface may be a model for other epithelia including lung, gut, and vascular endothelia that develop in relation to extracellular fluids (Bendall et al., 2007; Scadden, 2006). Extracellular fluid apparently regulates the microenvironment of hematopoietic stem cells, where Igf signaling regulates progenitor proliferation (Orkin and Zon, 2008; Zhang and Lodish, 2004). The differential capacity of Igf signaling to confer a proliferative advantage to stem cells may be regulated in part by Igf’s interactions with binding proteins or other secreted factors in the environment (Clemmons, 1997). Our experiments focused on the age-associated effects of CSF on survival and proliferation across the cortical ventricular zone. However, the distribution of CSF resident proteins, as well as the flow of the CSF, may also influence ciliary orientation and maturing ependymal cell polarity (Mirzadeh et al., 2010), which create activity gradients as has been shown for Slit (Sawamoto et al., 2006).
If a major component of the stem cell niche reflects secreted factors acting at long distances from their sources, modulation of the proteomic composition of extracellular fluids may also provide unexpected ways to regulate stem cell behavior in health and disease. For example, while Igf2 activity peaked in embryonic CSF, some CSF-borne Igf persisted in adulthood (Figure 3, Figure S3B, and data not shown). Igf2 and Igf1 in adult CSF may contribute to the retention of neural stem cell properties in the adult SVZ (Doetsch et al., 1999). Importantly, the regulation of CSF growth factors may also extend to pathologic states. Igf2 and other diffusible growth factors that drive neural progenitor proliferation during development are upregulated in some GBM patients (Louis, 2006; Soroceanu et al., 2007), and GBM patients have elevated Igf2 levels in their CSF. CSF Aβ1-42 and phosphorylated Tau levels were recently shown to assist in Alzheimer’s disease diagnosis (De Meyer et al., 2010). Thus, modulation of the proteomic composition of extracellular fluids together with the integration of cell autonomous determinants of self-renewal by the apical complex may ultimately provide unexpected ways to regulate stem cell behavior in health and disease.
EXPERIMENTAL PROCEDURES
Animals
Time pregnant Sprague Dawley, C57BL/6, and Swiss Webster dams were purchased from Charles River Laboratories and Taconic. Pals1loxP/loxP/NestinCre+/−, Pals1loxP/loxP/Emx1Cre+/−, Igf1RloxP/loxP/NestinCre+/− and GFAP:Igf_1Tg mice were obtained from heterozygous breedings, and PtenloxP/+/Pals1loxP/+/Emx1Cre+/− and PtenloxP/loxP/Pals1loxP/+/Emx1Cre+/− mice were obtained from PtenloxP/+/Pals1loxP/loxP/Emx1Cre+/− and PtenloxP/loxP/Emx1Cre−/− crosses (Groszer et al., 2001; Kim et al., 2010; Liu et al., 2009; Ye et al., 2004). Igf2−/− and control CSF was collected from embryos obtained from homozygous breedings. Igf2−/− and control P8 brains were obtained from homozygous crosses or paternal heterozygotes mated with homozygous knockouts (DeChiara et al., 1991). All animal experimentation was carried out under protocols approved by the IACUCs of Harvard Medical School, Children’s Hospital Boston, and UNC-Chapel Hill.
Antibodies
The following antibodies were purchased: Ctip2, Igf2 (for EM), Tbr2 (Abcam); BrdU (AbD Serotec); Ki67 (Abnova); Vimentin 4A4 (Assay Designs); Pax6 (Developmental Studies Hybridoma Bank); β-catenin, Cdc42 (BD Biosciences); AKT, phospho-AKT, Igf1R, phospho-Igf1R, CC3, and phospho-S6rp (Cell Signaling); GLAST (Chemicon); Tuj1 (Covance); HRP conjugated anti-Transferrin (Immunology Consultants Laboratory, Inc.); Igf2 (NAb; Millipore); Cux1, Igf2 (for WB) (Santa Cruz Biotechnology); and phospho-Histone H3 (Upstate). Tbr1 was a kind gift of R. Hevner.
CSF isolation
Embryonic rodent CSF, isolated as described (Zappaterra et al., 2007), was kept on ice during collection, centrifuged at 10,000g at 4°C for 10 min., and stored at −80°C. Human GBM and disease-free CSF samples were collected by lumbar puncture from patients undergoing clinical evaluation. The 56 GBM samples tested were obtained from 21 individuals representing the full-range of disease progression. The samples used in analyses of highest and lowest CSF Igf2 concentration were obtained from distinct individuals. All research was approved by the IRBs at BIDMC and Children’s Hospital Boston.
Cortical explants
The telencephalic wall was dissected onto polycarbonate membranes (Whatman; 13mm, 8.0um) and cultured for 24 hours as described in text. Artificial (A)CSF (NaCl 119mM, KCl 2.5mM, NaHCO3 26mM, NaH2PO4 1mM, Glucose 11mM, MgCl2 2mM, CaCl2 2.8mM) was supplemented with Igf2 (2ng/ml; US Biologicals) as indicated. Igf2 NAb antibody was incubated with E17 CSF for 1 hour at 4°C. Explants were pulsed with BrdU for 30 minutes and fixed (60% methanol, 30% chloroform, and 10% acetic acid; 10 minutes). For in vivo BrdU labeling, pregnant dams were administered a 3 hour BrdU (60mg/kg) pulse. Tissue was paraffin sectioned (5μm).
Supplementary Material
ACKNOWLEDGEMENTS
We thank A. Bonni, S. Gygi, R. Segal, and members of the Walsh laboratory for helpful discussions; H. Steen for assistance with mass spectrometry; D. Rubin and J. Sheng for pMSCVhyg-Igf2; U. Berger, J. Buchanan, M. Ericsson, Y. Lin, A. Peters, C. Kourkoulis, and S. White for technical assistance. This work was supported by a Sigrid Jusélius Fellowship, an Ellison/AFAR Postdoctoral Fellowship, and Award Number K99NS072192 from the NINDS (M.K.L); a Stuart H.Q. & Victoria Quan Fellowship (M.W.Z.), a NIH MSTP grant (M.W.Z. and Y.J.Y.); the Child Neurology Foundation (X.C.); A Reason To Ride research fund (M.L and E.T.W.), a NINDS grant (RO1 NS048868)(A.J.D. and P.Y.), a NICHD grant (RO1 HD008299)(A.J.D.), a NIH grant (HD029178) and an UNC-CH Reynolds Faculty Fellowship (A.S.L.); a NINDS grant (3 RO1 NS032457), the Manton Center for Orphan Disease Research, and the Intellectual and Developmental Disabilities Research Centers (CHB DDRC, P30 HD18655)(C.A.W.). C.A.W. is an Investigator of the Howard Hughes Medical Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ayer-le Lievre C, Stahlbom PA, Sara VR. Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development. 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- Binoux M, Lassarre C, Gourmelen M. Specific assay for insulin-like growth factor (IGF) II using the IGF binding proteins extracted from human cerebrospinal fluid. J Clin Endocrinol Metab. 1986;63:1151–1155. doi: 10.1210/jcem-63-5-1151. [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, Jr., LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev. 2009;23:1619–1624. doi: 10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Cohen E, Binet S, Meininger V. Ciliogenesis and centriole formation in the mouse embryonic nervous system. An ultrastructural analysis. Biol Cell. 1988;62:165–169. [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K, Evans C, Lai P, Lorscheider F, Malinowska D, Mollgard K, Saunders N. Proteins in cerebrospinal fluid and plasma of fetal rats during development. Dev Biol. 1981;83:193–200. doi: 10.1016/s0012-1606(81)80024-3. [DOI] [PubMed] [Google Scholar]
- Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283:23440–23449. doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Schwark HD. Neuronal primary cilia: a review. Cell Biol Int. 2004;28:111–118. doi: 10.1016/j.cellbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Hodge RD, D’Ercole AJ, O’Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PA, Cappello S, Gotz M. Stem cell ncihes during development - lessons from the cerebral cortex. Curr Opin Neurobiol. 2010;20:1–8. doi: 10.1016/j.conb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. doi: 10.1016/s0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- Kim S, Lehtinen MK, Sessa A, Zappaterra MW, Cho SH, Gonzalez D, Boggan B, Austin CA, Wijnholds J, Gambello MJ, et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 2010;66:69–84. doi: 10.1016/j.neuron.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia AS, Colbert MC, Linney E. Retinoic acid induction and regional differentiation prefigure olfactory pathway formation in the mammalian forebrain. Neuron. 1993;10:1035–1048. doi: 10.1016/0896-6273(93)90052-s. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. GDF3 at the crossroads of TGF-beta signaling. Cell Cycle. 2006;5:1069–1073. doi: 10.4161/cc.5.10.2771. [DOI] [PubMed] [Google Scholar]
- Liu W, Ye P, O’Kusky JR, D’Ercole AJ. Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. J Neurosci Res. 2009;87:2821–2832. doi: 10.1002/jnr.22129. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci. 2010;44:109–117. doi: 10.1016/j.mcn.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- Macdonald DR, C. T, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118:5157–5159. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- Martin C, Bueno D, Alonso MI, Moro JA, Callejo S, Parada C, Martin P, Carnicero E, Gato A. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev Biol. 2006;297:402–416. doi: 10.1016/j.ydbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Han Y-G, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. The Journal of Neuroscience. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parada C, Gato A, Bueno D. Mammalian embryonic cerebrospinal fluid proteome has greater apolipoprotein and enzyme pattern complexity than the avian proteome. J Proteome Res. 2005;4:2420–2428. doi: 10.1021/pr050213t. [DOI] [PubMed] [Google Scholar]
- Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- Purves D. A Trophic Theory of Neural Connections. Harvard University Press; Cambridge: 1988. Body and Brain. [DOI] [PubMed] [Google Scholar]
- Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Kharbanda S, Chen R, Soriano RH, Aldape K, Misra A, Zha J, Forrest WF, Nigro JM, Modrusan Z, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104:3466–3471. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulou F, Efstratiadis A, Herbert J, Pintar J. Pattern of the insulin-like growth factor II gene expression during rat embryogenesis. Development. 1988;103:497–506. doi: 10.1242/dev.103.3.497. [DOI] [PubMed] [Google Scholar]
- Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- von Stein W, Ramrath A, Grimm A, Muller-Borg M, Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132:1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- Weber MM, Melmed S, Rosenbloom J, Yamasaki H, Prager D. Rat somatotroph insulin-like growth factor-II (IGF-II) signaling: role of the IGF-I receptor. Endocrinology. 1992;131:2147–2153. doi: 10.1210/endo.131.5.1425415. [DOI] [PubMed] [Google Scholar]
- Wodarz A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol. 2005;17:475–481. doi: 10.1016/j.ceb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell. 2007;28:886–898. doi: 10.1016/j.molcel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Ye P, Hu Q, Liu H, Yan Y, D’Ercole A J. beta-catenin mediates insulin-like growth factor-I actions to promote cyclin D1 mRNA expression, cell proliferation and survival in oligodendroglial cultures. Glia. 2010;58:1031–1041. doi: 10.1002/glia.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D’Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res. 2007;6:3537–3548. doi: 10.1021/pr070247w. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.