Abstract
During exocytosis, neuroendocrine cells can achieve partial release of stored secretory products from dense core vesicles (DCVs) by coupling endocytosis directly at fusion sites and without full discharge. The physiological role of partial secretion is of substantial interest. Much is known about SNARE-mediated initiation of exocytosis and dynamin-mediated completion of endocytosis, but little is known about coupling events. We have used real-time microscopy to examine the role of secretory carrier membrane protein SCAMP1 in exo-endocytic coupling in PC12 cells. While reduced SCAMP1 expression is known to impede dilation of newly opened fusion pores during onset of DCV exocytosis, we now show that SCAMP1 deficiency also inhibits closure of fusion pores after they have opened. Inhibition causes accumulation of fusion figures at the plasma membrane. Closure is recovered by restoring expression and accelerated slightly by overexpression. Interestingly, inhibited pore closure resulting from loss of SCAMP1 appears to increase secondary fusion of DCVs to already-fused DCVs (compound exocytosis). Unexpectedly, reinternalization of expanded DCV membranes following compound exocytosis appears to proceed normally in SCAMP1-deficient cells. SCAMP1’s apparent dual role in facilitating dilation and closure of fusion pores implicates its function in exo-endocytic coupling and in the regulation of partial secretion. Secondarily, SCAMP1 may serve to limit the extent of compound exocytosis.
Keywords: neurosecretion, exocytosis, endocytosis, PC12 cells, dense core vesicle
In neuroendocrine cells, most secretory contents including neurotransmitters, hormones and proteins are stored in membrane-bounded dense core vesicles (DCVs) and released by exocytosis in response to stimulation. This is a highly regulated process in which DCVs and the plasma membrane contact one another and rearrange to create a fusion pore, through which the contents are expelled to the extracellular space. Following release, membrane is recovered by endocytosis for recycling. Fusion is driven by trans-complexes of SNARE proteins that link the DCV and plasma membrane (1–4). While much has been learned about the molecular events that bring the partner membranes into contact, set up the SNARE complexes, and regulate their fusion-ready state, much less is known about the ensuing events of fusion itself, including the composition of the fusion pore, how it expands, and ultimately how compensatory endocytosis is coupled to cause pore closure (5,6).
Recently, the calcium- and phosphoinositide-binding fusion regulator synaptotagmin, secretory carrier membrane proteins SCAMPs 1 and 2, myosin II, and the membrane lipid phosphatidylserine have been implicated in promoting opening, stabilization and expansion of nascent fusion pores (7–19). Also, physiological studies suggest that endocytic coupling may accommodate a range of mechanisms to provide signal-dependent control over the amount, composition and duration of secretion (20–23). For individual DCVs, their contents can be released in part or in full in a given exo-endocytic event (24,25) depending on whether the fusion pore flickers open and closed (“kiss-and-run”) or expands such that the DCV membrane flattens into the cell surface (“full fusion”). During flickering, small molecules, e.g., catecholamines, are released selectively and DCV membrane is recovered directly, whereas during full fusion, stored contents are released in entirety and the DCV membrane is recovered slowly and less directly by clathrin-mediated endocytosis (21). A third mode of coupling, cavicapture (26,27), has emerged as a major mechanism in neuroendocrine cells (26–31). It entails partial expansion of the fusion pore to an omega or U shape followed by direct constriction and recovery of the DCV membrane. In contrast to flickering, membrane reinternalization during cavicapture typically is delayed for seconds to minutes (26,28); content polypeptides are released at different rates and to differing extents (26,27,32), and DCV membrane proteins are retained and directly internalized with varying efficiency (e.g. 24,27,33,34). However, almost no information exists regarding membrane proteins that might link pore dilation and closure during cavicapture.
Because earlier studies implicated a function for SCAMPs in late events related to the process of fusion pore opening in both mast cells and neuroendocrine PC12 cells (9–12,35), we have been interested in the possibility that their roles might extend to fusion pore closure, particularly in PC12 cells where full fusion is rare and cavicapture is used almost exclusively for exo-endocytic coupling (26,36). In PC12 cells where the five known mammalian isoforms are all expressed, SCAMPs 1 and 2 concentrate in part near exocytotic sites in the plasma membrane in addition to marking intracellular membranes involved in recycling (10). SCAMPs are tetra-spanning integral membrane proteins that have a highly conserved core structure flanked by more variable cytoplasmic N- and C-terminal domains (37,38). The membrane core likely binds electrostatically to polyanionic phospholipids (39,40), a capability that would seem suitable for organizing polyphosphoinositides in support of both fusion pore opening and endocytic closure (8,27,41,42). Our previous findings in which we monitored exocytosis in real time using amperometry identified associations of both SCAMPs 1 and 2 in fusion pore formation and dilation (11,12). While depletion of SCAMPs 1 and 2 each impaired these processes, the effects for each isoform differed in part. Moreover, SCAMP2 specifically exhibited a more proximal role (12) thought to reflect its selective interactions with the small GTPase Arf6 and phospholipase D1 in support of local synthesis of phosphatidylinositol 4,5-bisphosphate (PIP2) (11). Herein, we use two-photon fluorescence and total internal reflection fluorescence microscopy to show that SCAMP1 but not SCAMP2 is required after pore dilation for fusion pore closure during exo-endocytosis in PC12 cells. In making this discovery, we have been able to use SCAMP1-deficient cells to evaluate effects on pore closure even though our earlier studies showed upstream pore dilation to be impaired. Quite interestingly, we also have found that inhibited fusion pore closure in SCAMP1-deficient cells increases the incidence of compound exocytosis by capitalizing on the sustained open fusion figures. Moreover, compound exocytosis in turn enables pore closure to proceed even in the absence of SCAMP1. These new insights into fusion pore dynamics emphasize the critical roles of SCAMPs 1 and 2 in the cavicapture process and highlight the pivotal contribution of SCAMP1 to pore closure in exo-endocytic coupling.
Results
SCAMP1 Knockdown Changes Kinetics of Coupled Exo-endocytosis in PC12 Cells
The development of two-photon extracellular polar-tracer imaging quantification (TEPIQ; 36) has enabled us to analyze fusion pore duration in relation to neuroendocrine secretion and in particular to evaluate candidate proteins that might regulate this process. In initiating this effort, we focused on SCAMP1. Exocytosis was triggered in PC12 cells by UV photolysis of internalized caged Ca2+ (NP-EGTA). Separate measurements using fura-2FF (43) showed that free intracellular Ca2+ rapidly peaked at ~50 µM and declined to ~20 µM during recording (Fig 1S). Cells were locally perfused with sulforhodamine B (SRB) before and throughout recording. We examined exocytosis at the base of the cells, and formation of fusion pores allowed influx of fluorescent dye into the lumens of opened DCVs and enabled their visualization against the dark non-fluorescent cytosol. Prior to experimentation, cells were transfected with control or SCAMP1-specific siRNAs, leading in the latter case to ≥ 90% loss of SCAMP1 (See Methods and Fig 2S) and without effect on intracellular Ca2+ kinetics (12). Before UV photolysis, limited low intensity SRB foci and a few bright foci were occasionally observed. These may reflect the uneven contour (folds) of the basal cell surface or SRB aggregates, respectively. They were excluded from our analysis, which focused only on photolysis-dependent puncta. Within the first few seconds after UV photolysis, focal fluorescent brightening appeared atop either the dark or modestly fluorescent background (Fig 1). In controls, the foci mostly brightened abruptly consistent with an exocytotic event then gradually decayed and nearly disappeared before perfusion ceased. For SCAMP1 KD, events began similarly but with slightly weaker signal; however, in the majority, there was a second extended phase of brightening that ultimately tapered off toward the end of recording (Fig 1A, C; Supplementary Movies 1 & 2). Our recording rate (1s/frame) only reports open vs. closed pores; rates of opening/closing of individual pores are not resolved because SRB (Mr 558D; 1.4 nm dia) will diffuse through 6-nm fusion pores of newly opened DCVs in < 1ms (36,43). However, sustained detection of fused DCVs enabled us to infer that events are mainly cavicapture (26,43). This behavior rules out full fusion/flattening into the surface, which occurs within ~20–40 ms (44) and is not a significant mechanism in PC12 cells (36). It also rules out fast kiss-and-run, where luminal staining through flickering ~2 nm pores is severely restricted (43). Indeed, previous analysis demonstrated simultaneous staining with 10kD dextran (6 nm dia) and SRB in PC12 cells (43).
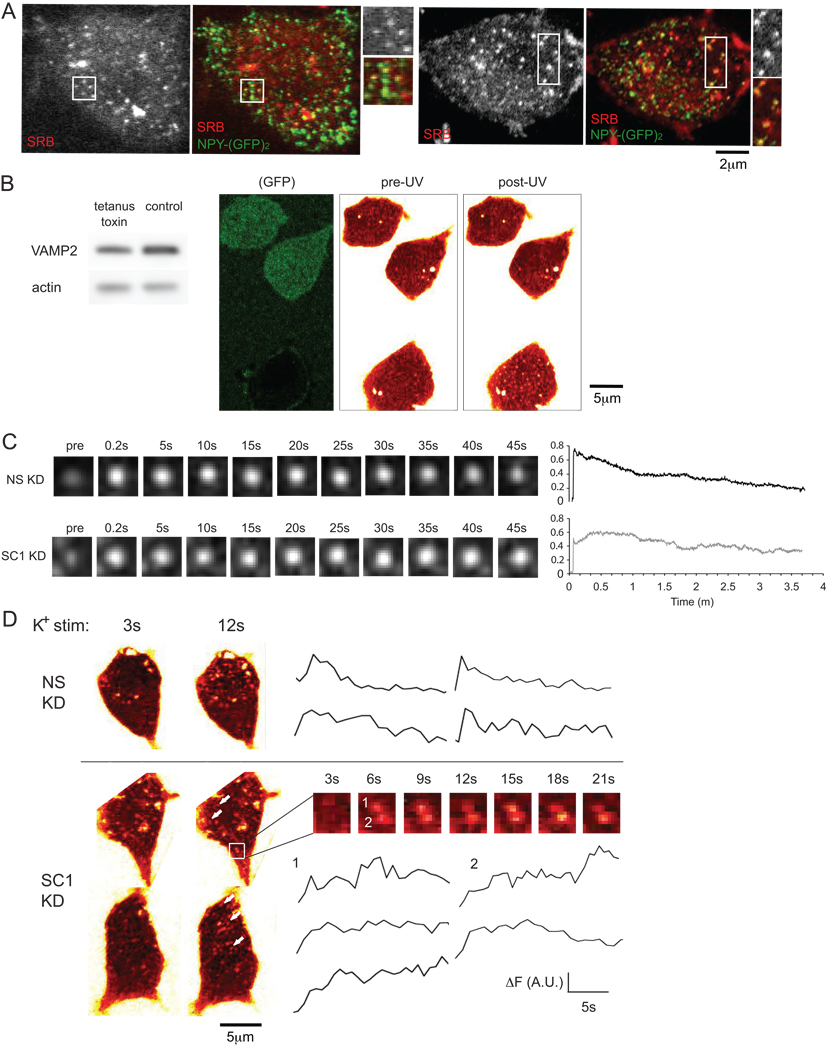
Figure 1. Effect of SCAMP1 knockdown on the kinetics of exocytosis as detected by TEPIQ and lack of effect of SCAMP2 knockdown.
(A) Sample images of the lower surfaces of control (NS KD) and SCAMP1 knockdown (SC1 KD) before (left) and 4 s (middle) and 40 s (right) after stimulation by UV photolysis of pre-loaded NP-EGTA (caged Ca2+). Cells were locally perfused with SRB-containing medium before and throughout recording, and images were obtained at 1 Hz. As previously (43), example images were displayed in Glow Scale color palette (as in Zeiss LSM Image Examiner) to remove background due to extracellular SRB, enabling visualization of exocytotic (cavicapture) events. Images are taken from Supplementary Movies 1 & 2. (B) Several examples of tracings of individual events showing fluorescence intensity change over time. Arrowheads indicate the beginning of the second step of signal increase within two-step events. (C) Fluorescence intensity change over time averaged for all events from SC1 KD and from NS KD cells; 375 events in 10 cells from 2 experiments for SC1 KD and 290 events in 7 cells from the same experiments for NS KD. Error bars indicate SEM. (D) Cumulative curves showing number of events per cell that had reached their peak fluorescence intensity by the indicated timepoint; two-step events were recorded only at the maximum of the second rise. (E) Proportion of one- vs. two-step events in SC1 KD and NS KD cells calculated from all events. In all experiments, only two massive multi-step events (43) were observed. (F) Examination of SCAMP2 knockdown. Fluorescence intensity change over time averaged from all events for SCAMP2 knockdown (SC2 KD) and control (NS KD) cells is shown; for both cell types, data were pooled from 6–10 cells over two experiments.
When the change in fluorescence intensity of individual foci over time was examined, two types of events were distinguished, and their relative incidence differed in control and KD cells. In controls, 59.1% of events showed a single-step rise in intensity upon stimulation; and in the rest (40.9%), the increased fluorescence was mainly two-step. For SCAMP1 KD, however, single-step changes accounted for only 21.5%, while two-step events increased to 78.5% of total events (Figure 1B, E). For single-step events, there was a significant difference in their shape. In controls the fluorescence post peak progressively decreased to baseline, which for the cavicapture mechanism indicates bleaching after pore closure (43). In SCAMP1 KD, fluorescence after the single-step rise mostly (> 80% of events) remained constant throughout recording. For the two-step events, further examination revealed a difference in the kinetics of stepwise increases in fluorescence in control and SCAMP1 knockdown cells. In controls, the second increase always occurred within 5 s after the initial rise, but in KD cells, it was substantially delayed (> 15 s) (Figure 1B). Notably, the second increase occurred step-wise and was incrementally greater than that observed in single-step events, consistent with occurrence of a second fusion event (43). The effect of knockdown on the overall response was clearly shown by a cumulative plot in which the time to attain peak fluorescence was recorded for all events (Fig 1D); in this analysis, two-step events were plotted only at the timepoint where they reached their highest intensity. This plot documents the two-phase nature of the response in both types of cells and at the same time emphasizes the higher incidence of two-step events, the slower rises for each step, and the prolonged period between steps in SCAMP1-deficient cells. The results likely reflect not only inhibitory effects of SCAMP1 deficiency on fusion pore opening (12) but also highlight impaired closure of the pore post-dilation as well as the possibility that this delay may enhance secondary fusion at the same sites (compound exocytosis).
TEP imaging, though advantageous for studying late events in fusion pore kinetics and shedding new light on the process, is an indirect way to detect exocytosis. In order to clarify that the events observed with TEP imaging were indeed DCV exocytosis, we tried to compare our SRB signal vs. DCV marker NPY-(mGFP)2. NPY-(mGFP)2 is a chimera of neuropeptide Y attached to two GFPs and due to its large size it is efficiently retained in DCVs during exocytosis (32). PC12 cells expressing NPY-(mGFP)2 were stimulated in the presence of SRB using the same method as in Fig 1. The dye was washed out 30s after stimulation and the signal resulting from SRB immediately after intracellular uptake was compared to NPY-(mGFP)2 by confocal microscopy. Quantification from fourteen cells in three separate experiments indicated that 82% of the SRB-labeled spots were marked by NPY-(mGFP)2 for the green image recorded at the same focal plane (Fig 2A). This implies that most SRB spots mark DCVs which have retained NPY-(mGFP)2 post cavicapture. To further confirm that SRB foci appearing upon photolysis correspond to DCV exocytosis, we transfected PC12 cells with the light chain of tetanus toxin using a bicistronic vector that also expresses GFP. In green toxin-expressing cells, NPE photolysis does not generate SRB foci most of the time (>80%) whereas non-expressing cells generally exhibit ample foci (Fig 2B).
Figure 2. Correlation between UV photolysis-triggered response and DCV exocytosis (A, B); defective closure of DCV fusion pores in SCAMP1 KD cells following K+ depolarization revealed by TIRF-M and by TEP imaging (C, D).
(A) Foci of internalized SRB colocalize with NPY-(mGFP)2, the retained DCV marker, at exo-endocytotic sites stimulated by NPE photolysis in PC12 cells -- SRB (left panel and upper enlarged image for each cell), corresponding merged image with NPY-(mGFP)2 (right panel and lower enlarged image for each cell). Data pooled from 14 cells in three experiments indicated 82% colocalization. (B) Inhibition of exocytosis by tetanus toxin light chain blocks appearance of SRB foci in NPE-loaded PC12 cells stimulated by UV photolysis. GFP-labeled (toxin expressing) cells are distinguished from unlabeled (nonexpressing) cells in the same field. Over 80% of toxin-expressing cells failed to respond to UV stimulation; ~30 cells from two separate experiments. Western blot (left panel) illustrates loss of VAMP2 (upper blot) consistent with expression of toxin in ~20% of cells (determined by fluorescence of coexpressed GFP); β-actin (lower blot) was used to normalize loads. (C) PC12 cell populations transfected with non-specific or SCAMP1-directed siRNA were each transfected with NPY-(mGFP)2, a secretory protein that concentrates in DCVs but is not released through fusion pores and is thus efficiently reinternalized by cavicapture as described in the text. Cells were stimulated by perifusion of high K+-depolarizing buffer. Images were recorded by TIRF-M at 5 Hz over 4 m. Exocytosis was identified in the recordings by abrupt increase in fluorescence intensity within one frame (0.2 s) due to exposure of the GFP to extracellular medium (> pH 7). A pre-stimulation image is shown at the left of each example. Traces (right) showed averaged change in fluorescence intensity of DCVs over time for control (NS KD, upper) and knockdown (SC1 KD); 10–15 events in 4–6 cells from 2 experiments were used for each cell type. For data processing, the fluorescence intensity (arbitrary units) of an individual event was first normalized to its pre-stimulation intensity and the net changes over time for all events were then pooled and averaged. (D) Control (NS KD) and SC1 KD cells stimulated by perifusion with high K+ depolarization were recorded using TEP imaging as described. Examples of cells stimulated for 3s and 12s are displayed in Glow scale (Zeiss, left). Representative traces of individual events from both cell types are shown on the right; selected examples among several exocytotic events are marked by arrows. A zoom up of two events in a SC1 KD cell (1, 2) is shown (right, middle panel) and traces of those events illustrate stepwise increase in their fluorescence intensity. Note that stained extracellular debris was masked from the image along the border of the upper SCAMP1 cell.
SCAMP2 Knockdown does not affect Cavicapture
Before considering the effects of SCAMP1 further, we were interested to determine whether knockdown of SCAMP2 caused a similar phenotype as SCAMP1 knockdown. RNAi-mediated knockdowns of SCAMP2 are as efficient as those of SCAMP1 (12). As shown in Fig 1F, the averages of multiple samples of control and SCAMP2 KD cells examined in parallel revealed no detectable difference. Therefore, the effects are SCAMP1-specific.
Delayed Pore Closure in SCAMP1-Deficient Cells in Response to Depolarization Visualized by both TIRF and TEPIQ
Because TEP imaging potentially detects exo-endocytotic events other than those related to DCVs (45, 46), we also used a separate approach that focuses selectively on DCVs. We expressed NPY-(mGFP)2 and monitored exocytosis by total internal reflection fluorescence (TIRF) microscopy. NPY-(mGFP)2 is stored in DCVs; during exocytosis it exhibits pH-dependent brightening, but because it is retained and internalized during cavicapture, its signal is decreased by reacidification of the closed vesicle (32). Cell populations transfected with non-specific or SCAMP1-directed siRNA were each transfected with NPY-(mGFP)2. Because the TIRF experimental set-up could not be combined with flash photolysis, the cells were stimulated by local perfusion of KCl while continuously imaging. The results (Fig 2C) corresponded well with those obtained by TEP imaging. In both samples, brightened foci appeared along the plasma membrane in the evanescent field; in controls, the intensity gradually decreased while in the knockdowns, it appeared sustained throughout the recording (left; series of images). Averaging of 10 – 15 events generalized the trends observed in individual image series (Fig 2C, right) and in addition revealed two other features. First, the abrupt fluorescence increase that signifies onset of exocytosis was higher in control than in SCAMP1 KD cells. This probably reflects a difference in the level of expression of NPY-(mGFP)2 in the separately transfected control and knockdown cells. Second, the abrupt fluorescence increase in SCAMP1 KD cells is followed by a second slower increase similar to what was observed by TEP imaging and over approximately the same timecourse (Fig 1C).
To further support that we were detecting and analyzing exocytosis of DCVs, we tested K+ depolarization in place of NPE photolysis in our TEPIQ procedure. To enable assay of multiple cells per experiment, K+ and SRB were applied together to a succession of individual cells by local perifusion. In each case, analysis was initiated when SRB level stabilized (~3 s) and thus reflected stimulation in progress. As shown in Fig 2D, stimulation induced the appearance of SRB foci of similar size and intensity as observed in Fig 1A, B. These foci occurred against a background that was variable from cell to cell. Generally, there were fewer stimulated events and they were less synchronized than with NPE photolysis. Their incidence and timing were consistent with observations of DCV exocytosis observed by TIRF and by amperometry in our previous studies (11, 12; see also Discussion). Examples of intensity traces of individual events (Fig 2D, right) reiterate the observations using NPE photolysis (Fig 1) and also the TIRF study (Fig 2C); fluorescence progressively declined post-peak in controls and remained elevated in SCAMP1 knockdowns, indicative of pore closure and sustained opening, respectively. Moreover, some events, particularly in SCAMP1 knockdowns, showed two-step brightening (see event 2, Fig 2D magnified inset) as observed and studied in detail following NPE photolysis. Finally, it should be noted that free Ca2+ in our depolarization experiments peaked at 26 µM (12) comparable to depolarization-induced Ca2+ in another study (30) and half the level elicited by NPE photolysis (Fig 1S), which was used in most of our experiments.
SCAMP1 Deficiency Inhibits Fusion Pore Closure
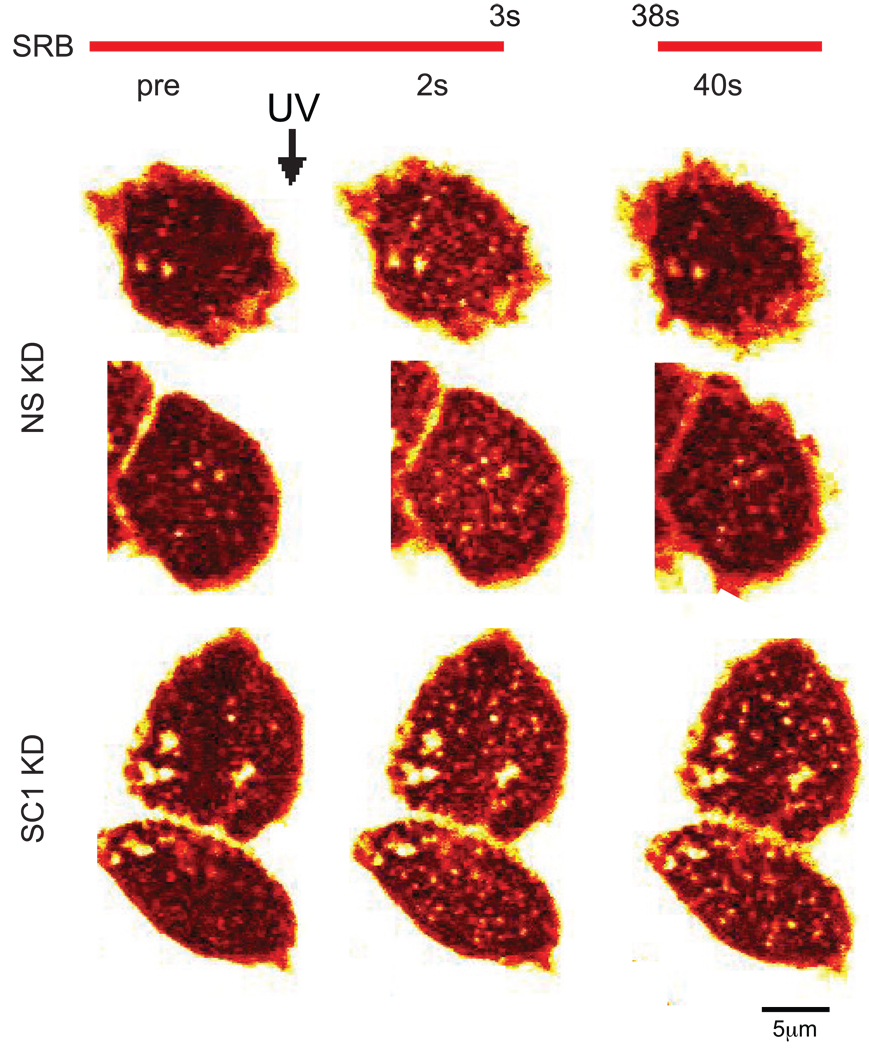
As shown above, single-step events in SCAMP1 knockdown cells showed little or no decline in fluorescence (Fig 1B), and this was accompanied by increased incidence of two-step events potentially signifying compound exocytosis (Fig 1B, E). Both observations suggest that fusion pore closure is inhibited. We tested this possibility further using a dye-delay protocol. Cells were first briefly perifused with SRB-containing solution (~ 5 s), and pre-stimulation images were obtained (Fig 3, left panels). UV flash was then performed, and initial post-stimulation images were recorded (Fig 3, middle panels). Dye perifusion was then stopped; after 30 s to allow dye to diffuse away, SRB was re-delivered, and images were captured a few seconds later (Fig 3, right panels). Control cells exhibited a burst of exocytotic events that had largely disappeared by the end of recording (Fig 3, top), suggesting that most all pores opened by stimulation had closed before SRB re-perfusion. In contrast, almost the same pattern of foci observed soon after UV flash of SCAMP1 depleted cells were observed after the delayed reapplication of dye (Fig 3 bottom), indicating that initially opened pores had largely remained open. In fact, many pores remained open when this interval was increased to 2 m (not shown), consistent with the sustained signal observed in Fig 1B. Thus SCAMP1 knockdown clearly extends pore lifetime and interferes with closure.
Figure 3. Delayed SRB perifusion documents that fusion pore closure is inhibited in SCAMP1 KD PC12 cells.
Examples of control (NS KD) and SCAMP1-deficient (SC1 KD) cells in which persistence of fusion pore opening was tested by a combination of initial and delayed perifusion with SRB. Initially, SRB was perifused on the cells of interest as described. After obtaining a pre-flash image (left), UV flashing was performed to uncage Ca2+ and a post-UV image was obtained immediately thereafter. SRB perifusion was then stopped to allow dye to diffuse away. After 35 s, SRB was reapplied, and te final images (right) were captured within 5 s. Differences between post-UV and final images were used to judge persistence of open fusion pores.
Two Populations of Exocytotic Events in PC12 Cells
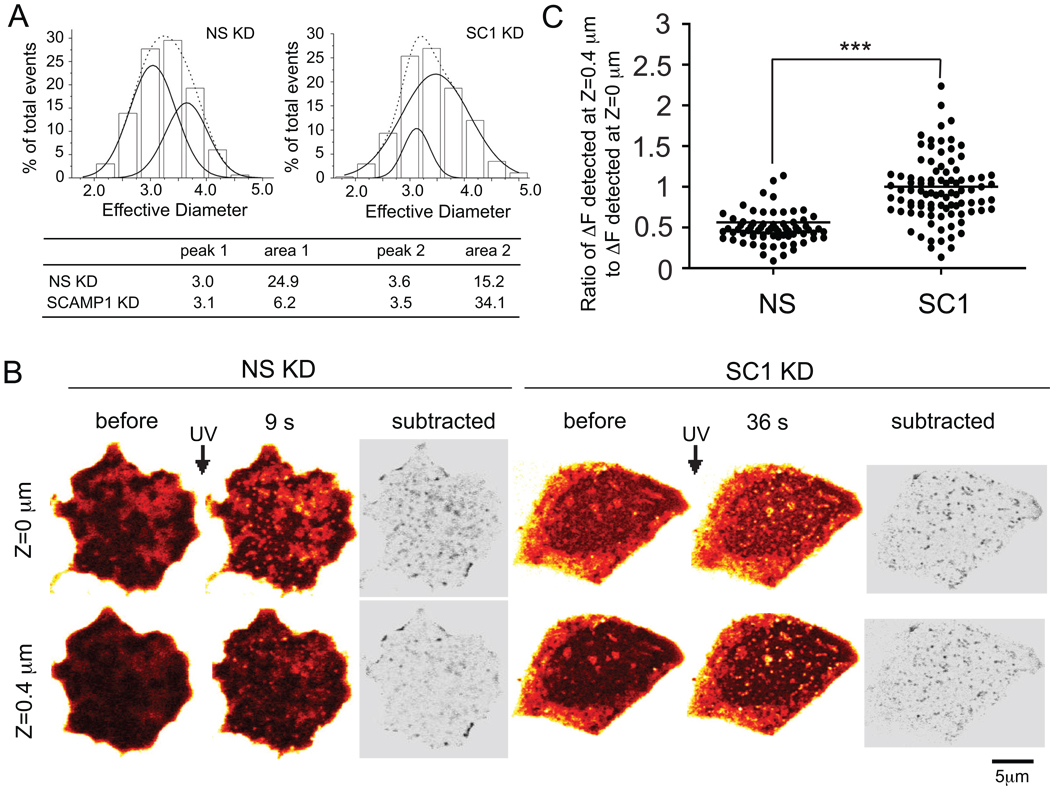
Demonstration of one- and two-step increases in signal led us to consider the fluorescence intensity distribution of the foci. SRB does not stain DCV membrane or matrix (43), so the dye likely fills the luminal space homogeneously. Assuming that dye excitation is uniform within the two-photon focal plane, peak fluorescence should be proportional to the volume of the vesicle. For DCVs in PC12 cells, the cube root of peak signal has been used to represent relative vesicle diameters and fit to Gaussian distributions (43,47). In performing a similar analysis, we found that control and SCAMP1 knockdown were best fit by two Gaussians (Fig 4A). As shown, the two peaks centered on the same two effective diameters (~3 (peak 1) and 3.5 (peak 2)) in both types of cells, yet the relative areas under the curves differed substantially between the samples (Fig 4A). In controls, 37.9% of total events belonged to the larger (peak 2) category while in SCAMP1 knockdowns, 84.6% did. Notably, these distributions reflect quite well the incidence of two-step events – 40% in control and 87.5% in SCAMP1 knockdown (Fig 1E). Thus the smaller diameter distribution likely reflects single-step events while the larger reflects two-step events. Though statistical analysis favors two-Gaussian fits for total events in both control and SCAMP1 KD cells, we note that the curve-fitting result for SCAMP1 knockdown is unusual in that the minor group of smaller events appears to fall inside the range of the major group of larger events. We think that the left tail of the distribution may include a third small group of events in SCAMP1 knockdown cells. Earlier data have shown that SCAMP1 depletion destabilizes fusion pores during their opening (12) resulting in prolonged flickering detected by capacitance measurements (9). During this period of rapid opening and closure, there may be limited SRB uptake leading to a fluorescent signal that under-represents DCV volume (and thus is non-Gaussian).
Figure 4. Evidence favoring two populations of different-sized exocytotic events; enhancement of the larger population upon SCAMP1 knockdown and its likely reflection of increased compound exocytosis.
(A) Top: Histograms of the distribution of effective diameters (calculated as cube root of peak fluorescence of individual events assuming a spherical shape) for all exocytotic events. Results of fitting the histograms to one or two Gaussians (using Origin software) are indicated by the dotted and solid lines, respectively. Chi2/DoF calculations (4.707 and 0.651 for 1- and 2-Gaussian fits for SC1 KD cells and 0.318 and 0.025 for 1- and 2-Gaussian fits of NS KD cells) favor the 2-Gaussian fit. Bottom: Parameters derived from the 2-Gaussian fit indicating that the two sizes of events resolved have the same effective diameters but different relative proportions in the two types of cells. (B) Before and after images of UV-triggered exocytotic events observed at different focal planes: the basal region of the cell adjacent to the coverslip (Z=0 µm) and a region deeper into the cytoplasm (Z=0.4 µm). Subtracted image: net response, post-stimulation minus pre-stimulation shown as inverted color map (see Methods). Post-stimulation timepoints (9 s, NS KD; 36 s SC1 KD) were selected to insure that two-step events had reached maximum levels in both samples (Fig 1D). (C) Scatter plot comparing distribution of the ratio of fluorescence intensities of individual events (DF Z=0.4 µm: DF Z=0 µm) in control and knockdown cells. *** indicates extremely significant, p<0.0001.
The Incidence of Compound Exocytosis is increased in SCAMP1 Knockdown Cells
Because the two populations of events deduced from curve fitting seem to equate to one- and two-step events, we thought that the smaller, one-step events might reflect exocytosis of single DCVs while the larger, two-step events would correspond to compound exocytosis, where a second DCV had fused sequentially to the omega-shaped membrane of an already-fused DCV. Compound exocytosis has been reported as a significant secretory route in PC12 cells (36), and we hypothesized that inhibited pore closure in SCAMP1 knockdown would amplify its occurrence. To address this possibility, we examined cells at times (9 s, control; 36 s, SCAMP1 KD) post-flash where two-step fusion events were nearly maximal (Fig 1D), and we made z-stack recordings using two focal planes. One was near the cell-coverslip interface (Z=0 µm, as above) to detect all events at the cell surface and the other was 0.4 µm deeper within the cell (Z=0.4 µm) to detect prospective compound events selectively. In setting the focal planes, we assumed that the average size of DCVs in PC12 cells was 220 nm (43). Images collected at Z = 0.4 µm had much less background than those at Z = 0 µm but also had reduced signals (Fig 4B). After subtracting pre-stimulation images for respective focal planes to remove background, remaining fluorescence intensities were measured. The ratio of intensity (Z = 0.4 µm): intensity (Z = 0 µm) was calculated and plotted for all events as a scatter plot (Fig 4C). Sustained fluorescence in the upper-layer images and corresponding increased ratio serve as potential qualitative and quantitative indicators of compound exocytosis. Both the subtracted images (Fig 4B) and the scatter plot (Fig 4C) indicated a substantial difference between control and SCAMP1 knockdown cells. These differences seem sufficiently large so as not to be compromised by limitations in two-photon resolution in the Z dimension. This analysis supports compound exocytosis as the probable cause of two-step events, which are enhanced by SCAMP1 knockdown to the point where they account for ~80% of total events. However, we acknowledge that our interpretation does not fully exclude possible contributions of larger-sized DCVs (48) or some sort of z-ward extension from plasma membrane. Notably, we have no evidence for enlarged single-step events.
SCAMP1 Overexpression Reduces the Incidence of Two-step Events
The finding that SCAMP1 knockdown delays fusion pore closure motivated us to test whether overexpression might have the opposite effect. We transfected PC12 cells with SCAMP1 together with GFP in a bicistronic vector and selected cells exhibiting moderate levels of GFP expression for analysis by TEPIQ. Cells expressing GFP alone in the same vector served as control. The latter responded similarly to the non-specific RNA knockdown cells (Fig 1A) with numerous fluorescent puncta appearing soon after UV flashing and gradually decaying towards the end of recording (Fig 5A). Cells overexpressing SCAMP1 also resembled controls (Fig 5A), and analysis of average fluorescence of total events and cumulative peak fluorescence supported the same conclusion (Fig 5B, C), although the cumulative plot showed slightly decreased resolution into two phases. Gaussian distributions of event size, however, detected a difference between overexpressors and controls. While two populations of events centered on 3 and 3.5 respectively were detected for both (Figure 5D, E), the proportion of larger (peak 2) events in control cells was 42.5% but was only 24.5% in SCAMP1 overexpressors. Thus increasing SCAMP1 caused a small acceleration of pore closure and somewhat reduced compound exocytosis.
Figure 5. SCAMP1 overexpression causes at most a small acceleration of fusion pore closure, but reduces the incidence of two-step events.
(A) Images using TEPIQ were obtained as described in Fig 1A on cells that expressed exogenous SCAMP1 and GFP or GFP alone using a bicistronic vector. Cells expressing medium levels of GFP within the overall range were selected for stimulation and imaging. (B) Fluorescence intensity change over time averaged from all events. For both cell types, events in 6–10 cells from 2 experiments were pooled together. Error bars indicate SEM. (C) Cumulative curves of the number of events per cell recorded at the time they reached peak fluorescence intensity; two-step events were recorded only after the second step. (D) Histograms of the distributions of effective diameters calculated as in Fig 2 for all exocytotic events; 1- or 2-Gaussian fits to the data (determined in Origin) are indicated by the dotted and solid curves, respectively. Parameters derived from the 2-Gaussian fit for each cell type are shown below.
Specificity of SCAMP1 Knockdown Effect on Fusion Pore Kinetics
To document the specificity of the siRNA-induced knockdown of SCAMP1, we conducted rescue experiments by expressing degradation-resistant SCAMP1 using the GFP/bicistronic vector in knockdown cells. Comparisons were made between prospective rescued cells (knockdown cells that express GFP and thus express the rescue construct), prospective unrescued cells (knockdown cells that express no detectable GFP and thus no resistant SCAMP1), and control cells (transfected non-specific siRNA). Results were pooled and analyzed as above. For unrescued cells, changes in fluorescence intensity averaged over all events document similar behavior to the earlier illustrated SCAMP1 knockdowns (compare Figs 6A and 1C). For rescued cells, the averaged fluorescence intensity profile appears very similar to that observed for controls (compare respective profiles in Fig 6A). Cumulative peak fluorescence plots (Fig 6B) also illustrate the rescue and in fact indicate slight over-recovery (acceleration of exocytosis) as compared to cells in which knockdown was performed with non-specific siRNA. Fig 6C shows a striking comparison of neighboring SCAMP1 knockdown cells that do (above) and do not (below) express GFP and thus siRNA-resistant SCAMP 1. Particularly in the lower set of images generated by background subtraction, rapid disappearance of fluorescent foci elicited by rescue (above) and sustained pore opening in the absence of rescue (below) are apparent. These results can also be viewed as a movie (Supplementary Movie 3). Taken together, the overexpression and rescue experiments indicate that pore closure rate reflects the quantity of SCAMP1.
Figure 6. Effect of SCAMP1 KD on exocytosis is rescued by siRNA-resistant SCAMP1 expressed in parallel with GFP using a bicistronic vector.
(A) Fluorescence intensity change over time averaged for all events from SC1 KD, NS KD cells and SC1 KD cells expressing the rescue construct. Error bars indicate SEM. (B) Cumulative curves showing number of events per cell that had reached their peak fluorescence intensity as a function of time. Data for control (NS KD), knockdown (SC1 KD) and resistant SCAMP1-expressing (SC1 KD rescue) cells were collected from 6–10 cells, 2 experiments. (C) An example of two cells side-by-side from SC1 KD rescue, but only one of which (upper cell) is expressing siRNA-resistant SCAMP1 as judged by its GFP fluorescence observed transiently before SRB perifusion and UV stimulation. Interval images (taken from the 1 Hz recorded series) are shown of both cells using glow scale. Below these images is shown the “net” response (displayed using inverted color map) obtained by subtracting the image obtained before UV flash (pre) from the post-stimulation images at each timepoint; subtraction was performed using Image Calculator in Image J. White arrows point out a few out of many events that exhibit characteristic kinetics in rescue and non-rescue cells. Images are taken from Supplementary Movie 3.
Electron Microscopic Correlates of Fusion Pores and Compound Exocytosis
Because the open time for fusion pores is prolonged following SCAMP1 knockdown, we decided to examine stimulated and fixed cells by EM to view exocytotic events at higher resolution. Control and SCAMP1 knockdown cells were stimulated by depolarization, fixed 20 or 40 s later and processed for transmission EM. PC12 cells typically contain numerous DCVs mostly with uniform dense cores accumulated in patches along the plasma membrane (Fig 7A) and separated by quite extensive DCV-free regions. This organization necessitates scanning profiles of large numbers of cells in search of evidence of exocytosis in progress, particularly where fusion figures can be visualized unambiguously. Because of this challenge, we have not identified a reliable means to compare specimens quantitatively and thus report semi-quantitative judgments in which we highlight differences in frequency of exocytotic images observed in local patches of surface area. In unstimulated cells, omega-shaped images along the plasma membrane were very rarely observed (not shown), suggesting that constitutive endocytotic events did not appreciably complicate our analysis. In stimulated control cells, exocytotic images were very infrequent in examining >100 cell profiles at each timepoint. Where we captured an event, it was always isolated with no others in the vicinity (Fig 7C). However, in these controls, we readily observed images of surface-associated closed vesicles in which cores were surrounded by haloes (Fig 7A), which were suggestive of closure by cavicapture before full core release. In SCAMP1 knockdown cells, open fusion pores were detected more frequently over a comparable number of cell profiles (Fig 7B), particularly at 40 s following stimulation where we commonly observed multiple fusion figures within the same local stretch of membrane (Fig 7D). As well, closed cavicapture images often exceeded the dimensions of individual DCVs, and residual cores were offset away from the plasma membrane (Fig 7B-bottom left). Two cores within the same closed vesicle (Fig 7B-bottom right) provided unambiguous evidence of cavicapture following compound exocytosis and also suggested that the other enlarged images were products of cavicapture following compound exocytosis but not before loss of the core closest to the plasma membrane. Vesicles with haloes were more frequent and larger in SCAMP1-deficient cells as compared to control (Fig 7E) leading us to hypothesize that smaller haloed vesicles reflected cavicapture after partial content loss during unitary fusion events whereas larger haloed vesicles reflected cavicapture after compound fusion (Fig 7F). We documented this difference quantitatively by measuring diameters of vesicles and their cores and constructing scatter plots of the core:vesicle diameter ratio as a function of vesicle diameter. As anticipated, SCAMP1-deficient cells are enriched in larger diameter vesicles with a low ratio (Fig 7G). Altogether, the EM images and results from TEPIQ support the view that SCAMP1 deficiency strongly inhibits fusion pore closure following DCV exocytosis; this enhances compound exocytosis, which in turn restores pore closure by cavicapture and thereby increases the number of enlarged haloed DCVs -- the final products of compound fusion.
Figure 7. Electron microscopy of PC12 cells fixed at 20 or 40 seconds following stimulation by K+ depolarization.
(A) Control (NS KD) cells at 20 s showing DCVs aligned along the plasma membrane; some (open arrowheads) exhibit increased size and peripheral halo. (B) SCAMP1 knockdown cells stimulated 20 s showing fusion figures (filled arrowheads) and enlarged vesicles with residual cores and prominent haloes (empty arrowheads). Images suggestive of compound exocytosis and of ensuing cavicapture are highlighted: elongate fusion figure (open circle, B, bottom middle); enlarged single-core vesicles (filled circles, bottom row A, B); and vesicle with two residual cores (asterisk, B bottom right). (C) control and (D) knockdown cells at 40 s showing occasional solitary fusion figures (arrows) in control and groups (3–5 in examples) observed in knockdowns. (E–G) DCV morphology at 40 s examined in unstained specimens to minimize sampling bias. (E) Examples of control and SCAMP1 knockdown (upper and lower two rows, respectively); one vesicle (asterisk, bottommost row) is continuous with the plasma membrane documenting an exo-endocytotic relationship. (F) Scatter plots illustrating differences in DCV structure (equal # DCVs/sample). X-axis: full diameters measured from all vesicles imaged, and Y-axis: ratio of core diameter to full diameter. The black rectangle initially drawn for control to include the majority of DCVs was copied onto the SCAMP1 knockdown plot to delineate the corresponding population. Percentages indicate the proportion of DCVs within the “group”. (G) Cartoon illustrating changes of DCVs following depolarization; cavicapture and compound exocytosis result in heterogeneity of DCV morphology.
Discussion
The central discovery in this work is that the integral membrane protein SCAMP1 regulates the closure of dilated fusion pores of DCVs during the terminal stage of exocytosis in PC12 cells. Thus the role of SCAMP1 extends beyond initial events preceding pore dilation where we observed that it served alongside SCAMP2 in regulating the opening of the incipient pore (9, 12). In fact, in a system where exo- and endocytosis are directly coupled without flattening of the fused DCV into the cell surface, SCAMP1’s contributions very likely span SNARE complex formation and dynamin-mediated scission suggesting an organizational role in coordinating multiple interactions.
In previous studies using amperometry to analyze secretion from K+-depolarized cells, we showed that the absence of SCAMP1, promoted closure rather than dilation of newly opened fusion pores, and this registered as a partial inhibition of DCV exocytosis (12). This is relevant to our present findings in at least three important ways. First, even though SCAMP1 knockdown has this effect early during fusion pore formation, it is still possible to analyze the behavior of fusion pores that successfully proceed through dilation as we have now shown using TEPIQ and TIRF. Second, the early inhibitory effect of SCAMP1 depletion on pore opening/dilation may be diminished when NPE photolysis, rather than depolarization, is used for stimulation. Indeed, we observed a rapid and robust response in both control and knockdown cells throughout our experiments with NPE. This probably reflects the two-fold higher free [Ca2+] generated by NPE (Fig 1S) as compared to depolarization (12). Because our results proved to be consistent between photolysis- and depolarization-stimulated cells, use of NPE appears to have been advantageous for bypassing the effects of SCAMP1 deficiency preceding pore dilation thereby enabling focus on late pore behavior. Moreover, the [Ca2+] employed is well below that which elicits endocytosis in neuroendocrine cells of a magnitude exceeding compensatory internalization of DCV membrane (49). Third, our previous and present studies now highlight apparent contradictory effects of SCAMP1 deficiency – increased closure of newly formed pores before they have dilated and inhibition of pore closure post-dilation. We suggest that these contrasting actions may reflect SCAMP1’s distinct effects on the machineries involved in the two different steps. Recent findings argue that membrane bending driven by synaptotagmin along the DCV - plasma membrane axis promotes pore opening and dilation (17–19). SCAMP1 may act within the plasma membrane to insure that deformation catalyzed by synaptotagmin membrane insertion is focused; absence of SCAMP1 would decrease the efficiency of deformation and thus promote closure rather than dilation of newly opened pores. (Notably, readily observed exocytosis in response to NPE-released Ca2+ in SCAMP1-deficient cells may reflect a Ca2+-elicited increase in synaptotagmin’s efficacy). In contrast, pore constriction post-dilation is probably driven by curvature generating machinery that encircles the dilated pore at the DCV – plasma membrane boundary (30; see 50 for a general discussion). SCAMP1 may play an essential role in organizing closure at this junction.
In focusing on the closure of dilated pores, TEPIQ performed on cells stimulated by uncaging Ca2+ proved to be especially useful for following the progress of an ensemble of exocytosed DCVs throughout recording. Our measured Ca2+ levels are comparable to or somewhat higher than in other previous studies (30,43) and may have slowed fusion pore kinetics (30). However, the slowed kinetics helped in distinguishing the importance of SCAMP1 for pore closure following unitary vesicle fusion and in revealing two-step events that were deduced to reflect compound exocytosis. Accordingly, we were also able to uncover striking relationships between an extended open state and the incidence of compound exocytosis and between compound exocytosis, ensuing pore closure and the accumulation of enlarged vesicles containing residual cores. At the same time, however, we acknowledge that slowed pore kinetics caused by our [Ca2+] may have led to overestimation of the effects of SCAMP1 deficiency. Even so, the differences between control and knockdown cells are clear and are also observed in cells stimulated by depolarization.
Notably, in SCAMP1-deficient cells, ~80% of one-step events appeared frozen in the open state as indicated by their stable fluorescence (Fig 1B) and their loading upon delayed SRB perfusion (Fig 3) and by the relative ease of identification by EM (Fig 7). Accumulation of open fusion figures is unlikely to reflect interruption of full collapse into the surface because such flattening occurs only rarely in stimulated PC12 cells (26,36). Rather, loss of SCAMP1 has the reverse effect of aborting pore closure; negative curvature generating machinery (e.g., amphiphysin (30)) leading to dynamin-mediated scission (51) during direct recovery may fail to act. The sustained U-shaped fusion figures that we have observed may provide a future means for addressing what type of surrounding infrastructure stabilizes the open state of cavicapture. Two candidates are dynamin, which has been detected at exo-endocytic sites and implicated in direct reinternalization (27,28,52,53) and actin, which extends from the plasma membrane onto fused granules in other cell types (15,36,54). As actin and dynamin are known to interact (55,56), they may function in tandem along with other pore-regulatory proteins (57) including myosin II (15,34).
Several aspects of interest stem from the realization that compound exocytosis is the likely source of two-step events and that its occurrence is a function of SCAMP1 expression. First, we observed a doubling of its frequency in SCAMP1-deficient cells (Fig 1E). Specificity as a SCAMP1-related effect was documented in a recovery experiment by expression of RNAi-resistant SCAMP1 (Fig 6) and by showing that SCAMP2 knockdown does not have a similar effect (Fig 1F). Interestingly, SCAMP1 overexpression decreased compound exocytosis somewhat (Fig 5C–E) but did not detectably change pore closure kinetics (Fig 5B). This could reflect limitations in our sensitivity to detect changes or could signal distinct functions of SCAMP1 related to pore opening and closure. Second, in addition to demonstrating an increased incidence of two-step events in SCAMP1-deficient cells, our TEPIQ analysis distinguished a substantial delay in the second step (Fig 1D). We suspect that the delay may reflect inhibition associated with opening the secondary fusion pore between DCV and fused DCV, which agrees with the protein’s role in fusion pore initiation/stabilization as revealed by amperometry (12). Third, EM images showed that while a few fusion figures in SCAMP1-deficient cells were sufficiently large to correspond to the surface area of two DCVs (e.g., Fig 7B, lower middle), most had dimensions similar to single DCVs (Fig 7C, D). Meanwhile, more enlarged DCVs with small residual cores (in one case two cores) were observed in SCAMP1-deficient cells. This may reflect the observation that compound exocytosis enhances membrane reinternalization (Fig 1C) thereby reducing the ability to observe the larger figures. Possibly the infrastructure that stabilizes the U-shaped figure resulting from the first fusion relaxes, reorganizes or dissociates following the second fusion. Such a change may be sufficient to enable vesicle rounding and pore closure regardless of the presence of SCAMP1.
What is the potential significance of our findings that SCAMP1 is apparently required for pore closure in cavicapture-mediated neurosecretion and that its deficiency promotes compound exocytosis which in turn generates enlarged recycled vesicles containing residual cores? The presence of SCAMP1 insures close coupling of exo- and endocytosis when partial (sub-quantal) secretion is the rule as in PC12 cells, and it may be essential in other cell types to limit the extent of basal secretion elicited by low-level stimulation. Enhanced compound exocytosis elicited by SCAMP1 deficiency raises the possibility that SCAMP1 activation/inactivation normally may serve as a switch between sub- and super-quantal secretion through pore closure control that depends on the intensity of stimulation. Finally, the enlarged recycled vesicles produced by endocytosis after compound exocytosis may be a source of size heterogeneity and increased catecholamine storage capacity in the PC12 cell’s regulated secretory pathway. Such vesicles are unlikely to accumulate constitutively because PC12 cells continually proliferate and typically are not stimulated. Whether there is any relationship to the different-sized populations of DCVs present in adrenal chromaffin cells (58) or to the presumed products of granule-granule fusion (22) is unclear. In any event, it will be interesting to investigate whether virgin and recycled DCVs are mobilized equivalently for exocytosis, especially in response to low-level stimulation, and whether size differences affect direct versus indirect endocytic coupling.
In conclusion, we have implicated SCAMP1 in events that span exocytosis and endocytosis in neurosecretion and have highlighted important roles in controlling the magnitude of secretion. The ongoing challenges are to define what are likely to be multiple interaction partners in coordinating these activities and to test for active/inactive states of SCAMP1.
Materials and Methods
siRNAs and DNA constructs
Short interfering RNA (siRNA) duplexes targeting SCAMPs 1 and 2 were against coding regions and have been characterized previously (12); SCAMP1: 5’-TCTCGCTCGTCATGTTTAA-3’ and SCAMP2: 5’-GCATTCAGTGACTCTGTTT – 3’. RNAs were synthesized by Invitrogen (Carlsbad, CA). Non-specific RNAi used as control was obtained from Dharmacon (siCONTROL 2). The SCAMP1 construct used for overexpression was made by subcloning rat SCAMP1 into a pIRES-EGFP vector at XhoI and EcoRI sites. The rescue construct used to preserve SCAMP1 expression in siRNA-treated cells and the construct for tetanus toxin light chain expression were made similarly. DNA encoding the light chain protease of tetanus toxin was the kind gift of Thomas Binz (Institut fur Biochemie/Physiolische, Hannover, Germany). Nucleotide replacement was introduced for I277/S278/L279/V280 to make the DNA resistant to the siRNA used. These constructs were kindly provided by Anna Castle. The bicistronic MSCV-based pIRES-EGFP vector was reported previously (11) and was from Derek Persons (St. Jude’s Children’s Research Hospital, Memphis, TN). The construct encoding NPY-(mGFP)2 constructed by Felix Felmy was obtained with permission from Wolf Almers (Vollum Institute, Portland, OR).
Cell culture and transfection
Rat pheochromocytoma PC12 cells originally obtained from Edwin Chapman (University of Wisconsin, Madison, WI) were cultured in DMEM containing 5% horse serum, 5% iron-supplemented calf serum at 10% CO2. Knockdowns were done by nucleofection electroporation (12). Equipment and supplies were from Amaxa (Lonza, Germany). Briefly, 2 × 106 cells together with 1 µg of siRNA were resuspended in 100 µl electroporation buffer (Amaxa Cell Line Nucleofector Kit V) and transfected using program U-29 (Amaxa Nucleofector II electroporator). Combined transfection of SCAMP1 siRNA and siRNA-resistant SCAMP + GFP was achieved simultaneously using the same electroporation protocol. In both cases, cells were used within 72–96 h after electroporation. To overexpress SCAMP1 or tetanus toxin light chain, cells were transfected with bicistronic vector containing the relevant DNA and GFP. The transfection was done by conventional electroporation (single 7-ms pulse, 235 V, 2-mm cuvette; ECM830 Electrosquare Porator) using 10 µg of DNA per 1 × 107 cells in 350 ml of electroporation buffer (47). Cells were used 48–72 hours later. For TIRF and confocal microscopy, cells were transfected with NPY-(mGFP)2 by electroporation (ECM 830); knockdown with siRNA for SCAMP1 or control RNAi was done on the second day by nucleofection (as above) and cells were used within 96 h (total). Previously, we have validated use of the selected siRNA to knock down SCAMP1 (12); using the identical procedure for all of the present experiments, we have confirmed in at least 10 analyses by quantitative western blotting that expression was reduced ≥90% (Figure 2S). Further, immunofluorescent staining showed that knockdown was effective in >95% of the cells (Data not shown, but see (12)). SCAMP1 knockdown did not affect the expression level of other SCAMPs (12) or the levels of expression of a variety of other proteins involved in neurosecretion (9).
Two-photon Extracellular Polar-Tracer Imaging Quantification (TEPIQ)
TEPIQ was performed on PC12 cells essentially as described (43). Transfected cells were plated on glass coverslips coated with 50 µg/ml collagen (Molecular Probes) and 100 µg/ml poly-D-lysine (Sigma). 30 µM nitrophenyl-EGTA (NPE)- acetoxymethyl ester (AM) (Molecular Probes) was loaded into the cells prior to the experiment. Cells were bathed in Solution A consisting of 140 mM NaCl, 5 mM KCl, 2mM CaCl2, 1 mM MgCl2, 10 mM glucose and 10 mM Hepes-NaOH (pH 7.4). Sulforhodamine B (SRB; 0.5 mM) (Molecular Probes) was locally delivered to the cells of interest through a glass pipette before and extending throughout recording. Imaging was carried out at room temperature on an inverted microscope (Zeiss LSM510 Meta confocal/2p) through a 63×, oil objective lens. Photolysis of NPE was triggered through the objective with an Arc lamp (FluoArc BP-1) outfitted with a DAPI filter. In the depolarization experiment (Fig 2), cells were bathed in a solution with very low concentration of SRB (0.025 mM) all time to enable location of the focal plane of interest. A depolarizing buffer that contained 105 mM KCl, 45 mM NaCl, 1 mM NaH2PO4, 0.7 mM MgCl2, 2 mM CaCl2 and 10 mM Hepes (adjusted to pH 7.3 final) (11,12,60) plus higher concentration of SRB (0.25mM) was locally delivered to individual cells after beginning to record and extending throughout recording. The fluorescent probe (SRB) was excited by a titanium sapphire 2-photon laser at the wavelength of 830 nm and emission signals were collected in the red channel (585nm–615nm). Images were exported as stacked tiff files and analyzed in Image J. The pre-stimulation image was subtracted from the whole stack as background using Image Calculator. Individual events represented by discrete fluorescent spots were recognized and determined to be NPE photolysis-dependent by eye and selected using the Rectangular Selection tool. The selected area was always minimized so that it just covered the fluorescent spot. Fluorescence intensity of the events was measured as the mean grey value of the selected areas in plug-in ROI/Multi Measure. Data were then transferred into Excel and analyzed. Statistical analysis on the results was done using Origin. For figure display, we masked out portions of non-target cells in same fields. Also, subtracted images in Figs 3, 5 are presented as inverted color maps for clarity.
Free intracellular calcium measurement
PC12 cells were cultured on imaging dishes coated with poly-D-lysine/collagen and bathed in Solution A. 10 µM fura-2FF (A. G. Scientific, San Diego, CA) was loaded into the cells together with NPE prior to the recording. Cell stimulation and imaging were done on the same setup as TEP imaging. The signal of fura-2FF was collected at the blue channel (435–485 nm). Before and after stimulation pictures were obtained from PC12 cells loaded with NPE and fura-2FF. Fluorescence measured before stimulation was used as the maximum fluorescence and the minimum fluorescence was taken from cells treated with 10 µM ionomycin (Sigma-Aldrich) for 3 min in solution A. Images collected from 10–15 cells for each group were exported as tiff files and then analyzed in Image J. A maximal rectangular area excluding the nucleus was selected for each cell, and fluorescence intensity of that area was measured as described. The resulting data were then used to calculate intracellular calcium concentration (59).
Electron microscopy
After transfection, cells were plated on plastic coverslips (Electron Microscopy Sciences, PA) coated with poly-D-lysine/collagen as above for two-photon microscopy. Stimulation was done at room temperature by incubating the cells with a depolarizing buffer that contained 105 mM KCl, 45 mM NaCl, 1 mM NaH2PO4, 0.7 mM MgCl2, 2 mM CaCl2 and 10 mM Hepes (adjusted to pH 7.3 final) (11,12,60) for 20 and 40 s. At the timepoints, the coverslips were immediately immersed in ice-cold aldehyde fixative (0.5% glutaraldhyde, 2% paraformaldhyde (stocks from Electron Microscopy Sciences, Hatfield, PA) in PBS) and then fixed 30 min more in the same solution at 4 degrees. Samples were post-fixed in 2% osmium tetroxide, 1.5% potassium ferrocyanide in H2O for 45 min at 4° C, followed by 1% osmium tetroxide in 0.1 M cacodylate buffer, pH 7.2 for 1 hour at room temperature. After rinsing three times with 0.15 M NaCl, the fixed cells were scraped off the plastic coverslips in PBS containing 1 mg/ml BSA, and after sedimenting were diluted 50:50 with 3.5% low-melting point agarose (BioWhittaker Molecular Applications, Rockland, ME), quickly mixed by pipeting and pelleted at 2000 × g. Pellets were dehydrated in ethanol and embedded in Epon resin according to standard procedures. Sections stained with uranyl acetate and lead citrate were examined and photographed on a Joel 1230 TEM equipped with a digital camera.
Total internal reflection fluorescence (TIRF) microscopy and data analysis
Knockdown cells (NS KD and SC1 KD) transfected with NPY-(mGFP)2 were plated on imaging dishes coated with poly-D-lysine and collagen. Cells were stimulated by local perfusion with a depolarizing high K+ buffer (see above under Electron microscopy) through a glass pipette using a pressure injector with micromanipulator mounted on an inverted microscope (IX70, Olympus Microscope) with a 60×/1.45NA oil PlanApo TIRF objective (Olympus). GFP was excited using the 488 nm laser line of an Argon ion laser, and emission signal was collected through a dichroic mirror (HQ485/30). Images were acquired with a charge-coupled device camera (Retiga Exi; Qimaging, Canada) at the speed of 200 ms per frame. Data were exported as tiff stacks and analyzed in Image J, as described for TEPIQ. Small differences in fluorescence change for events in control and knockdown cells were observed but do not affect conclusions regarding fusion pore kinetics.
Confocal microscopy
PC12 cells transfected with NPY-(mGFP)2 were plated on coverslips as described. 30 µM nitrophenyl-EGTA (NPE)-acetoxymethyl ester (AM) was loaded into the cells prior to the experiment. Exocytosis was triggered by UV flashes exactly as in the TEPIQ study, in presence of 25 µM SRB. 30s after stimulation the dye-containing solution in the recording chamber was replaced with Solution A by perifusion within 5 seconds. Images were then taken at the bottom focal plane of the cells, to detect SRB uptake vs. NPY-(mGFP)2. The fluorophores were excited using an Argon laser and emission signals were collected in the red channel (585nm–615nm, for SRB) and the blue channel (500nm–550nm, for GFP), respectively. Images in the right panel in Fig 2A were contrast-adjusted in Image J to match signal level in the two channels.
Statistical analysis
Gaussian fitting for the distribution of effective diameter from TEPIQ study in Fig 3A was done in Origin. Chi value resulting from 1- or 2-Gaussian fit was used as an indication of satisfaction of the fit. Comparison between control and SCAMP1 KD cells in the z-stack study in Fig 4C was done in Prism. A nonparametric t test (Mann-Whitney test) was performed on the data and p < 0.05 was considered statistically significant.
Supplementary Material
Figure S1 Intracellular calcium level change in PC12 cells triggered by photolysis of pre-loaded NP-EGTA. Data collected from around 40 cells, 2 experiments. Error bars indicate s.e.m.
Figure S2 Effects of SCAMP1 knock down in PC12 cells. Figure shows one example of a Western blot from cell lysate collected from PC12 cells transfected with siRNA against SCAMP1 or a non-target sequence. Upper panel shows the amount of SCAMP1 in the cell lysate and lower panel shows the amount of γ-adaptin as a control.
A movie taken on a NS KD PC12 cell that was triggered for exocytosis by UV photolysis of pre-loaded NP-EGTA. A short blackout right after beginning of the movie indicates UV flashes as stimulation. Displayed in Glow scale color palette from Zeiss. Movie plays at 7 frames/s.
A movie taken on a SC1 KD PC12 cell that was triggered for exocytosis by UV photolysis of pre-loaded NP-EGTA. A short blackout right after beginning of the movie indicates UV flashes as stimulation. Displayed in Glow Scale color palette from Zeiss. Movie plays at 7 frames/s.
A movie taken on two PC12 cells side by side within the same field of view. Cells were stimulated by UV photolysis of pre-loaded NP-EGTA to trigger exocytosis. A short blackout right after beginning of the movie indicates UV flashes. SiRNA against SCAMP1 was introduced to abolish endogenous SCAMP1 in both cells, while the upper cell was expressing a siRNA-resistent SCAMP1 construct at the same time (indicated by co-expression of GFP; not shown). Displayed in Magenta Hot color palette in Image J. Movie plays at 7 frames/s.
Acknowledgments
We thank colleagues mentioned under Methods who provided reagents. In addition we are very grateful to Ammasi Periasamy from the Keck Imaging Center for advice on two-photon fluorescence microscopy; Anna Castle and Candice Meuleners for DNA constructs used in the study; Rick Horwitz and Alexia Bachir for advice regarding total internal reflection fluorescence microscopy; the Center for Advanced Imaging for EM specimen preparation; and Mary Kate Worden, Dorothy Schafer, Bettina Winckler, Volker Kiessling, Anna Castle, Jim Casanova, and Ian Macara for advice regarding experimental strategies and presentation. These studies were supported by a grant (DK073380) from the NIH.
References
- 1.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 2.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–439. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 3.Pobbati AV, Stein A, Fasshauer D. N- to C- terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 4.Domanska MK, Kiessling V, Stein A, Fasshauer D, Tamm LK. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J Biol Chem. 2009;284:32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahn R, Scheller RH. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu. Rev Cell Dev Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Bai J, Chang PY, Chapman ER, Jackson MB. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: fusion pore opening and fusion pore dilation. J Physiol. 2006;570:295–307. doi: 10.1113/jphysiol.2005.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James DJ, Khodthong C, Kowalchyk JA, Martin TFJ. Phophatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Chacon R, de Toledo GA, Hammer RE, Sudhof TC. Analysis of SCAMP1 function in secretory vesicle exocytosis by means of gene targeting in mice. J Biol Chem. 1999;274:32551–32554. doi: 10.1074/jbc.274.46.32551. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Guo Z, Tieu Q, Castle A, Castle D. Role of secretory carrier membrane protein SCAMP2 in granule exocytosis. Mol Biol Cell. 2002;13:4266–4278. doi: 10.1091/mbc.E02-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Liao H, Castle A, Zhang J, Casanova J, Szabo G, Castle D. SCAMP2 interacts with Arf6 and phospholipase D1 and links their function to exocytotic fusion pore formation in PC12 cells. Mol Biol Cell. 2005;16:4463–4472. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao H, Zhang J, Shestopal S, Szabo G, Castle A, Castle D. Nonredundant function of secretory carrier membrane protein isoforms in dense core vesicle exocytosis. Am J Physiol Cell Physiol. 2008;294:797–809. doi: 10.1152/ajpcell.00493.2007. [DOI] [PubMed] [Google Scholar]
- 13.Neco P, Fernanez-Peruchena C, Navas S, Gutierrez LM, de Toledo GA, Ales E. Myosin II contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008;283(16):10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- 14.Doreian BW, Fulop TC, Meklemburg RL, Smith CB. Cortical F-actin, the exocytic mode, and neuropeptide release in mouse chromaffin cells is regulated by myristoylated alanine-rich C-kinase substrate and myosin II. Mol Biol Cell. 2009;20:3142–3154. doi: 10.1091/mbc.E09-03-0197. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bhat P, Thorn P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell. 2009;20:1795–1803. doi: 10.1091/mbc.E08-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Hui E, Chapman ER, Jackson MB. Phosphatidylserine regulation of Ca2+-triggerred exocytosis and fusion pores in PC12 cells. Mol Biol Cell. 2009;20:5086–5095. doi: 10.1091/mbc.E09-08-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316(5828):1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 18.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaprotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell. 2009;138(4):709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch KL, Gerona RR, Kielar DM, Martens S, McMahon HT, Martin TF. Synaptotagmin-1 utilizes membrane bending and SNARE bending to drive fusion pore expansion. Mol. Biol. Cell. 2008;19(12):5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceridono M, Ory S, Momboisse F, Chasserot-Golaz S, Houy S, Calco V, Haeberle A-M, Demais V, Bailly Y, Bader M-F, Gasman S. Select5ive recapture of secretory granule components after full collapse exocytosis in neuroendocrine chromaffin cells. Traffic. 2011;12:72–88. doi: 10.1111/j.1600-0854.2010.01125.x. [DOI] [PubMed] [Google Scholar]
- 22.Pothos EN, Mosharove E, Liu KP, Setlik W, Haburcak M, Baldini G, Gershon MD, Tamir H, Sulzer D. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. J Physiol. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi T, Rutter GA. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13:563–567. doi: 10.1016/s0960-9822(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 25.Graham ME, O’callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Nati Acad Sci. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuboi T, McMahon HT, Rutter GA. Mechanism of dense core vesicle recapture following “kiss-and-run” (“cavicapture”) exocytosis in insulin-secreting cells. J Biol Chem. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- 28.Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proc Natl Acad Sci. 2002;99:16806–16811. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrais D, Kleppe IC, Takaska JW, Almers W. Recapture after exocytosis causes differential retention of protein in granules in bovine chromaffin cells. J Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llobet A, Wu M, Lagnado L. The mouth of a dense-core vesicle opens and closes in a concerted action regulated by calcium and amphiphysin. J Cell Biol. 2008;182:1017–1028. doi: 10.1083/jcb.200807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cochilla AJ, Angleson JK, Betz WJ. Differential regulation of granule-to-granule and granule –to-plasma membrane fusion during secretion from rat pituitary lactotrophs. J Cell Biol. 2000;150:839–848. doi: 10.1083/jcb.150.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felmy F. Modulation of cargo release from dense core granules by size and actin network. Traffic. 2007;8:983–997. doi: 10.1111/j.1600-0854.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 33.Taraska JW, Almers W. Bilayers merge even when exocytosis is transient. Proc Natl Acad Sci. 2004;101:8780–8785. doi: 10.1073/pnas.0401316101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Z, Liu L, Cafiso D, Castle D. Perturbation of a very later step of regulated exocytosis by a secretory carrier membrane protein (SCAMP2)-derived peptide. J Biol Chem. 2002;277(38):35357–35363. doi: 10.1074/jbc.M202259200. [DOI] [PubMed] [Google Scholar]
- 36.Kasai H, Kishimoto T, Nemoto T, Hatakeyama H, Liu TT, Takahashi N. Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev. 2006;58:850–877. doi: 10.1016/j.addr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard C, Singleton D, Rauch M, Jayasinghe S, Cafiso D, Castle D. The secretory carrier membrane protein family: structure and membrane topology. Mol Biol Cell. 2000;11:2933–2947. doi: 10.1091/mbc.11.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Chacon R, Sudhof TC. Novel SCAMPs lacking NPF repeats: ubiquitous and synaptic vesicle-specific forms implicate SCAMPs in multiple membrane-trafficking functions. J Neurosci. 2000;20:7941–7950. doi: 10.1523/JNEUROSCI.20-21-07941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellena JF, Moulthrop J, Wu J, Rauch M, Jaysinghne S, Castle JD, Cafiso DS. Membrane position of a basic aromatic peptide that sequesters phosphatidylinositol 4,5 bishosphate determined by site-directed spin labeling and high-resolution NMR. Biophy J. 2004;87:3221–3233. doi: 10.1529/biophysj.104.046748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao H, Ellena J, Liu L, Szabo G, Cafiso D, Castle D. Secretory carrier membrane protein SCAMP2 and phosphatidylinositol 4,5-bisphophate interaction in the regulation of dense core vesicle exocytosis. Biochem. Biochem;46:10909–10920. doi: 10.1021/bi701121j. [DOI] [PubMed] [Google Scholar]
- 41.Milosevic I, Sorensen J, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4.5-bisphosphate level regulates the releaseable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 43.Kishimoto T, Liu TT, Hatakeyama H, Nemoto T, Takahashi N, Kasai H. Sequential compound exocytosis of large dense-core vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J Physiol. 2005;568:905–915. doi: 10.1113/jphysiol.2005.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen JB. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Eur J Physiol. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- 45.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8(13):740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 46.Racchetti G, Lorusso A, Schulte C, Gavello D, Carabelli V, D'Alessandro R, Meldolesi J. Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargosome. J. Cell Sci. 2010;123:165–170. doi: 10.1242/jcs.059634. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Lu J, Bai J, Chang PY, Martin TFJ, Chapman ER, Jackson MB. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- 48.Westerink RHS, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol (Oxf) 2007;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neher E, Zuker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 50.Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion. Trends Biochem. Sci. 2010 Jul 15; doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida Y, Kinuta M, Abe T, Liang S, Araki K, Cremona O, Di Paolo G, Moriyama Y, Yasuda T, De Camilli P, Takei K. The stimulatory action of amphiphysin on dynamin duaion is dependent on lipid bilayer curvature. EMBO J. 2004;23(17):3483–3491. doi: 10.1038/sj.emboj.7600355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 53.Fulop T, Doreian B, Smith C. Dynamin I plays dual roles in the activity-dependent shift in exocytic mode in mouse adrenal chromaffin cells. Arch Biochem Biophy. 2008;20:1–9. doi: 10.1016/j.abb.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 55.Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee E, De Camilli P. From the cover: dynamin at actin tails. Proc Nati Acad Sci USA. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu HE, Bement WM. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat Cell Biol. 2007;9:149–159. doi: 10.1038/ncb1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grabner CP, Price SD, Lysakowski A, Fox AP. Mouse chromaffin cells have two populations of dense core vesicles. J Neurophysiol. 2005;94:2093–2104. doi: 10.1152/jn.00316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Lino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3:253–259. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Grishanin R, Earles CA, Chang PY, Martin TFJ, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Intracellular calcium level change in PC12 cells triggered by photolysis of pre-loaded NP-EGTA. Data collected from around 40 cells, 2 experiments. Error bars indicate s.e.m.
Figure S2 Effects of SCAMP1 knock down in PC12 cells. Figure shows one example of a Western blot from cell lysate collected from PC12 cells transfected with siRNA against SCAMP1 or a non-target sequence. Upper panel shows the amount of SCAMP1 in the cell lysate and lower panel shows the amount of γ-adaptin as a control.
A movie taken on a NS KD PC12 cell that was triggered for exocytosis by UV photolysis of pre-loaded NP-EGTA. A short blackout right after beginning of the movie indicates UV flashes as stimulation. Displayed in Glow scale color palette from Zeiss. Movie plays at 7 frames/s.
A movie taken on a SC1 KD PC12 cell that was triggered for exocytosis by UV photolysis of pre-loaded NP-EGTA. A short blackout right after beginning of the movie indicates UV flashes as stimulation. Displayed in Glow Scale color palette from Zeiss. Movie plays at 7 frames/s.
A movie taken on two PC12 cells side by side within the same field of view. Cells were stimulated by UV photolysis of pre-loaded NP-EGTA to trigger exocytosis. A short blackout right after beginning of the movie indicates UV flashes. SiRNA against SCAMP1 was introduced to abolish endogenous SCAMP1 in both cells, while the upper cell was expressing a siRNA-resistent SCAMP1 construct at the same time (indicated by co-expression of GFP; not shown). Displayed in Magenta Hot color palette in Image J. Movie plays at 7 frames/s.