Abstract
Critically ill preterm infants experience multiple stressors while hospitalized. Morphine is commonly prescribed to ameliorate their pain and stress. We hypothesized that neonatal stress will have a dose-dependent effect on hippocampal gene expression, and these effects will be altered by morphine treatment. Male C57BL/6 mice were exposed to 5 treatment conditions between postnatal day 5 and 9: 1) Control, 2) mild stress + saline, 3) mild stress + morphine, 4) severe stress + saline and 5) severe stress + morphine. Hippocampal RNA was extracted and analyzed using Affymetrix Mouse Gene 1.0 ST Arrays. Single gene analysis and gene set analysis were used to compare groups with validation by qPCR. Stress resulted in enrichment of genes sets related to fear response, oxygen carrying capacity and NMDA receptor synthesis. Morphine downregulated gene sets related to immune function. Stress plus morphine resulted in enrichment of mitochondrial electron transport gene sets, and down-regulation of gene sets related to brain development and growth. We conclude that neonatal stress alone influences hippocampal gene expression, morphine alters a subset of stress-related changes in gene expression and influences other gene sets. Stress plus morphine show interaction effects not present with either stimulus alone. These changes may alter neurodevelopment.
Introduction
The effects of stress on health and development may be either positive or negative. For example, short-term acute stress can boost the immune system, but prolonged inescapable stress can have deleterious effects on learning, development, the immune system, and may increase susceptibility to degenerative diseases (1-3). Preterm infants in the NICU are exposed to prolonged inescapable stress. On average, a 28 week gestation infant spends 10-12 weeks (approximately the last trimester of pregnancy) in a NICU. During this time of rapid brain development they are separated from their mothers, handled repeatedly, exposed to multiple painful procedures, and they may also be mechanically ventilated, gavage fed, and exposed to repeated periods of oxidative stress. Neurodevelopmental outcomes following extreme prematurity remain poor, with moderate to severe impairment occurring in close to 50% of extremely low birth weight infants (4). Autism, attention deficit disorder, and school failure also occur more frequently NICU survivors (5). While some degree of impairment might be inevitable, it is likely that the stress and treatments these infants undergo impact neurologic outcome. Improved understanding of these factors will provide the basis of better treatments and subsequent improvement in outcomes.
Many preterm infants receive opiates for sedation or analgesia during their NICU stay. Treatment occurs during a period of intense brain development, with brain weight tripling during the last trimester of pregnancy (6). Neuronal differentiation, migration, and synapse formation are active processes through term gestation, as is glial proliferation (7). Complex neural networks form in regionally specific ways (8, 9), controlled by families of netrins, ephrins, semaphorins, and Slits (10). In the rat, neonatal stress disrupts subsequent adult learning and maturation of the adrenal stress response (1-3). Morphine exposure may have additional consequences in the newborn, affecting both immune function and neurodevelopment (11-14). The combined effects of stress and morphine have not been well studied, yet are of vital importance since the at-risk period when critically ill infants are exposed to morphine is also a time during which they are experiencing inescapable stress (15).
Brain development in the third trimester of human gestation generally corresponds to the first two weeks of postnatal life in mice (16). Use of neonatal rodents to model preterm brain development is therefore reasonable, despite differences in brain complexity. We developed a rodent model of neonatal stress which simulates many of the experiences of a hospitalized preterm infant. We previously reported short-term hippocampal gliosis (17), and long term neurobehavioral effects of stress and morphine exposure in our mouse model of neonatal stress (18). To further investigate the mechanism by which these changes occur, we now hypothesize that repeated neonatal stress will have a dose-dependent effect on hippocampal gene expression, and that these effects will be altered by morphine treatment.
Materials and Methods
Animals
Adult wild-type C57BL/6 mice were purchased (Harlan) and housed under a 12 h light-dark cycle with free access to food and water. All animal procedures were approved by the Animal Care and Use Committee at the University of Washington.
Treatment Groups
Male mice were exposed to 5 treatment conditions between postnatal day (P)5 and P9 (n=3/group), with birth recorded as P1. Litters were culled to n=7 maximum per dam. Groups included: 1) Untreated controls (CC), 2) mild stress + saline (MSS), 3) mild stress + morphine (MSM), 4) severe stress + saline (SSS) and 5) severe stress + morphine (SSM). Only males were used so as to eliminate sex-related genetic variability. Untreated control animals underwent minimal handling on P5 and at euthanasia on P9. All animals were killed on P9. Groups 2-5 received s.c. 10 μL injections of either saline or morphine twice daily at 08:00 h and 15:30 h. The morphine (Baxter, USA) dose was 2 mg/kg in a 10 μL volume and based on the daily average litter weight. This dose produces circulating morphine levels that approximate the range measured in human preterm infants given standard intermittent i.v. morphine boluses, or continuous i.v. morphine infusion (19). Severely stressed pups (groups 4 and 5) were separated from the dam and isolated in individual containers within a veterinary warmer at 32°C from 08:00 h until 16:00 h, thus experiencing both maternal and littermate separation. Pups were gavage fed 50 - 150 μL of rodent milk substitute at 10:00, 12:00, and 14:00 h, using a 24 gauge animal feeding needle (Popper & Sons, New Hyde Park, NY). To simulate the oxidant stress of apnea, pups were exposed to hypoxia (100% nitrogen 1 min followed by hyperoxia (100% oxygen 5 min) twice daily (08:00 and 15:30 h). Mice were then returned to the dam with their concurrent unstressed littermates each evening and allowed to nurse overnight ad lib.
RNA methods
RNA from the right hippocampus was isolated (Cartagen Molecular Systems, San Carlos, CA). RNA quality was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies Inc. Palo Alto, CA). Only RNA samples with appropriate size distribution, quantity, and an A260:A280 ratio of 1.8 – 2.1 were used for analysis.
Microarray processing
The manufacturer's protocols for the GeneChip platform by Affymetrix (Santa Clara, CA) were used. Methods included synthesis of first- and second-strand cDNAs, the purification of double-stranded cDNA, synthesis of cRNA by in vitro transcription, recovery and quantitation of biotin-labeled cRNA, fragmentation of this cRNA and subsequent hybridization to the microarray slide, post-hybridization washings, and detection of the hybridized cRNAs using a streptavidin-coupled fluorescent dye. Hybridized Affymetrix arrays were scanned with an Affymetrix GeneChip® 3000 scanner. Image generation and feature extraction were performed using Affymetrix GCOS Software.
Statistical analysis and data normalization for Affymetrix Mouse Gene 1.0 ST Arrays
Raw microarray data were processed with Bioconductor (20). Probes were normalized with Robust Multi-Array (RMA) (21). From the normalized data, genes with significant evidence for differential expression were identified using the Bioconductor limma package (22). P-values were calculated with a modified t-test in conjunction with an empirical Bayes method to moderate the standard errors of the estimated log-fold changes. P-values were adjusted for multiplicity with the Bioconductor package q-value (23), which allows for selecting statistically significant genes while controlling the estimated false discovery rate.
Single gene analysis
The experimental design, with the specific main effects and the interaction effects that we wished to examine are shown in Figure 1 and Table 1. Figure 1 shows the basis for our comparisons, and what specific questions they answer. We define a 1) stress-affected group of genes as the union of the mild and severe stress response genes (MSS-CC union with SSS-MSS) and 2) a morphine-affected group of genes as the union of the morphine response genes in the context of mild and severe stress (MSM-MSS union with SSMSSS), and 3) interaction effects of severe-stress with morphine as genes that react differently to morphine in the presence of severe stress than with mild stress.
Figure 1. Stress morphine comparison groups.
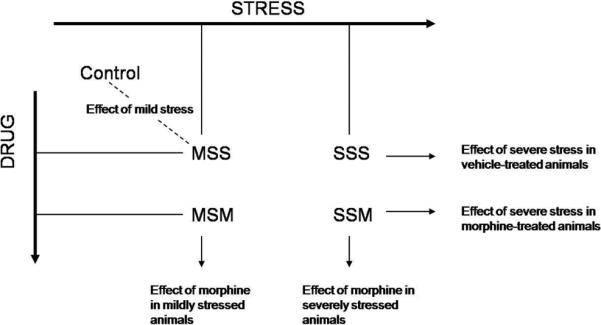
These comparisons allow us to answer the following questions: 1) What is the effect of mild stress? Answer: MSS-CC; 2) Is there a dose-dependent effect of stress? Answer: SSS-MSS; 3) How does severe stress affect morphine-treated animals? Answer: SSM-MSM; 4) How does morphine affect mildly stressed animals? Answer: MSM-MSS; 5) What does morphine do to severely stressed animals? Answer: SSM-SSS; 6) Does morphine act different in severely stressed animals? Answer: Stress X Morphine interaction
Table 1.
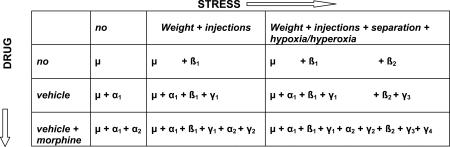
|
α1 = effect of vehicle
α2 = effect of morphine
β1 = effect of mild stress
β2 = effect of severe stress
γ1 = interaction effect of vehicle, α1, with mild stress, β1, probably zero
γ2 = interaction effect of morphine, α2, with β1+γ1, probably negligible
γ3 = interaction effect of α1+γ1 with severe stress, β2
γ4 = interaction effect of α2+γ2 and β2+γ3
Table 1 outlines the mathematical considerations we took into account when making group comparisons. For example, to answer the question “which genes increased by stress (|β1+ β2 | >>1) does morphine affect?” we look for genes that satisfy the equation: α1 + α2 + β1 + β2 + γ1 + γ2 + γ3 + γ4 ~ 0.
Gene Set Analysis (GSA)
To investigate categories of genes where the constituent genes show coordinated changes in expression over the experimental conditions, we used GSA, a type of biological category analysis (20, 24). GSA software is available as R code (24, 25) (http://www-stat.stanford.edu/~tibs/GSA/) to calculate separate gene set analyses. GSA considers all the genes in the experiment and allows for the identification of gene sets with strong cross-correlation by boosting the signal-to-noise ratio, making it possible to detect modest changes in gene expression. The term “gene set” refers to genes that are grouped together based on function. For example, each gene ontology category (e.g. apoptosis) is a gene set. We used four gene set databases for GSA: Biological Process, Molecular Function, and Cellular Component from Gene Ontology (26), and the C2 gene set from the Molecular Signature Database (25) (http://www.broad.mit.edu/gsea/msigdb/msigdb_index.html.
Validation of microarray data using fluorogenic 5' nuclease-based assay and quantitative RT-PCR (qPCR)
RNA from separate, additional animals were used for validation. Quantitation of specific mRNA levels were determined as previously described (27) using fluorogenic 5’ nuclease-based assays on an ABI 7900 Sequence Detection System (28).
Results
Forty-seven genes were affected by mild stress, and 156 genes were affected by severe stress (up or down fold change > 1.3 fold and p-value < 0.05) including Dnajb6 (Hsp40 homolog), aminolevulinic acid synthase 2 (Alas2), and secreted phosphoprotein 1 (Spp1). Morphine had effects on 3 broad categories of genes: 1) morphine-responsive genes which were unaffected by stress (n=92); 2) morphine-responsive genes which were also stress-responsive (n=8); and 3) genes which responded only when both morphine and stress were present together, i.e. a morphine X stress interaction effect (n=104). Morphine-responsive genes were defined as those which changed expression more than 1.3-fold (up or down) with p<0.05 in either the mild stress (MSM-MSS) or the severe stress (SSM-SSS) contrasts. A subset of morphine-responsive genes are shown in Table 2. In comparison, a subset of the interaction-specific genes are shown in Table 3. Examples of how treatment conditions affect gene expression are illustrated in Figure 2 using qPCR. The relationship between genes affected by mild and severe stress are shown in the Venn diagram in Figure 3.
Table 2.
Selected Morphine-responsive genes*, unaffected by stress (92 genes)
| Gene Symbol | Gene Description | MSM-MSS (log2FC)** | MSM-MSS p-value† | SSM-SSS (log2FC)** | SSM-SSS p-value† |
|---|---|---|---|---|---|
| Accn4 | amiloride-sensitive cation channel 4 | 0.400 | 0.114 | 0.605 | 0.024 |
| Aqpl | aquaporin 1 | 1.187 | 0.030 | 0.260 | 0.600 |
| Avprla | arginine vasopressin receptor 1A | -0.704 | 0.024 | -0.160 | 0.570 |
| Bex4 | brain expressed gene 4 | 0.015 | 0.901 | 0.389 | 0.006 |
| Carhspl | calcium regulated heat stable protein 1 | 0.221 | 0.300 | 0.477 | 0.037 |
| Cdh22 | cadherin 22 | 0.026 | 0.861 | 0.405 | 0.016 |
| Cldn2 | claudin 2 | 0.744 | 0.029 | 0.245 | 0.430 |
| Ctnnall | catenin (cadherin associated protein), alpha-like 1 | 0.415 | 0.021 | 0.137 | 0.396 |
| Cux2 | cut-like homeobox 2 | 0.653 | 0.013 | 0.007 | 0.977 |
| Dlx5 | distal-less homeobox 5 | 0.088 | 0.752 | 0.614 | 0.044 |
| Dock10 | dedicator of cytokinesis 10 | 0.122 | 0.445 | 0.410 | 0.027 |
| Gabra4 | gamma-aminobutyric acid (GABA-A) receptor, subunit alpha 4 | 0.101 | 0.385 | 0.392 | 0.004 |
| Gad2 | glutamic acid decarboxylase 2 | 0.231 | 0.424 | 0.609 | 0.0495 |
| Gal | galanin | 0.408 | 0.033 | 0.074 | 0.669 |
| Gh | growth hormone | -0.189 | 0.514 | -0.854 | 0.010 |
| Glra2 | glycine receptor, alpha 2 subunit | 0.171 | 0.414 | 0.441 | 0.049 |
| Mpeg1 | macrophage expressed gene 1 | 0.017 | 0.870 | 0.384 | 0.003 |
| Npy | neuropeptide Y | 0.305 | 0.052 | 0.539 | 0.002 |
| Reln | reelin | -0.097 | 0.532 | -0.491 | 0.007 |
| Rgs6 | regulator of G-protein signaling 6 | 0.439 | 0.010 | 0.066 | 0.657 |
| Tac1 | tachykinin 1 | 0.083 | 0.860 | 1.374 | 0.012 |
| Trh | thyrotropin releasing hormone | 0.700 | 0.029 | 0.275 | 0.350 |
Columns 1 and 2 list Gene symbols and gene descriptions. Columns 3 [MSM-MSS (log2FC)] and 5 [SSM-SSS (log2FC)] provide the log2 transformed change in expression caused by mild-stress-morphine (MSM) treatment relative to the mild-stress-saline (MSS) treatment, and the severe-stress-morphine (SSM) treatment relative to severe-stress-saline (SSS) treatment respectively. Columns 4 (MSM-MSS p-value) and 6 (SSM-SSS p-value) list the p-values associated with the aforementioned contrasts.
morphine responsive genes are defined as changing >1.3-fold (up or down) with p<0.05 in either the MSM-MSS or the SSM-SSS contrast
fold change is provided as log2, positive numbers indicate gene was expressed at higher level in MSM vs. MSS or SSM vs. SSS respectively, whereas negative numbers indicate the opposite, bold print indicates a > 1.3-fold absolute change
bold print indicates p<0.05.
Table 3.
Selected genes responding only when Stress and Morphine are present together (104 genes total).
| Gene Symbol | Gene Description | Stress × Morphine Interaction (log2FC)* | Stress × Morphine Interaction p-Value |
|---|---|---|---|
| C1ql3 | C1q-like 3 | 0.497 | 0.038 |
| Calbl | calbindin-28K dehydrogenase/reductase (SDR family) | 0.557 | 0.011 |
| Dhrs7b | member 7B | 0.396 | 0.011 |
| Dnahc3 | dynein, axonemal, heavy chain 3 | -0.440 | 0.013 |
| Dynclhl | dynein cytoplasmic 1 heavy chain 1 | -0.385 | 0.005 |
| Ephx2 | epoxide hydrolase 2, cytoplasmic | 0.384 | 0.004 |
| Fbnl | fibrillin 1 | -0.412 | 0.013 |
| Hs6st2 | heparan sulfate 6-O-sulfotransferase 2 | 0.4345 | 0.012 |
| Htr3a | 5-hydroxytryptamine (serotonin) receptor 3A low density lipoprotein receptor-related | 0.591 | 0.018 |
| Lrp1 | protein 1 malic enzyme 3, NADP(+)-dependent, | -0.445 | 0.012 |
| Me3 | mitochondrial | 0.401 | 0.035 |
| Myo19 | myosin XIX | -0.392 | 0.001 |
| Nav1 | neuron navigator 1 | -0.385 | 0.015 |
| Pknox1 | Pbx/knotted 1 homeobox | 0.434 | 0.001 |
| Rab11b | RAB11B, member RAS oncogene family ribonucleotide reductase M2 B (TP53 inducible) | 0.392 | 0.019 |
| Rrm2b | 0.384 | 0.010 | |
| Sst | somatostatin | 0.497 | 0.006 |
| Syt5 | synaptotagmin V | 0.379 | 0.014 |
| Trhr | thyrotropin releasing hormone receptor | 0.487 | 0.046 |
| Ttll11 | tubulin tyrosine ligase-like family, member 11 thioredoxin domain containing 4 | 0.441 | 0.008 |
| Txndc4 | (endoplasmic reticulum) | 0.381 | 0.019 |
| Zfp239 | zinc finger protein 239 | 0.380 | 0.009 |
fold change is provided as log2, positive numbers indicate that the Stress X Morphine interaction effect increased gene expression, whereas a negative number indicates the opposite.
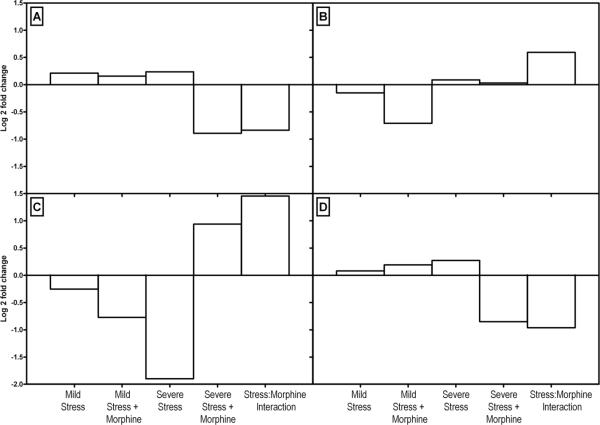
Figure 2. qPCR of selected hippocampal genes by treatment condition.
qPCR results are normalized to GAPDH. The X axis shows the conditions: mild stress, mild stress + morphine, severe stress, severe stress + morphine and interaction effects of stress. The Y axis shows the log 2 fold change in gene expression. Expression of Nav1 (Panel A), Wnt2 (Panel B), Spp1 (Panel C), and Reln (Panel D) differ by treatment group, demonstrating that the effect of morphine is not necessarily predictable.
Figure 3. Venn diagram.
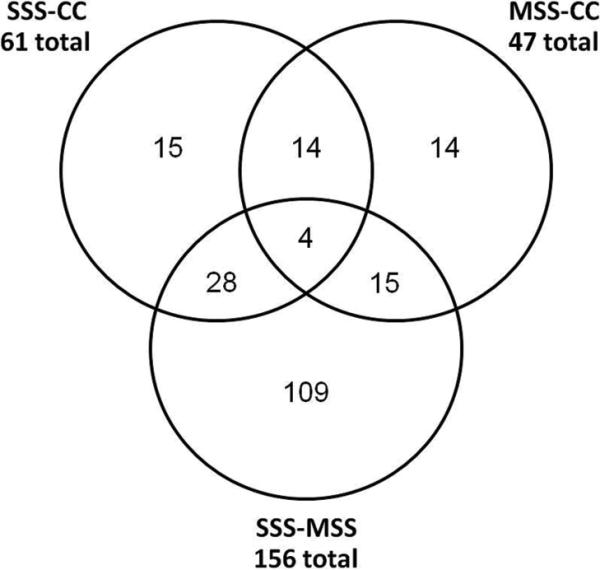
This figure shows the relationship between genes affected by severe stress (SSS-CC), mild stress (MSS-CC) and the difference between mild and severe stress (SSS-MSS). Criteria for inclusion include |fold| > 1.3, p < 0.05.
GSA allowed us to examine patterns of gene expression associated with each treatment group. Mild stress resulted in upregulation of many gene sets related to immune function including: IFNγ production, IL-1, 2 and 12 synthetic processes, defense response to gram-positive bacterium, regulation of T-helper cell differentiation, positive regulation of cell killing and leukocyte mediated cytotoxicity. Other upregulated gene sets included: positive regulation of systemic arterial blood pressure and glucocorticoid metabolic process. Down regulated gene sets included those related to DNA and cellular maintenance.
When animals undergoing severe stress were compared to controls, gene sets consistent with stress such as multicellular organismal response to stress and fear response were overexpressed. Unexpected, however, were the many upregulated gene sets related primarily to neurodevelopment and inflammation: dendrite morphogenesis, microtubule bundle formation, synaptic vessel exocytosis, cerebral cortex cell migration, axon regeneration, neurite development, wnt signaling pathway, ionotropic glutamate receptor signaling pathway, axon regeneration, nerve growth factor receptor signaling pathway, cell proliferation in forebrain, regulation of synapse structure and activity; IL-6 production, T cell signaling pathway, and regulation of cytokine production (GSA p-value < 0.001, false discovery rate < 0.001). Severe stress downregulated gene sets related to glutamine family amino acid catabolic process, glutamate metabolic process, mismatch repair, nuclear mRNA splicing, phagocytosis, histone modification, DNA metabolic process, folic acid synthesis, and telomere maintenance. Supplementary Tables S1A and SB (http://links.lww.com/PDR/XXX) give more details of severe stress related gene changes.
When comparing animals exposed to mild stress plus morphine (MSM) with mildly stressed animals (MSS), there was down-regulation of many gene sets related to immune function, particularly T and B cell function, IL-1 and IL-12 synthesis. Overexpressed gene sets were not as thematic, but included glutamine and dopamine metabolism, hyperosmotic response, and growth hormone secretion. Supplementary Tables 2A and B (http://links.lww.com/PDR/XXX) give more complete lists of gene sets whose expression is modified by mild stress plus morphine. Note that it is not possible to determine the effects of morphine in the absence of stress, since handling the animals to give injections is a form of stress. Therefore, the comparison MSM versus MSS provides the best estimation of the morphine effect alone.
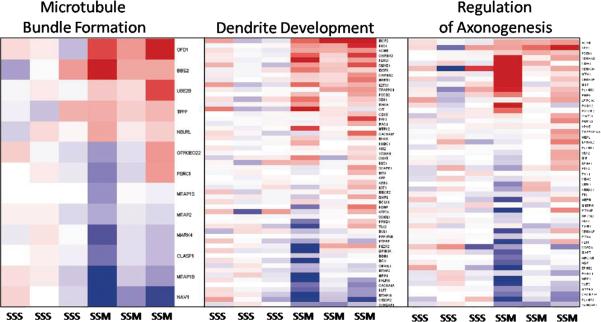
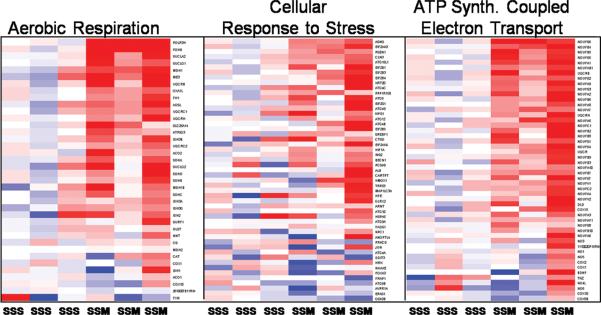
In contrast to mild stress plus morphine, severe stress plus morphine positively enriched gene sets were related in large part to mitochondrial electron transport, oxygen transport, ATP synthesis, ATP coupled electron transport, oxidative phosphorylation, cellular response to stress, regulation of dendrite morphogenesis, Notch signaling pathway, regulation of apoptosis, amyloid precursor metabolic process, and regulation of acute inflammatory response. Downregulated gene sets in this comparison included many gene sets involving neuronal development: regulation of axon extension, synaptic vesicle exocytosis, hippocampal development, cerebral cortex cell migration, dendrite development as well as several gene sets related to histone modification. Examples of these changes are shown in Figures 4 and 5; Figure 4 depicts the effects of combined severe stress and morphine on genes involved in microtubule bundle formation, dendrite development and regulation of axonogenesis, Figure 5 shows genes involved in aerobic respiration, cellular response to stress and ATP coupled electron transport. Supplementary Table S3,A and B (http://links.lww.com/PDR/XXX) give more complete lists of gene sets whose expression is modified by severe stress plus morphine.
Figure 4. Neonatal stress and morphine treatment effects on selected neuronal activities.
These composite heat maps show examples of three biological process gene sets related to neuronal development which differ by treatment group (GSA p-value< 0.0001). Each row represents a specific gene in the gene set, and each column shows the pattern of RNA expression from an individual animal, arranged by treatment group labeled across the bottom. Individual animal variability can be seen, as well as group differences. Relative gene expression is denoted by color, with red illustrating gene expression that is higher than the mean in the control reference group, blue indicating the opposite and white indicating no change in expression. Expression levels for each gene are shown relative to the average expression of that gene in control animals. Since the number of genes in the gene sets precludes adequate visibility, supplementary table S4 (http://links.lww.com/PDR/XXX) is provided listing the gene names in the same order as they appear in the heatmaps (from top to bottom).
Figure 5. Neonatal stress and morphine treatment effects on cellular metabolism gene sets.
Composite heat maps from severely stressed animals compared to severely stressed animals treated with morphine. Highlighted are gene sets related to energy metabolism. While there is some variability from animal to animal (columns), and by individual gene (rows), the treatment of stress with morphine clearly increased gene expression in many of the genes in each family relating to aerobic respiration, cellular response to stress, and ATP synthesis coupled electron transport (GSA p-value < 0.0001). Since the number of genes in the gene sets precludes adequate visibility, supplementary table S5 (http://links.lww.com/PDR/XXX) is provided listing the gene names in the same order as they appear in the heatmaps (top to bottom).
We selected a subset of genes to validate by qPCR. Figure 6 shows the comparison of qPCR vs. microarray data. The correlation varies somewhat by condition, but overall the correlation is excellent.
Figure 6. Validation of Microarray Results by qPCR.
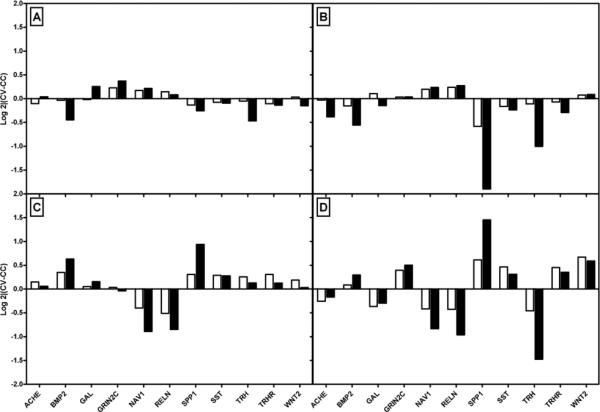
Microarray results were validated in 16 genes in all comparison conditions. A subset of 11 genes and conditions are shown in this figure. Panel A shows effects of mild stress; Panel B the effect of severe stress; Panel C shows effects of severe stress combined with morphine, and Panel D shows the stress: morphine interaction effects. Microarray results are shown in clear bars, and qPCR results in black for 11 genes. Gene names are listed on the X-axis. The Y axis shows the log 2 fold change in gene expression.
Discussion
As survival of the smallest infants improves, optimizing their neurodevelopmental potential becomes paramount. While some degree of impairment following extreme prematurity might be inevitable, it is just as likely that over time, research will uncover pre and postnatal treatments to protect the developing brain and improve outcomes. Most preterm infants receive analgesics and/or sedatives during their NICU stay, sometimes for weeks (29). During this period of neuronal network development, factors that influence neuronal excitation and inhibition result in profound changes in the balance of excitatory and inhibitory pathways as well as in cell survival (30). Clearly, both stress and opiate-exposure have this potential. Further delineation of the individual and interactive effects of opiates with stress is of vital importance since the at-risk period when critically ill infants are exposed to morphine is concurrent with severe stress (15).
In this study, we show that repeated neonatal stress in mice has a dose-dependent effect on hippocampal gene expression, and that the interactions of stress with morphine are complex. While morphine does down-regulate some stress-related changes in gene expression, this effect is not consistent across all stress-related genes. Depending on the gene in question and the degree of stress, treatment with morphine can have quite different and unpredictable effects. Figure 2 shows some examples of this: Wnt and Spp1 expression are changed in the presence of morphine, but the direction of change depends on the level of stress. These changes in hippocampal gene expression may significantly impact neurodevelopment, and subsequently, behavior.
Mild stress tended to increase genes related to immune function, blood pressure, and glucocorticoid expression while decreasing those related to cell maintenance. Severe stress had less immune modulatory effects, but increased expression of gene sets having to do with neurodevelopment and inflammation, while down regulating gene sets related to cellular repair such as telomere maintenance, mismatch repair, DNA metabolic process, folic acid synthesis, nuclear mRNA splicing, and histone modification.
Morphine exposure had different effects on hippocampal gene expression in the presence of mild vs. severe stress. Treatment of mild stress with morphine increased oxygen transport, mitochondrial membrane maintenance and increased expression of specific gene sets related to neurodevelopment. Cholesterol, proteoglycan and steroid synthesis were downregulated. In contrast, treatment of severe stress with morphine markedly up-regulated expression in several gene sets regulating energy metabolism, oxidative phosphorylation and aerobic respiration. Thus it is apparent that the modulatory effects of morphine on hippocampal gene expression are dependent upon the level of neonatal stress. Of particular concern, there was marked down-regulation in gene sets involving RNA processing and neurodevelopmental processes. These data suggest a mechanism by which decreased brain growth and other deleterious effects of morphine might be mediated.
This study is limited in that we could not assess the isolated effects of morphine, since morphine injections are by themselves stressful, and we also did not differentiate between the stressful stimuli of maternal separation, pain, formula feeding by gavage, and hypoxia/hyperoxia in the severely stressed group. These stimuli were combined to simulate the experiences preterm infants are exposed to. To assess isolated morphine effects we compared mildly-stressed morphine-treated animals with mildly-stressed animals. This comparison showed upregulation of estrogen metabolism, blood volume regulation, growth hormone synthesis, and glutamine metabolism whereas immune function, urea cycle metabolism and cell movement processes (microtubule polymerization) were down regulated.
Our data are consistent with previous work showing differential expression of Dnajb1 (Hsp40), and glutamate receptor ionotropic kainate 2 (Grik2) in the C57BL6 striatum after morphine treatment (31). Our results in regards to Neuropeptide Y (NPY) are also similar to a recent study showing 4 day morphine administration to mice caused an up-regulation of hypothalamic NPY gene expression (32). In addition, we found an up-regulation of galanin gene expression after morphine administration that is consistent with previous work demonstrating an increase in galanin gene expression in mouse locus ceruleus and ventral tegmental region tissues after chronic morphine treatment (33). Erabi et al showed that neonatal stress (isolation) resulted in long term changes the IGF-IR and IGFBP-2 in the hippocampus in response to adulthood restraint stress, suggesting that epigenetic modifications occurred at the time of neonatal isolation (34). In our study, we found, but did not validate, changes in gene sets that relate to histone modification, which is a primary mechanism of epigenetic modification.
This study has provided a great deal of information about the neonatal hippocampal response to clinically relevant neonatal conditions: minor stress, severe stress including oxidative stress, and the interaction of morphine treatment with these conditions. We have validated differential expression of 16 genes of the 156 identified by microarray. We used RNA from a separate set of animals because we reasoned that if the findings were reproducible in these new animals, it not only validated the initial findings, vis a vis the validation of the technique, but also provided information regarding the reproducibility of the findings in animals treated with stress and/or morphine. Some of the differentially regulated genes are of uncertain importance, but others are likely to be of great importance and should be further studied. Many questions have been generated by this study. Why are genes and gene families related to energy metabolism upregulated by the combination of stress and morphine? Is this beneficial or deleterious? What is the mechanism by which morphine and stress alter neurogenesis, synaptogenesis, axon formation and neuronal differentiation? Are these effects long lasting? Are they dose-dependent? Is there a dose of morphine which does not impair these functions, or perhaps alternative sedatives that do not have the same developmental effects? Most importantly, the findings from this study must make us question whether the current practice in the NICU is best for our patients. While the treatment of unavoidable stress and pain in critically ill neonates is ethically mandated, the drugs we currently use may not, in fact, be effective in this population due to immaturity of the nervous system (35), and they may also cause harm to the developing brain by interrupting normal developmental functions.
Supplementary Material
Acknowledgments
This work was supported by funding from NIH/NIDA, Grant number R21DA022573, and from NIH/NICHD, Grant number P30HD002274
Abbreviations
- GSA
Gene Set Analysis
- MSM
Mild stress + morphine
- MSS
Mild stress + saline
- NPY
Neuropeptide Y
- SSM
Severe stress + morphine
- SSS
Severe stress + saline
- P
Postnatal day
- qPCR
Quantitative PCR
- CC
Untreated controls
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pedresearch.org).
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roma PG, Huntsberry ME, Riley AL. Separation stress, litter size, and the rewarding effects of low-dose morphine in the dams of maternally separated rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:429–433. doi: 10.1016/j.pnpbp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Mesquita AR, Pego JM, Summavielle T, Maciel P, Almeida OF, Sousa N. Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience. 2007;147:1022–1033. doi: 10.1016/j.neuroscience.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 5.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156:525–531. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Guihard-Costa AM, Larroche JC. Differential growth between the fetal brain and its infratentorial part. Early Hum Dev. 1990;23:27–40. doi: 10.1016/0378-3782(90)90126-4. [DOI] [PubMed] [Google Scholar]
- 7.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Mrzljak L, Uylings HB, Van Eden CG, Judas M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- 10.Dickson BJ. Molecular Mechanisms of Axon Guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 11.Saurer TB, Ijames SG, Carrigan KA, Lysle DT. Neuroimmune mechanisms of opioid-mediated conditioned immunomodulation. Brain Behav Immun. 2008;22:89–97. doi: 10.1016/j.bbi.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine's effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157:70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handelmann GE, Dow-Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides. 1985;6:29–34. doi: 10.1016/0196-9781(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 15.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 16.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 17.Vien TN, Gleason CA, Hays SL, McPherson RJ, Chavkin C, Juul SE. Effects of Neonatal Stress and Morphine on Kappa Opioid Receptor Signaling. Neonatology. 2009;96:235–243. doi: 10.1159/000220763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boasen JF, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology. 2009;95:230–239. doi: 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- 19.McPherson RJ, Gleason C, Mascher-Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology. 2007;92:33–41. doi: 10.1159/000100084. [DOI] [PubMed] [Google Scholar]
- 20.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irizarry RA, Gautier L, Cope L. An R package for analyses of affymetrix oligonucleotide arrays. In: Parmigiani G, Garrett E, Irizarry R, Zeger S, editors. The Analysis of Gene Expression Data. Springer; London: 2003. pp. 102–119. [Google Scholar]
- 22.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 23.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to transcriptional responses to ionizing radiation. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani R. On testing the significance of sets of genes. Ann Appl Stat. 2007;1:107–129. [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camon E, Magrane D, Barrell V, Lee E, Dimmer D, Maslen JB, Harte N, Lopez R, Apweiler R. The Gene Ontology annotation (GOA) database: sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 2004;32:D262–D266. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65:485–492. doi: 10.1203/PDR.0b013e31819d90c8. [DOI] [PubMed] [Google Scholar]
- 28.Díaz D, Krejsa CM, White CC, Keener CL, Farin FM, Kavanagh TJ. Tissue specific changes in the expression of glutamate-cysteine ligase mRNAs in mice exposed to methylmercury. Toxicol Lett. 2001;122:119–129. doi: 10.1016/s0378-4274(01)00341-1. [DOI] [PubMed] [Google Scholar]
- 29.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 30.Macrì S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Korostynski M, Piechota M, Kaminska D, Solecki W, Przewlocki R. Morphine effects on striatal transcriptome in mice. Genome Biol. 2007;8:R128. doi: 10.1186/gb-2007-8-6-r128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anghel A, Jamieson CA, Ren X, Young J, Porche R, Ozigbo E, Ghods DE, Lee ML, Liu Y, Lutfy K, Friedman TC. Gene expression profiling following short-term and long-term morphine exposure in mice uncovers genes involved in food intake. Neuroscience. 2010;167:554–566. doi: 10.1016/j.neuroscience.2010.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erabi K, Morinobu S, Kawano K, Tsuji S, Yamawaki S. Neonatal isolation changes the expression of IGF-IR and IGFBP-2 in the hippocampus in response to adulthood restraint stress. Int J Neuropsychopharmacol. 2007;10:369–381. doi: 10.1017/S1461145706006675. [DOI] [PubMed] [Google Scholar]
- 35.Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115:1494–1500. doi: 10.1542/peds.2004-1425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.