Abstract
Objective
We implemented a set of processes of care measures for bipolar disorder that reflect psychosocial, patient preference, and continuum of care approaches to mental health, and examined whether veterans with bipolar disorder receive care concordant with these practices.
Method
Data from medical record reviews were used to assess key processes of care for 433 VA mental health outpatients with bipolar disorder. Both composite and individual processes of care measures were operationalized.
Results
Based on composite measures, 17% had documented assessment of psychiatric symptoms (e.g., psychotic, hallucinatory), 28% had documented patient treatment preferences (e.g., reasons for treatment discontinuation), 56% had documented substance abuse and psychiatric comorbidity assessment, and 62% had documentation of adequate cardiometabolic assessment. No-show visits were followed up 20% of the time and monitoring of weight gain was noted in only 54% of the patient charts. In multivariate analyses, history of homelessness (OR=1.61; 95% CI=1.05-2.46) and nonwhite race (OR=1.74; 95%CI=1.02-2.98) were associated with documentation of psychiatric symptoms and comorbidities, respectively.
Conclusions
Only half of patients diagnosed with bipolar disorder received care in accordance with clinical practice guidelines. High quality treatment of bipolar disorder includes not only adherence to treatment guidelines but also patient-centered care processes.
Keywords: mood disorders-bipolar, quality of care, quality improvement, co-occurring conditions
BACKGROUND
Bipolar disorder is a chronic illness affecting up to 5.5% of the population1 and is associated with substantial functional limitations2, 3 and health care costs.4, 5 Persons with bipolar disorder often require intensive pharmacologic and psychosocial treatment 2, because the illness is uniquely characterized by alternating periods of mania and depression, which can lead to treatment interruptions and self-medication with substance abuse that impede overall treatment adherence.6 Bipolar disorder is also one of the top ten causes of disability worldwide.7 Therefore, improving quality and subsequent outcomes of care for this illness is a priority.
Despite the availability of efficacious treatments and evidenced-based care guidelines for bipolar disorder within the past several years,2, 8-10 outcomes for bipolar disorder remain suboptimal. Reasons for suboptimal outcome may include poor processes of care, defined as measures in which providers have the most control over in changing care. Prior studies using administrative data have concluded that 37-54% of patients diagnosed with bipolar disorder are not receiving adequate mood stabilizers11, 12 or drug level safety monitoring 13 based on American Psychiatric Association 8 clinical guidelines for the treatment of bipolar disorder. 8 In a separate study14 based on Medicaid administrative claims data, about a third received antimanic agents or psychotherapy in a given year, and enrollees presenting with concurrent depression or anxiety diagnoses had a higher likelihood of receiving pharmacotherapy discouraged by guidelines.
Well-validated quality of care indicators can help to identify gaps in care and ultimately, improve care. Quality measures need to assess clinically relevant processes of care over which providers have control so that they can inform quality improvement initiatives.15 Previous studies regarding quality of care for bipolar and other mental disorders in routine care have only focused on adherence to medication treatment guidelines16 or relied solely on administrative data, which are relatively easy to ascertain.11, 14, 17 One of the limitations of administrative data is the lack of information on patient symptoms, provider decision-making, and details regarding psychosocial treatments, all of which are necessary for constructing patient-centered measures. Medical record reviews have been used to ascertain quality of care when administrative data are not detailed enough, yet most studies assessing quality of care for bipolar disorder based on medical record review have been limited to efficacy trials, employed restrictive exclusion criteria (e.g., bipolar I only, no co-existing substance use disorder), or focused exclusively on pharmacotherapy.16, 18
Nonetheless, quality measures that reflect other biopsychosocial aspects of clinical practice have not been fully operationalized. Measures based on the biopsychosocial model that integrate medication, psychosocial, and patient preference indices, such as assessment of medication side effects, no-show follow-ups, and comorbidities, are critical to the delivery of quality care. A more comprehensive set of quality indicators for bipolar and other mental disorders are needed in order to inform the next generation of electronic data capture. The purpose of this study was to apply a comprehensive set of operationalized quality indicators for chart review that reflect the integration of psychosocial and patient preference indices, and to evaluate the patient factors associated with lower performance on these indicators in a large, naturalistic study of patients receiving care for bipolar disorder.
METHOD
Study Population and Sample
We analyzed data from a longitudinal, naturalistic, population-based study of 435 veterans with mood disorders.19 The target population was patients being treated for bipolar disorder presenting for inpatient or outpatient care during a two-year period (July 2004-July 2006) at a large VA mental health facility. Patients who were clinically diagnosed with bipolar disorder (including bipolar I disorder as well as the spectrum disorders including bipolar II or schizoaffective disorder-bipolar subtype) were eligible. Bipolar subtype was garnered from consulting with the patients’ primary psychiatrist prior to enrollment. We chose this method of diagnosis ascertainment to mirror as closely as possible real-world treatment settings, which typically do not perform routine structured diagnostic interviews. Exclusion criteria included unstable acute medical conditions, acute psychiatric symptoms, or significant cognitive impairment that precluded informed consent.
Patients were approached at the time of their outpatient mental health appointment, or if hospitalized, at the point of reaching psychiatric stability based on clinician assessment, and asked to complete a baseline survey. All enrollees provided informed consent to be surveyed and to have data from their medical records and administrative files ascertained. Chart review and administrative data on utilization, quality of care, and clinical status were collected two years prior to the baseline survey, and between the baseline and follow-up surveys. Administrative data on utilization, including pharmacotherapy, lab tests, and visits were obtained from the VA National Patient Care Database. Of 435 patients, 433 had complete baseline chart data. This study was reviewed and approved by local IRBs.
Measures
Dependent Variables and Composite Measures
We developed a list of processes of care for bipolar disorder adapted from treatment guidelines for bipolar disorder developed by the American Psychiatric Association,20 the Standards for Bipolar Excellence (STABLE) project,21 and the RAND-Altarum national evaluation of VHA mental health programs.22 From this list, we selected measures for which data could be reasonably abstracted from standard medical records and applied to the entire at-risk population of interest. We also included additional measures focused on the integration of psychosocial and patient preferences that have not been previously operationalized, including assessment of symptoms and co-occurring conditions, documented reasons for patients discontinuing medications, and no-show follow-ups (Table 1). These measures also reflect important aspects of anticipatory care reflected in the Chronic Care Model, which was recently operationalized and implemented as a biopsychosocial approach to bipolar disorder care. 23 At the same time these additional measures are population-based (i.e., applicable to an entire cohort of patients diagnosed with bipolar disorder) and not reflective of a single treatment modality.
Table 1.
Summary of Process of Care Indicators for Bipolar Disorder and Source
| Process of Care Indicatorsa | Source |
|---|---|
| Symptom assessments | |
| Monitor change in symptom complex (assess psychotic, hallucinatory, and delusional symptoms) | STABLEb |
| Assess suicidal ideation | STABLE; APAc |
| Comorbidity assessments | |
| Assess illicit drug use | STABLE; RANDd |
| Assess alcohol use | STABLE; RAND |
| Assess tobacco use | RAND |
| Assess PTSD | RAND |
| Cardiometabolic assessment | |
| Record weight, blood pressure, pulse | RAND |
| Monitor for weight gain | STABLE; RAND |
| Documentation of patient treatment experience | |
| Record side effects from medications (monitor extrapyramidal symptoms) | STABLE; RAND; APA |
| Follow up with patient after a no-show visit (enhance treatment compliance) | APA |
| Note reason if patient voluntarily discontinues medication (enhance treatment compliance) | APA |
Indicators were applied using minimally adequate treatment criteria: patients had to have received the care on or within one year timeframe prior to their baselines assessment
STABLE = Standards for Bipolar Excellence
RAND = RAND-Altarum Evaluation of VA Mental Health Quality
APA = American Psychiatric Association
Indicators were categorized into four categories: symptom assessment, substance use/psychiatric comorbidity assessment, cardiometabolic assessment, and treatment experience (Table 1). To reflect minimum necessary standards of care (i.e., minimally adequate treatment for mental health conditions as described elsewhere),24 indicators were applied over a one-year period, from one year prior up to the date of the patient baseline survey. Specifically, patients were considered to have received adequate care for each indicator of the care was received within this one-year time frame. We chose to us this broad definition in order to account for patient no-shows and clinic cancellations that are difficult to capture reliably from existing data sources. For example, patients had to have been screened for substance abuse, or have treatment preferences discussed at least one within the one-year time window.
Based on the aforementioned individual measures, composite measures of quality were also developed from the individual measures based on the above-mentioned four categories: symptom assessment, including suicidal ideation, substance use/psychiatric comorbidity assessment, cardiometabolic assessment, and treatment experience. These categories are based on a framework for measuring quality of mental health care, 19 which outlined key areas for which mental health providers are expected to have some accountability, including assessment of psychiatric symptoms and side effects, assessment of co-occurring conditions such as substance abuse, and patient treatment preferences for psychotropic medications. These composite measures represent items that can be ascertained together from the same data source and are conceptually similar from the standpoint of providers (e.g., history and physical for co-occurring conditions, cardiometabolic measures involving lab tests and physical exams). Symptom assessment measures included a chart notation for each of the following symptoms/experiences: psychosis, hallucinatory symptoms, delusional symptoms, and suicidal ideation. Substance use and psychiatric comorbidity was defined as a chart notation of an assessment of illicit drug use, alcohol use, smoking, and post-traumatic stress disorder (PTSD). Cardiometabolic assessment included chart notation of weight, blood pressure, and pulse, as well as documentation of monitoring of weight gain. Documentation of patient's treatment experience included chart notation of (a) side effects from prescribed medications, (b) a follow-up phone call or letter if a no-show visit occurred, and (c) a reason if a patient voluntarily discontinued any medication.
For each composite measure, all of the definitions had to be satisfied in order to count towards appropriate care. We defined inadequate care for each process category if there was no documentation of at least one of the individual processes of care within a category.
Independent Variables
We used data from the baseline survey to assess key confounders of receiving adequate processes of care: age, sex, and race (white compared other due to small sample sizes of non-white veterans). We also assessed several patient-level variables that that are potentially mutable and could also influence receipt of important processes of care, including substance use (defined as hazardous drinking or illicit drug use disorder) as well as lifetime history of homelessness based on patient self-report. Life time history of homelessness was defined as whether the patient self-reported ever being without a permanent home, and staying overnight in a shelter/park/abandoned building/on the street. Hazardous drinking was assessed using a single survey question from the Alcohol Use Disorders Identification Test (AUDIT), which asked about having 6 or more drinks on a single occasion in the past month. 25, 26 Illicit drug use was assessed using a survey question concerning past year use of marijuana, cocaine, stimulants, or other illicit drugs.27 Social support was assessed with a variable indicating whether the respondent lived alone or with others. Since individuals with significant medical burden may access the healthcare system more often, we included VA service connection, which is an indicator of serious medical need. Any non-VA healthcare use within the past year was also ascertained based on self-report. Finally, we included recruitment into the study during an inpatient stay (indicator of severity), as certain processes of care (e.g. recording of weight, pulse, and blood pressure) may be more likely to occur during inpatient visits.
Analysis
The study used a cross-sectional design in which we assessed patient characteristics at baseline and cumulative data on quality of care were ascertained from baseline back to two years prior to the assessment. We used logistic multiple regression models to examine the effect of key independent variables on appropriate processes of care, including demographics (age, sex, race), alcohol or illicit drug use, lifetime history of homelessness, living alone, VA service connection, non-VA use, and inpatient visit at time of enrollment.. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Cary, NC). Using bivariate analyses (Chi-sq tests), we also compared the performance of our measures of appropriate processes of care to a medication-based quality metric for bipolar disorder: a prescription for any anti-manic medication, defined as any prescription for lithium, divalproex, valproic acid, carbamazepine, lamotrigine, or second generation antipsychotic (olanzapine, aripiprazole, quetiapine, risperidone, ziprasidone, or clozapine) that that occurred from the year prior and up to the enrollment date.18
Results
The sample (N=433) was 86% male, 77% white, and had a mean age of 49 (range 21-78). Overall, 74% of the sample was diagnosed with bipolar I, 9% diagnosed with bipolar II, and 17% diagnosed with schizoaffective disorder-bipolar subtype. Half (54%) reported a history of homelessness and 28% reported a history or illicit drug use (Table 2).
Table 2.
Sample Characteristics of Patients with Bipolar Disorder (N=433)
| N | Mean (SE) | |
|---|---|---|
| Age (mean, in years) | 433 | 49.38 (0.51) |
| N | % | |
| Male | 371 | 85.68 |
| Race | ||
| Black | 58 | 13.39 |
| White | 334 | 77.14 |
| Other | 41 | 9.47 |
| Diagnosis subtype | ||
| Bipolar I | 313 | 74 |
| Bipolar II | 39 | 9 |
| Schizoaffective disorder- bipolar subtype | 72 | 17 |
| Hazardous drinking (>=6 or more drinks-single occasion) | 92 | 21.30 |
| Any illicit drug use reported | 122 | 28.18 |
| Ever been homeless | 235 | 54.40 |
| Currently live alone | 153 | 35.33 |
| Any service connection for a medical condition | 171 | 41.50 |
| Any non-VA health services use | 211 | 48.96 |
| Inpatient visit at time of enrollment | 98 | 22.53 |
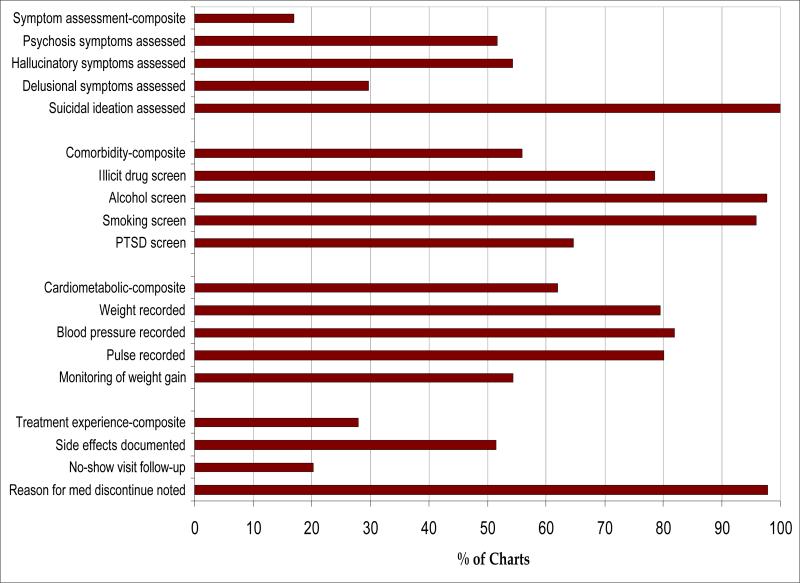
The denominator was the same for each indicator (N=433). Overall, 17% had documented assessment of all symptoms (delusional, psychotic, hallucinatory), 56% had documentation of substance abuse and psychiatric comorbidity assessment, 62% had documentation of adequate cardiometabolic assessment, and 28% had important aspects of the treatment experience recorded in their chart on or within one year prior to their assessment.
All patients (100%) had evidence of assessment of suicidal ideation (Figure 1), although only 20% of those with a no-show visit had documentation that the no-show was followed-up by phone or letter. Monitoring of weight gain was observed in 54% of the cohort. Other side effects were documented 52% of the time, while about half had documented hallucinatory or psychotic symptoms assessed. Delusional symptoms were assessed only 30% of the time.
Figure 1.
Patient-centered Processes of Care Results: Individual and Composite Measures (N=433)
Overall, 72% received appropriate anti-manic medication. Receipt of anti-manic medication was not significantly associated with three of the four composite scores: symptom assessment (Chi-sq=0.18; df=1; P=.67), comorbidity assessment (Chi-sq=3.96; df=1; P=.47), cardiometabolic assessment (Chi-sq=0.62; df=1; P=.43), and treatment experience (r=.71; P=.40).
In multivariate analyses, patients who had a history of homelessness (OR=1.84; 95% CI: 1.01-3.35; P=0.04) or had a service-connected disability (OR=1.87; 95% CI: 1.07-3.27; p=0.03) had an increased odds of having their bipolar symptoms assessed (Table 3). We also found that non-white individuals were more likely to have substance use and psychiatric comorbidity assessed (OR=1.74; 95% CI: 1.02-2.98; p=0.04). Patients who were enrolled as inpatients were more likely to have their bipolar symptoms (OR=2.61; 95% CI: 1.48-4.61; p=0.001), comorbidities (OR=4.61; 95% CI: 2.54- 8.35; P<0.001), and cardiometabolic risk factors assessed (OR=3.24; 95% CI: 1.81-5.81; P<0.001) after adjustment. Self-reported substance use risk factors, notably binge drinking and illicit drug use as reported by patients were not associated with comorbidity (i.e.. substance use) screening.
Table 3.
Patient Factors Associated with Adequate Processes of Care based on Composite Quality Measures: Multivariable Results (N=433)
| Symptomsa | Comorbidityb | Cardiometabolicc | Treatment Experienced | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.01 (0.98-1.03) | .68 | 1.00 (.98-1.02) | .89 | 1.04 (1.01-1.06) | .002 | 1.00 (.98-1.02) | .93 |
| Female | 1.00 (.46-2.20) | .99 | .70 (.38-1.28) | .24 | 1.48 (.80-2.74) | .21 | .85 (.45-1.63) | .63 |
| Non-White | 1.76 (.95-3.25) | .07 | 1.74 (1.02-2.98) | .04 | .62 (.37-1.04) | .07 | .97 (.55-1.70) | .91 |
| Binge drinking | 1.09 (.57-2.09) | .80 | 1.15 (.67-1.97) | .61 | 1.68 (.96-2.92) | .07 | .79 (.44-1.42) | .43 |
| Illicit drug use hx | 1.48 (.80-2.72) | .21 | 1.31 (.79-2.17) | .30 | 1.54 (.92-2.58) | .10 | .87 (.50-1.48) | .60 |
| Homelessness hx | 1.84 (1.01-3.35) | .04 | 1.03 (.67-1.58) | .91 | 1.05 (.68-1.63) | .81 | .78 (.49-1.23) | .28 |
| Live alone | 1.01 (.57, 1.80) | .97 | .81 (.52, 1.26) | .35 | .99 (.64, 1.56) | .99 | 1.35 (.86, 2.14) | .20 |
| Service connection | 1.87 (1.07-3.27) | .03 | 1.47 (.95-2.27) | .09 | 1.04 (.67-1.61) | .87 | 1.50 (.95, 2.36) | .08 |
| Any non-VA use | 1.59 (.92, 2.78) | .10 | .85 (.55, 1.29) | .43 | 1.01 (.66, 1.54) | .96 | 1.10 (.70, 1.70) | .68 |
| Inpatient stay | 2.61 (1.48, 4.61) | .001 | 4.61 (2.54, 8.35) | <.001 | 3.24 (1.81, 5.81) | <.001 | .99 (.58, 1.69) | .96 |
Symptoms assessment composite score was defined as a chart notation for each of the following symptoms/experiences: psychosis, hallucinatory symptoms, delusional symptoms, and suicidal ideation.
Comorbidity assessment composite score was defined as a chart notation of an assessment of illicit drug use, alcohol use, smoking, and post-traumatic stress disorder (PTSD).
Cardiometabolic assessment composite score was defined as a chart notation of weight, blood pressure, pulse, and any documentation of weight gain monitoring.
Treatment experience composite score was defined as a chart notation of (a) side effects from prescribed medications, (b) a follow-up phone call or letter if a no-show visit occurred, and (c) a reason if a patient voluntarily discontinued any medication.
Discussion
To our knowledge this is one of the few studies to apply operationalized processes of care that represent a wider range of services beyond medication use for patients with bipolar disorder, and determine the patient factors associated with receipt of adequate processes of care in this group. This is also one of the first studies to propose patient-centered composite measures for assessing quality of care for bipolar disorder. We found that although certain aspects of bipolar care were performed at a very high rate (e.g., everyone had suicidal ideation assessed) there is room for improvement in a number of domains. In particular, this study highlights the need for quality improvement in the area of symptom assessment, following up with patients who have a no-show visit, and documentation of side effects from antipsychotic medications, including obesity.
Our findings illustrate the usefulness of composite quality measures. By defining processes of care that should or could be conducted conjointly, it is possible to identify where in the treatment process to intervene to improve the overall quality of care. Composite measures for processes of care have been previously implemented in general medical care, primarily for hospitalized patients of for those with chronic disease,28-30 but few prior studies have developed and implemented composite measures for mental health quality of care in general. Hepner (2007) developed composite measures for quality of depression care based on expert panel input, which rated indicators by “high”, “medium” or ”low” clinical need; however, associations between these need-based measures and clinical outcomes was not assessed.31 In contrast, we developed composite measures to reflect a cluster of common assessments that would be practical to obtain from the same query or data sources (assessment of symptoms, history of comorbidities, cardiometabolic monitoring, and treatment preferences). By including processes of care indicators, these composite measures also reflect important aspects of care that represent services for which providers have most control.
Based on the application of our composite measures, we found that only one in 6 patients was assessed for symptoms related to bipolar disorder. In contrast, 100% received screening for suicidal ideation. This finding might reflect the VA's ongoing efforts to reduce the risk of suicide in patients diagnosed with mental disorders. However, a more comprehensive assessment of symptoms is warranted given the substantial levels of comorbidity experienced by patients with bipolar disorder. Furthermore, although the majority of patients had documented reasons for discontinuation of medications, potential warning signs of nonadherence, including side effects and follow-ups to no-shows were not routinely recorded. Although between 65% and 98% of records reviewed met criteria for each individual comorbidity assessment (e.g., illicit drug, alcohol, smoking, or PTSD screen), only a little over half were assessed for all four co-occurring conditions. These conditions are considered the rule rather than the exception in bipolar disorder, with over two-thirds with a lifetime substance use disorder diagnosis and one-third experiencing at least three medical comorbidities at a given point in time, both of which lead to poor functional outcomes.32
In addition, about two-thirds received all four cardiometabolic screenings, weight, blood pressure, pulse, and weight gain. This contrasts with non-VA settings where Golden et al.21 found that less that 20% received adequate monitoring of cardiometabolic side effects. The increased rates of cardiometabolic monitoring observed in our study perhaps reflect the VA's priority in chronic disease management. Nonetheless, this finding reflects the usefulness of composite assessments of cardiometabolic risk. Given the increased use of atypical antipsychotics as mood stabilizers, it is imperative to ensure that all four cardiometabolic screenings are completed. In fact, the American Diabetes Association has released a call for composite measures based on guidelines for cardiometabolic management.
Composite measures were not associated with guideline-concordant medication use, with the exception of symptom assessment. Anti-manic medication prescription use as a performance measure may not reflect other aspects of patient-centered care that are potentially associated with adverse outcomes, including comorbidity or functional impairment. Surprisingly, treatment preference measures were not significantly associated with guideline-concordant receipt of bipolar disorder medications as well. This finding illustrates the need for a more comprehensive set of measures to assess adequate processes of care, given that a collaborative decisionmaking process between patients and providers is considered an important component of care for bipolar disorder.18 Moreover, most quality measures based on guideline-concordant medication use are based on purely administrative data, where patient preference, contraindications and tolerability are not typically assessed. In contrast, our study applied operationalized quality indicators that reflected these other aspects of care.
VA patients who were nonwhite or homeless were more likely to be assessed for comorbidities or symptoms, respectively, perhaps due to selection effects. Notably, individuals who were homeless were recruited at an encounter rather than in the community, and hence, these findings may not represent those who are not engaged in treatment. Moreover, perhaps clinicians perceived nonwhite patients as having greater illness complexity, and hence, were more likely to screen for substance use. Evidence suggests that racial/ethnic minorities are less likely diagnosed with bipolar disorder and more likely diagnosed with a psychotic disorder compared to whites, and clinical judgment rather than symptoms presentation is likely the main reason for this trend33, 34 Hence, findings from our study may reflect potential selection effects, in which nonwhites who have been diagnosed with bipolar disorder are already receiving better care because they have been properly diagnosed.35 In contrast, patient self-reported substance use risk factors were not associated with the comorbidity assessment screening by providers, reflecting that providers may not be aware of these patient risk factors.
Our findings suggest that in order to present a more patient-centered view of quality, processes of care for bipolar disorder cannot be distilled into a single measure; but rather, a series of patient-centered composite indicators. Standard quality measures for the treatment of bipolar disorder have primarily focused on adequate type, dose, and duration of medications.14, 16-18 While this is certainly important, focusing only on medication-based quality measures may result in “not seeing the forest for the trees” – missing the bigger picture of quality care. Some have worried that recent policy efforts, such as pay-for-performance programs, would encourage physicians to attend to only those processes of care that were being measured.36 For example, Busch14 found that among patients with bipolar I disorder, guideline-concordant pharmacotherapy for bipolar disorder (i.e., receiving any Lithium, valproate, or carbamazepine prescription) increased 68-77% from 1991 to 1999, while psychosocial care was decreasing during the same time period (94% to 69% from 1991 to 1999). There is little evidence that quality improvement efforts focused on medication prescribing also improve non-measured critical processes of care.
Despite the comprehensive assessment of quality of care in bipolar disorder, the results from this study need to be interpreted with caution. First, quality of care was only assessed at a single point in time at a single site. The cross-sectional nature of this study precluded us from determining the extent to which these indicators were associated with patient outcomes over time. Some services documented may not reflect actual provision of care. In addition, while composite measures can be used to summarize complex data and can identify general areas for quality improvement, they run the risk of loss of key information (e.g., what particular processes are driving good or poor quality). Moreover, because we did not rely on structured clinical interviews to ascertain bipolar disorder diagnosis and subtype, we may have not identified all patients with bipolar disorder, nor differentiated subtype accurately, especially for bipolar II. Limited information from the medical records also precluded us from comprehensively assessing manic or depressive symptoms, as well as their treatment, and hence, we were unable to develop a quality metric for these episodes.
Finally, the generalizability of this study may be limited to large outpatient clinics within the VA. Nonetheless, this study serves as a starting point for improving electronic data capture of quality of care data. With its national network of providers, single EMR, and large patient population, the VA does not reflect the status of electronic medical records in the real world. In a recent study, only 12% of U.S. hospitals had electronic physician's notes37 and in general, the fragmentation of claims data across different services38 and payers (e.g., Medicaid, private) precludes many community-based practices from assessing quality of care for all of their patients. As a first step, this study applied a set of quality indicators that went beyond medication usage that were measurable using medical record data. Further developing this set of measures through the establishment of an “ontology” that establishes definitions and relationships among the various concepts, data elements and potential data sources available to community-based providers is an important next step in implementing quality measurement in mental health. In computer science, an ontology is a rigorous organization of a knowledge domain (e.g., evidence-based mental health care) that is usually hierarchical and contains all the relevant entities, their definitions, and information sources. Measurement ontologies are increasingly being used, notably in ICD-11, to facilitate the uptake of quality measurement across practices with varying degrees of information technology implementation. The biopsychosocial model-based quality assessment implemented as part of this study can form the basis for adaptation of an ontology tool to multiple health record environments ranging from paper charts to varying degrees of EMR adoption, independent of available information technology. Ultimately, this could allow providers across different sites to become more familiar with electronic data capture, moving them towards EMR use over time.
Overall, substantial gaps in care exist for patients with bipolar disorder, especially for patient-centered processes such as symptom assessment and treatment experience. Application of composite measures provides quality improvement opportunities that are comprehensive, patient-centered, system-oriented, and feasible to track over time. Future quality improvement efforts must focus on a common strategy of data collection as well as a more holistic approach to treatment.
ACKNOWLEDGEMENTS
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (IIR 02-283) and the National Institute of Mental Health (MH 74509; MH 79994, T32 MH19986). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. All authors warrant having no actual or perceived conflicts of interest- financial or non-financial- in the procedures described in this manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
REFERENCES
- 1.Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. Journal of Affective Disorders. 2003;73:123–131. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M, Unutzer J, Pincus HA, Lawson WB. Bipolar disorder. Mental Health Services Research. 2002;4:225–229. doi: 10.1023/a:1020968616616. [DOI] [PubMed] [Google Scholar]
- 3.Bauer MS, Kirk GF, Gavin C, Williford WO. Determinants of functional outcome and healthcare costs in bipolar disorder: a high-intensity follow-up study. Journal of Affective Disorders. 2001;65(3):231–241. doi: 10.1016/s0165-0327(00)00247-0. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Unutzer J. Health care utilization and costs among patients treated for bipolar disorder in an insured population. Psychiatric Services. 1999;50:1303–1308. doi: 10.1176/ps.50.10.1303. [DOI] [PubMed] [Google Scholar]
- 5.Peele PB, Xu Y, Kupfer DJ. Insurance expenditures on bipolar disorder: clinical and parity implications. American Journal of Psychiatry. 2003;160:1286–1290. doi: 10.1176/appi.ajp.160.7.1286. [DOI] [PubMed] [Google Scholar]
- 6.Keck PE, Jr., McElroy SL, Strakowski SM, et al. 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. American Journal of Psychiatry. 1998;155:646–652. doi: 10.1176/ajp.155.5.646. [DOI] [PubMed] [Google Scholar]
- 7.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nature Medicine. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiaric Association Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002 Apr;159(4 Suppl):1–50. [PubMed] [Google Scholar]
- 9.Dennehy EB, Bauer MS, Perlis RH, Kogan JN, Sachs GS. Concordance with treatment guidelines for bipolar disorder: data from the systematic treatment enhancement program for bipolar disorder. Psychopharmacol Bull. 2007;40(3):72–84. [PubMed] [Google Scholar]
- 10.Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002 Apr;63(4):288–299. doi: 10.4088/jcp.v63n0404. [DOI] [PubMed] [Google Scholar]
- 11.Unutzer J, Simon G, Pabiniak C, Bond K, Katon W. The use of administrative data to assess quality of care for bipolar disorder in a large staff model HMO. Gen Hosp Psychiatry. 2000;22:1–10. doi: 10.1016/s0163-8343(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 12.Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA. Trends in the treatment of bipolar disorder by outpatient psychiatrists. American Journal of Psychiatry. 2002;159(6):1005–1010. doi: 10.1176/appi.ajp.159.6.1005. [DOI] [PubMed] [Google Scholar]
- 13.Marcus SC, Olfson M, Pincus HA, Zarin DA, Kupfer DJ. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. American Journal of Psychiatry. 1999;156:1014–1018. doi: 10.1176/ajp.156.7.1014. [DOI] [PubMed] [Google Scholar]
- 14.Busch AB, Huskamp HA, Landrum MB. Quality of care in a Medicaid population with bipolar I disorder. Psychiatric Services. 2007;58:848–854. doi: 10.1176/ps.2007.58.6.848. [DOI] [PubMed] [Google Scholar]
- 15.Donabedian A. Explorations in Quality Assessment and Monitoring. Health Administration Press; Ann Arbor, MI: 1980. The Definition of Quality and Approaches to its Assessment. [Google Scholar]
- 16.Duffy FF, Narrow W, West JC, et al. Quality of care measures for the treatment of bipolar disorder. Psychiatric Quarterly. 2005;76(3):213–230. doi: 10.1007/s11126-005-2975-4. [DOI] [PubMed] [Google Scholar]
- 17.Busch AB, Frank RG, Sachs G. Bipolar-I depression outpatient treatment quality and costs in usual care practice. Psychopharmacol Bull. 2008;41:24–39. [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer MS, Biswas K, Kilbourne AM. Enhancing multiyear guideline concordance for bipolar disorder through collaborative care. American Journal of Psychiatry. 2009 Nov;166(11):1244–1250. doi: 10.1176/appi.ajp.2009.09030342. [DOI] [PubMed] [Google Scholar]
- 19.Kilbourne AM, Fullerton C, Dausey D, Pincus HA, Hermann RC. A framework for measuring quality and promoting accountability across silos: the case of mental disorders and co-occurring conditions. Qual Saf Health Care. 19:113–116. doi: 10.1136/qshc.2008.027706. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision). American Journal of Psychiatry. 2002;159:1–50. [PubMed] [Google Scholar]
- 21.Golden WE, Hermann RC, Jewell M, Brewster C. Development of evidence-based performance measures for bipolar disorder: overview of methodology. J Psychiatr Pract. 2008;14(Suppl 2):18–30. doi: 10.1097/01.pra.0000320123.91799.e4. [DOI] [PubMed] [Google Scholar]
- 22.Horvitz-Lennon ME WK, Pincus HA, Shugarman LR, Stein BD, Mattox T, Mannle TE., Jr . Veteran's Health Administration Mental Health Program Evaluation Technical Manual. Rand Health; Santa Mondiac: 2009. [Google Scholar]
- 23.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial. Psychiatric Services. 2006;57:927–936. doi: 10.1176/ps.2006.57.7.927. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Patten SB, Williams JV, et al. Help-seeking behaviours of individuals with mood disorders. Can.J.Psychiatry. 2005;50(10):652–659. doi: 10.1177/070674370505001012. 09. [DOI] [PubMed] [Google Scholar]
- 25.Saunders JB, Aasland OG, Babor TF, de lFJR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II 4. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. 06. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AJ, Maisto SA, McNeil M, et al. Three questions can detect hazardous drinkers. Journal of Family Practice. 2001;50:313–320. [PubMed] [Google Scholar]
- 27.Kessler RC, Andrews G, Mroczek D, Üstün TB, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berwick DM. Toward an applied technology for quality measurement in health care. Med Decis Making. 1988 Oct-Dec;8(4):253–258. doi: 10.1177/0272989X8800800405. [DOI] [PubMed] [Google Scholar]
- 29.Glickman SW, Boulding W, Roos JM, Staelin R, Peterson ED, Schulman KA. Alternative pay-for-performance scoring methods: implications for quality improvement and patient outcomes. Med Care. 2009 Oct;47(10):1062–1068. doi: 10.1097/MLR.0b013e3181a7e54c. [DOI] [PubMed] [Google Scholar]
- 30.Tu JV, Donovan LR, Lee DS, et al. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA. 2009 Dec 2;302(21):2330–2337. doi: 10.1001/jama.2009.1731. [DOI] [PubMed] [Google Scholar]
- 31.Hepner KA, Rowe M, Rost K, et al. The effect of adherence to practice guidelines on depression outcomes. Ann Intern Med. 2007 Sep 4;147(5):320–329. doi: 10.7326/0003-4819-147-5-200709040-00007. [DOI] [PubMed] [Google Scholar]
- 32.Bauer MS, Altshuler L, Evans DR, Beresford T, Williford WO, Hauger R. Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. Journal of Affective Disorders. 2005 Apr;85(3):301–315. doi: 10.1016/j.jad.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Strakowski SM, Keck PE, Jr., Arnold LM, et al. Ethnicity and diagnosis in patients with affective disorders. Journal of Clinical Psychiatry. 2003 Jul;64(7):747–754. doi: 10.4088/jcp.v64n0702. [DOI] [PubMed] [Google Scholar]
- 34.Neighbors HW, Trierweiler SJ, Ford BC, Muroff JR. Racial Differences in DSM Diagnosis Using a Semi-Structured Instrument: The Importance of Clinical Judgment in the Diagnosis of African Americans. Journal of Health and Social Behavior. 2003;44(3):237–256. [PubMed] [Google Scholar]
- 35.Kilbourne AM, Bauer MS, Han X, et al. Racial differences in the treatment of veterans with bipolar disorder. Psychiatric Services. 2005;56:1549–1555. doi: 10.1176/appi.ps.56.12.1549. [DOI] [PubMed] [Google Scholar]
- 36.Hayward RA, Kent DM. 6 EZ steps to improving your performance: (or how to make P4P pay 4U!). JAMA. 2008 Jul 16;300(3):255–256. doi: 10.1001/jama.2008.69. [DOI] [PubMed] [Google Scholar]
- 37.Jha AK, Desroches CM, Campbell EG, et al. Use of Electronic Health Records in U.S. Hospitals. New England Journal of Medicine. 2009;360:1–11. doi: 10.1056/NEJMsa0900592. [DOI] [PubMed] [Google Scholar]
- 38.Guo JJ, Keck PE, Jr., Corey-Lisle PK, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. Journal of Clinical Psychiatry. 2006 Jul;67(7):1055–1061. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]