Abstract
Behavioral sensitization involves increases in the magnitude of a response to a stimulus after repeated exposures to the same response initiator. Administration of psychomotor stimulants and the induction of appetitive motivational states associated with natural reinforcers like sugar and salt are among experimental manipulations producing behavioral sensitization. In rats, repeated administration of the mineralocorticoid agonist deoxycorticosterone acetate (DOCA) initially induces incremental increases in daily hypertonic saline consumption (i.e., sensitization of sodium appetite) in spite of the retention of sodium. The present studies investigated whether sodium appetite sensitization induced by DOCA shares mechanisms similar to those of psychomotor stimulant-induced sensitization, and whether there is evidence for reciprocal cross-sensitization. In Experiments 1 and 3, rats received control or cocaine treatments to induce locomotor sensitization. A week later DOCA (or vehicle) was administered to generate a sodium appetite. Animals pretreated with cocaine showed a greater sodium appetite. In Experiment 2, the order of the putative sensitizing treatments was reversed. Rats first received either a series of DOCA or vehicle treatments either with or without access to saline and were later tested for sensitization of the locomotor response to cocaine. Animals pretreated with DOCA without access to saline showed greater locomotor responses to cocaine than animals receiving vehicle treatments. Together these experiments indicate that treatments generating a sustained salt appetite and producing cocaine-induced psychomotor responses show reciprocal behavioral cross-sensitization. The underlying mechanisms accounting for this relationship may be the fact that psychostimulants and an unresolved craving for sodium can act as potent stressors.
Keywords: Salt-appetite, Psychostimulants, Sensitization, Natural-rewards, Stress, Deoxycorticosterone-acetate
1. Introduction
Both physiological and behavioral plasticity allow organisms to respond to a changing environment. Behavioral sensitization is an example of effector system plasticity that is defined as the enhancement of responses induced as a consequence of repeated experiences with an identical initiating stimulus. Both sensitization and tolerance can be considered to be examples of non-associative learning as indicated by an increase or decrease, respectively, in the magnitude of a response after its repeated elicitation. In the context of drugs, behavioral sensitization can be demonstrated by an increment in the effectiveness of an agent after intermittent administration (Stewart and Badiani 1993). A substantial body of experimental evidence from studies on the mechanisms of addictive behaviors demonstrates that repeated administration of many drugs of abuse results in response sensitization (Crombag et al., 1999; Gulley et al., 2003; Kuczenski and Segal 1999; Stewart and Badiani 1993).
Behavioral sensitization is not limited to addictive drugs, but can also be induced by the presence of strong motivational or affective states (e.g., hunger or thirst) that are coupled to consummatory behaviors associated with natural rewards (e.g., food, salt, water or access to a mate), putative physiological/physical insults or perceived potential threats to survival (e.g., restraint, social defeat or predator order). In the current series of studies we investigated the nature of reciprocal cross-sensitization of locomotion produced by cocaine with an ingestive behavior involving the consumption of normally non-palatable saline solutions, which in this case was induced by treatment with a mineralocorticoid agonist, deoxycorticosterone acetate (DOCA).
Sodium appetite (a.k.a. salt appetite) is often operationally defined by observing a significant increase in the consumption of sodium chloride solutions with concentrations sufficiently high (usually hypertonic) so that under fluid replete, satiated conditions they would be eschewed. A strong sodium appetite in animals can be evoked experimentally by using various challenges (e.g., treatment with a diuretic/natriuretic, hypovolemic hemorrhage or its experimental simulation) to induce sodium depletion. Using the diuretic/natriuretic furosemide, Clark and Bernstein (2004) demonstrated that rats with a history of periodic sodium depletion-induced salt appetite evidenced sensitized amphetamine-induced locomotor responses when compared to control animals. This result demonstrates cross-sensitization (i.e., a given treatment enhancing the response induced by another treatment). Cross-sensitization was also obtained by the same authors when animals were first repeatedly treated with amphetamine, and then tested for salt appetite induced by sodium depletion (Clark and Bernstein 2004). Taken together, these results demonstrate reciprocal cross-sensitization.
There are methods to induce salt appetite without producing a body sodium deficit. Administration of mineralocorticoid agonists, such as aldosterone or DOCA, provokes a robust salt appetite in the face of sodium and water retention (Tomita et al., 1985). The sodium appetite induced by DOCA treatment is characterized by a progressive escalation of hypertonic saline intake for several days until it reaches an asymptote (Wolf 1965). In other words, repeated DOCA treatment can be viewed as a progressive sensitization of sodium appetite. With DOCA treatments, sodium reabsorption occurs in the kidney to minimize sodium loss, and animals become sodium loaded. Therefore, DOCA administration provides a method to test whether it is the presence of a sodium appetite per se or a sodium deficit per se that induces sensitization, cross-sensitization with other treatment-induced responses, and reciprocal cross-sensitization.
Cocaine and amphetamine induce similar patterns of behavioral locomotor responses. Although both of these psychomotor stimulants provoke increases in synaptic dopamine, norepinephrine and serotonin, the cellular and molecular mechanisms associated with these effects are different (Jones et al., 1998; Kahlig and Galli 2003; Robertson et al., 2009). In the present experiment we chose to use cocaine to allow an assessment of the generality of efficacy of different psychomotor stimulants in relation to the cross-sensitization phenomenon involving salt appetite.
On the basis of this background the first objective of the present studies was to test whether the locomotor response produced with the psychomotor stimulant cocaine cross-sensitizes with DOCA-induced sodium appetite. A second objective of the present studies was to test whether the sensitization of DOCA-induced sodium appetite cross-sensitizes with psychomotor stimulants. The outcomes of studies addressing both these objectives provide evidence for reciprocal cross-sensitization for the treatments which induce a psychomotor response and salt appetite. In addition, the results indicate that cocaine is similar to amphetamine in inducing reciprocal cross-sensitization with a treatment that induces salt related appetitive behaviors.
2. General Methods
2.1 Animals
Sprague-Dawley male rats (n = 88 ; Harlan, Indianapolis, IN), weighing between 250 to 275 g at the beginning of the experiments, were housed in individual stainless steel cages on a 12h light-dark cycle (lights on at 0500h) with an ambient temperature of 22° C and a controlled relative humidity (45–55%). Animals had access to either distilled water plus 0.3 M NaCl solution (hypertonic saline) or only to distilled water. All animals were allowed free access to Teklad chow (0.31% NaCl; Harlan Teklad, Madison, WI). All procedures were conducted in accordance with the National Institutes of Health (1986) Guides for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee.
2.2 Drugs
Deoxycorticosterone acetate (DOCA; Sigma, St. Louis, MO) was dissolved in propylene glycol (Fisher Scientific, Pittsburgh, PA) and administered s.c. in a dose of 10 mg/kg in a volume of 1 ml/kg. Cocaine HCl (Coc, Sigma) was dissolved in isotonic saline for administration at the following doses: 7, 15 and 30 mg/kg. All cocaine doses were administered in a volume of 1 ml/kg. Control treatments for DOCA and cocaine administration were 1 ml/kg injections of the vehicles, propylene glycol and isotonic saline, respectively.
2.3 Locomotor Activity Monitoring
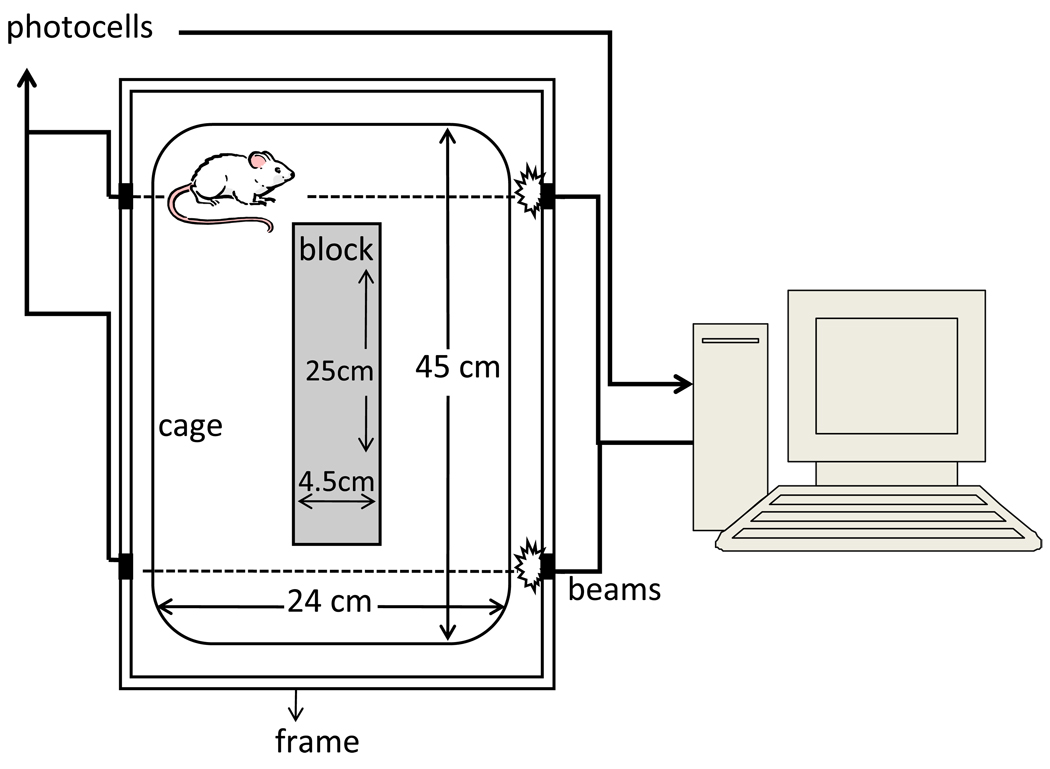
Locomotion was assessed after the administration of saline (vehicle) or cocaine. Locomotion was determined by placing the animals into activity monitor units (AMUs, constructed at The University of Iowa Department of Psychology Instrumentation Shop, see Figure 1). AMUs were constructed of standard translucent “shoe box” cages (45 cm length × 24 cm wide × 20 cm high) with plastic inserts (islands of 25 cm length × 4.5 cm wide × 20 cm high). Two sets of photocells and infrared beams were located at the front and back of the cages (5 cm from the back or front wall and 2 cm above the floor). A computer connected to the photocells recorded the number of times an animal traversed the cage to sequentially interrupt the two beams (i.e., crossovers). The AMUs contained commercial bedding material (Sani Chip, Harlan, Indianapolis, IN).
Figure 1.
2.4 Cocaine-induced behavioral sensitization
The cocaine sensitization procedure consisted of repeated daily i.p. administrations of either 15 mg/kg cocaine hydrochloride (cocaine group) or isotonic saline (saline group). Over the period of 7 days, animals were placed in the AMUs for 30 min for habituation to the apparatus. Then the animals received either cocaine or saline injections and were returned to the AMUs where their locomotion was recorded for another 60 min. After each vehicle/cocaine session, animals were returned to their home cages.
2.5 Statistics
Data were analyzed using ANOVAs with Group (i.e., for independent groups) and Time/Session (i.e., with repeated measures) as fixed factors, and rats as a nested random factor (i.e., a factor that is not controlled by the experimenter) with residual/maximum likelihood method of fitting data (Cobb 1998; Searle et al., 1992). Specifically, in Experiments 1 and 3 the Group factor (cocaine or saline treatments) was administered over repeated sessions (Session factor), and DOCA or vehicle induced 0.3 M NaCl intakes (Group factor) were measured repeatedly over sessions (Session factor). In Experiment 2 the Group factor (DOCA or vehicle treatment) was administered repeatedly over sessions (Session factor) and sodium and water intakes measured. In Experiment 2, three-min blocks of activity for the DOCA or vehicle treated animals (Group factor) was repeatedly measured over time (Time factor). Where it was appropriate, planned pair-wise contrasts were used. All the statistical analyses were performed in JMP (version 8; SAS Institute, Cary, SC). Alpha was set at 0.05.
2.6 Experiment 1: The effect of cocaine pretreatment on DOCA-induced sodium appetite with 2h of daily sodium access
2.6.1 Experimental Protocol
Animals had ad libitum access to the 0.3 M NaCl and distilled water for 3 days before the experiments began. The rats were matched on the basis of their spontaneous (ad libitum) hypertonic saline intake and divided into 4 experimental subgroups. Two subgroups (Cocaine = Coc, n=5/group; see Table 1 for a summary of the groups employed and the abbreviations to designate groups for all of the experiments presented in this paper) received a daily cocaine sensitization treatment (15 mg/kg i.p.) administered over the course of 7 days. [This procedure and dose of cocaine has been shown previously to induce locomotor sensitization (Bell et al., 2000).] A second pair of subgroups received vehicle (isotonic saline = Sal, n=5/group) treatments. When the cocaine sensitization procedure was complete, animals were left undisturbed for 7 days before a DOCA-induced salt appetite phase started. Then, one of the cocaine subgroups and one of the saline subgroups received daily DOCA (D, i.e., Coc/D+Na and Sal/D+Na, respectively) injections (10 mg/kg) at 0800h. The remaining two subgroups (Coc-Veh+Na and Sal-Veh+Na) received vehicle injections. During DOCA treatment, animals had continuous access to distilled water but were also given access to hypertonic saline (0.3 M NaCl) for 2h each day starting at 0900h. Sodium appetite was defined as a significant increase in the amount of hypertonic saline solution consumed in comparison to that ingested on the first day after DOCA or vehicle treatment.
Table I.
Group nomenclature according to treatments provided to animals during experiments. There is a week off between first and second treatment.
| Group | First Treatment | Second Treatment | |
|---|---|---|---|
| Experiment 1 | |||
| Sal/Veh+Na | 7 × saline | 8 × Vehicle with sodium access | |
| Sal/D+Na | 7 × saline | 8 × DOCA with sodium access | |
| Coc/Veh+Na | 7 × cocaine | 8 × Vehicle with sodium access | |
| Coc/D+Na | 7 × cocaine | 8 × DOCA with sodium access | |
| Experiment 2 | |||
| Veh+Na | 8 × Vehicle with sodium access | 1 × cocaine | |
| D+Na | 8 × DOCA with sodium access | 1 × cocaine | |
| D+NoNa | 8 × DOCA with no sodium access | 1 × cocaine | |
| Experiment 3 | |||
| Sal/Veh+Na | 7 × saline | 8 × Vehicle with sodium access | |
| Sal/D+NoNa | 7 × saline | 8 × DOCA with no sodium access except after the last DOCA treatment | |
| Sal/D+Na | 7 × saline | 8 × DOCA with sodium access | |
| Coc/Veh+Na | 7 × cocaine | 8 × Vehicle with sodium access | |
| Coc/D+NoNa | 7 × cocaine | 8 × DOCA with no sodium access except after the last DOCA treatment | |
| Coc/D+Na | 7 × cocaine | 8 × DOCA with sodium access | |
2.6.2 Results and Discussion
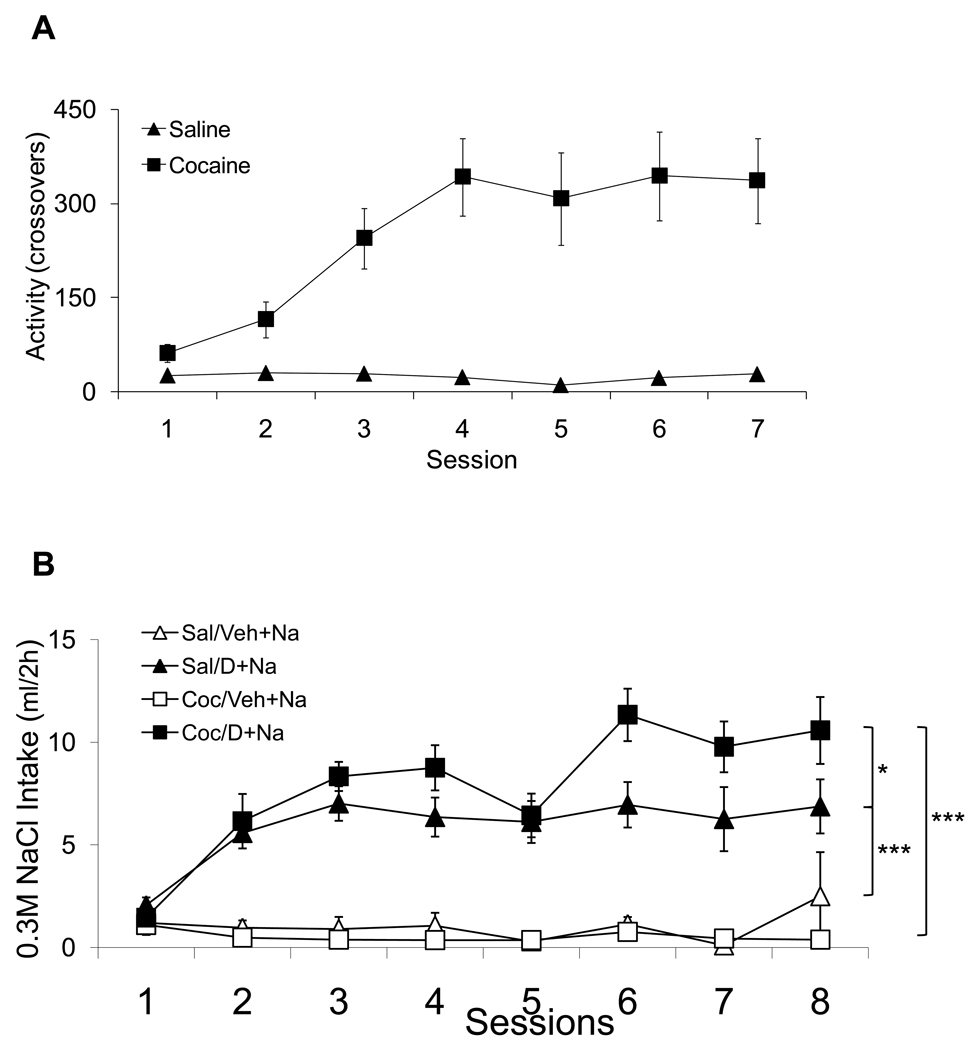
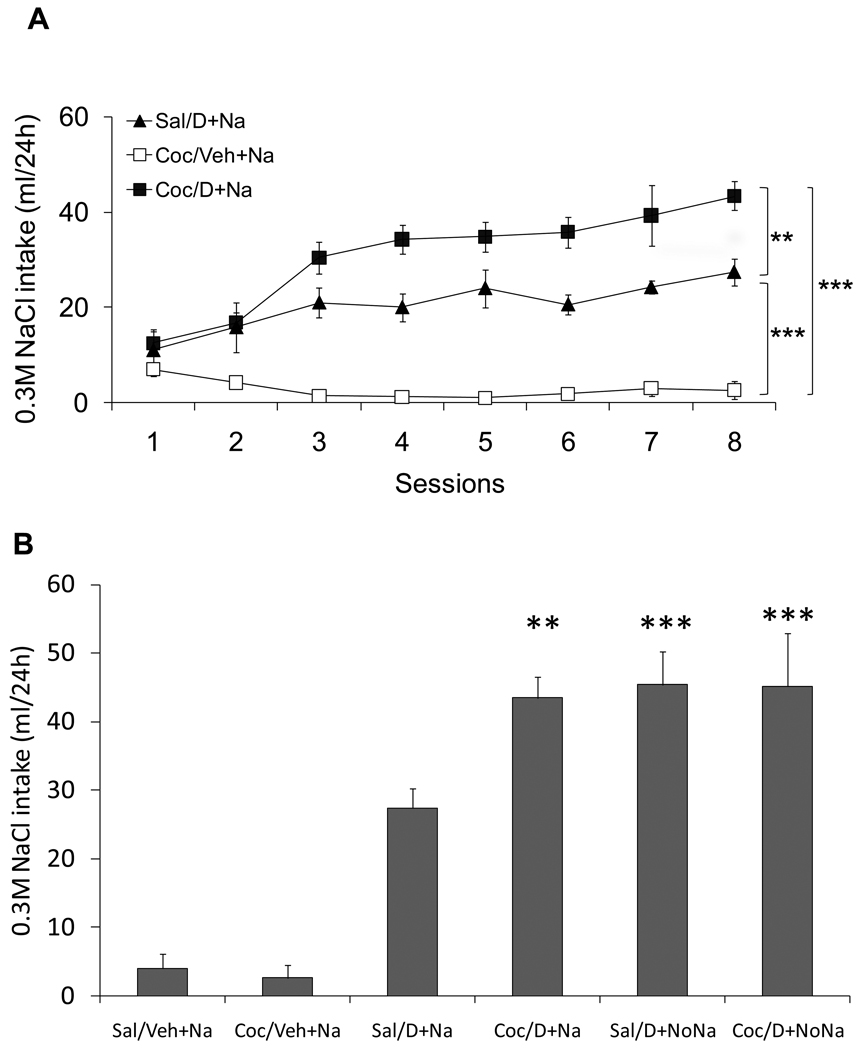
Figure 2A shows the mean daily locomotor responses induced by repeated administration of either 15 mg/kg of cocaine or isotonic saline. The ANOVA with Group and Session as fixed factors and Subject as a random factor found significant effects of Group and Session as well as significant interaction (F(1,27)= 41.12, F(6,162)= 6.51, F(6,162)= 7.13, respectively; p<0.0001). Figure 2B shows the 0.3 M NaCl intakes during DOCA or vehicle treatments in animals that have been pretreated with either cocaine or saline. The ANOVA with Group and Session as fixed factors and Subject as a random factor found significant effects of Group and Session as well as significant interaction (F(3,16)= 42.59, F(7,112)= 9.83, F(21,112)= 4.67, respectively; p<0.001). Planned pair-wise contrast comparisons indicated that the Coc/D+Na group consumed a greater amount of sodium than the Sal/D+Na group across sessions (Figure 2B: F(1,16)= 6.20, p<0.05). Both Coc/D+Na and Sal/D+Na groups increased their sodium intake when compared with Coc/Veh+Na (F(1,16)= 87.24, p<0.001) and Sal/Veh+Na (F(1,16)= 38.80, p<0.001), respectively. Specifically, cocaine pretreatment sensitized the salt appetite of DOCA treated rats. It should also be noted that both DOCA treated groups significantly increased their sodium intake compared to vehicle treated animals from the second day on, which is much sooner than the 3 to 5 day period before the onset of a significant salt appetite commonly reported in the literature.
Figure 2.
2.7 Experiment 2: The effects of DOCA pretreatment on cocaine-induced locomotion
2.7.1 Rationale
In order to test for reciprocal cross-sensitization between cocaine-induced locomotion sensitization and DOCA-induced sodium appetite sensitization, it was necessary to reverse the order of the treatments. In this experiment sodium appetite was induced by repeated administration of DOCA, and a week later the animals were tested to determine if the locomotor response to cocaine was sensitized. We have observed in previous studies in our laboratory (Morris et al., 2006; Morris et al., 2010) that the development of sodium appetite during DOCA treatment does not depend on animals having access to hypertonic saline throughout the mineralocorticoid treatment period. Consequently a DOCA treated group without access to hypertonic solution was included as part of the experimental design.
2.7.2 Experimental Protocol
Rats were allowed free access to distilled water and 0.3 M NaCl solution for 3 days prior to beginning the experiment. Animals were matched on the basis of their spontaneous sodium intake and divided among 3 experimental groups. Then, over the course of 8 days at 0800h, two of the groups received daily injections of DOCA (10 mg/kg) or propylene glycol vehicle (1 ml/kg). One group of DOCA treated animals (n=16) had access to 0.3 M NaCl plus distilled water (DOCA-sodium group = D+Na), but the second group had access only to distilled water (DOCA-No Sodium group = D+NoNa, n=16). A third group of animals received vehicle injections and had access to both 0.3 M NaCl and distilled water (Veh+Na, n=16). The amount of fluid that each animal ingested was recorded daily.
The DOCA treatment phase was followed by a week of rest where animals were left undisturbed. Then a cocaine challenge was administered employing the AMUs described above. The challenge consisted of a treatment comprised of three progressively increasing doses of cocaine. We chose to employ the procedure of administering increasing cocaine doses because we were uncertain which dose would be sufficient to demonstrate a cross-sensitization effect. The cocaine sensitivity test began with a 30 min habituation period where animals were allowed to explore the AMUs and their locomotion was recorded. Then the rats received an i.p. injection of isotonic saline, followed 60 min later by 7.5 mg/kg of cocaine, followed 60 min later by 15 mg/kg of cocaine, and finally 60 min later by 30 mg/kg of cocaine. After each injection, locomotion was recorded for 60 min.
2.7.3 Results and Discussion
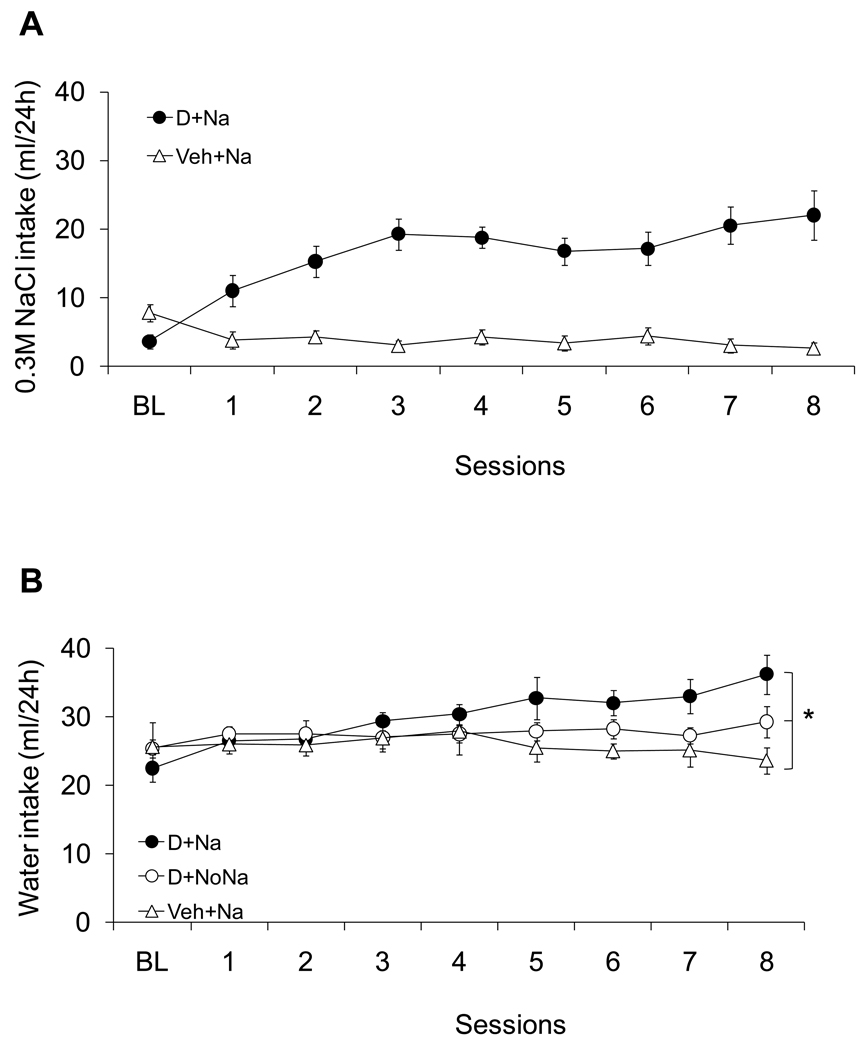
Figure 3 shows the daily sodium (panel A) and water (panel B) intakes of DOCA-treated animals with and without access to sodium. The D+Na group showed increased sodium appetite across the DOCA treatment period and when compared with the Veh+Na group. An ANOVA with Group and Session as fixed factors and Subject as a random factor found significant effects of group and session as well as a significant interaction (F(7,196)= 11.52, F(1,28)= 101.78, F(7,196)= 14.22 for the Group and Session factor effects and the interaction effect, respectively; p<0.01). Water intake (Figure 3B) was also significantly greater for the D+Na group compared to Veh+Na as indicated by ANOVA with Group and Session as fixed factors and Subject as a random factor (F(7,308)= 6.89; F(2,44)= 6.45; F(14,308)= 7.98 for the Group and Session factor effects and the interaction effect, respectively; p<0.05).
Figure 3.
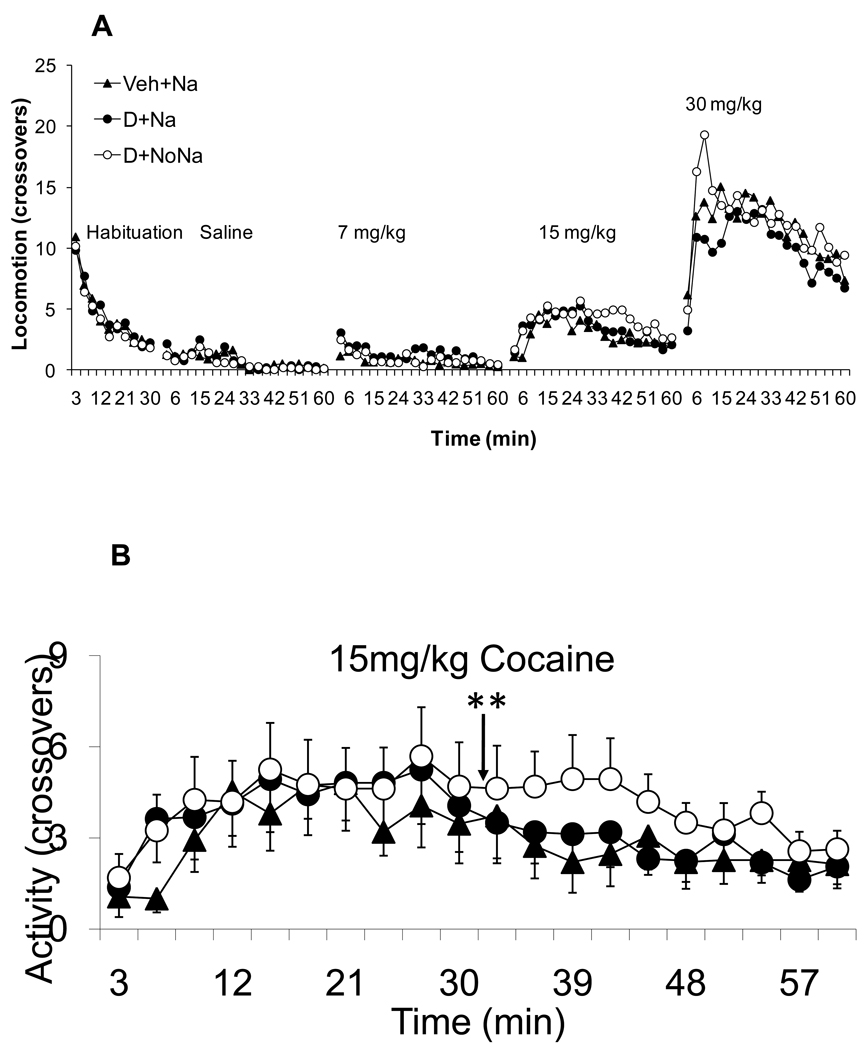
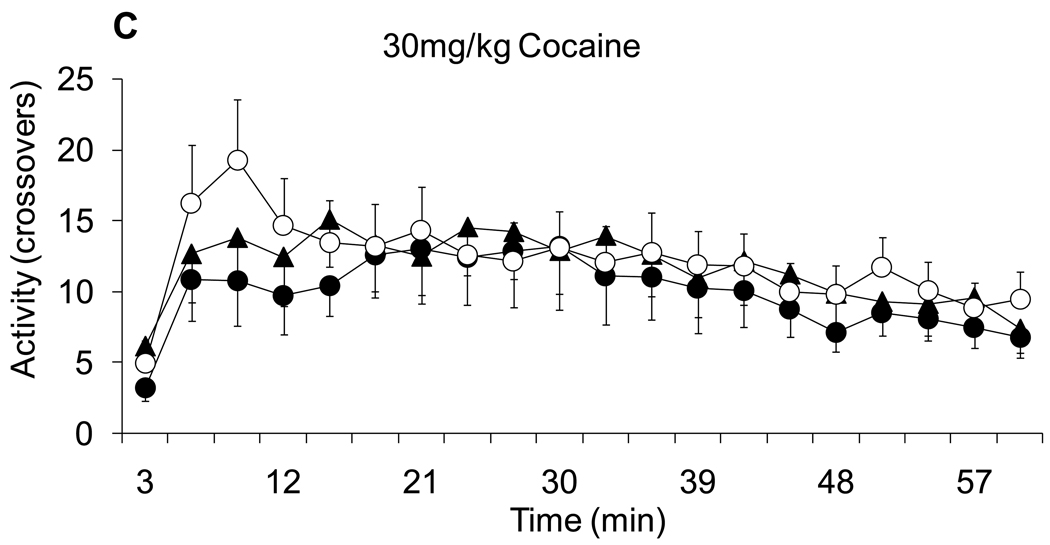
As noted above, after the DOCA treatment protocol, animals were left undisturbed for a week before cocaine testing was conducted. Figure 4 shows the locomotor responses (crossovers) during habituation and after administration of the progressively increasing doses of cocaine. Figure 4A shows the locomotor activity during habituation, after saline treatment and after each of the graded doses of cocaine. After saline and 7 mg/kg of cocaine rats did not increase activity across time nor did activity differ among groups. Higher resolution graphs of locomotion during the 15 and 30 mg/kg doses of cocaine are shown in panels 4B and 4C, respectively. ANOVA with Group and Time as fixed factor and subjects as nested random factor, found a significant effect of Group and Time (F(2,880)= 4.90, F(19,880)= 2.09 for Group and Time factor effects, respectively; p<0.008) during the 15 mg/kg cocaine dose. A planned pair-wise contrast comparison found significant differences between the D+NoNa and Veh+Na groups (F(1,800)= 9.4, p<0.002). A similar analysis was conducted for the locomotor response after 30 mg/kg (Figure 4C). ANOVA with Group and Time as a fixed factor and subject as a nested random factor found a significant effect of Group and Time factors (F(2,856)= 3.83 for Group and Time factor effects, respectively; p<0.02). However, the planned pair-wise comparison only found significant differences in the comparison of the D+Na and D+NoNa groups (F(1,856)= 5.00, p<0.02). In other words, the previous difference between the D+NoNa and Veh+Na groups was lost at the highest dose of cocaine. The fact that D+NoNa showed statistically significant differences when compared to D+Na for the 15 and 30 mg/kg doses of cocaine, suggests that the absence of salt during DOCA treatment is more effective in cross-sensitizing with the cocaine-induced locomotor response. Apparently not having access to saline in the presence of a sodium appetite represents a state that is more effective in producing sensitization than when a sodium appetite can be assuaged by consuming salt.
Figure 4.
2.8 Experiment 3: The effects of cocaine pretreatment on DOCA-induced sodium appetite with 24h sodium access
2.8.1 Rationale
Experiment 2 demonstrated that animals treated with DOCA to induce a sodium appetite showed better cross-sensitization with the cocaine-induced locomotor response if they did not have access to a saline solution to drink throughout the period of mineralocorticoid treatment. This prompted us to test the effect of cocaine-induced locomotor sensitization on DOCA treated animals that had no access to saline. To accomplish this, we added a Sal/D+NoNa group and a Coc/D+NoNa group to our original design of Experiment 1, and we extended the period of sodium access to 24h for the Coc/D+Na, Sal/D+Na and Veh+Na groups, in order to attempt to enhance possible differences between Coc/D+Na and Coc/D+NoNa groups.
2.8.2 Experimental protocol
Animals were allowed free access to distilled water and hypertonic saline solution for 3 days before the experiments were begun. Animals were then matched on the basis of their spontaneous (ad libitum) saline solution intake and divided into 6 experimental groups. A set of three groups of animals (Cocaine = Coc; n=5/group) received a cocaine sensitization treatment over 7 days. The other 3 groups of animals received drug vehicle (isotonic saline = Sal, n=5/group) treatment. The cocaine sensitization procedure consisted of repeated daily i.p. administration of either 15 mg/kg of cocaine or isotonic saline. After each daily injection, animals were placed in the AMU and their locomotion was recorded for 90 min. When the cocaine sensitization procedure was completed, animals were rested for 7 days before a DOCA-induced salt appetite phase started. Then each of two sets of three groups of animals received seven daily injections of either DOCA or vehicle (Sal/Veh+Na and Coc/Veh+Na) and had 24h access to either distilled water only (Coc/D+NoNa and Sal/D+NoNa) or distilled water and 0.3 M NaCl solution (Coc/D+Na and Sal/D+Na). The day after the seventh DOCA injection, all animals received an eighth DOCA or vehicle treatment and access to 0.3 M NaCl (plus water and food) for the next 24h, at which time saline was recorded.
2.8.3 Results and Discussion
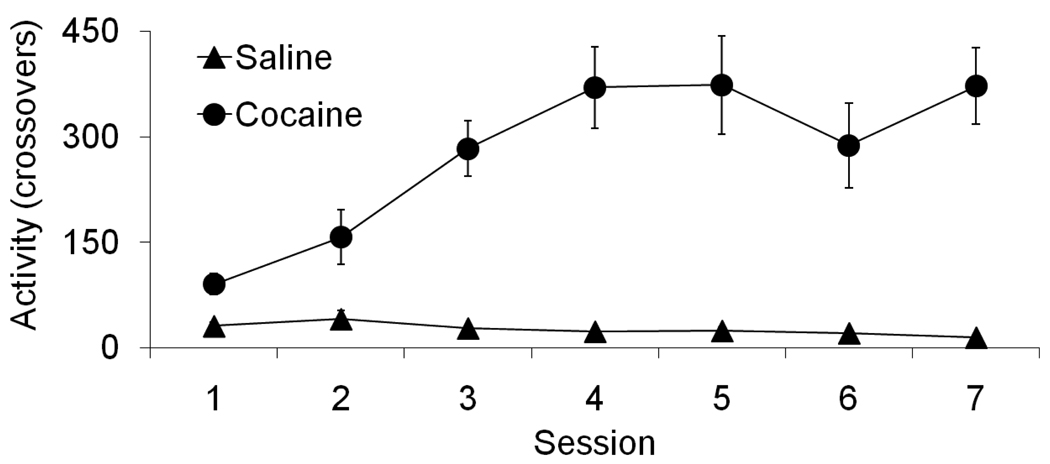
Figure 5 shows the locomotor response induced by repeated administration of either 15 mg/kg of cocaine or isotonic saline. An ANOVA with Group and Session as fixed factors and Subject as a random factor found a significant effect of Group and Session as well as a significant interaction (F(1,58)= 122.56, F(6,58)= 10.14, F(6,58)= 11.92, respectively; p<0.001). A planned pair-wise contrast comparison showed that the locomotor response of animals in the cocaine group increased from the first session to the last session (F(1,338)= 65.81, p<0.001).
Figure 5.
Figure 6A shows the 24h sodium intake values after daily administration of either 10 mg/kg of DOCA or vehicle. The sodium intake of the Sal/Veh+Na and the Coc/Veh+Na groups overlapped; therefore for the sake of clarity only the data of the Coc/Veh+Na group is shown in figure 6A. The sodium intake of the D+Na groups with a pretreatment of either saline or cocaine (Sal/D+Na and Coc/D+Na, respectively) increased across time. The ANOVA with Group and Session as fixed factors and Subject as a random factor, found a significant effect of Group and Session as well as a significant interaction (F(3,17)= 45.33, F(7,119)= 12.79, F(21,119)= 8.66, respectively; p<0.001).
Figure 6.
Because Experiment 1 showed that cocaine pretreated animals drank greater amounts of hypertonic saline with 2h daily access, we expected to observe a similar effect with 24h access. A planned pair-wise contrast comparison indicated that the intake of 0.3 M NaCl by the Sal/D+Na and Coc/D+Na groups were greater than their controls, Sal/Veh+Na (F(1,17)= 31.45, p<0.001) and Coc/Veh+Na (F(1,17)= 95.99, p<0.001), respectively. In addition, the Coc/D+Na group showed greater sodium intake across sessions compared to the Sal/D+Na group (F(1,17)= 13.22, p<0.01). In other words, DOCA treatment increased salt appetite in all groups. This increase, however, was more pronounced in the Coc/D+Na group. This replicates the basic findings from Experiment1 demonstrating that the cocaine pretreatment that increased DOCA-induced sodium appetite, when measured in a 2h access test, was also effective when the duration of the intake test was extended to 24h.
Figure 6B shows the 24h 0.3 M NaCl consumed by all six experimental groups during the last intake test day. A full-factorial ANOVA with Cocaine (Cocaine, Saline) and DOCA (D+Na/D+NoNa/Veh+Na) as factors, found a significant main effect of DOCA (F(2,25)= 53.22, p<0.0001), indicating that DOCA treatment affected sodium intake. The main effect of Cocaine was not significant. Although Cocaine×DOCA interaction failed to reach significance (F(2,30)= 2.78, p = 0.08), we had hypothesized that absence of access to saline during DOCA treatment would produce higher cross-sensitization. Planned pair-wise contrast comparisons indicated that the intakes of 0.3 M NaCl by Sal/D+NoNa and Coc/D+NoNa groups were statistically greater than Sal/D+Na group (F(1,25)= 8.89, p<0.006 and F(1,25)= 8.69; p<0.007, respectively). In other words, the absence of access to salt during DOCA treatment (Sal/D+NoNa group) resulted in enhanced sodium intake. However, 0.3 M NaCl intake was not increased in the Coc/D+NoNa compared to Coc/D+Na group. Possibly the sensitization produced by cocaine pretreatment attenuated any potential enhancement produced by having no access to sodium, thereby resulting in similar high sodium intakes in the 24h test. The failure to see an enhancement in 0.3 M NaCl intake by Coc/D+NoNa compared to Coc/D+Na group, might reflect a ceiling effect due to inhibitory mechanisms which limit sodium ingestion. In addition, the results of Experiment 3 replicate those of Experiment 1 by finding significantly higher sodium intake in the Coc/D+Na group as compared to the Sal/D+Na group (F(1,25)= 7.76, p<0.01).
3. General Discussion
The major result of the present experiments demonstrates a reciprocal cross-sensitization between DOCA-induced salt appetite when access to sodium is denied with the locomotor response induced by cocaine. Other important new findings of our studies are that: 1) cross-sensitization between sodium appetite and cocaine-induced behavioral responses can be more readily produced if access to hypertonic sodium chloride solution is prevented during DOCA treatment, and 2) repeated DOCA treatment sensitizes sodium appetite regardless of whether animals had constant access to hypertonic saline for consumption.
The primary goals of the present studies were to: 1) determine if the sodium appetite component, associated with reciprocal cross-sensitization of behaviors between salt appetite and a psychomotor stimulant, was due to repeated epochs of sodium deficiency, or a result of recurrent periods of a hunger for salt; and 2) whether the cross-sensitization phenomenon generalized between amphetamine and cocaine, another psychomotor stimulant. The fact that cocaine was effective in producing cross-sensitization supported the latter aim. To achieve the first goal we employed a mineralocorticoid agonist, DOCA, which induces a robust salt appetite in spite of actually increasing body sodium. The results indicate that the sodium appetite cross-sensitization phenomena investigated here are not dependent upon the status of body sodium homeostasis but rather the manifestation of a drive to consume salt. In other words, it may be the actual presence of a salt appetite which accompanies DOCA treatment that underlies the sensitization phenomena. In other work from our laboratory (Grippo et al., 2006b; Morris et al., 2006; Morris et al., 2010) we found that rats with a sodium appetite induced by either sodium depletion or DOCA treatment, evidenced anhedonia if they were prevented from drinking a hypertonic saline solution. Anhedonia is a psychological state characterized by the inability to experience pleasure to normally positive or pleasurable stimuli or events. Anhedonia is a core component of psychological depression and can be induced experimentally by exposure to a series of mild stressors (Grippo et al., 2006a; Willner et al., 1992) and is present in animals with experimental heart failure (Grippo et al., 2003). The anhedonia accompanying an unresolved sodium appetite is resolved by giving salt hungry rats access to a 1.8% sodium chloride solution (Grippo et al., 2006b; Morris et al., 2006; Morris et al., 2010).
It is well established that animals undergoing repetitive psychomotor stimulant and opioid treatments show progressive increases in locomotor responses over the course of administration (Anagnostaras and Robinson 1996; Badiani et al., 2000; Cador et al., 1999; Crombag et al., 1999). Such drug-related phenomena meet the definition of behavioral sensitization (Stewart and Badiani 1993). Similarly, appetitive behaviors directed towards acquiring natural rewards, such as food, water, salt or a mate, show behavioral sensitization with repeated experiences (Avena et al., 2005; Bradley and Meisel 2001; Falk 1965; Kohlert and Meisel 1999; Sakai et al., 1989).
It is widely accepted that reinforcement-motivated behaviors associated with natural rewards, such as palatable substances or access to a sexual partner, are associated with an increase in the release of dopamine and opiates in several limbic structures, including the nucleus accumbens and medial prefrontal cortex (Kohlert and Meisel 1999; Lucas et al., 1999; Lucas et al., 2000; Pecina and Berridge 2000; Rada et al., 2005). Psychomotor stimulants, opiates and alcohol elicit their reinforcing effects by increasing dopamine release in the same limbic areas as natural reinforcers (Badiani et al., 1998; Ding et al., 2009; Miller et al., 2005; Zocchi et al., 1998). Consequently, it has been hypothesized that behaviors produced by drugs of abuse and natural rewards share common neural substrates (Fiorino and Phillips 1999; Lucas et al., 2003; Nocjar and Panksepp 2002; Pecina et al., 2006; Robinson and Kolb 1997; Roitman et al., 2002).
Previous work has shown that there is reciprocal cross-sensitization between sodium depletion-induced salt appetite and two commonly abused substances, the psychomotor stimulant amphetamine (Clark and Bernstein 2004; Clark and Bernstein 2006b) and the opioid morphine (Na et al., 2009). In a series of studies Bernstein and colleagues established the relationship between the sensitization of salt appetite motivated by repeated sodium depletions and psychomotor sensitization induced by amphetamine (Clark and Bernstein 2004; Clark and Bernstein 2006b; Roitman et al., 2002). These studies explored whether repeated sodium depletion increases salt appetite by sensitizing motivational mechanisms underlying sodium seeking and intake behaviors just as psychomotor stimulants sensitize the motivational mechanisms underlying drug consumption (Bernstein 2003; Clark and Bernstein 2004; Clark and Bernstein 2006a; Clark and Bernstein 2006b; Roitman et al., 2002). In addition, members of the Bernstein laboratory demonstrated that sodium depletion-induced salt appetite reciprocally cross-sensitize with an amphetamine-induced psychomotor response (Clark and Bernstein 2004; Roitman et al., 2002). Bernstein and colleagues interpreted their demonstrations of reciprocal cross-sensitization between amphetamine-induced locomotion and sodium depletion-induced salt appetite in terms of common underlying motivational mechanisms. An alternative interpretation might view the work of Bernstein and colleagues and the present studies from the perspective that psychostimulant drug treatment and experimentally induced salt appetite both share common stress-related mechanisms.
Sodium is essential for survival, and consequently sodium metabolism is normally maintained by multiple physiological and behavioral controls that permit strict homeostatic regulation of the amount and distribution of this cation in the body. A complex pattern of reflexes and behaviors are activated in states of sodium deficiency. Critical immediate responses to sodium loss are activation of the sympathetic nervous system and mobilization of the renin-angiotensin-aldosterone system. These rapidly acting reflex stress-related responses mainly target the kidneys to stem the rate of sodium and water loss (Falk 1965; Fitzsimons 1998) and the cardiovascular system to maintain cardiac output and blood flow to critical vascular beds (Sciarretta et al., 2009; Triposkiadis et al., 2009). The reflexive autonomic and endocrine responses can be viewed as serving temporizing roles against circulatory collapse in order to provide time for the mobilization of vital appetitive and consummatory behaviors to seek out and consume sodium (Fitzsimons 1980; Johnson and Thunhorst 1997; Johnson and Thunhorst 2007; Thunhorst et al., 2007). Ultimately it is only salt appetite driven sodium consumption that will allow sodium homeostasis to be fully restored. The loss of body sodium is a strong stressor with both physiological and psychological components. The physiological neural and endocrine responses provoked by sodium deficiency are among those most commonly identified as neuro-hormonal mediators of the stress response (Brooks et al., 2005; Denton 1982; Denton et al., 1999; O'Donaughy et al., 2006; Toney et al., 2003; Vallee et al., 1995). Such observations suggest that there might be an important role of stress mechanisms in the sensitization phenomena associated with sodium appetite.
The stress response is considered a generalized activation, promoted by any stimulus that is novel, threatening, creates conflict, or causes homeostatic imbalance (Ursin 1978). States induced by stressors can be physiologically (physiological stressors) and/or emotionally (psychological stressors) challenging, and both types of stressors can activate adaptive processes aimed to either reestablish homeostasis (McEwen 2007) or prevent or buffer against a perceived, impending disruption in homeostasis (Herman et al., 2005; Ulrich-Lai and Herman 2009). Common physiological stressors are deprivation of food, water, and sleep, as well as extreme hyper- or hypothermia. Sodium depletion is clearly within the realm of such physiological stressors. Psychological stressors such as restraint, isolation, noxious light or sound which are not likely to cause immediate physical injury or cause frank pain are also effective in eliciting stress responses.
Because of their artificial nature, it may not be clear as to whether drugs act as physiological or psychological stressors, but psychomotor stimulants have also been shown to serve as effective antecedents for inducing stress responses. In light of the many studies on various cross-sensitization phenomena, it has been proposed that drugs such as amphetamine might be best considered as stressors (Antelman et al., 1980; Antelman and Chiodo 1983). In addition to their well-recognized actions as sympathomimetics (Knuepfer 2003; Lange and Hillis 2001; Maraj et al., 2010; Tella et al., 1993; Triposkiadis et al., 2009), psychomotor stimulants have been shown to increase blood levels of corticosterone, adrenocorticotropin releasing hormone, β-endorphin, and corticotropin releasing factor [CRF]. Preventing CRF from activating brain stress circuitry by central administration of a CRF1 receptor antagonist prevents sensitization of the locomotor response induced by repeated administration of cocaine (Erb and Brown 2006). Commonly employed psychological stressors, such as restraint, forced swimming and social defeat, have been shown to cross-sensitize with psychomotor stimulant-induced behavioral responses (Araujo et al., 2003; Nikulina et al., 2004).
In the present experiment, a DOCA-induced salt appetite showed reciprocal cross-sensitization especially when access to NaCl solution was denied. The present results indicate that the presence of a sustained, unresolved sodium appetite, which can be viewed as a severe psychological stressor, is sufficient to elicit the sensitization of salt appetite and to produce cross-sensitization with psychomotor stimulants. The finding that a sustained salt appetite is more stressful than one that can be periodically quenched by having constant access to saline is consistent with our studies mentioned above which indicate that preventing access to saline in the presence of a salt appetite will produce anhedonia (Grippo et al., 2006b; Morris et al., 2006; Morris et al., 2010).
Extensive research has focused on the identification of brain structures and neurohumoral systems associated with both sensitization and stress. Among the many limbic structures and brain neuro-transmitters/modulators implicated in sensitization or stress, it is notable that the nucleus accumbens and dopamine are likely to have a role in both. The nucleus accumbens is a structure which receives dopaminergic projections from the ventral tegmental area, and which has been extensively associated with motivational and reward processes underlying both natural (e.g., food or water) and pharmacological (e.g., cocaine or morphine) reinforcers (Ikemoto and Panksepp 1999; Nicola et al., 2000; Schultz 1999; Schultz 2002). Furthermore, the nucleus accumbens has been shown to be morphologically and functionally modified by stressors (Araujo et al., 2003; Barrot et al., 2000; Brake et al., 2000; Cabib and Puglisi-Allegra 1994; Cabib and Puglisi-Allegra 1996; Doherty and Gratton 2007; Doherty and Gratton 1996; Kalivas and Duffy 1995). Sensitization induced by both sodium depletion and psychomotor stimulants are associated with similar changes in dendritic arborization in nucleus accumbens (Robinson and Kolb 1997; Roitman et al., 2002), suggesting that both treatments can induce similar changes in neuronal plasticity. These and other alterations in the nucleus accumbens, including changes in indices of dopamine metabolism, have been associated with sodium appetite induced by either DOCA treatment or sodium deficiency (Lucas et al., 2000; Lucas et al., 2003; Morris et al., 2008; Na et al., 2007; Roitman et al., 1999; Roitman et al., 2002).
In addition to the nucleus accumbens, other limbic structures involved in the regulation of stress responses include the amygdala and the medial prefrontal cortex (mPFC). The amygdala is associated with modulation of both autonomic (the central nucleus of the amygdala) and neuroendocrine (the medial and the basolateral amygdala nuclei) stress responses (Bhatnagar et al., 2004; Davis 1992; Dayas et al., 1999). Components of the mPFC exert differential control over neuroendocrine responses to stressors. Lesions to the dorsal mPFC enhance Fos expression in the stress-related neurosecretory cells of the hypothalamic paraventricular nucleus (PVN) in response to restraint stress (Radley et al., 2006). In contrast, destruction of neurons of the ventral mPFC decreases Fos protein in PVN parvocellular neurosecretory neurons, but increases Fos in the region of this nucleus containing neurons with descending projections to a hindbrain region affecting sympathetic tone (Radley et al., 2008). In the prelimbic area of the mPFC norepinephrine acts to inhibit activity in neurons involved in the neuroendocrine stress response to restraint (Radley et al., 2006; Radley et al., 2008). In rats given repeated sodium depletions, both the mPFC and the basolateral nucleus, but not the central nucleus of the amygdala show increased Fos immunoreactivity (Na et al., 2007). With repeated DOCA administration, rats provided access to hypertonic saline show increased Fos immunoreactivity in the medial nucleus of the amygdala (Pietranera et al., 2001).
In summary, the results of the present experiments indicate that both repeated psychomotor stimulant treatment and reoccurring DOCA administration sufficient to induce a salt appetite may sensitize brain circuitry to stress responses. From this point of view, pre-exposure to one stressor (e.g., psychomotor stimulant) facilitates the response to a different stressor (e.g., DOCA-induced sodium appetite) resulting in cross-sensitization. In turn the psychological stressor of an unresolved sodium appetite and the stress response generated by the administration of a psychomotor stimulant may activate the same stress-related neuronal and/or neurohumoral networks within the limbic system (Lucas et al., 2000; Na et al., 2007; Pietranera et al., 2001; Uslaner et al., 2001). The results from our studies also indicate that methods for studying psychomotor stimulant-induced locomotion and DOCA-induced sodium appetite provide promising tools for studying basic mechanisms of behavioral sensitization. Such approaches are likely to provide unique perspectives on how psychological and pharmacological stressors influence behavioral sensitization and how potentially addictive drugs and stress mechanisms may interact with processes underlying motivation and conventional rewards.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute HL 14388 and HL 098207, the National Institute of Diabetes and Digestive and Kidney Diseases DK 66086 and the National Institute of Mental Health MH 80241 to AKJ, and the American Heart Association (0625661Z) to MJA. The authors thank Dr. Olga Lazareva (Drake University) for assistance during the statistical analysis, and Keith Miller and Lloyd Floyd (The University of Iowa) for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Chiodo LA. Amphetamine as a stressor. In: Creese I, editor. Stimulants: Neurochemical, Behavioral, and Clinical Perspectives. New York: Raven Press; 1983. pp. 269–299. [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Araujo AP, DeLucia R, Scavone C, Planeta CS. Repeated predictable or unpredictable stress: effects on cocaine-induced locomotion and cyclic AMP-dependent protein kinase activity. Behav Brain Res. 2003;139:75–81. doi: 10.1016/s0166-4328(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Robinson TE. Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacology (Berl) 2000;151:273–282. doi: 10.1007/s002130000447. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le MM, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bernstein IL. Interaction between natural motivational systems and those which respond to drugs of abuse. Appetite. 2003;41:333–334. doi: 10.1016/j.appet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann NY Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Flores G, Francis D, Meaney MJ, Srivastava LK, Gratton A. Enhanced nucleus accumbens dopamine and plasma corticosterone stress responses in adult rats with neonatal excitotoxic lesions to the medial prefrontal cortex. Neuroscience. 2000;96:687–695. doi: 10.1016/s0306-4522(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Brooks VL, Qi Y, O'Donaughy TL. Increased osmolality of conscious water-deprived rats supports arterial pressure and sympathetic activity via a brain action. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1248–R1255. doi: 10.1152/ajpregu.00638.2004. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Opposite responses of mesolimbic dopamine system to controllable and uncontrollable aversive experiences. J Neurosci. 1994;14:3333–3340. doi: 10.1523/JNEUROSCI.14-05-03333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Bernstein IL. Reciprocal cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav. 2004;78:691–698. doi: 10.1016/j.pbb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Bernstein IL. A role for D2 but not D1 dopamine receptors in the cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav. 2006a;83:277–284. doi: 10.1016/j.pbb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Bernstein IL. Sensitization of salt appetite is associated with increased "wanting" but not "liking" of a salt reward in the sodium-deplete rat. Behav Neurosci. 2006b;120:206–210. doi: 10.1037/0735-7044.120.1.206. [DOI] [PubMed] [Google Scholar]
- Cobb GW. Introduction to design and analysis of experiments. New York: Springer-Verlag; 1998. [Google Scholar]
- Crombag HS, Mueller H, Browman KE, Badiani A, Robinson TE. A comparison of two behavioral measures of psychomotor activation following intravenous amphetamine or cocaine: dose- and sensitization-dependent changes. Behav Pharmacol. 1999;10:205–213. doi: 10.1097/00008877-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Denton DA. The Hunger for Salt. An Anthropological, Physiological, and Medical Analysis. New York: Springer-Verlag; 1982. [Google Scholar]
- Denton DA, Blair-West JR, McBurnie MI, Miller JA, Weisinger RS, Williams RM. Effect of adrenocorticotrophic hormone on sodium appetite in mice. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1033–R1040. doi: 10.1152/ajpregu.1999.277.4.R1033. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Gratton A. Differential involvement of ventral tegmental GABA(A) and GABA(B) receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Res. 2007;1150:62–68. doi: 10.1016/j.brainres.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the mesoaccumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–364. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Falk JL. Water intake and NaCl appetite in sodium depletion. Psychol Rep. 1965;16:315–325. doi: 10.2466/pr0.1965.16.1.315. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin and other peptides in the control of water and sodium intake. Proc R Soc Lond B Biol Sci. 1980;210:165–182. doi: 10.1098/rspb.1980.0126. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006a;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R666–R673. doi: 10.1152/ajpregu.00430.2002. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Beltz TG, Johnson AK. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behav Neurosci. 2006b;120:1133–1143. doi: 10.1037/0735-7044.120.5.1133. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology, neurochemistry and molecular biology of thirst and salt appetite. In: Lajtha A, Blaustein JD, editors. Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. 3rd Edition. New York: Springer; 2007. pp. 641–687. [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479:153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol Ther. 2003;97:181–222. doi: 10.1016/s0163-7258(02)00329-7. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther. 1999;288:699–709. [PubMed] [Google Scholar]
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–358. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Grillo CA, McEwen BS. Involvement of mesolimbic structures in short-term sodium depletion: in situ hybridization and ligand-binding analyses. Neuroendocrinology. 2003;77:406–415. doi: 10.1159/000071312. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Pompei P, McEwen BS. Correlates of deoxycorticosterone-induced salt appetite behavior and basal ganglia neurochemistry. Ann N Y Acad Sci. 1999;897:423–428. doi: 10.1111/j.1749-6632.1999.tb07912.x. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Pompei P, McEwen BS. Salt appetite in salt-replete rats: involvement of mesolimbic structures in deoxycorticosterone-induced salt craving behavior. Neuroendocrinology. 2000;71:386–395. doi: 10.1159/000054559. [DOI] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, Lynn MD. Cocaine and the heart. Clin Cardiol. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Miller AD, Forster GL, Yeomans JS, Blaha CD. Midbrain muscarinic receptors modulate morphine-induced accumbal and striatal dopamine efflux in the rat. Neuroscience. 2005;136:531–538. doi: 10.1016/j.neuroscience.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Grippo AJ, Johnson AK. The effects of deoxycorticosterone-induced sodium appetite on hedonic behaviors in the rat. Behav Neurosci. 2006;120:571–579. doi: 10.1037/0735-7044.120.3.571. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Johnson AK. Salt craving: the psychobiology of pathogenic sodium intake. Physiol Behav. 2008;94:709–721. doi: 10.1016/j.physbeh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Johnson AK. Mineralocorticoid receptor antagonism prevents hedonic deficits induced by a chronic sodium appetite. Behav Neurosci. 2010;124:211–224. doi: 10.1037/a0018910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Johnson AK. Behavioral cross-sensitization between morphine-induced locomotion and sodium depletion-induced salt appetite. Pharmacol Biochem Behav. 2009;93:368–374. doi: 10.1016/j.pbb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Johnson RF, Beltz TG, Johnson AK. The neural substrates of enhanced salt appetite after repeated sodium depletions. Brain Res. 2007;1171:104–110. doi: 10.1016/j.brainres.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- O'Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48:658–663. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietranera L, Saravia FE, McEwen BS, Lucas LL, Johnson AK, De Nicola AF. Changes in Fos expression in various brain regions during deoxycorticosterone acetate treatment: relation to salt appetite, vasopressin mRNA and the mineralocorticoid receptor. Neuroendocrinology. 2001;74:396–406. doi: 10.1159/000054706. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-11-j0001.2002. RC225 (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Patterson TA, Sakai RR, Bernstein IL, Figlewicz DP. Sodium depletion and aldosterone decrease dopamine transporter activity in nucleus accumbens but not striatum. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1339–R1345. doi: 10.1152/ajpregu.1999.276.5.R1339. [DOI] [PubMed] [Google Scholar]
- Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need-free sodium intake of the rat. Behav Neurosci. 1989;103:186–192. doi: 10.1037//0735-7044.103.1.186. [DOI] [PubMed] [Google Scholar]
- Schultz W. The reward signal of midbrain dopamine neurons. News Physiol Sci. 1999;14:249–255. doi: 10.1152/physiologyonline.1999.14.6.249. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, Volpe M. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond) 2009;116:467–477. doi: 10.1042/CS20080390. [DOI] [PubMed] [Google Scholar]
- Searle SR, Casella G, McCulloch CE. Variance components. New York: John Wiley and Sons; 1992. [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. Cocaine: cardiovascular effects in relation to inhibition of peripheral neuronal monoamine uptake and central stimulation of the sympathoadrenal system. J Pharmacol Exp Ther. 1993;267:153–162. [PubMed] [Google Scholar]
- Thunhorst RL, Beltz TG, Johnson AK. Glucocorticoids increase salt appetite by promoting water and sodium excretion. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1444–R1451. doi: 10.1152/ajpregu.00294.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest. 1985;76:132–136. doi: 10.1172/JCI111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin H. Activation, coping and psychosomatics. In: Ursin H, Baade E, Levine S, editors. Psychobiology of Stress: A Study of Coping Man. New York: Academic Press; 1978. pp. 201–228. [Google Scholar]
- Uslaner J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Vallee SM, Grillo CA, Gonzalez S, Cosen-Binker L, de Kloet ER, McEwen BS, De Nicola AF. Further studies in deoxycorticosterone acetate treated rats: brain content of mineralocorticoid and glucocorticoid receptors and effect of steroid antagonists on salt intake. Neuroendocrinology. 1995;61:117–124. doi: 10.1159/000126832. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wolf G. Effect of deoxycorticosterone on sodium appetite of intact and adrenalectomized rats. Am J Physiol. 1965;208:1281–1285. doi: 10.1152/ajplegacy.1965.208.6.1281. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82:521–528. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]