Description
Bisphosphonates (BPs) are potent inhibitors of osteoclast-mediated bone resorption. They are widely used in the management of osteoporosis and other diseases of high bone turnover [1]. BPs’ pronounced affinity for bone, but not other tissues, makes them the ideal candidates for treatment of bone diseases. BPs have been shown to increase bone mineral density (BMD), reduce bone turnover markers, and reduce the risk of osteoporotic fractures. They are currently the most important and effective class of drugs for metabolic bone disorders such as osteoporosis, Paget’s disease, and osteogenesis imperfecta [1–3]. Intravenous BPs are also used in the treatment of malignant hypercalcemia, osteogenesis imperfecta, and prevention of skeletal-related events in patients with multiple myeloma or breast and prostate cancers [3–7].
Chemically, BPs are synthetic analogs of pyrophosphate, an endogenous regulator of bone mineralization that contains a nonhydrolysable P-C-P backbone with two side chains (R1 and R2) [8]. The two phosphonate groups are required for both binding to bone mineral and for antiresorptive potency. Modification to one or both phosphonate groups significantly reduced the binding affinity as well as the antiresorptive potency of BPs [9]. The R1 and R2 side chains are responsible for a wide range of activities. Acting together with the two phosphonate groups, the presence of a hydroxyl group (-OH) or an amino group (-NH2) rather than an H group in the R1 chain enhances binding to calcium minerals. The presence of a nitrogen or amino group in the R2 side chain significantly increases the antiresorptive potency of BPs and also affects binding to hydroxyapatite [9–11].
BPs can be broadly classified into two major classes with distinct mechanisms of action: the nonnitrogen containing class and the nitrogen-containing class. The earlier nonnitrogen containing BPs (e.g. clodronate, tiludronate, and etidronate) act by incorporation into ATP, whereas the newer, more potent nitrogen-containing BPs (N-BPs, e.g. pamidronate, alendronate, ibandronate, riserdronate, and zoledronate) act by inhibiting farnesyl diphosphate synthase (FDPS) in the mevalonate pathway [12].
Pharmacokinetics
Pharmacokinetic studies showed that all BPs have high affinity for bone mineral and poor intestinal absorption [13]. The amount of BPs taken up by the skeleton depends on several factors including renal function, rate of bone turnover and on the affinity for bone mineral. The skeleton has a high capacity to retain BPs and the major route of elimination is through kidney [8]. Renal transporters for BPs have not been elucidated. Potential candidate transporters for BPs are the sulfate transporters and phosphate transporters that belong to SLC22A, SLC17A, and SLC13A families [14,15].
The two classes of BPs are metabolized differently. The simple, nonnitrogen-containing BPs are metabolized to cytotoxic, nonhydrolysable analogs of ATP [16,17]. Accumulation of these toxic byproducts interferes with mitochondrial function and ultimately leads to apoptosis of osteoclasts. However, the more potent nitrogen-containing BPs are not metabolized and are excreted unchanged through the kidney. BPs are typically given when fasting, as food reduces the bioavailability of oral nitrogen-containing BPs [18].
Pharmacodynamics
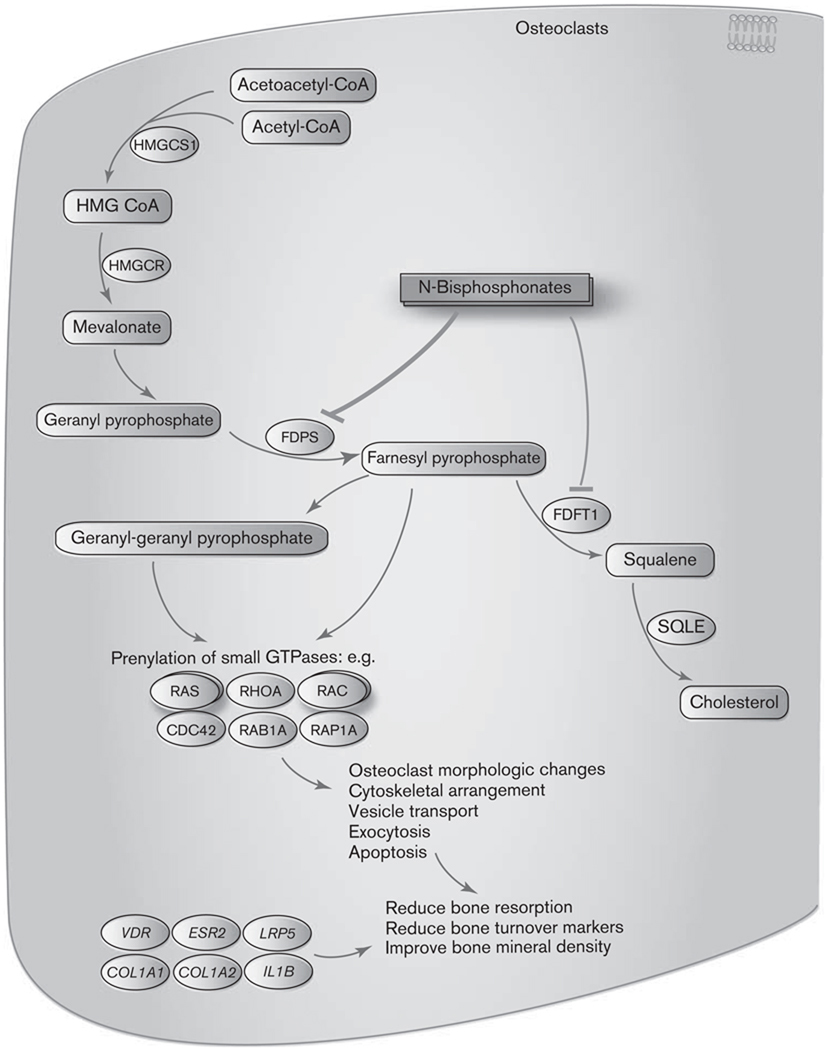
BPs preferentially bind to the surface of bone at sites of active remodeling and become internalized into osteoclasts through endocytosis [19]. Two distinct mechanisms of action have been revealed. The nonnitrogen containing BPs inhibit bone resorption by generating the cytotoxic analogs of ATP which interfere with mitochondrial function and induce apoptosis of osteoclasts [16,17]. In contrast, the more potent nitrogen-containing BPs bind to and inhibit key enzymes of the intracellular mevalonate pathway, thereby preventing the prenylation and activation of small GTPases that are essential for the bone-resorbing activity and survival of osteoclasts (Fig. 1, BP pathway, https://www.pharmgkb.org/do/serve?objId=PA154423660&objCls=Pathway) [1,20–22]. The mevalonate pathway is a biosynthetic pathway responsible for production of cholesterol, other sterols and isoprenoid lipids [23]. Some of these isoprenoid lipids (e.g. farnesyl pyrophosphate and geranyl-geranyl pyrophosphosphate) are essential for the prenylation and activation of the small GTPases such as Ras, Rho, Rac, Rab, and Cdc42 [23–25]. The small GTPases are important signaling proteins regulating osteoclast morphology, cytoskeleton arrangement, membrane ruffling, trafficking, and cell survival [26,27]. Inhibition of enzymes along the mevalonate pathway may impair the prenylation process and lead to loss of function of the small GTPases. Several enzymes along the mevalonate pathway have been studied as potential molecular targets for nitrogen-containing BPs. Earlier studies showed that incadronate and ibandronate, but not other BPs, are inhibitors for squalene synthase (FDFT1), an enzyme required for cholesterol biosynthesis in the mevalonate pathway. Inhibition of FDFT1, however, does not lead to loss of protein prenylation [28,29], suggesting that other enzymes upstream in the pathway may be inhibited by N-BPs. The main target protein of the N-BPs is currently considered to be FDPS, a key regulatory enzyme catalyzing the production of isoprenoid lipids. Multiple studies demonstrated that FDPS is inhibited by all the N-BPs and the antiresorptive potency of various N-BPs correlates with their ability to inhibit FDPS [23–25]. X-ray crystallography study also confirmed that the potent N-BPs (risedronate and zoledronate respectively) bind and inhibit the active site of FDPS through the nitrogen atoms [30]. Inhibition of FDPS by N-BP blocks the synthesis of farnesyl pyrophosphate and geranyl-geranyl pyrophosphosphate, which in turn prevents prenylation of small GTPases and disrupts normal osteoclast function. These studies clearly indicate that inhibition of FDPS is a central mechanism by which N-BPs inhibit bone resorption.
Fig. 1.
Bisphosphonates pathway: Mechanism of action of N-bisphosphonates in osterclasts.
Bisphosphonates are currently the most widely used and effective antiresorptive therapy for osteoporosis. They are well tolerated by most; however, the efficacy and safety of BPs vary among patients [31]. It is estimated that around 5–10% patients fail to respond to BP therapy [32,33]. In addition, some patients with intravenous BP treatment experienced adverse events such as atrial fibrillation [34,35], acute phase response [36–38], renal insufficiency, and osteonecrosis of the jaw (ONJ) [39,40]. Despite the large amount of genetic evidence elucidated for the susceptibility to osteoporosis, the understanding about genetic factors contributing to osteoporosis treatment response is still limited. The majority of published pharmacogenetic studies for BP response to date have focused on candidate genes related to BMD, bone turnover and osteoporotic fracture risk [8,41–44]. These include the vitamin D receptor gene (VDR), the estrogen receptor beta gene (ESR2), the type 1 collagen gene (COL1A1 and COL1A2), and the low-density lipoprotein receptor-related protein 5 gene (LRP5). Common polymorphic variation in the VDR gene (BsmI genotype, rs1544410) has been reported to contribute to individual response to etidronate [45] and alendronate [46,47] in Caucasian postmenopausal women, with the bb genotype associated with lower increase in BMD after BP treatment. It has also been observed that the BsmI (rs1544410) and TaqI (rs731236) polymorphisms of VDR and − 511 C/T polymorphism (rs16944) of IL1B were significantly associated with acquired resistance to BP treatment in Caucasian patients with Paget’s disease of the bone [48,49]. The COL1A1 Sp1 polymorphism (rs1800012) has been associated with decrease in bone mass and osteoporotic fractures. The SS genotype of this polymorphism was associated with a better response to etidronate treatment in terms of femoral neck BMD but not lumbar spine BMD, indicating site-specific BMD response influenced by COL1A1 genotype to cyclical etidronate therapy [50]. Variations in ESR2 (RsaI) and LRP5 (rs3736228 and rs4988321) were also evaluated for their influence on response to alendronate and risedronate respectively, however, no association was found [51,52]. As BPs modulate the mevalonate pathway, polymorphisms in genes encoding target enzymes of the mevalonate pathway may modulate the response to N-BP treatments. Marini et al. [53] has demonstrated that a polyphorphism in FDPS, a target of N-BPs, impacted the response to long-term N-BP treatment in Danish postmenopausal women. Patients with the CC genotype for rs2297480 showed a decreased response of bone turnover markers to N-BP therapy. Moreover, a recent genome-wide association study identified a polymorphism of CYP2C8 (rs1934951) to be associated with BPrelated ONJ [54]. Individuals homozygous for the Tallele showed an increased risk for developing ONJ. Results from all the studies described above highlight the possibility of using genetic testing to tailor BP treatments for optimal response. However, further population analysis involving larger patient cohorts, different ethnic backgrounds and examining all functional gene variants simultaneously is required to validate the existing findings and to fully elucidate the mechanisms underlying the variation in the treatment response to BPs.
Acknowledgements
PharmGKB is supported by the NIH/NIGMS Pharmaco-genetics Research Network and Database (UO1GM61374).
References
- 1.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005088.pub2. Art. No.: CD005088. [DOI] [PubMed] [Google Scholar]
- 3.Milner RJ, Farese J, Henry CJ, Selting K, Fan TM, de Lorimier LP. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597–604. doi: 10.1892/0891-6640(2004)18<597:bac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 5.Naidu A, Dechow PC, Spears R, Wright JM, Kessler HP, Opperman LA. The effects of bisphosphonates on osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:5–13. doi: 10.1016/j.tripleo.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Yoneda T, Hashimoto N, Hiraga T. Bisphosphonate actions on cancer. Calcif Tissue Int. 2003;73:315–318. doi: 10.1007/s00223-002-0025-x. [DOI] [PubMed] [Google Scholar]
- 7.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 8.Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44:551–570. doi: 10.2165/00003088-200544060-00001. [DOI] [PubMed] [Google Scholar]
- 9.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 10.Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068:367–401. doi: 10.1196/annals.1346.041. [DOI] [PubMed] [Google Scholar]
- 11.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 12.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–312. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022–1033. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 14.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch. 2004;447:629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- 15.Ullrich KJ, Rumrich G, Burke TR, Shirazi-Beechey SP, Lang H. Interaction of alkyl/arylphosphonates, phosphonocarboxylates and diphosphonates with different anion transport systems in the proximal renal tubule. J Pharmacol Exp Ther. 1997;283:1223–1229. [PubMed] [Google Scholar]
- 16.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 17.Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 18.Porras AG, Holland SD, Gertz BJ. Pharmacokinetics of alendronate. Clin Pharmacokinet. 1999;36:315–328. doi: 10.2165/00003088-199936050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Thompson K, Rogers MJ, Coxon FP, Crockett JC. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol. 2006;69:1624–1632. doi: 10.1124/mol.105.020776. [DOI] [PubMed] [Google Scholar]
- 20.Schindeler A, Little DG. Bisphosphonate action: revelations and deceptions from in vitro studies. J Pharm Sci. 2007;96:1872–1878. doi: 10.1002/jps.20904. [DOI] [PubMed] [Google Scholar]
- 21.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 22.Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2:571–577. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- 23.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 24.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 25.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–1678. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 26.Coxon FP, Rogers MJ. The role of prenylated small GTP-binding proteins in the regulation of osteoclast function. Calcif Tissue Int. 2003;72:80–84. doi: 10.1007/s00223-002-2017-2. [DOI] [PubMed] [Google Scholar]
- 27.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–1476. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 28.Amin D, Cornell SA, Gustafson SK, Needle SJ, Ullrich JW, Bilder GE, Perrone MH. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J Lipid Res. 1992;33:1657–1663. [PubMed] [Google Scholar]
- 29.Amin D, Cornell SA, Perrone MH, Bilder GE. 1-Hydroxy-3-(methylpentylamino)-propylidene-1,1-bisphosphonic acid as a potent inhibitor of squalene synthase. Arzneimittelforschung. 1996;46:759–762. [PubMed] [Google Scholar]
- 30.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006;103:7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122:S14–S21. doi: 10.1016/j.amjmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Delmas PD. The use of bisphosphonates in the treatment of osteoporosis. Curr Opin Rheumatol. 2005;17:462–466. doi: 10.1097/01.bor.0000163448.51661.87. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TV, Eisman JA. Pharmacogenomics of osteoporosis: opportunities and challenges. J Musculoskelet Neuronal Interact. 2006;6:62–72. [PubMed] [Google Scholar]
- 34.Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356:1895–1896. doi: 10.1056/NEJMc076132. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 36.Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- 37.Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–139. doi: 10.1016/8756-3282(95)00448-3. [DOI] [PubMed] [Google Scholar]
- 38.Thiebaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, Green JR, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–392. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- 39.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Farrugia MC, Summerlin DJ, Krowiak E, Huntley T, Freeman S, Borrowdale R, Tomich C. Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope. 2006;116:115–120. doi: 10.1097/01.mlg.0000187398.51857.3c. [DOI] [PubMed] [Google Scholar]
- 41.Carbonell Sala S, Masi L, Marini F, Del Monte F, Falchetti A, Franceschelli F, Brandi ML. Genetics and pharmacogenetics of osteoporosis. J Endocrinol Invest. 2005;28:2–7. [PubMed] [Google Scholar]
- 42.Massart F, Marcucci G, Brandi ML. Pharmacogenetics of bone treatments: the VDR and ERalpha gene story. Pharmacogenomics. 2008;9:733–746. doi: 10.2217/14622416.9.6.733. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen TV, Center JR, Eisman JA. Pharmacogenetics of osteoporosis and the prospect of individualized prognosis and individualized therapy. Curr Opin Endocrinol Diabetes Obes. 2008;15:481–488. doi: 10.1097/MED.0b013e32831a46be. [DOI] [PubMed] [Google Scholar]
- 44.Marini F, Brandi ML. Pharmacogenetics of osteoporosis: future perspectives. Calcif Tissue Int. 2009;84:337–347. doi: 10.1007/s00223-009-9226-x. [DOI] [PubMed] [Google Scholar]
- 45.Marc J, Prezelj J, Komel R, Kocijancic A. VDR genotype and response to etidronate therapy in late postmenopausal women. Osteoporos Int. 1999;10:303–306. doi: 10.1007/s001980050231. [DOI] [PubMed] [Google Scholar]
- 46.Palomba S, Numis FG, Mossetti G, Rendina D, Vuotto P, Russo T, et al. Effectiveness of alendronate treatment in postmenopausal women with osteoporosis: relationship with BsmI vitamin D receptor genotypes. Clin Endocrinol (Oxf) 2003;58:365–371. doi: 10.1046/j.1365-2265.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 47.Palomba S, Orio F, Jr, Russo T, Falbo A, Tolino A, Manguso F, et al. BsmI vitamin D receptor genotypes influence the efficacy of antiresorptive treatments in postmenopausal osteoporotic women. A 1-year multicenter, randomized and controlled trial. Osteoporos Int. 2005;16:943–952. doi: 10.1007/s00198-004-1800-5. [DOI] [PubMed] [Google Scholar]
- 48.Mossetti G, Gennari L, Rendina D, De Filippo G, Merlotti D, De Paola V, et al. Vitamin D receptor gene polymorphisms predict acquired resistance to clodronate treatment in patients with Paget’s disease of bone. Calcif Tissue Int. 2008;83:414–424. doi: 10.1007/s00223-008-9193-7. [DOI] [PubMed] [Google Scholar]
- 49.Corral-Gudino L, del Pino-Montes J, Garcia-Aparicio J, Corral E, Montilla CA, Gonzalez-Sarmiento R. −511 C/T IL1B gene polymorphism is associated to resistance to bisphosphonates treatment in Paget disease of bone. Bone. 2006;38:589–594. doi: 10.1016/j.bone.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Qureshi AM, Herd RJ, Blake GM, Fogelman I, Ralston SH. COLIA1 Sp1 polymorphism predicts response of femoral neck bone density to cyclical etidronate therapy. Calcif Tissue Int. 2002;70:158–163. doi: 10.1007/s00223-001-1035-9. [DOI] [PubMed] [Google Scholar]
- 51.Arko B, Prezelj J, Komel R, Kocijancic A, Marc J. No major effect of estrogen receptor beta gene RsaI polymorphism on bone mineral density and response to alendronate therapy in postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2002;81:147–152. doi: 10.1016/s0960-0760(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 52.Kruk M, Ralston SH, Albagha OM. LRP5 Polymorphisms and response to risedronate treatment in osteoporotic men. Calcif Tissue Int. 2009;84:171–179. doi: 10.1007/s00223-008-9207-5. [DOI] [PubMed] [Google Scholar]
- 53.Marini F, Falchetti A, Silvestri S, Bagger Y, Luzi E, Tanini A, et al. Modulatory effect of farnesyl pyrophosphate synthase (FDPS) rs2297480 polymorphism on the response to long-term amino-bisphosphonate treatment in postmenopausal osteoporosis. Curr Med Res Opin. 2008;24:2609–2615. doi: 10.1185/03007990802352894. [DOI] [PubMed] [Google Scholar]
- 54.Sarasquete ME, Garcia-Sanz R, Marin L, Alcoceba M, Chillon MC, Balanzategui A, et al. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: a genome-wide single nucleotide polymorphism analysis. Blood. 2008;112:2709–2712. doi: 10.1182/blood-2008-04-147884. [DOI] [PubMed] [Google Scholar]