1. Introduction
Gram-negative and Gram-positive bacteria have evolved elaborate machinery to biosynthesize and respond to diverse small-molecule signals. As bacteria grow, these signals accumulate in the extracellular environment until a particular concentration is reached, usually at a specific cell density or “quorum”, activating a regulatory cascade that controls some type of cellular process. This phenomenon is generally referred to as “quorum-sensing” and has been the subject of many excellent review articles1-3. The general paradigm is that Gram-negatives recognize small chemical compounds called N-acyl homoserine lactones that are membrane permeable and bind to a cytoplasmic receptor in order to exert a regulatory output. In contrast, the Gram-positives recognize peptides with diverse post-translational modifications using either a membrane-bound histidine kinase or cytoplasmic receptors.
Amongst the Gram-positives, the size and structure of these peptide signals vary widely depending on function and the producing bacterium, and published examples of these regulatory mechanisms have become abundant. As notable examples, Streptococcus pneumoniae regulates competence with a 17-residue linear peptide4. Bacillus subtilis regulates sporulation and competence with a series of linear peptides5, one of which is post-translationally modified6. Bacillus cereus regulates the expression of virulence factors and Enterococcus faecalis controls plasmid-mediated conjugation with various linear peptides7-8. As this quick overview demonstrates, peptides are regulating an impressive array of cellular events, and this list continues to grow as additional systems are being discovered.
One of the more intriguing classes of peptide signals are the cyclic lactones and thiolactones. The first of these cyclic peptide signals was discovered in Staphylococcus aureus and is the focus of this review article. The peptide signal controls an autoactivation circuit and hence is referred to as an autoinducing peptide or “AIP”. With the surge of studies on quorum-sensing and bacterial genome sequencing, it is now evident that the AIP scaffold and autoactivation circuitry is conserved among many Gram-positive bacteria9. Notably, all of the staphylococcal species make similar AIP structures10-11, and in recent studies, related signals have been identified in Enterococcus faecalis12-14, Lactobacillus plantarum15-16, Listeria monocytogenes17-18, Clostridium perfringens19-20 and C. botulinum21. Genome mining has revealed additional agr-like systems in other Gram-positives 9, such as the outbreak C. difficile 027 strain22 and in some species of Bacillus.
In this review, we will focus on the accessory gene regulator or “agr” quorum-sensing system in S. aureus as a paradigm model. We will describe what is known about the function of each gene product in the agr locus and the mechanism of signal production. Signal sensing and output will be reviewed, along with the contribution of other regulatory inputs to agr function. We will also describe the current status of agr in biofilms and pathogenesis, and outline the latest advances in agr-targeted therapies. Finally, the similarities and differences of the agr system in other Staphylococci will be described.
2. Overview of agr
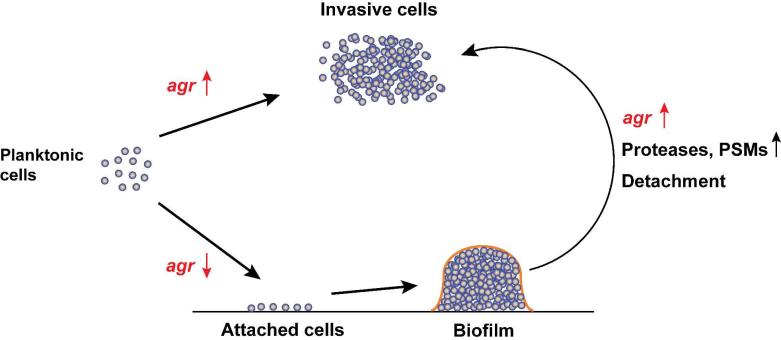
S. aureus is a remarkable bacterial pathogen that is known for causing a diverse array of acute and chronic infections23-24. This pathogen secretes an impressive arsenal of virulence determinants to combat the host, including pore-forming toxins, tissue degrading enzymes, and immune evasion factors. It has been appreciated for many years that this secreted assortment of proteins is temporally regulated by growth phase25. Early studies on S. aureus mutants provided preliminary indications that a master regulator of virulence factor expression might exist. Mutants were isolated based on the loss of a single secreted factor (e.g. protease, alpha-toxin) and were subsequently found to have altered regulation of other proteins26-29. In follow-up analysis of the mutants, reduced levels of toxins and the proteolytic enzymes was apparent, while at the same time production of surface proteins, such as protein A, was increased27,29. The chromosomal region responsible for these changes was given various names including exp, hla, and finally agr26-28.
The agr genetic locus was mapped using transformation and linkage analysis, and localized to a region between the S. aureus purB and ilv-129 genes26,30. Sequencing the flanking regions of the transposon insertions identified the agrA gene, later found to encode a response regulator31. Early studies also indicated that agr temporal control was most pronounced at the post-exponential and stationary phases of growth28. In a critical development, the spent media could activate the system independently of growth phase, indicating a secreted signal was modulating agr function32. By fractionating the spent media, a single active peak was isolated and mass spectrometry combined with peptide sequencing revealed the cyclic thiolactone AIP as the functional signal33-34. This pioneering work demonstrated that agr is a peptide quorum-sensing system and opened the doors to extensive follow-up analysis to examine the molecular and biochemical mechanism of the system, variation across clinical isolates, function in pathogenesis and biofilms, and parallel peptide systems in other Gram-positives. In this section, we will overview the basic molecular features of agr and virulon controlled by this system, as well as structure function studies on the AIP signal.
2.1 Molecular arrangement of the agr locus
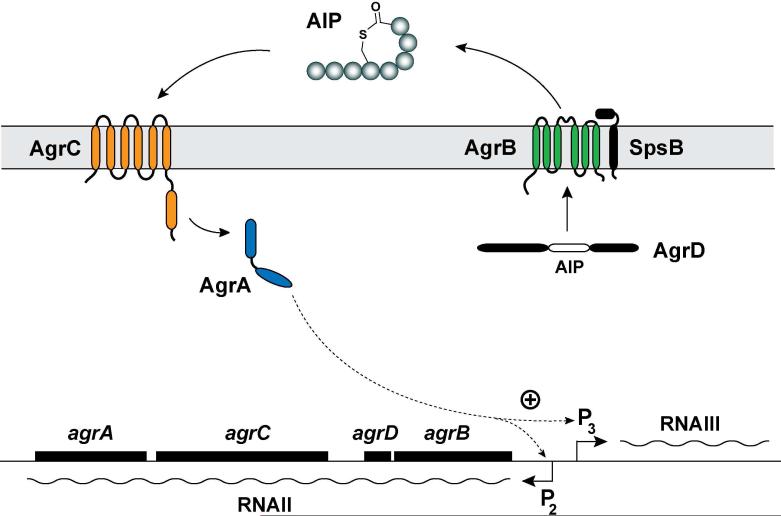
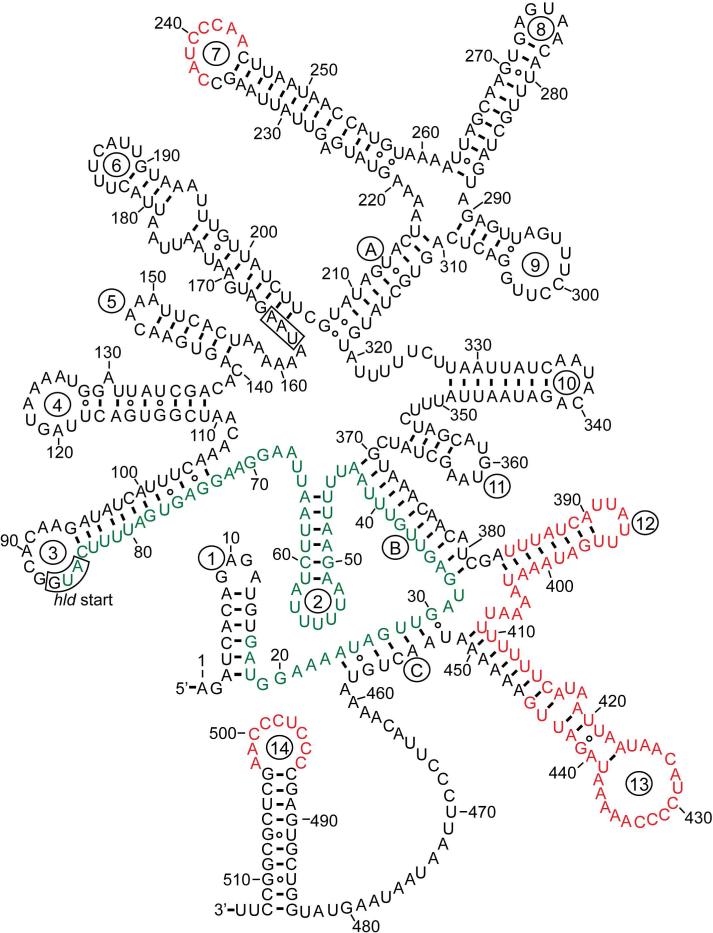
The agr locus is located on the S. aureus chromosome and is considered to be part of the core genome and not a pathogenicity island. The locus is known to contain two divergent transcripts named RNAII and RNAIII35-36. The RNAII transcript is an operon of four genes, agrBDCA, that encode factors required to synthesize AIP and activate the regulatory cascade35 (Fig. 1). Briefly, AgrD is the precursor peptide of AIP, and AgrB is an integral membrane endopeptidase essential to biosynthesize AIP. AgrC and AgrA form a two-component pair where AgrC is the membrane histidine kinase and AgrA is a response regulator37. Upon binding of AIP, AgrC phosphorylates AgrA, which in turn activates the P2 and P3 promoters to autoactivate the agr system and upregulate RNAIII transcription. RNAIII is the major downstream effector of the agr system that post-transcriptionally regulates expression of virulence factors and the Rot transcriptional regulator38. AgrA can additionally activate transcription from two promoters for expression of phenol soluble modulins39 (PSMs). In early agr studies, a third transcript called RNAI was identified that is controlled by the P1 promoter31. The P1 promoter is weak and RNAI transcript only encodes agrA and is not considered a significant player in agr function.
Figure 1.
Schematic of the agr system in Staphylococci. The locus is composed of two divergent transcripts called RNAII and RNAIII, driven by the P2 and P3 promoters, respectively. The RNAII transcript is an operon of four genes, agrBDCA, which encodes the core machinery of the system. AgrD is the peptide precursor and is processed and exported through AgrB and SpsB action at the cytoplasmic membrane. SpsB is the house-keeping type I signal peptidase. At the threshold concentration, autoinducing peptide (AIP) binds to the AgrC receptor, a membrane-bound histidine kinase. AIP binding activates the AgrC kinase, resulting in phosphorylation of the AgrA response regulator and activation of the P2 and P3 promoters. AgrA also activates the PSMα and PSMβ promoters (not shown). This figure is an adaption of the version reported by Thoendel et al.66. Reprinted with permission from reference 66. Copyright 2009 The American Society for Biochemistry and Molecular Biology, Inc.
2.2 agr regulon
The agr system regulates a diverse range of genes in S. aureus (see Table 1). Regulation can result in either increased or decreased expression and can occur at the level of transcription, mRNA stability, and translation initiation. This regulation can occur through direct AgrA binding, RNAIII positive action through binding to target mRNA, or indirectly through RNAIII-mediated inhibition of translation of the repressor of toxins (Rot). While the upregulation of secreted virulence factors and downregulation of surface proteins are the most well recognized effects of agr activation, many other genes involved in various metabolic pathways, transport, and at least one regulatory sRNA are also affected by this system.
Table 1.
The agr regulon
| Genes Upregulated by agr | ||||
|---|---|---|---|---|
| Gene | Product | Function | Notes | Reference |
| Secreted Enzymes | ||||
| aur | Aureolysin | Metalloprotease | C | 287-288 |
| splA-F | Spl proteases | Serine proteases | C | 289 |
| sspA | V8 protease | Serine protease | C | 287-288 |
| sspB | Ssp proteases | Cysteine protease | C | 287 |
| scpA | Staphopain | Cysteine protease | C | 287 |
| sak | Staphylokinase | Plasminogen activator | C | 28 |
| lip | Lipase | Fatty acid breakdown | C | |
| geh | Glycerol ester hydrolase | Fatty acid breakdown | C | |
| ureA-G | Urease | Urea neutralization | C | |
| plc | PI-Phospholipase C | Phosphatidyl-inositol hydrolysis | C | 290 |
| fme | Fatty acid modifying enzyme | Bactericidal fatty acid neutralization | C | 291 |
| Toxins | ||||
| hla | Alpha-toxin | Cytolysin, pore-forming | A | 28,42, 128 |
| hlb | Beta-hemolysin | Cytolysin, sphingomyelinase | C | 28 |
| hlgBC | Gamma-hemolysin | Cytolysin, two-component pore-forming | C | 292 |
| hld | Delta-toxin | Cytolysin, pore-forming | B | 28,36 |
| lukD/E | Leukocidin | Cytolysin, two-component pore-forming | C | 292 |
| lukS/F | Panton-Valentine leukocidin | Cytolysin, two-component pore-forming | C | 292 |
| lukG/H | Leukocidin | Cytolysin, two-component pore-forming | C | |
| seb | Enterotoxin B | Superantigen | C | 293,133 |
| sec | Enterotoxin C | Superantigen | C | 294 |
| sed | Enterotoxin D | Superantigen | C | 295 |
| tst | Toxic shock syndrome toxin-1 | Superantigen | C | 28 |
| etaAB | Exfoliative toxins | Desmoglein cleavage (Scalded-skin syndrome) | C | 296,297 |
| Immunomodulatory Peptides | ||||
| PSMα1-4 | Alpha PSMs | Cytolysin, PMN chemotaxis, inflammatory | B | 39 |
| PSMβ1-2 | Beta PSMs | Inflammatory | B | 39 |
| Regulators | ||||
| agrBDCA | agr regulator | Quorum sensing | B | 35 |
| arcR | Transcriptional regulator | Arginine catabolism regulation | C | |
| Regulatory RNAs | ||||
| RNAIII | RNAIII | Gene regulation | B | 36 |
| rsaE | RsaE sRNA | Gene regulation | C | 46 |
| Surface Factors | ||||
| cap5 | Polysaccharide capsule type 5 | Antiphagocytic | C | 298 |
| cap8 | Polysaccharide capsule type 8 | Antiphagocytic | C | 299 |
| Genes Downregulated by agr | ||||
| Secreted proteins | ||||
| ssl5,8 | Staphylococcal superantigen-like proteins 5 and 8 | Ssl5 inhibits PMN adherence | C | 300 |
| Surface Proteins | ||||
| fnbAB | Fibronectin binding proteins A/B | Fibrinogen and fibronectin adhesin | A | 301 |
| spa | Protein A | Antibody Fc-region binding | A | 28,115,128 |
| coa | Coagulase | Plasminogen to plasmin conversion | A | 117,302 |
| SA1000 | Surface protein | Fibrinogen and fibronectin adhesion | A | 114 |
| Regulators | ||||
| rot | Rot transcription factor | Gene regulation | A | 40,114 |
2.2.1 Genes regulated by RNAIII/Rot
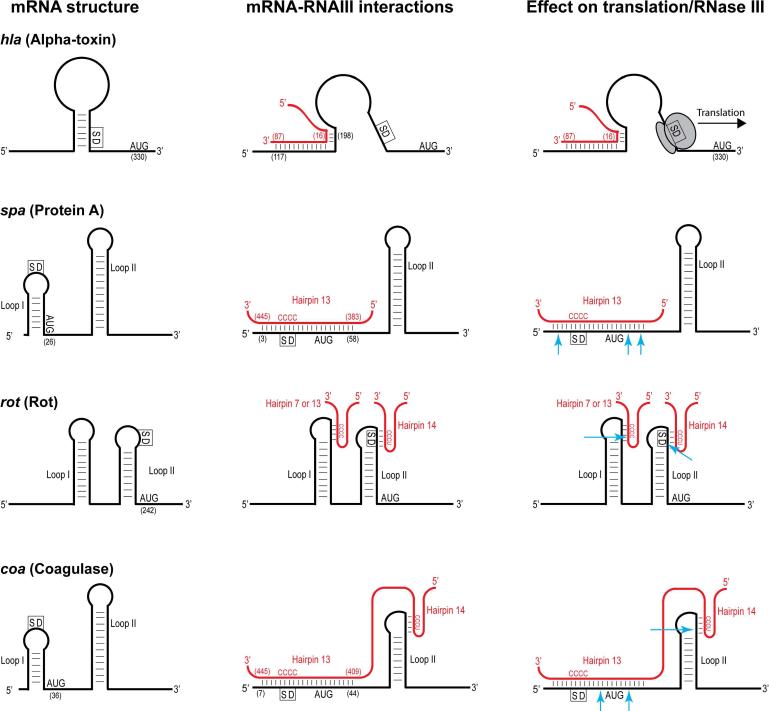
RNAIII is the agr-induced regulatory RNA that is the primary effector of the system. RNAIII is capable of regulating gene expression at the post-transcriptional level by affecting mRNA stability and promoting or inhibiting mRNA translation (see section 6 for more information). Much of RNAIII's effect on gene expression, particularly at the transcriptional level, comes through its inhibition of the Rot transcriptional regulator40.
Secreted virulence factors are a well-recognized class of targets that are RNAIII regulated. These include toxins (alpha, beta, delta, and bi-component classes), proteases, lipases, enterotoxins, superantigens (TSST-1, SEB, SEC, SED), and urease have all been shown to be upregulated in a RNAIII-dependent manner41. In some cases, such as alpha-toxin, this upregulation is a direct result of RNAIII action, and in other cases, it is indirectly through Rot function42-43 (see Table 1). Surface virulence factors responsible for functions such as adhesion (fibronectin binding proteins) and antibody binding (protein A) are well recognized to be down-regulated by agr activation41. Additionally many cellular functions, such as genes involved in nutrient transport and amino acid metabolism, are also downregulated by agr activation and Rot inhibition43.
2.2.2 Genes regulated directly by AgrA
AgrA was the first gene discovered in the agr operon and was recognized early on as transcriptional regulator. AgrA was found to activate the agr P2 and P3 promoters, driving transcription of the agrBDCA quorum-sensing genes and the RNAIII regulatory RNA respectively35,38. With the discovery of RNAIII as the major effector of agr regulation, AgrA was overlooked as a potential regulator of other genes outside of the core agr system. This oversight was likely due the challenges of separating direct AgrA regulation and AgrA effects mediated through RNAIII. Further, searching for AgrA binding sites across the S. aureus genome failed to reveal other potential targets44.
This perception changed when Queck et al. published a report on direct regulation of genes by AgrA39. By searching for genes that were differentially regulated in the agrA mutant but not in the RNAIII mutant (both compared to wild-type strain MW2), they were able to identify genes regulated by AgrA independently of RNAIII. The most notable upregulated genes are the phenol-soluble modulins (PSMs). PSMs are short amphipathic peptides that have been shown to be important for pathogenesis as they have chemotactic, proinflammatory, and leukolytic activity45. S. aureus contains seven PSMs: four short (21-23 residues) alpha-type PSMs in one operon, two longer (44 residues) beta-type PSMs in another operon, and delta-toxin encoded within RNAIII. The alpha and beta PSM transcripts are directly upregulated by AgrA independently of RNAIII action. AgrA binding sites were identified within the alpha and beta-PSM promoters, and AgrA binding was confirmed using electrophoretic mobility assays (EMSAs)39, demonstrating that AgrA activates at least four promoters: P2, P3, PPSMα, and PPSMβ.
Interestingly, AgrA was found to downregulate more genes than it upregulated (85 downregulated vs approximately 15 upregulated)39. Given the large number of downregulated genes, it is unlikely that AgrA directly binds and regulates expression of each gene. It appears more probable that an additional regulator is impacted by AgrA and potential candidates were uncovered in the AgrA microarray. Or alternatively, AgrA could affect metabolic pathways in a manner that leads to secondary changes in regulation. The downregulated genes fall under categories of carbohydrate metabolism (transport and utilization), amino acid metabolism (histidine degradation and arginine synthesis) and staphyloxanthin synthesis. To date, no studies have examined AgrA interaction with the promoters of these genes.
2.2.3 The sRNA RsaE is upregulated by agr
A recent report regarding the presence of small noncoding RNAs (sRNAs) in S. aureus identified 11 candidates (RsaA-K) with potential to regulate gene expression46. One of these sRNAs, RsaE, was positively regulated by agr. RsaE is a 100 nucleotide RNA transcript located in an intergenic region (between SA0859 and SA0860). RsaE forms two hairpin structures, one of which contains a 5’-CCCC-3’ sequence in the loop portion similar to the 5’-UCCC-3’ motifs found in the 3’ functional hairpins of RNAIII. RsaE is well-conserved in staphylococcal species and other low G+C Gram-positive bacteria, including Macrococcus, Geobacillus, and numerous Bacillus species. RsaE regulates the expression of numerous gene targets likely in a mechanism similar to RNAIII with extended base-pairing with the targeted mRNAs. In the case of three of RsaE's targets, oppB, sucD, and SA0873, the formation of the RsaE-mRNA complex prevents formation of the translation initiation complex as determined by toeprint analysis. In genome-wide transcriptome analysis, RsaE upregulated and downregulated approximately equal numbers of genes that fall under categories of (i) amino acid transport; (ii) nucleotide, lipid, and carbohydrate transport and metabolism; (iii) cell wall/membrane biogenesis; and (iv) inorganic ion transport. Exactly how RsaE is regulated by agr remains an open question.
2.3. AIP signal
The agr system responds to the extracellular concentration of the AIP signal. The original identified AIP from strain RN6390 is an eight-residue peptide (YSTCDFIM) with the last five residues constrained as a thiolactone macrocycle between the cysteine side chain and the terminal carboxylate33-34. This peptide fragment is derived from the internal region of AgrD through a series of AgrB-dependent processing events (see section 3.3). Why Gram-positive bacteria like S. aureus have evolved to biosynthesize a cyclic peptide signal instead of adopting linear structures is not known. Cyclization does improve stability through resistance to proteolytic cleavage. Evidence has been reported that the AIPs are resistant to thermolysin, chymotrypsin, proteinase K, and V8 protease47. This metabolic resistance can extend functional lifetime of the signal in the host. In an animal model, the AIP lifetime has been reported at 3 hr48, which is exceptionally long for a fast-growing bacterial pathogen. The other advantage of constraining the peptide structure through cyclization is the limitation on conformational entropy. 1H NMR structural analysis of AIP demonstrates the signal adopts a constrained conformation, and the protons are solvent shielded47. These properties facilitate the molecular recognition and binding affinity for the receptor. Most of the reported binding constants are in the 10-30 nM range (EC50) for AIP bound to the AgrC receptor49-50, making the system exquisitely sensitive to trace amounts of the signal. This remarkable sensitivity has advantages in particular environments, such as in the harsh locales of the host, where the S. aureus cells can still detect and respond to fleeting amounts of AIP in scenarios that do not conform to the classic quorum-sensing response51.
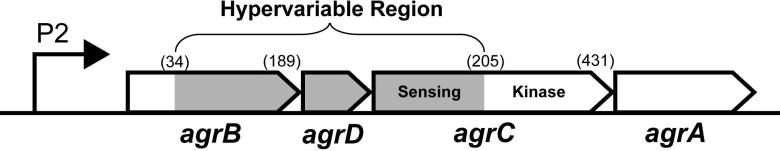
While the elucidation of the first AIP signal structure was fascinating, it was immediately apparent that diversity in the scaffold had evolved. Even across S. aureus strains, many important agr differences are known. These differences have been linked to hypervariable regions that range across the agrBDCA operon and encoded protein products52 (Fig. 2). In terms of operon order, the “hypervariable region” consists of the 3’ end of agrB, the agrD gene, and the 5’ end of agrC, where numerous basepair changes have occurred over time to establish the different agr groups. For instance, the first 34 N-terminal amino acid residues of the different AgrB's are completely conserved, while the rest of the protein shows substantial differences. Perhaps most notably, agrD gene variation results in divergent AIP structures33. Similarly, the N-terminus of AgrC shows considerable variation, where the AIP binding pocket is located, while the C-terminal kinase domain is highly conserved.
Figure 2.
Hypervariable region of the agrBDCA operon. White areas represent highly conserved sections of genes. The gray regions mark where residue changes have occurred to establish different AIPs (AgrD), group-specific AIP processing (AgrB) and group-specific receptor recognition (AgrC). Numbers in parentheses indicate amino acid residue numbers marking the border of the variable regions.
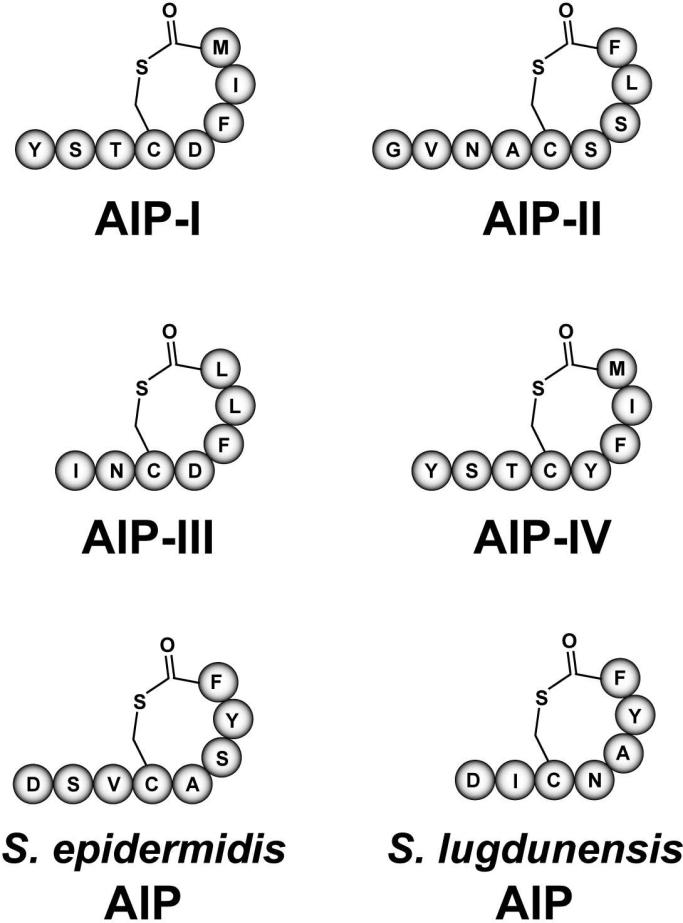
The unique features of the divergent agrBDCA operons in S. aureus isolates lead to the classification of four agr systems (referred to as agr-I, agr-II, agr-III, and agr-IV). Each agr system recognizes a different AIP structure, and to maintain grouping, these signals are termed AIP-I through AIP-IV. AIP-I, -II, and –III were identified first33, and a few years later AIP-IV was independently reported by several laboratories50,53-54. The overall structure of the four S. aureus AIPs is similar. Each contains a five-residue thiolactone ring based on linkage of the cysteine sulfhydryl to the C-terminal carboxylate, whereas the N-terminus is variable with 2-4 residue extensions from the macrocycle. The molecular weight of these AIPs has been confirmed by mass spectrometry11,33,53. Figure 3 shows the residues in each of the four AIP structures.
Figure 3.
Structures of staphylococcal autoinducing peptides (AIPs). The four S. aureus AIPs representing agr Types I-IV are shown. The most common S. epidermidis (Type I) AIP and the predicted structure of the S. lugdunensis AIP are also shown.
2.4. agr interference
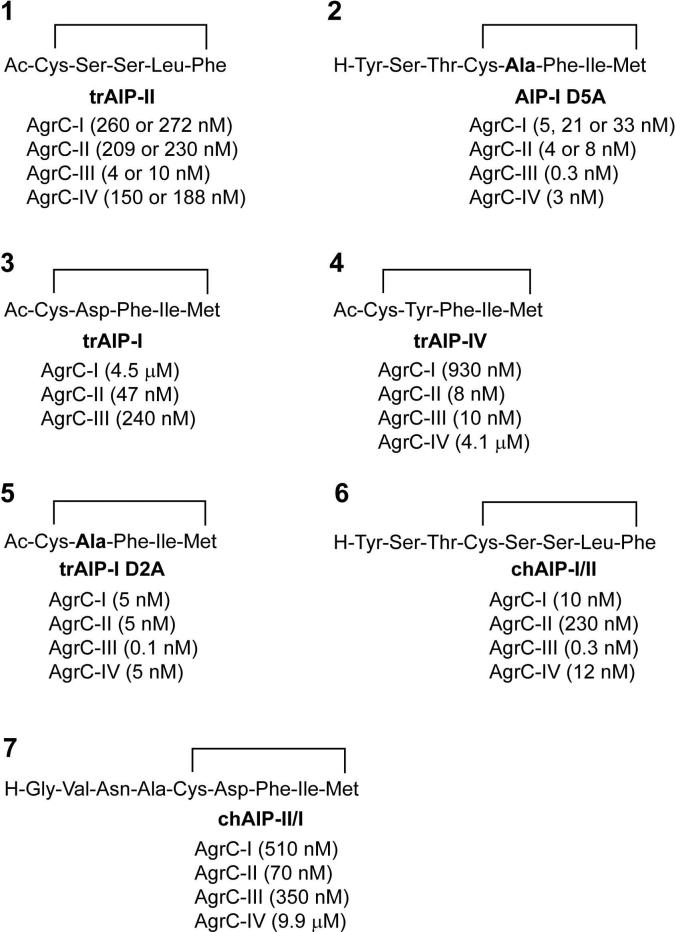
Through a fascinating mechanism of chemical communication, these different AIP signals cross-inhibit each others activity with surprising potency, a phenomenon called “agr interference”33. The interference is mediated by three sub-classes of cross-inhibitory groups: AIP-I/IV, AIP-II, and AIP-III. Since AIP-I and AIP-IV differ by only one amino acid and function interchangeably53, they are grouped together in the classification scheme, although this assignment has been controversial55. The potency of the cross-inhibition is impressive, as each of the three AIP groups bind to alternative AgrC receptors with affinity constants in the low nanomolar range49,56. The biological role of agr interference has remained elusive, but there are proposals that this phenomenon gives a competitive advantage to the producing strain57. How S. aureus has evolved this elaborate mechanism of cross-talk with agr group variation has been debated58-59 and is beyond the scope of this review. For further insight on this intriguing topic, we refer the interested reader to the discussion by Novick and Geisinger and references therein59.
2.5 AIP structure-activity relationships
Numerous studies have examined the essential structural features of the AIP signals. Methods for AIP chemical synthesis were developed and extensive structure-activity relationship (SAR) studies of the AIP scaffold were performed49-50,56,60-61. These studies uncovered critical properties of the AIP structure that are important for function. Notable findings are summarized below and the reader is referred to excellent reviews for more information (see 47,62):
The presence of the macrocyclic ring is critical for AIP function and agr activation. Synthetic linear peptides do not activate agr and hydrolysis of the thioester blocks function33.
Removal of the N-terminal residues in AIPs eliminates agr activation but not inhibition. The AIP-I, AIP-II, or AIP-IV macrocyclic ring without the tail can serve as a universal inhibitor of the system49,61. These and other SAR studies have lead to the proposal that the ring is the molecular “address” for receptor recognition and the tail is the “message” region needed for agr activation.
The presence of the AIP thioester bond versus an ester is critical for S. aureus agr activation. This finding is based on the failure of a synthetic lactone to activate, which initially lead to the hypothesis that acylation of the AgrC receptor is an important step in agr activation56. However, later studies demonstrated that AIP-binding interactions can be competed out with an antagonist, indicating reversible binding60. Further, the observation that a synthetic lactam will activate agr50,56 and the subsequent identification of lactone-containing AIPs63, both go against the acylation mechanism and provide a convincing demonstration that AIP binding to the AgrC receptor is reversible.
The methionine side-chain on AIP-I is labile. McDowell et al. initially reported that the methionine oxidizes to a sulfoxide under aerobic conditions, rendering the AIP-I non-functional in agr activation50. Neutrophils can exploit this labile residue and accelerate the deactivation of AIP-I using the oxidative burst64. However, the oxidized AIP-I is still effective at interference with the agr-II and III systems. Replacement of the labile methionine with isoleucine resulted in a functional AIP-I (M8I) in agr activation, but whether or not the molecule is more resistant to oxidative damage is unclear49. None of the other AIPs are reported to have a labile residue, although there is a methionine in a similar position in AIP-IV.
Examples of mutations that convert an AIP structure into an activator are rare. In one of the only reported successes, an AIP-I D5N structure gained agonist activity for the agr-III system (EC50 = 360 nM)49.
3. AIP Production
The section will outline the properties of AgrD and AgrB and summarize the current knowledge of the AIP biosynthetic mechanism.
3.1. AgrD
AgrD is a ribosomally-produced propeptide that is the precursor to the final AIP signal (Fig. 4). The AgrD peptide consists of 46 amino acids (when using S. aureus agr-I as the example) and can be divided into three general regions: (1) the N-terminal 24 residues form an amphipathic helix that is capable of targeting the peptide to the cell membrane65; (2) the middle section of AgrD encodes the final eight residue AIP-I molecule34; and (3) the C-terminal 14 residues appear to form a helix and tend to have numerous negatively charged residues66.
Figure 4.
S. aureus AgrD sequences and domains. The four S. aureus AgrDs are aligned and separated into three domains that include an N-terminal amphipathic leader, AIP region, and C-terminal charged tail. Conserved resides across the four sequences are shown in red. Potential -1 and -3 signal peptidase cleavage sites are shown in green. Boxed residues in the C-terminal tail are essential for AgrB endopeptidase activity and AIP production.
Among all staphylococcal species, each of these AgrD features is conserved59,67 (Fig. 5). The N-terminal helix can vary significantly in sequence with only a single glycine residue being conserved, however the amphipathic properties are maintained. This helix is capable of targeting the AgrD propeptide to the cell membrane, presumably to associate with AgrB for processing65. Using AgrD-I as a model, truncation of the first 12 residues can be tolerated with some AIP-I still being produced, but deletion of 14 residues prevents AIP-I synthesis. If the removed section is replaced with an artificial amphipathic helix, AIP-I production can be rescued, suggesting the function of this section is membrane targeting, but not necessarily to mediate any specific interactions with AgrB. Replacement of the N-terminus with a classic Sec signal sequence prevented AIP-I production and the speculation here is that AgrB processing was bypassed.
Figure 5.
Sequence alignments of AgrD. Known AgrD sequences from various Gram-positive species are aligned and split into three sections that consist of an N-terminal leader, central AIP sequence (bolded), and charged C-terminal tail. Residues showing strong sequence conservation are colored red. AgrD's whose final AIP product has been confirmed are marked with an asterisk and all others are depicted as eight residues by default, except for variants from within the same species with known structures. Species type numbers reflect variants as reported by Dufour et al.52.
The AIP-encoding section of AgrD shows considerable divergence across species (Fig. 5), although some properties remain consistent. The only conserved residue is the cysteine required for formation of the thiolactone ring structure of AIP. Among staphylococcal strains, the exceptions to this rule are the S. intermedius, S. pseudintermedius, and S. delphini species, which have a serine in place of the cysteine and produce an AIP containing a lactone bond. In S. aureus, the AgrD cysteine is essential for making AIP, but not necessary for AgrB cleavage of the C-terminal tail66.
The C-terminal tail is the most conserved portion of AgrD, especially the first nine residues52, and this tail is predicted to be negatively charged and adopt an alpha-helical conformation66. The presence of these nine residues is necessary for full AIP production66. Across the Staphylococci, aspartate and glutamate are conserved as the first two tail residues, but this conservation is not maintained in AgrD's from other Gram positives (Figs. 4 and 5). Mutations in either the aspartate or glutamate in S. aureus AgrD-I prevents AgrB cleavage activity and AIP production, as will mutation of the leucine at the ninth residue66. Proline residues are conserved in the third and sixth position of the tail, with the carboxy-proximal proline being absolutely conserved in every AgrD sequence (Fig. 5).
3.2. AgrB
AgrB is a 22 kDa protein that localizes to the cell membrane68 and is the primary enzyme for processing AgrD into the final AIP product. Supporting this statement, expression of only agrB and agrD is sufficient for AIP production in an agr deletion mutant34. Further, heterologous expression of agrBD in E. coli or B. subtilis resulted in functional AIP66, demonstrating these are the only unique genes of the system required to make AIP. Multiple studies have demonstrated that AgrB has endopeptidase activity that can remove the AgrD C-terminal tail66,69-70. Two residues were identified as being essential for proteolytic activity, His-77 and Cys-84, suggesting that AgrB acts as a cysteine protease70. These two residues remain absolutely conserved in every AgrB-like protein, including those in non-staphylococcal species, highlighting their importance in AgrB function. Additional mutations in well-conserved serine and histidine residues did not inhibit AIP production, further implicating His-77 and Cys-84 as forming the catalytic center.
In many respects, AgrB is the most unique feature of the staphylococcal agr system based on its lack of sequence similarity with other quorum-sensing proteins. AgrB does not share homology to cysteine proteases or other proteins in the database35. The only AgrB-like proteins that have been identified are those in related cyclic peptide signaling systems in other Gram-positive bacteria (Fig. 6). This uniqueness makes AgrB a valuable genome mining tool to uncover new peptide systems9,71. When comparing the different AgrB sequences among S. aureus agr types, there is a significant amount of variation between strains. Overall properties such as hydrophobic sections that make up transmembrane sections are conserved, though the sequences of these regions are diverse (a common feature of integral-membrane protein homologs). Interestingly, the N-terminal portion of AgrB is highly similar among staphylococcal species, with the first 34 residues being absolutely conserved among the four S. aureus agr types. This region is required for AgrB function; however its role is currently unknown70.
Figure 6.
Sequence alignments of AgrB. AgrB sequences from selected Gram-positive species were aligned using ClustalW308. Conserved residues are marked with gray boxes, and the essential histidine and cysteine residues are colored red. The colored lines above the sequence represent transmembrane regions as reported by Zhang et al.69 (red) or predicted using TOPCONs309 (green and black bars represent the two most common predictions). The 34 N-terminal residues conserved among S. aureus agr types are boxed.
One study defined the topology of AgrB within the cytoplasmic membrane69. Using alkaline phosphatase fusions, the approximate positions of transmembrane spanning regions were determined in E. coli. This method generated a topology map with six transmembrane regions, in which both the N- and C-termini are predicted to be inside the cell and a 27-residue loop located outside the cell. Topological studies indicate that the catalytic residues are located on the cytoplasmic face of the membrane70.
3.3. Mechanism of processing
Since the original report of AIP production by AgrB and AgrD34, there have been only a limited number of studies examining the biosynthesis mechanism, especially in comparison to studies of agr regulation and role in virulence. This may reflect the difficult nature of performing biochemical studies on an integral membrane protein, combined with the fact that AgrB shows no homology to other characterized proteins. Nevertheless, there has been some progress made in understanding how AgrD is processed into AIP.
Zhang et al. were the first to demonstrate that AgrB acts as a protease to cleave AgrD69. Using epitope-tagged AgrD, they demonstrated AgrB-dependent cleavage of AgrD via immunoblots. The predominant product seen is AgrD without the C-terminal tail, indicating that removal of this tail is an early step in AgrD processing. This cleavage activity is abolished by mutation of either the His-77 and Cys-84 catalytic residues70. Interestingly, AgrB activity is inhibited by a subset of protease inhibitors, such as the serine protease inhibitors AEBSF, VFK-CMK, and TPCK. Other serine protease inhibitors (thrombin inhibitor, soybean trypsin inhibitor, and aprotinin) and cysteine protease inhibitors (E-64, E-64D, NCO-700, and Z-Phe-Gly-NHO-Bz) are not effective. The functional AgrB inhibitors (AEBSF, VFK-CMK, and TPCK) are known to block activity of some cysteine proteases, but the reason for lack of activity of the other serine and cysteine protease inhibitors against AgrB is unclear.
Beyond cleavage of the AgrD C-terminal tail, no studies have been reported for AIP thiolactone ring formation or transport across the membrane. A stable AgrB-AgrD structure is detectable, providing a clue to how the thiolactone bond might be formed66 (see below). AIP can be produced by E. coli when only AgrB and AgrD are provided, suggesting that AgrB is responsible for generating the thiolactone bond66. AgrB's role in transport of AIP across the membrane is less clear because other well-conserved transport mechanisms could mediate the process.
To make the final AIP molecule, the N-terminal leader must be removed from AgrD. Kavanaugh et al. investigated this process by purifying the activity capable of cleaving a fluorescein-labeled peptide mimicking the AgrD-I cleavage site72. The type I signal peptidase, SpsB, was identified as the enzyme capable of cleaving the AgrD N-terminal leader. Signal peptidases are membrane-bound, house-keeping proteases responsible for removing N-terminal signal peptides as proteins are being secreted through the Sec or Tat pathways73. In support of this proposal, signal-peptidase inhibitors were capable of preventing AIP production and agr activation in both agr-I and agr-II strains72. However, it remains untested whether signal peptidase is involved in AIP production in other staphylococcal strains. Signal peptidase cleavage sites typically contain small uncharged residues at the -1 and -3 positions of the cleavage site. S. aureus AgrD-I, II, and IV peptides fit this pattern, as do many other staphylococcal AgrDs, but some contain residues such as lysine or tyrosine at the predicted -1 position (Figs. 4 and 5), which could disrupt signal peptidase activity. Further investigation is necessary to verify the universal requirement of signal peptidase in AIP biosynthesis.
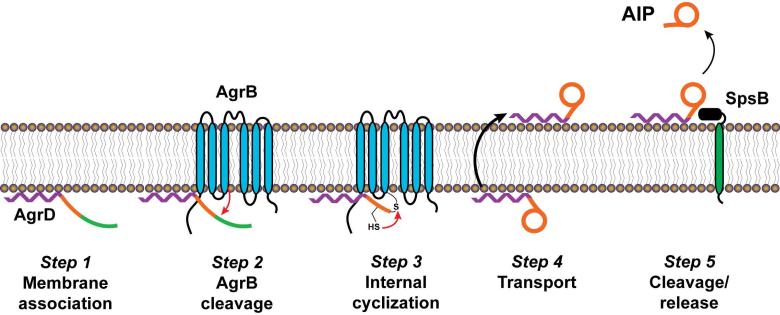
Using all the experimental findings outlined above, a model for AIP biosynthesis has been proposed66,71 (Fig. 7). The first step is association of AgrD with the cytoplasmic membrane via its N-terminal leader. At the membrane, AgrB carries out cysteine-dependent nucleophilic attack on AgrD, removing the C-terminal tail. This cleavage event results in the formation of a covalent intermediate in which AgrD and AgrB are linked through a thioester bond. To create the thiolactone ring, the AgrD internal cysteine carries out a thioester exchange with the bound AgrD-AgrB intermediate, removing the AIP precursor. At this point, the precursor is transported across the membrane, possibly by AgrB, and signal peptidase SpsB removes the N-terminal leader to release AIP into the extracellular environment.
Figure 7.
Model of AIP biosynthetic pathway. Step 1, AgrD is targeted to the cell membrane via its N-terminal amphipathic helix. Step 2, AgrB associates with AgrD and carries out a nucleophilic attack to remove the C-terminal domain of AgrD, resulting in formation of an AgrB-AgrD intermediate linked by a thioester bond. Step 3, the cysteine residue within the AIP-encoding portion of AgrD carries out a thioester exchange to form the thiolactone ring and release the AIP precursor from AgrB. Step 4, transport of the AIP precursor across the membrane, presumably by AgrB. Step 5, removal of the AgrD N-terminal domain by SpsB and release of the final AIP. This figure is an adaption of the version reported by Thoendel et al.66. Reprinted with permission from reference 66. Copyright 2009 The American Society for Biochemistry and Molecular Biology, Inc.
Many questions about the mechanistic details of AIP biosynthesis remain to be answered: (1) direct evidence for AgrD binding to AgrB is unavailable; (2) the role of AgrB in AIP transport has been suggested but never demonstrated; (3) the oligomeric state of the AgrB protein is unknown and the current AgrB topology map has numerous buried charges and exposed hydrophobic loops69, suggesting the membrane spanning regions should be reexamined; (4) most biosynthesis experiments were performed on the agr-I system and the consistency of findings in more divergent systems is unknown; and finally (5) the mechanism thiolactone bond formation has not been demonstrated.
3.4. Type specific processing
Similar to the AgrD sequence variability of the different agr types, the AgrB proteins also evolved in concert with each class of AIP signal. This coevolution resulted in group specificity that enables each AgrB protein to process the AgrD propeptide of the same agr type. When considering the group specificity, it is important to note that AgrB catalyzes multiple steps in the AIP biosynthetic mechanism that should be analyzed independently. However, this type of analysis has not been performed to date, and instead specificity assessments have been made using AIP bioassays. In this case, neither AgrB-I or AgrB-III could use AgrD-II as a substrate for AIP production33. Similarly, AgrB-II could only process its cognate AgrD. Taken together, these findings highlight that group specific processing events do exist, but the step in the AgrB mechanism mediating the specificity remains in question. Some leniency in the group specificity is apparent since the AgrD-I and III classes are similar enough to be functionally interchangeable.33
To define AgrB regions responsible for group specificity, chimeras were generated with different sections of AgrB-I and AgrB-II74. For AgrD-I to be processed, AgrB needs to contain a section encompassing the first transmembrane segment of Type I sequence (residues 43-66), while the rest of the protein could be Type II. In contrast, AgrD-II required one of two AgrB segments (residues 67-75 or 126-141) to be Type II. These regions correspond to either the second and fourth transmembrane regions according to the published topology map69, or two cytoplasmic sections based on computer prediction models (Fig. 6). Both of these sections are fairly well conserved between Types I and II, with six out of nine identical residues in the first segment and 12 out of 16 in the second, narrowing down possible critical residues in dictating specificity. Again, considering AIP production was measured as the output, it is unclear whether the specificity differences are due to AgrD peptide recognition, cleavage, thiolactone ring formation, or transport of the peptide after processing.
4. AgrC
S. aureus AgrC is part of a growing family of peptide-inducible histidine protein kinases (HPK's) that regulate various group behaviors in gram-positive bacteria by sensing the presence of inducer peptide pheromones (IP's) that are produced by the bacteria themselves. This family includes AgrC homologs in other Staphylococcal species52, Clostridium perfringens19,75, Listeria monocytogenes17, Enterococcus faecalis76, and Lactobacillus plantarum16 that bind cyclic IP's, either lactones or thiolactones, and function as quorum-sensing systems that regulate bacterial virulence and/or adhesion. The family also includes HPK's that bind linear IP's and regulate the production of specific secreted factors, and thus we will refer to this greater class of peptide signals as “IP” in this section. Examples of such HPK's are PlnB77, SppK78, PlsK79, and SapK80 that regulate production of bacteriocins in Lactobacillus, CbnK81 that regulates peptide antibiotic production in Carnobacterium, as well as ComD82 that regulates production of competence stimulating factor by Streptococcus. In this section, we have delved deeper into the unique features of AgrC-like HPK's with a hope of presenting a more insightful understanding of AgrC structure and function.
As their cognate IP's accumulate to a critical concentration, AgrC and other IP-responsive HPK's undergo ATP-dependent autophosphorylation in response to signal binding37. After autophosphorylation, they activate their respective response regulator (RR), AgrA in the case of AgrC, through phosphotransfer to an aspartic acid residue on the RR. The phosphorylated RR's in turn activate transcription by directly binding to regulatory DNA elements. In this way the IP-responsive HPK/RR pairs function as classic two-component systems. However, since the RR's of the IP-based quorum systems directly upregulate the expression of genes responsible for IP biosynthesis, the HPK's, RR's and IP's collectively function as three-component systems that afford bacteria a means of measuring population density. Understanding the specificity of IP binding, and how structural changes associated with binding propagate through the membrane and into the cytoplasmic domain, is fundamental to understanding the HPK's signal transduction mechanism.
As outlined in this section, AgrC and the other IP-responsive HPK's are membrane-associated polytopic receptors that share significant structural homology, implying a shared stereochemical mechanism for transmembrane signal transduction. For several members of the family, it has been found that the determinants of IP binding specificity localize to similar regions of the N-terminal sensory domain, as do mutations that cause increased or constitutive activity (see section below). Together these findings lend further support to the idea that the IP-responsive HPK's employ a common transmembrane signal transduction mechanism. There are likely to be differences between this mechanism and those employed by HPK's of the most common HPK subfamilies. Sequence analysis indicates the IP-responsive HPK's are missing a region of the cytoplasmic domain that is thought to play a role in modulating the relative levels of autokinase, phosphotransfer, and phosphatase activity. The allosteric regulation of AgrC enzymatic activity is achieved by modifying the distribution of AgrC among these various conformational states in an IP-dependent manner. The recent demonstration that AgrC is a dimer that undergoes symmetric signal transduction83, implies that IP is bound with positive cooperativity. If this inference proves true, it has important implications for the efficiency and timing of virulence factor expression in vivo, since positive cooperativity would allow a dramatic increase in virulence factor production to occur upon signal binding.
4.1 Structural homology of AgrC family members
Comparison of the primary amino acid sequences of the IP-responsive HPK's, whether they are activated by cyclic or linear IP's, reveals a shared structural organization that is unique to the group84-86. Using primary amino acid sequences of AgrC from the four S. aureus agr groups53, the shared features of the IP-responsive HPK family are revealed using a COBALT87 generated multiple sequence alignment (see Fig. S1). In this section, Figure S1 will be used to summarize the current knowledge of the structures of the IP-responsive HPK's.
Like all HPK's88, the IP-responsive HPK's are composed of the following structural features: (1) N-terminal sensor domain (residues 1-205 for AgrC-I) that contains membrane spanning α-helices (TM's, predicted using HMMTOP89-90 and highlighted in gray in Fig. S1); (2) cytoplasmic domain (residues 206-430 for AgrC-I) consisting of a dimerization and histidine phosphotransfer (DHp) subdomain; (3) autophosphorylation site, a conserved histidine (H239 for AgrC-I) that is located within a region that has high propensity for coiled-coil formation (as predicted with COILS91 and highlighted in blue in Fig. S1); and (4) C-terminal catalytic and ATP binding (CA) subdomain that contains the nucleotide binding site. It is generally accepted that both the DHp and CA subdomains are necessary for autokinase activity, whereas only the DHp subdomain is required for phosphotransfer and phosphatase activities88.
4.2 Structural features of HPK10 cytoplasmic domains
When HPK's are assigned to subfamilies based on the presence or absence of conserved sequence elements (homology boxes) in their DHp and CA subdomains, all the IP-responsive HPK's belong to the HPK10 subfamily84-86. As noted by Grebe and Stock85, HPK's in this subfamily are characterized by CA domains that have several distinguishing features: they (1) lack discernable D-boxes; (2) have a single N-box asparagine (N339 in AgrC-I) instead of the more common two; (3) possess DHp domains whose H-boxes have a tyrosine (Y241 in AgrC-I) two residues downstream of the conserved phosphoaccepting histidine; and (4) are missing a proline that is located five residues C-terminal of the phosphoaccepting histidine in all HPK's of subfamilies one through four (i.e. in the majority of HPK's).
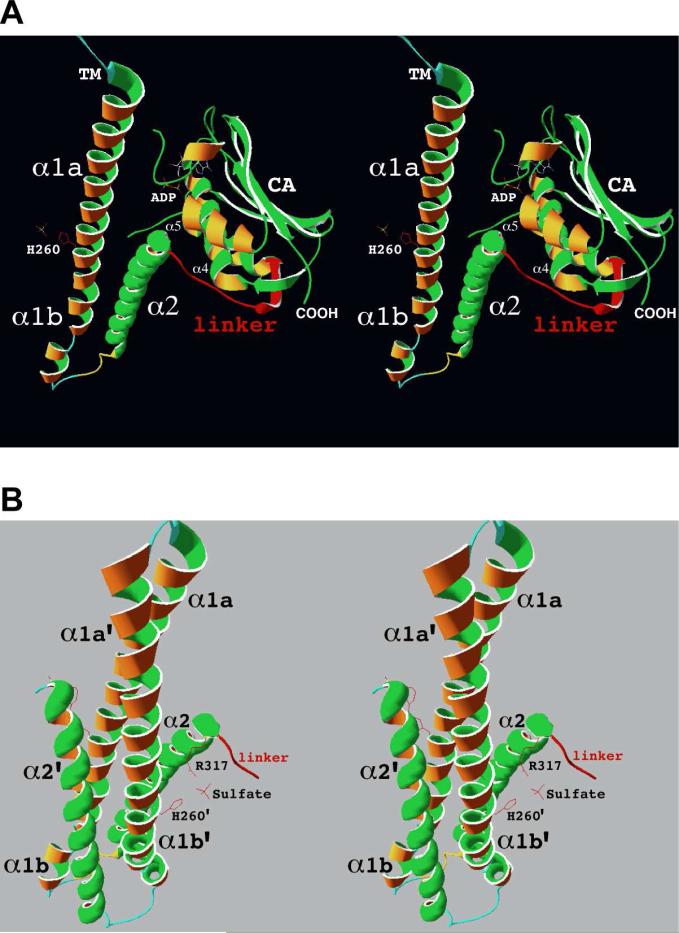
In order to gain insight into the structure of the cytoplasmic domain, a hypothetical AgrC-I structure was generated using the PHYRE server92. The AgrC-I amino acid sequence was threaded onto the crystal structure of the cytoplasmic domain of HK853 from Thermotoga maritima93, a member of the HPK of subfamily 1a, which is the only available structure of the intact cytoplasmic domain. Using the hypothetical structure, regions can be identified that are likely to have high structural similarity as well as others likely to have significant structural differences with potential functional implications. Figure 8 shows the sequence alignment of AgrC-I with HK853, and the cytoplasmic domains of E. coli HPK's EnvZ and PhoQ, which belong to HPK subfamilies 2b and 3a, respectively. While the overall identity between the cytoplasmic domains of AgrC-I and HK853 is a modest 18%, the relative spacing of the homology boxes is maintained and conserved residues are distributed throughout the sequence. There are several regions where multiple conserved residues are found within individual secondary structure elements of HK853 (α-helices are underlined and β-strands are enclosed in boxes). Collectively these regions, which include the α5 helix, the C-terminal half of α3 through βB, and the C-terminal half of α4 through βE, form the nucleotide binding site and the core of the CA subdomain as seen in the ribbons drawing of the HK853 structure (Fig. 9A). This suggests the portion of the AgrC-I CA subdomain not making direct contact with the DHp subdomain is likely to have a structure that is very similar to that of HK853 CA subdomain.
Figure 8.
Alignments of cytoplasmic domains of HPKs. PHYRE310 was used to align the sequence of AgrC-I, HK853 from Thermotoga maritima, and EnvZ and PhoQ from E. coli. Residues that are highly conserved in HPK10's are highlighted in red and residues that are highly conserved in HK853, EnvZ and PhoQ are highlighted in yellow. AgrC-I residues highlighted in blue have a high propensity for coiled-coil formation, and AgrC-I residues highlighted in green are positions where mutations result in constitutive activity. EnvZ residues highlighted in green are positions where mutations eliminate phosphatase activity without altering autokinase activity. Underlined HK853 residues are within α-helices and boxed HK853 residue are within β-strands. The locations of the DHp subdomain and the X-region are indicated. Protein and bacteria names as well as specific residue numbering are indicated in the margins, and a residue count line is provided at the top of each panel.
Figure 9.
Structure of the cytoplasmic domain of HK853 from Thermotoga maritima. A. Stereo diagram of HK853 drawn with Swiss-PDB Viewer311 and PDB312 entry 2C2A. Inter-subdomain and βA residues that are predicted to be deleted in the cytoplasmic domains of HPK10's are drawn in red. The side chain of phosphoaccepting His260 also is shown in red extending from α-helix β1a of the DHp subdomain helical-hairpin located at the far left of the figure. B. Stereo diagram of the four-helix bundle formed by the helical-hairpins from the DHp subdomains of two HK853 monomers. The side chains of phosphoaccepting His260’ and Arg317, as well as an ordered sulfate ion are drawn in red.
Given the putative coiled-coil (highlighted in blue in Figures S1 and 8) and the spacing of conserved hydrophobic residues in the region corresponding to α2 of HK853, it is likely that the DHp subdomain of AgrC forms a helical-hairpin domain similar to those observed in the x-ray crystal structure of HK85393 (Fig. 9A) and the NMR structure of EnvZ94. It is also likely that AgrC dimerization involves formation of a four-helix bundle by the DHp subdomains as has been observed in HK853 (Fig. 9B), EnvZ, and other HPK's88.
There are likely to be some structural differences between the AgrC1 and HK853 DHp subdomains due to the absence of the H-box proline (Pro265 in HK853) and a 6-amino acid deletion corresponding to HK853 residues Thr275 through Leu280 (Fig. 8). In HK853, and all HPK's of subfamilies 1 through 4, the conserved H-box proline is located 5 residues, or approximately 1.5 helical turns, C-terminal of the phosphoaccepting histidine where it places a kink in the first α-helix of the DHp subdomain, such that the helix is split into two helices referred to as α1a and α1b (Figures 8 and 9A). Lacking this proline, the α1 helix of the AgrC-I and the other HPK10's most likely will not be kinked. Also, the AgrC-I helical-hairpin is probably shorter than the HK853 helical-hairpin due to the 6-amino acid deletion. Aligning each of the HPK10's with HK853 using the PHYRE server reveals deletions ranging from 4 to 10 amino acids at this same position (data not shown), indicating that a shorter helical-hairpin is a feature that can be generalized to the entire HPK10 subfamily. These structural differences have the potential to affect phosphotransfer reactions between the HPK10's and their response regulators. The recent x-ray crystal structure of the complex between HK853 and its response regulator, RR46895, shows that the four-helix bundle formed by the DHp subdomains is integral to the HK-RR interaction, with the α1 helix of DHp subdomain making extensive contacts with the α1 helix and β5- α5 loop of the RR (see Figure 1c of 95). In light of this, a detailed characterization of AgrC-AgrA interactions and their effect on the phosphotransfer reaction is a necessary part of a full understanding of AgrC's role in quorum-sensing.
It is important to note that the PHYRE produced alignment identifies a 13-amino acid deletion in AgrC-I corresponding to HK853 residues Agr317 through Leu329 (Fig. 8). This deletion eliminates the inter-subdomain linker and the first β-strand (βA) of the CA subdomain (drawn in red in Figure 9A). PHYRE alignments between the cytoplasmic domains of the other HPK10's and the cytoplasmic domain of HK853 identify similar deletions of 11 to 20 amino acids at this position (data not shown). This region is present in other HPK's, such as EnvZ and PhoQ from E. coli (Fig. 8), suggesting that the missing residues represent deletions in the HPK10's as opposed to an insertion in HK853. The deleted residues constitute a significant portion of the interface between the DHp and CA subdomain (Fig. 9A), and they appear to play in an important role in modulating the various activities of HK853 and EnvZ. Based on these differences with the HPK10's, it is reasonable to expect their absence may have a substantial impact on the structures and activities of the HPK10's.
Elimination of 11 to 20 amino acids from the inter-subdomain linker region will restrict the conformational degrees of freedom available to the HPK10's. Thus, the trans autophosphorylation observed for AgrC may be a consequence of AgrC's inability to access a conformation similar to that seen in the HK-RR complex of HK85383. Since there is no equivalent to Arg317 (of HK853; Arg389 of EnvZ) in the HPK10's due to the deletion, and this arginine residue is required for phosphatase activity96, it is tempting to speculate that AgrC and other HPK10's lack phosphatase activity. It is interesting to note that although the literature on AgrC and the other quorum-sensing HPK's is fairly extensive, there are no reports of these proteins having phosphatase activity. If AgrC does indeed lack phosphatase activity, this has important implications for the regulation of AgrA phosphorylation state by AgrC.
Beyond the differences noted above, the DHp domains of IP-responsive HPK's also are unique owing to the absence of a HAMP (histidine kinase, adenylyl cyclase, methyl-accepting chemotaxis proteins and phosphatase) domain84,86. HAMP domains form a four-helix, parallel coiled-coil that is important for symmetric transmembrane signal transduction97-98. The domain is approximately 50 amino acids in length and normally links the coiled-coil region of the DHp domain to the last TM of the sensory domain93, which is shown as interactions between the N-terminal portions of α1and α1'helices in Figure 9B. In the HPK10 subfamily, the last TM of the sensory domain (TM6) is essentially contiguous with the coiled-coil region of the DHp subdomain (illustrated in Fig. S1). If the structures depicted in Figure 9 were AgrC, the next turns of the α1 helices located at the top of the figures would be in the membrane. Importantly, the continuity between the DHp and TM6 implies that TM6 is also part of the dimerization domain in HPK10's.
4.3 Atypical topology of the HPK10 sensory domains
Whereas the prototypical sensory domain consists of a single, large extra-cytoplasmic loop flanked by two TM's88,99, the sensory domains of HPK10's are predicted to be polytopic84,86. Published hydropathy analyses of AgrC-I37, PlnB77, and ComD182 predict that the sensory domains of these proteins contain five to seven TM's with two to three intervening extracellular loops. The polytopic nature of the sensory domains has been confirmed by β-galactosidase (lacZ) and/or alkaline phosphatase (phoA) fusion analyses for S. aureus AgrC37 and PlnB82. The AgrC-phoA fusions studies confirm that residues 33, 105, and 176 (black boxes in Fig. S1) are located on the extracellular side of the membrane, and residue 142 (red box in Fig. S1) is located on the cytoplasmic side of the membrane. Interestingly, the phoA and lacZ fusion analyses reveal topographical inconsistencies within the N-terminal halves of the AgrC and PlnB sensory domains (TM1 through TM3). Specifically, Ser33 reacts with extracellular nature in both AgrC-I and AgrC-II, whereas Asn35 of PlnB reacts with cytoplasmic nature, implying that the orientations of TM1 and TM2 are opposite in the two proteins. In PlnB the reactivity of Gly64, located on the second predicted intervening loop, is consistent with an extracellular localization, which also supports an inside-to-outside orientation of TM2 in PlnB. In light of these discrepancies, it is important to note that topology prediction programs generate divergent models for the number and orientation of TM's, as Jensen et al. noted for AgrC sensory domain100. For example, MEMSTAT-SVM and MEMSTAT3101 predict 7 TM's for AgrC and PlnB instead of the 6 TM's predicted by HMMTOP89 (Fig. S1). In both cases, the extra TM is the result of splitting TM2 into two TM's.
To obtain more accurate topographical description of the HPK10 sensory domains, we have analyzed all sixteen sequences using TOPCONS102, a program that generates a consensus topology by averaging the topology predictions generated by five programs. Figure S2 shows the TOPCONS generated consensus residue topology predictions and plots of residue reliability value versus residue number for each of the sixteen representative HPK10 sequences. The ambiguity in TM prediction noted above for the N-terminal portions of the AgrC-I and PlnB sensory domains can be generalized to all of the HPK10 sensory domains. The N-terminal third of nearly all the HPK10's contain regions with low reliability values, indicating poor correlation between the topology predictions produced by the individual prediction programs. In contrast, the reliability values corresponding to the C-terminal two-thirds of each sensory domain are all ~0.8 or higher, indicating good correlation between the predictions produced by the individual prediction programs. Moreover, comparison of the bars indicating the TOPCONS residue topologies suggests that within this region the relative positions and orientations of the individual TM's show significant conservation between the various HPK10 sensory domains.
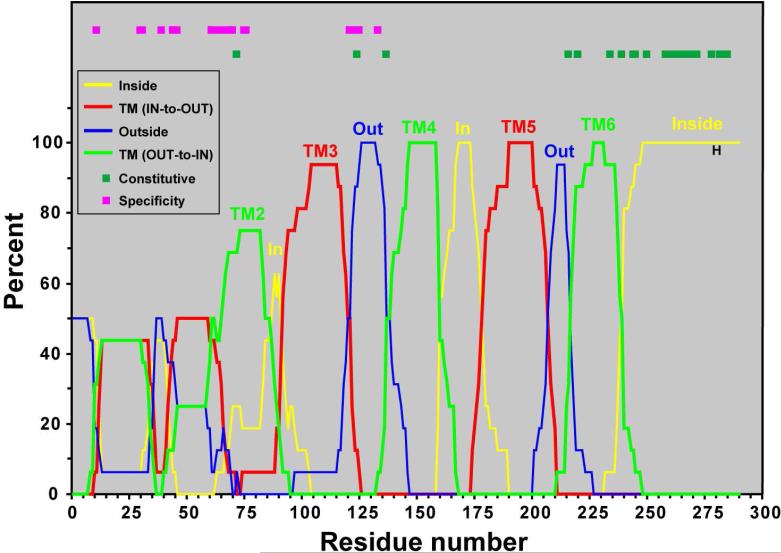
In order to obtain a quantitative measure of this conservation, we have created a consensus HPK10 topology plot (Fig. 10) by counting the frequency with which each of the TOPCONS residue topologies occurs at each residue, and plotting the frequencies versus residue number. The resulting consensus HPK10 topology plot identifies four highly conserved TM's (labeled TM3 through TM6) within the C-terminal two-thirds of the sequence that have the same orientation in all sixteen HPK10's. The plot also identified a fourth TM (labeled TM2), but the position of this helix is less conserved. The positions of these five TM's correspond well with the HMMTOP predictions (Fig. S1). In contrast, the region of the plot corresponding to TM1 does not show very high conservation between the 16 HPK10's, with this region forming either one or two TM's in the various HPK10's. The positions of mutations that confer ligand specificity and constitutive activity are identified in Figure 10. The ligand specificity determinants are localized within the less conserved N-terminal halves of AgrC37,100,103-104 and other HPK10 sensory domains77,105-107. In contrast, mutations that result in constitutive activity localize within the more highly conserved C-terminal halves of AgrC108 and other HPK1 sensory domains77-78,107. This suggests that the N-terminal halves of the HPK10 sensory domains have evolved so they can respond to different ligands, while the C-terminal halves of the HPK10 sensory domains have maintained a structure that is important for efficient transmembrane signaling.
Figure 10.
Consensus HPK10 topology plot. TOPCONS residue topologies were determined for each HPK10 family member (see Fig. S2). The frequency of a particular topology at each residue is plotted as a percentage versus residue number. Labeling on the plot is as follows: outside, thin blue line; TMOUT-to-IN, thick green line; inside, thin yellow line; TMIN-to-OUT, thick red line). TM is an abbreviation for transmembrane. Across the top of the plot, boxes indicate residue mutations in HPK's that result in constitutive activity (dark green) or ligand specificity (magenta).
The HPK10 consensus topology plot (Fig. 10) is also consistent with the bioinformatic analysis of the agr loci from 71 divergent staphylococcal strains reported by Dufour et al. 52. These authors further note that in three variant sequences, S. xylosus, S. arlettae, and S. epidermidis 1, the TM5's “include a perfect four-element leucine zipper motif” and speculate this helix may be involved in homodimerization (S. epidermidis leucines shown as bold, underlined, red letters in Fig. S1). In addition, Dufour et al. point out that the other 21 variants all have hydrophobic residues in the positions corresponding to the repeat leucines52. Comparison to the four S. aureus AgrC sequences shows that leucine is conserved at 11 of the 16 positions corresponding to the zipper motif, and that the other positions contain either phenylalanine, isoleucine, or methionine. Interestingly, the first leucine of the repeat is predicted to be located within the cytoplasm, a prediction that was confirmed by phoA fusion analyses, as noted above. These observations suggest that TM5 helix extends into the cytoplasm where it participates in coiled-coil type interactions. If this hypothesis is correct, the cytoplasmic extension of TM5 is most likely making coiled-coil interactions with the N-terminal region of the α1 helix of the DHp subdomain, since TM6 is contiguous with the DHp domain in the HPK10's and TM5 is predicted to form inter-helix contacts with TM6 (see below). The continuity between the TM6 and the α1 helix of the DHp subdomain suggests there is a high likelihood that TM6 is part of the AgrC dimerization. This observation also raises the possibility that TM5 may contribute to the homodimer interface by participating in a four-helix bundle consisting of TM5, TM6, and TM5’ and TM6’ from the other AgrC subunit, consistent with the speculation of Dufour and colleagues52.
Since the AgrC mutational and chimera studies indicate that IP's interact with N-terminal portion of the HPK10 receptors, it is important to understand the linkage between the N-terminal and C-terminal regions of the HPK10 receptors, especially considering the C-terminal portion of the HPK10 receptors is responsible to transmembrane signaling. More specifically, it is important to identify the helix-helix contacts that occur between the TM's of the sensory domain as receptor activation is likely triggered by AIP-binding induced changes in these interactions.
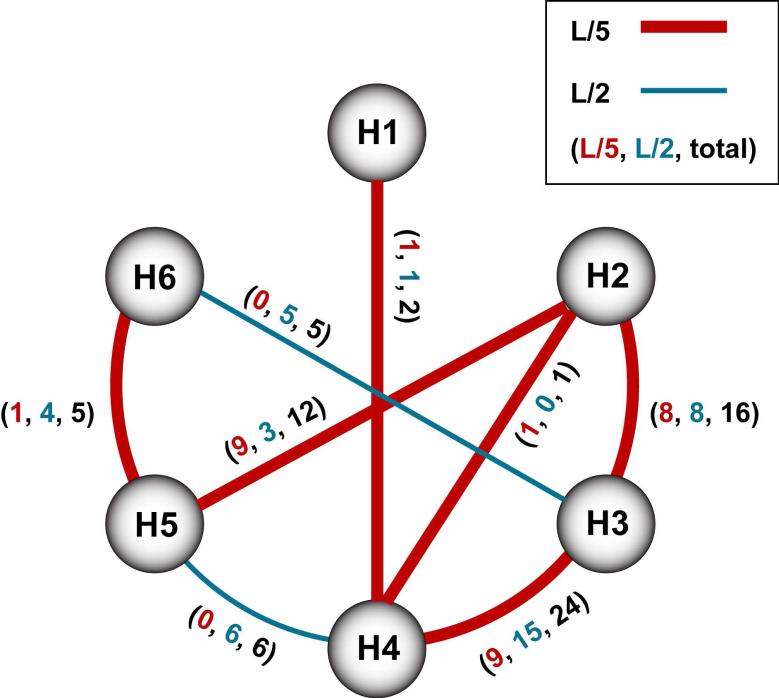
In the absence of atomic resolution data about the three-dimensional structure of the AgrC sensory domain, we have used the program TMHit109 to predict helix-helix contacts and generated a contact map that is shown in Figure 11. The analysis predicts extensive interactions for three different TM helical pairs in AgrC-I. These interactions include (1) TM2-TM3 with 8 high confidence interactions; (2) TM2-TM5 with 9 high confidence interactions; and (3) TM3-TM4 with 9 high confidence interactions. When TMHit was tested against a dataset consisting of TM helices from membrane proteins with known structures, predicted helix-helix contacts with 8 high confidence interactions were identified with 82.4% accuracy, suggesting the predicted TM2-TM3, TM2-TM5, and TM3-TM4 interactions shown in Figure 11 likely exist in AgrC-I. Interestingly the program predicts only limited interactions between TM5 and TM6. This observation suggests that the principle helix-helix interactions between TM5 and TM6 may be across the homodimer interface with the other AgrC monomer. Importantly, since the primary determinants of AIP-I binding specificity are located on the extracellular loop between TM's 3 and 4, and on TM2100,103,110, the extensive helix-helix contacts that TM2 is predicted to make with TM3 and TM5 provide a potential linkage between the IP binding site and the dimerization/trans-membrane signaling domain.
Figure 11.
Helix-Helix interaction map for AgrC-I. TMHit 313 generated transmembrane (TM) helices are indicated as circles and numbered according to AgrC-I topology37. Interactions between the helices are indicated by the lines (or arcs). The presence of a line/arc indicates that at least one predicted atomic interaction pair exists between the helices, and the absence of a line/arc means that the program predicts no contact pairs. An interaction pair is defined as a pair of residues that have at least one pair of atoms that are separated by less than the sum of their van der Waals radii plus 0.6 Å. Contact pairs can be predicted at a higher confidence level (called “L/5” and depicted by thick, red lines/arcs) or at a lower confidence level (called “L/2” and depicted by thin, blue lines). Next to each lines/arcs are three numbers listed in parentheses, and these numbers are TMHit predicted interaction pairs in order of high confidence, low confidence, and total.
4.4 Ligand specificity determinants
There is fairly extensive literature concerning the location of ligand specificity determinants within the sensory domain of AgrC. Initial evidence that the AIP binding site is located within the N-terminal transmembrane domain came from studies in which maltose-binding protein (MBP) was fused to C-terminal portions of AgrC-I37. A MPB fusion protein containing only the cytoplasmic domain of AgrC (residue 199 through the C-terminus) spontaneously autophosphorylated, whereas, a MPB fusion protein containing the portion of AgrC from residue 173 through the C-terminus autophosphorylated only in the presence of agr+ supernatants or purified AIP. This finding indicated that the region between residues 173 and 199, which corresponds to the most C-terminal extracellular loop and TM of the sensory domain, contained at least some AIP binding determinants.
Using chimeric AgrC's that combined sensory domains of one agr type with DHp and CA subdomains of another agr type, it was possible to demonstrate that the AIP binding site is contained wholly within the N-terminal transmembrane domain60-61. More recent studies using a chimeric sensory domain consisting of the N-terminal half of AgrC-I and the C-terminal half of AgrC-IV were able to further localized the specificity determinants to the second extracellular loop (located between TM3 and TM4 in Fig. 10)104. Most recently, three research groups independently confirmed that extracellular loop 2 is a primary site of specificity determinants that differentiates between AIP's I and IV100,103,110. Within this loop the sequences of AgrC's I and IV differ at only four positions (indicated in magenta text in Fig. S1), and all the groups were able to show that swapping these residues in several different combinations significantly alters the relative EC50's for AIP-I and AIP-IV. Significantly, these results imply that when AIP binds to AgrC the thiolactone ring interacts with extracellular loop 2 since the sequences of AIP-I and AIP-IV differ only at the first position in the ring (Asp in AIP-I and Tyr in AIP-IV). In additional site-directed studies, mutations in the region between residues 35-45100 and 49-59110 affected the degree to which AgrC receptors could activate, suggesting that the first extracellular loop may contribute to receptor activation by interacting with the N-terminal tail of AIP.
These findings are consistent with SAR investigations of AIP's that demonstrated the presence of the tail region is an absolute requirement for receptor activation. In particular the finding that tailless AIP-I and AIP-I-D5A both inhibit AgrC-I activity suggests that receptor activation requires AIP-I to simultaneously bind both extracellular loops 1 and 2. Since Asn3 is a critical determinant of AIP-II activity50,56, and that AIP-II-N3A is an antagonist of AgrC-II49,61, demonstrates that AIP-II must also bind extracellular loops 1 and 2 in order to activate its cognate receptor. Collectively these findings imply that the structural rearrangements necessary for receptor activation are initiated when the tail of AIP binds to the first extracellular loop, triggering a displacement of TM2 that could be propagated to the DHp subdomain through alterations of TM's 5 and 6.
There is also substantial literature concerning the location of specificity determinants within the sensory domains of other HPK10's that is consistent with localization to the first and second extracellular loops. Chimera studies utilizing SppK and PlnB77, similar to those conducted on AgrC-I/AgrC-IV sensory domains104, indicate that ligand specificity determinants for these receptors are localized within the N-terminal half of the sensory domains. Additionally, alanine scanning mutagenesis of PlnB identified two residues, Asp54 and Ser58 (magenta text in Figure S1), that are required for IP activation. If PlnB were to adopt the consensus HPK10 structure, these two residues would be located on the first extracellular loop. In the case of S. pneumoniae106-107, there are two major pherotypes of competence-stimulating factor, CSP-1 and CSP-2, that cross activate the ComD1 and ComD2 receptors with EC50's that are significantly higher than those of their cognate receptors105. There are only 12 amino acid differences between the sequences of ComD1 and ComD2 and they are all located within the first 80 residues of the sensory domain (see Fig. S1). The first five occur within the poorly defined N-terminus of the consensus HPK10 structure and may just reflect the low sequence and structural conservation of this region. The other seven differences cluster in the region between residues 47 and 59, which again would place them on the first extracellular loop of the consensus HPK10 structure.
4.5 Constitutive mutations
To identify residues that increased HPK10 activity or made the receptor constitutive, error-prone PCR and assays were performed on AgrC-I108, SppK78, and ComD1107. In all three proteins, mutations with constitutive activity were identified at multiple sites (highlighted in green in Figures S1 and 10). The constitutive mutants were found most frequently in the DHp subdomain, with a total of 3, 7, and 4 mutants mapping to the DHp subdomains of AgrC-I, SppK, and ComD1, respectively. These finding reflect the important role of the DHp subdomain in activation of the HK domain. Importantly, all of the constitutive AgrC-I mutants in the DHp subdomain were resistant to reverse agonism by AIP derivatives that function as antagonists of wild-type receptors, suggesting the structures of these receptors are locked in “on” conformations as a result of the mutations. In AgrC-I, five mutations (R180W, S183F, T197K, L205R, and L205H) were located on the flanks of TM6. Importantly, all of these mutants were sensitive to reverse agonsim, suggesting the AIP derivatives that function as antagonists can shift the structures of these mutants back to an inactive conformation. Interestingly, given the nature of these mutations, charged/polar → hydrophobic on the N-terminal end of TM6 and either polar → charged or non-polar → charged on the C-terminal end of TM6, it has been proposed that these mutations will cause TM6 to be displaced in an extracellular to intracellular direction108. Similarly in SppK, two mutants W213L and F218C are found on the C-terminal side of TM678. Two additional mutations were found in the second extracellular loop of SppK, I110V and T125A, but these both occur in a triple mutant in which the third mutation (Q237R) is located within the DHp subdomain.
4.6 Symmetric transmembrane signal transduction
George and colleagues have recently reported that AgrC forms ligand-independent dimers that activate via trans-autophosphorylation upon interaction with AIP83. Co-immunoprecipitation was used to demonstrate the existence of ligand-independent dimers, and trans-autophosphorylation was demonstrated through a series of intermolecular complementation experiments. When AgrC's bearing mutations either in their G-box (G394A/G396A double mutant) or their H-box (H239Q) were expressed in the reporter strain, no activity was detected in response to AIP addition. To explain this result, in the case of the H-box mutant, AgrCHis, the site of histidine autophosphorylation has been deleted, and in the case the G-box mutant, AgrCKin, the nucleotide binding has been disrupted. However, when the AgrCHis and AgrCKin mutants were co-expressed in the reporter strain, dose-dependent activity was detected in response to AIP addition, with an EC50 of 40 nM that compared well to wild-type (EC50 = 10 nM). This activity is only possible if the mutant protomers complement each other's defect through trans-autophosphorylation within AgrCHis/AgrCKin heterodimers.
Having demonstrated trans-autophosphorylation, George et al. further demonstrated that binding of AIP to a single sensory domain is sufficient for receptor activation and that receptor activation is symmetric83. Dose-dependent activity was detected when AIP-I was added to reporter strain in which full length AgrCHis was co-expressed with AgrCKin in which the AIP binding site was eliminated by deleting first 135 amino acids of the sensory domains (extracellular loops 1 and 2). The activity was significantly lower than that of AgrCHis/AgrCKin heterodimers (~1/3), which could have been due to weaker membrane association of the dimers containing the sensory domain deletion. To address this possibility, an analogous experiment was conducted using AgrCKin containing mutations in the second extra-cellular loop of the sensory domain (T104V/S107A/S116I) that reduce the sensitivity to AIP-1 approximately 50-fold103. When this mutant, AgrCSensor,Kin, was co-expressed with AgrCHis, dose-dependent activation was observed. The activity was equivalent to that of AgrCHis/AgrCKin heterodimers, confirming that binding of AIP to a single sensory domain is sufficient for receptor activation. Similar activation was observed when AgrCSensor,His was co-expressed with AgrCKin, indicating that receptor activation is symmetric. In other words, binding AIP to a single sensory domain is capable of activating the catalytic domain of either protomer, which implies that AgrC dimers bind AIP with positive cooperativity. Alternatively, symmetric signaling could be due to AIP binding to a composite binding site made up regions from both sensory domains of the tetramer. In order to distinguish between these possible explanations, experiments were conducted with intermolecular complementation in which activation was accomplished by introducing a constitutive mutation rather than AIP addition. Introduction of a mutation into one protomer was found to activate the wild-type protomer in the absence of AIP, consistent with the idea that symmetric signaling is the result of ligand binding induced structural changes being propagated across the dimer interface. This raises the possibility that AgrC binds AIP with positive cooperativity59, which may be advantageous for S. aureus to survival in host since it would allow for very rapid induction of virulence factor expression.
5. AgrA
AgrA has sequence similarity to response regulators and is essential for activation of the agr P2 and P3 promoters35,38. Initial progress on AgrA characterization was slowed by technical challenges, but success in recent years has provided much insight on AgrA function. In this section, we briefly summarize the DNA-binding and structural studies that have been performed on this essential part of the agr regulatory system.
5.1 P2/P3 and PSM promoter binding
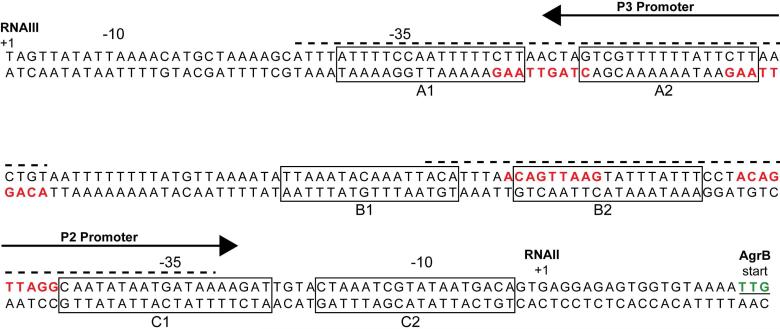
While initial analysis of AgrA indicated that it functioned as a transcriptional regulator35, skepticism over AgrA's role as a DNA-binding protein grew when experiments were unable to demonstrate AgrA binding to DNA probes representing the P2-P3 promoter region, likely due to purification challenges111. Koening et al. overcame this challenge and used purified AgrA in DNA-binding experiments to confirm that AgrA is indeed a transcriptional regulator44. EMSA experiments with the P2 promoter sequence indicated AgrA bound as a dimer with high affinity (Kd = 0.16 nM). This interaction occurred with 10-fold higher affinity compared to unphosphorylated AgrA (Kd = 3.8 nM). DNaseI footprinting assays of the P2-P3 promoter region demonstrated two regions of protection, both overlapping the AgrA binding site direct repeats in each of the promoters (Fig. 12). AgrA binds to P2 with higher affinity than the P3 promoter (Kd 0.16 nM vs 1.7 nM). This difference can be attributed to two basepair changes in the downstream direct repeat of the P3 promoter (AC-to-CT difference). Mutation of these two basepairs to the idealized “AC” results in a P3 promoter with an even higher affinity for AgrA than P2. Interestingly, the P3 promoter also has an unusually long spacer of 20 bp, in contrast the normal 16-17 bp spacer length, and shortening of the P3 spacer by 3 bp elevates RNAIII levels111. The modified P3 promoter also bypasses the requirement for AgrA transcriptional activation, as demonstrated through RNAIII expression in an agrA mutant, presumably due to recognition by the housekeeping sigma factor.
Figure 12.
The agr P2-P3 intergenic region. RNAII and RNAIII transcriptional start sites are marked with a +1. Direct repeats recognized by AgrA are colored red while areas protected from DNaseI cleavage by AgrA are indicated with a dashed line above the sequence. Boxes indicate SarA binding sites as reported by Rechtin et al.143.
For many years, the possible role of AgrA interacting with other promoters besides the agr P2 and P3 went unaddressed. In 2008, Queck et al used DNA microarrays to demonstrate that AgrA is capable of upregulating three chromosomal operons in addition to the agr P2 and P3 promoters39. These include the PSMα, PSMβ, and MW0370/0372 operons (see section 2.2.2). Closer examination of the promoters for these genes revealed potential AgrA binding sites. EMSAs and DnaseI footprinting experiments identified an AgrA protected region immediately downstream of the direct repeats in the psmα promoter at a site overlapping the -35 and -10 regions. Using the same approaches on the psmβ promoter, AgrA protected regions were found overlapping the direct repeats. This study also identified other genes that were downregulated by AgrA independently of RNAIII, but whether AgrA inhibits transcription of these genes through promoter binding will require further investigation.
5.2 Structural studies
S. aureus AgrA is the only member of the LytTR class of response regulator for which a structure has been determined112. Due to challenges with full-length protein expression, Sidote et al. purified the C-terminal domain (residues 137-238) and used this protein for structural studies. Using EMSAs, a 15-bp minimum DNA sequence was identified that maintained optimal AgrA-DNA interactions. This sequence contains the consensus 9-bp binding site in addition to 3 bp flanking this site on either side. The crystal structure was solved in co-complex with the oligonucleotide duplex at 1.6 Å resolution and demonstrated that AgrA has a novel 10 β-stranded topology that includes a two-turn α helix. Interaction of AgrA with the oligonucleotide duplex results in a ~38° bend that creates a concave DNA-binding surface, and due to this distortion, AgrA makes contacts with successive major grooves along a single face of the DNA. In the current model of binding to tandem repeats, AgrA interacts with the repeats as a dimer, but only the AgrA N-terminal regions form protein-protein interactions, while the C-terminal domains are spaced ~10 Å apart. In the C-terminal region, only two residues, H169 and R233, make specific DNA contacts in the major grooves, while a third (N201) interacts with water in the minor groove. Surprisingly, these residues are not strictly conserved in LytTR family members, potentially due to the significant sequence variation among family members. Mutations of residues H169 and R233 to alanines increased Kd values 40-90 fold, indicating they are essential for AgrA DNA-binding affinity112. Unfortunately, the AgrA structure has not provided significant insight on the non-functional mutations that occur spontaneously in S. aureus strains at the AgrA C-terminus113. Sidote et al. suggest that the mutations could render AgrA unstable or result in the loss of other interactions or contacts with transcriptional machinery.
6. RNAIII
Following the discovery of the agr system, it was unclear how a single two-component regulator could have such a pleiotropic effect on gene expression, especially when some genes are being upregulated and others downregulated. The answer to this question came from mutations that occurred upstream of the agr P2 promoter. A second promoter, later named P3, was discovered close to the P2 promoter facing in the opposite direction36. Analysis of the transcript (named RNAIII) revealed a 0.5 kb mRNA containing a short gene, hld, which encodes the 26 amino-acid delta-toxin (also called delta-hemolysin). Surprisingly, when hld was deleted the mutants showed the same phenotype as agr mutants, suggesting a regulatory role for delta-toxin or its transcript. When the RNAIII region was complemented under the control of an inducible promoter, it was able to rescue the phenotype of an agr null mutant38. This complementation occurred regardless of whether hld was present, suggesting that the RNAIII transcript itself was serving as the regulatory molecule. Following this critical observation, numerous studies have investigated RNAIII's function as an effector molecule of the agr system40,42,114-117. In addition to encoding delta-toxin, RNAIII is able to directly promote or inhibit translation of multiple virulence factors, while also inhibiting the translation of a regulatory protein, Rot. The basic properties and mechanism of action of this extraordinary regulatory RNA will be outlined in this section.
6.1. Structure
At 514 nucleotides, S. aureus RNAIII is one of the largest regulatory RNAs found in prokaryotes. Structural studies using chemical and enzymatic probes combined with RNA-folding algorithms revealed a molecule consisting of 14 hairpins (Fig. 13) 116. The folded molecule also contains three sites of long range interaction in which distant nucleotides come together to form helices. These long range interactions divide the RNA into three distinct domains. The 5’ domain is composed of the first 31 nucleotides and contains hairpin 1. The large central domain (nt 32-382) consists of hairpins 2-11 and contains multiple features. It encodes delta-toxin (nt 85-165), contains most of the region necessary for initiating alpha-toxin translation (highlighted green in Fig. 13), while also possessing hairpin 7 which is involved in negative post-transcriptional regulation of genes such as rot. The 3’ domain (nt 383-514) makes up helices 12-14 which are primarily involved in negatively regulating translation of targeted genes.
Figure 13.
Secondary structure of S. aureus RNAIII. RNAIII hairpins are designated with circled numbers while letters signify long range interactions that establish general domains. hld start and stop codons are boxed. Nucleotides that basepair with and activate hla translation are colored green. Nucleotides demonstrated to inhibit translation of genes are red. This figure is an adaptation from Benito et al.116. Reprinted with permission from Reference 116. Copyright 2000 Cold Spring Harbor Laboratory Press.
In S. aureus, RNAIII is expressed during post-exponential phase when cultured, consistent with its activation by agr36. While the P3 promoter is activated after P2, it is upregulated to a much higher extent118. The delayed activation can be attributed to the higher affinity of phosphorylated AgrA for the P2 compared to P344. After transcription RNAIII has a long half-life of up to 45 minutes115,119.
When comparing RNAIII sequences of different staphylococcal species, the secondary structure is well conserved while the primary sequence is conserved only in the first 50 and last 150 nucleotides120. The size can vary significantly with some molecules being up to 700 nucleotides in length. Despite the difference in sequence, the function of the RNAIII's are conserved as the molecules from various staphylococcal species are able to complement RNAIII-deficient S. aureus.
6.2. Delta-toxin
In addition to the RNAIII role as a regulatory RNA, within the transcript is the hld gene that encodes delta-toxin. The gene is located towards the 5’ end of the transcript at nucleotide positions 85-165 (agr Type I numbering). Most staphylococcal species contain a copy of hld in their RNAIII, however Staphylococcus lugdunensis RNAIII does not carry the gene, while Staphylococcus warneri contains two hld genes that differ by eight amino acids120-121. S. aureus delta-toxin is a 26-residue peptide that forms an amphipathic helix in solution122. It is capable of lysing cells by oligomerizing and inserting into targeted membranes to form pores, or at high enough concentrations by acting as surfactant and disrupting the membrane and forming micelles (the reader is directed to Verdon et al. for a comprehensive review of delta-toxin function123).
Translation of hld is typically delayed one hour after RNAIII is synthesized124. The mechanism behind the translation delay is unclear. The hld SD sequence is located at the base of hairpin 3 (Fig. 13), suggesting that when this hairpin is present that the ribosome initiation complex might have trouble recognizing the sequence. However, toeprint studies suggest that ribosomes are able to bind native RNAIII116. Additionally, the hld Shine-Dalgarno (SD) sequence and start codon are part of the RNAIII segment that basepairs with hla mRNA to promote alpha-toxin translation. Interestingly, a deletion of 155 nucleotides of RNAIII (including hairpins 11-14) at the 3’ end is able to relieve the hld translation delay124. This deletion prevents the long range association that divides RNAIII into its three domains, while also preventing negative regulation by RNAIII on Rot expression and other targets. It remains unclear whether the effect on hld translation is due to altered RNAIII structure versus changes in other gene expression that might influence translation.
6.3. Mechanism of action
RNAIII can regulate protein expression by basepairing with the mRNA of the target gene. In order for translation to occur, the 30S ribosomal subunit must be able to recognize the ribosome binding site of the mRNA. This recognition occurs through the 16s rRNA base-pairing with the SD sequence of the single-stranded mRNA. If the SD sequence is hidden by the formation of double-stranded RNA, either by forming a stem-loop structure or base-pairing with another RNA, then translation initiation will be inhibited.
RNAIII is capable of promoting or inhibiting translation of various mRNAs by exploiting this mechanism. RNAIII can promote translation by base-pairing and relieving a stem-loop structure that normally masks the SD sequence42. In contrast, multiple RNAIII stem loops (notably 7, 13, and 14) are capable of base-pairing with target gene SD sequences, forming double-stranded mRNA structures unrecognizable by ribosomes40,114-115,117. To carry out this function each of the three stem loops contains the sequence 5’-UC(3-4)A-3’ which allows for direct basepairing with SD sequences40. Notably, this motif has been found in a variety of other small noncoding RNAs in S. aureus that are thought to act via a similar mechanism46. Through these interactions, RNAIII is capable of both positively and negatively regulating gene expression at the translational level.
Another mechanism of post-transcriptional regulation carried out by RNAIII is mediated through RNase III. RNase III is an endoribonuclease that carries out cleavage of double-stranded RNAs. Rnase III specifically binds RNAIII is the absence of a target mRNA, enabling RNAIII to act as a shuttle to target the enzyme to different mRNAs115. By basepairing with the target mRNA, RNAIII acts to generate a cleavable substrate for RNase III. This cleavage leads to both the destabilization of the mRNAs as the half-lives are significantly shortened, and in some cases results in the removal of the SD sequence to prevent ribosome binding of the mRNA115,117. This combination adds yet another level of post-transcriptional regulation for RNAIII to control gene expression.
Hfq is a well-conserved protein that is important for the function of many small regulatory RNAs in prokaryotes125. By binding to small RNAs, Hfq is able to both stabilize and promote the interaction with targeted substrates. Hfq is important in virulence for multiple bacteria, but its role in S. aureus, particularly in regards to RNAIII function, is less clear. In one study by Bohn et al., there was virtually no change in gene expression in an Hfq knockout126. However a recent study by Liu et al. demonstrated decreased protease and nuclease expression while producing more pigment127. These mutants also displayed decreased cytotoxicity and virulence in a mouse peritonitis model. The reason for the discrepancies between the studies remains unclear. Hfq can be co-purified with RNAIII and does bind in EMSA experiments115. However multiple studies have shown that deletion of Hfq does not affect RNAIII stability or activity in regards to translational control or RNase III recruitment, suggesting that Hfq is not important for RNAIII function114-115,117.
6.4. Positive regulation of alpha-toxin
One of the first RNAIII functions identified was the positive regulation of hla gene translation into alpha-toxin (also called alpha-hemolysin)42,128. Alpha-toxin is a secreted virulence factor that forms heptameric pores in cell membranes, resulting in lysis of the targeted cell. A post-transcriptional level of regulation for alpha-toxin was suggested when a RNAIII mutant was isolated that produced significant levels of hla mRNA with little alpha-toxin product42. In studies by Morfeldt et al, RNAIII was found to complex with hla transcript in vitro and in vivo using gel-shift experiments42. RNase protection studies indicate the 5’ end of RNAIII, including nucleotides 16-86, base pairs with the 5’ untranslated region of the hla mRNA (~75% complementary). When no RNAIII is present, the 5’ region of hla mRNA forms a hairpin loop which masks the SD sequence in the stem, resulting in a confirmation that cannot be translated. RNAIII binding to the mRNA relieves this stem loop structure, allowing the ribosome to recognize the SD sequence and carry out translation (Fig. 14). This was one of the first examples where a regulatory RNA was shown to promote translation of its targeted protein. This regulatory switch allows a second level of control over alpha-toxin production, in conjunction with the transcriptional control by other regulatory factors, such as Rot, SaeRS, and SarA43,129-130.
Figure 14.
RNAIII mechanisms of post-transcriptional regulation on hla, spa, rot, and coa. RNAIII activates hla translation by disrupting the stem-loop and allowing ribosomes to access the Shine-Dalgarno (SD) sequence. RNAIII inhibits the translation of genes (spa, rot, and coa) by basepairing with the mRNA, blocking recognition of the SD sequence, and promoting RNaseIII cleavage. Targeted mRNAs are shown in black while RNAIII sections are red. Numbers reflect nucleotide position from 5’ end of transcripts. Blue arrows indicate sites of cleavage by RNaseIII.
6.5. Negative regulation of protein A
In contrast to its role in promoting hla translation, RNAIII inhibits the translation of multiple proteins. One of the first examples of inhibition came from the regulation studies on the translation of the spa gene into protein A. Protein A is a cell-wall anchored surface protein known for its ability to bind the Fc region of antibodies with high affinity. During lab culture, it is expressed during exponential growth, but protein A production is reduced during the transition to post-exponential phase, suggesting a role for agr in downregulation of the protein.
Early studies identified the 3’ region of RNAIII as being important for repression of spa transcription, though this was probably due in part to this domains role in regulating Rot (see below)116,128. The recognition of RNAIII as a translational regulator of hla, along with its complementary sequence to spa mRNA, led to the hypothesis that RNAIII was inhibiting protein A expression at the level of translation115,131. To test this question, Huntzinger et al generated translational fusions with the 5’ end of the spa mRNA fused in-frame with a β-galactosidase reporter. Using an agr-independent promoter, a significant decrease in reporter activity was apparent when RNAIII was expressed, indicating control at the post-transcriptional level115. The authors went on to show that stable RNAIII-spa complexes can be isolated and this complex formation is dependent on hairpin 13 of RNAIII. RNAIII binds to spa mRNA with an initial binding rate of 3 × 105 M-1s-1 and apparent Kd of 31 × 10-9 M, consistent with values obtained from other sRNA interactions. Translational inhibition occurs through the formation of an extended duplex of more than 30 base pairs between RNAIII and the spa mRNA, including the SD sequence and start codon (Fig. 14). This leads to masking of the SD sequence and prevents formation of the transcription initiation complex.
RNAIII also represses protein A expression by altering the stability of the spa transcript. In the absence of RNAIII the spa mRNA was found to have an unusually long half-life of 15 minutes115. The 5’ untranslated region contains a stem-loop structure in which the SD sequence is located in the external loop of the hairpin. Ribosomal binding and stalling at this structure is thought to help stabilize the transcript by interfering with initial degradation. When RNAIII binds the spa mRNA, it disrupts the 5’ hairpin through the formation of an extended duplex. This duplex serves as a substrate for RNase III, which cleaves the spa mRNA at two sites just downstream from the start codon. Cleavage at the first site also results in the base-paired RNAIII being cut, while the second site is in an internal loop and therefore does not result in RNAIII cleavage. The role of RNase III in spa regulation is further highlighted by the observation that full repression of spa expression cannot be achieved in an RNase III knockout. These findings support the idea that RNAIII works in concert with RNase III in order to suppress expression of targeted genes.
6.6. Negative regulation of Rot
Perhaps RNAIII's greatest impact on gene expression is through its regulation of Rot. Rot is a global transcriptional regulator in the SarA family of regulators. It was originally identified when a Rot transposon mutant was able to restore hemolysis and protease production to an agr mutant132. Rot is best known for its ability to downregulate many secreted virulence factors including the alpha-, beta-, and gamma-toxins, extracellular proteases, urease, lipase, and enterotoxin B43,133. Rot also acts to upregulate the transcription of genes encoding virulence factors, including protein A, coagulase, and clumping factor B43. Additionally, Rot is able to upregulate or downregulate a large number of other genes involved in various cellular processes, such as transport, cell wall biosynthesis, and metabolism of amino acids and carbohydrates. Not surprisingly, there is considerable overlap in the genes identified as being agr regulated and those identified as Rot regulated41,43. Nevertheless some differences are observed, highlighting the fact that agr gene regulation is not due simply to inhibition of Rot.
There are parallels between RNAIII regulation of Rot and protein A expression (Fig. 14). The rot mRNA contains a large 5’ untranslated region that extends 293 base pairs upstream of the start codon134. This region contains multiple stem loop structures including loops I and II just upstream of the start codon, in which the outer loop of loop II contains the rot SD sequence. Isolated RNAIII hairpins were tested for their ability to bind the rot loops and protect them from hydrolysis by RNase T1114. This revealed that RNAIII hairpin 14 bound Rot loop II with the highest affinity while hairpins 7 and 13 bound best to loop I. Two of the three RNAIII hairpins are required for sufficient binding and inhibition Rot translation, no single hairpin appears to be required. This binding is mediated by the RNAIII 5’-UCCC-3’ motifs interacting with the 5’-UUGGGA-3’ sequences in loops I and II. Therefore rather than forming an extended duplex as with other mRNA targets, RNAIII instead inhibits Rot translation by forming two loop-loop interactions, with the interaction involving Rot loop II blocking the SD sequence.
RNAIII also promotes the degradation of rot mRNA in a mechanism involving RNase III. Translational fusions of Rot with β-galactosidase revealed that RNAIII is not able to fully inhibit the reporter when RNase III is knocked out, indicating a role for RNase III in Rot downregulation114. Multiple studies have indicated that the presence of RNAIII does not decrease the rot mRNA half-life, however, Northern blots indicated the presence of cleaved rot transcript when RNAIII and RNase III are both present40,114,134. The major cleavage site is located in the rot SD sequence and results in a non-translatable transcript114. This cleavage is dependent on the presence of RNAIII hairpin 14, while a second cleavage event in the upstream loop I is mediated by hairpin 7. These interactions are thought to result in coaxial stacking between the loops, which provides a substrate that RNase III is able to recognize and cleave.
6.7. Regulation of other targets
To uncover additional genes regulated by RNAIII action, a bioinformatics search of the S. aureus N315 genome was carried out to identify mRNAs whose 5’ regions were predicted to basepair with hairpin 13 of RNAIII114. The mRNA predicted to bind RNAIII with the lowest free energy was SA1000, which encodes a fibrinogen binding protein. Similar to rot, SA1000 mRNA is predicted to form two stem-loops near the 5’ end, although the 5’ loop I of SA1000 mRNA contains the SD sequence and start codon. Using similar techniques as outlined above, hairpin 13 was found to interact with loop I via its SD sequence, followed by the rapid formation of a 40 bp extended duplex, including the SD sequence, which disrupts the loop I structure. This mechanism has the dual effect of inhibiting formation of the translation initiation complex and allowing RNase III to cleave the SA1000 mRNA at two sites on either side of the start codon. The result is an mRNA that cannot be translated and a decrease in SA1000 mRNA levels in the cell.
Another virulence factor that is directly downregulated by RNAIII is coagulase. Coagulase is a secreted protein that promotes the formation of clots by complexing with prothrombin to activate fibrin formation in plasma. Similar to SA1000, RNAIII hairpin 13 binds to a 5’ coa mRNA stem-loop containing the SD sequence (Fig. 14), resulting in the formation of an imperfect duplex that consists of two base-paired regions flanking an internal bulge117. In addition to this interaction, RNAIII hairpin 7 binds to a third coa stem-loop approximately 50 nucleotides downstream of the start codon. This interaction is reminiscent of the RNAIII-rot mRNA complex in that the loop-loop interaction results in coaxial stacking rather than a long duplex formation and results in cleavage at the site of interaction. This seemingly extra loop-loop interaction is considered to be important both for specificity of RNAIII targeting and in destabilizing the coa mRNA by disrupting the stem-loop structure located within the coding sequence.
7. Regulation of agr
Many different environmental, host, and transcriptional regulators factors have been shown to impact agr system function. Due to the abundance of transcriptional regulators that have been identified with agr phenotypes, we have summarized these regulators in Table 2. Some of the best described examples of environmental, positive and negative regulation are outlined in this section. Host factors also modulate agr function and these interactions will be addressed later in section 10.
Table 2.
Transcriptional regulators that modulate agr function.
7.1. Environmental cues
Following the discovery of agr, there was interest in how the system responded to various environmental factors or stresses. One of the earliest factors identified that modulates agr function is pH. Initial studies by Regassa and Betley examined how both high and low pH affected RNAIII expression135. In one study, the pH of the culture was maintained at intervals from 6.5 to 8.0 and RNAIII expression was monitored through Northern blots. Expression was optimal at pH 7.0 and then continually decreased until the media pH reached 8.0.
Similar agr inhibitory effects are observed when S. aureus is grown under conditions that generate acidic pH. When S. aureus is grown aerobically in media supplemented with sufficient metabolites to accumulate excess acetyl-CoA (e.g. glucose), the cells release acetate as an overflow byproduct to generate ATP through substrate level phosphorylation and recycle CoA136. The accumulation of acetate results in a drop in the extracellular pH of the local environment. As the primary metabolites are consumed, S. aureus transitions into post-exponential phase growth and assimilates acetate as a carbon source, causing the extracellular pH to increase. As indicated above, low pH represses the agr system and buffering of culture media prevents this phenotype. The environment of certain colonization sites, such as the skin and vaginal mucosa, can have a local pH < 5137, which is low enough to inhibit agr activation and virulence factor expression138. Currently, molecular and biochemical mechanisms through which pH changes affect agr activation remain unclear, but they could have an important impact on S. aureus pathogenesis in the host.
While glucose supplementation can inhibit agr function, glucose also activates carbon catabolite repression, a regulatory system that modulates gene expression to adapt to preferred carbon source utilization. In low G + C Gram-positive bacteria, this process is mediated by three main proteins; the phosphotransferase HPr, a bifunctional HPr kinase-phophatase, and the transcriptional regulator CcpA139. In the presence of glucose, HPrK/P phosphorylates HPr, facilitating the formation of an Hpr-CcpA complex that can bind to catabolite-responsive elements (CREs) of promoter regions. Given the effects of glucose on virulence factor expression, CcpA's role in regulating agr was investigated. Interestingly, a ccpA knockout displayed a dramatic decrease in RNAIII expression in the presence or absence of glucose at a constant pH140, which suggests that CcpA activates agr in the presence of glucose. When examining the agr P2/P3 region there is no obvious CRE sequence, implying that the regulatory effect of CcpA may be indirect and in need of further investigation.
7.2. Positive regulation
7.2.1. SarA
Perhaps one of the best studied regulators of agr activation is the staphylococcal accessory regulator (sarA). SarA was originally identified as a transposon mutant that displayed altered exoprotein production130. It was evident early on that sarA mutants shared some characteristics of agr mutants such as decreased toxin production, but other changes were divergent, such as increased extracellular protease activity and decreased fibronectin-binding protein production. Molecular analysis confirmed that the sarA gene was indeed independent from the agr locus130,141.
Initial experiments on sarA mutants demonstrated that agr RNAII and RNAIII transcript production was diminished compared to wild-type strains142. SarA activates transcription in the agr locus by binding to the P2/P3 promoter region142-143 (Fig. 12). The identification of the SarA binding site has been the focus of multiple studies. Using DNase I footprinting assays with GST-tagged protein, Chien et al. identified a 29 bp protected region between the P2 and P3 promoters144. This protected region was later refined by aligning promoters of SarA regulated genes to a consensus 26 bp recognition site, which overlapped the original 29 bp site by 22bp145. Similarly, Rechtin et al. performed DNAse I footprint studies, in this case using untagged SarA, and found that SarA protects three paired regions within the agr P2/P3 region: (1) boxes A1 and A2 overlap the -35 region and AgrA binding sites of P3; (2) B1 and B2 overlap the AgrA binding site of P2; and (3) C1 and C2 overlap the -10 and -35 regions of P2.143. When compared with Chien's SarA binding site, there is overlap with the B1 box. The concept of multiple SarA binding sites in agr was further corroborated by Sterba et al. using a technique called SELEX (systematic evolution of ligands by exponential enrichment). Briefly, 98 bp double-stranded oiligonucleotides containing 50 bp of random sequence underwent selection for binding to SarA by EMSA146. By cloning and sequencing the enriched DNA fragments, a conserved 7 bp sequence (ATTTAT) was identified and deemed important for SarA binding. When compared to Rechtin's SarA boxes, six out of seven matches in this 7 bp sequence were found in boxes A2, B1, and twice in C1, lending further evidence that these indeed are SarA binding sites.
Crystal structures of SarA indicate that the protein is a winged-helix dimer. When complexed with DNA, SarA distorts the DNA helix to appear overwound, resulting in tighter packing of nucleotides147. The result is a shortening the spacing between the -35 and -10 elements of promoter. Interestingly, these elements in the P2 and P3 promoters are spaced further apart than in normal promoters (20 bp vs 16 bp), lending support to the hypothesis that SarA activates by overwinding the helix and tightening the -35 and -10 spacing for idealized sigma factor recognition. However, this hypothesis is complicated by the proximity, and in some cases overlap, of AgrA binding sites to those for SarA.
7.2.2. MgrA
MgrA (also known as Rat) is a small transcriptional regulator related to the SarA family of regulators. MgrA regulates cell wall turnover and activates the production of secreted toxins and proteases148-149. These parallels indicated an overlap with proteins induced during agr activation. In initial experiments, Ingavale et al. showed that a transposon insertion in mgrA caused a small decrease in agr RNAII and RNAIII levels148. Follow-up studies using an mgrA knockout demonstrated a complete loss of RNAIII production and no activation of an agr-P3 reporter fusion150. However, the same deletion in S. aureus strain Newman had a minor effect150, indicating strain-to-strain variation. Purified MgrA does shift agr promoter fragments in an EMSA, but the specificity of the MgrA interaction with the P3 promoter is in need of further investigation. Microarray analysis confirmed that MgrA activates toxin and protease production, along with many other genes151, but it failed to identify the agr genes or RNAIII as being differentially regulated, potentially due to the use of strain Newman150. Further analysis is necessary to clarify strain-to-strain discrepancies and define the MgrA and agr interconnection.
7.3. Negative regulation
7.3.1. Sigma Factor B
Sigma B (SigB) is an alternative sigma factor associated with the extracellular stress response in Gram-positive bacteria. In S. aureus, SigB is responsible for activating transcription of surface proteins and pigment production, while downregulating production of secreted toxins and proteases152-156. Given the opposing regulatory output compared to agr, perhaps it is not surprising that SigB also downregulates agr. SigB knockouts in a variety of backgrounds show increased RNAIII levels and stronger activation of P3 reporter fusions157-158. The molecular mechanism through which SigB inhibits RNAIII expression is unclear. There are no identifiable SigB binding sites within the agr locus and attempts to link the two global regulator systems through an unknown intermediate have been unsuccessful157,159. These observations point to a yet unidentified factor under SigB control that is responsible for the agr effect.
7.3.2. CodY
The link between cell metabolism and virulence factor production has been recognized for many pathogens. Similarly with S. aureus, the metabolic state can have dramatic effects on the expression of virulence genes160. One regulator responsible for mediating this interaction is CodY, a transcription factor found in low G+C Gram-positive bacteria that plays a role in adapting to metabolic stress and nutrient limitation. CodY also has been implicated in regulating virulence factors by sensing nutrient availability through binding cellular GTP and branched chain amino acids161-162. During nutrient limitation these pools become depleted, resulting in CodY being unable to bind DNA and relieve repression of target genes163.
The agr system is among the many genes regulated by CodY. A codY knockout elevated RNAII and RNAIII levels during exponential growth, indicating negative regulation when nutrients levels are high164. During stationary phase growth, nutrient pools are depleted and CodY does not have an effect. In S. aureus, the major CodY ligand is the amino acid isoleucine. Wild-type cells grown in the absence of isoleucine show elevated RNAIII expression, similar to the levels seen in a codY knockout, further suggesting a role for this protein in downregulating agr during exponential phase165.
The mechanism of agr repression through CodY has been investigated. Putative CodY binding sites have been determined in other Gram-positive species 166,167. However, the closest match in the agr region is located near hld within the RNAIII transcript, potentially too far to have a direct affect on RNAII transcription. There is a second CodY binding site located within the RNAII transcript in the agrC coding sequence. Pull-down experiments using His6-tagged CodY identified the agrC gene among the enriched fragments of DNA168. Two explanations of CodY repression on agr have been proposed. First, CodY binding could downregulate transcription from the original P1 promoter, reducing levels of RNAI transcript that encode AgrA. Whether downregulation of P1 is significant enough to inhibit overall RNAII synthesis is unknown.
Alternatively, CodY could act as a transcription roadblock within RNAII, preventing transcription of the 3’ end of agrC and agrA. CodY binding sites have been identified in the middle of other operons (cap and ica), and the effect on agr could be a parallel mechanism of action. While progress has been made, the CodY interconnection with agr is in need of further investigation to clarify these possible mechanisms.
7.3.3. Rsr
One of the most recently identified regulators of agr activity is Rsr (for repressor of sarR).169 The rsr gene is located between the regulators sarY and sarR, however, the rsr gene is transcribed independently. Rsr is a 108 amino acid protein that shares no homology with proteins outside of the Staphylococci. When rsr was deleted in multiple S. aureus backgrounds, an increase in SarR and the RNAII and RNAIII transcripts was observed, leading to an upregulation of the agr regulon169. Conversely, rsr overexpression inhibits RNAIII production. A deletion of rsr increased S. aureus virulence in a subcutaneous murine model, indicating the agr effect correlated in vivo. Rsr's effect on agr was independent of the SarR phenotype, as an increase in RNAIII was seen even in an rsr sarR double mutant. Considering the Rsr protein does not bind to the agr P2-P3 region, it remains unclear how loss of the protein increases RNAIII levels. Interestingly, the authors report that the agr mRNA sequence has features consistent with a small antisense RNA, leaving open the possibility that the RNA itself is regulating agr activation169.
7.4. Other regulators that affect agr function
Another potential regulator of agr function in the staphylococcal respiratory response genes SrrAB. SrrA and SrrB form a two-component regulatory pair in which SrrB is membrane-bound histidine kinase and SrrA is a response regulator. SrrAB acts in response to anaerobic environments to regulate gene expression. In initial experiments, Yarwood et al. reported that srrAB mutants possessed an agr regulatory effect170. Follow-up studies demonstrated SrrA binding to the agr P2 and P3 promoters, as well as tst and spa, by EMSA, suggesting a direct regulation by SrrA on these promoters171. Overexpression of srrAB in various S. aureus strains made them less virulent in a rabbit endocarditis model, likely attributable in part to a repressed agr system. These findings are somewhat complicated by further studies using the clinical isolate MN8. In this strain, antisense suppression of srrA had no discernable effect on RNAIII levels 172, while other regulation was maintained. These conflicting findings on the Srr and agr interactions could be a consequence of strain-to-strain variation.
In addition to the examples described above, numerous other additional regulators have been characterized and described to affect agr function (Table 2). For the sake of brevity, a full description of these results will not be discussed. As examples, ArlRS173, SarU174 and SarZ175 are reported to activate agr, while SarT176 and SarX177 downregulate agr function. In many cases, it remains undetermined whether these regulators directly effect agr transcription or if the mechanism is indirect through another factor.
8. Interconnection of agr and biofilms
Biofilms are defined as communities of bacteria attached to a surface that are enclosed in an extracellular matrix. Biofilm development is especially important in the setting of a chronic infection where the multicellular aggregates exhibit increased resistance to antibiotics and host immune defenses. For more information, the reader is referred to numerous excellent reviews that describe the topic of biofilms in more detail178-182. In terms of S. aureus and other Staphylococci, biofilm formation has been implicated as a virulence mechanism in chronic infections that include endocarditis183-184, osteomyelitis185-186, and indwelling medical device infections187-188. The complexity of biofilm maturation and dispersal, along with the potential for the development of improved therapeutics, has lead to a surge in interest in biofilm research over the past decade.
8.1. Effect of agr mutations on biofilms
The interconnection of quorum-sensing and biofilm formation is a topic that has drawn considerable interest189-191. It only seems natural that a cell-density dependent mechanism would play an important role in controlling the development of a multicellular community, a topic that has been coined “sociomicrobiology”192. Seminal early studies on Pseudomonas aeruginosa homoserine lactone signaling demonstrated an important link between the communication mechanism and biofilm maturation193. This finding and numerous follow-up studies led to the widely held belief that bacterial biofilms require quorum-sensing for maturation. Thus, it was somewhat surprising in preliminary studies by Vuong et al. that the agr system in S. aureus was not required to form a biofilm 194. In fact, most clinical S. aureus isolates that could form a biofilm were agr defective, suggesting a functional agr system impeded biofilm maturation (Fig. 15). This important discovery has been confirmed by other laboratories138,195 and has been used as a way to assess agr function196. This finding has even fueled debate that agr is a poor target for therapeutic intervention due to the potential for promoting chronic infection197. However, agr mutation does not enhance biofilm formation under all conditions, especially in the presence of a biotic surface198-200, suggesting this is a topic that needs further investigation. Adding to the complexity of the interpretation is the tremendous variation in agr dynamics across S. aureus strains (see section 9.4).
Figure 15.
Schematic of the S. aureus agr system as a regulator of biofilm lifestyle. Under normal conditions with a functional agr system, S. aureus will secrete invasive factors and remain planktonic. When the agr system is inhibited or deactivated, S. aureus will preferentially adhere to surfaces and develop a biofilm if growth conditions permit. If the situation reverses in the biofilm and the agr system reactivates, the cells can detach in a protease and PSM-dependent manner and return to the planktonic state. It is important to note this schematic is based on the results of in vitro experiments and has not been confirmed in vivo.
8.2. Biofilm dispersal with agr activation
Yarwood et al. introduced an alternative model to explain the surprising role of agr in biofilms that sets S. aureus apart from other bacterial systems201. In the model, agr activation occurs in pockets of cells at the biofilm surface, and the cells detach from the community and disperse into the surrounding environment before the cycle repeats (Fig. 15). Time lapse flow cell studies using agr-dependent GFP reporters, coupled with flow sorting, were used to establish the concept201. This intriguing model was confirmed through addition of biosynthesized AIP to intact biofilms, resulting in agr activation across the entire biofilm and complete detachment138,202. The agr-mediated dispersal mechanism was conserved across multiple S. aureus strains and functions on diverse substratum chemistries138,203.
During normal biofilm development, bacterial cells undergo mechanical and active detachment processes in which they leave the biofilm and return to the planktonic state181. The discovery of agr-mediated dispersal demonstrates that S. aureus possesses a molecular mechanism to actively control biofilm detachment and seed new sites138,201. How is this complex multicellular structure disassembled to return the cells to a planktonic state? The logical conclusion is that the agr activation must induce regulatory changes that allow cells to detach from the extracellular matrix. The problem with this scenario is that the presence and relative contribution of matrix components to the S. aureus biofilm are controversial.
Early studies on S. aureus biofilms pointed to a key role in polysaccharide intercellular adhesin (PIA) as a matrix component204. However, the role of PIA in S. aureus biofilms is largely strain dependent138,195,205, with many recent clinical isolates developing PIA-independent biofilms203,206-207. In contrast, low-specificity proteases, such as Proteinase K and Trypsin, can disperse the biofilms of these PIA-independent S. aureus clinical isolates, leading to a recent surge in studies on protein-dependent biofilm formation158,198,208-211. Linking these observations back to agr-mediated biofilm dispersal, a well known output of agr activation is the production of extracellular proteases. In S. aureus, at least 10 extracellular proteases are under agr control (see Table 1). Boles et al. demonstrated that the addition of a serine protease inhibitor abrogated AIP-mediated biofilm dispersal, and in parallel, genetic inactivation of the metalloprotease aureolysin (aur) and a serine protease cluster (splABCDEF) markedly reduced the dispersal mechanism138. While the possibility that extracellular proteases could be mediating dispersal is intriguing (Fig. 15), the targets of the proteolytic action remain to be determined. As one potential target, studies have demonstrated that the surface-localized fibronectin-binding proteins (FnBPs) are important for biofilm formation208, and the S. aureus SspA (V8) protease is known to cleave these structures212.
Another class of agr-regulated proteins thought to be important in biofilm dispersal is the delta-toxin and PSM peptides. Given their amphipathic nature, PSMs possess surfactant-like properties, and surfactants play an important role in biofilm remodeling and dispersal in other bacterial species213-215. This same function has been proposed for S. aureus194,216 (Fig. 15). This proposal is based on the observation that purified delta-toxin has a negative impact on S. aureus biofilm formation in microtiter plates194. However, the addition of delta-toxin at the measured wild-type concentration of 1 μg/ml only had a minor effect on biofilm formation. Whether other S. aureus PSM peptides will display improved activity against biofilms, and whether gene knockouts of these peptides will affect biofilm maturation, remains to be determined. In summary, agr-mediated biofilm dispersal is a fascinating but complicated mechanism with many unanswered questions. Considering the dispersal of biofilms restores antibiotic susceptibility138,203, an improved understanding of the mechanism warrants further investigation for the development biofilm infection therapies.
9. Role of agr in S. aureus pathogenesis
S. aureus is one of the most common causes of nosocomial and community-associated bacterial infections. This pathogen can cause a diverse spectrum of infections that can be acute, chronic, or toxin-mediated and affect most areas of the human body25,217. The rising prevalence of methicillin-resistant S. aureus (MRSA), and emergence of these strains in the community23, is amplifying the healthcare problem. Much of the acute disease manifestations have been attributed to the arsenal of secreted virulence factors induced by the agr system. Therefore, it is not surprising that the agr system plays an important role when examining S. aureus pathogenesis. In this section, we will define CA-MRSA and summarize the contribution of agr in ex-vivo studies with phagocytes and in-vivo animal model studies. We will also outline the role of agr in colonization and the challenges of strain variation.
9.1 Community-associated MRSA
The epidemic waves of antimicrobial resistance in S. aureus are well documented and particularly concerning (reviewed in23). In the late 1990s, the MRSA strains expanded from healthcare settings and began infecting otherwise healthy individuals in the community. These strains were coined “community-associated” MRSA (CA-MRSA) for their new properties and have become the most recent epidemic wave of resistance in S. aureus23-24. While the global spread has been extraordinary, perhaps more concerning is that these strains are hypervirulent. Of the CA-MRSA stains, the USA300 pulse field gel type has emerged as the most common218-219. While the exact reason for this expansion and hypervirulence of the isolates is unknown, it is apparent that USA300 strains have an agr Type I system that functions at a very high basal rate. Evidence for this phenotype is clear when comparing RNAIII transcript levels to other strains220-221. The mechanism behind the enhanced agr dynamic range is not known, but it might explain the high level of toxins and extracellular enzymes produced by this strain.
9.2. Neutrophil and macrophage interactions
Polymorphonucleur leukocytes (PMNs or neutrophils) are professional phagocytes that are the first wave of defense at the site of infection222. The ability of S. aureus to evade phagocytosis or survive after engulfment is a critical determinant of virulence. Over time the bacteria are capable of replicating within the neutrophils and inducing cell death, allowing the bacteria to escape and spread in the host. S. aureus employs an arsenal of virulence factors and strategies to avoid destruction by neutrophils (see 24,222 for more information). The role of agr in these immune evasion mechanisms has been the focus of multiple studies. Shortly after phagocytosis, RNAIII expression is upregulated by S. aureus within the cells, even when there is only a single bacterium within a phagosome, suggesting that the agr activation can function in a confined intracellular space51. This activation occurs despite the presence of neutrophil-produced ROS capable of inactivating AIP-I through oxidation of the methionine in the macrocycle64. An agr mutant shows a reduced ability to lyse neutrophils, and this effect has been linked to the agr induced alpha-toxin and a group of low-molecular weight toxins called PSMs. Individual knockouts of the hla gene encoding alpha-toxin51 or the PSMα and PSMβ genes223 results in reduced neutrophil lysis. The effect of eliminating the PSM toxins shows a more pronounced lysis defect compared to the alpha-toxin studies223. However, comparison of clinical isolates indicates there is considerable variation in neutrophil lysis phenotypes224, making study-to-study comparisons challenging.
Macrophages are another professional phagocyte that are capable of controlling S. aureus infections. Macrophages do exhibit longer lifespans than neutrophils, potentially serving as reservoirs for S. aureus dissemination within the host. Kubica et al. recently demonstrated survival of S. aureus for 5 days, following which the bacteria replicated and induced host cell lysis225. Isogenic strain studies demonstrated this mechanism was agr dependent. In subsequent analysis of potential agr regulated factors, alpha-toxin and aureolysin mutants showed survival defects225. These studies highlight the important role of agr in S. aureus interactions with phagocytes and provide additional clues to how this system contributes to these initial responders at sites of infection.
9.3 Animal models of infection
Many animal model studies have compared agr mutants to isogenic parental strains to monitor changes in disease progression. Others have focused on the therapeutic value of inhibiting an otherwise functional agr-positive strain. Across numerous animal infection models, more often than not agr dysfunction is associated with a decrease in virulence. Some examples of these studies are presented.
9.3.1 Skin and soft tissue infections
Skin and soft tissue infections are the most common type caused by S. aureus226-227. These range from minor inflammatory conditions to more invasive infection, and most of these cases are associated with the formation of abscesses, the hallmark of a S. aureus infection. The importance of the agr system in abscess formation has been demonstrated using mutants and small-molecule inhibitors of the system48,56,196,228-229. In time course studies using a murine skin infection model, activation of agr within an abscess can be observed with an agr P3-lux reporter within three hours of infection, followed by a decrease in reporter activity, and eventual reporter reactivation 48 hours later48. Abscess lesions of agr mutants are consistently smaller than wild type and have reduced bacterial load229. The bulk of the phenotype is due to agr-dependent secreted virulence factors as demonstrated with studies on sterile supernatants from wild type and agr mutant strains48. The role of agr in abscess formation has also been investigated using various inhibitors of the regulatory system. Interference of AIP signaling through the use of competing AIPs or AIP-sequestering antibodies decreased abscess formation48,56,196. These findings provide direct support for the notion agr-targeted therapies could be an option for the development of skin infection treatments.
9.3.2 Pneumonia
S. aureus is a common cause of community and healthcare-pneumonia230. The role of agr in pneumonia has been investigated using infection models in which high bacterial loads are inoculated intranasally and followed for up to 72 hours. Strains defective in agr displayed attenuated virulence in neonatal mice, showing both a decrease in the establishment of pneumonia and mortality231. These findings were corroborated using an adult mouse pneumonia model232-233. An alpha-toxin knockout was also unable to cause significant mortality, suggesting a critical role for this toxin during pneumonia progression232. Other agr-regulated factors, such as protein A and PVL, have also been implicated in pneumonia pathogenesis, and the altered levels of these proteins may contribute to the agr mutant phenotype234.
In addition to acute pneumonia infections, S. aureus is problematic for causing chronic lung infections in individuals with cystic fibrosis. Cystic fibrosis is a genetic disease in which a defective ion channel predisposes individuals to chronic lung infections by pathogens including Haemophilus influenzae, Pseudomonas aeruginosa, and S. aureus235. In contrast to its role in pneumonia, the agr system is not essential for maintaining these chronic infections. Goerke et al. measured transcripts from cystic fibrosis sputum samples and found RNAIII to be expressed at low levels236. Interestingly, they also observed low expression of spa, a gene downregulated by agr activation, while alpha-toxin expression was variable depending on the sample. Even though RNAIII levels are low in this context, the downstream pattern of gene expression is somewhat similar to agr-activated cells. Some of these conflicting phenotypes could be due to small colony variants (SCVs) that often arise during chronic infections. SCVs accumulate various mutations that slow growth by limiting different metabolic pathways, such as oxidative phosphorylation or thymidine synthesis237. SCVs show a gene expression pattern consisting of a downregulated agr system and decreased toxin and pigment production238. These adaptations make SCVs more resistant to killing by antibiotics and non-professional phagocytes, making it difficult to clear infections in these individuals. Taken together, the agr system is important for acute lung infections, but is dispensable in the context of chronic persistence within the cystic fibrosis lung.
9.3.3 Infective endocarditis and osteomyelitis
S. aureus is the leading cause of infective endocarditis239. This disease is characterized by S. aureus growth on the cardiac valves or endothelium of the chambers, leading to the formation of vegetations consisting of bacteria, platelets, fibrin, and inflammatory cells. It is often associated with bacteremia and treatment requires prolonged antibiotic therapy lasting weeks or in other cases surgery to replace the damaged valves. A rabbit model is often used to replicate infective endocarditis disease progression. When an agr mutant strain was compared to its wild-type parent in this model, there was a decrease in the frequency in which the agr mutant established endocarditis240. Vegetations formed by agr mutants contained fewer bacteria compared to the wild type and led to reduced dissemination to the kidneys, a common secondary infection site241. All of these effects were dose-dependent, as higher inoculums diminished the difference between strains240,241. Deletion of rot, a downstream effector in the cascade, restored the virulence of the agr mutant in this model241. These findings indicate an important role for agr-regulated genes in establishing endocarditis.
Osteomyelitis is an infection of the bone caused by hematogenous seeding or direct infection from nearby tissue. S. aureus is the most common cause of osteomyelitis and often leads to chronic infections that require extended prophylaxis and sometimes surgery for treatment242. Virulence factors have been identified as being important for causing osteomyelitis, such as the fibronectin binding proteins and collagen adhesin243. There is also evidence that S. aureus can persist in the osteoblasts responsible for generating the bone matrix, providing a protective niche from immune cells and antibiotics244. Similar to endocarditis, agr mutants displayed a decreased ability to establish osteomyelitis245-246. When infection did occur, less tissue damage was observed with the agr mutant. Future studies will be necessary to determine the relative contributions of agr-regulated proteins to the pathogenesis mechanisms of infective endocarditis and osteomyelitis.
9.4 Association with diseases and colonization
From an epidemiology perspective, one of the more intriguing observations is that the different agr classes show a correlation with disease. In the early stages, the correlations were rough assignments based on limited strain groups. The agr-I class was considered common and linked to many disease types, while agr-III and IV isolates were rare and linked to toxic shock syndrome and exfoliative toxin related syndromes, respectively247. With the changing epidemiology, more comprehensive strain collections and the advent of rapid typing techniques have provided further insight on the correlations between agr, nasal carriage, and disease. In a small sampling of recent studies that used multi-locus sequencing and other typing methods, the most common nasal carriage isolates fell into the categories called clonal complex 30 (CC30) or USA200 pulse field gel type (PFGE)248-249. Interestingly, both the CC30 and USA200 strains are agr-III and possess the tst gene encoding toxic shock toxin. Looking at one collection of invasive MSSA bloodstream isolates, the most common group identified was CC8, which have the agr-I allele and is the same grouping as USA300249-250. In a large US population-based study of invasive MRSA isolates, the USA100 (agr-II) group predominated at 53.6%, while USA300 (agr-I) was second at 31.6%251. It is important to note that this is only a sampling of studies, but some obvious trends are apparent, some that fit with older assignments and others that show divergence. Consistently, agr-I isolates are some of the most frequently linked to invasive disease, which parallels older observations247. Also consistent, the frequency of isolating an agr-IV strain continues to be a rare event249. What is emerging from recent samplings is that agr-II and III classes are underappreciated. Previously, there was common belief that agr-III was relatively limited to toxic shock syndrome cases, but this group may be quite abundant in nasal carriage. Similarly, agr-II is often ignored as being rare, common in bovine mastitis and other animal infections252-253, but the incredible abundance of USA100 in invasive infections suggests agr-II function should be evaluated in these isolates.
9.5 agr variation across strains
One agr phenotype that is often not appreciated is the variation in system dynamics across strains. As prominent examples, it is known that recent CC30 isolates, such as model strains UAMS-1 and MRSA252, have weak agr systems for reasons that are not clear254. This phenotype is not a general feature of agr-III alleles as USA400 strains possess a robust agr system220. Similarly, many historical agr-I isolates like COL have a muted output, while more recent, related isolates have systems that operate at a high level221. This strain-to-strain variation creates significant challenges for investigators in interpreting the agr contribution to experimental results. If a strain group is selected with a weak or defective agr system, little is learned through mutant analysis. Similarly, the use of strains with known chromosomal defects that distort RNAIII levels, such as NCTC8325 or RN6390, further complicates agr interpretations. Unfortunately in some early agr mutant studies, the strain classification and agr allele are unknown, making it hard to compare the effect of a knockout to more recent studies. As indicated above, the agr system is also hyper susceptible to mutation in many genetic backgrounds255, which has clouded the literature with misassignments of phenotypes. The recurring theme is that studies need to be performed in strains, preferably multiple strains in parallel, where there is adequate information to compare to the field in order to make accurate conclusions.
10. Mechanisms of agr inhibition
The requirement for peptide signaling mechanisms in pathogenesis has raised considerable interest in quorum-quenching approaches. Similar to the Gram-negative acyl-homoserine lactones, nature has already evolved strategies to inhibit the agr system, and some of those mechanisms are outlined below. Antagonist discovery for the AgrC receptors has been and continues to be a fertile area for targeted approaches due to their extracellular exposure and lack of outer membrane barriers, diversifying the functional chemistry that can be employed in inhibitor design256. Other approaches, such as antibody sequestration of the signal and inhibition of the biosynthetic machinery196,257, are preliminary but gaining interest. The underlying complicating factor in agr-targeted therapy is the ongoing acute versus chronic dilemma. The general dogma is that agr inhibition tends to make S. aureus more adherent and “biofilm-like”, and thus more recalcitrant to host defenses and antimicrobial therapy. While this concern has been raised197, it clearly is in need of further evaluation. Most of the in vitro enhancement of agr mutant biofilms appears limited to abiotic surfaces, and the difference is muted using a biotic surface198-200. Perhaps more importantly, agr mutants show defects in chronic biofilm infections in vivo, such as in osteomyelitis and infective endocarditis241,245, suggesting the biofilm enhancement phenotype could be restricted to abiotic surfaces or that the function of agr-dependent virulence factors is dominant in vivo. Nevertheless, the evidence that agr inhibition can attenuate the most severe invasive disease, and the hyperactive nature of agr in the outbreak MRSA strains, supports the continued efforts to identify effective agr-targeted therapies.
10.1. Host factors that inhibit agr
While the prospect of discovering quorum-quenching drugs is gaining popularity, not surprisingly nature is a step ahead and mechanisms of inhibition already exist in the host. Low pH was one of the earliest identified environmental cues that inhibits agr135,137. Many common sites of S. aureus colonization, such as the skin and vaginal tract, are in the pH range of 4.2-6, and the agr system does not function at pH levels of 5.5 and below (see section 7.1). While blood is maintained at neutral pH, multiple host factors in blood can inhibit agr activation. Initially, Yarwood et al. identified that increasing concentrations of serum could quench the agr system in a clinical TSS isolate258. In a related study, Peterson et al. observed that lipoprotein-deficient serum failed to inhibit agr using S. aureus reporter strains. Through follow-up fractionation of lipoprotein components, apolipoprotein B (ApoB) was identified as the primary factor mediating the inhibition259-260. ApoB is an integral component of the low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) particle, which circulate in high concentrations in blood. ApoB has two alpha-helical domains that anchor the protein in the lipoprotein micelle and a globular amino terminus that protrudes from the lipoprotein complex and can interact with soluble blood components. Considering ApoB could bind native AIP-I, but not linear, and this binding was inhibited by AIP-II and AIP-IV, Peterson et al. hypothesized that ApoB can sequester AIPs of all S. aureus classes259. Importantly, mice deficient in ApoB were more susceptible to wild-type S. aureus infection than an agr mutant, suggesting ApoB may have a host defense function against invasive bacterial infection.
Another abundant blood component, hemoglobin, is known to inhibit the agr system. Schlievert et al. found that human blood inhibited TSST-1 production, while simultaneously enhancing Protein A levels, suggestive of an agr interaction261. In fractionation experiments, both the α and β chains of hemoglobin inhibited delta-toxin production, indicating repression of RNAIII. Whether the repression effect is due to direct or indirection action of the globin fragments is not clear, but Schlievert et al. speculate that the positive charge of the globin chains could be interacting with the negatively charged S. aureus phospholipids or surface-exposed residues on the AgrC membrane histidine kinase.
10.2 AgrC receptor antagonists
Structure-activity relationship studies provided critical details for the design of AgrC receptor antagonists (using numbering in Fig. 16). Initially, the AIP-II scaffold drew considerable interest as an antagonist starting point, in part due to the interference activity of this AIP against the other three agr systems and the success of attenuating a murine skin abscess resulting from agr-I strain infection33,56. Based on the initial AIP – AgrC receptor interaction results56, an N-terminal truncation of AIP-II (1) was reasoned to have inhibitory properties61. The success was striking with universal inhibitory properties against all four agr types in the low nanomolar range. Site-directed mutagenesis also provided an important lead as alanine-scanning on AIP-I uncovered the D5A variant (2) as a potent inhibitor of agr-I (IC50 = 33 nM)50, later shown to be a universal inhibitor49. These early clues lead to additional antagonist design approaches using all four AIP scaffolds49. Truncations of AIP-I (3) and AIP-IV (4) displayed impressive inhibitory properties, although trAIP-I still served as a weak agr-I activator. Combining the truncation and an aspartate to alanine mutation (5) resulted in an especially potent inhibitor with IC50's of 0.1-5 nM for all four agr systems. The generation of chimeric AIPs (6,7) also created universal inhibitors, although not of the same potency of the truncation derivatives or AIP-I D5A.
Figure 16.
Global antagonists of S. aureus AgrC. Seven antagonists are shown based on SAR studies of the AIPs. IC50 values from S. aureus reporter strains for each AgrC type are shown underneath the structure.
10.3 Other strategies of agr inhibition
While targeting AgrC has been fruitful, the eventual challenge of this approach is the narrow application of a receptor antagonist towards one species of bacterial pathogen. Resistance also could be a complicating factor considering the hypermutability of the agr system and the likelihood that AgrC point mutations could reverse function of an inhibitor to an activator, as was observed in studies with AIP-I D5A and mutants of AgrC-IV100.
As a counter to this problem, researchers have stepped back further in the quorum-sensing mechanism and focused on the peptide signals themselves. The appeal of this strategy is that it resembles the host defense mechanisms where inactivating or sequestering the signal is the focal point. One promising therapeutic strategy was the preparation of monoclonal antibodies to the S. aureus AIP-IV signal196. The challenge of this approach is that AIPs are poor antigens due to their small size and labile nature. This challenge was averted by synthesizing a stable hapten of the AIP-IV, coupling to carrier proteins, and immunizing mice with the conjugate. Following screening, one of the Abs possessed high specificity for AIP-IV (KD = 90 nM). In vitro quorum-quenching using prototype agr-IV strains, such as RN4850 and NRS168, was effective, with RNAIII production markedly reduced, alpha-toxin production eliminated by immunoblot and PARP cleavage assays, and protein A production increased, all signs of agr inhibition. Most importantly, murine skin abscess formation was inhibited and passive immunization against intraperitoneal infection was successful196.
Besides signal sequestration, there are several examples of small-molecule inhibition of cyclic peptide signal biosynthesis. For agr-I strains, linear peptide inhibitors of Type I signal peptidase were identified and found to reduce AIP-I production and quench agr function72. Signal peptidase is an essential enzyme and also a promising antimicrobial target due to the extracellular location of the active site. One of the most notable examples of biosynthesis inhibition is the discovery of ambuic acid in the screening of natural product libraries for E. faecalis fsr signaling inhibitors through reduction in the secretion of gelatinase biosynthesis-activating pheromone (GBAP)257. At 10 μM concentration, ambuic acid resulted in a 50% inhibition of gelatinase production, an output of the fsr system. Ambuic acid is a polyketide-derived epoxyquinone natural product made by Pestalotiopsis spp. and Monochaetia sp. Fungi. The molecule contains a cyclohexenone moiety that has similarities to those in the antibiotic tetracycline and was found to have anti-fungal properties262. The ambuic acid mechanism of action was investigated, and the compound inhibits processing of the FsrB enzyme, an AgrB homologue, at levels of 100 μM or greater. The inhibition was evaluated using a tagged FsrD substrate and monitoring processing of the peptide precursor257. Perhaps most interestingly, Nakayama et al. evaluated the bioactivity of the compound against other Gram-positives with AgrB-like enzymes. Using signal production assays, the authors concluded that 100 μM ambuic acid could inhibit AIP biosynthesis in a S. aureus agr-I strain and Listeria innocua257, suggesting potential for general applicability towards quorum-quenching in Gram positives.
11. Staphylococcus epidermidis
Staphylococcus epidermidis is an abundant human commensal found on the skin whose role as a human pathogen, until recently, has largely been underestimated. Though less of a problem in healthy individuals, S. epidermidis can cause severe disease in immunocompromised patients and has been identified as a common cause of nosocomial infections. These infections are typically associated with indwelling medical devices, including catheters and prosthetic implants, resulting in difficult to treat chronic infections263-265. The device infections are recalcitrant to host defenses and antimicrobial therapy due to the ability of S. epidermidis to form biofilms188. Currently, S. epidermidis and S. aureus rank as the most common causes of these types of infections on indwelling devices. The increasing use of indwelling devices, and the ubiquitous presence of S. epidermidis on the skin, has created a situation where S. epidermidis could be considered an “accidental” pathogen that is a consequence of our own medical progress188. Like other Staphylococci, S. epidermidis contains a functional agr quorum-sensing system266 and many roles for this system have been reported. In this section, basics of the system, AIP structure and interference properties, and role in biofilms will be covered. The agr system has been linked to many other S. epidermidis phenotypes, such as production of the lantibiotic epidermin267, physiological adaptation268-269, and exo-enzyme production269 that are beyond the scope of this review.
11.1 S. epidermidis agr system
The S. epidermidis agr system has many parallels to its S. aureus cousin with an agrBDCA operon encoding the core components, AIP signal, and RNAIII regulatory RNA that encodes delta-toxin266. Proteome analysis and expression profiling have shown that autoinduction of the S. epidermidis agr system occurs during late-exponential phase and results in the downregulation of cell-surface associated proteins and upregulation of secreted toxins and exoproteins268-270. There is speculation that deactivation of agr alters growth rate and metabolic profile268, but not every strain has a growth defect270, suggesting this issue is strain dependent and in need of clarification.
11.2 AIP structure
Based on the similarities to the S. aureus paradigm, it was assumed that the S. epidermidis AIP structure is composed of a five-residue thiolactone ring with an N-terminal extension. A series of SAR studies were performed on the AIP to identify the preferred length of the N-terminal tail and other allowed modifications to the scaffold10,271. These studies were performed on strain Tu3298 (DSM 3095), which has an agr system that parallels the one found in the commonly used strain 1457 (AIP Type I, see Fig. 3). Although other classes (Type II and III, see Fig. 5) of S. epidermidis agr have been identified52, they are rarely the cause of human infections272.
To determine the preferred size of the S. epidermidis AIP (Type I), synthetic AIPs were generated with N-terminal extensions of two, three, or four residues from the macrocycle and were tested using a biological reporter assay. Based on these experiments, an octapeptide “DSVCASYF” with a thioester linkage of the cysteine to the terminal carboxylate was the preferred inducer10. As anticipated, a linear version of the AIPs that lacked any chemical modification had no biological activity. Interestingly, replacement of the AIP thiolactone with a lactam or lactone also conferred no activity271. This result contrasts S. aureus where the AIP lactam derivatives are active at high concentrations50,60. The structure of the alternative S. epidermidis AIPs (class II and III) are predicted to be quite divergent in sequence52, but have not been verified.
11.3 agr interference
Using synthetic S. epidermidis AIP, it was observed that this signal could inhibit the agr system in S. aureus Type I strains. At 100 nm AIP, S. aureus delta-toxin production was eliminated, while at 1 M AIP, alpha-toxin and other exo-protein levels were reduced as protein A levels increased on the surface271. In studies with other S. aureus agr types, synthetic S. epidermidis AIP inhibited the Type II and III systems, but surprisingly not the Type IV system. In the converse experiment, only the S. aureus AIP-IV inhibited S. epidermidis agr, but the amount of inhibition was limited54. Considering agr Type IV system is often found in S. aureus strains that cause skin infections, such as scalded skin syndrome53, it has been suggested that S. epidermidis and the Type IV strains occupy a similar environmental niche and have evolved competition mechanisms54. In future evaluations of this niche competition proposal, it would be worthwhile to test the cross-species effects of S. epidermidis class II and III AIPs on S. aureus agr Type IV strains.
11.4 agr regulation of biofilms
Analysis of the S. epidermidis genome indicates there are fewer toxins, degradative enzymes, and surface proteins compared to S. aureus 273. This finding, along with the observations that S. epidermidis infections are less acute than S. aureus, and often associated with indwelling medical devices, has led to the conclusion that biofilm formation is the primary virulence mechanism187-188. S. epidermidis biofilms are a complex association of cells that exist within an extracellular matrix composed of polysaccharides, proteins and DNA. Initial attachment to either host matrix proteins or an abiotic surface, such as a catheter or prosthetic implant, is mediated by a diverse range of surface adhesins and the autolysin Atl188,205. While both S. aureus and S. epidermidis express versions of Atl, some notable differences exist. S. epidermidis studies have shown that atlE transcription is growth-phase dependent and absent in an agr mutant274. This result contrasts S. aureus where atl expression is not under the control of the agr system194. Therefore, AtlE in S. epidermidis appears to play a role in initial attachment of this bacterium to synthetic polymers and is regulated by agr.
Multiple lines of evidence indicate that S. epidermidis biofilm detachment is controlled by agr. Similar to S. aureus, an S. epidermidis agr mutant forms thicker biofilms and the mutant has a detachment defect274-275. The agr activity also is limited to expression at the surface of the biofilm. It has been suggested that the detergent activity of PSMs mediate the detachment mechanism, while both PSMs and extracellular proteases have been linked to detachment in S. aureus138,194. Again similar to S. aureus223, PSM production is strictly under agr control in S. epidermidis276. Taken together, agr-mediated biofilm detachment is a conserved mechanism across Staphylococci, but the specific agr regulated factors controlling the mechanism may be species dependent.
12. Other Staphylococci
The presence of loci similar to agr have been identified by PCR, Southern blot and Northern blot analysis in many other coagulase-negative Staphylococci52,120,277, but only a limited number of studies have been performed to validate the existence and function of these loci. The first species examined in some detail was S. lugdunensis, an emerging pathogen that was first described in 1988278. Unlike other coagulase-negatives, S. lugdunensis is known for causing severe infections, such as skin and soft tissue infections, brain abscesses, acute endocarditis, and bacteremia279-280. In the pre-genomics era, S. lugdunensis was one of the first non-aureus Staphylococci to be investigated for the agr system. The agr locus was identified through Southern blot and sequencing, and it was found to contain an agrBDCA operon and RNAIII, although no delta-toxin was encoded in the RNAIII transcript281, despite the fact that S. lugdunensis produces a delta-toxin like activity282. Later studies demonstrated that the toxin activity was mediated by a three-gene cluster called SLUSH, an acronym for S. lugdunensis synergistic hemolysin283. Similar to the PSMs223,276, the expression of SLUSH is controlled by the agr system277. The RNAIII transcript was investigated and demonstrated to be functional through heterologous complementation of a S. aureus RNAIII mutant121. In later studies, the S. lugdunensis agrBD genes were cloned and shown to make a functional AIP signal33. The AIP structure is proposed as a heptapeptide composed of the residues DICNAYF with the last five residues constrained as a thiolactone. Similar to the other Staphylococci, the linear form of the peptide does not activate agr in S. lugdunensis33. Despite the recent interest in this emerging pathogen, an agr mutant has not been constructed to assess phenotypes and the agr regulon remains unknown.
The only other staphylococcal agr system that has drawn significant attention is that of the S. intermedius group. The interest in the S. intermedius group stems from the fact that the AIP structure is a nonapeptide (RIPTSTGFF) with a lactone ring instead of the usual thiolactone in other staphylococcal AIPs11,52,63. Since this discovery, a total of four agr classes have been identified in the S. intermedius group, all containing the identical lactone macrocycle (STGFF) with variant N-terminal extensions284-285. This group includes S. intermedius, as well as the more recently defined species S. pseudintermedius and S. delphini284. Thus, it is not surprising that isolates labeled as S. intermedius are sometimes mislabeled and belong to these other species. S. pseudintermedius is a member of the normal flora of dogs and a common cause of canine pyoderma284. Whether the lactone-containing AIP evolved in this group of Staphylococci due to the particular animal host environment or the commensal flora is not clear. The replacement of the AIP thioester bond with an ester reduces the reactivity of the carbonyl and should enhance the stability of the S. intermedius AIP signal.
In mechanism studies on S. intermedius agr, the AIP was shown to autoactivate RNAIII production in vitro, and the RNAIII transcript was demonstrated to encode a delta-toxin63, unlike the RNAIII of S. lugdunensis. Similar to S. epidermidis, the S. intermedius AIP inhibits all the S. aureus agr systems, with S. aureus agr Type IV showing the most resistance63. For AIP biosynthesis, the S. intermedius AgrB will process its cognate AgrD, and interestingly also the serine-to-cysteine substitution, demonstrating this enzyme can produce both lactone and thiolactone-containing signals. In contrast, S. aureus AgrB cannot process an AgrD sequence with a serine substitution63. What features of S. intermedius AgrB have evolved to accommodate the less reactive serine nucleophile and ester formation are not clear. The AgrB residues or domains involved in AIP ring formation are unknown, but these S. intermedius changes must have evolved in conjunction with the lactone AIP to facilitate catalysis using a serine nucleophile.
13. Conclusions and future perspectives
The agr peptide quorum-sensing system is a fascinating mechanism of cell-to-cell communication that has drawn tremendous interest from staphylococcal researchers. The dramatic effects of agr on exoprotein expression, pathogenesis, and biofilm formation, coupled with the surprising variation that has evolved into the system, has made agr the focal point of hundreds of research papers. Perhaps more importantly, studies on other Gram positives and genome mining has revealed that AIP-mediated communication is a conserved signaling mechanism9. In our current appreciation of evolutionary dynamics, all species of the genera Staphylococcus, Enterococcus, Listeria, and Clostridium appear to possess the machinery to make a cyclic peptide signal. While exceptions to this rule undoubtedly will be discovered, the conservation of agr-like systems across this group that includes some of the most clinically important bacterial pathogens highlights the emerging significance of cyclic peptide signaling. A variety of other Gram positives also possess agr machinery, but notable exceptions include those of the genera Streptococcus, Bacillus, and Mycoplasma9.
Novick and Geisinger elegantly put forth several possibilities to explain the function of quorum-sensing in Staphylococci59. The suggested roles include (1) a bacterial response mechanism to host-specific signals; (2) autoactivation circuitry that is exponentially coupled to a regulatory output enabling exquisite control in variant growth conditions; and (3) a self versus non-self recognition mechanism for autocrine regulation in mixed populations. We agree with Novick and Geisinger's concerns regarding the default view that the agr system should conform with the textbook presentation of quorum-sensing as a cell-density dependent regulatory mechanism59. The recent work of Pang et al. demonstrate that S. aureus can activate agr in a human neutrophil phagosome51, while extracellular S. aureus could not, suggesting that autoactivation can transcend the classical paradigm observed during standard in vitro culture. The confined space of the phagosome (1.2 femtoliters), each with ≥1 cocci, must facilitate the enhanced local concentration of AIP, allowing the signal to surpass the AgrC receptor threshold. The exponential coupling of this ligand-receptor interaction with the cooperative response of AgrC dimers enables S. aureus to differentially regulate accessory genes in this intracellular milieu51. This response is not limited to the neutrophil as similar agr effects are seen with S. aureus confinement in synthetic lipid spheres286.
Going forward, there are still many agr-related questions remaining to be addressed. In terms of the basic signaling mechanism, numerous holes remain in our understanding of AIP biosynthesis in Staphylococci and this extends further into all Gram-positive agr systems. A focal point of open questions is the catalytic function of the AgrB enzyme. This integral membrane endopeptidase is one the most conserved and unique features of agr-like systems but has only been the target of limited study. Multiple studies have confirmed that AgrB catalyzes AgrD cleavage, and AgrB has been proposed to carry out thiolactone ring formation and AIP transport. However, almost no information is available on both the mechanism of ring formation and signal transport steps. Numerous other features of the enzyme remain poorly understood, such as oligomeric state and the surprising concentration of charged resides in proposed transmembrane regions69. A three-dimensional structure of AgrB would answer many of these questions, but this endeavor could be challenging in light of difficulties in purifying integral membrane proteins.
Beyond the basic mechanism, the variation in agr dynamics that occurs across staphylococcal strains is another topic in need of investigation. The best example of this variation is the hyperactivity of the agr system in the outbreak USA300 CA-MRSA220-221. The mechanism behind the tremendous levels of RNAIII generated in these isolates versus other S. aureus remains to be determined. The difference could be at the level of AIP biosynthesis, AgrC-AgrA two-component signaling, P3 promoter activation, RNAIII stability, or possibly some other unexpected mechanism. The increased toxin and exo-enzyme output due to this elevated agr system has been suggested as a possible reason for the hypervirulence of USA300220-221,223. In the converse, the low RNAIII output in CC30 type isolates is also not understood254. With the high frequency of these isolates in nasal carriage249, the muted function of agr could have an underappreciated role in persistent carriage mechanisms.
With the surge in CA-MRSA infections over the past decade, the significance of the agr system has reemerged as a pathogenesis regulator. There is renewed interest in targeting this regulatory mechanism as a means of quenching acute infection. Currently, the bulk of quorum-quenching initiatives focus on development of AgrC antagonists, leaving room for the pioneering of innovative strategies to sequester the AIP signal, breakdown AIP, and inhibit biosynthesis machinery. Some initial successes indicate there is potential in these alternative quenching approaches196,257. For all the agr targeting strategies, structural studies on the AgrC receptor kinase and AgrB biosynthetic enzyme would aid our understanding of current antagonists and facilitate development of new leads. With the considerable interest in quorum-quenching approaches, it will also be important to reevaluate the possibility that agr inhibition will promote chronic infection.
In the current era of genomics, the uncovering of countless new cyclic peptide signaling systems across the Gram positives highlights our current limited understanding of this emerging type of quorum-sensing. In most of these bacterial species, the agr-like systems have not been inactivated to assess the contribution to cellular processes or virulence of pathogenic strains. While the S. aureus agr system has served as the paradigm model for studies on signal production and two-component machinery, the outputs of related systems in other Gram positives is likely to show tremendous variation that remains to be deciphered. With the increasing availability of improved genetic and molecular tools for less tractable organisms, there are opportunities for significant future discoveries in the area of peptide quorum-sensing.
Supplementary Material
Acknowledgements
M. Thoendel and C. E. Flack were supported by NIH Training Grant No. T32 AI07511 and GM077973, respectively. Research in the laboratory of A R. Horswill was supported by award AI078921 from the National Institute of Allergy and Infectious Diseases.
Biography
 Matthew Thoendel
Matthew Thoendel
Matthew “Magnet” Thoendel was born in David City, Nebraska. He received his B.S. in microbiology at the University of Iowa while studying acid sensing ion channels and Pseudomonas aeruginosa biofilms. He is currently earning his M.D./Ph.D. at the University of Iowa studying various aspects of Staphylococcus aureus physiology in Dr. Alexander Horswill's laboratory. While his work primarily focuses on the biosynthesis of signaling peptides, he also investigates other areas such as biofilm formation, stress response systems and host-pathogen interactions. His background in microbiology has also lead to the interesting hobbies of homebrewing and charcuterie.
 Jeffrey S. Kavanaugh, Ph.D.
Jeffrey S. Kavanaugh, Ph.D.
Jeffrey S. Kavanaugh received degrees in biochemistry from the University of Vermont (B.S., 1986) and the University of Iowa (Ph.D., 1992). As a graduate student in the laboratory of Dr. Arthur Arnone, he trained as an x-ray crystallographer and conducted high-resolution x-ray crystallographic analyses on mutant human hemoglobins. Upon completion of his graduate work, he remained in the Arnone lab as a research scientist to continue studies on hemoglobin's stereochemical mechanism with several internationally renowned researchers in the field of hemoglobin allosterism. In 2005, he joined Dr. Alexander Horswill's laboratory in the Microbiology Department at the University of Iowa, and he is involved in many aspects of the group's investigations on virulence regulation and biofilm formation by Staphylococcus aureus.
 Caralyn E. Flack
Caralyn E. Flack
Caralyn E. Flack earned a degree in Physics from Bemidji State University (B.S., 2006). During her undergraduate studies, she participated in an astronomy research experience for undergraduates (REU) at the University of Wisconsin – Madison studying the interstellar medium and a physics REU at the University of California – Davis studying gravitational lens systems. Though she enjoys astronomy research, she also has a strong interest in pathogenic bacteria. She is currently a graduate student at the University of Iowa in Dr. Alexander Horswill's laboratory and is studying Staphylococcus aureus signal transduction.
 Alexander R. Horswill, Ph.D.
Alexander R. Horswill, Ph.D.
Alexander R. Horswill was born in 1973 in Rochester, Minnesota. He studied bacteriology at the University of Wisconsin-Madison as an undergraduate and continued on as a graduate student in the laboratory of Dr. Jorge Escalante-Semerena. He completed his Ph.D. in 2001 researching short-chain fatty acid catabolism in Salmonella. For post-doctoral studies, he joined the group of Dr. Stephen J. Benkovic at Pennsylvania State University and studied enzyme engineering and peptide cyclization with inteins. In 2005, he joined the Microbiology Department at the University of Iowa as an Assistant Professor. His research program focuses on Staphylococcus aureus, with an emphasis on quorum-sensing mechanisms and inhibition, biofilm maturation and dispersal, and host-pathogen interactions.
Footnotes
Supporting Information Available
Figure S1. COBALT generated alignment of AgrC and representative AgrC homologs (HPK10's)
Figure S2. TOPCONS consensus topology predictions
This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Fuqua C, Greenberg EP. Nat. Rev. Mol. Cell. Biol. 2002;3:685. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua C, Parsek MR, Greenberg EP. Annu. Rev. Genet. 2001;35:439. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua C, Winans SC, Greenberg EP. Ann. Rev. Microbiol. 1996;50:727. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 4.Havarstein LS, Coomaraswamy G, Morrison DA. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11140. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazazzera BA. Peptides. 2001;22:1519. doi: 10.1016/s0196-9781(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, Sakagami Y. Nat. Chem. Biol. 2005;1:23. doi: 10.1038/nchembio709. [DOI] [PubMed] [Google Scholar]
- 7.Slamti L, Lereclus D. EMBO J. 2002;21:4550. doi: 10.1093/emboj/cdf450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clewell DB, Francia MV, Flannagan SE, An FY. Plasmid. 2002;48:193. doi: 10.1016/s0147-619x(02)00113-0. [DOI] [PubMed] [Google Scholar]
- 9.Wuster A, Babu MM. J. Bacteriol. 2008;190:743. doi: 10.1128/JB.01135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto M, Sussmuth R, Jung G, Gotz F. FEBS Lett. 1998;424:89. doi: 10.1016/s0014-5793(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalkum M, Lyon GJ, Chait BT. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2795. doi: 10.1073/pnas.0436605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiguchi K, Nagata K, Tanokura M, Sonomoto K, Nakayama J. J. Bacteriol. 2009;191:641. doi: 10.1128/JB.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama J, Cao Y, Horii T, Sakuda S, Akkermans AD, de Vos WM, Nagasawa H. Mol. Microbiol. 2001;41:145. doi: 10.1046/j.1365-2958.2001.02486.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama J, Cao Y, Horii T, Sakuda S, Nagasawa H. Biosci. Biotechnol. Biochem. 2001;65:2322. doi: 10.1271/bbb.65.2322. [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Ingham C, Nakayama J, Beerthuyzen M, Kunuki R, Molenaar D, Sturme M, Vaughan E, Kleerebezem M, de Vos W. J. Bacteriol. 2008;190:7655. doi: 10.1128/JB.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturme MH, Nakayama J, Molenaar D, Murakami Y, Kunugi R, Fujii T, Vaughan EE, Kleerebezem M, de Vos WM. J. Bacteriol. 2005;187:5224. doi: 10.1128/JB.187.15.5224-5235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CG, Hill C. Mol. Microbiol. 2009;71:1177. doi: 10.1111/j.1365-2958.2008.06589.x. [DOI] [PubMed] [Google Scholar]
- 18.Autret N, Raynaud C, Dubail I, Berche P, Charbit A. Infect. Immun. 2003;71:4463. doi: 10.1128/IAI.71.8.4463-4471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu T. J. Bacteriol. 2009;191:3919. doi: 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal JE, Chen J, Li J, McClane BA. PLoS ONE. 2009;4:e6232. doi: 10.1371/journal.pone.0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooksley CM, Davis IJ, Winzer K, Chan WC, Peck MW, Minton NP. Appl. Environ. Microbiol. 2010;76:4448. doi: 10.1128/AEM.03038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. Genome Biol. 2009;10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers HF, Deleo FR. Nat. Rev. Microbiol. 2009;7:629. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLeo FR, Chambers HF. J. Clin. Invest. 2009;119:2464. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowy FD. N. Engl. J. Med. 1998;339:520. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 26.Brown DR, Pattee PA. Infect. Immun. 1980;30:36. doi: 10.1128/iai.30.1.36-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janzon L, Lofdahl S, Arvidson S. FEMS Microbiol. Lett. 1986;33:193. [Google Scholar]
- 28.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. Mol. Gen. Genet. 1986;202:58. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 29.Bjorklind A, Arvidson S. FEMS Microbiol. Lett. 1980;7:203. [Google Scholar]
- 30.Mallonee DH, Glatz BA, Pattee PA. Appl. Environ. Microbiol. 1982;43:397. doi: 10.1128/aem.43.2.397-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. J. Bacteriol. 1988;170:4365. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balaban N, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1619. doi: 10.1073/pnas.92.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji G, Beavis R, Novick RP. Science. 1997;276:2027. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 34.Ji G, Beavis RC, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 1995;92:12055. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. Mol. Gen. Genet. 1995;248:446. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 36.Janzon L, Lofdahl S, Arvidson S. Mol. Gen. Genet. 1989;219:480. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 37.Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick RP, Vandenesch F. Mol. Microbiol. 1998;28:655. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 38.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. EMBO J. 1993;12:3967. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. Mol. Cell. 2008;32:150. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. Mol. Microbiol. 2006;61:1038. doi: 10.1111/j.1365-2958.2006.05292.x. [DOI] [PubMed] [Google Scholar]
- 41.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. J. Bacteriol. 2001;183:7341. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morfeldt E, Taylor D, von Gabain A, Arvidson S. EMBO J. 1995;14:4569. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Said-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, Arvidson S, Foster TJ, Projan SJ, Kreiswirth BN. J. Bacteriol. 2003;185:610. doi: 10.1128/JB.185.2.610-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. J. Bacteriol. 2004;186:7549. doi: 10.1128/JB.186.22.7549-7555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Nat. Med. 2007;13:1510. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 46.Geissmann T, Chevalier C, Cros MJ, Boisset S, Fechter P, Noirot C, Schrenzel J, Francois P, Vandenesch F, Gaspin C, Romby P. Nucleic Acids Res. 2009;37:7239. doi: 10.1093/nar/gkp668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan WC, Coyle BJ, Williams P. J. Med. Chem. 2004;47:4633. doi: 10.1021/jm0400754. [DOI] [PubMed] [Google Scholar]
- 48.Wright JS, 3rd, Jin R, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1691. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyon GJ, Wright JS, Muir TW, Novick RP. Biochemistry. 2002;41:10095. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 50.McDowell P, Affas Z, Reynolds C, Holden MT, Wood SJ, Saint S, Cockayne A, Hill PJ, Dodd CE, Bycroft BW, Chan WC, Williams P. Mol. Microbiol. 2001;41:503. doi: 10.1046/j.1365-2958.2001.02539.x. [DOI] [PubMed] [Google Scholar]
- 51.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. J. Innate. Immun. 2010;2:546. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, Etienne J, Lina G. J. Bacteriol. 2002;184:1180. doi: 10.1128/jb.184.4.1180-1186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarraud S, Lyon GJ, Figueiredo AM, Gerard L, Vandenesch F, Etienne J, Muir TW, Novick RP. J. Bacteriol. 2000;182:6517. doi: 10.1128/jb.182.22.6517-6522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otto M, Echner H, Voelter W, Gotz F. Infect. Immun. 2001;69:1957. doi: 10.1128/IAI.69.3.1957-1960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goerke C, Kummel M, Dietz K, Wolz C. J. Infect. Dis. 2003;188:250. doi: 10.1086/376450. [DOI] [PubMed] [Google Scholar]
- 56.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1218. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fleming V, Feil E, Sewell AK, Day N, Buckling A, Massey RC. J. Bacteriol. 2006;188:7686. doi: 10.1128/JB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson DA, Monk AB, Cooper JE, Feil EJ, Enright MC. J. Bacteriol. 2005;187:8312. doi: 10.1128/JB.187.24.8312-8321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novick RP, Geisinger E. Annu. Rev. Genet. 2008;42:541. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 60.Lyon GJ, Wright JS, Christopoulos A, Novick RP, Muir TW. J. Biol. Chem. 2002;277:6247. doi: 10.1074/jbc.M109989200. [DOI] [PubMed] [Google Scholar]
- 61.Lyon GJ, Mayville P, Muir TW, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13330. doi: 10.1073/pnas.97.24.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyon GJ, Novick RP. Peptides. 2004;25:1389. doi: 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Ji G, Pei W, Zhang L, Qiu R, Lin J, Benito Y, Lina G, Novick RP. J. Bacteriol. 2005;187:3139. doi: 10.1128/JB.187.9.3139-3150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothfork JM, Timmins GS, Harris MN, Chen X, Lusis AJ, Otto M, Cheung AL, Gresham HD. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13867. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Lin J, Ji G. J. Biol. Chem. 2004;279:19448. doi: 10.1074/jbc.M311349200. [DOI] [PubMed] [Google Scholar]
- 66.Thoendel M, Horswill AR. J. Biol. Chem. 2009;284:21828. doi: 10.1074/jbc.M109.031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novick RP. Mol. Microbiol. 2003;48:1429. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 68.Saenz HL, Augsburger V, Vuong C, Jack RW, Gotz F, Otto M. Arch. Microbiol. 2000;174:452. doi: 10.1007/s002030000223. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Gray L, Novick RP, Ji G. J. Biol. Chem. 2002;277:34736. doi: 10.1074/jbc.M205367200. [DOI] [PubMed] [Google Scholar]
- 70.Qiu R, Pei W, Zhang L, Lin J, Ji G. J. Biol. Chem. 2005;280:16695. doi: 10.1074/jbc.M411372200. [DOI] [PubMed] [Google Scholar]
- 71.Thoendel M, Horswill AR. Adv. Appl. Microbiol. 2010;71:91. doi: 10.1016/S0065-2164(10)71004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kavanaugh JS, Thoendel M, Horswill AR. Mol. Microbiol. 2007;65:780. doi: 10.1111/j.1365-2958.2007.05830.x. [DOI] [PubMed] [Google Scholar]
- 73.Paetzel M, Karla A, Strynadka NC, Dalbey RE. Chem Rev. 2002;102:4549. doi: 10.1021/cr010166y. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Ji G. J. Bacteriol. 2004;186:6706. doi: 10.1128/JB.186.20.6706-6713.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyristis M, Bryant AE, Sloan J, Awad MM, Nisbet IT, Stevens DL, Rood JI. Mol. Microbiol. 1994;12:761. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 76.Qin X, Singh KV, Weinstock GM, Murray BE. Infect. Immun. 2000;68:2579. doi: 10.1128/iai.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnsborg O, Diep DB, Nes IF. J. Bacteriol. 2003;185:6913. doi: 10.1128/JB.185.23.6913-6920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathiesen G, Axelsen GW, Axelsson L, Eijsink VG. Arch. Microbiol. 2006;184:327. doi: 10.1007/s00203-005-0049-5. [DOI] [PubMed] [Google Scholar]
- 79.Stephens SK, Floriano B, Cathcart DP, Bayley SA, Witt VF, Jimenez-Diaz R, Warner PJ, Ruiz-Barba JL. Appl. Environ. Microbiol. 1998;64:1871. doi: 10.1128/aem.64.5.1871-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Axelsson L, Holck A. J. Bacteriol. 1995;177:2125. doi: 10.1128/jb.177.8.2125-2137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohde BH, Quadri LE. BMC Microbiol. 2006;6:93. doi: 10.1186/1471-2180-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Havarstein LS, Gaustad P, Nes IF, Morrison DA. Mol. Microbiol. 1996;21:863. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 83.George EA, Cisar G, Geisinger E, Muir TW, Novick RP. Mol. Microbiol. 2009;74:44. doi: 10.1111/j.1365-2958.2009.06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolanin PM, Thomason PA, Stock JB. Genome Biol. 2002;3:REVIEWS3013. doi: 10.1186/gb-2002-3-10-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grebe TW, Stock JB. Adv. Microb. Physiol. 1999;41:139. doi: 10.1016/s0065-2911(08)60167-8. [DOI] [PubMed] [Google Scholar]
- 86.Mascher T, Helmann JD, Unden G. Microbiol. Mol. Biol. Rev. 2006;70:910. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papadopoulos JS, Agarwala R. Bioinformatics. 2007;23:1073. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
- 88.Gao R, Stock AM. Ann. Rev. Microbiol. 2009;63:133. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tusnady GE, Simon I. Bioinformatics. 2001;17:849. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 90.Tusnady GE, Simon I. J. Mol. Biol. 1998;283:489. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 91.Lupas A. Methods Enzymol. 1996;266:513. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 92.Kelley LA, Sternberg MJ. Nat. Protoc. 2009;4:363. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 93.Marina A, Waldburger CD, Hendrickson WA. EMBO J. 2005;24:4247. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomomori C, Tanaka T, Dutta R, Park H, Saha SK, Zhu Y, Ishima R, Liu D, Tong KI, Kurokawa H, Qian H, Inouye M, Ikura M. Nat. Struct. Biol. 1999;6:729. doi: 10.1038/11495. [DOI] [PubMed] [Google Scholar]
- 95.Casino P, Rubio V, Marina A. Cell. 2009;139:325. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Qin L, Yoshida T, Inouye M. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7808. doi: 10.1073/pnas.97.14.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, Martin J, Schultz JE, Lupas AN, Coles M. Cell. 2006;126:929. doi: 10.1016/j.cell.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 98.Zhu Y, Inouye M. J. Biol. Chem. 2004;279:48152. doi: 10.1074/jbc.M401024200. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Z, Hendrickson WA. J. Mol. Biol. 2010;400:335. doi: 10.1016/j.jmb.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jensen RO, Winzer K, Clarke SR, Chan WC, Williams P. J. Mol. Biol. 2008;381:300. doi: 10.1016/j.jmb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 101.Jones DT. Bioinformatics. 2007;23:538. doi: 10.1093/bioinformatics/btl677. [DOI] [PubMed] [Google Scholar]
- 102.Bernsel A, Viklund H, Hennerdal A, Elofsson A. Nucleic Acids Res. 2009;37:W465. doi: 10.1093/nar/gkp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geisinger E, George EA, Muir TW, Novick RP. J. Biol. Chem. 2008;283:8930. doi: 10.1074/jbc.M710227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wright JS, 3rd, Lyon GJ, George EA, Muir TW, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16168. doi: 10.1073/pnas.0404039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnsborg O, Kristiansen PE, Blomqvist T, Havarstein LS. J. Bacteriol. 2006;188:1744. doi: 10.1128/JB.188.5.1744-1749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnsborg O, Godager LH, Nes IF. Arch. Microbiol. 2004;182:450. doi: 10.1007/s00203-004-0728-7. [DOI] [PubMed] [Google Scholar]
- 107.Martin B, Prudhomme M, Alloing G, Granadel C, Claverys JP. Mol. Microbiol. 2000;38:867. doi: 10.1046/j.1365-2958.2000.02187.x. [DOI] [PubMed] [Google Scholar]
- 108.Geisinger E, Muir TW, Novick RP. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1216. doi: 10.1073/pnas.0807760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lo A, Chiu YY, Rodland EA, Lyu PC, Sung TY, Hsu WL. Bioinformatics. 2009;25:996. doi: 10.1093/bioinformatics/btp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen LC, Tsou LT, Chen FJ. J. Microbiol. 2009;47:572. doi: 10.1007/s12275-009-0004-2. [DOI] [PubMed] [Google Scholar]
- 111.Morfeldt E, Tegmark K, Arvidson S. Mol. Microbiol. 1996;21:1227. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 112.Sidote DJ, Barbieri CM, Wu T, Stock AM. Structure. 2008;16:727. doi: 10.1016/j.str.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Traber K, Novick R. Mol. Microbiol. 2006;59:1519. doi: 10.1111/j.1365-2958.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 114.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, Gaspin C, Vandenesch F, Romby P. Genes Dev. 2007;21:1353. doi: 10.1101/gad.423507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Lina G, Etienne J, Ehresmann B, Ehresmann C, Jacquier A, Vandenesch F, Romby P. EMBO J. 2005;24:824. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benito Y, Kolb FA, Romby P, Lina G, Etienne J, Vandenesch F. RNA. 2000;6:668. doi: 10.1017/s1355838200992550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chevalier C, Boisset S, Romilly C, Masquida B, Fechter P, Geissmann T, Vandenesch F, Romby P. PLoS Pathogens. 2010;6:e1000809. doi: 10.1371/journal.ppat.1000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiong YQ, Van Wamel W, Nast CC, Yeaman MR, Cheung AL, Bayer AS. J. Infect. Dis. 2002;186:668. doi: 10.1086/342046. [DOI] [PubMed] [Google Scholar]
- 119.Janzon L, Arvidson S. EMBO J. 1990;9:1391. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tegmark K, Morfeldt E, Arvidson S. J. Bacteriol. 1998;180:3181. doi: 10.1128/jb.180.12.3181-3186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benito Y, Lina G, Greenland T, Etienne J, Vandenesch F. J. Bacteriol. 1998;180:5780. doi: 10.1128/jb.180.21.5780-5783.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Colacicco G, Basu MK, Buckelew AR, Jr., Bernheimer AW. Biochim. Biophys. Acta. 1977;465:378. doi: 10.1016/0005-2736(77)90087-6. [DOI] [PubMed] [Google Scholar]
- 123.Verdon J, Girardin N, Lacombe C, Berjeaud JM, Hechard Y. Peptides. 2009;30:817. doi: 10.1016/j.peptides.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 124.Balaban N, Novick RP. FEMS Microbiol. Lett. 1995;133:155. doi: 10.1111/j.1574-6968.1995.tb07877.x. [DOI] [PubMed] [Google Scholar]
- 125.Valentin-Hansen P, Eriksen M, Udesen C. Mol. Microbiol. 2004;51:1525. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- 126.Bohn C, Rigoulay C, Bouloc P. BMC Microbiol. 2007;7:10. doi: 10.1186/1471-2180-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y, Wu N, Dong J, Gao Y, Zhang X, Mu C, Shao N, Yang G. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. EMBO J. 1993;12:3967. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giraudo AT, Cheung AL, Nagel R. Arch. Microbiol. 1997;168:53. doi: 10.1007/s002030050469. [DOI] [PubMed] [Google Scholar]
- 130.Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6462. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Novick RP. Mol Microbiol. 2003;48:1429. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 132.McNamara PJ, Milligan-Monroe KC, Khalili S, Proctor RA. J Bacteriol. 2000;182:3197. doi: 10.1128/jb.182.11.3197-3203.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tseng CW, Stewart GC. J. Bacteriol. 2005;187:5301. doi: 10.1128/JB.187.15.5301-5309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hsieh HY, Tseng CW, Stewart GC. J. Bacteriol. 2008;190:546. doi: 10.1128/JB.00536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Regassa LB, Novick RP, Betley MJ. Infect. Immun. 1992;60:3381. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Somerville GA, Said-Salim B, Wickman JM, Raffel SJ, Kreiswirth BN, Musser JM. Infect. Immun. 2003;71:4724. doi: 10.1128/IAI.71.8.4724-4732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weinrick B, Dunman PM, McAleese F, Murphy E, Projan SJ, Fang Y, Novick RP. J. Bacteriol. 2004;186:8407. doi: 10.1128/JB.186.24.8407-8423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boles BR, Horswill AR. PLoS Pathogens. 2008;4:e1000053. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Titgemeyer F, Hillen W. Antonie Van Leeuwenhoek. 2002;82:59. [PubMed] [Google Scholar]
- 140.Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bachi B, Bischoff M. Antimicrob. Agents Chemother. 2006;50:1183. doi: 10.1128/AAC.50.4.1183-1194.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheung AL, Projan SJ. J. Bacteriol. 1994;176:4168. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heinrichs JH, Bayer MG, Cheung AL. J. Bacteriol. 1996;178:418. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rechtin TM, Gillaspy AF, Schumacher MA, Brennan RG, Smeltzer MS, Hurlburt BK. Mol. Microbiol. 1999;33:307. doi: 10.1046/j.1365-2958.1999.01474.x. [DOI] [PubMed] [Google Scholar]
- 144.Chien Y, Cheung AL. J. Biol. Chem. 1998;273:2645. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 145.Chien Y, Manna AC, Projan SJ, Cheung AL. J. Biol. Chem. 1999;274:37169. doi: 10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 146.Sterba KM, Mackintosh SG, Blevins JS, Hurlburt BK, Smeltzer MS. J. Bacteriol. 2003;185:4410. doi: 10.1128/JB.185.15.4410-4417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schumacher MA, Hurlburt BK, Brennan RG. Nature. 2001;409:215. doi: 10.1038/35051623. [DOI] [PubMed] [Google Scholar]
- 148.Ingavale SS, Van Wamel W, Cheung AL. Mol. Microbiol. 2003;48:1451. doi: 10.1046/j.1365-2958.2003.03503.x. [DOI] [PubMed] [Google Scholar]
- 149.Luong TT, Newell SW, Lee CY. J. Bacteriol. 2003;185:3703. doi: 10.1128/JB.185.13.3703-3710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL. Infect. Immun. 2005;73:1423. doi: 10.1128/IAI.73.3.1423-1431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY. J. Bacteriol. 2006;188:1899. doi: 10.1128/JB.188.5.1899-1910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gertz S, Engelmann S, Schmid R, Ziebandt AK, Tischer K, Scharf C, Hacker J, Hecker M. J. Bacteriol. 2000;182:6983. doi: 10.1128/jb.182.24.6983-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nicholas RO, Li T, McDevitt D, Marra A, Sucoloski S, Demarsh PL, Gentry DR. Infect. Immun. 1999;67:3667. doi: 10.1128/iai.67.7.3667-3669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kullik I, Giachino P, Fuchs T. J. Bacteriol. 1998;180:4814. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan PF, Foster SJ, Ingham E, Clements MO. J. Bacteriol. 1998;180:6082. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bachi B, Projan S. J. Bacteriol. 2004;186:4085. doi: 10.1128/JB.186.13.4085-4099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. J. Bacteriol. 2002;184:5457. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. Infect. Immun. 2009;77:1623. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shaw LN, Aish J, Davenport JE, Brown MC, Lithgow JK, Simmonite K, Crossley H, Travis J, Potempa J, Foster SJ. J. Bacteriol. 2006;188:6070. doi: 10.1128/JB.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Somerville GA, Proctor RA. Microbiol. Mol. Biol. Rev. 2009;73:233. doi: 10.1128/MMBR.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, Andrew PW, Roberts IS. Mol. Microbiol. 2007;63:1453. doi: 10.1111/j.1365-2958.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 162.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Mol. Microbiol. 2007;66:206. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 163.Shivers RP, Sonenshein AL. Mol. Microbiol. 2004;53:599. doi: 10.1111/j.1365-2958.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 164.Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL. J. Bacteriol. 2008;190:2257. doi: 10.1128/JB.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, Goerke C, Schrenzel J, Wolz C. J. Bacteriol. 2009;191:2953. doi: 10.1128/JB.01492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. J. Biol. Chem. 2005;280:34332. doi: 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
- 167.Belitsky BR, Sonenshein AL. J. Bacteriol. 2008;190:1224. doi: 10.1128/JB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL. J. Bacteriol. 2010;192:2861. doi: 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tamber S, Reyes D, Donegan NP, Schwartzman JD, Cheung AL, Memmi G. Infect. Immun. 2010;78:4384. doi: 10.1128/IAI.00401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yarwood JM, McCormick JK, Schlievert PM. J. Bacteriol. 2001;183:1113. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. J. Bacteriol. 2004;186:2430. doi: 10.1128/JB.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pragman AA, Ji Y, Schlievert PM. Biochemistry. 2007;46:314. doi: 10.1021/bi0603266. [DOI] [PubMed] [Google Scholar]
- 173.Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji Y. J. Bacteriol. 2005;187:5486. doi: 10.1128/JB.187.15.5486-5492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Manna AC, Cheung AL. Infect. Immun. 2003;71:343. doi: 10.1128/IAI.71.1.343-353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tamber S, Cheung AL. Infect. Immun. 2009;77:419. doi: 10.1128/IAI.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schmidt KA, Manna AC, Gill S, Cheung AL. Infect. Immun. 2001;69:4749. doi: 10.1128/IAI.69.8.4749-4758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manna AC, Cheung AL. J. Bacteriol. 2006;188:4288. doi: 10.1128/JB.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Davey ME, O'Toole G,A. Microbiol. Mol. Biol. Rev. 2000;64:847. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.del Pozo JL, Patel R. Clin. Pharmacol. Ther. 2007;82:204. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 180.Davies D. Nat. Rev. Drug Discov. 2003;2:114. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 181.Hall-Stoodley L, Costerton JW, Stoodley P. Nat. Rev. Microbiol. 2004;2:95. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 182.Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 183.Parsek MR, Singh PK. Ann. Rev. Microbiol. 2003;57:677. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 184.Frank KL, Del Pozo JL, Patel R. Clin. Microbiol. Rev. 2008;21:111. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Costerton JW. Clin. Orthop. Relat. Res. 2005:7. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 186.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. FEMS Immunol. Med. Microbiol. 2008;52:13. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 187.Rogers KL, Fey PD, Rupp ME. Infect. Dis. Clin. North Am. 2009;23:73. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 188.Otto M. Nat. Rev. Microbiol. 2009;7:555. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Horswill AR, Stoodley P, Stewart PS, Parsek MR. Anal. Bioanal. Chem. 2007;387:371. doi: 10.1007/s00216-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yarwood JM, Schlievert PM. J. Clin. Invest. 2003;112:1620. doi: 10.1172/JCI20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kjelleberg S, Molin S. Curr. Opin. Microbiol. 2002;5:254. doi: 10.1016/s1369-5274(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 192.Parsek MR, Greenberg EP. Trends Microbiol. 2005;13:27. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 193.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. Science. 1998;280:295. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 194.Vuong C, Saenz HL, Gotz F, Otto M. J. Infect. Dis. 2000;182:1688. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 195.Beenken KE, Blevins JS, Smeltzer MS. Infect. Immun. 2003;71:4206. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park J, Jagasia R, Kaufmann GF, Mathison JC, Ruiz DI, Moss JA, Meijler MM, Ulevitch RJ, Janda KD. Chem. Biol. 2007;14:1119. doi: 10.1016/j.chembiol.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Otto M. FEMS Microbiol. Lett. 2004;241:135. doi: 10.1016/j.femsle.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 198.Beenken KE, Mrak LN, Griffin LM, Zielinska AK, Shaw LN, Rice KC, Horswill AR, Bayles KW, Smeltzer MS. PLoS ONE. 2010;5:e10790. doi: 10.1371/journal.pone.0010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shenkman B, Varon D, Tamarin I, Dardik R, Peisachov M, Savion N, Rubinstein E. J. Med. Microbiol. 2002;51:747. doi: 10.1099/0022-1317-51-9-747. [DOI] [PubMed] [Google Scholar]
- 200.Pratten J, Foster SJ, Chan PF, Wilson M, Nair SP. Microbes Infect. 2001;3:633. doi: 10.1016/s1286-4579(01)01418-6. [DOI] [PubMed] [Google Scholar]
- 201.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. J. Bacteriol. 2004;186:1838. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Malone CL, Boles BR, Horswill AR. Appl. Environ. Microbiol. 2007;73:6036. doi: 10.1128/AEM.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. J. Orthop. Res. 2010;28:55. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 204.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. Infect. Immun. 1999;67:5427. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.O'Gara JP. FEMS Microbiol. Lett. 2007;270:179. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 206.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O'Gara JP. J. Clin. Microbiol. 2007;45:1379. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rohde H, Burandt EC, Siemssen N, Frommelt L, Burdelski C, Wurster S, Scherpe S, Davies AP, Harris LG, Horstkotte MA, Knobloch JK, Ragunath C, Kaplan JB, Mack D. Biomaterials. 2007;28:1711. doi: 10.1016/j.biomaterials.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 208.O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O'Gara JP. J. Bacteriol. 2008;190:3835. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Marti M, Trotonda MP, Tormo-Mas MA, Vergara-Irigaray M, Cheung AL, Lasa I, Penades JR. Microbes Infect. 2010;12:55. doi: 10.1016/j.micinf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 210.Boles BR, Thoendel M, Roth AJ, Horswill AR. PLoS ONE. 2010;5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tsang LH, Cassat JE, Shaw LN, Beenken KE, Smeltzer MS. PLoS ONE. 2008;3:e3361. doi: 10.1371/journal.pone.0003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McGavin MJ, Zahradka C, Rice K, Scott JE. Infect. Immun. 1997;65:2621. doi: 10.1128/iai.65.7.2621-2628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boles BR, Thoendel M, Singh PK. Mol. Microbiol. 2005;57:1210. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- 214.Irie Y, O'Toole G,A, Yuk MH. FEMS Microbiol. Lett. 2005;250:237. doi: 10.1016/j.femsle.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 215.Davey ME, Caiazza NC, O'Toole GA. J. Bacteriol. 2003;185:1027. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kong KF, Vuong C, Otto M. Int. J. Med. Microbiol. 2006;296:133. doi: 10.1016/j.ijmm.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 217.Gordon RJ, Lowy FD. Clin. Infect. Dis. 2008;46(Suppl 5):S350. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. N. Engl. J. Med. 2006;355:666. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 219.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, Ray SM, Blumberg HM. Clin. Infect. Dis. 2006;42:647. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 220.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. J. Infect. Dis. 2008;198:561. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 221.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5883. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nauseef WM. Immunol. Rev. 2007;219:88. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 223.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. Nat. Med. 2007;13:1510. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 224.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, Whitney AR, Sturdevant DE, Dorward DW, Holland SM, Kreiswirth BN, Musser JM, DeLeo FR. J. Innate. Immun. 2010;2:560. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J. PLoS ONE. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hersh AL, Chambers HF, Maselli JH, Gonzales R. Arch. Intern. Med. 2008;168:1585. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 227.Daum RS. N. Engl. J. Med. 2007;357:380. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 228.Stryjewski ME, Chambers HF. Clin. Infect. Dis. 2008;46(Suppl 5):S368. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 229.Schwan WR, Langhorne MH, Ritchie HD, Stover CK. FEMS Immunol. Med. Microbiol. 2003;38:23. doi: 10.1016/S0928-8244(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 230.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Chest. 2005;128:3854. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 231.Heyer G, Saba S, Adamo R, Rush W, Soong G, Cheung A, Prince A. Infect. Immun. 2002;70:127. doi: 10.1128/IAI.70.1.127-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bubeck Wardenburg J, Patel RJ, Schneewind O. Infect. Immun. 2007;75:1040. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Nat. Med. 2007;13:1405. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 234.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5587. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Renders N, Verbrugh H, Van Belkum A. Infect. Genet. Evol. 2001;1:29. doi: 10.1016/s1567-1348(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 236.Goerke C, Campana S, Bayer MG, Doring G, Botzenhart K, Wolz C. Infect. Immun. 2000;68:1304. doi: 10.1128/iai.68.3.1304-1311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. Nat. Rev. Microbiol. 2006;4:295. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 238.Kahl BC, Belling G, Becker P, Chatterjee I, Wardecki K, Hilgert K, Cheung AL, Peters G, Herrmann M. Infect. Immun. 2005;73:4119. doi: 10.1128/IAI.73.7.4119-4126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fowler VG, Jr., Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. JAMA, J. Am. Med. Assoc. 2005;293:3012. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 240.Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS. J. Clin. Invest. 1994;94:1815. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McNamara PJ, Bayer AS. Infect. Immun. 2005;73:3806. doi: 10.1128/IAI.73.6.3806-3809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Berendt T, Byren I. Clin. Med. 2004;4:510. doi: 10.7861/clinmedicine.4-6-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Johansson A, Flock JI, Svensson O. Clin. Orthop. Relat. Res. 2001:241. doi: 10.1097/00003086-200101000-00032. [DOI] [PubMed] [Google Scholar]
- 244.Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. Microb. Pathog. 1999;26:317. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 245.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. Infect. Immun. 1995;63:3373. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Blevins JS, Elasri MO, Allmendinger SD, Beenken KE, Skinner RA, Thomas JR, Smeltzer MS. Infect. Immun. 2003;71:516. doi: 10.1128/IAI.71.1.516-523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F. Infect. Immun. 2002;70:631. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tenover FC, McAllister S, Fosheim G, McDougal LK, Carey RB, Limbago B, Lonsway D, Patel JB, Kuehnert MJ, Gorwitz R. J. Clin. Microbiol. 2008;46:2837. doi: 10.1128/JCM.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Holtfreter S, Grumann D, Schmudde M, Nguyen HT, Eichler P, Strommenger B, Kopron K, Kolata J, Giedrys-Kalemba S, Steinmetz I, Witte W, Broker BM. J. Clin. Microbiol. 2007;45:2669. doi: 10.1128/JCM.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Holtfreter S, Roschack K, Eichler P, Eske K, Holtfreter B, Kohler C, Engelmann S, Hecker M, Greinacher A, Broker BM. J. Infect. Dis. 2006;193:1275. doi: 10.1086/503048. [DOI] [PubMed] [Google Scholar]
- 251.Limbago B, Fosheim GE, Schoonover V, Crane CE, Nadle J, Petit S, Heltzel D, Ray SM, Harrison LH, Lynfield R, Dumyati G, Townes JM, Schaffner W, Mu Y, Fridkin SK. J. Clin. Microbiol. 2009;47:1344. doi: 10.1128/JCM.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. PLoS ONE. 2007;2:e1120. doi: 10.1371/journal.pone.0001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Melchior MB, van Osch MH, Graat RM, van Duijkeren E, Mevius DJ, Nielen M, Gaastra W, Fink-Gremmels J. Vet. Microbiol. 2009;137:83. doi: 10.1016/j.vetmic.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 254.Cassat J, Dunman PM, Murphy E, Projan SJ, Beenken KE, Palm KJ, Yang SJ, Rice KC, Bayles KW, Smeltzer MS. Microbiology. 2006;152:3075. doi: 10.1099/mic.0.29033-0. [DOI] [PubMed] [Google Scholar]
- 255.Somerville GA, Beres SB, Fitzgerald JR, DeLeo FR, Cole RL, Hoff JS, Musser JM. J. Bacteriol. 2002;184:1430. doi: 10.1128/JB.184.5.1430-1437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gorske BC, Blackwell HE. Org. Biomol. Chem. 2006;4:1441. doi: 10.1039/b517681f. [DOI] [PubMed] [Google Scholar]
- 257.Nakayama J, Uemura Y, Nishiguchi K, Yoshimura N, Igarashi Y, Sonomoto K. Antimicrob. Agents Chemother. 2009;53:580. doi: 10.1128/AAC.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yarwood JM, McCormick JK, Paustian ML, Kapur V, Schlievert PM. J. Bacteriol. 2002;184:1095. doi: 10.1128/jb.184.4.1095-1101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Peterson MM, Mack JL, Hall PR, Alsup AA, Alexander SM, Sully EK, Sawires YS, Cheung AL, Otto M, Gresham HD. Cell Host Microbe. 2008;4:555. doi: 10.1016/j.chom.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Horswill AR, Nauseef WM. Cell Host Microbe. 2008;4:507. doi: 10.1016/j.chom.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 261.Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, Wang F, Brosnahan AJ, Mleziva JA, Peterson ML, Jones BE. Biochemistry. 2007;46:14349. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li JY, Harper JK, Grant DM, Tombe BO, Bashyal B, Hess WM, Strobel GA. Phytochemistry. 2001;56:463. doi: 10.1016/s0031-9422(00)00408-8. [DOI] [PubMed] [Google Scholar]
- 263.Vuong C, Otto M. Microbes Infect. 2002;4:481. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 264.Raad I, Alrahwan A, Rolston K. Clin. Infect. Dis. 1998;26:1182. doi: 10.1086/520285. [DOI] [PubMed] [Google Scholar]
- 265.Huebner J, Goldmann DA. Annu. Rev. Med. 1999;50:223. doi: 10.1146/annurev.med.50.1.223. [DOI] [PubMed] [Google Scholar]
- 266.Van Wamel WJ, van Rossum G, Verhoef J, Vandenbroucke-Grauls CM, Fluit AC. FEMS Microbiol. Lett. 1998;163:1. doi: 10.1111/j.1574-6968.1998.tb13018.x. [DOI] [PubMed] [Google Scholar]
- 267.Kies S, Vuong C, Hille M, Peschel A, Meyer C, Gotz F, Otto M. Peptides. 2003;24:329. doi: 10.1016/s0196-9781(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 268.Batzilla CF, Rachid S, Engelmann S, Hecker M, Hacker J, Ziebuhr W. Proteomics. 2006;6:3602. doi: 10.1002/pmic.200500732. [DOI] [PubMed] [Google Scholar]
- 269.Yao Y, Vuong C, Kocianova S, Villaruz AE, Lai Y, Sturdevant DE, Otto M. J. Infect. Dis. 2006;193:841. doi: 10.1086/500246. [DOI] [PubMed] [Google Scholar]
- 270.Vuong C, Gotz F, Otto M. Infect. Immun. 2000;68:1048. doi: 10.1128/iai.68.3.1048-1053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Otto M, Sussmuth R, Vuong C, Jung G, Gotz F. FEBS Lett. 1999;450:257. doi: 10.1016/s0014-5793(99)00514-1. [DOI] [PubMed] [Google Scholar]
- 272.Carmody AB, Otto M. Arch. Microbiol. 2004;181:250. doi: 10.1007/s00203-003-0644-2. [DOI] [PubMed] [Google Scholar]
- 273.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. J. Bacteriol. 2005;187:2426. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. J. Infect. Dis. 2003;188:706. doi: 10.1086/377239. [DOI] [PubMed] [Google Scholar]
- 275.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. J. Infect. Dis. 2004;190:1498. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 276.Vuong C, Durr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. Cell Microbiol. 2004;6:753. doi: 10.1111/j.1462-5822.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 277.Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F. FEMS Microbiol. Lett. 1997;151:139. doi: 10.1111/j.1574-6968.1997.tb12562.x. [DOI] [PubMed] [Google Scholar]
- 278.Fleurette J, Bes M, Brun Y, Freney J, Forey F, Coulet M, Reverdy ME, Etienne J. Res. Microbiol. 1989;140:107. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 279.Kleiner E, Monk AB, Archer GL, Forbes BA. Clin. Infect. Dis. 2010;51:801. doi: 10.1086/656280. [DOI] [PubMed] [Google Scholar]
- 280.Bocher S, Tonning B, Skov RL, Prag J. J. Clin. Microbiol. 2009;47:946. doi: 10.1128/JCM.01024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vandenesch F, Projan SJ, Kreiswirth B, Etienne J, Novick RP. FEMS Microbiol. Lett. 1993;111:115. doi: 10.1111/j.1574-6968.1993.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 282.Vandenesch F, Storrs MJ, Poitevin-Later F, Etienne J, Courvalin P, Fleurette J. FEMS Microbiol. Lett. 1991;62:65. doi: 10.1016/0378-1097(91)90256-a. [DOI] [PubMed] [Google Scholar]
- 283.Donvito B, Etienne J, Denoroy L, Greenland T, Benito Y, Vandenesch F. Infect. Immun. 1997;65:95. doi: 10.1128/iai.65.1.95-100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bannoehr J, Ben Zakour NL, Waller AS, Guardabassi L, Thoday KL, van den Broek AH, Fitzgerald JR. J. Bacteriol. 2007;189:8685. doi: 10.1128/JB.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sung JM, Chantler PD, Lloyd DH. Infect. Immun. 2006;74:2947. doi: 10.1128/IAI.74.5.2947-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, Brinker CJ. Nat. Chem. Biol. 2010;6:41. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shaw L, Golonka E, Potempa J, Foster SJ. Microbiology. 2004;150:217. doi: 10.1099/mic.0.26634-0. [DOI] [PubMed] [Google Scholar]
- 288.Oscarsson J, Tegmark-Wisell K, Arvidson S. Int. J. Med. Microbiol. 2006;296:365. doi: 10.1016/j.ijmm.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 289.Reed SB, Wesson CA, Liou LE, Trumble WR, Schlievert PM, Bohach GA, Bayles KW. Infect. Immun. 2001;69:1521. doi: 10.1128/IAI.69.3.1521-1527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Daugherty S, Low MG. Infect. Immun. 1993;61:5078. doi: 10.1128/iai.61.12.5078-5089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chamberlain NR, Imanoel B. J. Med. Microbiol. 1996;44:125. doi: 10.1099/00222615-44-2-125. [DOI] [PubMed] [Google Scholar]
- 292.Bronner S, Stoessel P, Gravet A, Monteil H, Prevost G. Appl. Environ. Microbiol. 2000;66:3931. doi: 10.1128/aem.66.9.3931-3938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gaskill ME, Khan SA. J. Biol. Chem. 1988;263:6276. [PubMed] [Google Scholar]
- 294.Regassa LB, Couch JL, Betley MJ. Infect. Immun. 1991;59:955. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bayles KW, Iandolo JJ. J. Bacteriol. 1989;171:4799. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.O'Toole PW, Foster TJ. Microb. Pathog. 1986;1:583. doi: 10.1016/0882-4010(86)90043-4. [DOI] [PubMed] [Google Scholar]
- 297.Sheehan BJ, Foster TJ, Dorman CJ, Park S, Stewart GS. Mol. Gen. Genet. 1992;232:49. doi: 10.1007/BF00299136. [DOI] [PubMed] [Google Scholar]
- 298.Dassy B, Hogan T, Foster TJ, Fournier JM. J. Gen. Microbiol. 1993;139(Pt 6):1301. doi: 10.1099/00221287-139-6-1301. [DOI] [PubMed] [Google Scholar]
- 299.Luong T, Sau S, Gomez M, Lee JC, Lee CY. Infect. Immun. 2002;70:444. doi: 10.1128/IAI.70.2.444-450.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pantrangi M, Singh VK, Wolz C, Shukla SK. FEMS Microbiol. Lett. 2010 doi: 10.1111/j.1574-6968.2010.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Saravia-Otten P, Muller HP, Arvidson S. J. Bacteriol. 1997;179:5259. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lebeau C, Vandenesch F, Greenland T, Novick RP, Etienne J. J. Bacteriol. 1994;176:5534. doi: 10.1128/jb.176.17.5534-5536.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. J. Infect. Dis. 2009;200:724. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ziebandt AK, Becher D, Ohlsen K, Hacker J, Hecker M, Engelmann S. Proteomics. 2004;4:3034. doi: 10.1002/pmic.200400937. [DOI] [PubMed] [Google Scholar]
- 305.Ballal A, Ray B, Manna AC. J. Bacteriol. 2009;191:1656. doi: 10.1128/JB.01555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kaito C, Morishita D, Matsumoto Y, Kurokawa K, Sekimizu K. Mol Microbiol. 2006;62:1601. doi: 10.1111/j.1365-2958.2006.05480.x. [DOI] [PubMed] [Google Scholar]
- 307.Bischoff M, Entenza JM, Giachino P. J. Bacteriol. 2001;183:5171. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Thompson JD, Higgins DG, Gibson TJ. Nucleic Acids Res. 1994;22:4673. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bernsel A, Viklund H, Hennerdal A, Elofsson A. Nucleic Acids Res. 2009;37:W465. doi: 10.1093/nar/gkp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kelley LA, Sternberg MJE. Nat. Protocols. 2009;4:363. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 311.Guex N, Peitsch MC. Electrophoresis. 1997;18:2714. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 312.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. Nucleic Acids Res. 2000;28:235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lo A, Chiu Y-Y, Roland EA, Lyu P-C, Sung T-Y, Hsu W-L. Bioinformatics. 2009;25:996. doi: 10.1093/bioinformatics/btp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.