Abstract
Primary auditory afferents are usually perceived as passive, timing-preserving, lines of communication. Contrasting this view, a special class of auditory afferents to teleost Mauthner cells, a command neuron that organizes tail-flip escape responses, undergoes potentiation of their mixed (electrical and chemical) synapses in response to high frequency cellular activity. This property is likely to represent a mechanism of input sensitization as these neurons provide the Mauthner cell with essential information for the initiation of an escape response. We review here the anatomical and physiological specializations of these identifiable auditory afferents. In particular, we discuss how their membrane and synaptic properties act in concert to more efficaciously activate the Mauthner cells. The striking functional specializations of these neurons suggest that primary auditory afferents might be capable of more sophisticated contributions to auditory processing than has been generally recognized.
Keywords: synaptic plasticity, LTP, gap junctions, persistent sodium current, electrical resonance, repetitive firing
1. Introduction
The operation of neural networks depends upon interactions among multiple nonlinear processes at many levels (Getting, 1989). Among these processes, specialized cellular and synaptic properties are generally recognized as major determinants of network operation (Getting, 1989). The auditory system is rich in cellular specializations, where morphological, biophysical and biochemical features converge to secure the preservation of acoustic timing information along the auditory pathway (Trussell, 1999). Such specializations include unconventional transduction mechanisms that provide outstanding temporal resolution through the absence of slow chemical processes (Hudspeth, 1997), the presence of particular potassium (K+) voltage-gated channels that impart brief membrane time constants and provide one-to-one spiking (Trusell, 1997), and synaptic mechanisms that guarantee the fast and safe transmission of auditory information through highly specialized contacts (Trusell, 1999; Glowatzki and Fuchs 2002; Trusell 2002).
In this context, primary auditory afferents are perceived as canonical passive lines of communication between peripheral receptors and second order sensory neurons located in the central nervous system that faithfully relay critical timing information for its processing along the auditory pathway. A special class of auditory afferents terminating as, single, “Large Myelinated Club Endings” on the goldfish Mauthner (M-) cells (Bartelmez, 1915), a pair of large reticulospinal neurons that mediate tail-flip escape responses in fish (Eaton et al. 2001; Korn and Faber, 2005), challenge this perception as their synapses undergo activity-dependent potentiation (Yang et al., 1990; for review see Pereda et al., 2004). Stimulation of these afferents with high frequency trains evokes a long-term potentiation of both components of their mixed, electrical (gap-junction mediated) and chemical, synaptic response (Yang et al., 1990). The plastic properties of these synapses likely represent a mechanism for input sensitization (Yang et al., 1990) and therefore an unusual specialization for a primary auditory afferent, which in this case provides a decision-making neuron (Eaton et al., 2001) with relevant sensory information that could be directly translated into a behavioral response essential for the survival of the fish.
Primary auditory afferents extend from their contacts with peripheral receptors to those with their target in the central nervous system. As a result of this unfavorable anatomical spread the membrane and synaptic properties of these neurons have been generally investigated in-vitro at either their central (Zhang and Trussell 1994) or peripheral ends (Santos-Sacchi 1993; Davis, 1996; Glowatzki and Fuchs 2002). In contrast, because of their advantageous experimental in vivo accessibility (where anatomical integrity and synaptic connectivity are preserved) and critical role in the initiation of the escape response, identifiable auditory afferents terminating as Large Myelinated Club endings on the M-cells provide an ideal opportunity to link cellular biophysical analysis with system-level analysis of information processing.
Here we review the anatomical and physiological specializations of these notable auditory afferents. More specifically, we discuss how intrinsic membrane and synaptic properties act in concert to more efficaciously activate the M-cells. The striking functional properties of these neurons suggest that primary auditory afferents might be capable, at least in lower vertebrates, of more sophisticated contributions to auditory processing than has been generally recognized.
2. The Mauthner cell system and the Club ending afferents
2.1. The Mauthner cell system
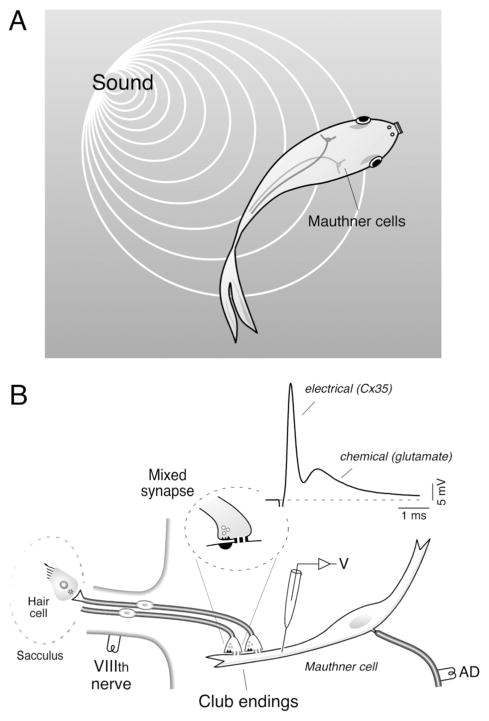
Fish can elude predatory attacks by producing a stereotyped escape behavior, which is characterized by a rapid and powerful unilateral bending of the body and tail that involves most of its somatic musculature (for review see Korn and Faber, 2005). This behavior has a characteristic short latency when triggered by abrupt acoustic stimuli, and it is initiated by the activation of the M-cell (Fig. 1A). The M-cells are a pair of reticulospinal neurons located in the medulla of teleost fish (Beccari, 1907). These uncommonly large cells are anatomically and physiologically identifiable and have historically constituted a valuable preparation for the study of the cellular correlates of behavior (Faber et al., 1989; Korn and Faber, 2005). Their characteristic large myelinated axons, first noticed by Mauthner (Mauthner, 1859), cross the midline to descend the length of the spinal cord, issuing axon collaterals that massively activate cranial and spinal motor systems via reliable (with high safety factor) chemical synapses (Faber et al., 1989). Such an anatomical arrangement allows a single action potential in this cell to initiate an escape response by producing a tail flip.
Figure 1. The Mauthner cell system and the Club ending afferents.
A, The M-cells mediate sound-evoked tail-flip escapes responses in teleost fish. B, Large identifiable auditory afferents innervate the rostral portion of the saccular macula (Sacculus), the main auditory component of the goldfish ear, and terminate as mixed (electrical and chemical) synapses known as Large Myelinated Club endings (“Club endings”) on the lateral dendrite of the M-cell. These terminals form mixed, electrical and chemical, synapses (Mixed synapse) with the distal portion of the M-cell lateral dendrite. Experimental arrangement used to obtain in vivo dendritic intracellular recordings of the M-cell. Stimulation of the VIIIth nerve, where Club endings run, elicits a mixed excitatory postsynaptic potential (EPSP) composed of an early, fast electrical component (electrical) which is followed by a delayed, longer lasting glutamatergic component (chemical).
Because of its dramatic behavioral consequences, this response has a characteristic high-threshold that is supported, amongst other factors, by the cellular properties of the M-cells. Together with a strong inhibitory control (Faber and Korn, 1978), the low input resistance, short time constant and hyperpolarized membrane potential (about −83 mV) of the M-cell prevents the spontaneous occurrence of this behavior as a consequence of weak, environmental, noise (Faber and Korn, 1978). Also adapted to their function, M-cells are unable to fire repetitively, an otherwise inconvenient feature for a cell in which a single action potential initiates an escape response that lasts several hundreds of milliseconds (Eaton et al., 1988; Korn and Faber 2005). This property was proposed to rely on the presence of K+ channels of the Kv1 family (Nakayama and Oda 2004), known to antagonize repetitive responses (Rathouz and Trusell 1998; Trussell 1999).
2.2. Auditory afferents terminating as Large Myelinated Club endings
Because of their large size, characteristic myelinization and dendritic localization, the Large Myelinated Club endings (Club endings) are the most recognizable synaptic input to the M-cells. First described by Bartelmez in 1915 in the catfish (Fig. 1A), and henceforth referred to as “Club ending afferents”, this population of about 100 large afferents 5–15 μm in diameter, originate in the rostral portion of the saccular macula (the main auditory component of fish ear; Popper and Fay, 1998) and run in the posterior branch of the VIIIth nerve of teleost fish. These afferents have bipolar cell bodies ellipsoid in shape covered by a myelin sheath (Rosenbluth and Palay, 1961; Sento and Furukawa, 1987). Such anatomical characteristics, shared with other fibers of escape networks across species (Bullock, 1977), support high-speed impulse conduction and suggest they provide the M-cells with critical auditory information for the initiation of the escape response.
Because of their large size and characteristic physiological properties Club ending afferents can be easily recognized during anatomical and physiological studies. The afferents can be recorded in vivo in the posterior root of the VIIIth nerve and unambiguously identified by the presence of the electrotonic coupling potential due to the passive dendritic depolarization produced by the antidromically-evoked M-cell action potential (Furshpan, 1964), their characteristic lack of spontaneous activity and high threshold for acoustic stimulation. Several studies showed that intracellular labeling of fibers exhibiting these properties invariably resulted in fibers that because of their size, prominent myelinization, dendritic distribution and saccular origin unambiguously corresponded to Club endings afferents (Lin and Faber 1988a; Smith and Pereda 2003).
While the study of these neurons mainly focused on the structure and physiological properties of their easily recognizable synaptic contacts (see section 3), less is known regarding the physiological properties of the afferent fibers themselves. The anatomical characteristics of the Club ending afferents (large diameter and characteristic myelinization) match those of saccular fibers originated from the rostral part of the sacculus, and designated as S1, according to the initial characterization of goldfish auditory afferents by Furukawa and Ishii (Furukawa and Ishii, 1967). In contrast with S2, a smaller type of fiber, S1 afferents were shown to respond to higher frequencies (>500 Hz; Furukawa and Ishii, 1967). More rigorous characterization of goldfish afferent responses confirmed the presence of both high frequency and low frequency afferent types (Fay 1978, 1995). Although in these studies physiological responses were not correlated with anatomical identification of the afferent fibers, both low and high frequency tuning were observed in afferents lacking spontaneous activity (Fay, 1978), a notable characteristic and identification criteria for Club ending afferents (Curti et al., 2008). Thus, it is likely that these larger afferents can respond to a broader range of frequencies. Anyway, the individual frequency selectivity of these afferent neurons is likely to be irrelevant for stimuli that initiate an escape response, as most saccular fibers will respond to any frequency within the goldfish’s effective range of hearing at sound levels 40 dB or more above best threshold (Fay, 1995; see below). Accordingly, strong acoustic stimuli are necessary for triggering an escape response in the M-cell system, which has a characteristic high threshold (Fay 1995).
3. Synaptic specializations
Although a Club ending afferent issues a few substantially thinner branches, likely targeting other neurons in the teleost brainstem, the primary axon characteristically terminates as a large single terminal (8–15 μm; about the same diameter of the axons) on the lateral dendrite of the M-cell (Fig. 1B). These unusually large contacts are easily identifiable under the light microscope and were the object of early investigations attempting to understand the nature of neuronal interconnectivity. Specifically, early studies of these terminals by the anatomical school of Chicago were designed to investigate the presence or absence of cytoplasmatic continuity between pre and postsynaptic neurons (Bartelmez, 1915), an issue that was finally settled by Bodian in his classic 1937 paper “The structure of the vertebrate synapse.” (Bodian, 1937; see Pereda et al., 2004 for review).
Together with its large diameter and heavy myelinization, the existence of a single terminal constitutes by itself a functional specialization. The lack of terminal branching provides safe impulse conduction, eliminating the possibility of branch point failures and of slower conduction through thinner processes, thus guaranteeing faster transmission and higher temporal fidelity. Consistent with these anatomical features the dendritic arborization of these afferents at the saccular macula is also significantly simpler and with thicker processes than those of other afferent types (S2 fibers; Sento and Furukawa, 1987). Thus, the relatively simpler anatomical features of Club ending afferents are likely to represent a functional specialization of these neurons, which seem advantageous to their vital behavioral role.
A wealth of anatomical and electrophysiological data shows that Club endings support both chemical and electrical modalities of transmission (Lin and Faber, 1988a; for review see Pereda et al., 2004). In fact, these contacts provided one of the first demonstrations of gap junction plaques (Robertson, 1963) and electrical transmission (Furshpan, 1964) in the vertebrate central nervous system. Detailed electron microscopic studies, including freeze-fracture techniques (Tuttle et al., 1986), have shown that while the specializations corresponding to chemical transmission lie in the periphery, gap junction plaques are located on most of the surface area of the terminal. The plaques range in number from 63 to 243 gap junctions, and the total area they occupy about 20 % of the synaptic area (Tuttle et al., 1986). These gap junctions are formed by connexin 35 (Pereda et al., 2003) the fish ortholog of the widely expressed mammalian connexin 36, that is responsible for electrical coupling at neocortical inhibitory interneurons and inferior olivary cells among many cell types (Connors and Long, 2004). From the electrophysiological point of view, a presynaptic impulse generates a mixed excitatory response. That is, stimulation of the posterior branch of the VIIIth nerve, where these fibers runs, evokes an electrical potential followed by a chemically mediated excitatory postsynaptic potential (Fig. 1B). Due to the fast time constant of the M-cell (~400 μs) both components can be easily distinguished (Fukami et al., 1965). Interestingly, it has been recently suggested that the dominant mode of transmission at Club ending synapses during natural stimulation is electrical (Szabo et al., 2006).
While electrical transmission is supported by the existence of an unusual number of gap junctions plaques, chemical transmission is mediated by the release of glutamate from ~15 release sites (Lin and Faber, 1988b), which are distributed in the periphery of the terminals, where the access resistance is low. Both the pharmacological characteristics and voltage dependence of the synaptic currents are consistent with glutamate being the transmitter at these contacts that activates both non-NMDA and NMDA receptors (Wolszon et al., 1997). Even at its relatively hyperpolarized resting potential (~ −80mV), the excitatory synaptic responses of the Club endings on the M-cell are normally mediated by the activation of both NMDA and non-NMDA receptors (the contribution of NMDA receptors depends on the size of the synaptic potential; see Wolszon et al., 1997). Surprisingly, the NMDA component is almost as fast as the non-NMDA component (Wolszon et al., 1997). In contrast to most primary auditory afferent synapses, which are known to depress in response to multiple stimuli (Zhang and Trussell, 1994), glutamatergic synapses at Club endings characteristically exhibit frequency-dependent facilitation (Pereda and Faber, 1996; Wolszon et al., 1997), suggesting the existence of unusual synaptic specializations in these teleost afferents. While mixed synapses are often found in lower vertebrates, the combination of these two forms of transmission is particularly beneficial for the function of Club ending afferents. Electrical transmission provides speed and reliability of transmission and the presence of chemical transmission, with relatively longer duration, allows temporal summation during repetitive responses in a cell in which the membrane time constant is unusually brief (Fig. 2B).
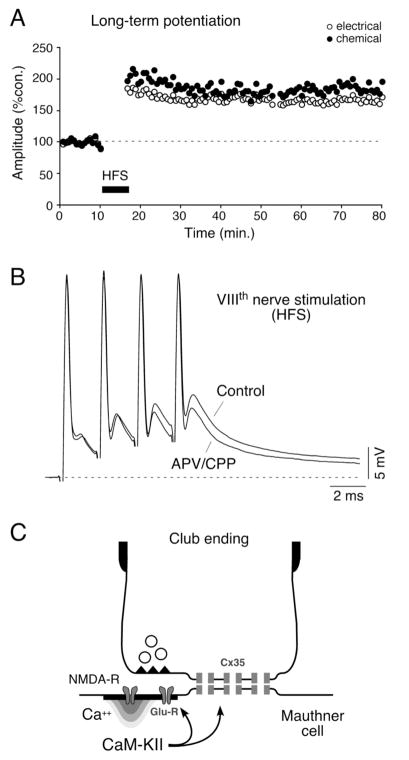
Figure 2. Activity-dependent modulation of synaptic transmission in Club endings.
A, Discontinuous high frequency stimulation (HFS; trains of 6 pulses at 500 Hz, every 2 seconds for 4 minutes) of the VIIIth nerve evokes persistent potentiation of both components of the EPSP. Plot illustrates the amplitudes of the electrical (open circles) and chemical (filled circles) components versus time (each point represents the average of 20 traces) for one experiment. B, Induction of the short-term potentiations of the synaptic responses depends on NMDA receptor activation. Superimposed synaptic responses evoked by a train of four VIIIth nerve stimuli, in control and after superfusing with saline containing the antagonists (APV/CPP). Note that while the first chemical synaptic response produced by the train does not produce a significant NMDA receptor mediated response the late facilitated components do. C, Schematic representation of the proposed potentiating mechanism for Club endings. Influx of Ca2+ through NMDA receptors activates CaM-KII that phosphorylates either glutamate receptors and connexins or regulatory molecules. Modified from Pereda et al. (2004), with permission.
The advantage and contribution of combining electrical and chemical synapses are emphasized by the existence of important functional interactions between these two modalities of transmission. The most remarkable of these interactions is the induction by glutamatergic synapses of long-term activity-dependent changes in the efficacy of both chemical and electrical synaptic transmission. That is, both components of the mixed synaptic response undergo activity-dependent potentiation of their synaptic strength in response to high frequency stimulation of the VIIIth nerve. The induction of activity-dependent long-term potentiation (LTP; Fig. 2A) requires a strong high frequency stimulation of the VIIIth nerve (brief trains of 2 to 8 stimuli at 500 Hz applied once every two seconds), is blocked by postsynaptic injections of BAPTA and by superfusion of the medulla with NMDA receptor antagonists (Yang et al., 1990; Pereda and Faber, 1996), and is specific to the tetanized pathway. Physiological and pharmacological evidence showed that this discontinuous stimulating pattern is essential to evoke potentiation; continuous high frequency stimulation, which rapidly depresses the chemical component, does not cause potentiation (Pereda and Faber, 1996). Thus, bursting facilitates release of glutamate and allows temporal summation of the synaptic responses for the postsynaptic depolarization presumably necessary to relieve Mg+ block of the NMDA receptors (Fig. 2B), whose activation is necessary for potentiation (Yang et al., 1990; Pereda and Faber, 1996).
It has been shown that the activation of NMDA receptors leads to a localized increased in the intracellular concentration of calcium (Ca++), which in turns activates the kinase Ca++-calmodulin-dependent kinase II (CaM-KII) (Pereda et al., 1998), whose activity is necessary for the potentiations (Fig. 2C). Simultaneous pre and postsynaptic recordings at these single terminals, demonstrated that such functional interaction takes place in the same ending, within a few micrometers (Smith and Pereda, 2003). Accordingly, confocal and freeze fracture immunolabeling of these terminals showed that the NR1 subunit of the NMDA glutamate receptor, proposed to be the key regulatory element in this phenomenon, is present at PSDs closely associated with gap junction plaques containing Cx35 (Pereda et al, 2003).
These synaptic plastic properties are unusual for synapses of a primary auditory afferent and they are likely to represent a form of sensory-motor processing. They probably represent a cellular specialization of these contacts whose function is to provide a decision-making neuron with essential sensory information. As mentioned above, the ability to trigger these synaptic changes relies on stimulation with brief trains. Interestingly, the initial selection of this pattern was inspired in the natural bursting properties of these afferents (Yang et al., 1990), which characteristically respond with multiple action potentials to brief or low frequency acoustic stimulation (Furukawa and Ishii, 1967; see below). This suggests that the synaptic properties of Club endings are adapted to the firing characteristics of the afferents and the requirements of the M-cell system, where the increased synaptic gain of these auditory nerve synapses will sensitize a vital escape response, lowering its threshold to acoustic stimuli (for review see Korn and Faber, 2005).
4. Intrinsic membrane properties underlying repetitive firing in Club ending afferents
In addition to being phase-locked to higher frequencies, Club ending afferents characteristically respond with multiple action potentials to brief (Fig. 3A) or low frequency (Furukawa and Ishii, 1967) acoustic stimulation. Consistent with this property, depolarizing pulses typically evoke an initial high frequency burst of action potentials, which is immediately followed by a complete absence of activity (Fig. 3B). In contrast, depolarization of the M-cell axon with a current pulse of equal (typically 1.5 the cell’s threshold; Curti et al., 2008) or stronger magnitude invariably results in only a single action potential, a property that has been linked to the presence of K+ channels of the Kv1 type (Nakayama and Oda, 2004). Taken together, these findings indicate that: 1) Club ending afferents are endowed with electrophysiological properties that favor the generation of high frequency bursts in response to strong depolarizations, and 2) because the M-cells are not capable of repetitive firing, rather than relaying timing information, their ability to generate high frequency bursts must represent a functional specialization of these afferents with the goal of providing the M-cell with adequate patterns of synaptic activation for the initiation of an escape response.
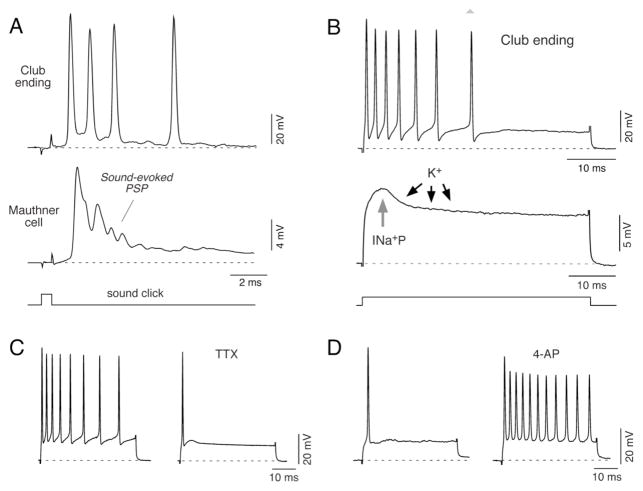
Figure 3. Intrinsic membrane properties underlying repetitive firing in Club ending afferents.
A, Electrophysiological responses obtained sequentially from the M-cell lateral dendrite (middle trace) and a Club ending (upper trace) in response to brief acoustic stimulation (500 μs duration sound click, lower trace). This stimulus evokes a high frequency burst of action potentials in the Club ending that temporally correlates with the sound-evoked synaptic potential (Sound-evoked PSP) recorded in the M-cell lateral dendrite, produced by the activity of this and an undetermined number of these afferents. B, Top: Direct intracellular activation of Club ending afferents with depolarizing current pulses (50 ms duration, lower trace) at 1.5 times its threshold (1.5 T) evokes a repetitive discharge consisting of a train of action potentials that exhibits marked frequency adaptation. Middle: Active mechanisms involved in near-threshold membrane responses. The figure shows a representative response of a Club ending to a depolarizing current pulse (represented by the lower trace; magnitude is different for upper and middle trace). At the beginning of the pulse the response is dominated by the activation of a persistent Na+ current (INa+P). The amplifying action of this Na+ current is counterbalanced by the delayed activation of an A-type K+ current (K+). C, Firing responses evoked by a depolarizing current pulse (3.3 nA, 50 ms duration) before (left) and after extracellular application of 1 μM TTX (right; TTX). The effects of TTX were observed within the time window in which the amplitude of the action potential of the afferents remained largely unaffected by the drug, suggesting that only persistent sodium channels were affected. D, Membrane responses to current pulses obtained before (left) and after extracellular application of 5mM 4-AP (right; 4-AP). The current pulse, which in control conditions was just sufficient to evoke a single action potential, was capable of inducing a vigorous repetitive discharge 5 minutes after 4-AP application. Modified from Curti et al. (2008), with permission.
Consistent with this view, detailed analysis showed that Club ending afferents show an intrinsic ability to respond with repetitive discharges of 200–600 Hz and exhibit a strong frequency adaptation (Fig. 3B). This property critically relies on the activation of a persistent sodium current (INa+P), which is counterbalanced by the delayed activation of an A-type potassium current (Curti et al., 2008). This interaction can also be observed at near-threshold membrane potentials (Fig. 3B), suggesting that the same active mechanisms are essential for determining both subthreshold and suprathreshold electrical behavior of these neurons. Intracellular injection of the derivative of local anesthetic QX-314 or extracellular application of tetrodotoxin (TTX), which block Na+ channels, abolished repetitive firing within a time window of 5 to 10 minutes in which the amplitude of action potentials were largely unaffected (Fig. 3C) (Curti et al., 2008). In contrast, when extracellular applications of 4-aminopyridine (4-AP) were used to reduce voltage-dependent K+ channels (Rudy, 1988; Storm, 1990; Jerng et al., 2004) the action of INa+P was unopposed resulting in prolonged repetitive discharges in response to suprathreshold pulses that in control conditions evoked only one spike (Fig. 3D). These observations indicate that the firing pattern of Club endings afferents critically relies on a balance between these two conductances (see Curti et al., 2008). Interestingly, unlike many neuronal types in the auditory system in which firing is controlled by low threshold K+ channels containing subunits of the Kv1 family (Mo et al., 2002, Rathouz and Trusell, 1998; Trussell 1999; Klug and Trussell, 2006), the insensitivity to α-DTX, sensitivity to 4-AP in the milimolar range and the time course of the recovery from inactivation all suggest the participation of subunits of the Kv4 family in this A-type current. Thus, the evidence suggest a lack of participation of Kv1 channels, a property that is likely to contribute to the ability of these afferents to fire repetitively (Curti et al., 2008).
The interaction of these two currents with the passive membrane properties of these afferents supports the presence electrical resonance, whose frequency preference is consistent with both the effective range of hearing in goldfish and the firing frequencies required for synaptic facilitation, an obligatory requisite for the induction of activity-dependent changes. Electrical resonance characterizes the frequency at which neurons respond best to depolarization and therefore describes its frequency-dependent properties, in particular how neurons process oscillatory inputs at subthreshold potentials and their propensity to fire within a frequency range (Hutcheon and Yarom, 2000). Accordingly, membrane oscillations were occasionally observed following the initial burst of action potentials evoked by depolarizing current pulses, suggesting that underlying oscillatory membrane mechanisms were responsible for repetitive firing at Club ending afferents (Fig. 4A). Subthreshold K+ conductances such as that found at Club ending afferents are responsible for the generation of electrical resonant behavior in various neuronal types (Hutcheon et al., 1996; Hutcheon and Yarom, 2000; Izhikevich, 2007), and they produce a characteristic “sag” in the subthreshold membrane response to depolarizing pulses (Fig. 4B).
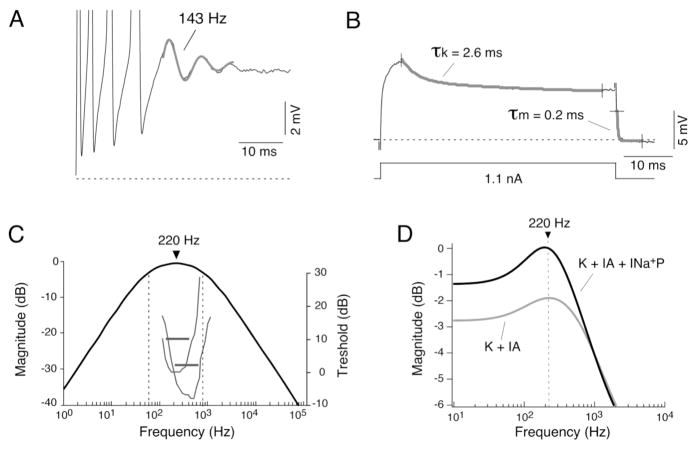
Figure 4. Club ending afferents exhibit resonant membrane properties.
A, Electrophysiological recordings revealed the presence of subthreshold membrane oscillations underlying repetitive responses. Trace illustrates the membrane response to a depolarizing current pulse, consisting of four action potentials followed by a damped oscillation of the membrane potential (trace represents the average of 5 single responses centered in the oscillation; action potentials are truncated). The frequency of this subthreshold oscillation was estimated fitting a function consisting in the sum of a sinusoidal and an exponential function (143 Hz, thicker trace). B, Indirect estimates of electrical resonance were obtained by calculating cutoff frequencies of the low-pass and high-pass filtering properties of the membrane from kinetics of subthreshold membrane responses. The time constant of a high-pass filter representing the activation of the A-type current (τk) was estimated by fitting a double-exponential function to the decaying portion of the near-threshold response about 5 ms after the pulse onset. Only the faster and most prominent time constant (30 fold the magnitude and 10 times faster than the second one), which likely represents the activation of the A-type current, is represented here and its value used for the estimates of electrical resonance. The time constant of a low-pass filter, representing passive membrane properties (τm), was accurately estimated by fitting a single exponential function to the decay that followed the cessation of current pulses of different polarities and amplitudes (only one of these pulses is illustrated in this example and indicated as τm). This estimate is influenced by both the passive membrane properties and the spread of the injected current along the axon, and represents the effective time constant of the afferent fiber. C, Bode plot (magnitude versus frequency) constructed using a linear model that combines both high- and low-pass filter properties of the membrane. The combination of membrane mechanisms with high-pass and low-pass filtering properties determines a band-pass filter with a bandwidth of 742 Hz and a maximum at 220 Hz. For comparison, the tuning curves of two representative saccular afferents are also illustrated in the same graph (gray traces; curves originally illustrated in Fay, 1995). Threshold in dB (right side ordinates) is plotted against sound frequency. Note that the characteristic frequencies and Q10dB responses (horizontal lines) of both tuning curves (corresponding to the responses of the two types of afferents found in goldfish) matched the estimated bandwidth of the electrical resonance. D, Computer simulations with NEURON revealed the relative contributions of A-type (IA) and persistent Na+ (INa+P) currents to membrane resonance. Based on available anatomical data, an ideal Club ending afferent fiber was modeled as a section consisting of a cylindrical process with passive properties (see Curti et al., 2008). Plot illustrates the computed input impedance (ordinates, normalized to the magnitude of the steady-state membrane response in the absence of any active mechanism) versus frequency (abscissa). When a delayed rectifier and an A-type current with the kinetics estimated experimentally for the ventral cochlear nucleus (Rothman and Manis, 2003) were added to the model, a clear resonant behavior appeared centered at 220 Hz (K + IA, gray trace). Note that the addition of a persistent Na+ current produces a significant amplification of the membrane resonance (K + IA + INa+P, black trace), without modifying the resonant frequency. Modified from Curti et al. (2008), with permission.
In contrast to other neurons in which resonant properties were explored directly (this approach was prevented by the filtering properties of the electrodes in our in-vivo recording conditions; see Curti el al., 2008) the frequency preference of Club ending afferents was estimated by determining the underlying resonant mechanisms from near-threshold membrane responses. Electrical membrane resonance is known to result from the interaction of two mechanisms with specific frequency-dependent properties: a low-pass filter that is determined by the passive membrane properties (membrane time constant) that attenuates responses to inputs with high frequency content, and a high-pass filter, which is set by slowly activating voltage-dependent K+ currents that opposes membrane depolarization and thereby attenuates voltage responses to inputs with low frequency content (“resonant currents”; Hutcheon and Yarom, 2000; Izhikevich, 2007). The approximate resonant properties can then be estimated if the values of the activation time constant of the “resonant K+ current” and the membrane time constant are determined experimentally. The low pass filter (membrane time constant) was measured by fitting a single exponential function to the decay that followed the cessation of current pulses of different polarities and amplitudes whereas the high pass filter (representing the activation of the A-type current) was estimated from the decaying portion of the near-threshold membrane response (Fig. 4B). Combined (Fig. 4C), these values determined a band-pass filter with a bandwidth of 742 Hz and a peak value at 220 Hz, suggesting the existence of resonant properties at Club ending afferents (Curti et al., 2008).
Computer simulations using parameters of A-type currents described for auditory neurons (Rothman and Manis, 2003) that are similar to those estimated for these afferents (comparable values of kinetics of activation and recovery from inactivation and similar pharmacological profile [lack of sensitivity to DTX and sensitivity to 4-AP in the mM range]) showed a clear resonant behavior with a peak at 220 Hz (Fig. 4D) (Curti et al., 2008). This approach also allowed us to evaluate the role of the persistent Na+ current in this resonant behavior. As previously shown (Hutcheon and Yarom, 2000), the addition of an INa+P produces a strong amplification (~35%) of the predicted membrane resonance without modifying the resonant frequency, suggesting that this conductance is likely to play a relevant functional role by allowing the full expression of this resonance (Fig. 4D). Thus, despite the low stringency of this model (reported values of currents with similar kinetics were used given the impossibility of obtaining direct measurements), computer simulations adequately reproduced the frequency range of the predicted membrane resonance, suggesting that IA has robust resonant properties in these neurons and indicated an essential role for INa+P in amplifying these properties (Curti et al., 2008).
If the interaction between “resonant” and “amplifying” currents is strong, it can destabilize the membrane potential allowing the generation of spontaneous oscillatory pacemaker-like activity (Hutcheon and Yarom 2000). Most commonly, this interaction is weak so the frequency preference of a given cell is latent and oscillatory activity is only revealed in the presence of its inputs. Such “weaker” resonance makes a neuron a “good listener” within a specialized frequency band (Hutcheon and Yarom 2000). This second possibility seems to be the case of Club ending afferents, in which oscillatory activity was observed only in response to depolarization (Fig. 4A). Remarkably, the bandwidth of the estimated resonance matched the effective range of hearing in goldfish, estimated to be 100 to 1000 Hz (Fay, 1995). The tuning curves of two fibers, representative of the “high” and “low” frequency types of broadly-tuned afferents identified in goldfish (Fay, 1978, 1995), are superimposed on the predicted Bode plot in Fig. 4C (examples taken from Fay, 1995). Thus, resonant mechanisms seem to endow Club ending afferents with a special sensitivity to a range of behaviorally relevant frequencies and to translate them into specific patterns of activity.
5. Interactions between membrane and synaptic properties
The most important consequence of electrical resonance is that it endows these afferents with the propensity to respond with high frequency (200 to 600 Hz) bursts of action potentials to strong depolarizing inputs (Curti et al., 2008). Bursts, which are often generated by the interaction of synaptic inputs with intrinsic membrane properties, are thought to represent reliable neural codes during information processing (Lisman, 1997; Izhikevich et al., 2003; Krahe and Gabbiani, 2004), and they play special roles in the induction of synaptic plasticity (Lisman, 1997). The generation of bursts in response to abrupt strong acoustic stimuli seems a particularly advantageous strategy for the M-cell system, and it could constitute an efficient and desirable code of information. That is, a burst of action potentials in these afferents would generate, as opposed to a single action potential, a prolonged synaptic response that can efficiently depolarize the large and unusually low input resistance M-cell (Faber and Korn, 1978).
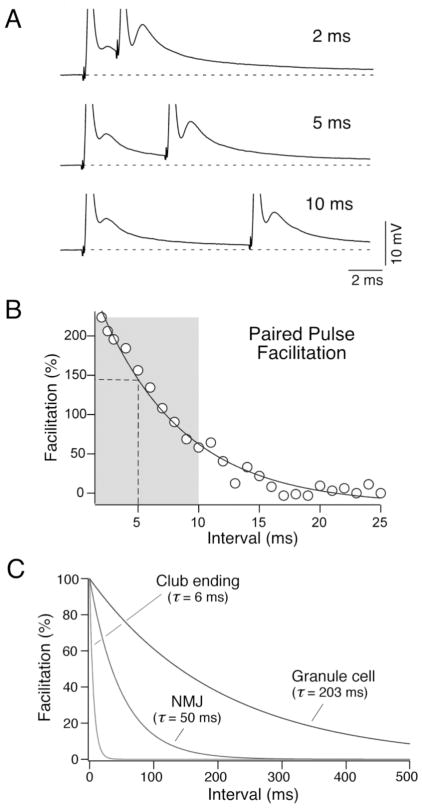
Interestingly, the optimal intervals required for the synaptic facilitation of the glutamatergic component of the mixed synaptic potential matched the band of frequency determined by the resonant properties of the membrane (Fig. 5). That is, this facilitation takes place within a narrow temporal window (Fig. 5B) and it does not extend beyond the frequency range determined by the firing properties of Club ending afferents, indicating that bursts of 200–600 Hz are optimal in producing facilitation of the chemical component of synaptic response (Curti et al., 2008). Further, the time course of the synaptic facilitation was extremely fast when compared with that of other well-characterized contacts (Fig. 5C), suggesting that firing characteristics of these auditory afferents are adapted to the requirements of synaptic transmission at Club endings (Curti et al., 2008).
FIGURE 5. Matching of membrane and synaptic properties.
A, Time course of the frequency-dependent facilitation of the chemical component with a two stimuli protocol (paired pulse facilitation). Stimuli were delivered at different intervals (2 ms, 5 ms and 10 ms are illustrated; the electrical components of the synaptic responses appear truncated). B, Facilitation of the chemical component, estimated as ((second EPSP amplitude − first EPSP amplitude)/first EPSP amplitude)*100, is plotted as a function of the paired pulse interval in a representative experiment. The data were fitted to a single exponential function (solid line) with a time constant in this case of 6.9 ms. Vertical dashed line and the gray rectangular area approximately indicate the peak value and bandwidth of the estimated electrical resonance, respectively. C, Time constants of paired pulse facilitation of chemical EPSP at Club endings, the neuromuscular junction (NMJ; Magleby 1987) and cerebellar granule to Purkinje cell synapse (granule cell; Atluri and Regehr 1996). Modified from Curti et al. (2008), with permission.
Because of the longer duration of the chemical component, high frequency bursts also allow temporal summation of the mixed synaptic responses (Fig. 2B, 5A), an otherwise unlikely possibility given the short duration of the electrical component and the brief membrane time constant of the M-cell. Thus, by promoting synaptic facilitation and allowing temporal summation of successive synaptic responses, high frequency bursts of action potentials are capable of leading to stronger and longer lasting depolarizations of the M-cell lateral dendrite. As previously mentioned, brief high frequency bursts of action potentials are also required for the induction of activity-dependent long-term potentiation of the mixed synaptic response (Yang et al., 1990), as they optimize the activation of NMDA receptors by providing enhanced glutamate release at more depolarized potentials, which is essential for the induction of the plastic changes (Pereda and Faber, 1996; Wolszon et al., 1997). Taken together our evidence suggests that the intrinsic membrane properties of Club ending afferents allow them to translate behaviorally relevant auditory signals into patterns of activity that seem to match the requirements of their fast and highly modifiable synapses.
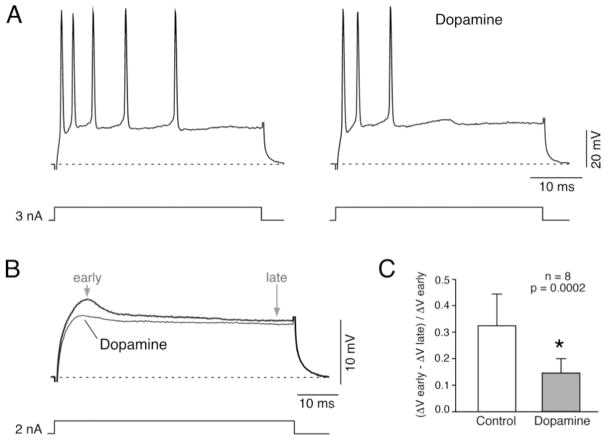
We showed that INa+P plays an essential role in shaping this afferent’s firing properties (Curti at al., 2008). These mixed synapses are surrounded by numerous dopaminergic fibers (Pereda et al., 1992) and one target of dopamine modulation via PKA-phosphorylation is Na+ channel function (Cantrell and Catterall, 2001). Indeed, our data shows that bath application of dopamine (100 μM) modifies the firing properties and near-threshold responses in a way that is consistent with a down-regulation of INa+P at these afferents (Fig. 6). Specifically, near-threshold responses to depolarizing current pulses were affected only in the early part of the response (Fig. 6B), which is dominated by INa+P (see Fig. 3B). In contrast, the late part of the voltage response, which is dominated by the activation of IA, remained largely unaffected (Fig. 6B). This effect was more clearly observed by comparing the ratio between the amplitudes of the early and late portions of the near-threshold responses, during control conditions and after Dopamine application (Fig. 6C). The effect of Dopamine on subthreshold responses was accompanied by a parallel reduction on the number of spikes and firing frequency during repetitive responses. Similar results were observed after bath application of forskolin (50 μM; not shown), which increases the intracellular concentration of cAMP. Although these results remain to be confirmed with more adequate experimental approaches, they suggest that modulation of INa+P could be a target for regulatory processes. Because of its impact on repetitive firing, regulation of INa+P could dramatically reduce dendritic depolarization and affect the induction of activity-dependent synaptic plasticity at Club ending mixed synapses (a potential metaplastic mechanism), endowing these auditory afferents with a dynamic mechanism for the processing of sensory information.
Figure 6. Effect of Dopamine on Club ending repetitive firing and near-threshold membrane responses.
A, Firing responses evoked by a depolarizing current pulse (3 nA, 50 ms duration) before (left) and after (right) extracellular application of 100 μM Dopamine. B, Near-threshold voltage response (2 nA, 50 ms duration) obtained before and after Dopamine application. The characteristic initial non-linear membrane response in the form of an apparent increase in the slope resistance, attributed to the activation of INa+P (Curti and Pereda, 2004; Curti et al, 2008) was substantially reduced by application of Dopamine, suggesting the down-regulation of INa+P by this modulator. C, This effect was quantified by comparing the ratio between early and late responses during control conditions and after Dopamine application. This ratio, estimated as (ΔV early − ΔV late)/ΔV early, averaged 0.33 ± 0.12 in control, and was significantly affected by dopamine application averaging 0.15 ± 0.05 (mean ± S.D.; p= 0.0002; n=8).
6. Electrical resonance as a mechanism of input synchronization
Club endings constitute a relatively homogenous population of ~100 afferents terminating on the lateral dendrite of the M-cell (Bartelmez 1915). These afferents were also shown to be relatively physiologically homogeneous, as all fibers exhibited similar biophysical properties (Curti et al., 2008). Mechanisms of frequency selectivity in lower vertebrates, including goldfish (Sugihara and Furukawa, 1989), are known to involve the contribution of resonant electrical membrane properties, which allow inner-ear hair cells to be tuned to stimuli of specific frequency content (Fettiplace and Fuchs, 1999). The reported tuning curves and characteristic frequencies of the two types of afferent response reported in goldfish (Fay 1978, 1995) fell within the band of electrical resonance estimated for Club ending afferents (Fig. 4C; Fay 1978, 1995), indicating that this mechanism is unlikely to underlie their individual frequency tuning.
Because resonant features seem to be uniformly distributed amongst Club ending afferents, rather than contributing to individual frequency tuning, these properties are likely to serve as a mechanism for the synchronization of the afferent population during strong acoustic stimulation. That is, because most saccular fibers will respond to any frequency within the goldfish’s effective range of hearing at sound levels 40 dB or more above best threshold (see Fig. 4C for the Q10dB [range of frequency response at 10dB above threshold] of each afferent; Fay, 1995), this resonant mechanism would act instead to synchronize this population of large afferents to loud acoustic stimuli of behavioral relevance by making them, regardless of their characteristic frequency, electrically tuned to the whole hearing range. This possibility is consistent with the fact that strong acoustic stimuli are necessary for triggering an escape response in the M-cell system, which has a characteristic high threshold (Fay, 1995).
7. A mechanism of lateral excitation contributes to afferent synchronization
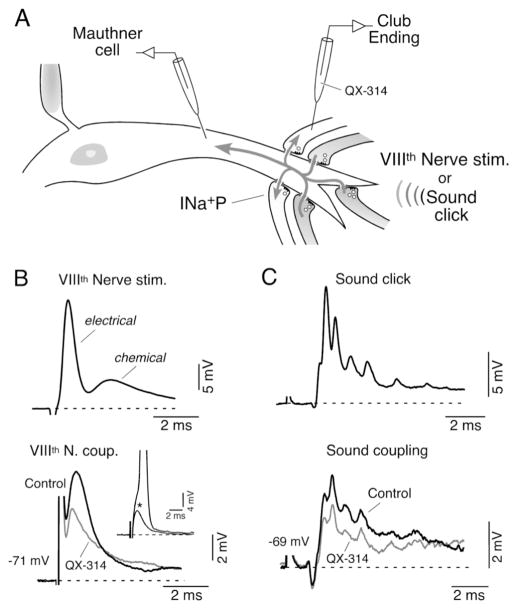
The synaptic contacts of Club endings afferents are tightly segregated to the distal portion of the M-cell lateral dendrite (Bartelmez, 1915). Despite the fact that the M-cell’s geometry and electrical properties favor the spread of postsynaptic depolarizations from this relatively remote dendritic site towards the lower impedance soma (Fig. 7A), the postsynaptic depolarization produced by the activation of some of these afferents can be recorded as a coupling potential in neighboring, inactive, terminals and can lead to backfiring of subthreshold terminals (Fig. 7B) (Pereda et al., 1995; Curti and Pereda, 2004). Thus, because their electrical synapses are bi-directional (Furshpan, 1964), these terminals are interconnected through the M-cell lateral dendrite thereby forming a functional compartment. The retrograde spread of dendritic depolarizations to the presynaptic Club endings influences their excitability (Pereda et al., 1995), promoting cooperativity between afferents (Fig. 7A).
Figure 7. A mechanism of lateral excitation contributes to afferent synchronization.
A, Dendritic synaptic potentials (Mauthner cell) evoked by suprathreshold electrical or natural stimulation of VIIIth nerve afferents (darker afferents; VIIIth Nerve stim. and Sound click; upper traces in B and C) can be recorded as coupling potentials, in neighbouring subthreshold terminals (light terminals; lower traces in B and C, respectively). INa+P (persistent, subthreshold, Na+ current. B, Mixed synaptic response (electrical and chemical) produced by extracellular electrical stimulation of the posterior branch of the VIIIth nerve (Upper, VIIIth Nerve stim.) evokes a retrograde coupling potential in a subthreshold terminal (lower, VIIIth Nerve coup.). Inset: amplitude of the VIIIth nerve coupling (asterisk) was adjusted to be at the threshold of the presynaptic afferent (truncated spike: 100 mV). Superimposed traces in the lower panel represent the amplitude of these retrograde responses obtained right after (control) and 5 minutes after the penetration of the terminal with an electrode containing QX-314. C, Sound-evoked synaptic potential (Sound click; 500 μs pulse) can also be recorded as a coupling potential in neighbouring inactive terminals (bottom trace; Sound coupling). Superimposed traces represent the retrograde responses obtained right after (Control) and 5 min. after the penetration of the synaptic terminal with an electrode containing QX-314. Note the reduction in amplitude of both retrograde coupling potentials observed after injection of QX-314 (grey traces). Modified from Curti and Pereda (2004), with permission.
Because of the unfavorable conditions mentioned above, this mechanism of retrograde communication is enhanced by properties of the Club ending afferents. That is, the amplitude of the coupling potential produced by the retrograde spread of signals from the postsynaptic Mauthner cell is dramatically enhanced by depolarization of the presynaptic terminal (Pereda et al., 1995). This voltage-dependent enhancement of electrical coupling does not represent a property of the junctions themselves but instead the activation of the INa+P present at presynaptic terminals that acts to amplify the synaptic response (Curti and Pereda, 2004). This amplification can also be observed at resting potential if signals are large enough to activate a significant fraction of INa+P channels, and it can be removed by intracellular application of QX314 (Fig. 7B). This amplification was also shown to occur under physiological conditions, using brief sound clicks (Fig. 7A,C) (Curti and Pereda, 2004). Figure 7C illustrates the intradendritic response to this brief auditory stimulus (top) and the coupling, obtained in sequential recordings, in one neighboring terminal for which the stimulus strength was subthreshold (bottom). This coupling was also suppressed by QX-314 indicating that amplification of retrograde coupling by INa+P operates under physiological conditions. Thus, amplification of retrograde transmission by INa+P is likely to play an essential physiological role in promoting the recruitment of subthreshold afferents during the activation of a population of these fibers. Because of its voltage-dependence, the integrative property of this amplifying mechanism would be particularly relevant in afferents that were subliminally activated by the stimulus (i.e., depolarization did not reach threshold; Fig. 7A). As a result, retrograde coupling acts as a mechanism of lateral excitation that enhances the effectiveness of an input when only a portion of these fibers are active by synchronizing that afferent population and promoting the recruitment of new fibers that are close to threshold (Pereda et al., 1995; Curti and Pereda, 2004). In addition, retrograde coupling may act to enhance transmitter release when afferents are simultaneously activated (Smith and Pereda, 2003; Pereda et al., 2004). This population mechanism is likely to boost synaptic transmission in situations in which a large number of these afferents are activated, such as during loud threatening sounds, and represents another example of a functional interaction between chemical and electrical synapses at these terminals (Pereda et al., 2004).
It is generally believed that patterns of lateral excitation play relevant physiological roles by amplifying the responses of sensory afferents tuned to qualitatively similar stimuli (Herberholz et al., 2002). In contrast to other examples such as retinal cones (DeVries et al., 2002), and crayfish locomotor system (El Manira et al., 1993) in which electrical synapses are located between the presynaptic neurons, Club endings are electrically coupled to each other through the M-cell lateral dendrite. This coupling arrangement would explain why while in some systems junctional properties prevent the antidromic spread of postsynaptically originated currents (Furshpan and Potter, 1959; Auerbach and Bennett, 1969; Ringham, 1975; Edwards et al., 1991) the physiological properties of the Club endings actually promote this phenomenon. Thus, by virtue of its bi-directional properties, electrical transmission plays an essential functional role by promoting synchronization between terminals and therefore enhancing the efficacy of the synaptic potential evoked by the population of afferents. Furthermore, changes in the efficacy of electrical synapses would further enhance this mechanism, as an additional way of increasing the impact of this input on the excitability of the M-cells.
8. Discussion
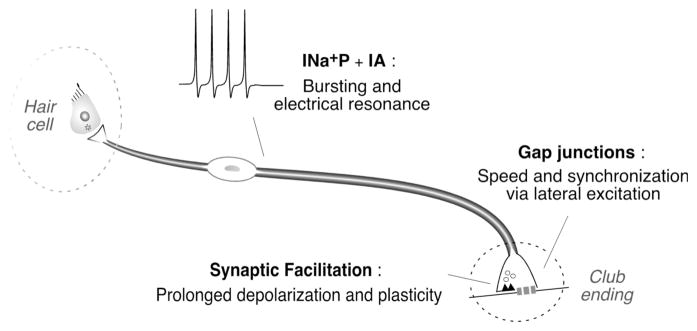
Club ending afferents exhibit several functional specializations that make them the ideal candidates to provide the M-cell with the essential information for the initiation of a behavioral response. Anatomical (large size and characteristic myelinization) and synaptic specializations (single terminals which combine strong electrical transmission with facilitating chemical synapses) guarantee speedy dendritic depolarization and high temporal fidelity by combining swift impulse conduction with fast and reliable synaptic transmission (Fig. 8). In addition, converging biophysical and synaptic properties contribute to the synchronization of the population of afferents, increasing the probability of evoking an escape response.
Figure 8. Membrane and synaptic specializations of Club ending afferents.
Anatomically simple Club ending afferents (dendritic arborization is not represented in this cartoon) are endowed with electrophysiological properties that allow these neurons to translate behaviorally relevant acoustic signals into patterns of activity that match the requirements of their fast and highly modifiable synapses. The properties of both electrical and chemical synapses contribute to generate adequate synaptic activation of the M-cell.
Perhaps the most remarkable specialization of Club endings is the ability of their synapses to undergo activity-dependent potentiation of both chemical and electrical synapses (Yang et al., 1990; Pereda and Faber, 1996; Smith and Pereda, 2003). Interestingly, there are not reports of plasticity in mammalian primary auditory afferents. Such property is presumably undesirable in initial stages of the auditory pathway, where synaptic connections should be reliable and specialized to transmit precise timing information. Strategies such as multivesicular release in hair cells (Glowatzki and Fuchs, 2002) or multiplicity of release sites and anatomical specializations in the Calyx of Held (Trussell, 1997) guarantee fast and secure relay transmission with high probability of triggering a postsynaptic spike and low jitter. Consistent with the high probability of release observed in synapses with high safety factors, the synaptic contacts of mammalian primary afferents onto cochlear nucleus neurons show a characteristic paired-pulse depression (Zhang and Trussell, 1994). Chemical synapses at Club endings on the M-cell, on the other hand, exhibit paired-pulse facilitation (Pereda and Faber, 1996; Wolszon et al., 1997), which is a requisite for the induction of plastic changes. These properties are consistent with the existence of unusual synaptic specializations. Thus, the unique synaptic properties of Club endings likely represent a sophisticated control of sensory-motor processing and endow these specialized teleost primary auditory afferents with significant power over a decision-making neuron.
Recently, a novel form of activity-dependent potentiation was reported at Club ending synapses using a different stimulating pattern (Cachope et al., 2007). In this case stimulation of the VIIIth nerve with 5 trains of 100 Hz and 1 second duration evoked a long-term enhancement of both components of the synaptic response (Cachope et al., 2007). This novel effect involves endocannabinoids and is indirectly mediated via the release of dopamine from nearby varicosities (Cachope et al., 2007), which in turn led to potentiation of the synaptic response via a cAMP-dependent protein kinase-mediated postsynaptic mechanism (Cachope et al., 2007; Pereda et al, 1994). The pattern of activity used to trigger this form of potentiation was chosen because is known to promote the release of endocannabinoids in other systems (Chevaleyre et al., 2006), and it was not inspired in the firing characteristics of these afferents. Thus, it is still unclear how endocannabinoid release is achieved under more physiological patters of activity.
The properties of these fast and modifiable mixed synapses are supported by biophysical specializations that allow Club endings to translate the goldfish’s effective range of hearing into adequate patterns of afferent activity. While the lack of repetitive M-cell firing is thought to be due to the presence of DTX-sensitive K+ channels of the Kv1 family (Nakayama and Oda 2004), our results show that the ability of Club ending afferents to respond with high frequency bursts of action potentials results from the interaction of a INa+P, which is required for repetitive responses, with an A-type K+ conductance. This INa+P plays an essential functional role as is required for the generation of repetitive responses and amplifies an otherwise weak electrical resonance. From the dynamical systems point of view the electrical behavior of these afferents corresponds to an Andronov-Hopf type of bifurcation (Izhikevich, 2007). Further, the lack of a bistability of this afferent’s electrical behavior (Curti et al., 2008) suggests that it more specifically corresponds to a supercritical Andronov-Hopf bifurcation (Izhikevich, 2007). Interestingly, Andronov-Hopf bifurcations can be reproduced in reduced models that combine a persistent Na+ current with a delayed K+ current (“persistent sodium plus potassium model”; Izhikevich 2007) indicating that the electrophysiological properties of Club ending afferents can be adequately explained by the interaction between these two currents.
Activation of the M-cell necessarily involves synchronized input from a number of afferent synapses, given the brief duration of the postsynaptic potentials. Consistent with this need, two mechanisms are known to contribute to the synchronization of this population of afferents. The first one, supported by their resonant properties, is their propensity to respond to any frequency within the goldfish hearing range during loud acoustic stimuli (Curti el al., 2008). The second is a mechanism of lateral excitation that promotes the coordinated activity of these afferents and is supported by the existence of bi-directional electrical synapses (Pereda et al., 1995; Curti and Pereda, 2004). INa+P was shown to play an essential role in amplifying retrograde synaptic communication via electrical synapses, thereby contributing to the synchronization of Club endings (Pereda et al., 1995; Curti and Pereda, 2004). Thus, biophysical and synaptic properties synergistically contribute to promote the synchronization of the afferent response, increasing the probability of evoking an action potential in the postsynaptic M-cell.
The presence of a relatively small number of Na+ channels lacking fast inactivation (INa+P) is essential for the function of a Club ending afferent and defines its electrophysiological phenotype, allowing these fibers to link behaviorally relevant auditory signals into patterns of activity that match the requirements of their fast and highly modifiable synapses (Fig. 8). Although INa+P generally represents a small (~1%) non-inactivating fraction of the total Na+ current it has a significant functional impact because it is activated about 10 mV negative to the transient Na+ current, where few voltage-gated channels are activated and neuron input resistance is high (Crill, 1996). As a result of this property, subthreshold Na+ currents play essential cellular roles, namely amplifying dendritic synaptic potentials, regulating repetitive firing, and producing depolarizing responses (Crill, 1996; Ogata and Ohishi, 2002). Persistent Na+ currents have been reported to be present in primary afferents (Kocsis and Waxman, 1983; Bowe et al., 1985; Honmou et al., 1994; Wu et al., 2005; Rush et al., 2007) where they are thought to mediate important functional and pathological roles (Stys et al., 1992, 1993). Consistent with these observations, Nav1.6 channels, thought to underlie persistent Na+ currents (Ogata and Ohishi, 2002; Rush et al., 2007), were found in primary auditory afferents (Hossain et al., 2005). It is possible that the transient Na+ channels underlying spikes could also contribute to the observed oscillatory behavior. Because some models of Na+ channel gating kinetics predict slow and incomplete inactivation of macroscopic current for depolarizations to subthreshold voltages, it has been proposed they could underlie subthreshold currents in some neuronal types (Taddese and Bean, 2002).
In summary, the biophysical properties of these afferents seem to be adapted to the M-cell system, where the increased synaptic gain of these auditory nerve synapses will sensitize a vital escape response, lowering its threshold to acoustic stimuli (Korn and Faber, 2005). Although anatomically simple, primary afferents can be endowed with complex membrane and synaptic properties (Fig. 8) and capable, at least in lower vertebrates, of more sophisticated contributions to auditory processing than has been generally recognized.
Acknowledgments
We thank Donald S. Faber for critically reading the manuscript and Jose Luis Peña for useful discussions. Supported by NIH grants DC03186 and NS0552827 to A. Pereda. S. Curti was partially supported by CSIC (Uruguay) and IBRO fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–71. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach AA, Bennett MVL. A rectifying synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969;53:183–210. doi: 10.1085/jgp.53.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez GW. Mauthner’s cell and the nucleus motorius tegmenti. J Comp Neurol. 1915;25:87–128. [Google Scholar]
- Beccari N. Richerche sulle cellule e fibre del Mauthner e sulle loro conessioni in pesci ed anfibii. Arch Ital Anay E Embr. 1907;T.6:660–705. [Google Scholar]
- Bodian D. The structure of the vertebrate synapse. A study of the axon endings on Mauthner’s cell and neighboring centers in the goldfish. J Comp Neurol. 1937;1:117–160. [Google Scholar]
- Bowe CM, Kocsis JD, Waxman SG. Differences between mammalian ventral and dorsal spinal roots in response to blockade of potassium channels during maturation. Proc R Soc Lond B Biol Sci. 1985;224:355–66. doi: 10.1098/rspb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Bullock T. Introduction to Nervous Systems. W.H. Freeman and Company; San Francisco: 1977. [Google Scholar]
- Cachope R, Mackie K, Triller A, O’Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–47. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–62. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Curti S, Pereda AE. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J Neurosci. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, Gomez L, Budelli R, Pereda AE. Subthreshold sodium current underlies essential functional specializations at primary auditory afferents. J Neurophysiol. 2008;99:1683–99. doi: 10.1152/jn.01173.2007. [DOI] [PubMed] [Google Scholar]
- Davis RL. Differential distribution of potassium channels in acutely demyelinated, primary-auditory neurons in vitro. J Neurophysiol. 1996;76:438–47. doi: 10.1152/jn.1996.76.1.438. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Qi X, Smith R, Makous W, Sterling P. Electrical coupling between mammalian cones. Curr Biol. 2002;12:1900–7. doi: 10.1016/s0960-9822(02)01261-7. [DOI] [PubMed] [Google Scholar]
- Eaton RC, DiDomenico R, Nissanov J. Flexible body dynamics of the goldfish C-start: implications for reticulospinal command mechanisms. J Neurosci. 1988;8:2758–68. doi: 10.1523/JNEUROSCI.08-08-02758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol. 2001;63:467–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Leise EM, Fricke RA. Postsynaptic modulation of rectifying electrical synaptic inputs to the LG escape command neuron in crayfish. J Neurosci. 1991;11:2117–29. doi: 10.1523/JNEUROSCI.11-07-02117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Manira A, Cattaert D, Wallen P, DiCaprio RA, Clarac F. Electrical coupling of mechanoreceptor afferents in the crayfish: a possible mechanism for enhancement of sensory signal transmission. J Neurophysiol. 1993;69:2248–51. doi: 10.1152/jn.1993.69.6.2248. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H. Electrophysiology of the Mauthner cell, basic properties, synaptic mechanisms, and associated networks. In: Faber DS, Korn H, editors. Neurobiology of the Mauthner cell. New York: Raven; 1978. pp. 47–131. [Google Scholar]
- Faber DS, Fetcho JR, Korn H. Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann NY Acad Sci. 1989;563:11–33. doi: 10.1111/j.1749-6632.1989.tb42187.x. [DOI] [PubMed] [Google Scholar]
- Fay RR. Coding of information in single auditory-nerve fibers of the goldfish. J Acoust Soc Am. 1978;63:136–46. doi: 10.1121/1.381705. [DOI] [PubMed] [Google Scholar]
- Fay RR. Physiology of primary saccular afferents of goldfish: implications for Mauthner cell response. Brain Behav Evol. 1995;46:141–50. doi: 10.1159/000113267. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–34. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Fukami Y, Furukawa T, Asada Y. Excitability changes of the Mauthner cell during collateral inhibition. J Gen Physiol. 1965;48:581–600. doi: 10.1085/jgp.48.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ. “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science. 1964;144:878–80. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Ishii Y. Neurophysiological studies on hearing in goldfish. J Neurophysiol. 1967;30:1377–403. doi: 10.1152/jn.1967.30.6.1377. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–54. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Herberholz J, Antonsen BL, Edwards DH. A lateral excitatory network in the escape circuit of crayfish. J Neurosci. 2002;22:9078–85. doi: 10.1523/JNEUROSCI.22-20-09078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–37. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain WA, Antic SD, Yang Y, Rasband MN, Morest DK. Where is the spike generator of the cochlear nerve? Voltage-gated sodium channels in the mouse cochlea. J Neurosci. 2005;25:6857–68. doi: 10.1523/JNEUROSCI.0123-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–50. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–22. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- Hutcheon B, Miura RM, Puil E. Subthreshold membrane resonance in neocortical neurons. J Neurophysiol. 1996;76:683–97. doi: 10.1152/jn.1996.76.2.683. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Dynamical Systems in Neuroscience: The geometry of excitability and bursting. Cambridge, MA: MIT Press; 2007. [Google Scholar]
- Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 2003;26:161–7. doi: 10.1016/S0166-2236(03)00034-1. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–69. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Klug A, Trussell LO. Activation and deactivation of voltage-dependent K+ channels during synaptically driven action potentials in the MNTB. J Neurophysiol. 2006;96:1547–55. doi: 10.1152/jn.01381.2005. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Long-term regenerated nerve fibres retain sensitivity to potassium channel blocking agents. Nature. 1983;304:640–2. doi: 10.1038/304640a0. [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I Characteristics of electrotonic and chemical postsynaptic potentials. J Neurosci. 1988a;8:1302–12. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II Plasticity of excitatory postsynaptic potentials. J Neurosci. 1998b;8:1313–25. doi: 10.1523/JNEUROSCI.08-04-01313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Magleby KL. Short-term changes in synaptic efficacy. In: Edelman GM, Einar W, Gall W, Cowan M, editors. Synaptic function. New York: John Wiley & Sons, Inc; 1987. pp. 21–56. [Google Scholar]
- Mauthner L. Untersuchungen uber den Bau des Ruckenmarkes der Fische. Eine vorlaufige Mitteilung. Sitzgsber Kaiserl Akad Wiss Wien, Math-Naturw Classe. 1859;34:31–36. [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K(+) currents contribute to accommodation in murine spiral ganglion neurons. J Physiol. 2002;542:763–78. doi: 10.1113/jphysiol.2002.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Oda Y. Common sensory inputs and differential excitability of segmentally homologous reticulospinal neurons in the hindbrain. J Neurosci. 2004;24:3199–209. doi: 10.1523/JNEUROSCI.4419-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Ohishi Y. Molecular diversity of structure and function of the voltage-gated Na+ channels. Jpn J Pharmacol. 2002;88:365–77. doi: 10.1254/jjp.88.365. [DOI] [PubMed] [Google Scholar]
- Pereda A, Bell T, Faber DS. Retrograde synaptic communication via gap junctions coupling auditory afferents to the Mauthner cell. J Neurosc. 1995;15:5943–5955. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Bell T, Chang B, Czernik A, Nairn A, Soderling T, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:3272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Faber DS. Activity dependent short-term plasticity of intercellular coupling. J Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Nairn A, Wolszon L, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosci The Journal of Neuroscience. 1994;14:3704–3712. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, O’Brien J, Nagy JI, Bukauskas F, Davidson KG, Kamasawa N, Yasumura T, Rash JE. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci. 2003;23:7489–503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Rev. 2004;47:227–44. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci USA. 1992;89:12088–12092. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper AN, Fay RR. The auditory periphery in fishes. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians (Springer Handbook of Auditory Research. New York: Springer-Verlag; 1998. pp. 43–100. [Google Scholar]
- Rathouz M, Trussell L. Characterization of outward currents in neurons of the avian nucleus magnocellularis. J Neurophysiol. 1998;80:2824–35. doi: 10.1152/jn.1998.80.6.2824. [DOI] [PubMed] [Google Scholar]
- Ringham GL. Localization and electrical characteristics of a giant synapse in the spinal cord of the Lamprey. J Physiol. 1975;251:395–407. doi: 10.1113/jphysiol.1975.sp011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J Cell Biol. 1963;19:159–99. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J, Palay SL. The fine structure of nerve cell bodies and their myelin sheaths in the eighth nerve ganglion of the goldfish. J Biophys Biochem Cytol. 1961;9:853–77. doi: 10.1083/jcb.9.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. Kinetic analyses of three distinct potassium conductances in ventral cochlear nucleus neurons. J Neurophysiol. 2003;89:3083–96. doi: 10.1152/jn.00126.2002. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–49. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner-ear. J Neurosci. 1993;13:3599–611. doi: 10.1523/JNEUROSCI.13-08-03599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sento S, Furukawa T. Intra-axonal labeling of saccular afferents in the goldfish, Carassius auratus: correlations between morphological and physiological characteristics. J Comp Neurol. 1987;258:352–67. doi: 10.1002/cne.902580304. [DOI] [PubMed] [Google Scholar]
- Smith M, Pereda AE. Chemical synaptic activity modulates nearby electrical synapses. Proc Natl Acad Sci USA. 2003;100:4849–54. doi: 10.1073/pnas.0734299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–87. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci USA. 1993;90:6976–80. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na+-Ca2+ exchanger. J Neurosci. 1992;12:430–9. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, Furukawa T. Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J Neurophysiol. 1989;62:1330–43. doi: 10.1152/jn.1989.62.6.1330. [DOI] [PubMed] [Google Scholar]
- Szabo TM, Weiss SA, Faber DS, Preuss T. Representation of auditory signals in the M-cell: role of electrical synapses. J Neurophysiol. 2006;95:2617–29. doi: 10.1152/jn.01287.2005. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Cellular mechanisms for preservation of timing in central auditory pathways. Curr Opin Neurobiol. 1997;7:487–92. doi: 10.1016/s0959-4388(97)80027-x. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–96. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Transmission at the hair cell synapse. Nat Neurosci. 2002;5:85–6. doi: 10.1038/nn0202-85. [DOI] [PubMed] [Google Scholar]
- Tuttle R, Masuko S, Nakajima Y. Freeze fracture study of the Large Myelinated Club Ending synapse on the goldfish Mauthner cell: special reference to the quantitative analysis of gap junctions. J Comp Neurol. 1986;246:202–211. doi: 10.1002/cne.902460206. [DOI] [PubMed] [Google Scholar]
- Wolszon LR, Pereda AE, Faber DS. A fast synaptic potential mediated by NMDA and non-NMDA receptors. J Neurophysiol. 1997;78:2693–706. doi: 10.1152/jn.1997.78.5.2693. [DOI] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic V neurons participate in burst generation and control of membrane excitability. J Neurophysiol. 2005;93:2710–22. doi: 10.1152/jn.00636.2004. [DOI] [PubMed] [Google Scholar]
- Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol. 1994;480:123–36. doi: 10.1113/jphysiol.1994.sp020346. [DOI] [PMC free article] [PubMed] [Google Scholar]