Summary
SIRT1 regulates energy homeostasis by controlling the acetylation status and activity of a number of enzymes and transcriptional regulators. The fact that NAD+ levels control SIRT1 activity confers a hypothetical basis for the design of new strategies to activate SIRT1 by increasing NAD+ availability. Here we show that the deletion of the poly(ADP-ribose) polymerase-1 (PARP-1) gene, encoding a major NAD+-consuming enzyme, increases NAD+ content and SIRT1 activity in brown adipose tissue and muscle. PARP-1−/− mice phenocopied many aspects of SIRT1 activation, such as a higher mitochondrial content, increased energy expenditure, and protection against metabolic disease. Also, the pharmacologic inhibition of PARP in vitro and in vivo increased NAD+ content, SIRT1 activity and enhanced oxidative metabolism. These data show how PARP-1 inhibition has strong metabolic implications through the modulation of SIRT1 activity, a property that not only could be useful in the management of metabolic diseases but also of cancer.
Keywords: caloric restriction, longevity, mitochondria, NAD+, oxidative phosphorylation, peroxisome proliferator activated receptor gamma coactivator 1α, poly(ADP-ribose) polymerase, SIRT1
Introduction
Intracellular NAD+ levels control the activity of the type III deacetylase SIRT1 allowing it to act both as a metabolic sensor and effector (Yu and Auwerx, 2009). Overexpression studies indicated how activation of SIRT1 or of its orthologs extends lifespan in lower eukaryotes and protects against high-fat diet (HFD)-induced metabolic disease in mice (Yu and Auwerx, 2009). These attractive properties spurred a quest to identify small molecule SIRT1 agonists that could be used in situations of metabolic stress and damage. This strategy identified compounds like resveratrol or SRT1720 (Howitz et al., 2003; Milne et al., 2007), whose ability to directly interact and activate SIRT1 is, however, debated (Pacholec et al., 2010). Therefore, a strong interest exists to develop alternative strategies to activate SIRT1. Given the NAD+-dependency of SIRT1, another potential way to activate it is by increasing NAD+ availability. This could be achieved by specifically inhibiting other NAD+-consuming activities.
Poly(ADP-ribose) polymerase (PARP)-1 is a major cellular NAD+ consumer (Sims et al., 1981). PARP-1 is activated upon binding to damaged or abnormal DNA (Durkacz et al., 1980), and catalyzes the formation of poly(ADP-ribose) polymers (PAR) onto different acceptor proteins, including PARP-1 itself (auto-PARylation), using NAD+ as substrate (Adamietz, 1987). PARP-1 activation depletes cellular NAD+ levels, using it to form PAR (Sims et al., 1981). This led us to test the influence of PARP-1 on SIRT1 activity and metabolic homeostasis. Our results show how a reduction/ablation of PARP-1 activity boosts NAD+ levels and SIRT1 activity, which, in turn, enhances mitochondrial content and function, culminating in a solid protection against metabolic disease.
Results
PARP-1−/− mice are leaner and have increased energy expenditure
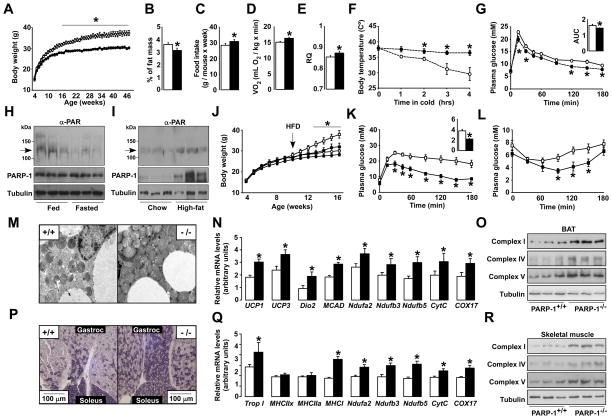
Chow fed PARP-1−/− mice (Menissier-de Murcia et al., 1997) weighed less (Fig.1A) and accumulated less fat than PARP-1+/+ littermates upon aging (Fig.1B) despite eating significantly more (Fig.1C). During indirect calorimetry, PARP-1−/− mice consumed more O2 (Fig.1D), suggestive of higher energy expenditure (EE). Resting EE was not different (Fig.S1A), indicating that the increase was due to changes at night, when mice are active. Accordingly, PARP-1−/− mice were more active at night (Fig.S1B). In addition, the respiratory quotient was also higher in PARP-1−/− mice during the dark phase, indicating enhanced glucose oxidation during the feeding period (Fig.1E). PARP-1−/− mice also maintained a higher body temperature upon cold exposure (Fig.1F), were more glucose tolerant (Fig.1G) and had a trend towards lower fasting blood glucose levels (4.30±0.17 mM in PARP-1+/+ mice vs. 3.98±0.18 mM in PARP-1−/− mice; p=0.058), despite similar insulin levels (data not shown). During euglycemic-hyperinsulinemic clamp, glucose infusion rates or hepatic glucose production were similar to PARP-1+/+ mice, but supporting their better glucose tolerance, glucose uptake in PARP-1−/− muscle trended up (Fig.S1C-E). In line with the lower fat mass and improved glucose tolerance, serum triglycerides (1.04±0.07 mM in PARP-1+/+ vs. 0.84±0.05 mM in PARP-1−/− mice; p=0.048) and free fatty acids (FFA) (0.93±0.09 mEq/L in PARP-1+/+ vs. 0.72±0.03 mEq/L in PARP-1−/− mice; p=0.040) were reduced in PARP-1−/− mice.
Figure 1. Phenotyping the PARP-1−/− mice.
(A) Body weight (BW) evolution in PARP-1+/+ and −/− mice (n=8/9). (B) Epidydimal white adipose tissue mass. (C) Average food consumption. (D) O2 consumption and (E) respiratory quotient (RQ) of PARP-1+/+ and −/− mice (n=9/9) determined by indirect calorimetry. (F) Body temperature after exposure to 4°C (n=6/5). (G) Oral glucose tolerance test (OGTT) (n=5/5) and the area under curve (AUC). (H) PARP-1 autoPARylation (arrow), analyzed in 100μg of protein extract from gastrocnemius muscles of 16 wk-old mice fed ad libitum or fasted (24h). PARP-1 and tubulin levels were checked using 50μg of protein extract. (I) Gastrocnemius from mice on CD or HFD (12 wk) were analyzed as in (H). (J) BW evolution in PARP-1+/+ and −/− mice (n=10/10) fed a CD (circles) or HFD (squares) from 8 wk of age. (K) OGTT and (L) an insulin tolerance test in HFD fed PARP-1+/+ and −/− mice at 16 wk of age (n=10/10). The AUC of the OGTT is shown on the top-right. (M-O) BAT from PARP-1+/+ and −/− mice on CD was extracted and mitochondrial biogenesis was analyzed by (M) transmission electron microscopy, (N) mRNA expression of the genes indicated and (O) the abundance of mitochondrial complexes in 25μg of total protein extracts. (P) SDH staining of sections from gastrocnemius and soleus of PARP-1+/+ and −/− mice on CD. (Q-R) Gastrocnemius was also used to analyze mRNA levels of the indicated genes (Q) and the abundance of mitochondrial complexes in 25μg of total protein extracts (R). White bars represent PARP-1+/+; black bars represent PARP1−/− mice. * indicates statistical difference vs. PARP-1+/+ at p<0.05. For abbreviations, see Table S1.
PARP-1 is induced by nutrient availability and contributes to HFD-induced diabesity
The metabolic impact of PARP-1 deletion made us evaluate whether nutrient scarcity (fasting) or overload (HFD) affects PARP-1 activity. Despite similar PARP-1 protein levels, a 24-hr fast sharply reduced PARP-1 autoPARylation levels, which reflect global PARP activity (Adamietz, 1987), suggesting a lower enzymatic activity (Fig.1H). In contrast, HFD robustly increased PARP-1 protein levels and activity (Fig.1I), indicating a positive correlation between PARP-1 activity and nutrient availability.
As nutrient availability induces PARP-1 activity and PARP-1 deletion prompts a leaner phenotype, we next explored how PARP-1−/− mice responded to HFD-induced metabolic disease. PARP-1−/− mice gained less weight after 2 months of HFD (Fig.1J), due to a lower fat accumulation (Fig.S1F). Moreover, PARP-1−/− mice on HFD were more glucose tolerant (Fig.1K), insulin sensitized (Fig.1L), and had lower serum FFAs (0.66±0.05 mEq/L vs. 0.53±0.03 mEq/L; p=0.026).
Mitochondrial activation in brown adipose tissue and muscle from PARP-1−/− mice
The above results suggested improved mitochondrial activity in key metabolic tissues of PARP-1−/− mice, such as skeletal muscle and brown adipose tissue (BAT). PARP-1−/− mice had a relative higher amount of BAT, with a more intense red appearance (Fig.S1G). Transmission electron microscopy revealed higher mitochondrial content in PARP-1−/− BAT (Fig.1M), which was further corroborated by the increased mitochondrial DNA content (Fig.S1H) and mRNA expression of genes involved in mitochondrial respiration (Ndufa2, Ndufb2, Ndufb5, Cyt C, COX17), uncoupling (UCP1, UCP3), fatty acid oxidation (MCAD), and thyroid hormone activation (Dio2). Mitochondrial biogenesis was also evidenced by the higher protein content of subunits from different respiratory complexes in the BAT from PARP-1−/− mice (Fig.10).
Also PARP-1−/− skeletal muscle had a marked oxidative profile. Succinate dehydrogenase (SDH) staining (Fig.1P) and the expression of muscle fiber isotype genes (Trop I, MHCI) (Fig.1Q) exposed an increase in oxidative fibers with a high mitochondrial content. As in BAT, the increased mitochondrial content was linked to an induction of the mRNA (Fig.1Q) and protein levels (Fig.1R) of mitochondrial components. In contrast to BAT and muscle, the expression of key metabolic genes was not altered in PARP-1−/− livers (Fig.S1I), reflecting a minor role of PARP-1 in the liver, probably due to its very low expression (Fig.S1J).
Higher NAD+ content and SIRT1 activity in BAT and muscle from PARP-1−/− mice
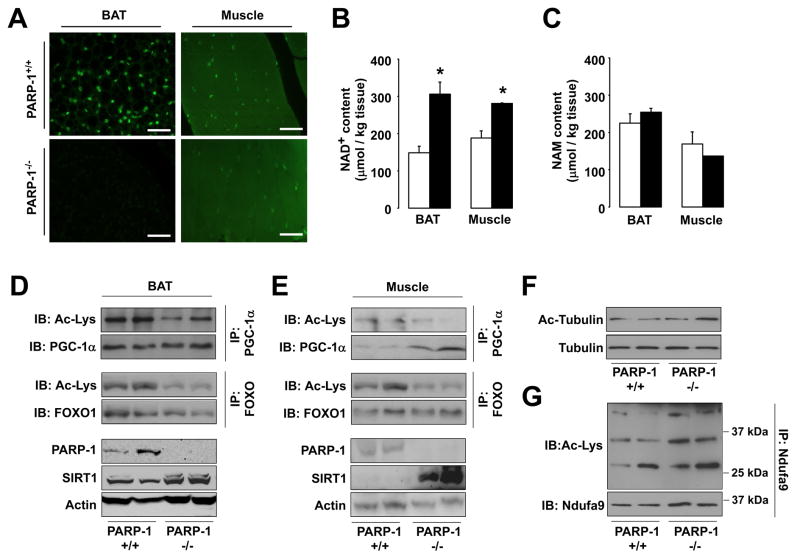
The PARP-1−/− mice phenocopy many features seen after SIRT1 activation (Yu and Auwerx, 2009). As PARP-1 is a major NAD+-consumer (Sims et al., 1981), we speculated that the lack of PARP-1 activity might increase NAD+ content, in turn activating SIRT1. Illustrating how PARP-1 drives most PARP activity, the ablation of PARP-1 reduced PARylation in both BAT and muscle (Fig.2A). The expression of the other PARP enzymes was not increased in PARP-1−/− BAT and muscle (Fig.S2A-B), explaining the lack of compensation on PARylation. Confirming previous studies (Allinson et al., 2003; Fong et al., 2009), terminal dUTP nick-end labeling indicated that DNA damage was not increased in PARP-1−/− tissues (data not shown). In line with the attenuated NAD+-consuming PARP activity, NAD+ content was robustly increased in PARP-1−/− BAT and muscle (Fig.2B), while the levels of nicotinamide (NAM), a NAD+-derived metabolite that inhibits sirtuin activity (Bitterman et al., 2002), remained unaffected (Fig.2C).
Figure 2. PARP-1 deletion raises NAD+ levels and activates SIRT1.
(A) Protein PARylation determined by α-PAR staining on formalin-fixed 7μm BAT and muscle tissue sections of PARP-1+/+ and −/− mice. White bar = 10μm. (B) NAD+ and (C) NAM levels in BAT and muscle from PARP-1+/+ (white bars) and PARP-1−/− (black bars) mice determined by mass spectrometry. (D-E) PARP-1, SIRT1 and actin protein content in BAT (D) and muscle (E) were determined by Western blot, using 100μg of protein lysate. PGC-1α and FOXO1 acetylation were examined by immunoprecipitation. (F) Tubulin and acetylated-tubulin levels were tested in PARP-1+/+ and −/− gastrocnemius. (G) The Ndufa9 subunit of mitochondrial complex I was immunoprecipitated from 400μg of total protein from gastrocnemius and acetylation levels were analyzed by Western blot. * indicates statistical difference vs. PARP-1+/+ mice at p<0.05.
We next tested if the increase in NAD+ correlated with SIRT1 activation. Indicative of SIRT1 activation, and supporting the higher mitochondrial content, PGC-1α acetylation levels in BAT and muscle of PARP-1−/− mice were reduced by ~40% and ~90%, respectively (Fig.2D-E). The acetylation of another SIRT1 target, forkhead box O1 (FOXO1), was also reduced by ~60% in BAT and ~40% in muscle (Fig.2D-E), supporting that PARP-1 deficiency leads to SIRT1 activation. Remarkably, SIRT1 protein was also robustly induced in PARP-1−/− BAT and muscle (Fig.2D-E), further amplifying SIRT1 activity.
As altered NAD+ levels could also potentially impact on other sirtuins, we also tested the activity of SIRT2 and SIRT3, which act as cytoplasmic and mitochondrial sirtuins, respectively. The acetylation level of tubulin, a SIRT2 target (North et al., 2003), was not altered in muscle from PARP-1−/− mice (Fig.2F). Likewise, the acetylation of Complex I, a target for SIRT3 (Ahn et al., 2008) even showed a slight tendency to increase in PARP-1−/− muscles (Fig.2G). These results indicate that not all sirtuins are activated in PARP-1−/− tissues.
Reduced PARP-1 activity in cellular models enhances oxidative metabolism
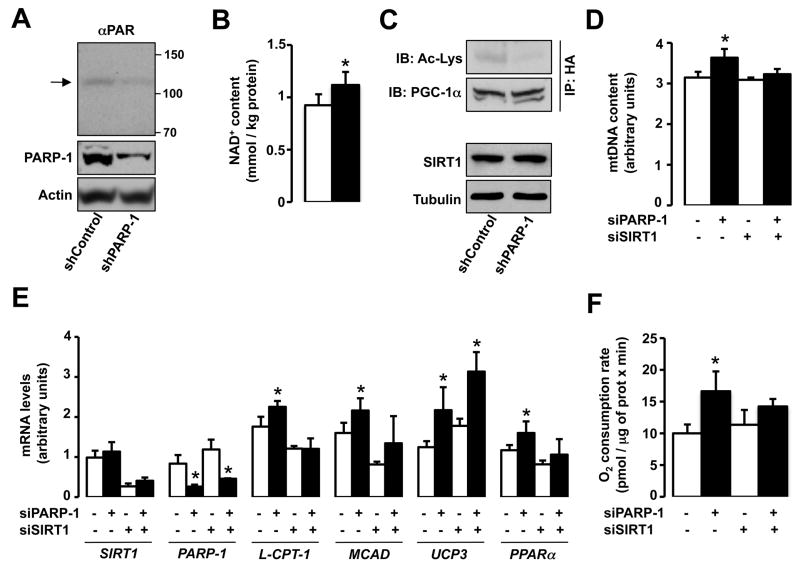
Next, we knocked-down PARP-1 in HEK293T cells to evaluate whether an acute reduction in PARP-1 activity enhances oxidative metabolism. In these conditions, PARP-1 protein levels were reduced by ~80% and the low PARP1 auto-PARyation demonstrated that PARP activity was largely blunted (Fig.3A). The reduced PARP activity was associated with enhanced NAD+ content and SIRT1 function, as illustrated by decreased PGC-1α acetylation (Fig.3B-C). Importantly, this happened despite unchanged SIRT1 protein levels (Fig.3C) or changes in the activity of SIRT2 or SIRT3, as manifested in tubulin, Ndufa9 or total mitochondrial acetylation levels (Fig.S3A-C). The induction of SIRT1 and PGC-1α activity culminated in a robust increase in mitochondrial DNA content (Fig.3D), mitochondrial-related gene expression (Fig.3E), and O2 consumption (Fig.3F). Importantly, most of the metabolic effects elicited by PARP-1 depletion were lost when SIRT1 was simultaneously knocked-down (Fig.3D-F).
Figure 3. PARP-1 knock-down promotes SIRT1 activity and oxidative metabolism.
(A-C) HEK293T cells were transfected with either scramble (control) or PARP-1 shRNA and HA-PGC-1α expression vector for 48h. Then, (A) PARP-1 protein levels and autoPARylation (arrowhead) were analyzed in total protein lysates. (B) Intracellular NAD+ was measured on total acid extracts. (C) PGC-1α acetylation was analyzed in HA immunoprecipitates. (D-F) HEK293T cells were transfected with either a pool of PARP-1 siRNAs, a pool of SIRT1 siRNAs, or different combinations of both using the corresponding scramble siRNAs as control (−). The cells were simultaneously transfected with HA-PGC-1α for 48h. Then, (D) relative mitochondrial DNA content, (E) mRNA levels of the genes indicated and (F) total O2 consumption was analyzed. * indicates statistical difference vs. respective control sh/siRNA-transfected cells at p<0.05.
In line with the results in HEK293T cells, the expression of genes involved in mitochondrial function, mitochondrial DNA content and O2 consumption were also induced in PARP-1−/− compared to PARP-1+/+ MEFs (Fig.S3D-F). In line with our observations in tissues from PARP-1−/− mice, SIRT1 protein was also induced in PARP-1−/− MEFs (Fig.S3G).
Pharmacological PARP inhibition enhances oxidative metabolism via SIRT1
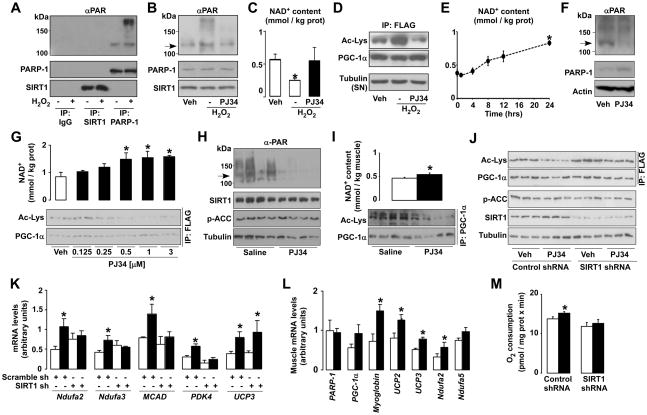
To test the relation between SIRT1 and PARP-1 activities, we exposed C2C12 myotubes to H2O2 (500 μM, 6h), a well-known inducer of PARP-1 activity (Schraufstatter et al., 1986). H2O2 treatment vigorously increased PARP-1 (Fig.4A) and global protein PARylation levels (Fig.4B), as manifested by the slow migrating bands, in the absence of changes in PARP-1 levels (Fig.4A-B). Importantly, SIRT1 was not PARylated in response to H2O2 (Fig.4A), indicating that it is not a PARylation substrate. As reported (Schraufstatter et al., 1986), the H2O2-induced increase in PARP-1 activity sharply depleted NAD+ content (Fig.4C), but did not affect SIRT1 protein levels (Fig.4A-B). This lower NAD+ availability limited SIRT1 activity, as reflected in PGC-1α hyperacetylation (Fig.4D). Interestingly, the inhibition of PARP activity with PJ34 (Garcia et al., 2001) rescued the drop in NAD+ and recovered SIRT1 function during H2O2 exposure (Fig.4B-D). These results indicate that PARP-1 activation restrains SIRT1 activity and that PARP inhibitors relieve this limitation.
Figure 4. PARP-1 inhibition enhances mitochondrial function through SIRT1.
(A-C) C2C12 myotubes expressing FLAG-HA-PGC-1α were treated for 6h with either PBS (vehicle (Veh)), H2O2 (500μM) or H2O2 and PJ34 (1μM). Then, (A) SIRT1, PARP-1 and unspecific IgG immunoprecipitates from 500 μg of protein extracts were used to test PARylation and the proteins indicated. (B) proteins were analyzed in total cell extracts and the arrow indicates PARP-1 autoPARylation. (C) NAD+ content was measured and (D) PGC-1α acetylation was tested in FLAG immunoprecipitates. Tubulin was checked on the supernatants as input. (E) C2C12 myotubes were treated with PJ34 (1μM) for the times indicated and NAD+ levels were evaluated in acidic extracts. (F-G) C2C12 myotubes expressing FLAG-HA-PGC-1α were treated for 24h with PBS (Veh) or with PJ34 (1 μM, unless stated). (F) PARP-1 protein and autoPARylation (arrow) were determined by Western blot and (G) NAD+ content and PGC-1α acetylation were measured. (H-I) 10-wk old mice received PJ34 (10mg/kg BID i.p.) or saline (Veh) for 5 days before sacrifice (n=10/10); then (H) PARP-1 autoPARylation (arrow), p-ACC and SIRT1 levels were determined in 100μg of total protein extracts from gastrocnemius and (I) NAD+ and PGC-1α acetylation were determined. PGC-1α was immunoprecipitated using 2mg of protein from gastrocnemius muscle and 5μg of antibody. (J-K) C2C12 myotubes expressing FLAG-HA-PGC-1α and either a control or a SIRT1 shRNA were treated with PJ34 for 48h. Then, (J) PGC-1α acetylation levels were quantified in FLAG immunoprecipitates and 50μg of total protein extracts were used to measure the other markers indicated; (K) mRNA levels of selected genes were quantified. (L) Mice were treated as in (H) and mRNA of selected genes was determined in gastrocnemius. (M) C2C12 were treated as in (J) and cellular O2 consumption was measured. White bars represent Veh; black bars represent PJ34 treatment. * indicates statistical difference vs. Veh group at p<0.05. For abbreviations, see Table S1.
PARP-1 activity is not necessarily linked to DNA-damage and it has been shown to fluctuate in a circadian fashion (Asher et al., 2010). Therefore, we wondered whether prolonged PARP inhibition, even in the absence of DNA damage, would favor NAD+ accumulation and, potentially, SIRT1 activity. Supporting this premise, PARP inhibition by PJ34 (Fig.4E) or a structurally unrelated compound, TIQ-A (data not shown), gradually raised NAD+ levels, becoming significant after 24hr. At that time, PARP activity, but not PARP-1 protein levels, was robustly decreased (Fig.4F). PJ34 increased NAD+ levels dose-dependently, in correlation with SIRT1 activity, as illustrated by PGC-1α deacetylation (Fig.4G). Similar effects also happened in vivo, as treatment of mice with PJ34 (10 mg/kg, BID for 5 days) blunted basal PARP activity in muscle (Fig. 4H), while increasing NAD+ and SIRT1 activity (Fig.4I). Despite the short duration of the treatment, serum triglyceride (1.21±0.08 mM Vehicle vs. 1.11±0.04 mM PJ34; p=0.08) and FFA levels (1.59±0.06 mEq/L vehicle vs. 1.44±0.03 mEq/L PJ34; p=0.03) were reduced in PJ34-treated mice. Of note, while compounds like resveratrol impact on SIRT1 through AMP-activated protein kinase (AMPK) (Canto et al., 2010), PJ34 did not alter AMPK activity, as reflected by the unchanged acetyl-CoA carboxylase (ACC) phosphorylation in either C2C12 myotubes (Fig.4J) or gastrocnemius muscle (Fig.4H). PJ34 neither affected SIRT1 protein levels (Fig. 4H and 4J), but robustly induced the expression of mitochondrial and lipid oxidation genes both in C2C12 myotubes and muscle (Fig.4K-L). This was consistent with PJ34-induced PGC-1α activation and its recruitment to target gene promoters (e.g. PDK4; Fig.1A) and culminated in higher O2 consumption rates (Fig.4M), testifying for enhanced oxidative metabolism.
The effect of PJ34 on PGC-1α acetylation in C2C12 myotubes was blunted upon SIRT1 knock-down (Fig.4J). The role of SIRT1 in mediating PJ34-induced PGC-1α deacetylation was further confirmed in SIRT1−/− MEFs, where PJ34 was unable to decrease PGC-1α acetylation (Fig.S4B). In line with impaired PGC-1α activation, mitochondrial gene expression and O2 consumption, were largely unresponsive to PJ34 upon SIRT1 depletion in C2C12 cells (Fig. 4K and 4M) and in SIRT1−/− MEFs (Fig.S4C-D), indicating that SIRT1 is a key mediator of PJ34 action. However, PJ34 also had SIRT1-independent effects, as reflected by the persistent increase in UCP3 mRNA even after the SIRT1 knock-down (Fig.4K). This could be explained by the fact that PJ34 does not regulate UCP3 expression by recruitment of PGC-1α to its promoter (Fig.S4A). The pharmacological inhibition of PARP recapitulates the phenotypic characteristics of the PARP-1−/− mice, and reveals that these effects are largely mediated by SIRT1.
Discussion
The difficulty to identify compounds that specifically and directly bind and activate SIRT1 led us to test whether the modulation of NAD+ availability could be an alternative path to activate SIRT1. Our present work supports this concept by showing how the attenuation of PARP-1, another NAD+-consuming enzyme, increases intracellular NAD+ levels and enhances SIRT1 activity. This prompts the deacetylation and activation of key metabolic transcriptional regulators such as PGC-1α and FOXO1, leading to increased mitochondrial content and metabolism.
Our data suggests that PARP-1 limits NAD+ availability for SIRT1 activation. This concept originates from the differences in the KM and Kcat/KM of both enzymes for NAD+, which indicate that PARP-1 is a faster and more efficient NAD+ consumer than SIRT1 (Knight and Chambers, 2001; Smith et al., 2009). Therefore, it is likely that PARP-1 activity maintains NAD+ at limiting levels for SIRT1 function. The prediction that PARP-1 deletion would increase NAD+ levels and activate SIRT1 is perfectly matched by our data. While previous work already speculated on a link between PARP-1 and sirtuin activities (Kolthur-Seetharam et al., 2006; Pillai et al., 2005), our study expands the consequences of this link to energy homeostasis. In apparent discrepancy, one report (Devalaraja-Narashimha and Padanilam, 2010) suggested that PARP-1−/− mice could be more susceptible to HFD-induced obesity. However, that study used mice on an SV129 background, which are less suited for metabolic studies than C57Bl/6J mice. The convergent results of our genetic, physiological, pharmacological and in vitro studies clearly support our conclusions.
Results from our lab indicate that the activation of SIRT1 after a bout of exercise or cold exposure is not linked to decreased PARP-1 activity (data not shown). Rather, only robust and/or protracted changes, such as pharmacological (PJ34) or genetic (knock-down or deletion) PARP-1 inhibition, influence SIRT1 activity. While PARP-1 might not always participate in the physiological modulation of SIRT1 activity, our data suggest that the interplay between both proteins could be exploited pharmacologically.
Our work illustrates how SIRT1 is a key mediator of the PARP-1 deficient phenotype, but the link between PARP-1 and SIRT1 activities is still unclear. Several models used in this work show that reducing PARP-1 activity controls SIRT1 function independent of changes in SIRT1 protein levels. In all these cases, the levels of NAD+, the rate-limiting coenzyme for SIRT1, correlated with SIRT1 activity, suggesting that NAD+ availability might influence SIRT1 activity. If it were true, boosting NAD+ content through alternative strategies should elicit similar metabolic phenotypes to those of the PARP-1−/− mice. Supporting this notion, the deletion of another NAD+ consumer, CD38, also activates SIRT1 (Aksoy et al., 2006), resulting in protection against HFD-induced obesity (Barbosa et al., 2007). Nutrient scarcity and AMPK activation also lead to increased NAD+ levels and SIRT1 activation coupled to the induction of oxidative metabolism (Canto et al., 2009; Canto et al., 2010). This correlative evidence indicates that the increased NAD+ availability might be a key mechanism by which PARP deficiency activates SIRT1. However, we cannot exclude the possibility that PARP inhibition also impacts on SIRT1 via other means, even though our results rule out direct PARylation of SIRT1 as the mechanism (Fig.4A). In addition, SIRT1 content was also increased in PARP-1−/− tissues and MEFs, further amplifying SIRT1 activity. The reason for this increase in SIRT1 levels remains elusive, but is independent of changes in SIRT1 mRNA (PB, CC and JA, unpublished observations).
Of note, PARP-1 depletion affects the activity of SIRT1, but not that of SIRT2 and SIRT3, which occupy non-nuclear compartments. If increased SIRT1 activity was mainly driven by changes in NAD+, the unchanged SIRT2 and SIRT3 activities in PARP-1−/− tissues suggest that the increase in NAD+ is either not enough to enhance SIRT2 and SIRT3 activities, or that it only happens in specific cellular compartments, supporting an independent regulation of different subcellular NAD+ pools (Yang et al., 2007). Alternatively, PARP-1 and SIRT1 activities might not be linked by changes in NAD+ and some yet unfound mechanism drives the specificity towards this sirtuin.
Some results indicate that the effects of PARP deficiency cannot be completely explained by SIRT1 (Fig.4K). Future research will have to clarify the nature of these SIRT1-independent effects of PARP inhibition on metabolism. It will be interesting to explore whether PARylation can directly modulate the activity of key metabolic transcriptional regulators, as PARP-1 may contribute to nuclear processes other than DNA repair (Krishnakumar and Kraus, 2010). PARP inhibitors are currently in clinical development as anti-tumoral drugs (Fong et al., 2009). While our data encourages a possible utilization of PARP inhibitors as therapeutic agents to activate SIRT1 and promote oxidative metabolism, this should be taken cautiously.
PARP-1 has key roles in genomic maintenance, and, while neither this nor previous studies (Allinson et al., 2003) detected enhanced DNA damage in PARP-1−/− mice under basal conditions, it cannot be ignored that PARP-1−/− mice are sensitive to ionizing radiation (Menissier-de Murcia et al.,1997). Hence, it will be important to analyze how ageing and metabolic disease impact on DNA damage to establish the therapeutic potential and limitations of PARP inhibition.
Experimental procedures
Detailed materials and procedures can be found as supplemental information.
Animal experiments
Pure C57Bl/6J male mice were used for the study. PARP-1+/+ and PARP-1−/− were described (Menissier-de Murcia et al., 1997). Mice were housed separately, had ad libitum access to water and chow (10 kcal% of fat, Safe, Augy, France) or HFD (60 kcal% of fat, Research Diets, New Brunswick, NJ, USA), and were kept under a 12h dark-light cycle. Animal experiments were carried out according to local national and EU ethical guidelines. Animals were sacrificed after a 6h of fast, and tissues were collected and processed as specified.
NAD+ and NAM determination
NAD+ levels in cultured cells were determined using an enzymatic method (Enzychrom, BioAssays Systems, CA), whereas for tissues (Fig.4) NAD+ and NAM levels were determined as described (Sauve et al., 2005).
Statistics
All data was verified for normal distribution. Statistical significance was assessed by Student’s t-test for independent samples. Values are expressed as mean +/− SEM unless otherwise stated.
Supplementary Material
Acknowledgments
This work was supported by fellowships by of Bolyai (PB), SNSF (PB), EMBO (CC), FEBS (AB) and NWO (RHH) as well as grants from the NKTH, OTKA (NFF78498, IN80481), Mecenatura (DE OEC Mec-1/2008), the NIH (DK59820), the ERC (2008-AdG-23118), the CNRS and the ANR.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamietz P. Poly(ADP-ribose) synthase is the major endogenous nonhistone acceptor for poly(ADP-ribose) in alkylated rat hepatoma cells. Eur J Biochem. 1987;169:365–372. doi: 10.1111/j.1432-1033.1987.tb13621.x. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- Allinson SL, Dianova II, Dianov GL. Poly(ADP-ribose) polymerase in base excision repair: always engaged, but not essential for DNA damage processing. Acta Biochim Pol. 2003;50:169–179. [PubMed] [Google Scholar]
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. Faseb J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja-Narashimha K, Padanilam BJ. PARP1 deficiency exacerbates diet-induced obesity in mice. J Endocrinol. 2010;205:243–252. doi: 10.1677/JOE-09-0402. [DOI] [PubMed] [Google Scholar]
- Durkacz BW, Omidiji O, Gray DA, Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N Engl J Med. 2009;24:24. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Garcia SF, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. NatMed. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Knight MI, Chambers PJ. Production, extraction, and purification of human poly(ADP-ribose) polymerase-1 (PARP-1) with high specific activity. Protein Expr Purif. 2001;23:453–458. doi: 10.1006/prep.2001.1513. [DOI] [PubMed] [Google Scholar]
- Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menissier-de Murcia J, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. ProcNatlAcadSciUSA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. JBiolChem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Moir RD, Schramm VL, Willis IM. Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell. 2005;17:595–601. doi: 10.1016/j.molcel.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Hinshaw DB, Hyslop PA, Spragg RG, Cochrane CG. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JL, Berger SJ, Berger NA. Effects of nicotinamide on NAD and poly(ADP-ribose) metabolism in DNA-damaged human lymphocytes. J Supramol Struct Cell Biochem. 1981;16:281–288. doi: 10.1002/jsscb.1981.380160308. [DOI] [PubMed] [Google Scholar]
- Smith BC, Hallows WC, Denu JM. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E10–19. doi: 10.1111/j.1749-6632.2009.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.