Abstract
Monocyte/macrophages and activated lymphocytes traffic through normal brain, and this trafficking is increased in inflammatory conditions such as HIV encephalitis (HIVE). HIVE is characterized in part by perivascular accumulations of macrophages. The earliest events in this process are poorly understood and difficult or impossible to address in humans. The SIV-infected macaque model of neuroAIDS has demonstrated migration of monocytes into the brain early in disease, coincident with peak SIV viremia. The chemotactic signals that initiate the increased emigration of mononuclear cells into the CNS have not been described. Here we describe astrocytes as a primary source of chemokines to facilitate basal levels of monocyte trafficking to CNS and that increased CCL7 production may be responsible for initiating the increased trafficking in neuroAIDS. We have previously published complementary in vivo work demonstrating the presence of MCP-3/CCL7 within the brain of SIV-infected macaques. Here we demonstrate that MCP-3/CCL7 is a significant chemokine produced by astrocytes, that basal monocyte migration may be facilitated by astrocyte-derived CCL7, that production of CCL7 is rapidly increased by TNF and thus likely plays a critical role in initiating neuroinvasion by SIV/HIV.
Keywords: Neuroimmunology, chemokine, cell trafficking, cytokines, chemotaxis, monocyte, HIV, pathology
Introduction
Astrocytes and other glial elements are known to modulate monocyte/macrophage migration into the brain. The glia limitans, formed predominantly by astrocyte foot processes, lines the basement membrane of microvascular brain endothelia. Thus, these cells are optimally situated to provide chemotactic signaling for emigration of monocytes/macrophages in to the brain. Bone marrow derived perivascular macrophages are the predominant cell type productively infected by HIV and SIV (30). Perivascular macrophages have relatively short half-lives and are replenished by circulating monocytes that traffic to the CNS. These cells are also known to traffic through brain (31). NeuroAIDS associated with SIV or HIV infection of macaques and humans respectively is characterized by perivascular accumulations of macrophages many of which are infected. Whether these cells are actively recruited or selectively retained in the brain is unclear. In late stage disease abundant expression of β-chemokines in brain parenchyma has been described which would be expected to contribute to increased recruitment of monocytes/macrophages (3). The signals involved in the initial recruitment/neuroinvasion of SIV/HIV infected monocytes/macrophages across the blood-brain barrier (BBB) are unknown. It is known, however, that viral infection of monocytes does not alter their chemotaxis (18), and that monocytes/macrophages, microglia (11) and astrocytes (13) can all secrete proinflammatory cytokines, and chemokines, to which monocyte/macrophages respond. It has been proposed that the initial neuroinvasion is mediated at least in part by astrocyte production of MCP-1/CCL2 (26). However, in vivo data examining MCP-1/CCL2 production in the brain parenchyma of SIV/HIV-infected individuals is conflicting (12, 20). Furthermore, MCP-1/CCL2 would also be expected to cause large numbers of lymphocytes to migrate into the CNS - an event rarely observed with either HIV or SIV infection or SIVE/HIVE.
It is difficult or impossible to examine these early events in vivo in humans, to confirm that the results have biological relevance. The premier in vivo model of HIV CNS infection is the SIV-infected rhesus macaque (8, 16, 20, 24, 29, 30). In vitro models have been developed to approximate early events of HIV-induced neurological disease (18, 26). We hypothesized that the initiation of neuroinvasion by monocyte/macrohpages is likely an exaggeration of the normal trafficking patterns to replace perivascular macrophages. We further hypothesized that chemokines secreted by astrocytes may be responsible for this basal level of monocyte turnover in the CNS based in part on observations that astrocytes under resting conditions produces chemokines capable of inducing monocyte migration which is enhanced by TNF-α (13). Until now, this basal migration induced by astrocyte conditioned media has been regarded as a nuisance for chemotactic studies, something that can be reduced by replacing the medium immediately before starting the chemotaxis assays (22).
In this study we demonstrate that this basal level monocyte migration is largely due to CCL7 released by astrocytes and that the production rapidly increases upon TNF-α stimulation. Furthermore, baseline production of CCL7 at the glia limitans is observed in vivo (20) and is likely responsible for the trafficking of mononuclear cells through the brain as described by Williams and Hickey (31). In addition to CCL7 we also demonstrate that stimulated astrocytes produce numerous chemokines specific for monocytes/macrophages and, thus are likely to play a major role in augmenting monocyte recruitment initiated by CCL7.
Materials & Methods
Culture of astrocytes
Astrocytes were cultured as described previously (10). In brief, frontal cortices from normal macaques were obtained at necropsy. Pooled supernatants from multiple astrocyte preparations were used in these studies to eradicate any variability between cultures. Meninges were removed and the tissue finely diced before incubation with PBS containing 0.25% trypsin (Invitrogen, Carlsbad, CA) and DNAse (4 U/ml) for 45 minutes at 37°C. Trypsin was inhibited with calf serum, and the resulting slurry filtered with 110μm pore filters. Filtrate was washed twice with culture media (M199 containing 5% glucose, 5% fetal calf serum, Penicillin, Streptomycin and fungizone) and plated in T25 flasks. Contaminating microglia were shaken free after 10–12 days. Residual microglia were killed using l-leucine methyl ester (10mM for 1hr). Astrocyte cultures were routinely >95% astrocytes as determined by glial fibrillary acidic protein (GFAP) staining.
Astrocyte supernatants
Astrocytes were cultured to near confluence in 75cm2 flasks (Corning) before incubation with 50, 100 or 500 U/ml TNF-α for 48 hours. This time was determined to induce maximal responses. Supernatants were decanted, centrifuged to remove cellular debris, and frozen at −80°C until use. Astrocytes were removed from flasks by trypsinization and prepared for molecular analysis.
Peripheral blood mononuclear cell migration induced by astrocyte supernatant
Frozen astrocyte supernatants were warmed to 37°C and used for migration assays. Control or TNF-α treated astrocyte conditioned medium (600μl) was added to lower compartments of 3μm pore multiwell insert filters (Becton Dickinson, Franklin Lakes, NJ). 100μl freshly isolated PBMCs at 106/ml from normal rhesus macaques were added to the upper compartment and allowed to transmigrate for 2 hours (to allow monocyte migration (26)) before preparation for flow cytometric analysis as described previously (24, 26). To determine the cell type and number of transmigrated cells, samples were labeled with CD11b (clone Bear1, Immunotech), CD14 (clone M5E2, BD Pharmingen) and CD3 (clone FN18, Biosource). This combination of antibodies in conjunction with forward vs. side scatter allows differentiation of T cells from monocytes. Samples of input cells were stained using the same antibodies to determine ratios of monocytes: T cells added to filters.
Gene array analysis for chemokine mRNA
Astrocytes were cultured to near confluence in 75cm2 flasks (Corning) before incubation with 50, 100 or 500 U/ml TNF-α for 48 hours. mRNA was extracted from astrocytes as described previously (15). Superarray™ GE chemokine kits were purchased and used according to manufacturers instructions. All samples were corrected to internal controls and graphed as relative units. A value of 2 or greater (relative to housekeeping genes) was determined to be significant.
Detection of CCL7 protein by fluorescence microscopy
Astrocytes were cultured in 75cm2 flasks as described above. After trypsinization, astrocytes were plated on 8 well coverslips and allowed to recover for 48 hours. Astrocytes were incubated in culture medium with or without TNF-α (Pharmingen, 100U/ml) for 48 hours. To inhibit secretion of chemokines, brefeldin A (10 μg/ml) was added to the media for the last 4 hours before fixation. Cells were fixed with 2% paraformaldehyde for 10 minutes and stained as described elsewhere (9). Numbers of CCL7 immunopositive vesicles were quantified using Image J. Statistical analyses between treatment groups was performed using the Kruzkal-Wallis Test in InStat (La Jolla, CA).
Ex vivo studies
Fresh brain tissues were obtained from normal macaques immediately post mortem at necropsy, sliced into 2mm thick sections using a custom fabricated brain slicer (Ted Pella Inc.) and maintained on filters in 6-well culture dishes (BD, Franklin Lakes, NJ). Macrophages were cultured from previously obtained bone marrow obtained from control macaques at necropsy as described (6). SIVmac251-infected (or control) macrophages were incubated with the brain slices for 4 hours either with, or without Brefeldin A to block protein transport and secretion (7). Following fixation in 2% paraformaldehyde and sucrose protection, the brain slices were embedded in OCT and sectioned at 50μm. Sections were then labeled for CCL7 and GFAP to detect astrocytes.
Results
Induction of mononuclear cell migration
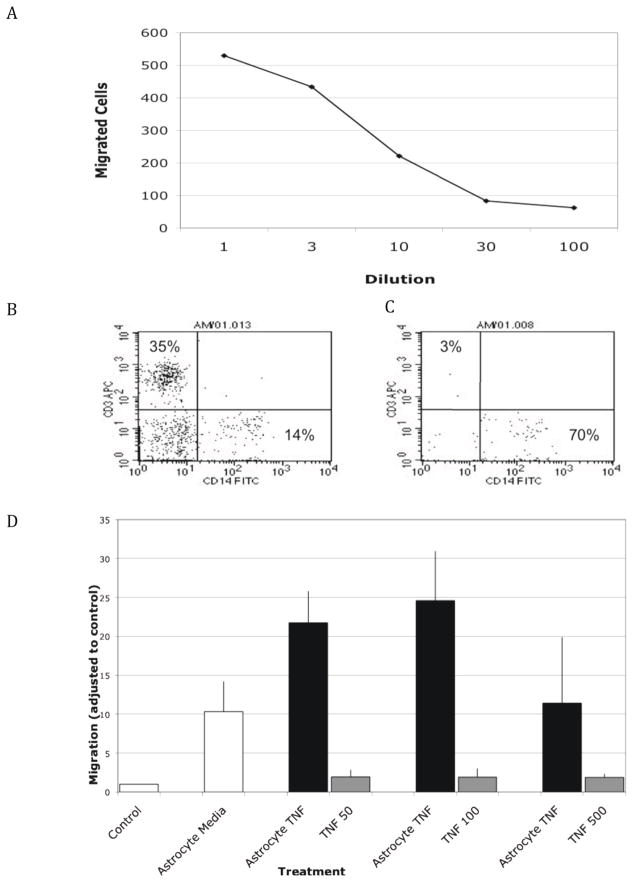
Astrocyte supernatants added to the lower compartment of 3μm pore multiwell insert filters were noted to induce chemotaxis of monocytes from PBMCs added to the upper chamber. To determine if this chemotaxis was dose-dependent, astrocyte conditioned supernatants were diluted with fresh medium. Figure 1A demonstrates that the induced chemotaxis was, indeed, dose-dependent, and not an artifact of this system. The transmigrated cells were determined to be monocytes by flow cytometry (Figure 1B, C) Cells added to the upper compartment were determined to contain approximately 35% lymphocytes and 14% monocytes (B). Following migration towards astrocyte-conditioned medium, it was determined that the cells comprised 70% monocytes and less than 3% lymphocytes, as determined by CD14 and CD3 staining, respectively (C).
Figure 1. Induction of monocyte/macrophage chemotaxis by resting astrocyte supernatants.
Monocytes/macrophages were observed to have increased chemotaxis in response to supernatants from astrocytes in culture. This was dose-dependent, and could be diluted out with astrocyte culture media (A). Migrated cells were determined to be macrophages by using forward and side scatter gating, followed by gating through CD11b. Cells added to the upper compartment contained approximately 35% lymphocytes and 14% monocytes. Cells that had migrated to astrocyte supernatant contained approximately 70% monocytes and less than 3% lymphocytes (C). Preincubation of astrocytes for 48 hours with TNF-α (D, grey bars) led to increased migration of PBMCs through the blood-brain barrier model over migration to cytokine alone (black bars) with migrated cells comprising 76% monocytes, and 3% lymphocytes.
We next sought to determine if exposure of astrocytes to TNF-α, the primary cytokine known to be elevated by the presence of HIV or SIV within the brain, would result in release of increased chemokine secretion. Treatment of astrocytes, cultured in 75cm2, flasks with 50, 100 or 500U/ml of TNF-α for 48 hours led to a significant increase in the number of migrating monocytes/macrophages (Fig. 1D, grey bars). Migrated cells comprised 76% monocytes, and 3% lymphocytes (data not shown). Within the concentration range used, there was no significant dose dependency. To ensure that the observed induction of chemotaxis was not induced directly by TNF-α, we performed control experiments whereby TNF-α was added to lower wells of 3μm pore multiwell inserts (Fig 1D, black bars).
Gene array analysis for chemokine mRNA
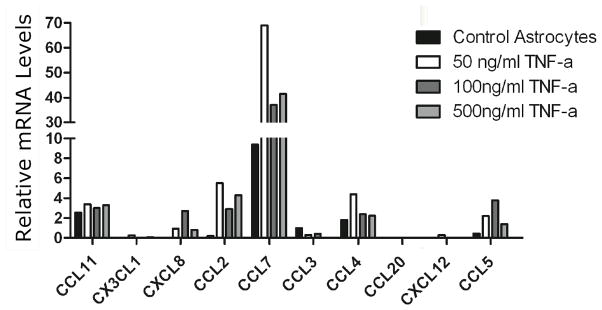
To determine which chemokines were being produced by resting astrocytes in culture we performed gene array analysis of chemokine mRNA. Analyses of data revealed that resting astrocytes in culture were transcribing large quantities of CCL7 mRNA, considerably smaller levels of CCL11 and CCL4, and insignificant levels of any other chemokine tested (Figure 2, black bars).
Figure 2. mRNA analysis of chemokine genes produced by astrocytes in culture.
Gene array analyses demonstrated that of the 23 chemokines tested, mRNA for CCL7 was produced in quantities significantly above all other genes tested by resting astrocytes (black bars). Following incubation with 50, 100 or 500 U/ml TNF-α, CCL7 was still produced at higher levels than any other chemokine on the array. Other monokines were expressed at significant levels, notably CCL2, CCL4, CCL5 and CCL11. Relative mRNA levels (compared with housekeeping genes) of genes of interest are shown in Table 1.
Considerably higher levels of CCL7 mRNA were produced by purified astrocytes in response to 50, 100 and 500 U/ml TNF-α than any other chemokine analyzed (Fig 2). Two-fold or greater elevations of target mRNA levels relative to control levels were considered to be significant. There were also substantial increases in levels of CCL2, CCL11 and CCL5 mRNAs following incubation with TNF-α compared to resting astrocytes. However, measured as proportion increase following TNF-α incubation, CCL2 was, by far, the most increased chemokine mRNA at 27 fold (Table 1). Other chemokines with notable increases were: CCL5 (8 fold), CCL4 (2.4 fold) and CCL7 (7.3 fold).
Table 1.
Chemokines with increased expression following TNF-α incubation
| Chemokine | Control Astrocytes | 50 U/ml TNF-α | 100 U/ml TNF-α | 500 U/ml TNF-α | Maximum fold change |
|---|---|---|---|---|---|
| CCL11 | 2.55 | 3.4 | 3 | 3.3 | 1.3 |
| CX3CL1 | 0 | 0.27 | 0 | 0.1 | 0.27 |
| CXCL8 | 0 | 0.93 | 2.7 | 0.8 | 2.7 |
| CCL2 | 0.2 | 5.53 | 2.9 | 4.3 | 27.65 |
| CCL7 | 9.4 | 69 | 37 | 41.5 | 7.34 |
| CCL3 | 1 | 0.32 | 0.43 | 0 | −3.125 |
| CCL4 | 1.8 | 4.4 | 2.38 | 2.25 | 2.2 |
| CCL20 | 0 | 0 | 0 | 0 | 0 |
| CXCL12 | 0 | 0.3 | 0 | 0 | 0.3 |
| CCL5 | 0.46 | 2.2 | 3.8 | 1.4 | 8.25 |
Localization of CCL7 production within astrocytes
To examine the presence of CCL7 protein in astrocytes, we performed immunofluorescence for CCL7 and the astrocyte specific protein glial fibrillary acidic protein (GFAP). Resting astrocytes had a low level of CCL7 staining (Fig. 3A). The number of CCL7 immunopositive vesicles was increased slightly following stimulation with TNF-α (100U/ml for 48 hours) although this increase was not significant (Fig 3B). We suspect this is due to secretion of the chemokine. Numerous small vesicles were observed over much of the cell area. Astrocytes stimulated with TNF-α and brefeldin A (Fig 3D) had significantly increased numbers of vesicles per cell compared with the brefeldin A control (Fig 3C) (p<.001), demonstrating that the chemokine is normally secreted by astrocytes. These data are presented graphically in Figure 3E.
Figure 3. Visualization of CCL7 protein within astrocytes in culture.
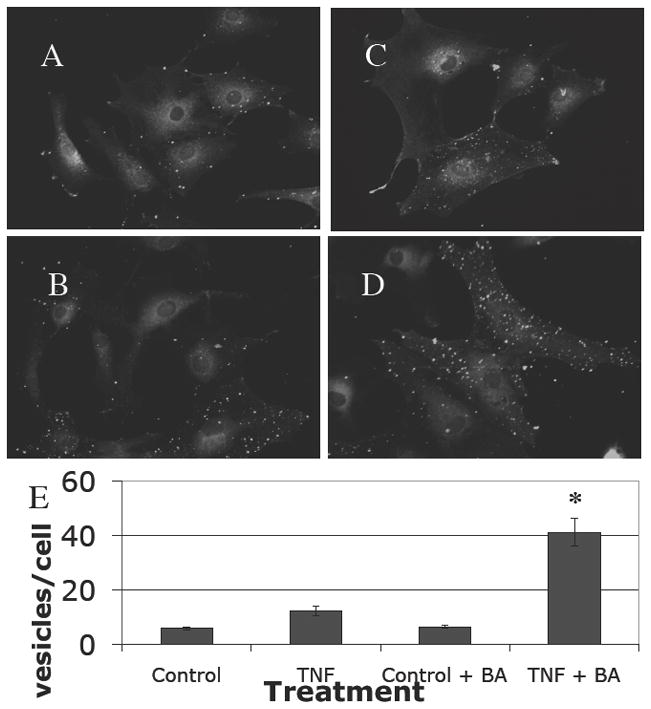
CCL7 expression was visible weakly in many astrocytes in culture (A). Stimulation with TNF-α increased the amounts of CCL7 detected slightly, with a characteristic punctate vacuolar distribution (B). This was augmented further when the cells were incubated with brefeldin A (C) or with TNF-α and brefeldin A (D), indicating that CCL7 is constitutively produced and released from cells, but that the rate of production can be increased by proinflammatory stimulus (demonstrated in graph E).
CCL7 production in situ
To examine the production of CCL7 in tissues in response to SIV-infected macrophages, we performed ex vivo studies. Expression of CCL7 (green, open arrows) is present in normal frontal cortex predominantly in cells morphologically consistent with endothelial cells (Fig 4A, closed arrow), rather than in parenchymal astrocytes (red). Figure 4B demonstrates colocalization of CCL7 (green) with von Willebrand’s Factor (blue), indicating that CCL7 is expressed in endothelial cells. Colocalization of blue and green (turquoise) is marked with a closed arrow. Following four hours incubation with SIVmac251-infected macrophages at (106/ml) increased CCL7 expression was evident in astrocytes (appearing yellow due to colocalization). Brefeldin A blocking forced the CCL7 to remain in vesicles along the length of astrocyte processes (Fig 4C).
Figure 4. CCL7 expression in situ.
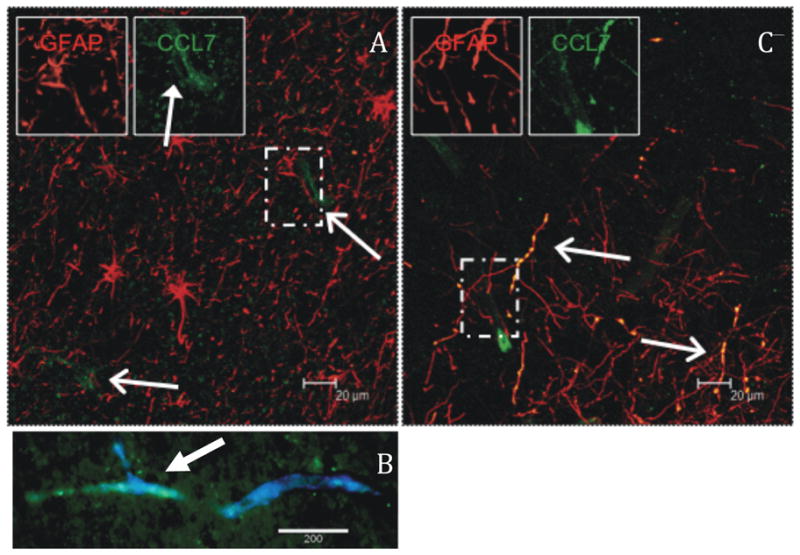
Brain slices were noted to have limited expression of CCL7 (green), predominately perivascular in localization (A). One such vessel double labeled with Von Willebrand’s Factor (blue) and CCL7 (green) is shown in B. After 4 hr incubation with SIVmac251-infected macrophages there was marked increase in CCL7 expression in astrocytes (B, red). The beaded pattern is due to blocking secretion with brefeldin A for four hours.
Taken together these results demonstrate that CCL7 is an important chemokine actively produced and secreted by astrocytes, and that this production can be increased by SIV-infected macrophages and cytokines found within the AIDS encephalitic brain.
Discussion
Our data indicate that CCL7 secretion by astrocytes increases in response to TNF-α stimulation (in vitro) or exposure to SIV infected macrophages (ex vivo), and that CCL7 actively recruits macrophage transmigration across 3 μm filters, and potentially across the blood-brain barrier.
Multiple lines of evidence implicate trafficking of monocyte/macrophages from the periphery to brain in neuroinvasion by HIV and SIV (12, 16, 18–20, 27, 30). How this process is initiated is however unclear. In this study we have begun to address this by examining chemokine production by resting astrocytes in the hope of elucidating processes that initiate infiltration by HIV/SIV-infected cells into the brain. Our data implicate CCL7 production by resting astrocytes in this process with other mediators such as CCL2, CCL4 and CCL5 playing a much greater subsequent role as has been suggested previously (5, 11–13, 20, 26, 27). The data further suggest that CCL7 may play a major role in the basal level of trafficking of monocytes across the BBB.
These data generated from an in vitro model of monocyte chemotaxis complement existing knowledge of very early in vivo events in SIV-infected macaques (19). Of particular relevance, in vivo data from macaques infected with neurovirulent SIV demonstrate TNF-α expression in the brain concurrent with increased endothelial adhesion molecule expression, chemokine production, mononuclear cell infiltration into the brain and SIV neuroinvasion (19, 20, 23, 28). TNF-α has similarly been implicated in the neuropathogenesis of AIDS (23, 28). In SIV and HIV-associated encephalitis, and other encephalitides, the proinflammatory stimulus usually originates within the parenchyma of the brain, rather than in the circulation. Therefore, we added TNF-α to astrocytes which are a key parenchymal component of the BBB.
The current study suggests CCL7 is responsible for normal trafficking of monocytes/macrophages through the BBB (into the parenchyma), and, thus, for initiating SIV/HIV neuroinvasion. This interpretation is supported by the presence of CCL7 in normal brain tissues of both humans and rhesus macaques (20). Furthermore, molecular analysis of the chemokine mRNA produced by resting astrocytes in culture showed CCL7 was the predominant chemokine produced. This mRNA was translated into protein, as supernatants of astrocytes in culture contained chemokines capable of inducing chemotaxis in resting monocytes (yet not in lymphocytes). Taken together, these data suggest that previously observed trafficking of hematogenous cells through brain may be due in very large measure to production of CCL7 by astrocytes, most probably at foot processes as the majority of these mononuclear cells are immediately adjacent to the BBB (31). Further evidence for this story is given by a demonstration by Chen and colleagues that CCR2, a receptor for CCL7 is required to be present on monocytes/macrophages for migration into virally infected brain (1).
We also investigated the role of CCL7 and other chemokines produced by astrocytes after stimulation by TNF-α. We focused on TNF-α because there is an initial increase in levels of TNF-α within brains of monkeys infected with SIVmac251 associated with SIV neuroinvasion (14). Astrocytes are known to produce chemokines under the influence of proinflammatory cytokines such as TNF-α (5, 13, 25–27). Data from several groups indicate that HIV-infected monocyte/macrophages are activated and produce significant quantities of TNF-α (24, 26). Thus we expected to find an increase in chemokine production in astroctyes treated with TNF-α. This was observed, but the effect was not global, with striking increase in the mRNA levels of chemokines (such as CCL2, CCL7, CCL5 and CCL4) associated with monocyte recruitment. Elevations of each of these chemokines has previously been associated with neuroAIDS in humans or macaques (2, 17, 20, 21). The probable synergistic, or at the very least sequential release of these chemokines would be expected to increase numbers of monocytes/macrophages to migrate through the BBB, and thus augment neuroinvasion and the formation of perivascular cuffs prominent in neuroAIDS.
In conclusion, we suggest that CCL7 may be responsible for initial entry of HIV-infected cells into the brain, probably during ‘normal’ turnover of perivascular macrophages (4, 31). Subsequent production of TNF-α in the parenchyma by these infected, activated macrophages stimulates astrocytes to produce increased levels of CCL7 and additional chemokines, notably CCL2, CCL5 and CCL4 that are important for further recruitment of monocyte/macrophage to the CNS.
Acknowledgments
We thank Jodi Zarycki for gene array analyses. We also thank Maury Duplantis and Christina Polizzi for excellent sample recovery at necropsy, and Desiree Waguespack for flow cytometry assistance. This work was supported by public health service grants NS30769, MH61192, AA13828, MH077544, RR20159, RR00164 and RR00168. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award. Nicole Renner is a Louisiana Board of Regents Scholar LEQSF(2007-12)-GF-15.
References
- 1.Chen BP, Kuziel WA, Lane TE. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J Immunol. 2001;167:4585–92. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- 2.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 4.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–37. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz AA, Lyman WD, Berman JW. Tumor necrosis factor alpha and transforming growth factor beta upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol. 1995;57:193–8. doi: 10.1016/0165-5728(95)00011-p. [DOI] [PubMed] [Google Scholar]
- 6.Ivey NS, Renner NA, Moroney-Rasmussen T, Mohan M, Redmann RK, Didier PJ, Alvarez X, Lackner AA, Maclean AG. Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization. J Neurovirol. 2009:1–12. doi: 10.1080/13550280902998413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson CL. Brefeldin A revealing the fundamental principles governing membrane dynamics and protein transport. Subcell Biochem. 2000;34:233–72. doi: 10.1007/0-306-46824-7_6. [DOI] [PubMed] [Google Scholar]
- 8.Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–46. [PubMed] [Google Scholar]
- 9.MacLean AG, Orandle MS, Alvarez X, Williams KC, Lackner AA. Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells. J Neuroimmunol. 2001;118:223–32. doi: 10.1016/s0165-5728(01)00348-4. [DOI] [PubMed] [Google Scholar]
- 10.MacLean AG, Orandle MS, MacKey J, Williams KC, Alvarez X, Lackner AA. Characterization of an in vitro rhesus macaque blood-brain barrier. J Neuroimmunol. 2002;131:98–103. doi: 10.1016/s0165-5728(02)00256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManus CM, Brosnan CF, Berman JW. Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J Immunol. 1998;160:1449–55. [PubMed] [Google Scholar]
- 12.McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and Chemokine-Receptor Expression in Human Glial Elements: Induction by the HIV Protein, Tat, and Chemokine Autoregulation. Am J Pathol. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- 14.Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76:5797–802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orandle MS, Williams KC, MacLean AG, Westmoreland SV, Lackner AA. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J Virol. 2001;75:4448–52. doi: 10.1128/JVI.75.9.4448-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persidsky Y, Gendelman HE. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997;62:100–6. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- 17.Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–510. [PubMed] [Google Scholar]
- 19.Sasseville VG, Lane JH, Walsh D, Ringler DJ, Lackner AA. VCAM-1 expression and leukocyte trafficking to the CNS occur early in infection with pathogenic isolates of SIV. J Med Primatol. 1995;24:123–131. doi: 10.1111/j.1600-0684.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 20.Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996;93:700–4. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanimirovic D, Zhang W, Howlett C, Lemieux P, Smith C. Inflammatory gene transcription in human astrocytes exposed to hypoxia: roles of the nuclear factor-kappaB and autocrine stimulation. J Neuroimmunol. 2001;119:365–76. doi: 10.1016/s0165-5728(01)00402-7. [DOI] [PubMed] [Google Scholar]
- 23.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 24.Veazey RS, I, Tham C, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss JM, Berman JW. Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J Neuroimmunol. 1998;91:190–7. doi: 10.1016/s0165-5728(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–903. [PubMed] [Google Scholar]
- 27.Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up- regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–9. [PubMed] [Google Scholar]
- 28.Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–82. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- 29.Westmoreland SV, Williams KC, Simon MA, Bahn ME, Rullkoetter AE, Elliott MW, deBakker CD, Knight HL, Lackner AA. Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol. 1999;155:1217–28. doi: 10.1016/S0002-9440(10)65224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular Macrophages Are the Primary Cell Type Productively Infected by Simian Immunodeficiency Virus in the Brains of Macaques. Implications for the neuropathogenesis of aids. J Exp Med. 2001;193:905–16. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KC, Hickey WF. Traffic of hematogenous cells through the central nervous system. Curr Top Microbiol Immunol. 1995;202:221–45. doi: 10.1007/978-3-642-79657-9_15. [DOI] [PubMed] [Google Scholar]