Abstract
Electrophysiological studies showed that mesostriatal dopamine (DA) neurons increase activity in response to unpredicted rewards. With respect to other functions of the mesostriatal dopaminergic system, dopamine’s actions show prominent laterality effects. Whether changes in DA transmission elicited by rewards also are lateralized, however, has not been investigated. Using [11C]raclopride-PET to assess the striatal DA response to unpredictable monetary rewards, we hypothesized that such rewards would induce an asymmetric reduction of [11C]raclopride binding in the ventral striatum, reflecting lateralization of endogenous dopamine release. In 24 healthy volunteers, differences in the regional D2/3 receptor binding potential (ΔBP) between an unpredictable reward condition and a sensorimotor control condition were measured using the bolus-plus-constant-infusion [11C]raclopride method. During the reward condition subjects randomly received monetary awards while performing a “slot-machine” task. The ΔBP between conditions was assessed in striatal regions-of-interest and compared between left and right sides. We found a significant condition × lateralization interaction in the ventral striatum. A significant reduction in binding potential (BPND) in the reward condition versus the control condition was found only in the right ventral striatum, and the ΔBP was greater in the right than the left ventral striatum. Unexpectedly, these laterality effects appeared to be partly accounted for by sex differences, as our data showed a significant bilateral BPND reduction in women, while in men the reduction reached significance only in the right ventral striatum. These data suggest that DA release in response to unpredictable reward is lateralized in the human ventral striatum, particularly in males.
Keywords: positron emission tomography, dopamine, hemispheric, motivation
Introduction
The mesostriatal dopaminergic system plays a major role in the neural processing underlying motivated and reward-related behavior. With respect to some functions of this system, dopamine (DA) transmission in the striatum shows prominent laterality effects. In rodents, these laterality effects depended upon the direction of motor behavior and the type of reinforcement schedule in some experimental conditions (Zimmerberg et al., 1974; Glick et al., 1980; 1981; Yamamoto et al., 1982; Yamamoto & Freed, 1984; Szostak et al., 1986; Morice et al., 2005), whereas in others the lateralization of DA release appeared fixed with respect to function or task (Besson & Louilot, 1995; Silva et al., 2007). The observed lateralization effects have been hypothesized to be attributable to an asymmetry in the nigrostriatal projections (Kelly & Moore, 1977; Pycock & Marsden, 1978) and have been conceptualized as one of the biological bases for lateralized behavior (Rodriguez et al., 1994; Alonso et al., 1997).
In humans laterality effects in elements of the DA systems appear regionally selective. In the putamen, DA transporter (DAT) and D1-receptor binding were higher on the left than the right, whereas in the caudate and/or accumbens DAT, D1-receptor and D2/3-receptor binding and DA synthesis capacity were higher on the right than the left (Hietala et al., 1999; Laakso et al., 2000; van Dyck et al., 2002; Vernaleken et al., 2007; Cannon et al., 2009). A meta-analysis of Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) studies on D2 binding in healthy humans (Larisch et al., 1998) indicated a preponderance of DA D2 receptors on the right side in the striatum. In addition, the resting asymmetry in D2/3-receptor availability was associated with greater positive incentive motivation (Tomer et al., 2008). Whether hemispheric asymmetries exist in the mesostriatal DA response to rewarding stimuli, however, has not been investigated.
In response to unpredicted rewards, mesostriatal DA neurons emit a phasic burst of electrophysiological spike activity (Horvitz et al., 2007; Schultz, 2007) which in the accumbens is associated with an elevation of intrasynaptic DA concentrations (Garris et al., 1994). PET studies showed that the binding of [11C]raclopride, a radiotracer that is sensitive to changes in intrasynaptic DA concentrations, decreased in the ventral striatum while playing a video game for points relative to rest (Koepp et al., 1998) or in response to large monetary rewards versus large monetary losses (Pappata et al., 2002), and decreased in the caudate in response to unpredictable monetary rewards (Zald et al., 2004), putatively reflecting the effects of increased DA release.
Here, we applied a bolus plus constant-infusion (B/I) method for administering [11C]raclopride to obtain BP values that are measured under equilibrium conditions (Lassen, 1992; Carson et al., 1997). We tested for the first time the hypothesis that the ventral striatal DA response to reward is lateralized. Because the previous PET-studies of reward-related DA release did not control for lateralization effects, it was not clear on which side of the ventral striatum this increase would occur.
METHODS AND MATERIALS
Subjects
Twenty-four right-handed healthy volunteers (12 women) between ages 20 and 46 (mean=32±8.1) years participated. Male and female participants were group-matched for age (mean age men: was 32.1years, SD: 9.2; and mean age women: 33.5 years, SD: 7.2). There was no significant age difference between men and women (t=0.4, p<0.6). Volunteers were screened via medical history, physical examination, laboratory testing (including drug screening), neuromorphological MRI, structured (Structured Clinical Interview for DSM-IV; (Spitzer et al., 2002) and unstructured psychiatric interviews, and intelligence testing (Wechsler Abbreviated Scale of Intelligence; mean IQ=123±15.0). Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971). Volunteers were excluded if they had: a current or past psychiatric disorder; a major medical or neurological disorder; exposure to drugs likely to affect cerebral physiology, monoaminergic neurotransmitter function, or vascular function within 3 weeks; illicit drug use or alcohol abuse within one year; lifetime history of alcohol or drug dependence; tobacco use within 1 year; gambling history or pathological gambling behavior as assessed with the lie/bet screen (Gotestam et al., 2004) and South Oaks Gambling Screen (SOGS, Stinchfield, 2002), intelligence quotient <85; current pregnancy or breast feeding; general MRI exclusions. Participants provided written informed consent after receiving a full explanation of the study procedures and risks, as approved by the NIMH IRB and in accordance with the declaration of Helsinki (Rickham, 1964).
Behavioral Conditions
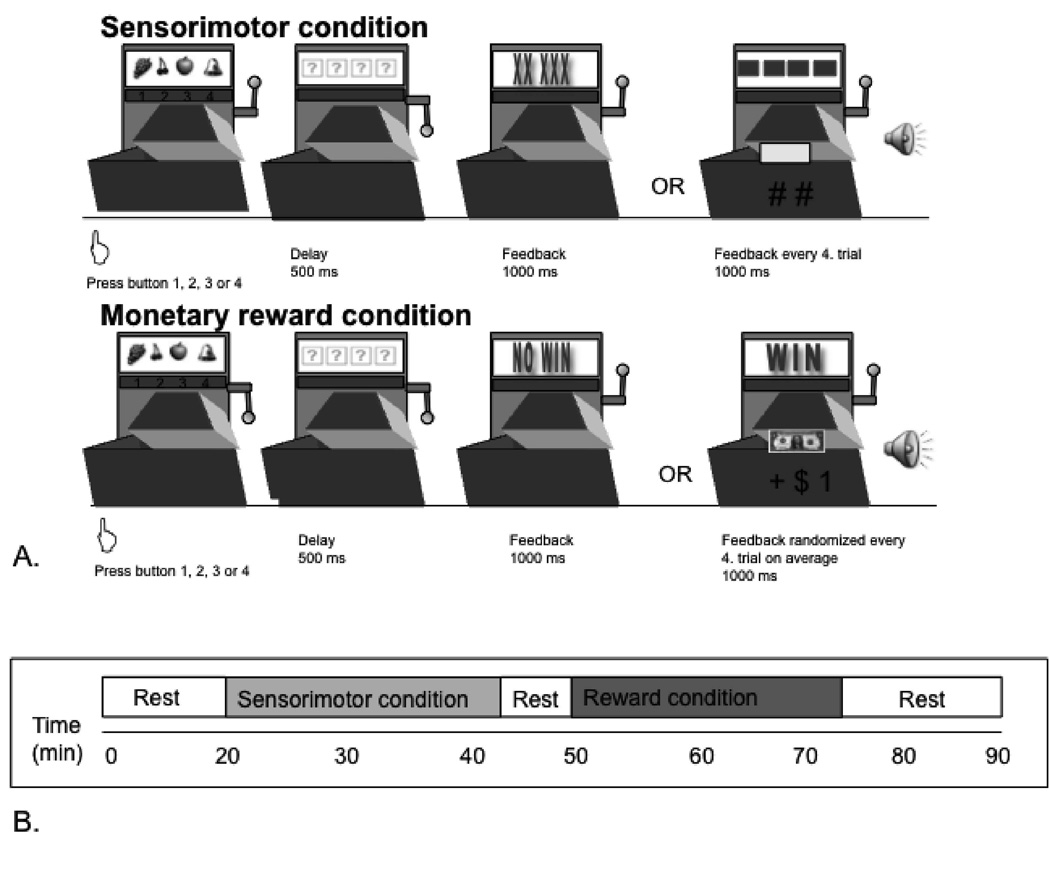
The slot machine task involved two conditions, each consisting of 180 trials, each of average duration 8 sec. In the monetary-reward condition subjects received financial rewards unpredictably, in a pseudo-randomized order with an average of one reward per four trials. In the sensorimotor control condition subjects performed the same task without receiving rewards, to control for motor activation or other nonspecific aspects of task-performance (figure 1a).
Figure 1. Illustration of the experimental task.
A. Illustration of a sensorimotor and a rewarded trial. During each trial, subjects were presented four distinct pictures (apple, grape, cherry, bell) presented in a “slot-machine” motif. Subjects were asked to choose one of the four with a button press on a four-button response box using their right hand. This response was followed by a 500 msec delay. In the rewarded trials a one dollar bill appeared for 1,000 msec and subjects heard the characteristic sound of an opening cash-register door. These monetary gains were provided in a pseudo-randomized order with an average of one reward per every fourth trial. In the sensorimotor control trials, subjects instead were presented a meaningless symbol accompanied by a “click” sound on every fourth trial. After receiving the trial outcome subjects were presented their running total of earnings for 1,000 msec. Displaying the actual balance account prevented rapid discounting of the rewards presented. At the end of each trial subjects viewed a blank screen for 1,000 msec. During the reward task subjects were unaware of which trial or picture would lead to the receipt of a reward, except that the same picture could not provide a reward in two consecutive trials. Subjects thus were instructed not to select the same picture more than twice in a row (selection of the same picture twice-in-a-row led to interruption of the task, and the task continued only after another picture was selected).
B. Timeline of the experiment. Subjects rested for the initial 20 min to allow [11C]raclopride to approach equilibrium. During the subsequent 24.6±2.0 min subjects performed the sensorimotor control task. Beginning 50 min after the start of the [11C]raclopride infusion, subjects performed the monetary reward condition for 24.1±1.7 min. The timing of the tasks relative to scanning was based upon previous optimization studies for PET-[11C]raclopride imaging using the bolus plus constant infusion approach (Watabe et al. 2000; Garraux et al. 2007).
Before scanning, subjects performed a short practice session of each task. During scanning subjects rested for the initial 20 min to allow [11C]raclopride to approach equilibrium. During the subsequent 24.6±2.0 min subjects performed the sensorimotor control task. Beginning 50 min after the start of the [11C]raclopride infusion, subjects performed the monetary reward condition for 24.1±1.7 min (fig. 1b). This timing allowed the distribution of [11C]raclopride to come into equilibrium for each of the two task conditions during the scanning epochs described below. During the 24 min epochs that corresponded to each task condition, subjects alternated between two-min periods in which they actively performed the task and one-min periods when they rested to minimize fatigue. Each subject won a total of $33 during the rewarded condition, which they received by check following the study.
Image Acquisition
PET scans were acquired using a GE-Advance scanner in 3D-mode (3D resolution=6 mm full-width at half-maximum). During scanning the subject’s head was immobilized using a thermoplastic mask. The PET data were reconstructed using a Hanning-filter and Gaussian-fit scatter-correction method. A transmission scan was acquired using rotating 68Ge/68Ga rods to perform attenuation-correction of the emission scans.
The [11C] raclopride (20 mCi) was administered as an initial bolus over 60 seconds after Watabe et al (Watabe et al., 2000), followed by a maintenance infusion over the remainder of the scanning session (a total of 90 minutes) using a computer-operated pump (Harvard Instruments, Natick, MA). Dynamic emission scanning (27 frames of increasing length) was initiated with injection of the [11C] raclopride bolus.
MRI images were acquired at 3 or 1.5 Tesla, using T1 weighted sequences to provide an anatomical framework for image analysis (in-plane resolution: 0.86mm, slice thickness: 1.2mm).
PET data analyses
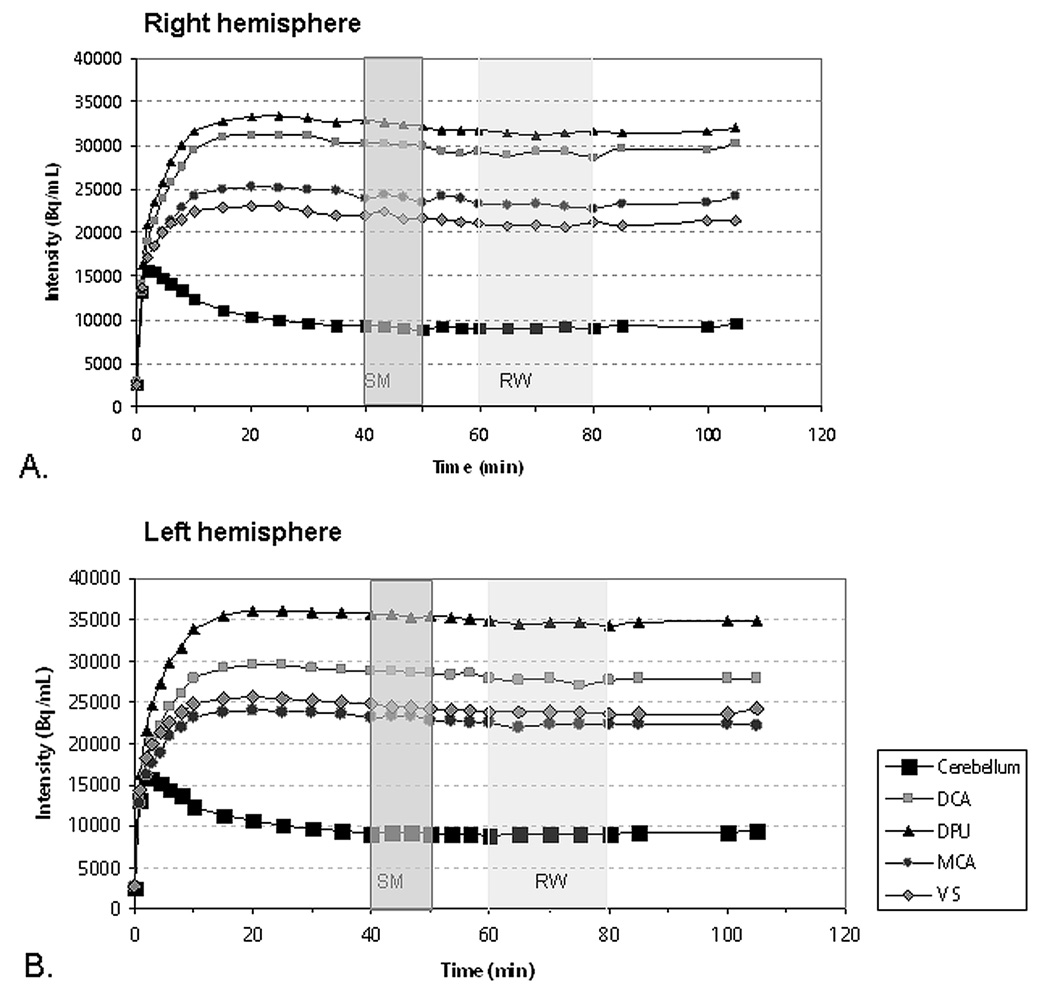
Corrections for subject motion during the 100 min PET acquisition were performed with a mutual-information-registration of each image-frame to a standard frame (10–15 min after injection) before attenuation-correction. Based on the calculated motion, the transmission images were resliced and projected for final attenuation-correction, reconstruction and realignment. The realigned frames acquired during the first eight min of scanning, during which the radiotracer distribution was most sensitive to CBF so that cortical outlines were sufficiently evident to guide image co-registration, were summed to generate an image that was co-registered to the corresponding MRI image using FLIRT (FMRIB, University of Oxford, UK). The derived transformation matrix was applied to the composite images consisting of 1) the baseline (frames acquired between 40 and 50 min) and 2) the reward condition (frames acquired between 60 and 80 min) images, which were acquired under equilibrium conditions achieved as subjects performed the sensorimotor control and monetary reward tasks (figure 2), respectively, after Garraux et al. (2007).
Figure 2.
Decay-corrected, tissue radioactivity concentrations across time obtained using PET in the predefined regions-of-interest within the right (A) and left hemispheres (B), with indication of the time epochs in which PET data were analyzed to reflect the sensorimotor (darker shading) and reward conditions (lighter shading). The timing of the image acquisition for each task condition was optimized for PET-[11C]raclopride imaging studies applying the bolus plus constant infusion approach by Watabe et al. (2000). Abbreviations: Bq - becquerels; VS - ventral striatum; DPU - dorsal putamen; MCA - middle caudate; DCA - dorsal caudate. SM: sensorimotor condition. RW: reward condition.
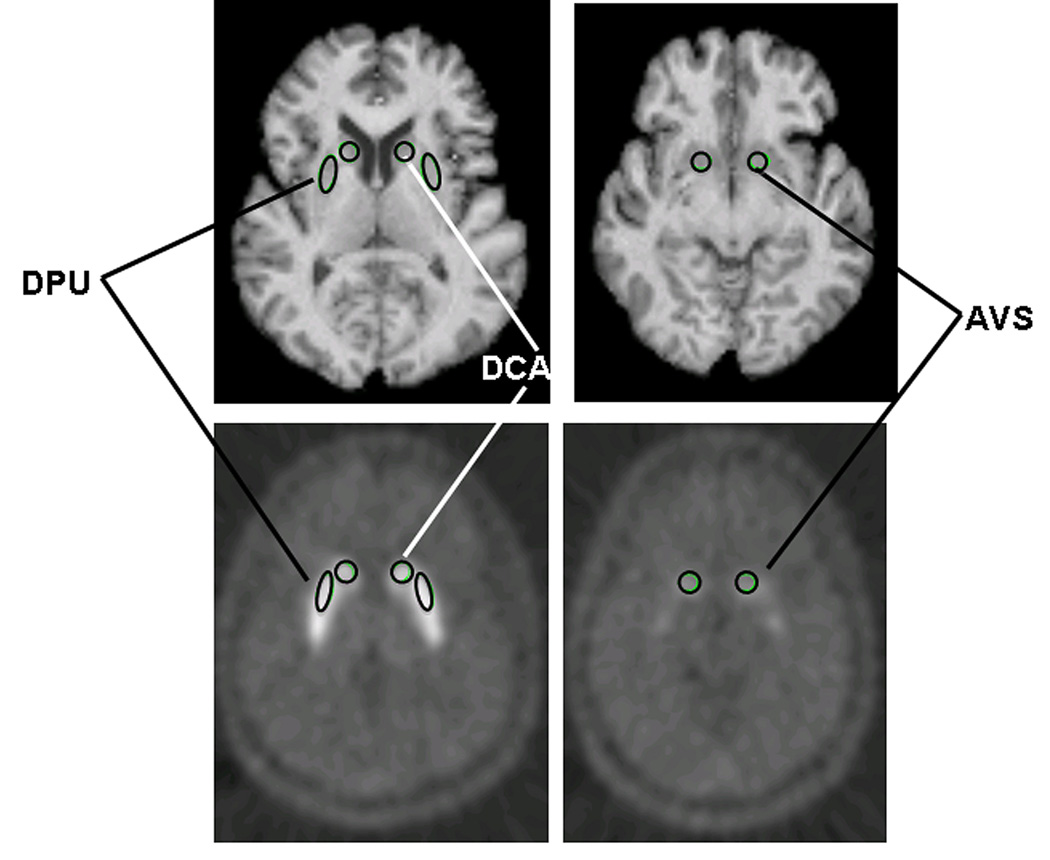
Mean tissue radioactivity concentrations from the baseline and reward images were extracted using MRI-based regions-of-interest (ROI), defined on a template MRI image using MEDx software (Medical Numerics, Sterling, VA) in the anteroventral striatum, ventral putamen, dorsal putamen, middle caudate, dorsal caudate and cerebellum (figure 3), after Drevets et al (1999; 2001). Each individual’s MRI was registered to the template brain and the ROIs were repositioned as needed to accommodate individual differences in anatomy. The anatomical accuracy and symmetry of each set of individual ROI was verified by a neuroscientist familiar with striatal anatomy (W. Drevets). These ROIs then were back-transformed into the subject’s native MRI space and applied to the co-registered PET images. To improve sensitivity the anteroventral striatum and ventral putamen ROI were combined into a single ventral striatal ROI. In primates the cells with histochemical and connectional features of the nucleus accumbens shell implicated in reward learning are scattered through the accumbens area, ventromedial caudate and anteroventral putamen (Heimer & Alheid, 1991), and previous PET studies of amphetamine-induced DA release showed that euphoria correlated with DA release in both the anteroventral striatum and the ventral putamen (Drevets et al., 2001; Martinez et al., 2003). Results for these ROI considered separately were addressed post hoc (see Supplementary Data).
Figure 3.
Location of the MRI-based regions-of-interest (ROIs) defined a priori in the putamen, caudate and anteroventral striatum (after Drevets et al 2001). Regional PET measures were extracted using from each ROI and compared between conditions. Abbreviations: DPU: dorsal putamen; DCA: dorsal caudate, AVS: anteroventral striatum.
Decay-corrected, tissue radioactivity concentrations (C) were obtained from each ROI using a calibrated phantom standard to convert tomographic counts to Bq/ml. Mean radioactivity in the reference region (cerebellum; C’) was used to factor out the effects of free and non-specifically bound [11C]raclopride. The percent change in [11C]raclopride binding was computed as the difference in BPND (C/C’−1 for each ROI) between baseline and reward images (Watabe et al., 2000):
The a priori hypothesis was tested using two-factorial ANOVA’s with task conditions and lateralization as factors on the BPND obtained in the ventral striatum, with a null hypothesis that there is no effect of condition, and no interaction between condition and laterality. Where the ANOVA results indicated significant laterality-by-condition effects, specific contrasts between sides and conditions were performed using paired t-tests with the p-values corrected (Bonferroni) for comparisons in the left, right and right-versus-left ventral striatum (i.e. significance threshold set at alpha=0.0167) for two-tailed p-values.
The significance of ΔBP and laterality differences in other ROI was assessed post hoc to evaluate the specificity of the ventral striatal findings. A three factorial ANOVA with region, task condition and laterality was performed to assess differential responses between regions. Additional post hoc analyses explored laterality effects in the baseline BPND values, correlations between age and ΔBP, and sex differences in ΔBP (McGeer et al., 1977; Antonini & Leenders, 1993; Pohjalainen et al., 1998; McCormack et al., 2004). A four factorial ANOVA with region, task condition, laterality and gender was performed to test for an effect of gender on the differential responses between regions.
Finally, to more specifically localize the changes in [11C]raclopride BP between conditions, we performed a voxel-wise analysis post hoc using Statistical Parametric Mapping Software (SPM8b; Wellcome Department of Imaging Neuroscience) implemented within Matlab 7.7. (MathWorks). The two condition-specific composite images of raclopride BPND were spatially normalized to the Montreal Neurological Institute spatial array (http://www.bic.mni.mcgill.ca) and smoothed using a 6 mm FWHM Gaussian kernel. Processed raclopride binding images then were analyzed voxel-wise within the general linear model framework using a paired t-computation that tested for a change in [11C]raclopride BPND during the reward versus the baseline conditions. MNI coordinates were non-linearly translated to the stereotaxic spatial array of Talairach and Tournoux (1988) (http://www.bioimagesuite.org/Mni2Tal/index.html). The analysis was constrained by searching only areas where receptor-specific binding exceeded a BPND threshold≥1.2, which essentially limited the search area to the striatum. We report results that remained significant after applying the Small Volume Correction for multiple comparisons using the cluster test (provided within SPM software; Worsley et al., 1996) within an atlas-based, masked image for the entire striatum (right-and left) with the voxel t-value threshold for defining clusters-of-interest set at p<0.01.
RESULTS
Regions-of-Interest Analyses
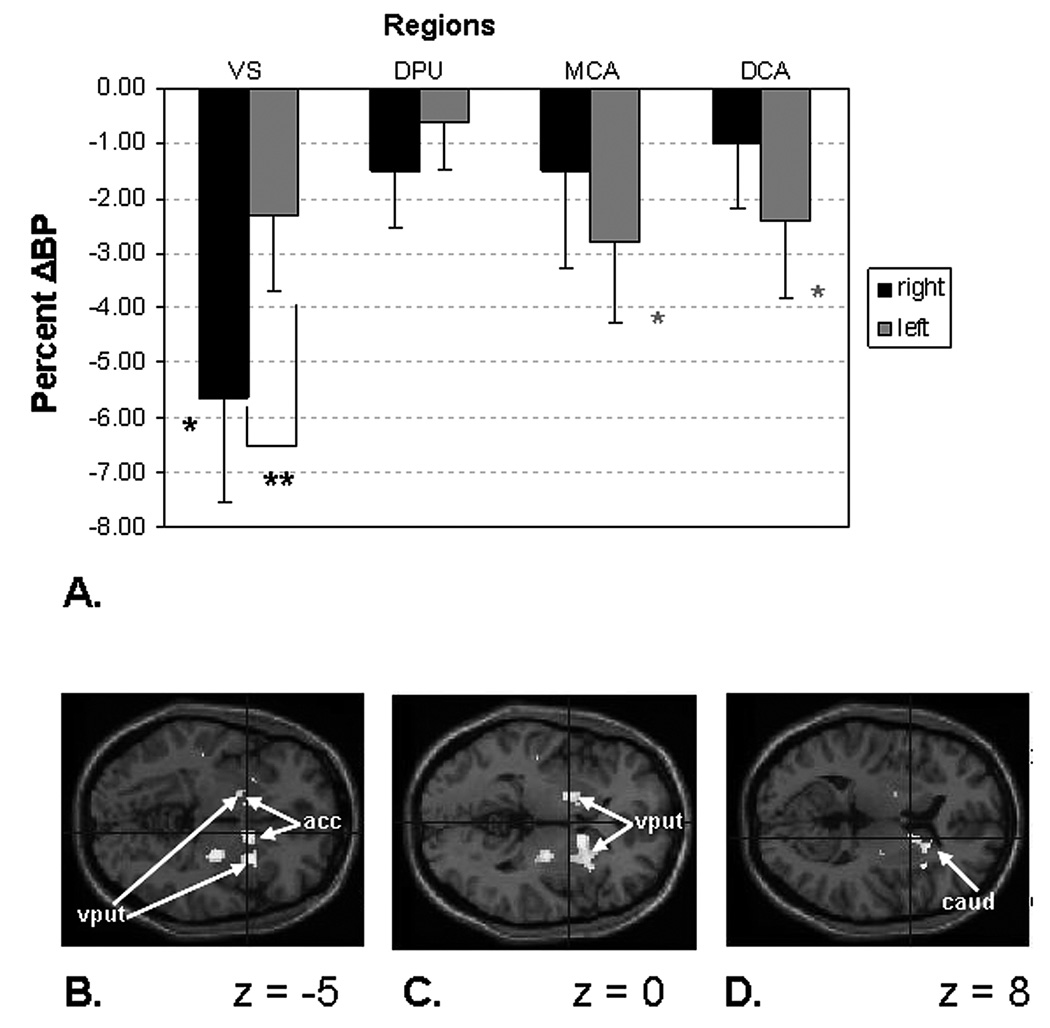
Mean BP and ΔBP values appear in Table 1 and figure 4a. The three factorial ANOVA of region × laterality × condition showed significant main effects of region (F3,69=62.1, p<0.001) and condition (F1,23=4.37, p<0.005), as well as a significant region × laterality interaction (F3,69=45.9, p<0.001). Subsequent paired t-tests showed a trend for ΔBP differences between the reward and the sensorimotor control conditions at a Bonferroni-corrected level of significance of p<0.004 (alpha level of 0.05 corrected for 12 comparisons) in the following comparisons: right ventral striatum versus right middle caudate (p<0.005), right ventral striatum versus right dorsal caudate (p<0.007) and right ventral striatum versus right dorsal putamen (p<0.01).
Table 1. Mean regional binding potential (BPND) values for each condition and mean changes in BPND (Δ BP, in percent) between conditions.
The regions in which the a priori hypotheses were tested appear in bold font. Levels of significance (two-tailed) and t-values are given for the one-sample t-tests of Δ BP.
| Region | Regional BPND Control Mean (SD) |
Regional BPND Reward Mean (SD) |
% difference (ΔBP) Mean (SD) |
t-value | p-value |
|---|---|---|---|---|---|
| Right ventral striatum | 1.90 (0.63) | 1.82 (0.68) | −5.65 (9.53) | 2.9 | 0.008 |
| Left ventral striatum | 2.20 (0.56) | 2.1 (0.62) | −2.32 (6.74) | 1.69 | 0.1 |
| Right dorsal putamen | 2.78 (0.36) | 2.73 (0.35) | −1.51 (5.03) | 1.47 | 0.15 |
| Left dorsal putamen | 2.92 (0.36) | 2.89 (0.30) | −0.62 (4.2) | 0.72 | 0.47 |
| Right middle caudate | 1.94 (0.53) | 1.92 (0.59) | −1.52 (8.59) | 0.86 | 0.39 |
| Left middle caudate | 1.79 (0.51) | 1.74 (0.5) | −2.81(7.22) | 1.9 | 0.06 |
| Right dorsal caudate | 2.48 (0.34) | 2.45 (0.32) | −1.00 (5.81) | 0.84 | 0.4 |
| Left dorsal caudate | 2.26 (0.33) | 2.20 (0.32) | −2.43 (6.83) | 1.746 | 0.09 |
Figure 4. Reward-related changes in regional binding potentials for [11C]-raclopride.
A. Plot of mean percent of change in binding potential (ΔBP) in the regions of interest analyses (ROI). The mean ΔBP value was significant in the right ventral striatum and we found a non-significant trend in the left middle caudate and left dorsal caudate (table 1). A significant laterality difference was evident in the ventral striatum. Abbreviations: VS - ventral striatum; DPU - dorsal putamen; MCA - middle caudate; DCA - dorsal caudate. * p< 0.01, ** p< 0.005, * trend 0.05 < p< 0.1
B–D. Statistical parametric maps of voxel t-values corresponding to the reduction in BPND in the monetary reward task condition versus the sensorimotor control condition. This analysis was conducted post hoc to more precisely locate the areas of greatest effect size, so all voxels for which p<.01 are displayed. Results are shown superimposed on a T1-weighted MRI image (provided within SPM software) on horizontal slices situated parallel to a plane containing both the anterior and posterior commissures, located 5 mm below (z = −5), at (z = 0) or 8 mm above (z=8) this reference plane in B, C, and D, respectively. The coordinates for the peak voxel t-values are provided in table 3. The right side is toward the page bottom. Abbreviations: acc – accumbens area (in ventral striatum); caud – caudate nucleus, put - putamen
Ventral striatum
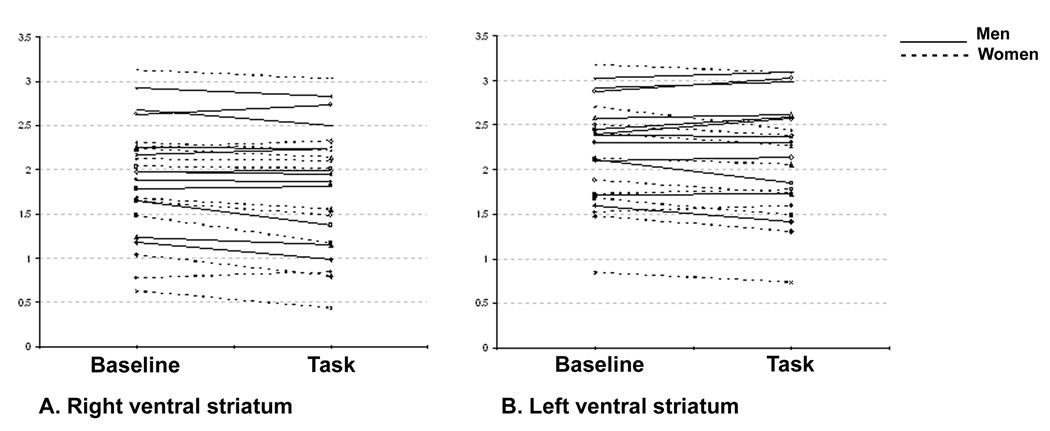
The test of the a priori hypothesis showed significant effects of lateralization (F1,23=57.8, p<0.001), condition (F1,23=5.50, p<0.05) and lateralization × condition interaction (F1,23 = 6.22, p<0.05) in the ventral striatum. Mean BP was lower in the right than the left ventral striatum both at baseline and during reward (both p<0.001). The BP decreased significantly during the reward versus the sensorimotor conditions in the right, but not the left ventral striatum (p=0.003 and 0.2 respectively). The mean ΔBP was greater on the right than the left (p<0.005). These differences remained significant at the Bonferroni-corrected threshold of p< 0.0167. Individual raclopride BP data for the sensorimotor versus the reward condition in the right and left ventral striatum is illustrated in figure 5.
Figure 5.
Individual raclopride binding potential (BP) values in the sensorimotor and the reward tasks in the A. right and B. left ventral striatum.
Dorsal putamen
Post hoc analyses additionally showed an effect of lateralization in the dorsal putamen (F1,23=20.5, p<0.001). The mean BP was lower in both conditions on the right than the left (baseline: p<0.01; reward: p<0.005). Neither the effect of condition (F1,23=1.9, p<0.17) nor the interaction between condition and lateralization (F1,23=1.3, p<0.24) reached significance.
Middle caudate
The main effect of lateralization was significant in the middle caudate (F1,23=9.4, p<0.005), neither the effect of condition (F1,23=3.1, p<0.08) nor the interaction between condition and lateralization reached significance (F1,23=0.8, p<0.37). The mean BP was lower in both conditions on the left than the right (baseline: p<0.01; reward: p<0.004).The decrease of BP during the reward versus the sensorimotor conditions showed a trend on the left side (p< 0.06).
Dorsal caudate
showed an effect of lateralization in the dorsal caudate (F1,23= 36.6, p<0.001)
The mean BP was lower in both conditions on the left than the right in dorsal caudate (baseline: p<0.001; reward: p<0.001). Neither the effect of condition (F1,23=3.1, p<0.09) nor the interaction between condition and lateralization (F1,23=0.8, p<0.36) reached significance.
Influence of gender and age
Using gender as a between-subjects factor in the four factorial ANOVA demonstrated significant interactions for region × gender (F3,66=4.6, p<0.005), condition × gender (F1,22=4.31, p<0.05), and region × laterality × condition × gender (F3,66=2.5, p<0.05). Subsequent separate paired t-tests for men and women showed a trend for ΔBP differences between the reward and the sensorimotor control conditions at a Bonferroni-corrected significance level of p<0.004 (alpha = 0.05 corrected for 12 comparisons) in men for the following comparisons: right dorsal putamen versus right ventral striatum (p<0.01), right middle caudate versus right ventral striatum (p< 0.05), right dorsal caudate versus right ventral striatum (p<0.005), left dorsal putamen versus left middle caudate (p< 0.02). We found no significant regional difference in women.
The region by region ANOVA’s yielded significant results in the dorsal putamen for the condition × gender interaction (F1,21=5.73, p<0.05) and a non-significant trend in the ventral striatum for the gender × condition × lateralization interaction (F1,21=3.56, p<0.07)(Tables 2). The mean ΔBP was greater in women than in men in the left and right dorsal putamen (left: p<0.01; right; p<0.05; two-tailed, figure 6) and showed a non-significant trend toward being higher in left ventral striatum (p<0.07). The ΔBP between conditions was significant in right and left ventral striatum (p<0.05), right and left dorsal putamen (p<0.005 bilaterally) right and left dorsal caudate (p<0.005 on the right and p<0.03 on the left) in women, but only in right ventral striatum (p< 0.05) in men. The comparisons of condition in men respectively women alone indicated significant difference in ΔBP between the right and left ventral striatal ROI in men (p<0.005, two-tailed) but not in women (p<0.17).
Table 2.
Mean regional binding potential (BPND) values for each condition and mean changes in BPND (Δ BP, in percent) between conditions for men and women. Levels of significance (two-tailed) and t-values are given for the one-sample t-tests of Δ BP for each gender analyzed separately and for independent samples t-test for the Δ BP comparison between men and women.
| Women | Men | Group comparison |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Regional BPND Control Mean (SD) |
Regional BPND Reward |
%difference (∆BP) Mean (SD) |
t-value | p-value | Regional BPND Control Mean (SD) |
Regional BPND Reward Mean (SD) |
%difference (∆BP) Mean (SD) |
t-value | p-value | p-value |
| Right ventral striatum | 1.77 (0.72) | 1.67 (0.75) | − 7.65 (11.52) | 2.30 | 0.04 | 2.02 (0.54) | 1.97 (0.58) | − 3.65 (6.9) | 1.81 | 0.08 | 0.315 |
| Left ventral striatum | 2.03 (0.64) | 1.95 (0.64) | − 4.7 (6.47) | 2.56 | 0.02 | 2.37 (0.44) | 2.39 (0.53) | 0.14 (6.3) | 0.07 | 0.94 | 0.072 |
| Right dorsal putamen | 2.81 (0.37) | 2.7 (0.37) | −3.74 (4.3) | 2.99 | 0.01 | 2.74 (0.37) | 2.75 (0.35) | 0.7 (4.8) | 0.5 | 0.62 | 0.02 |
| Left dorsal putamen | 2.97 (0.43) | 2.88 (0.39) | −2.69 (3.1) | 2.94 | 0.01 | 2.86 (0.3) | 2.9 (0.2) | 1.44 (4.1) | 1.2 | 0.25 | 0.01 |
| Right middle caudate | 1.79 (0.53) | 1.73 (0.57) | −3.83 (9.8) | 1.34 | 0.2 | 2.09 (0.52) | 2.11 (0.57) | 0.79 (6.7) | 0.41 | 0.68 | 0.193 |
Figure 6.
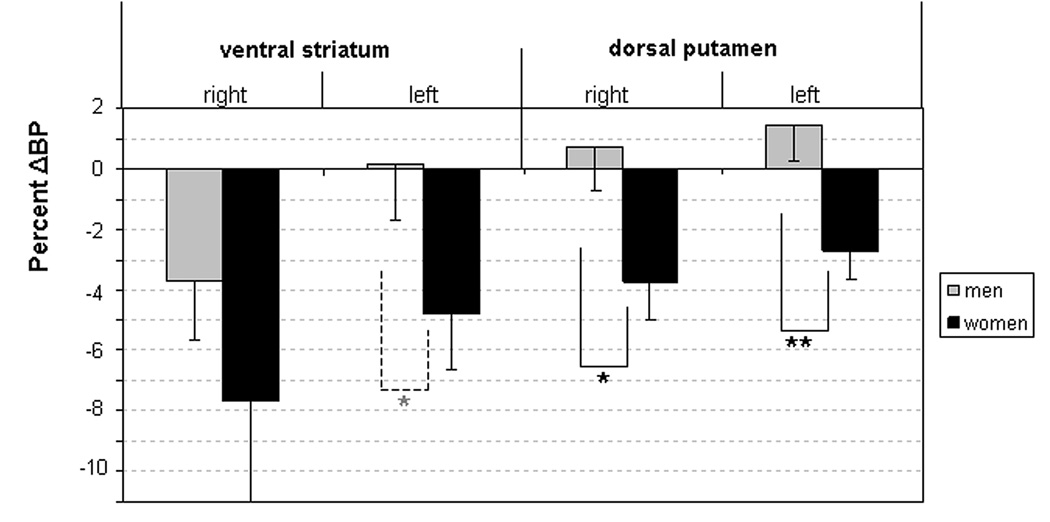
Plot of the mean percent change in binding potential (ΔBP) in regions where significant gender differences were found. Women showed greater ΔBP values than men in the right and left dorsal putamen and showed a nonsigificant trend toward higher ΔBP values in the left ventral striatum. Considered separately, the mean ΔBP in the left ventral striatum as well as in the right and left dorsal putamen was significant in women only. Right-left differences in ΔBP were significant in the ventral striatum only in men (p<0.001).
* p< 0.05, ** p< 0.01, * trend
Age did not correlate significantly with ΔBP in any ROI.
Whole brain analysis
The voxel-wise analysis showed four clusters of voxels in which BP decreased significantly in the reward condition relative to the control condition. These clusters localized to subregions of the right ventral striatum (which included portions of the accumbens, ventromedial caudate and anteroventral putamen), the left anterolateral caudate nucleus (in an area encompassed by our left middle caudate ROI), and the right caudal putamen (figure 4b–d, Table 3; Talairach & Tournoux, 1988; Mai et al., 2004). In contrast, no areas were identified where BP was significantly higher in the reward condition relative to the baseline condition at the specified significance threshold.
Table 3. Areas identified in the voxel-wise analysis conducted post hoc to more precisely localize changes in the [11C]raclopride binding potential (BPND) in the monetary reward task condition relative to the sensorimotor control condition.
Cluster sizes correspond to the number of contiguous voxels (of dimension 2 mm3 after reslicing the images within SPM8) with similarly valenced t-values for which the voxel t-value corresponded to p<0.01. The coordinates and t-values for voxels containing the peak effect sizes are indicated for each group. Coordinates reflect the stereotaxic spatial system of Talairach and Tournoux (Talairach et al, 1988), in which each coordinate reflects the distance in mm from the anterior commissure, with positive x, y, and z indicating right, anterior, and superior, respectively.
| Brain Regions | Cluster size (voxels) |
Coordinates (x, y, z) |
t-value |
|---|---|---|---|
| R accumbens area | 233 | 11, 5, −2 | 2.32 |
| R anteroventral putamen | 27, 6, 0 | 2.25 | |
| R accumbens area/ anteroventral putamen | 13, 7, 1 | 2.50 | |
| R anterior caudate nucleus | 11, 5, 9 | 2.25 | |
| R anterior caudate nucleus | 17, 17, 8 | 2.16 | |
| R caudal ventral putamen | 24 | 27, −13, 0 | 2.22 |
| L caudal ventral putamen | 12 | −27 10 −3 | 2.42 |
| L dorsal anterior caudate nucleus | 12 | −17, 14, 16 | 2.46 |
Abbreviations: R-right, L-left
DISCUSSION
We confirmed the a priori hypothesis that unpredicted monetary rewards elicit a lateralized increase in DA transmission in the ventral striatum. The significant interaction between task-condition and lateralization in this region specifically was accounted for by a reduction in BP in the reward versus the sensorimotor conditions in the right ventral striatum, where the mean ΔBP was more prominent on the right versus the left side. The reduction in [11C]raclopride binding in the reward versus the sensorimotor control condition presumably reflected greater endogeneous DA release in response to unpredictable monetary reward. The specificity of this finding is supported by the lack of significant difference in [11C]raclopride binding in the reward versus the sensorimotor control condition in the other striatal regions and by the non-significant trends evidenced in the comparisons between ΔBP in the right ventral striatum and the other right-sided striatal regions. These data thus constitute the first indication that the elevation in dopaminergic transmission in response to unpredicted reward shows a laterality effect toward the right ventral striatum in right-handed humans.
Baseline laterality Differences in the Regional Binding Potentials
In addition, the mean BP values were lower both at baseline and during reward on the right versus the left in the ventral striatum and dorsal putamen, but lower on the left versus the right in the dorsal caudate. The laterality effect on baseline BP observed in the caudate was consistent with the previously reported finding of Vernaleken et al (2007) that mean D2/3 receptor binding was lower in the left than the right caudate and is consistent overall with the asymmetries reported in DA receptor binding studies under resting conditions (Larisch et al., 1998). Notably, the lateralization in the ventral striatum under the baseline condition appears complementary to previous results showing that D1-receptor binding in the ventral putamen is lower on the right than the left side (Cannon et al., 2009).
Gender differences
The effect of sex on ΔBP in the putamen was novel and unexpected (figure 6, Table 2, supplemental data). In the dorsal putamen women--but not men--showed a significant difference in BP between conditions bilaterally, and the magnitude of the mean ΔBP was greater in women than men. In the ventral striatum the gender-by-condition interaction showed a nonsignificant trend, as ΔBP was significant in women bilaterally but only on the right side in men. Moreover, the right-to-left difference in ventral striatal ΔBP was significant in men (p<0.005) but not in women (p<0.17). The analysis of subregions within the ventral striatum (i.e., ventral putamen versus accumbens area) more specifically revealed that the gender-by-condition-by-laterality interaction was highly significant in the ventral putamen (supplemental data). Additionally, the comparisons of ΔBP between the different striatal regions evidenced non-significant trends between the ΔBP in the right ventral striatum relative to the ΔBP in other striatal regions tested only in men. Our data thus suggest that unpredicted rewards elicit DA release over a wider area of the striatum in women than men and that reward-elicited DA release in the ventral striatum occurs bilaterally in women, but unilaterally in men. However, while the mean ΔBP values reached significance in women they showed a nonsignificant trend in men, possibly related to the small sample sizes of the gender based subgroups.
Taken together, these data add to other evidence for sex effects on the striatal response to reward, as previous studies reported that the hemodynamic response to reward in the striatum is influenced by menstrual phase (Dreher et al., 2007) and differs between for men and women (Spreckelmeyer et al., 2009), that striatal DAT binding is higher in women than men (Lavalaye et al., 2000) and that dopaminergic transmission in the accumbens is modulated by estrogen and progesterone levels in women (Becker, 1999). Laterality effects also have been reported in experimental animals, as striatal DA D2 receptor binding in pigs (Cumming et al., 2003) and accumbens DA concentrations in rats show sex effects (Duchesne et al., 2009). Finally, these results are in line with previous findings showing gender differences in the neural processing of emotional stimuli (Canli et al., 2002; Wrase et al., 2003).
Several limitations of our study design merit comment. First, the phase of menstrual cycle in which female subjects were imaged was not fixed and we did not evaluate differential effects of distinct menstrual phases on reward-induced DA release. Second, since the PET-[11C]raclopride B/I approach permits imaging in only two conditions per scan session, we did not measure BP in a resting state, so we could not assess whether BP differed between the sensorimotor condition and the resting state. Thus the effect of condition observed in our analyses is specific to the reward condition versus the sensorimotor control condition. Finally, due to limitations in spatial resolution the regional BPND values in each ROI were influenced by the tissue radioactivity concentrations in adjacent ROI via partial-volume averaging effects. Demonstrating differential regional sensitivity to reward thus depended partly on showing that the magnitude of ΔBP was greater in the right ventral striatum than in adjacent ROI (Table 1; Drevets et al., 1999; Drevets et al., 2001). Nevertheless, in the gender-based analyses, the apparent changes in BP in the dorsal putamen were at least partly influenced by partial volume effects from the more prominent changes in the adjacent ventral putamen (see Supplemental Materials; Drevets et al., 1999; 2001). Thus the differences in BP observed in the dorsal putamen could not be resolved from the corresponding changes in ventral putamen, because the latter were greater in magnitude. In the left striatum, however, the magnitude of ΔBP was highest in left middle caudate, and relatively lower in adjacent left ventral striatum and dorsal caudate. These data thus appeared compatible with those of Zald et al. (2004), in which the reduction in BP within the left striatum during unpredictable reward showed the greatest effect size in left middle caudate. Finally, the lack of significant age effect could be related to the small age range of the participants studied.
Although previous PET-[11C]raclopride studies of DA release during reward processing did not specifically address laterality effects, our data appeared partly compatible with their results (Pappata et al., 2002; Zald et al., 2004). Pappata et al (2002) showed that [11C]raclopride binding decreased in the ventral striatum bilaterally in response to large monetary rewards versus large monetary losses (n=8, all males). Consistent with our observations in the left middle caudate (figure 4a, Table 3), Zald et al (2004) found that [11C]raclopride binding decreased in response to unpredictable rewards in a left “medial caudate” area that overlapped our “middle caudate” ROI (n=10, 4 males). However, Zald et al (2004) also reported that [11C]raclopride binding increased in the left lateral putamen (at x=−30, y=−2, z=0; coordinates interpreted as in Table 3) in response to unpredictable reward, which we did not confirm. This group subsequently reported that [11C]raclopride binding also increased in the caudate and putamen in response to unpredicted rewards (n=12, all males; Hakyemez et al., 2008), although the passive monetary reward task used in this study differed from the active behavioral engagement required in our task. Our sample size (n=25) was substantially larger than those in previous studies (in which n ranged from 8 to 12). Another major methodological difference across studies was that these previous studies relied on the bolus method for infusing [11C]raclopride while ours applied the B/I method.
Binding measures obtained following bolus administration of [11C]raclopride can be influenced by CBF changes, and these previous studies employed tasks likely to increase striatal CBF (Delgado et al., 2000; Berns et al., 2001). In contrast, the B/I method allows neuroreceptor quantitation under equilibrium conditions (Lassen, 1992; Breier et al., 1997; Marenco et al., 2004), such that measures of BP are relatively insensitive to changes in local CBF (Cumming et al., 2003; Rosa-Neto et al., 2004). Our findings might show a greater specificity for reflecting intrasynaptic DA release.
A novel finding of our study was that in right-handed humans the dopaminergic response to unpredictable rewards in the ventral striatum appears right-lateralized. Our findings appear consistent with preclinical evidence for lateralization of DA release during motor activation and motor learning (Yamamoto & Freed, 1982; Morice et al., 2005; Silva et al., 2007; Lappin et al., 2009), and follow-up studies must address the contributions of motor dominance (“handedness”) and interactions between behavioral incentive and motor response. Nevertheless, our data regarding both reward-related DA release and baseline DA D2/3 receptor binding converge with a variety of evidence indicating that several elements of the mesostriatal dopaminergic system are lateralized in humans.
Interestingly, we found significant gender differences that influence the lateralization observed in the ventral striatum in response to reward. Men showed a significant lateralization effect in this region and appeared to increase DA release only in right ventral striatum during unpredicted reward, while women evidenced a significant increase of DA release in the ventral striatum bilaterally, although the magnitude of this effect trended toward being greater on the right. These results may illuminate the significance of laterality differences in striatal function in mood disorders (Starkstein et al., 1989; Drevets et al., 1992; Steffens et al., 1998) characterized by disturbances of hedonic capacity and motivation, which show lateralized abnormalities in striatal D1 receptor binding that are specific for women (Cannon et al., 2009). These findings could also relate to the gender differences observed in the association between depression and pathological gambling (Desai & Potenza, 2008). Our data further suggest that previously reported associations between depression and lateralized abnormalities of striatal function (Starkstein et al., 1989; Drevets et al., 1992; Steffens et al., 1998) or between schizophrenia and the disruption of interhemispheric asymmetries in the DA function (Hietala et al., 1999; Laakso et al., 2000) require re-examination for sex effects.
In conclusion, our data further suggest a gender specific hemispheric asymmetry in the mesostriatal DA response to rewarding stimuli that should be taken into account in future studies on the neural basis of reward processes.
Supplementary Material
Acknowledgment
We thank the staff f the PET Department staff for for study execution help, and Joan Williams and Michele Drevets for assistance in identifying research volunteers and evaluating their suitability for study participation. This study was supported by National Institute of Mental Health Intramural Research Program (Z01-MH002812; Wayne Drevets PI) and a NARSAD Young Investigator Award (Chantal Martin PI).
Footnotes
Address from which the work originated: Section on Neuroimaging in Mood and Anxiety Disorders, NIMH, NIH, Bethesda, MD, USA
Statement of Interest
None
References
- Alonso SJ, Navarro E, Santana C, Rodriguez M. Motor lateralization, behavioral despair and dopaminergic brain asymetry after prenatal stress. Pharmacol Biochem Behav. 1997;58:443–448. doi: 10.1016/s0091-3057(97)00009-9. [DOI] [PubMed] [Google Scholar]
- Antonini A, Leenders K. Dopamine D2 receptors in normal human brain: effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Ann N Y Acad Sci. 1993;695:81–85. doi: 10.1111/j.1749-6632.1993.tb23033.x. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson C, Louilot A. Asymmetrical involvement of mesolimic dopminergic neurons in affective perception. Neuroscience. 1995;68:963–968. doi: 10.1016/0306-4522(95)00255-h. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. PNAS. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc. Nath. Acad.Sci. USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Klaver JM, Peck SA, Rallis-Voak D, Erickson K, Drevets WC. Dopamine Type-1 Receptor Binding in Major Depressive Disorder Assessed Using Positron Emission Tomography and [(11)C]NNC-112. Neuropsychopharmacology. 2009;34:1277–1287. doi: 10.1038/npp.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11C] Raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Cumming P, Rosa-Neto P, Watanabe H, Smith D, Bender D, Clarke PB, Gjedde A. Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage. 2003;19:1127–1136. doi: 10.1016/s1053-8119(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Desai RA, Potenza MN. Gender differences in the associations between past-year gambling problems and psychiatric disorders. Soc Psychiatry Psychiatr Epidemiol. 2008;43:173–183. doi: 10.1007/s00127-007-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, Mathis C. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Dufresne M, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Garraux G, Peigneux P, Carson RE, Hallett M. Task-related interaction between basal ganglia and cortical dopamine release. J Neurosci. 2007;27:14434–14441. doi: 10.1523/JNEUROSCI.1595-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Weaver LM, Meibach RC. Lateralization of reward in rats: differences in reinforcing thresholds. Science. 1980;207:1093–1095. doi: 10.1126/science.7355277. [DOI] [PubMed] [Google Scholar]
- Glick SD, Weaver LM, Meibach RC. Amphetamine enhancement of reward asymmetry. Psychopharmacology (Berl) 1981;73:323–327. doi: 10.1007/BF00426459. [DOI] [PubMed] [Google Scholar]
- Gotestam KG, Johansson A, Wenzel HG, Simonsen IE. Validation of the lie/bet screen for pathological gambling on two normal population data sets. Psychol Rep. 2004;95:1009–1013. doi: 10.2466/pr0.95.3.1009-1013. [DOI] [PubMed] [Google Scholar]
- Hakyemez HS, Dagher A, Smith SD, Zald DH. Striatal dopamine transmission in healthy humans during a passive monetary reward task. Neuroimage. 2008;39:2058–2065. doi: 10.1016/j.neuroimage.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Eronen E, Ruotsalainen U, Salokangas RK. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Choi WY, Morvan C, Eyny Y, Balsam PD. A "good parent" function of dopamine: transient modulation of learning and performance during early stages of training. Ann N Y Acad Sci. 2007;1104:270–288. doi: 10.1196/annals.1390.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Moore KE. Mesolimbic dopamine neurons: Effecs of 6-hydroydopamine-induced destruction and receptor blockade on drug-induced rotation in rats. Psychopharmacology. 1977;55 doi: 10.1007/BF00432814. [DOI] [PubMed] [Google Scholar]
- Koepp M, Gunn R, Lawrence A, Cunningham V, Dagher A, Jones T, Brooks D, Bench C, Grasby P. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Rakkolainen V, Syvalahti E, Hietala J. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- Lappin JM, Reeves SJ, Mehta MA, Egerton A, Coulson M, Grasby PM. Dopamine release in the human striatum: motor and cognitive tasks revisited. J Cereb Blood Flow Metab. 2009;29:554–564. doi: 10.1038/jcbfm.2008.146. [DOI] [PubMed] [Google Scholar]
- Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nuclear medicine communications. 1998;19:781–787. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Neuroreceptor quantitation in vivo by the staedy-state principle using constant infusion or bolus injection of radioactive tracers. J Cereb Blood Flow Metab. 1992;12:709–716. doi: 10.1038/jcbfm.1992.101. [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. London, San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology. 2004;29:259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA, Delfani K, Irwin I, DeLanney LE, Langston AM. Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol. 2004;471:387–395. doi: 10.1002/cne.20036. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki JS. Aging and exptrapyramidal function. Arch. Neurol. 1977;34:33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- Morice E, Denis C, Macario A, Giros B, Nosten-Bertrand M. Constitutive hyperdopaminergia is functionally associated with reduced behavioral lateralization. Neuropsychopharmacology. 2005;30:575–581. doi: 10.1038/sj.npp.1300570. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A. In vivo detection of striatal dopamine release during reward: a PET study with [(11)C]raclopride and a single dynamic scan approach. Neuroimage. 2002;16:1015–1027. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Mnagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Marsden CD. The rotating rodent: A two component system? Eur J Pharmacol. 1978;47:167–175. doi: 10.1016/0014-2999(78)90388-6. [DOI] [PubMed] [Google Scholar]
- Rickham PP. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Martin L, Santana C. Ontogenic develpment of brain asymetry in dopaminergic neurons. Brain Res Bull. 1994;33:163–171. doi: 10.1016/0361-9230(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Gjedde A, Olsen AK, Jensen SB, Munk OL, Watanabe H, Cumming P. MDMA-evoked changes in [11C]raclopride and [11C]NMSP binding in living pig brain. Synapse. 2004;53:222–233. doi: 10.1002/syn.20053. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Silva MA, Topic B, Lamounier-Zepter V, Huston JP, Tomaz C, Barros M. Evidence for hemispheric specialization in the marmoset (Callithrix penicillata) based on lateralization of behavioral/neurochemical correlations. Brain Res Bull. 2007;74:416–428. doi: 10.1016/j.brainresbull.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Spitzer MB, Gibbon M, Williams JBW. Stuctured Clinical Interview for DSM-IV-TR, Research Version, Patient Edition. (SCID-I/P) New York: New York State Biometrics Institute; 2002. [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Grunder G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Honig MA, Parikh RM, Joselyn J, Price TR. Mood changes after right-hemisphere lesions. Br J Psychiatry. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Tupler LA, Ranga K, Krishnan R. Magnetic resonance imaging signal hypointensity and iron content of putamen nuclei in elderly depressed patients. Psychiatry Res. 1998;83:95–103. doi: 10.1016/s0925-4927(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Stinchfield R. Reliability, validity, and classification accuracy of the South Oaks Gambling Screen (SOGS) Addict Behav. 2002;27:1–19. doi: 10.1016/s0306-4603(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Szostak C, Jakubovic A, Phillips AG, Fibiger HC. Bilateral augmentation of dopaminergic and serotonergic activity in the striatum and nucleus accumbens induced by conditioned circling. J Neurosci. 1986;6:2037–2044. doi: 10.1523/JNEUROSCI.06-07-02037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Innis RB. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10:36–43. [PubMed] [Google Scholar]
- Vernaleken I, Weibrich C, Siessmeier T, Buchholz HG, Rosch F, Heinz A, Cumming P, Stoeter P, Bartenstein P, Grunder G. Asymmetry in dopamine D(2/3) receptors of caudate nucleus is lost with age. Neuroimage. 2007;34:870–878. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C] raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–530. [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Herrman N, Flor H, Mann K, Braus DF, Heinz A. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imagign study. Neurosci Lett. 2003;348:41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Freed CR. The trained circling rat: a model for inducing unilateral caudate dopamine metabolism. Nature. 1982;298:467–468. doi: 10.1038/298467a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Freed CR. Asymmetric dopamine and serotonin metabolism in nigrostriatal and limbic structures of the trained circling rat. Brain Res. 1984;297:115–119. doi: 10.1016/0006-8993(84)90547-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Lane RF, Freed CR. Normal rats trained to circle show asymmetric caudate dopamine release. Life Sci. 1982;30:2155–2162. doi: 10.1016/0024-3205(82)90289-2. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Glick SD, Jerussi TP. Neurochemical correlate of a spatial preference in rats. Science. 1974;185:623–625. doi: 10.1126/science.185.4151.623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.