Abstract
Objective
In a recent study, performance on a certain kind of prospective memory task (PM), labeled focal PM, was sensitive to the very early stages of Alzheimer’s disease (AD; Duchek, Balota, & Cortese, 2006). The present study sought to replicate and extend these findings by investigating both focal and non-focal PM, as well as possible influences of alleles of the apolipoprotein E (APOE) gene.
Method
Thirty-five healthy older adults and 33 adults in the very earliest stages of AD, as determined by the clinical dementia rating scale, completed both focal and non-focal PM tasks. Performance on these tasks has been linked to qualitatively different cognitive processes (Scullin, McDaniel, Shelton, & Lee, 2010), thereby providing leverage to illuminate the specific processes underlying PM failures in very early AD. Approximately half of the adults in each group were APOE e4 carriers and half were non-carriers. We also obtained participants’ scores on a battery of standard psychometric tests.
Results
There was a significant interaction between the type of PM task and dementia status, p < .05. η2p = .12, demonstrating that the AD-related decline was more robust for focal than for non-focal PM. Further, focal PM performance significantly discriminated between the very earliest stages of AD and normal aging, explaining variance unique to that explained by typical psychometric indices. APOE status, however, was not associated with PM performance.
Conclusions
The pronounced deficit observed in the focal PM task suggests that spontaneous retrieval processes may be compromised in very early AD.
Keywords: Prospective memory, Alzheimer’s Dementia, apolipoprotein E gene
Event-based prospective memory (PM) refers to people’s ability to perform an intended action in the future in response to a specific event. Previous studies have demonstrated that PM is disrupted in patients with dementia of the Alzheimer’s type (AD; Duchek, Balota, & Cortese, 2006; Huppert, Johnson, & Nickson, 2000; Maylor, Smith, Della Sala, & Logie, 2002). However, these initial studies have investigated only one type of event-based PM (cf. McDaniel & Einstein, 2007), and few studies have focused on the consequence of very mild AD on PM. In the present study we examined several types of event-based PM performance in very mild AD patients and normal controls, and we also investigated the effect of APOE status and its possible interaction with AD status on event-based PM. Before reporting the study, we first describe a distinction between two laboratory event-based PM tasks, develop theoretical predictions, and then consider the initial empirical findings regarding PM and AD from this perspective.
In typical laboratory PM paradigms, participants are busily engaged in an ongoing task, and they are also instructed to try to remember to perform a particular response (e.g., press the F1 key) when a particular target event appears. According to the theoretical approach we adopted herein, we suggest that in some paradigms the ongoing task encourages processing of the attributes of the target event that were processed during initial encoding of the intention (see McDaniel & Einstein, 2007; Scullin, McDaniel, Shelton, & Lee, 2010, for theoretical details and supportive evidence; we will labeled a focal PM task to be consistent with the literature). For example, consider an ongoing task in which pairs of words are presented and subjects decide whether one word is a member of the category represented by the other word. In this case, specifying a particular word as a target event—e.g., “tortoise”— would create a focal PM task. A real-world example of a focal PM task would be remembering to give a colleague a message when you encounter them at work. In encoding this intention, you would likely process attributes of your colleague (the target event), such as her name and perhaps her physical appearance. Upon passing your colleague in the hallway at work, your normal ongoing activity would be to notice her appearance and greet her by name. In this case, then, processing of the target features (your colleague) is focal to your ongoing activity upon encountering the event (your colleague).
By contrast, in other paradigms the ongoing task does not direct attention to processing the designated PM target event (here labeled a non-focal PM task). As an example, for the above ongoing category decision task, specifying a particular syllable—e.g., “tor”— as the target event would create a non-focal PM task. This PM task would be considered non-focal because syllabic information is not an attribute normally attended to when words are processed for meaning, as required in the ongoing activity. Returning to the previous real-world example, perhaps your PM task was to remember to post a message on a bulletin board if you happen to be in a particular place in the hallway. At some later time while you are in the hallway classes let out, you attend to peoples’ faces, notice their appearances, navigate around them, recognize the instructor as a colleague, and greet her. Now the features of the PM target (the place in the hallway with the bulletin board) would be non-focal to the information pertinent to your ongoing activities. Importantly, a number of studies suggest that focal PM tasks stimulate reliance on more spontaneous retrieval processes to support PM performance, whereas non-focal PM tasks require attention demanding monitoring strategies to support PM performance (Brewer, Knight, Marsh, & Unsworth, 2010; Einstein et al., 2005; Scullin, McDaniel, & Einstein, 2010; Scullin et al., 2010).
Implications for Prospective Memory in AD
In the present study, we considered performances on both focal and non-focal PM tasks to provide a nuanced analysis of the kinds of PM deficits that might be associated with very mild AD. One possible prediction can be based on the observations that non-focal PM tasks are relatively more challenging than focal PM tasks (because they require more strategic attentional deployment), and that with normal aging PM deficits are generally robust for non-focal PM tasks but not for focal PM tasks (Rendell, McDaniel, Forbes, & Einstein, 2007; see McDaniel & Einstein, 2007 and Kliegel & Jäger, 2008, for reviews and a metaanalysis). Reasoning that very mild AD will primarily penalize more challenging cognitive tasks, one might expect that PM declines in very mild AD would be significantly greater for non-focal PM than for focal PM (a pattern that has been reported for Parkinson Disease patients; Foster, McDaniel, Repovš, & Hershey, 2009; see also Blanco-Campal, Coen, Lawlor, Walsh, & Burke, 2009, for a similar pattern with mildly cognitive impaired participants).
A second prediction is based on the straightforward assumption that spontaneous prospective memory retrieval (reflected at least in part in focal PM but not non-focal PM tasks) may primarily involve a reflexive associative system that Moscovitch (1994) proposes is subserved by medial temporal (e.g., hippocampal) structures. Hippocampal decline is considered to be a hallmark of AD (e.g., Buckner, 2004; Head, Snyder, Girton, Morris, & Buckner, 2005; see also Jack et al., 2008). Consequently, on this analysis we would expect that very mild AD will produce a robust disruption in focal prospective memory tasks relative to normally aging older adults. Note that this prediction anticipates a signature decline in prospective memory for very mild AD adults, as normally aging older adults tend to show equivalent performance to younger adults on the focal PM task used in the present study (McDaniel et al., 2008). Accordingly, in this study, we also explore the degree to which focal (and non-focal) PM performance can provide significant improvement in discriminating very mild AD from normal aging, relative to established neuropsychological indices.
Initial evidence is consistent with our expectation of PM deficits in very mild AD for focal PM tasks. In a recent study by Duchek et al. (2006), participants performed a PM task in which the PM cues (i.e., sentences about presidents) were embedded within a set of general knowledge questions. This task might be classified as focal because the processing required by the ongoing task of answering general questions presumably directed attention to the semantic features of the PM cues (a question about a president). PM performance was substantially worse in the very mildly demented relative to the other groups. Similarly, Huppert et al. (2000) and Maylor et al. (2002) used apparently focal PM tasks in a group of older adults with very mild dementia and in AD patients at various stages of disease progression, respectively. In both cases, there were significant declines in PM performance for the AD participants relative to healthy older adults (see also Blanco-Campal et al., 2009, for focal PM decline in mild cognitive impairment of suspected AD etiology).
There were, however, several features of the above studies that may limit interpretation of their findings. First, in the two studies that focused on individuals in the early stages of AD (Duchek et al., 2006; Huppert et al., 2000) it is clear that retrospective memory failures, rather than PM failures per se, were partly responsible for PM performance deficits observed in the AD group. In the Huppert et al. study, of the participants who met criteria for AD diagnosis, 60% did not recall being told to perform the PM task. In Duchek et al, 17 out of the 22 individuals with AD who scored 0% on the PM task were queried, and of these, 29% did not remember anything about the PM task, although excluding these individuals from the analyses did not change the pattern (Duchek, 2010, personal communication). Further, the demands of the ongoing task may have been more difficult for the AD participants than the healthy older adults, as suggested by significantly worse performance on the general knowledge task for AD participants (Duchek et al.). It may be that the increased difficulty of the ongoing task for the very mild AD participants could have contributed to their lower accuracy on the PM component of the task.
The present experiment attempted to remedy these potential shortcomings by excluding data from participants who did not demonstrate memory of the PM task (memory for the target item and the instructed response) at the conclusion of the experiment, and by adopting the category decision ongoing task (described earlier), a task that we expected very mild AD participants to perform with high accuracy, albeit overall more slowly (e.g., Nebes, Martin, & Horn, 1984). Another advantage of the category-decision ongoing task is that response times to make the category decision appear to be sensitive to participants’ use of strategic monitoring to support prospective remembering (McDaniel et al., 2008). Because strategic monitoring is assumed to require attentional resources (see McDaniel & Einstein, 2007), a footprint of strategic monitoring is increased decision latency on the category decision task when the PM task is present relative to when the PM task is absent (in a control block of trials; Einstein et al., 2005).
We examined the cost of the PM task on the category-decision ongoing activity for several purposes. We wanted to demonstrate that the non-focal PM task, but not the focal PM task, produced significant costs to the ongoing activity, thereby implying that the non-focal PM performance was supported by strategic attentional processes (presumably related to executive control) and the focal PM task supported more so by spontaneous retrieval processes. Second, the cost measure would provide insights into whether very mild AD disrupts such strategic monitoring (in the non-focal PM task) relative to healthy older adults.
A final objective of this study was to investigate the possible influence of the APOE 4 allele on PM. The presence of the APOE 4 allele is a known genetic risk factor for AD, and there is interest in determining whether APOE 4 might relate to memory decline in both healthy old and in very mild AD (see Small, Rosnick, Fratiglioni, & Bäckman, 2004, for a review). With regard to APOE 4 and PM, there are apparently discrepant findings. Driscoll, McDaniel, and Guynn (2005) reported PM decrements in a group of older adult APOE 4 carriers who were classified as healthy, whereas, Duchek et al. (2006) failed to find a detrimental effect of APOE 4 in PM performance of healthy older adults. Duchek et al. suggested that these discrepant findings might have been a consequence of improper classification of a healthy cognitive status for at least some of the participants in the Driscoll et al. study. To more carefully examine the possible influence of APOE 4 on PM in healthy older adults and in very mild AD, we determined the APOE status for all participants, and following Duchek at al. we used a highly sensitive index of dementia, the Clinical Dementia Rating Scale, to identify groups of healthy older adults and those with very mild AD.
Method
Design and Participants
The design was a 2 × 2 × 3 mixed-factor design, with the participant’s APOE 4 genotype status (carriers or non-carriers) and their clinical dementia rating (CDR: 0 or 0.5) as between-subjects factors. Task block (control, focal PM, non-focal PM) in the category decision task served as the within-subjects factor. Seventy-two older adults were recruited for participation via the Washington University Alzheimer’s Dementia Research Center (ADRC). Table 1 displays the number of participants in each group. Four participants had to be dropped due to retrospective memory failures (being unable to recall the PM targets when queried), resulting in 68 participants in the final sample.
Table 1.
Mean age, education, and MMSE as a function of CDR and ApoE 4 status.
| CDR 0 | CDR 0.5 | |||
|---|---|---|---|---|
| e4 Positive | e4 Negative | e4 Positive | e4 Negative | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Education | 15.94 (3.70) | 14.44 (2.45) | 14.94 (3.03) | 13.63 (2.06) |
| MMSE | 29.00 (1.12) | 28.72 (1.49) | 26.71 (1.93) | 27.69 (1.54) |
| Age | 75.60 (8.33) | 78.03 (7.05) | 75.99 (8.59) | 81.67 (8.12) |
| n | 17 | 18 | 17 | 16 |
Participants were screened at the ADRC for a variety of disorders including depression, hypertension, and reversible dementias. The CDR scale was used to classify dementia severity, which is based on a 90-minute clinical interview with the patient and a collateral source. A CDR rating of 0 reflects no dementia and a CDR rating of 0.5 characterizes people as being in the very earliest stages of AD. The reliability and validity of this scale have proven to be excellent with a 93% level of diagnostic accuracy (Berg et al., 1998). The age range for the 68 participants retained in the sample (40 females) was 60–93 (M=77.77, SD=8.20). A breakdown of age, education, and mini-mental status exam (MMSE) scores is presented in Table 1. A few differences were 10 observed between the experimental groups on these variables. Higher MMSE scores were observed in the healthy relative to very mildly demented adults, F (1, 64) = 19.72, p < .01; however, mean MMSE scores would be considered relatively high in both groups. The APOE 4 non-carriers were older, F (1, 64) = 4.33, p = .04, and less educated, F (1, 64) = 4.04, p = .05, than carriers. However, the age and education ranges for the APOE 4 noncarriers and carriers overlapped considerably, suggesting a high degree of comparability across these groups on these two demographic factors (for APOE 4 noncarriers, age ranged from 62–93 and the years of education ranged from 10–18; for the APOE 4 carriers age ranged from 60–94 and the years of education ranged from 10–23).
In addition to the laboratory-based tasks completed as part of the current experiment, all participants had completed a battery of standard psychometric tests administered as part of their participation in the Washington University ADRC project. The psychometric tests were completed within one year of the prospective memory test. The psychometric tests that were incorporated into the present study are presented in Table 2. Participants were compensated ten dollars for each hour of participation in the laboratory tasks comprising the experiment.
Table 2.
Mean psychometric test scores as a function of CDR status
| CDR 0 | CDR 0.5 | |
|---|---|---|
| M (SD) | M (SD) | |
| n | 35 | 33 |
| Animal Naming | 18.80 (7.35)* | 15.12 (5.34)* |
| Word Fluency | 29.54 (10.05) | 26.21 (9.16) |
| Associative Memory | 15.07 (3.78)** | 10.33 (4.72)** |
| Selective Reminding Test | 29.69 (5.96)** | 16.70 (9.33)** |
| Digit Forwards | 6.51 (1.52) | 6.36 (1.17) |
| Digit Backwards | 4.94 (1.64) | 4.55 (1.25) |
| Digit Symbol | 43.80 (13.31) | 39.00 (11.62) |
| Boston Naming Test | 56.63 (3.26)** | 52.42 (6.52)** |
| Crossing Off | 169.66 (40.19)** | 144.85 (30.44)** |
Note. Differences between CDR 0’s and .5’s are indicated by
p < .05,
p < .01.
Procedure
The laboratory tasks were completed on a PC and the tasks were constructed using E-Prime software (Schneider, Eschman, & Zuccolotto, 2002). Participants were first given instructions and practice on the category decision task. Each trial of the category decision task consisted of item and category pairings. Participants were asked to determine if the word on the left side of the computer screen in lowercase letters was a member of the category presented on the right hand side of the computer screen in uppercase letters (e.g., green COLOR). Item and category pairings were taken from Einstein et al. (2005). Three lists of 106 pairings were used and counterbalanced across task blocks, with half of the pairings yielding a correct “yes” answer, and half yielding a correct “no” answer. Thus, there were 106 trials in the control block of the category decision task, and 109 trials in the focal PM and non-focal PM blocks of the task, with 3 trials being PM target trials. PM targets always appeared as exemplar items rather than category names. Following initial work (Einstein & McDaniel, 1990), we used low target frequency to parallel everyday prospective memory, in which the intended activity is typically retrieved for execution a single time. We acknowledge that doing so may limit the psychometric power. However, to achieve strict psychometric properties can require about 30 PM trials (see Kelemen, Weinberg, Alford, Mulvey, & Kaeochinda, 2006), which arguably could change the processes recruited to perform the “PM” task (and may even fundamentally change it to a vigilance task). Our preference was to attempt to capture memory processes representative of everyday PM. Because the PM target in the focal condition (a specific word) was presented 3 times in the focal PM block of the category decision task, 11 non-target items were presented 3 times, and 9 were presented 2 times to reduce the distinctiveness of the PM target (exemplar item--category label pairs were not repeated). There was no theoretical reason for choosing these particular repetition rates; the objective was to include 20 non-target items in the list that repeated a comparable number of times as the PM target items.
The order in which the control, focal PM, and non-focal PM blocks was presented was counterbalanced across participants (see Einstein & McDaniel, 2010, for details of the effectiveness of this procedure for controlling for possible order effects). For all counterbalancing orders, participants first received instructions for the category decision task and were told to press the key labeled “Yes”, if the item belonged to the given category, or the key labeled “No”, if the item did not belong to the given category. The instructions specified: “Equally important in this task are your speed and accuracy in responding to each trial. We are looking at your response time for each pair of words. Therefore it is critical that you respond to the word as fast as you can without sacrificing accuracy.” Participants practiced the category decision task for six trials followed by six additional practice trials with feedback provided to encourage speed and accuracy in responding.
Prior to the focal PM block, participants were instructed to press the “Q” key during the category decision task if they ever saw a particular word. There were three potential PM targets (tortoise, raspberry, or aluminum), which were counterbalanced across participants. For the non-focal block of the category decision task, participants were instructed to press the “Q” key if they ever saw a word containing a particular syllable. The three targets that were used were ‘tor’ (appeared in words tortoise, history, and motorcycle), ‘ras’ (appeared in words raspberry, harassment, and grasshopper) and ‘min’ (appeared in words peppermint, aluminum, and minister)1, and they were counterbalanced across participants. The counterbalancing scheme ensured that participants did not receive focal and non-focal targets containing exactly the same items (e.g., ‘tortoise’ in the focal and ‘tor’ in the non-focal).
In both PM blocks participants were told that if they forgot to press the “Q” key, they could do so as soon as they remembered. However, the instructions emphasized that “in this experiment, we are primarily interested in how individuals categorize words.” Participants were required to summarize the PM instructions to the experimenter. If participants were uncertain about the instructions, or if they reported the instructions incorrectly, they were asked to re-read the instructions and then report to the experimenter again. Participants were not allowed to progress unless the experimenter was confident in their comprehension.
After the PM instructions (or after the category decision instructions in the control block), participants were informed that prior to the category decision task they would perform an unrelated task. The purpose of including these unrelated tasks in PM experiments is to create a delay between intention formation and execution. In the delay period of the present study participants completed a combination of the Deese, Roediger, and McDermott (DRM) associative memory task (Roediger & McDermott, 1995), the visual array comparison task (Cowan et al., 2005) and a physical activity survey. Each task completed during the delay period lasted approximately five minutes. These data will not be considered here. After completing the unrelated task, participants were briefly reminded of the instructions for the category decision task, with no mention made of the PM instructions. They next proceeded with the category decision task. Items in this task were presented in random order, with the only constraint being that PM targets occurred on trials 31, 72, and 102 (this was true for both the focal and non-focal PM blocks).
Upon completion of each of the PM blocks, participants were informed (unless it was the final block of the experiment) that they would never see another PM target word (focal condition) or an item containing the target syllable (non-focal condition). Thus, they no longer had to commit to the instruction to press the “Q” key for that target item. The control block was similar in procedure to the PM blocks except that there were no PM instructions. If the control block occurred after either of the PM blocks participants were instructed that for this block of the task they would only be performing the category decision task and there was no additional task.
After all three blocks of the category decision task were completed, participants were asked to complete the retrospective memory survey. The survey read as follows, “In the category decision task you were asked to press a key when you either saw a specific word or a specific syllable.” Participants were then asked three questions: 1) “what was the word,” 2) “what was the syllable,” and 3) what key were you supposed to press when you saw them?” Incorrect responses to any of these questions led to the exclusion of that person’s data due to retrospective memory failures. Finally, participants were thanked, debriefed, and paid for their participation.
Results
We first report analyses of PM performance followed by analyses of ongoing-task (word categorization) performance. In a final section we explore whether distinguishing between focal and non-focal PM tasks provides leverage on discriminating between healthy older adults and the very mildly demented. We also consider the extent to which PM measures improve discrimination between healthy aging and very mild dementia relative to standard psychometric indices. For all statistical tests, an alpha level of .05 was used to determine significance. Effect sizes are given by partial eta squared.
Prospective Memory Performance
We calculated the proportion of times that each participant remembered to press the Q key on presentation of the focal and the non-focal PM targets2. Consistent with previous work in these laboratory PM paradigms (Scullin, McDaniel, Shelton, & Lee, 2010) late responses were very rare. Indeed, there was only one instance where a participant responded late to a PM target item; this response occurred within two trials of the PM target and was coded as correct. We entered these proportions into a 2 (PM task: focal/non-focal) x 2 (CDR status: 0/0.5) x 2 (APOE 4 status: carrier/non-carrier) mixed analysis of variance (ANOVA), with PM task as the within-subjects variable and CDR status and APOE status as the between-subjects variables. A parallel analysis of covariance (ANCOVA) with age and education as covariates3 produced identical patterns; accordingly, we report just the ANOVA results.
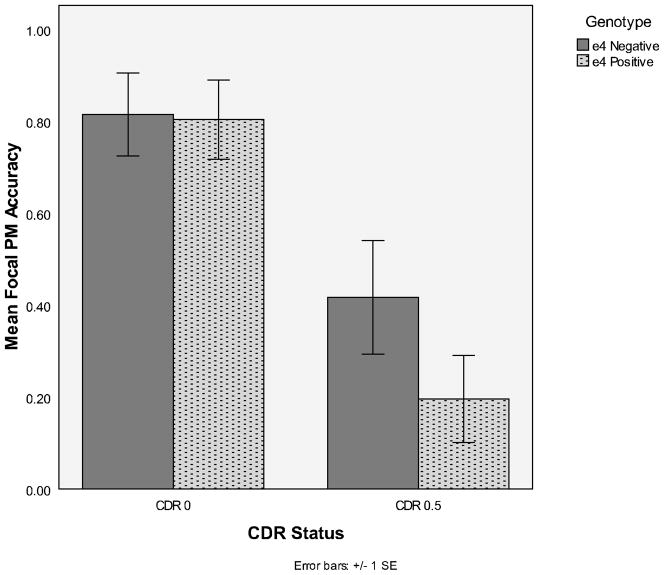
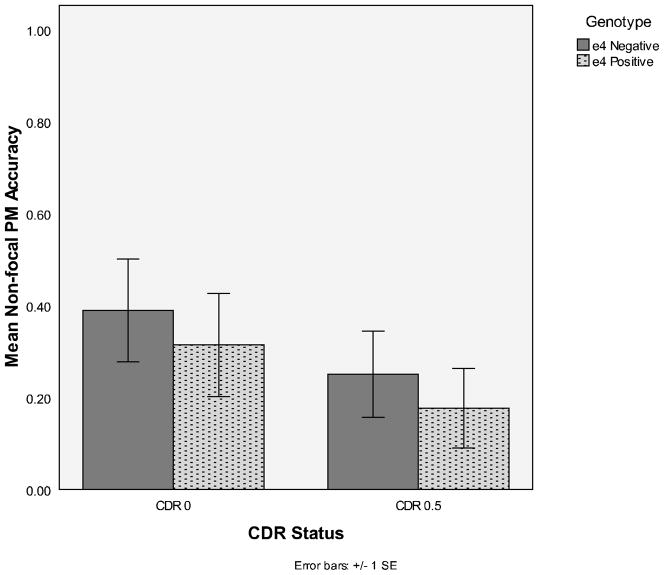
There was no main effect of APOE status nor did this variable interact with the other variables (largest F = 1.46). As expected, PM levels were higher for the focal task than for the non-focal task, F(1, 64) = 19.19, MSE = .134, η2p = .23. Also, the very mildly demented participants generally demonstrated significantly worse PM than the healthy older controls, F(1, 64) = 16.60, MSE = .210, η2p = .21. However, this main effect was qualified by a significant interaction with type of PM task, F (1, 64) = 8.41, MSE = .134, η2p = .12. The very mildly demented participants were significantly impaired relative to healthy controls on the focal PM task (Ms = .30 and .81, respectively; see Figure 1), F (1, 64) = 21.15, η2p = .21. In fact, 64% of CDR 0.5’s never responded to a focal PM target, while only 14% of CDR 0’s never responded to focal PM targets. Conversely, significant performance differences were not observed between healthy older adults and the very mildly demented on the non-focal PM task (Ms = .35 and .21, respectively), F (1, 64) = 1.59, η2p = .21 (see Figure 2).
Figure 1.
Mean focal PM accuracy as a function of CDR status (0 or 0.5) and genotype (APOE status: e4 positive or negative)
Figure 2.
Mean non-focal PM accuracy as a function of CDR status (0 or 0.5) and genotype (APOE status: e4 positive or negative)
One potential difficulty in interpreting the above interaction is that non-focal PM performance was relatively low, possibly limiting the opportunity to observe a deficit in non-focal PM for the very mildly demented. To circumvent this interpretational difficulty, we limited the next analyses to high performing participants. We calculated an overall PM performance index collapsed across focal and non-focal tasks. Only those participants who were above the median (with the median calculated separately for the CDR 0 and CDR 0.5 groups) were included in the subsequent analyses. There were no significant education differences between the high-performing CDR 0’s (n = 14, M = 14.79, SD = 2.75) and CDR 0.5’s (n = 16, M = 14.31, SD = 2.92; t < 1). There were also no significant age differences between the high-performing CDR 0’s (M = 74.38, SD = 6.68) and CDR 0.5’s (M = 78.80, SD = 9.61; t (28) = 1.41, p = .16).
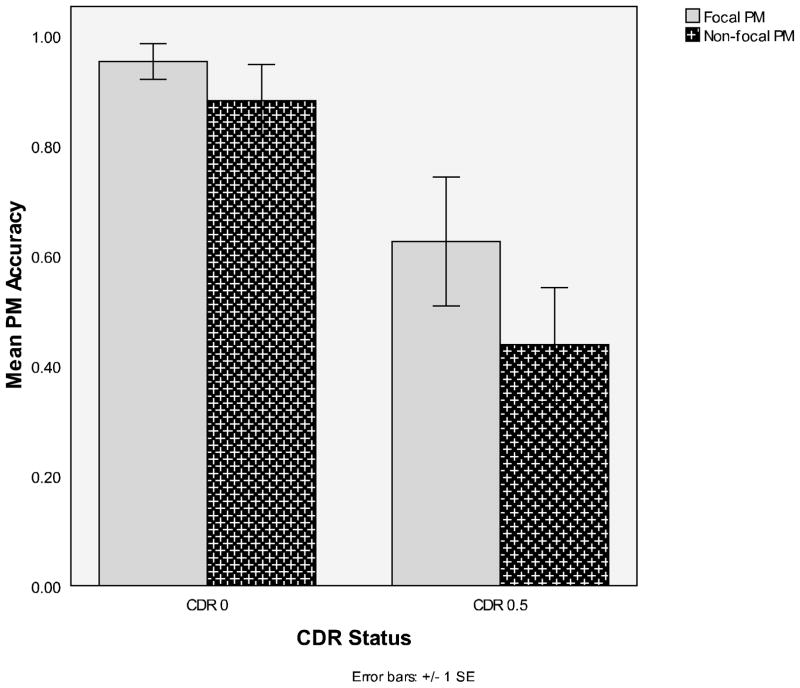
PM for these above-median performers was analyzed with a 2 (PM type: focal/non-focal) x 2 (CDR status: 0/0.5) mixed ANOVA (see Figure 3 for mean performances). There was no PM type main effect, which is not surprising given this analysis only included people who performed well across the focal and non-focal conditions. There was a CDR main effect, F (1, 28) = 31.07, MSE = .071, η2p = .53, reflecting superior performance in the CDR 0 group, and there was no PM type x CDR interaction (F < 1). Planned contrasts reinforced this pattern, with the very mildly demented older group showing significant decline relative to the healthy older adult group for focal PM (Ms = .63 and .95, respectively), F (1, 28) = 10.78, η2p = .53, and for non-focal PM (Ms = .44 and .88, respectively), F (1, 28) = 20.38, η2p = .53. This latter finding suggests that the absence of a difference in non-focal PM performance between CDR 0’s and 0.5’s in the entire sample was at least partly a consequence of overall low performance in the non-focal condition.
Figure 3.
Mean focal and non-focal PM accuracy for the high-performers as a function of CDR status (0 or 0.5)
Category Decision Task Performance
Table 3 provides the mean accuracy and response times (RTs) for each group across the three task blocks (control, focal PM, non-focal PM). The average proportion correct on the category decision task trials was entered into a 3 (task block) x 2 (CDR status) x 2 (APOE 4 status) mixed ANOVA, with task block as the within-subjects variable and CDR and APOE status as the between-subjects variables. There were no significant main effects or interactions (all F’s < 1.6). In general, performance accuracy was high and not affected by the presence of a PM task demand nor by CDR status.
Table 3.
Ongoing task accuracy and Reaction time (in milliseconds: control/focal/non-focal) x 2(CDR status: 0/0.5), x 2 (ApoE status: e4 positive/negative)
| CDR 0 | CDR 0.5 | |||
|---|---|---|---|---|
| e4 Positive | e4 Negative | e4 Positive | e4 Negative | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Ongoing Task accuracy | ||||
| Control | .95 (.04) | .95 (.02) | .94 (.02) | .94 (.03) |
| Focal | .95 (.02) | .95 (.02) | .95 (.02) | .95 (.03) |
| Non-focal | .95 (.02) | .95 (.03) | .95 (.03) | .95 (.02) |
| Reaction Time | ||||
| Control | 1671 (349) | 1479 (254) | 2057 (705) | 1811 (495) |
| Focal | 1748 (334) | 1573 (311) | 2093 (686) | 1781 (387) |
| Non-focal | 1825 (660) | 1809 (504) | 2207 (843) | 2022 (677) |
| n | 17 | 18 | 17 | 16 |
Mean RT across trials of each task block (excluding the PM target trials) was used for the RT analyses. In addition, two participants from the CDR 0.5/APOE 4carrier group had mean RT’s in each task block that exceeded 3 SD’s of their group mean. The mean RT’s for these participants was replaced with the value reflecting 3 SD’s above the group mean for each task block. A 3 (task block) x 2 (CDR status) x 2 (APOE status) mixed ANOVA on the RTs revealed a main effect of task block, F(2, 128) = 12.14, MSE = 69450.41, η2p = .16. Planned contrasts between each PM block and the control block indicated that RT’s in the non-focal task block were significantly slower than RT’s in the control block, F(1, 128) = 22.23, η2p = .16). By contrast, there was not a significant slowdown for the category decisions in the focal PM block relative to the control block (F = 1.04). The slower responding on the ongoing task trials when performed in the presence of the non-focal PM task is consistent with the notion that participants were monitoring for PM targets in the non-focal PM task. For the focal PM task there was no evidence of significant levels of monitoring, despite high power to detect a small size effect (power > .99). There was also a CDR main effect, F (1, 64) = 6.49, MSE = 757945.55, η2p = .09, demonstrating that CDR 0.5’s generally responded more slowly to the ongoing task trials. No other main effects or interactions were observed (largest F = 2.36).
In light of the age and education differences observed in the APOE status groups, age and education were treated as covariates in an ANCOVA3 paralleling the above ANOVA, which provides a more sensitive test of APOE effects. In addition to the effects reported above, a main effect of APOE status emerged, F (2, 126) = 5.61, MSE = 696853.75, η2p = .08. The APOE 4 non-carriers displayed slower RT’s on all task blocks relative to the APOE 4 carriers. APOE status did not interact with any of the other variables examined (largest F = 2.10).
Prediction of Dementia Status
The means and SD’s for all of the psychometric tests used in the following analyses are presented in Table 34. Data were collapsed across APOE 4 groups because none of the psychometric tests discriminated between carriers and non-carriers (with the exception of the Selective Reminding Test in the CDR 0.5 group).
A series of logistic regression analyses were conducted with CDR status as the dependent measure to determine if focal PM performance could discriminate between CDR 0’s and 0.5’s above and beyond the psychometric indices. Several psychometric composite indices were constructed based on the confirmatory factor analyses conducted by Tse, Balota, Yap, Duchek, and McCabe (2010) who used a large sample of healthy and very mildly demented older adults. All of the individual psychometric test scores in the present study were converted to standardized scores based on performance in the healthy older adult sample of Tse et al., and these standardized scores were used to construct the composite indices. First, an episodic/semantic memory composite was constructed based on average performance in Logical Memory, Associative Memory, and Animal Naming. Second, a processing speed composite was constructed based on the standardized scores of Digit Symbol and Word Fluency. Finally, 38 participants (15 CDR 0.5’s and 23 CDR 0’s) completed 3 complex working memory span tests (reading span, computation span, and letter rotation span) and standardized scores from these tests were used to construct an index representing the working memory composite.
In the first analysis, focal PM performance was entered alone into the regression equation and it successfully classified the CDR status for 75% of participants, p < .001; however, when non-focal PM performance was entered alone into the equation, CDR status was only successfully classified for 54% of participants, p > .10. Next, the episodic/semantic memory composite was entered into the first block of the regression analysis and it successfully classified 70 % of participants into the appropriate CDR group, p < .001. Focal PM performance was entered into the second block of the regression equation and it classified an additional 11% of participants, p = .01. The processing speed composite index only classified 62 % of participants according to their CDR status, p = .08. Finally, the working memory composite successfully discriminated between CDR 0’s and 0.5’s (classified 74 %, p < .02) and focal PM performance was able to further discriminate between the two groups (classified an additional 3 %, p = .02). Thus, focal PM performance successfully discriminated between healthy older adults and the very mildly demented and was able to do so above and beyond the psychometric and working memory tests5.
Discussion
The present experiment reinforces and significantly extends the patterns reported in the nascent literature on prospective memory in very mildly demented older adults. In line with the studies of Duchek et al. (2006) and Huppert et al. (2000), we demonstrated large decrements in PM performance for older adults in the very early stages of dementia relative to a group of healthy older adults. Interpretation of these previous dementia-related declines in PM performance as a consequence of PM deficits per se has been somewhat clouded, however. As noted earlier, in several existing studies, at least some of the PM failures reported are retrospective memory failures (Duchek et al.; Huppert et al.), failures that are not necessarily distinguishable from retrospective memory deficits that are commonly reported for individuals with dementia (or probable dementia). In the present study, we confirmed that all of the participants had intact retrospective memory for the PM task. Thus, the significant PM decline observed for the very mild AD group decisively indicates that this group exhibits a deficit in the PM component of prospective remembering.
The PM deficit associated with very mild AD participants for the focal PM task is especially striking and important on several grounds. First, this finding represents a significant departure from focal PM patterns related to both normal aging and other types of pathological aging. Older adults typically show little if any decline on focal PM tasks relative to younger adults (see McDaniel et al., 2008, using the PM task and ongoing activity adopted herein; for a similar pattern of minimal or no-age related decline in focal PM see Einstein & McDaniel, 1990; Einstein, Holland, McDaniel, & Guynn, 1992; Einstein, McDaniel, Richardson, Guynn, & Cunfer, 1995; Rendell et al., 2007; see Kliegel & Jäger, 2008 for a metaanalysis). Further, adults with Parkinson’s disease also show spared focal PM performance relative to healthy controls (Foster et al., 2009). This dissociation between the present decline in focal PM for very mild AD individuals and the spared focal PM reported for Parkinson’s patients is fairly direct because the focal PM task and ongoing activities were the same in both studies. Thus, robust declines in focal PM performance (at least in the kind of laboratory paradigm used here) appear to be uniquely manifested in very early AD relative to normal older adults and older adults with Parkinson’s disease pathology.
Second, the patterns noted above, taken in conjunction with the ongoing task results, provide leverage on revealing possible cognitive mechanisms involved in the PM component that appear to be compromised in very mild AD. Findings from the basic PM literature suggest that the focal PM task but not the non-focal task encourages reliance on spontaneous retrieval processes (for supporting evidence see Einstein et al., 2005, using the same PM tasks and ongoing activity incorporated into the present study; see also Scullin et al., 2010). A behavioral marker suggesting that prospective remembering in a focal PM task can be supported by spontaneous retrieval is that the presence of the focal PM task does not significantly attenuate performance (either in terms of accuracy or response latency) on the ongoing activity in which the PM task is embedded (see Einstein et al., 2005, and Einstein & McDaniel, 2010, for theoretical amplification). In the present study, we demonstrated just this pattern. The implication is that participants were relying minimally, if at all, on strategic monitoring processes to support retrieval of the intention at the appropriate point (i.e., during presentation of the PM target). Thus, we can infer that a more spontaneous retrieval process was involved in PM performance for the focal PM task.
In the context of the above reasoning, our finding that the very mild AD participants displayed a substantial decrement in focal PM performance relative to healthy controls illuminates a possible locus for the AD-related PM deficit. Specifically, this pattern suggests that the very early stages of AD compromise PM processes related to spontaneous retrieval of an intended action. Several related theoretical and empirical observations add currency to this conclusion. First, spontaneous associative retrieval is assumed to be supported by medial temporal structures, including the hippocampus (Moscovitch, 1992; 1994), and these are structures that display decline in early AD (Buckner, 2004; Head et al., 2005; Jack et al., 2008). Further converging on the tentative idea that spontaneous retrieval in PM and medial-temporal functioning are related, in Parkinson’s disease prefrontal structures are assumed to be primarily impacted as opposed to medial-temporal structures; thus, Parkinson’s patients should not be at risk for focal PM deficits. As mentioned earlier, Parkinson’s disease patients display high levels of focal PM performance, levels that are equivalent to that displayed by healthy controls (Foster et al., 2009).
It is also important to note that if PM performance in the focal PM paradigm used here (and in Foster et al., 2009) was instead supported by strategic monitoring processes (as opposed to spontaneous retrieval), then according to existing theoretical accounts, prefrontal processes should be involved (McDaniel & Einstein, 2007; see Burgess, Scott, & Frith, 2003, for evidence with PET; McDaniel, Glisky, Rubin, Guynn, & Routhieaux, 1999, for neuropsychological related evidence; Reynolds, West, & Braver, 2009, for fMRI evidence). Therefore, pathologies like Parkinson’s disease that impact prefrontal structures would be expected to produce robust deficits on the focal task (and they do not). However, Parkinson’s patients do display decline on the non-focal task, a task that clearly requires strategic monitoring (Foster et al., 2009). These patterns reinforce our interpretation that the focal PM task declines for AD were related, at least in part, to impaired spontaneous retrieval processes.
The results for the non-focal PM task were more equivocal in terms of outcomes related to very mild AD. This task was designed to require the use of strategic monitoring processes to search for PM target items. We observed robust costs to the ongoing task (in terms of slower RT’s to nontarget trials) as a consequence of having to perform the non-focal PM task, suggesting that the participants were recruiting attention demanding (monitoring) processes to perform the non-focal PM task. Further, the healthy older participants demonstrated relatively low PM performance on the non-focal task (much lower than for the focal PM task), a finding that is not uncommon for PM tasks that would be considered non-focal in nature (e.g., Maylor, 1996; Park, Hertzog, Kidder, Morrell, & Mayhorn, 1997; see McDaniel & Einstein, 2007, for a review). The novel finding from the present experiment is that the very mild AD participants did not show significant decline on the non-focal PM task relative to the healthy participants. One interpretation is that very mild AD does not further compromise monitoring processes over that produced by normal aging, at least in the context of the relatively short-term PM tasks investigated here.
The above interpretation is clouded, though, because the low performance of the healthy participants (in the non-focal PM task) leaves less room to detect a decline. Along these lines, it should be noted that when the analysis was restricted to high PM performers, a significant deficit in non-focal PM was observed in the very mildly demented group, and was numerically larger in the non-focal PM task (.44) than the focal PM task (.32). Additionally, Blanco-Campal et al. (2009) recently reported that non-focal PM performance was particularly impaired for individuals with mild cognitive impairment (suspected of AD) relative to normal control adults (with control adults showing nearly perfect non-focal PM performance). As these authors mentioned, this pattern suggests that very mild AD may compromise strategic monitoring processes. This is not surprising given the widespread evidence of attentional/strategic control deficits in early stage AD (e.g., Balota & Faust, 2001; Castell et al., 2009; Perry & Hodges, 1999; Tse et al., 2010).
Interestingly, however, in the present study, the very mild AD participants demonstrated increased RTs in their category decision responses in the presence of the non-focal PM task (this was also the case for the high PM performers alone; M = 2190 ms for RTs on the non-focal PM block vs. M = 1828 ms for the control block), suggesting that they were engaging monitoring but not doing so effectively (see Foster et al., 2009, for a similar pattern with Parkinson’s patients). These RT costs associated with the non-focal PM task also imply that the very mild AD (and healthy older adults) did not just forget they had a prospective memory task to do; they apparently remembered there was a prospective memory task but did not monitor consistently enough to remember at the appropriate moment (cf. Tse et al., 2010; West, 2001).
Accordingly, from a theoretical perspective more work remains to understand the contexts in which strategic monitoring processes required for non-focal PM tasks are substantially compromised in very mild AD individuals relative to healthy age-matched controls. Yet from an applied perspective the present non-focal PM results hold import. Given that healthy older adults display robust deficits in non-focal PM (see Kliegel & Jäger, 2008; McDaniel & Einstein, 2007, for reviews), relatively impaired non-focal PM performance may not prove useful in signaling very mild AD (although see Blanco-Campal et al., 2009, who reported extremely high non-focal PM performances in old-adult controls, and in this context non-focal PM was useful for detecting mild cognitive impairment believed related to AD disease). We turn to this point in the next section.
Prospective Memory as a Marker of Very Mild AD
The present findings have potential practical implications in terms of identifying individuals who are in the very early stages of AD. We found that focal PM performance alone provided sensitive detection of individuals in the very early stages of this disease. By contrast, non-focal PM performance provided little if any discrimination between healthy older adults and those in very early stages of AD. Further, detection sensitivity was not enhanced when focal and non-focal PM performances were combined (70.6% classification achieved) relative to when focal PM alone was considered (75% classification achieved); indeed, detection sensitivity was better for focal PM alone. These patterns are consistent with the above idea that PM assessments that do not distinguish between types of event-based PM tasks or that do not incorporate focal PM tasks (e.g. Cambridge Behavior Prospective Memory Test; Groot, Wilson, Evans, & Watson, 2002), may not be maximizing sensitivity to various pathological declines such as very mild dementia.
It is also noteworthy, that focal PM was able to discriminate between healthy and very mildly demented adults as well or better than any of the other psychometric measures often used in memory and cognitive assessments to detect dementia-related decline. In addition, when focal PM performance was considered in concert with standard psychometric tests, the accuracy of discriminating between healthy and demented individuals significantly improved relative to that obtained with standard psychometric tests alone. The preliminary implication is that focal PM might be a valuable measure to assist detection of very mild AD. In this regard, it is favorable that the testing time for the focal PM task was about 5 minutes, comparable to that of the psychometric tests, which ranged from approximately 2 to 10 minutes.
Prospective Memory and APOE Influences
Another goal of this study was to further explore the possible relation between APOE 4 status and PM performance, in particular focal PM performance. Only two published studies have been reported in the literature regarding a possible effect of APOE 4 status on (focal) PM performance, and the results were mixed (Driscoll et al., 2005; Duchek et al., 2006). Consistent with Duchek et al., we found no hint of a general relation between APOE 4 status and focal PM performance. Thus, as Duchek et al. argued, the decrease in PM associated with the presence of the APOE 4 allele reported in Driscoll et al. might parsimoniously be interpreted as related to a higher incidence of very mild AD in the Driscoll et al. APOE 4-carrier sample than in the non-carrier sample.
In closing, the findings from the present study offer insight into the specific types of PM deficits observed in the very early stages of AD and the cognitive mechanisms underlying these deficits. Specifically, focal PM tasks appear to be particularly sensitive to the very earliest stages of AD. These tasks apparently rely on spontaneous retrieval (the focal PM tasks did not penalize performance on the ongoing category decision activity). Thus, very mild AD may compromise spontaneous PM retrieval. The non-focal PM task required strategic monitoring, as indicated by the (RT) costs to the ongoing activity when the non-focal task was present. As indexed by these costs, very mild AD participants appeared to engage monitoring, but the analysis of high PM performers hinted that the monitoring of AD participants was less effective than that of control participants.
Acknowledgments
This research was supported in part by National Institute on Aging Grants P50AG05681 (Alzheimer’s Disease Research Center) and P01AG03991. Preparation of this article was supported in part by National Institute on Aging Grant RC1AG036258, and Jill Shelton’s participation was supported by National Institute on Aging Grant 5T32AG00030. Portions of this research were presented at the Armadillo Conference, Trinity University, San Antonio, October, 2007 and at the Cognitive Aging Conference, Atlanta, April, 2010.
We would like to thank Janet Duchek and Martha Storandt for their assistance with the psychometric data, and Fergus Craik for helpful comments on an earlier version of the paper. We thank the team at the Washington University Alzheimer’s Disease Research Center (ADRC) for facilitating this project, including John Morris, Virginia Buckles, Anne Canamucio, and Mary Coats. We are also extremely grateful to Alison Goate and the ADRC Genetics Core for the APOE data, and to those enrolled in the ADRC subject pool who kindly volunteered to participate in the study.
Footnotes
Clearly, in some cases the target syllable was retained as a syllable in some of the words but not in other words. To confirm that participants were not biased against responding to words in which the target syllables were not retained as syllables per se, we evaluated whether PM performance differed for the words in which target syllables were actually syllables from those words in which target syllables did not appear as syllables per se. PM performance did not significantly differ across these two situations.
False alarms to non-target items were infrequent, with only 5 commission errors made in both the focal and non-focal conditions. As expected, no PM responses were observed in the control block of the category decision task.
The covariates were mean centered as suggested by Delaney and Maxwell (1981) when conducting mixed-design ANCOVA (i.e., testing within-subjects effects).
A portion of the participants completed an older version of the Logical Memory test, while the rest of the participants completed the newer version of the test. The scales are different between the two tests, accordingly the raw scores were converted to z scores. The standardized score for this test reflects values from different reference groups and this score was used for all analyses. Due to the difference in raw scores between the old and new version of this test, descriptive statistics were not presented in Table 3. In addition, there was one participant (CDR 0, ApoE 4+) whose Logical Memory score was not available.
Additional logistic regression analyses were conducted to examine how well each individual psychometric test discriminated between healthy older adults and the very mildly demented, and in each of these analyses focal PM performance was entered in the second block of the regression equation to determine if it could account for additional variance above and beyond the individual psychometric tests. The psychometric tests that were able to classify the CDR status of a significant number of participants were Logical Memory, Animal Naming, Associative Memory, Selective Reminding Test, Boston Naming, and Crossing Off. Focal PM scores were able to classify a significant number of participants beyond each of these psychometric tests, p ≤ .05.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
Contributor Information
Mark A. McDaniel, Department of Psychology, Washington University
Jill Talley Shelton, Department of Psychology, Washington University.
Jennifer E. Breneiser, Department of Psychology, Valdosta State University
Sarah Moynan, Department of Psychology, Washington University.
David A. Balota, Department of Psychology, Washington University
References
- Balota DA, Faust ME. Attention in dementia of the Alzheimer’s type. In: Boller F, Cappa S, editors. The handbook of neuropsychology: Aging and dementia. 2. New York: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Blanco-Campal A, Coen RF, Lawlor BA, Walsh JB, Burke TE. Detection of prospective memory deficits in mild cognitive impairment of suspected Alzheimer’s disease etiology using a novel event-based prospective memory task. Journal of the International Neuropsychological Society. 2009;15:154–159. doi: 10.1017/S1355617708090127. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Knight JB, Marsh RL, Unsworth N. Individual differences in event-based prospective memory: Evidence for multiple processes supporting cue detection. Memory & Cognition. 2010;38:304–311. doi: 10.3758/MC.38.3.304. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Scott SK, Frith CD. The role of the rostral frontal cortex (area 10) in prospective memory: A lateral versus medial dissociation. Neuropsychologia. 2003;41:906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP. Memory efficiency and the strategic control of attention at encoding: Impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology. 2009;23:297–306. doi: 10.1037/a0014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Sauls JS, More CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention. Its estimation and its role in working memory capacity and cognitive aptitudes. Cognitive Psychology. 2005;51:40–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney HD, Maxwell SE. On using analysis of covariance in repeated measures designs. Multivariate Behavioral Research. 1981;16:105–123. doi: 10.1207/s15327906mbr1601_6. [DOI] [PubMed] [Google Scholar]
- Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19:28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Cortese M. Prospective memory and apolipoprotein E in healthy aging and early Alzheimer’s disease. Neuropsychology. 2006;20:633–644. doi: 10.1037/0894-4105.20.6.633. [DOI] [PubMed] [Google Scholar]
- Einstein GO, Holland LJ, McDaniel MA, Guynn MJ. Age-related deficits in prospective memory: The influence of task complexity. Psychology and Aging. 1992;7:471–478. doi: 10.1037//0882-7974.7.3.471. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory and what costs do not reveal about retrieval processes: A commentary on Smith, Hunt, McVay, and McConnell (2007) Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1082–1088. doi: 10.1037/a0019184. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR. Aging and prospective memory: Examining the influences of self-initiated retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovš G, Hershey T. Prospective memory In Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23:347–358. doi: 10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot YCT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8:645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cerebral Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Johnson T, Nickson J. High prevalence of prospective memory impairment in the elderly and early-stage dementia: Findings from a population-based study. Applied Cognitive Psychology. 2000;14:S63–S81. [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen WL, Weinberg WB, Alford HSY, Mulvey EK, Kaeochinda KF. Improving the reliability of event-based laboratory tests of prospective memory. Psychonomic Bulletin & Review. 2006;13:1028–1032. doi: 10.3758/bf03213920. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jäger T. Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging. 2008;23:203–208. doi: 10.1037/0882-7974.23.1.203. [DOI] [PubMed] [Google Scholar]
- Maylor EA. Age-related impairment in an event-based prospective memory task. Psychology and Aging. 1996;11:74–78. doi: 10.1037//0882-7974.11.1.74. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Smith G, Della Sala S, Logie RH. Prospective and retrospective memory in normal aging and dementia: An experimental study. Memory and Cognition. 2002;30:871–884. doi: 10.3758/bf03195773. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- McDaniel MA, Einstein GO, Rendell PG. The puzzle of inconsistent age-related declines in prospective memory: A multiprocess explanation. In: Kliegal M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. Mahwah, NJ: Erlbaum; 2008. [Google Scholar]
- McDaniel MA, Glisky EL, Rubin SR, Guynn MJ, Routhieaux BC. Prospective memory: A neuropsychological study. Neuropsychology. 1999;13:103–110. doi: 10.1037//0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In: Shacter DL, Tulving E, editors. Memory Systems. Cabmvridge: MT Press; 1994. pp. 269–310. [Google Scholar]
- Nebes RD, Martin DC, Horn LC. Sparing of semantic memory in Alzheimer’s disease. Journal of Abnormal Psychology. 1984;93:321–330. doi: 10.1037//0021-843x.93.3.321. [DOI] [PubMed] [Google Scholar]
- Park DC, Hertzog C, Kidder DP, Morrell RW, Mayhorn CB. Effect of age on event-based and time-based prospective memory. Psychology and Aging. 1997;12:314–327. doi: 10.1037//0882-7974.12.2.314. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Rendell PG, McDaniel MA, Forbes RD, Einstein GO. Age-related effects in prospective memory are modulated by ongoing task complexity and relation to target cue. Aging, Neuropsychology, and Cognition. 2007;14:236–256. doi: 10.1080/13825580600579186. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology; Learning, Memory, and Cognition. 1995;4:803–814. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychological Software Tools; 2002. [Google Scholar]
- Scullin MK, McDaniel MA, Einstein GO. Control of cost in prospective memory: Evidence for spontaneous retrieval processes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:196–203. doi: 10.1037/a0017732. [DOI] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH. Focal/nonfocal cue effects in prospective memory: Monitoring difficulty or different retrieval processes? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:736–749. doi: 10.1037/a0018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse CS, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- West R. The transient nature of executive control processes in younger and older adults. European Journal of Cognitive Psychology. 2001;13:91–105. [Google Scholar]