Abstract
There has been a resurgence of interest in synthetic and plant-derived flavonoids as modulators of γ-amino butyric acid-A (GABAA) receptor function influencing inhibition mediated by the major inhibitory neurotransmitter GABA in the brain. Areas of interest include (i) flavonoids that show subtype selectivity in recombinant receptor studies in vitro consistent with their behavioural effects in vivo, (ii) flumazenil-insensitive modulation of GABAA receptor function by flavonoids, (iii) the ability of some flavonoids to act as second-order modulators of first-order modulation by benzodiazepines and (iv) the identification of the different sites of action of flavonoids on GABAA receptor complexes. An emerging area of interest is the activation of GABAA receptors by flavonoids in the absence of GABA. The relatively rigid shape of flavonoids means that they are useful scaffolds for the design of new therapeutic agents. Like steroids, flavonoids have wide-ranging effects on numerous biological targets. The challenge is to understand the structural determinants of flavonoid effects on particular targets and to develop agents specific for these targets.
Keywords: ionotropic, GABAA receptors, flavonoids, allosteric modulation
Introduction
Flavonoids are ubiquitous in plants and as a result constitute the most common group of polyphenolic compounds in the human diet. It is estimated that human daily consumption of flavonoids ranges from tens of milligrams to over one gram. Dietary flavonoids are predominantly derived from fruits, vegetables, chocolate and beverages such as tea, coffee and red wine as well as herbal preparations. In particular, fruits vegetables, cereals, green tea and coca are rich sources of flavonoids (Manach et al., 2004). Although the absorption and distribution of flavonoids is not well understood, and their bioavailability is a topic of considerable debate (Manach et al., 2004), the lipophilicity of some flavonoids allows them to cross the blood–brain barrier (BBB) (Youdim et al., 2004), and thus, it is likely that diet-derived flavonoids are found in brain.
Flavonoids have been the focus of intense research, displaying many interesting biological activities (Cushnie and Lamb, 2005; Clarke and Wiseman, 2008; Andres et al., 2009; Rathee et al., 2009), and are believed to play a significant role in reducing the risks of age- and lifestyle-related diseases such as cancer (Kale et al., 2008; García-Lafuente et al., 2009), diabetes (Cazarolli et al., 2008), cardiovascular disease (García-Lafuente et al., 2009) and declining neurological function (Spencer, 2009; Spencer et al., 2009). Many flavonoids have well-established antioxidant and free-radical-scavenging activities, and it was initially believed that their protective effects were as a direct result of these actions. However, it is now accepted that in addition to their ability to prevent damage caused by oxidative stress, flavonoids also exert their biological effects through direct actions on enzymes, receptors and signalling pathways (Williams et al., 2004). More recently, the actions of flavonoids on the central nervous system have attracted much attention. Flavonoids are believed to prevent neurodegeneration associated with Parkinson's and Alzheimer's disease and improve cognitive function (Spencer, 2009; Spencer et al., 2009). Additionally, flavonoids have demonstrated anxiolytic, sedative and anticonvulsant activities. Although their actions in the central nervous system occur through a variety of interactions with different receptors and signalling pathways, it is believed that some of these effects are mediated by ionotropic GABA, in particular GABAA receptors.
This has led to a resurgence of interest in flavonoids as modulators of GABAA receptor function influencing inhibition mediated by the major inhibitory neurotransmitter GABA in the brain. Areas of interest include (i) flavonoids that show subtype selectivity in recombinant receptor studies in vitro consistent with their behavioural effects in vivo, (ii) flumazenil-insensitive modulation of GABAA receptor function by flavonoids, (iii) the ability of some flavonoids to act as second-order modulators of first-order modulation by benzodiazepines and (iv) the identification of the site(s) of action of flavonoids on GABAA receptor complexes.
GABAA receptors
GABAA receptors are the most important inhibitory receptors in the central nervous system (Chebib and Johnston, 2000; Whiting, 2003; Johnston, 2005; Carter et al., 2010). They are members of the cys-loop superfamily of ligand-gated ion channels (LGICs) that encompasses both cationic [nicotinic acetylcholine and 5-hydroxytryptamine family 3 (5-HT3)] and anion (GABAA, GABAC and glycine) receptors. These channels are membrane bound, structurally similar and considered to be composed of pentamers formed from distinct subunit combinations. Each subunit has a large N-terminal domain that encompasses the ligand binding domain and the cys-loop motif. The cys-loop consists of two disulphide bond–forming cysteines separated by 13 amino acid residues, several of which are highly conserved to form a signature sequence. There are four transmembrane domains termed TM1 to TM4 that traverses the membrane, and the TM2 lines the channel lumen. A large intracellular loop exists that contains sites for phosphorylation, anchoring and channel clustering. The GABA agonist binding site (the orthosteric site) is located at the interface between β-α subunits and is formed from loops that occur in the N-terminal region of the subunit (reviewed in Akabas, 2004).
Several classes of subunits with multiple isoforms have been identified by sequence homology (including α1–α6, β1–β3, γ1–γ3, δ) (Alexander et al., 2009). These subunits can ‘mix and match’ to form receptor subtypes. Given the large number of subunits and possible combinations, the total number of potential receptor subtypes would be huge. However, only 10 distinct subunit combinations have been conclusively identified to be physiologically relevant with the majority of GABAA receptors in the brain formed from two α-, two β- and one single γ- or δ-subunit (Whiting, 2003).
Despite great understanding of how GABA binds to these receptors, the complex pharmacology of GABAA receptors is not well characterized due to the diversity of subunit combinations and a lack of subtype selective agents. In general, GABAA receptors are activated by GABA and selectively blocked by the alkaloid bicuculline. Of the agonists known, most are not selective for any particular subtype of the GABAA receptor. However, GABAA receptors incorporate a large number of allosteric modulatory sites, agents known to modulate GABAA receptors, including benzodiazepines, barbiturates, neurosteroids, general anaesthetics such as etomidate, and propofol, ethanol, the sedative and anticonvulsant loreclezole and flavonoids, amongst others.
Modulation of GABAA receptors
Since their discovery in the mid-1970s, benzodiazepines have been some of the most widely prescribed drugs. They have a range of wanted (including anticonvulsant, sedative–hypnotic and anxiolytic) and unwanted (including sedative, memory impairment and physical dependence) effects. These agents have no direct action on mammalian GABAA receptors but modulate (either positively or negatively) the action of GABA via an allosteric site.
The effects of benzodiazepines on GABAA receptors are complex and dependent on receptor subunit composition. In general, the γ2-subunit is required for the most widely observed effects of benzodiazepines on native GABAA receptors (Puia et al., 1991; Wafford et al., 1993), with the α-subunit determining benzodiazepine sensitivity. Benzodiazepine agonists such as diazepam and flunitrazepam enhance the action of GABA with high affinity (nM activity) for GABAA receptors consisting of α1-3,5 such as α1βγ2, α2/3βγ2 and α5βγ2 receptors. In contrast, receptors containing α4- or α6-subunits are insensitive to diazepam (Wieland et al., 1992).
Walters et al. showed that benzodiazepines can act on GABAA receptors via ‘two distinct and separable mechanisms’ (Walters et al., 2000). At nanomolar concentrations, benzodiazepines act in a classic flumazenil-sensitive manner to enhance the action of GABA, while at micromolar concentrations, benzodiazepines act in a flumazenil-insensitive manner. They showed that specific mutations in the second membrane spanning domains of α-, β- and γ-subunits abolished the micromolar but not the nanomolar component. Furthermore, the biphasic action of benzodiazepines apparent in recombinant α1β2γ2S GABAA receptors was absent in receptors consisting of α1β2 subunits, with these receptors exhibit only the micromolar component. Given that plasma levels of benzodiazepines can reach micromolar concentrations (Bond et al., 1977) and there is evidence for the occurrence of GABAA receptors consisting of only α- and β-subunits (Olsen and Sieghart, 2009), especially during epilepsy, hormone treatment, ethanol intake, or during development, the flumazenil-insensitive, low-affinity benzodiazepine site may have pharmacological relevance.
Recently the use of transgenic mice (knock-out and knock-in) has provided new knowledge towards our understanding of the physiological role of the various α-subunit containing receptors (Rudolph and Mohler, 2004; Atack, 2005; Whiting, 2006). Comparison of drug-induced behavioural responses in the mutated and wild-type mice has allowed the identification of diazepam effects that were missing, or reduced, in the mutant mice. With this approach, it was demonstrated that the α1-subunits mediate sedation and serve as targets for sedative–hypnotics, while α2- and/or α3-containing receptors mediate anxiolysis and α5-containing receptors are related to memory (Rudolph and Mohler, 2006).
Many intravenous and volatile anaesthetics potentiate the action of GABA at clinically relevant concentrations. At least two binding sites exist: a high-affinity site whereby the action of GABA is potentiated by low micromolar concentrations of anaesthetic and a low-affinity site whereby high micromolar concentrations of anaesthetics directly activate the receptor. Recombinant GABAA receptor studies, chimeric and mutagenesis studies evaluating anaesthetic activity along with transgenic mice studies have identified sites within the β- and α-subunits to be the target for a number of anaesthetics (reviewed in Rudolph and Antkowiak, 2004).
The site of action for general anaesthetics is complex and differs for volatile versus intravenous anaesthetics (reviewed in Franks, 2008). Irrespective of the type of anaesthetic, the general consensus for the binding site is within the TM2 and TM3 domains of the receptor. Specifically, mutation of sites on the α-subunit reduce or eliminate the effects of volatile anaesthetics (Krasowski et al., 1997; Mihic et al., 1997; Jenkins et al., 2001) but not the effects of intravenous agents (Krasowski et al., 1998; Schofield and Harrison, 2005). In contrast, sites on the β-subunit (Belelli et al., 1997; Mihic et al., 1997) are critical for allosteric modulation of GABAA receptors by intravenous anaesthetics such as etomidate, propofol, enflurane, isoflurane and other modulators such as loreclezole and ethanol (reviewed in Franks, 2008). Studies using knock-in transgenic mice (Drexler et al., 2009) and a photoreactive analogue of etomidate, [3H]azietomidate (Li et al., 2010) suggest that general anaesthetics do not necessarily bind to a common binding site but may bind to an overlapping binding site or bind to separate sites but use a common transduction mechanism.
Barbiturates exert anxiolytic and hypnotic actions, and although most are no longer used clinically, pentobarbitone is still used as an anaesthetic and phenobarbitone as an anticonvulsant. Like general anaesthetics, the pharmacology of barbiturates is complex. Pentobarbitone for example has several actions: low micromolar concentrations potentiate GABA, high micromolar concentrations directly activate GABAA receptors while millimolar concentrations inhibit the receptor (Thompson et al., 1996; Drafts and Fisher, 2006). The various effects seen by pentobarbitone indicate several sites may be involved in mediating the various such effects. Both the α- and β-subunits are important in determining the affinity and efficacy of barbiturates, with the α-subunit having more pronounced effects (Hadingham et al., 1993; Thompson et al., 1996).
Neurosteroids, for example allopregnanolone and tetrahydrocorticosterone, are physiologically important allosteric modulators of GABAA receptors, acting with highest potencies on α5- and δ-subunit containing receptors (Hosie et al., 2007). Neurosteroids have two distinct actions on GABAA receptors, at nanomolar concentrations such as those experienced during stress they act as positive modulators and at higher (µM) concentrations such as those that occur during pregnancy that directly activate GABAA receptor. These actions are mediated by two distinct binding sites in the transmembrane region of the receptor. The modulatory site is located in a lipophillic pocket between the highly conserved M1 domain and M4 domain of the α-subunit (Hosie et al., 2009). The binding site for direct activation is at the α–β interface between the M1 domain of the α-subunit and the M2 domain of the β-subunit (Hosie et al., 2006; 2009;).
Flavonoids
Flavonoids are characterized by a phenylbenzopyran chemical structure. The basic structure includes a C15 (C6-C3-C6) skeleton consisting of two aromatic rings and an oxygen containing heterocyclic benzopyran ring. The fused aromatic ring is known as the A ring, phenyl constituent as the B ring and the benzopyran ring as the C ring. Flavonoids are subdivided in to eight different classes, defined according to the oxidative status and the number and type of substituents on the heterocyclic ring. These are flavones, flavanones, flavonols, flavanonals, flavan-3-ols (catechins), flavan-3,4-diol, isoflavones and anthocyanins (Figure 1).
Figure 1.
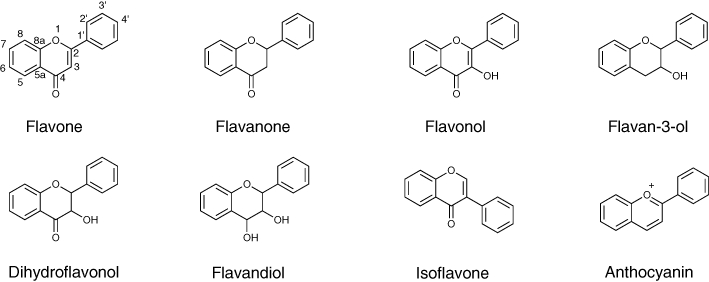
Different classes of flavonoids.
The presence of the double bond in the C ring of flavones, flavonols and isoflavones results in the A ring and C ring being planar. The absence of this double bond in flavanones, flavan-3-ols and flavandiols means that the two rings are not planar, the C ring existing in a puckered conformation with the C2 and C3 carbons lying on opposite sides of the plane of the A ring. In addition, the absence of the double bond means these non-planar compounds are chiral at the C2 and/or C3 centres, existing as stereoisomers.
There is an abundance of natural flavonoids, with over 6000 different members of the flavonoid family reported. Extensive modification of the parent structure through hydroxylation accounts for many of these compounds. However flavonoids may also be C-prenylated or O-methylated. In nature, many flavonoids are also found as O- or C-glycosides (Bohm, 1998). Flavonoids may also exist in polymereric form with dimers being the most common, through a variety of different C–C and C–O–C linkages. For example, apigenin (4′,5,7-trihydroxyflavone) forms the natural products 6,8"-biapigenin (agathisflavone), 3′8"-biapigenin (amentoflavone), 8,8"-biapigenin (cupressiflavone), 3′6"-biapigenin (robustaflavone) through C–C linkages and 4′,6"-biapigenin (hinokiflavone) through a C–O–C linkage.
Flavonoids as modulators of GABAA receptors
The search for the elusive endogenous benzodiazepine receptor ligand led to the first report of flavonoids interacting with benzodiazepine receptors. The isoflavones S-(–)-equol, 4′-hydroxy-7-methoxyisoflavone and 3′,7-dihydroxyisoflavone (Figure 2) isolated from bovine urine were found to displaced 3[H]-diazepam binding in rat brain. S-(–)-Equol was found to be most potent with an IC50 of 80 µM but reported to have no significant pharmacological effects in vivo (Luk et al., 1983). However, it was the pioneering work of Marder, Medina, Paladini and their coworkers in Argentina during the 1990s drew attention to flavonoids as ‘a new family of benzodiazepine receptor ligands’ (Medina et al., 1997; Paladini et al., 1999; Marder and Paladini, 2002).
Figure 2.
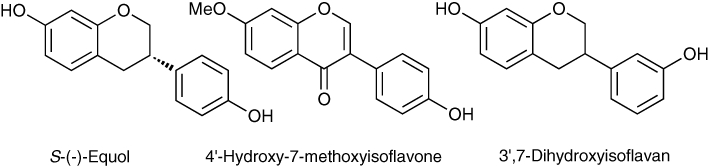
Naturally occurring flavonoids shown to displace diazepam binding.
As benzodiazepines were at that time amongst the most widely prescribed pharmaceuticals, a wide range of natural and synthetic flavonoids were investigated in vitro and in vivo as potential leads for new benzodiazepine ligands. Structure activity studies led to development of synthetic flavonoid ligands with the high affinity for the classical benzodiazepine binding site, including 6-bromoflavone, 6,3′-dinitroflavone, 6-bromo-3′-nitroflavone and 6-chloro-3′-nitroflavone (Figure 3). The relatively rigid shape of flavonoids means that they are useful scaffolds for the design of new therapeutic agents. Flavonoids have been used as scaffolds in the design of a variety of combinatorial libraries for drug discovery (Yao et al., 2007). The use of a combinatorial library of synthetic flavones provided further evidence that substitution at the 6- and/or 3′-positions with electronegative moieties were important determinants of high affinity at benzodiazepine receptors (Marder et al., 1988).
Figure 3.
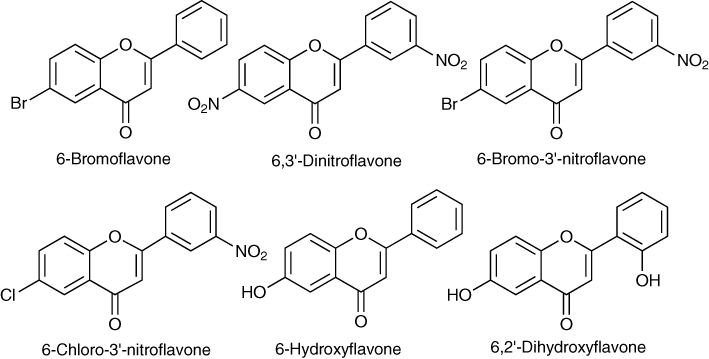
Some synthetic flavonoids that displace benzodiazepine binding.
Using GABA ratios determined by the impact that ligand binding has on the GABA binding, it was established that the flavones exhibited the full spectrum of biological activities at benzodiazepine receptors. 6-Bromoflavone with a GABA ratio of 1.6–2.0 was identified as a full agonist (Marder et al., 1996); 6-bromo-3′-nitroflavone with a GABA ratio of 1.38 as a partial agonist (Wolfman et al., 1998); and 6-chloro-3′-nitroflavone with a GABA ratio of 2.0 as an antagonist (Viola et al., 2000). One study found 6-bromo-3′-nitroflavone was a potent anxiolytic in the elevated plus maze (Marder et al., 2001), and that this effect could be antagonized by flumazenil (Griebel et al., 1999). However, anticonflict activity was not antagonized by flumazenil, and 6-bromo-3′-nitroflavone was found to lack anticonvulsant activity but failed to substitute of these compounds for the stimulus produced by chlordiazepoxide (Griebel et al., 1999). Although these results suggest that 6-bromo-3′-nitroflavone may affect anxiolysis via a mechanism other than through the benzodiazepine receptor, it is possible that the effects of 6-bromo-3′-nitroflavone are subtype selective.
Recent studies have identified 6-hydroxyflavone (Figure 3) as a subtype selective partial positive allosteric modulator at the flumazenil-sensitive benzodiazepine site. 6-Hydroxyflavone displayed significant preference for α2- and α3- compared with α1- and α5-containing receptors expressed in HEK 293T cells. In vivo 6-hydroxyflavone exhibited anxiolytic effects in the elevated plus-maze test, with no sedation, cognitive impairment, myorelaxation, motor incoordination or anticonvulsant effects at anxiolytic doses (Ren et al., 2010).
In functional electrophysiological studies, 6,2′-dihydroxyflavone (Figure 3) was characterized as a flumazenil-sensitive partial inverse agonist or negative modulator at α1β3γ2, α2β3γ2, and α5β3γ2, but not α3β3γ2 receptors, demonstrating subtype selectivity. As expected for an inverse agonist 6,2′-dihydroxyflavone elicited anxiogenic effects in the elevated plus-maze test and enhanced cognitive performance in the step-through passive avoidance test. Surprisingly, no proconvulsant effects were noted, and this was attributed to the fact that it may be α3β3γ2 receptors that are responsible for convulsant/anticonvulsant activity (Wang et al., 2007).
Wogonin (Figure 4), a flavone found in Scutellaria baicalensis Georgi, an important medicinal herb used in traditional Chinese medicine, has been reported to elicit anxiolysis in mice when administered orally (7.5 to 30 mg·kg−1) (Hui et al., 2002) and to significantly block convulsions induced by pentylenetetrazole and electroshock but not strychnine when administered i.p. (10 mg·kg−1) (Park et al., 2007). No sedative or myorelaxation effects were observed, and both the anxiolytic and anticonvulsant actions could be blocked by co-administration of flumazenil. Another flavonoid isolated from S. baicalensis Georgi, oroxylin A, identified as an antagonist at the benzodiazepine site, selectively abolished the anxiolytic, myorelaxant and motor incoordination, but not the sedative and anticonvulsant effects of diazepam (Huen et al., 2003b). These reports suggest that both compounds act with some subtype selectivity; however, further studies using specific recombinant receptors are needed to verify this.
Figure 4.
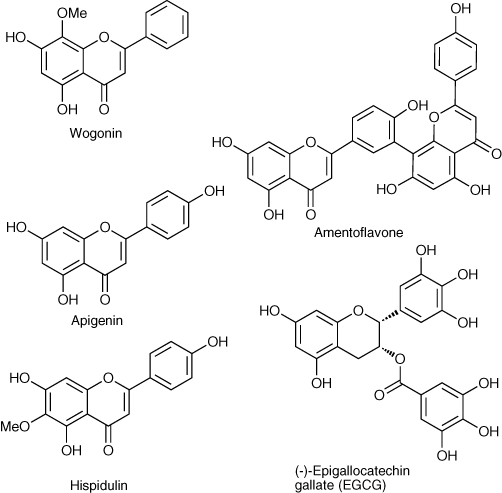
Naturally occurring flavonoids that influence the activation of GABAA receptors. GABAA, γ-amino butyric acid-A.
Using the abundant biological data afforded by the radioligand binding studies, a number of quantitative structure activity studies have been carried out to define the structural requirements for high-affinity binding of flavonoid ligands at the benzodiazepine binding site. Incorporation of flavonoids (flavones) into the unified pharmacophore model of Cook (Zhang et al., 1995) using the non-benzodiazepine high-affinity partial agonist CGS-9896 as a template gave rise to a model in which the flavones lie between the two hydrogen bond-donating sites and with the carbonyl groups of the flavones and benzodiazepines overlap, interacting with a hydrogen bond–donating site (H1) in essentially the same manner. The ether oxygen of the flavones making similar H-bond interactions to the benzodiazepine N1 with a second hydrogen bond–donating site (H2), and the fused aromatic ring of benzodiazepines and the B ring of flavones superimpose. The A-ring of the flavones occupies the lipophilic region (L1) occupied by 5-phenyl substituent of benzodiazepines. However, this model does not explain the fact that 6-methyl-3′-nitroflavone acts as an inverse agonist, but does not interact with a H-bond acceptor site (A2) required in the Cook model for benzodiazepine inverse agonist activity (Dekermendjian et al., 1999).
Further three-dimensional studies using comparative molecular field analysis (CoMFA), comparative molecular similarity indices (CoMSIA) and hologram quantitative structure activity relationship (HQSAR) approaches with flavone as a structural template afforded a similar pharmacophore to Dekermendjian's and proposed specific S1 and S2 subsites relating to electrostatic interaction of substituents in 6-position of flavone (Huang et al., 2001). Refinement of their model by the Danish group using a larger dataset of compounds led to the identification of two addition regions of steric repulsive interactions around the 4′- and 5′-regions of the B-ring (Kahnberg et al., 2002). Using benzodiazepine itself as a template, Marder et al. (2001) proposed an alternative model for the interaction of flavones with the benzodiazepine-binding site. In this model, the carbonyl group of the flavones interacts only with a bifunctional H2/A3 site, and the B-ring is positioned close to the lipophilic region L2. The A-ring in the flavones superimposes the 5-phenyl ring in diazepam; there are no interactions between the flavones and the H1 site and no possibility for interactions with A2. This model does not account for the increased affinity of compounds with a 2′-OH group (Huen et al., 2003a) or the strong decrease in affinity due to a 4′-methyl substituent.
Although these models can predict the binding affinity of the synthetic flavones, none of these studies have accounted for the different pharmacological activities reported of flavones. Unlike the Cook model, which qualitatively accounts for the relative affinities, efficacies and functional effects displayed by various ligands at the benzodiazepine receptor (Zhang et al., 1995), the pharmacophores proposed for flavones have only accounted for relative binding affinities of flavones. In addition, none of the studies include the biflavone, amentoflavone (Figure 4), that binds to the benzodiazepine site with an affinity similar to that of diazepam. Kahnberg et al. suggest that as it is a biflavone it must bind to other receptor sites and not be compared directly with the flavones used in the pharmacophore studies (Kahnberg et al., 2002). However, given its high affinity for the benzodiazepine binding site, it is likely that it does interact with this site, and a subsequent study postulated that one monomer of the biflavone binds in a manner similar to apigenin (Figure 4), and the increased affinity of amentoflavone is due to the second monomer forming additional binding interactions in the interface between the α- and γ-subunits (Svenningsen et al., 2006).
Concurrent with the elegant structure–activity studies relating the interactions of flavonoids with the high-affinity benzodiazepine binding site of GABAA receptors, many actions of flavonoids in vivo and in vitro were found to be insensitive to the classical benzodiazepine antagonist flumazenil. In particular, functional electrophysiological studies indicated that flavonoids act on GABAA receptors via two separate mechanisms involving a flumazenil-sensitive high-affinity site and an alternative site that may be the flumazenil-insensitive low-affinity benzodiazepine site.
In addition, certain flavonoids were found to modulate the flumazenil-sensitive modulatory effects of benzodiazepine ligands on GABAA receptors under conditions where the flavonoids alone had no detectable modulatory effects on GABA responses. This gave rise to the concept of second-order modulation by flavonoids of first-order modulation by benzodiazepines (Campbell et al., 2004; Fernandez et al., 2005; Vignes et al., 2006). The second-order positive modulation of diazepam enhancement of GABA responses by apigenin and EGCG was observed under conditions of the maximal flumazenil-sensitive enhancement of the action of low doses of GABA. It is unlikely that apigenin acts by enhancing diazepam binding as it is known to inhibit such binding. Furthermore, apigenin does not influence the binding of muscimol, a potent GABAA agonist. The observed second-order modulation may result from alterations in the coupling of the flumazenil-sensitive benzodiazepine allosteric sites with the orthosteric GABA sites on GABAA receptors. This action was selective for diazepam modulation as it was not observed for enhancement by pentobarbitone or allopregnanolone. Second-order modulation (or metamodulation) of receptor activation has been noted in other systems (Mesce, 2002; Ribeiro and Sebastiao, 2010) and may represent a new, albeit obscure, mechanism of drug action deserving of further investigation. It is not easy to study as it involves the dose-dependent interactions between three ligands and thus may be difficult to observe. Apigenin and EGCG may serve as lead compounds for the development of more selective agents for the study of second-order modulation of GABAA receptors.
Flumazenil-insensitive effects of flavonoids on GABAA receptors
Avallone and colleagues found that the inhibitory action of apigenin on locomotor activity in rats was not influenced by pretreatment with flumazenil (Avallone et al., 2000; Zanoli et al., 2000). In vitro studies showed that apigenin was a relatively weak inhibitor (IC50 250 µM) of the binding of labelled flumazenil to rat cerebellar membranes. Furthermore, they showed that flumazenil (1 µM) was able to antagonise the inhibitory effect of apigenin (1–10 µM) on the action of GABA on cultured cerebellar granule cells. In contrast to other studies (Viola et al., 1995; Salgueiro et al., 1997; Viola et al., 1998), Avallone and colleagues were unable to observe any anxiolytic action of apigenin (Avallone et al., 2000). They concluded that ‘the sedative action of apigenin cannot be ascribed to an activation of GABAA receptors’ (Zanoli et al., 2000).
Hispidulin (Figure 4), which differs from apigenin only by the addition of a 6-methoxy substituent, is a ligand at benzodiazepine binding sites with an IC50 value of 1.3 µM and has demonstrated anticonvulsant activity in a model of epilepsy in seizure-prone Mongolian gerbils. In functional studies on recombinant receptors, expressed oocytes hispidulin is inactive when applied alone (Figure 5A) and found to be approximately equipotent and exhibit a biphasic activity (Figure 5B) at α1,2,3,5,6β2γ2S receptors, enhancing at low concentrations (EC50 0.8–5 µM) and inhibiting at higher concentrations (>30 µM), was only partially blocked by flumazenil but was inactive at α1β2 receptors at a concentration of 10 µM (Figure 5C) (Kavvadias et al., 2004). This is biphasic action, and the fact that hispidulin is active at α6-containing receptors that are benzodiazepine insensitive suggests that hispidulin may act via more than one binding site on GABAA receptors. Interestingly, previous studies indicated that a range of natural and synthetic flavones had no affinity for recombinant α6β3γ receptors (Marder et al., 2001).
Figure 5.
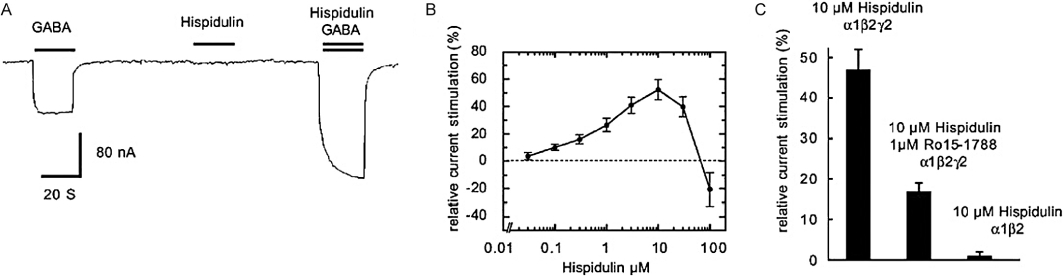
(A) Hispidulin at 10 mM by itself did not elicit any current but potentiated currents elicited by 4 µM GABA at recombinant α1β2γ2S GABAA receptors expressed in X. leavis oocytes. (B) Concentration dependence of allosteric potentiation by hispidulin demonstrating a biphasic response with potentiation at lower concentrations and inhibition at higher concentrations (>10 µM) of hispidulin. (C) The potentiation of currents elicited by GABA EC2-5 by hispidulin (10 µM) at α1β2γ2S GABAA receptors is partially inhibited by co-application of the BZD receptor antagonist Ro15-1788 (flumazenil, 1 µM). The GABA EC2-5 response at benzodiazepine insensitive α1β2 GABAA receptors is not potentiated by 10 µM hispidulin. From Kavvadias et al. 2004. GABAA, γ-amino butyric acid-A.
The first definitive description of flumazenil-insensitive actions of flavonoids on recombinant GABAA receptors reported that micromolar concentrations of apigenin and quercetin inhibited the action of GABA on α1β2γ2S GABAA receptors expressed in oocytes in the presence of 0.1–1 µM flumazenil (Goutman et al., 2003). Other investigators reported similar flumazenil-insensitive negative modulatory actions of apigenin and genistein on recombinant GABAA receptors (Campbell et al., 2004). These flavonoids, however, had similar negative modulatory actions on a variety of other transmitter-gated ion channels, and such actions were thus not specific to GABAA receptors.
The biflavone amentoflavone was initially identified as a high-affinity benzodiazepine receptor ligand through the investigation of the pharmacologically active constituents of the plant extract Karmelitter Geist® (Nielsen et al., 1988) and later Hypericum species (Baureithel et al., 1997). Displaying an affinity similar to that of diazepam, amentoflavone is the most potent non-nitrogen containing benzodiazepine ligand known; in contrast, I3, II8-biapigenin has no effect at concentrations of 1 µM. Despite later being reported to cross the BBB by passive diffusion in the porcine brain endothelial capillary cells model of BBB (Gutmann et al., 2002), amentoflavone displayed only poor inhibition of [3H]-flunitrazepam binding in vivo following i.v. administration (Nielsen et al., 1988). Later studies demonstrated that amentoflavone in fact displays a biphasic activity acting as a benzodiazepine antagonist at nanomolar concentrations (Hansen et al., 2005) and flumazenil-insensitive negative modulator at higher concentrations (Hanrahan et al., 2003; Hansen et al., 2005).
In 2004, Hall et al. showed that 6-methylflavone acted as a flumazenil-insensitive positive modulator of recombinant α1β2γ2L and α1β2 GABAA receptors expressed in oocytes (Hall et al., 2004). Subsequently, these authors found that 6-methylflavanone (Figure 6) was also a fluamazenil-insensitive positive modulator of recombinant GABAA receptors that, unlike 6-methylflavone, was subtype selective being a more efficacious positive modulator at α2β2γ2L receptors that at α1β2γ2L and α1β2 receptors (Hall et al., 2005). 6-Methylflavanone acted at micromolar concentrations, producing a maximal enhancement of 417% of the action of GABA on α2β2γ2L compared with 120% at α1β2γ2L receptors. This subtype-selective efficacy, together with the finding that neither flavonoid influenced ρ1 GABAC receptors, indicates that these actions are relatively selective for GABAA receptors. Targeting specific GABAA receptors through subtype-selective efficacy as distinct from potency has been validated for other modulators of GABAA receptors, including the non-sedating anxiolytic TPA023 and the cognitive enhancer alpha 5IA (Atack, 2008).
Figure 6.
Some synthetic flavonoids with subtype-selective actions on the activation of GABAA receptors. GABAA, γ-amino butyric acid-A.
6-Methylflavanone differs from 6-methylflavone in having a single rather than a double bond at C2–C3. This results in changes in the delocalization of π-electrons throughout the structure such that 6-methylflavanone is relatively non-planar compared to 6-methylflavone where the phenyl and quinone rings are effectively planar (Hall et al., 2001). The C2–C3 single bond is crucial to the observed subtype-selective efficacy of 6-methylflavanone.
In general, flavanones have shown little or no activity in benzodiazepine-binding studies (Haberlein et al., 1994; Paladini et al., 1999; Marder and Paladini, 2002) and have not been considered in molecular modelling of benzodiazepine binding sites on GABAA receptors (Dekermendjian et al., 1999; Huang et al., 2001; Clayton et al., 2007). 6-Bromoflavanone and 5-methoxy-6,8-dibromoflavanone have been show to act as anxiolytics in mice, but no details are available as to their possible interactions with GABAA receptors (Ognibene et al., 2008). (2S)-5,7,8,4′-Tetrahydroxyflavanone and 2,5-dihydroxy-7-methoxy-6,8-dimethylflavan-3-one have been shown to inhibit flunitrazepam binding (IC50s 21 and 1 µM respectively), but no in vivo data on these compounds have been reported (Wang et al., 2002; Ognibene et al., 2008). Narigenin, the flavanone analogue of apigenin, has been shown to only weakly inhibit (IC50 2.6 mM) flumazenil binding (Jager et al., 2007).
Epigallocatechin gallate (EGCG; Figure 4), a flavanol constituent of green tea, demonstrates dose-dependent anxiolytic, sedative–hypnotic and amnesiac activity, with evidence that these activities are mediated at least in part by GABAA receptors (Adachi et al., 2006; Vignes et al., 2006). Using 6-methylflavanone and EGCG as lead compounds, structure–activity studies led to the discovery of Fa131 (2S,3R-3-acetoxy-4′-methoxyflavan) (Figure 6) as the most efficacious compound in a series of flavanol esters with positive modulatory activity of GABAA receptors (Fernandez et al., 2008; Mewett et al., 2009). Interestingly, similar to barbiturates, Fa131 also acts as weak partial agonist α1β2γ2L receptors and at higher doses exerts a negative modulation (Fernandez et al., 2008).
Fa131 was found to show subtype selectivity for receptors containing α2-subunits on the basis of efficacy (Fernandez et al., 2008) being more than twice as efficacious on α2β2γ2L receptors (EC50 14 µM; 2100% enhancement) as on α1β2γ2L receptors (EC50 14 µM; 850% enhancement) and still less efficacious on α3β2γ2L and α5β2γ2L receptors (Figure 7A). The efficacy of 2100% exceeds the highest efficacy yet recorded of 1250% by (+)-borneol at these receptors (Granger et al., 2005). The actions of Fa131 were flumazenil insensitive (Figure 7B) and could be observed on α1β2 receptors that lack high-affinity benzodiazepine receptors. It was inactive on ρ1 GABAC receptors (Fernandez et al., 2008).
Figure 7.
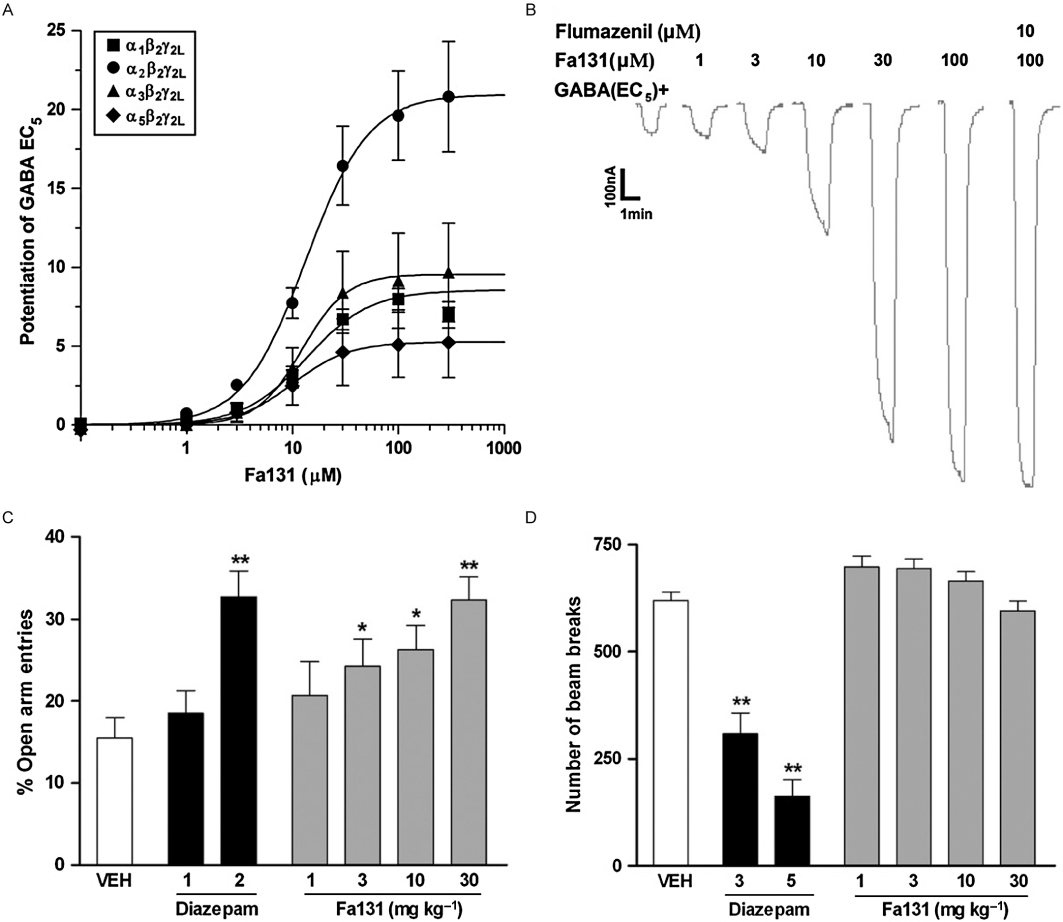
(A) Fractional potentiation of GABA EC5 current response by Fa131 at human recombinant GABAA receptors expressed in Xenopus oocytes. Concentration-response curves on α1β2γ2L, α2β2γ2L, α3β2γ2L, α5β2γ2L receptors. (B) Potentiation of GABA EC5 by Fa131 at GABAA α1β2γ2L could not be blocked by coapplication of the benzodiazepine antagonist Ro15-1788 (flumazenil, 10 µM). (C) Effect of diazepam (1 and 2 mg·kg−1) and Fa131 (1–30 mg·kg−1) on the behaviour of mice in the elevated plus maze. Bars represent mean ± SEM of the % open arm entries % time spent on the open arms recorded over a 5 min session, 20 min after an i.p. injection of drugs or vehicle. *P < 0.05 or **P < 0.01 compared with vehicle group, anova followed by Dunnett's multiple comparison test. (d) Effect of diazepam (3 and 5 mg·kg−1) and Fa131 (1–30 mg·kg−1) on the behaviour of mice in the actimeter test. Bars represent mean ± SEM of number of beam breaks recorded over a 5 min session, 20 min after an i.p. injection of drugs or vehicle, showing a significant decrease in the number of beam breaks induced by diazepam at doses of 3 and 5 mg·kg−1 (P < 0.01). Fa131 did not produce any changes in this parameter at doses up to 30 mg·kg−1. *P < 0.05 or **P < 0.01 compared with vehicle group, anova followed by Dunnett's multiple comparison test. From Fernandez et al. 2008. GABA, γ-amino butyric acid.
In mice, Fa131 (1–30 mg·kg−1 i.p.) induced anxiolytic-like action in two unconditioned models of anxiety: the elevated plus maze (Figure 7C) and the light/dark paradigms. No sedative or myorelaxant effects were detected using the hole board, actimeter (Figure 7D) and horizontal wire tests, and only weak barbiturate-potentiating effects on the loss of righting reflex test. Fa131 thus demonstrated improved segregation of anxiolytic and sedative doses when compared with the nonselective agonist diazepam (2 mg·kg−1 i.p.) consistent with a preferential enhancement at α2 containing receptors (Fernandez et al., 2008). Fa131 is the first positive modulator to distinguish between α2- and α3-subunit containing GABAA receptors highlighting the potential of targeting flumazenil-insensitive allosteric sites in the search for new anxioselective drugs.
An emerging area of interest is the activation of GABAA receptors by flavonoids in the absence of GABA, similar to such activation produced by barbiturates and steroids. There is preliminary evidence for 2′-methoxy-6-methylflavone (Figure 6) activating recombinant GABAA receptors as micromolar concentrations in the absence of GABA in a flumazenil-insensitive manner (Karim et al., 2010). This activation is blocked by the GABAA receptor antagonists bicuculline and gabazine and is subtype selective being observed with α2β2/3γ2L and α4βδ receptors. This suggests that there is a further flavone site on certain GABAA receptors that mediates opening of the chloride channel in the absence of GABA.
Conclusions
It is now over 25 years since binding studies first demonstrated that flavones are ligands for benzodiazepine binding sites on GABAA receptors. Since that time, it has become apparent that the actions of flavonoids on these receptors are far more complex than a single action at this one site. It addition to the relatively well-characterized flumazenil-sensitive benzodiazepine-binding sites, there is significant interest in flumazenil-insensitive binding sites for flavonoids. Then there is the question of the site(s) involved in the second-order modulation of benzodiazepine first-order modulation by certain flavonoids and the activation of some GABAA receptor subtypes by flavonoids in the absence of GABA.
Areas for further study include:
Much more work needs to be done on the subtype specificity of flavonoid actions on GABAA receptors.
Single channel studies may shed light on the different mechanisms of action of flavonoids on GABAA receptors.
Identification of the various flavonoid binding sites and their possible overlap with binding sites for other agents acting on GABAA receptors, for example etomidate and loreclezole.
Flavonoids that bind to GABAA receptors but show no intrinsic activity in functional studies should be investigated as possible antagonists.
As significant constituents of our diet, it is important that we understand how natural flavonoids might influence brain function. Understanding the structural determinants of flavonoid effects on GABAA receptors and developing agents specific for GABAA receptor subtypes is a key challenge. Synthetic flavonoids are attractive leads for not only drugs to treat brain dysfunction but also for the development of molecules that can be used to study and investigate the role of the modulatory sites at GABAA receptors and further the development of GABAA subtype-selective agents.
Acknowledgments
The authors would like to acknowledge their coworkers and students who have contributed to this work.
Glossary
Abbreviations
- BBB
blood–brain barrier
- CoMFA
comparative molecular field analysis
- CoMSIA
comparative molecular similarity indices analysis
- EGCG
epigallocatechin gallate
- Fa131
trans-(2R,3S)-3-acetoxy-4′-methoxyflavan
- GABA
γ-amino butyric acid
- GABAA
γ-amino butyric acid-A receptor
- HEK
human embryonic kidney
- HQSAR
hologram quantitative structure activity relationship
- i.p
intraperitoneal injection
- TM
transmembrane domain
- TPA023
7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Adachi N, Tomonaga S, Tachibana T, Denbow DM, Furuse M. (–)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur J Pharmacol. 2006;531:171–175. doi: 10.1016/j.ejphar.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. doi: 10.1016/S0074-7742(04)62001-0. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres A, Donovan SM, Kuhlenschmidt MS. Soy isoflavones and virus infections. J Nutrit Biochem. 2009;20:563–569. doi: 10.1016/j.jnutbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABA(A) receptor subtype-selective efficacy: TPA023, an alpha2/alpha3 selective non-sedating anxiolytic and alpha5IA, an alpha5 selective cognition enhancer. CNS Neurosci Ther. 2008;14:25–35. doi: 10.1111/j.1527-3458.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. J Chem Inf Model. 2000;59:1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Baureithel KH, Buter KB, Engesser A, Burkard W, Schaffner W. Inhibition of benzodiazepine binding in vitro by amentoflavone, a constituent of various species of Hypericum. Pharm Acta Helv. 1997;72:153–157. doi: 10.1016/s0031-6865(97)00002-2. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm BA. Introduction to Flavonoids. 1st edn. Amsterdam: Taylor & Francis; 1998. [Google Scholar]
- Bond AJ, Haily DM, Lader MH. Plasma concentrations of benzodiazepines. Br J Clin Pharmacol. 1977;4:51–56. doi: 10.1111/j.1365-2125.1977.tb00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Chebib M, Johnston GAR. The dietary flavonoids apigenin and (–)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochem Pharmacol. 2004;68:1631–1638. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Carter C, Kozuska J, Dunn S. Insights into the structure and pharmacology of GABAA receptors. Future Med Chem. 2010;2:859–875. doi: 10.4155/fmc.10.178. [DOI] [PubMed] [Google Scholar]
- Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MSRB, Folador P, Damazio RG, et al. Flavonoids: cellular and molecular mechanism of action in glucose homeostasis. Mini Rev Med Chem. 2008;8:1032–1038. doi: 10.2174/138955708785740580. [DOI] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. GABA-activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem. 2000;43:1427–1447. doi: 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- Clarke D, Wiseman H. Phytoestrogens. In: Gilbert J, Senyuva HZ, editors. Bioactive Compounds in Food. Oxford: John Wiley & Sons; 2008. pp. 173–198. [Google Scholar]
- Clayton T, Chen JL, Ernst M, Richter L, Cromer BA, Morton CJ, et al. An updated unified pharmacophore model of the benzodiazepine binding site on gamma-aminobutyric acid(a) receptors: correlation with comparative models. Curr Med Chem. 2007;14:2755–2775. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekermendjian K, Kahnberg P, Witt M-R, Sterner O, Nielsen M, Liljefors T. Structure–activity relationships and molecular modeling analysis of flavonoids binding to the benzodiazepine site of the rat brain GABAA receptor complex. J Med Chem. 1999;42:4343–4350. doi: 10.1021/jm991010h. [DOI] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. Identification of structures within GABAA receptor alpha subunits that regulate the agonist action of pentobarbital. J Pharmacol Exp Ther. 2006;318:1094–1101. doi: 10.1124/jpet.106.104844. [DOI] [PubMed] [Google Scholar]
- Drexler B, Jurd R, Rudolph U, Antkowiak B. Distinct actions of etomidate and propofol at beta3-containing gamma-aminobutyric acid type A receptors. Neuropharmacology. 2009;57:446–455. doi: 10.1016/j.neuropharm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Wasowski C, Paladini AC, Marder M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur J Pharmacol. 2005;512:189–198. doi: 10.1016/j.ejphar.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Mewett KN, Hanrahan JR, Chebib M, Johnston GAR. Flavan-3-ol derivatives are positive modulators of GABAA receptors with higher efficacy for the ?2 subtype and anxiolytic action in mice. Neuropharmacology. 2008;55:900–907. doi: 10.1016/j.neuropharm.2008.06.069. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Waxemberg MD, Donate-Oliver F, Pomata PE, Calvo DJ. Flavonoid modulation of ionic currents mediated by GABAA and GABAC receptors. Eur J Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- Granger RE, Campbell EL, Johnston GAR. (+)- And (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Tan S, Schoemaker H, Sanger DJ. Pharmacological studies on synthetic flavonoids: comparison with diazepam. Neuropharmacology. 1999;38:965–977. doi: 10.1016/s0028-3908(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Gutmann H, Bruggisser R, Schaffner W, Bogman K, Botomino A, Drewe J. Transport of amentoflavone across the blood–brain barrier in vitro. Planta Med. 2002;68:804–807. doi: 10.1055/s-2002-34401. [DOI] [PubMed] [Google Scholar]
- Haberlein H, Tschiersch KP, Schafer HL. Flavonoids from Leptospermum scoparium with affinity to the benzodiazepine receptor characterized by structure activity relationships and in vivo studies of a plant extract. Pharmazie. 1994;49:912–922. [PubMed] [Google Scholar]
- Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, et al. The role of the beta subunit in determining the pharmacology of GABAA receptors. Mol Pharmacol. 1993;44:1211–1218. [PubMed] [Google Scholar]
- Hall BJ, Hanrahan JR, Johnston GAR, Hambley TW, Hibbs DE. 6-Methylflavone. Acta Crystallogr Sect E Struct Rep Online. 2001;57:o592–o593. [Google Scholar]
- Hall BJ, Chebib M, Hanrahan JR, Johnston GAR. Flumazenil-independent positive modulation of γ-aminobutyric acid action by 6-methylflavone at human recombinant α1β2γ2L and α1β2 GABAA receptors. Eur J Pharmacol. 2004;491:1–8. doi: 10.1016/j.ejphar.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Chebib M, Hanrahan JR, Johnston GAR. 6-Methylflavanone, a more efficacious positive allosteric modulator of gamma-aminobutyric acid (GABA) action at human recombinant α2β2γ2L than at α1β2γ2L and α1β2 GABA(A) receptors expressed in Xenopus oocytes. Eur J Pharmacol. 2005;512:97–104. doi: 10.1016/j.ejphar.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Hanrahan JR, Chebib M, Davucheron NLM, Hall BJ, Johnston GAR. Semisynthetic preparation of amentoflavone: a negative modulator at GABAA receptors. Bioorg Med Chem Lett. 2003;13:2281–2284. doi: 10.1016/s0960-894x(03)00434-7. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Paulsen I, Davies M. Determinants of amentoflavone interaction at the GABAA receptor. Eur J Pharmacol. 2005;519:199–207. doi: 10.1016/j.ejphar.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu T, Gu J, Luo X, Ji R, Cao Y, et al. 3D-QSAR model of flavonoids binding at benzodiazepine site in GABAA receptors. J Med Chem. 2001;44:1883–1891. doi: 10.1021/jm000557p. [DOI] [PubMed] [Google Scholar]
- Huen MSY, Hui K-M, Leung JWC, Sigel E, Baur R, Wong JT-F, et al. Naturally occurring 2′-hydroxyl-substituted flavonoids as high-affinity benzodiazepine site ligands. Biochem Pharmacol. 2003a;66:2397–2407. doi: 10.1016/j.bcp.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Huen MSY, Leung JWC, Ng W, Lui WS, Chan MNS, Wong JT-F, et al. 5,7-Dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonistic properties. Biochem Pharmacol. 2003b;66:125–132. doi: 10.1016/s0006-2952(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Hui KM, Huen Michael SY, Wang Hong Y, Zheng H, Sigel E, Baur R, et al. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol. 2002;64:1415–1424. doi: 10.1016/s0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- Jager AK, Almquist JP, Vangsoe SAK, Stafford GI, Adsersen A, van Staden J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S Afr J Bot. 2007;73:518–521. [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, et al. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GAR. GABA(A) receptor channel pharmacology. Curr Pharm Des. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- Kahnberg P, Lager E, Rosenberg C, Schougaard J, Camet L, Sterner O, et al. Refinement and evaluation of a pharmacophore model for flavone derivatives binding to the benzodiazepine site of the GABAA receptor. J Med Chem. 2002;45:4188–4201. doi: 10.1021/jm020839k. [DOI] [PubMed] [Google Scholar]
- Kale A, Gawande S, Kotwal S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother Res. 2008;22:567–577. doi: 10.1002/ptr.2283. [DOI] [PubMed] [Google Scholar]
- Karim N, Gavande N, Johnston GA, Hanrahan JR. Chebib M 2′-Methoxy-6-methylflavone: a novel allosteric activator of α4β1/3δ GABAA receptors. In: Neuroscience Meeting Planner, editor. Program No 3385/E26 2010. San Diego, CA: Society for Neuroscience; 2010. Online. [Google Scholar]
- Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, et al. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, O'Shea SM, Rick CE, Whiting PJ, Hadingham KL, Czajkowski C, et al. Alpha subunit isoform influences GABA(A) receptor modulation by propofol. Neuropharmacology. 1997;36:941–949. doi: 10.1016/s0028-3908(97)00074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Koltchine VV, Rick CE, Ye Q, Finn SE, Harrison NL. Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type A receptor distinct from that for isoflurane. Mol Pharmacol. 1998;53:530–538. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–8620. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Stern L, Weigele M, O'Brien RA, Spirst N. Isolation and identification of ‘diazepam-like’ compounds in bovine brain. J Nat Prod. 1983;46:852–861. doi: 10.1021/np50030a005. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Marder M, Paladini AC. GABAA-receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2:853–867. doi: 10.2174/1568026023393462. [DOI] [PubMed] [Google Scholar]
- Marder M, Viola H, Bacigaluppo JA, Colombo MI, Wasowski C, Wolfman C, et al. Detection of benzodiazepine receptor ligands in small libraries of flavone derivatives synthesized by solution phase combinatorial chemistry. Biochem Biophys Res Commun. 1988;249:481–485. doi: 10.1006/bbrc.1998.9146. [DOI] [PubMed] [Google Scholar]
- Marder M, Viola H, Wasowski C, Wolfman C, Waterman PG, Cassels BK, et al. 6-Bromoflavone, a high affinity ligand for the central benzodiazepine receptors is a member of a family of active flavonoids. Biochem Biophys Res Commun. 1996;223:384–389. doi: 10.1006/bbrc.1996.0903. [DOI] [PubMed] [Google Scholar]
- Marder M, Estiu G, Blanch LB, Viola H, Wasowski C, Medina JH, et al. Molecular modeling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABAA receptor complex. Bioorg Med Chem. 2001;9:323–335. doi: 10.1016/s0968-0896(00)00250-9. [DOI] [PubMed] [Google Scholar]
- Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, et al. Flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–425. doi: 10.1023/a:1027303609517. [DOI] [PubMed] [Google Scholar]
- Mesce KA. Metamodulation of the biogenic amines: second order modulation by setroid hormones and amine cocktails. Brain Behav Evol. 2002;60:339–349. doi: 10.1159/000067793. [DOI] [PubMed] [Google Scholar]
- Mewett KN, Fernandez SP, Pasricha AK, Pong A, Devenish SO, Hibbs DE, et al. Synthesis and biological evaluation of flavan-3-ol derivatives as positive modulators of GABAA receptors. Bioorg Med Chem. 2009;17:7156–7173. doi: 10.1016/j.bmc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Froekjaer S, Braestrup C. High affinity of the naturally-occurring biflavonoid, amentoflavone, to brain benzodiazepine receptors in vitro. Biochem Pharmacol. 1988;37:3285–3287. doi: 10.1016/0006-2952(88)90640-5. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Bovicelli P, Adriani W, Saso L, Laviola G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6,8-dibromoflavanone as anxiolytic compounds. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:128–134. doi: 10.1016/j.pnpbp.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH. Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol. 1999;51:519–526. doi: 10.1211/0022357991772790. [DOI] [PubMed] [Google Scholar]
- Park HG, Yoon SY, Choi JY, Lee GS, Choi JH, Shin CY, et al. Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur J Pharmacol. 2007;574:112–119. doi: 10.1016/j.ejphar.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl– currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- Ren L, Wanga F, Xua Z, Chana WM, Zhaoa C, Xue H. GABAA receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem Pharmacol. 2010;79:1337–1344. doi: 10.1016/j.bcp.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM. Modulation and metamodulation of synapses by adenosine. Acta Physiologica. 2010;199:161–169. doi: 10.1111/j.1748-1716.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Salgueiro JB, Ardenghi P, Dias M, Ferreira MB, Izquierdo I, Medina JH. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol Biochem Behav. 1997;58:887–891. doi: 10.1016/s0091-3057(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Schofield CM, Harrison NL. Transmembrane residues define the action of isoflurane at the GABAA receptor alpha-3 subunit. Brain Res. 2005;1032:30–35. doi: 10.1016/j.brainres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Spencer JPE. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JPE, Vauzour D, Rendeiro C. Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys. 2009;492:1–9. doi: 10.1016/j.abb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Svenningsen AB, Madsen KD, Liljefors T, Stafford GI, van Staden J, Jaeger AK. Biflavones from Rhus species with affinity for the GABAA/benzodiazepine receptor. J Ethnopharmacol. 2006;103:276–280. doi: 10.1016/j.jep.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br J Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Maurice T, Lanté F, Nedjar M, Thethi K, Guiramand J, et al. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG) Brain Res. 2006;1110:102–115. doi: 10.1016/j.brainres.2006.06.062. [DOI] [PubMed] [Google Scholar]
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silvera R, Medina AE, et al. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- Viola H, Marder M, Wolfman C, Wasowski C, Medina JH, Paladini AC. Central nervous system effects of natural and synthetic flavonoids. Anales de la Asociacion Quimica Argentina. 1998;86:229–236. [Google Scholar]
- Viola H, Wolfman C, Marder M, Goutman JD, Bianchin M, Wasowski C, et al. 6-Chloro-3′-nitroflavone is a potent ligand for the benzodiazepine binding site of the GABA(A) receptor devoid of intrinsic activity. Pharmacol Biochem Behav. 2000;65:313–320. doi: 10.1016/s0091-3057(99)00199-9. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Whiting PJ, Kemp JA. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993;44:437–442. [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KDW, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosi. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Wang H, Hui K-M, Chen Y, Xu S, Wong JT-F, Xue H. Structure-activity relationships of flavonoids, isolated from Scutellaria baicalensis, binding to benzodiazepine site of GABAA receptor complex. Planta Med. 2002;68:1059–1062. doi: 10.1055/s-2002-36357. [DOI] [PubMed] [Google Scholar]
- Wang F, Xu Z, Yuen CT, Chow CY, Lui YL, Tsang SY, et al. 6,2′-Dihydroxyflavone, a subtype-selective partial inverse agonist of GABAA receptor benzodiazepine site. Neuropharmacology. 2007;53:574–582. doi: 10.1016/j.neuropharm.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6:24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evancs C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wolfman C, Viola H, Marder M, Ardenghi P, Wasowski C, Schroder N, et al. Pharmacological characterization of 6-bromo-3′-nitroflavone, a synthetic flavonoid with high affinity for the benzodiazepine receptors. Pharmacol Biochem Behav. 1998;61:239–246. doi: 10.1016/s0091-3057(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Yao N, Song A, Wang X, Dixon S, Lam KS. Synthesis of flavonoid analgues as scaffolds for natural product-based combinatorial libraries. J Comb Chem. 2007;9:668–676. doi: 10.1021/cc070009y. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37:1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zanoli P, Avallone R, Baraldi M. Behavioral characterization of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71:S117–S123. doi: 10.1016/s0367-326x(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koehler KF, Zhang P, Cook JM. Development of a comprehensive pharmacophore model for the benzodiazepine receptor. Drug Des Discov. 1995;12:193–248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


