Abstract
Purpose
To determine the tear oxygen tension under a variety of conventional and silicone hydrogel contact lenses in human subjects.
Methods
Three hydrogel and five silicone hydrogel lenses (Dk/t = 17 to 329) were coated on the back surface with an oxygen sensitive, bovine serum albumin-Pd meso-tetra (4-carboxyphenyl) porphine complex (BSA-porphine). Each lens type was placed on the right eye of 15 non-contact lens wearers to obtain a steady-state open eye tear oxygen tension using oxygen sensitive phosphorescence decay of BSA-porphine. A closed-eye oxygen tension estimate was obtained by measuring the change in tear oxygen tension after 5 min of eye closure. In separate experiments, a goggle was placed over the lens wearing eye and a gas mixture (PO2 = 51 torr) flowed over the lens to simulate anterior lens oxygen tension during eye closure.
Results
Mean open eye oxygen tension ranged from 58 to 133 torr. Closed eye estimates ranged from 11 to 42 torr. Oxygen tension under the goggle ranged from 8 to 48 torr and was higher than the closed eye estimate for six out of the eight lenses, suggesting that the average closed eye anterior lens surface oxygen tension is <51 torr. For Dk/t >30, the measured tear oxygen tension is significantly lower than that predicted from previous studies.
Conclusions
The phosphorescence decay methodology is capable of directly measuring the in vivo post lens PO2 of high Dk/t lenses without disturbing the contact lens or cornea. Our data indicate that increasing Dk/t up to and beyond 140 continues to yield increased flux into the central cornea.
Keywords: silicone hydrogel contact lenses, cornea, oxygen tension, phosphorescence
Corneal hypoxia secondary to contact lens wear is a well-recognized clinical risk factor for corneal edema, red eye, and microbial keratitis.1–4 Corneal hypoxia is significantly greater during overnight wear of contact lenses, and this is associated with increased incidence of clinical problems.2–4 These factors were the major impetus for the contact lens industry to develop highly oxygen permeable materials and resulted in the introduction of silicone hydrogel (SiH) lenses several years ago. Although there have been a number of studies examining clinical complications of SiH lens use relative to conventional hydrogels, and measuring the oxygen transmissibility of SiH lenses,5–7 there has been very little measurement data gathered for the “on eye” oxygen delivery performance of SiH lenses.
Mathematical modeling of central corneal oxygenation has suggested that increases in oxygen transmissibility (Dk/t) of SiH lenses above 50 yields very little gain in performance, where performance is gauged by predicted changes in oxygen flux into the cornea or changes in corneal oxygen consumption.8,9 However, direct measurements of tear oxygen tension under rigid lenses in rabbits10 and under a SiH lens in humans11 are lower than that predicted by modeling. Similarly, using the oxygen uptake approach in human subjects, significant decreases in “oxygen shortfall” were measured after RGP lens wear up to Dk/t = 135.12 These studies suggest that corneal oxygen flux saturates at a higher level than previously thought or that the parameters used in the modeling of oxygen distribution (e.g., corneal Dk, O2 consumption, boundary conditions) may not adequately represent the actual conditions of the cornea.
Direct measurement of tear oxygen levels while wearing a lens of known Dk/t, enables the determination of oxygen flux and human corneal Q (oxygen consumption) in vivo. Using a small set of lenses (Dk/t = 4.2, 12, and 99), our laboratory previously found that corneal flux and Q increased with increasing tear oxygen tension to levels that were higher than expected based on previous in vitro corneal Q measurements.11 To confirm and expand on our earlier work, the current study was undertaken to provide direct, on eye, non-contact tear oxygen measurements using a greater range and variety of SiH and conventional hydrogel lenses than used previously.
MATERIALS AND METHODS
Subjects
Fifteen subjects (eight male, seven female, median age 24 years, range, 21 to 53) free of ocular and systemic disease and who had not worn contact lenses for at least 6 months participated in this study. The research followed the tenets of the Declaration of Helsinki and was approved by the Indiana University Human Subjects Committee.
Lenses
Table 1 lists the lenses used in the study. The Dk values listed are those reported by the manufacturer. Using a new approach, a recent study showed that these Dk values were consistent except for Lotrafilcon A (140), which gave a significantly different value (180).7 All lenses had back surface radii of ~8.6 mm, diameter of 13.5 mm, and −0.50 D power to achieve uniform thickness. An ET-1 thickness gauge (Rehder Development Co., Castro Valley, CA) was used to verify lens thickness. All lenses are commercially available except for the Night & Day Ultrathin, obtained directly from Ciba Vision.
TABLE 1.
Lens parameters
| Dk | Thickness (cm) |
Dk/t (×10−9 Barrer/cm) |
kq (torr−1) | |
|---|---|---|---|---|
| Biomedics | 19.7 | 0.115 | 17.1 | 370 |
| Acuvue2 | 28 | 0.105 | 27 | 327 |
| Advance | 60 | 0.071 | 85 | 372 |
| Purevision | 112 | 0.09 | 124 | 310 |
| Optix | 110 | 0.08 | 138 | 187 |
| Oasys | 103 | 0.062 | 166 | 372 |
| N&D | 140 (1807) | 0.08 | 175 (226) | 187 |
| N&D UT | 140 (1807) | 0.055 | 255 (329) | 187 |
Tear PO2
Tear oxygen tension (PO2) under the lenses in the open eye and closed eye was measured with a time-domain phosphorimeter as previously described11,13 with some modification. In the first modification, lenses were clamped at the edge between two plastic rings that stood upright in a small well. Bovine serum albumin-Pd meso-tetra (4-carboxyphenyl) porphine complex (BSA-porphine) solution was pipetted onto the back surface only and lenses were incubated at room temperature overnight. The next day, the solution was discarded and the lens washed extensively with Unisol. This procedure avoided phosphorescence from the front surface. The lens coated with the oxygen sensitive phosphorescent dye on the back surface was then inserted onto the right eye of the subject and allowed to settle for 10 min before the steady-state (SS) open eye tear PO2 was measured. SS measurements were continuous for at least 60 s (~75 intensity measurements). The subject blinked normally. Data collection and analysis were obtained using a Medical Systems phosphorimeter and software.11 Each reported data point is a calculated mean of 10 phosphorescence exponential decay rates. If the associated correlation of an individual decay rate to the exponential model was <0.9, that point was discarded.
The eyes were then closed for 5 min and PO2 measured (1.25 Hz) on eye opening and continually over the next minute. The resultant PO2 data were fit to a first order exponential model and extrapolated to time = 0, which was used as the estimate for closed eye PO2. A tight fitting goggle was then placed over the eyes and a calibrated gas mixture (6.7% oxygen, ~51 torr/balance nitrogen) flowed over the right eye. Phosphorescence decay was then measured through the goggles to obtain a simulated eye closure PO2. After this procedure, the goggle gas was switched to air and PO2 measurements were made. We found that the PO2 estimates under the lens without wearing a goggle were not significantly different from those while wearing the goggle when perfused with air (data not shown). This indicated that the goggle procedure did not interfere with the PO2 estimate.
All subjects were tested with each of the eight lenses. New lenses were used for each trial. Lenses were always placed on the right eye. Subjects fixated a light emitting diode about 10 feet away using the left eye. There was at least 24 h between trials.
Calibration
The manufacturer’s surface treatment of the lenses altered the phosphorescence quenching characteristics and in some cases the adherence of the BSA-porphine. Therefore, we devised a modified calibration procedure. Lotrafilcon A (Night & Day) & B (O2Optix) were coated with polyacrylic acid to overcome the low protein affinity of the plasma treated surface and enable the BSA-porphine to adhere. In solution, the quenching constant (kq) for this dye complex is 310 [Torr−1 sec−1].13 This value was used in our previous study.11 To determine the apparent kq for each lens type, lenses were stained on the front surface and placed on the eye of a subject so that temperature and tear pH were similar to the experimental condition. Because the phosphorescence decay rate in air is very rapid and difficult to measure, we placed a goggle over the eye and exposed the front surface to a slow stream of a calibrated gas mixture (6.7% oxygen) and determined the kq using the Stern-Vollmer equation:
| (1) |
where τ is the measured phosphorescence decay time constant, τ0 is τ at 0% oxygen (626 ms), and (O2) is the oxygen tension. In practice, the flow rate in the goggle was slowly adjusted upward until a positive pressure could be discerned by observing bubbles emanating from the goggle outflow tube within 1 cm of water.
Statistics
Summary statistics for the measured parameters are expressed as the mean and standard deviation.
RESULTS
Table 1 lists the results of the calibration procedure. Purevision and Acuvue 2 lenses showed very little deviation of kq from that in solution, whereas the Oasys, Advance and Biomedics lenses showed positive deviation and the polyacrylic acid-coated lotrafilcon lenses showed a negative deviation. In other words, if left uncorrected, the oxygen tension under the polyacrylic acid coated lenses would be underestimated, whereas for the positively deviating lenses PO2 would be overestimated. For example, if the uncorrected kq was used for the positively deviating lenses, the open eye oxygen tension was calculated to be near 200 torr, which is impossible.
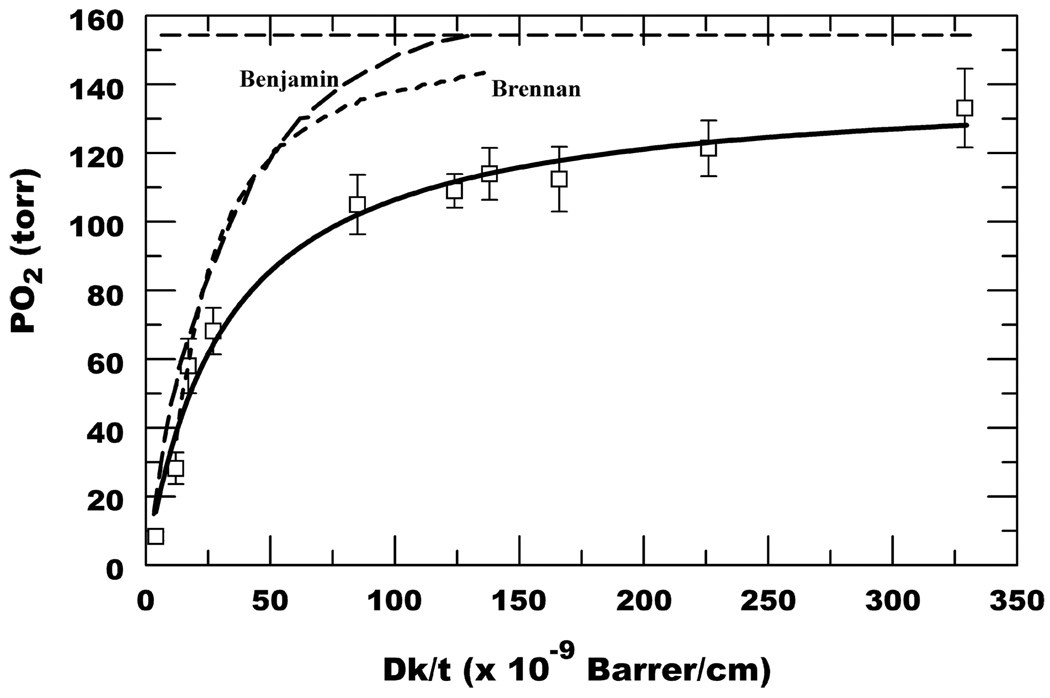
Table 2 lists the calculated open eye, closed eye, and goggle PO2 using the kq determined in the calibration procedure. The Table also includes the calculated open and goggle (simulated closed eye) flux (J) values assuming Pa = 155 and 51 torr, respectively, where J = (Dk/t) × (Pa − Pcl) and Pa is anterior and Pcl is posterior lens surface PO2. The open eye SS PO2 as a function of Dk/t is plotted in Fig. 1, which also includes the data from the two polymacon lenses (Dk/t = 4.2 and 12, kq = 310) used in our previous study.11 Fig. 1 shows that lenses with widely different kq, but similar Dk/t, show similar open eye oxygen tensions indicating internal consistency in the calibration and measurement procedure. Fig. 1 also shows the predicted PO2 based on Equivalent Oxygen Percentage (EOP) data from Benjamin14 and the model by Brennan.9 The direct PO2 estimates from our study are significantly less than that predicted by the earlier work, especially for Dk/t >30.
TABLE 2.
Summary of tear PO2
| Dk/t | Open PO2 (torr) |
Closed PO2 (torr) |
Goggle PO2 |
|---|---|---|---|
| 17.1 | 58.0 ± 7.9 | 11.2 ± 2.7 | 7.8 ± 1.1 |
| 27 | 68.1 ± 6.8 | 12.4 ± 3 | 9.9 ± 1.2 |
| 85 | 105.0 ± 8.6 | 30.0 ± 5.9 | 37.0 ± 1.8 |
| 124 | 109.0 ± 4.9 | 24.0 ± 6 | 40.3 ± 3.6 |
| 138 | 114.0 ± 7.5 | 37.5 ± 6.9 | 41.0 ± 3.4 |
| 166 | 112.4 ± 9.4 | 38.2 ± 7 | 45.7 ± 2.7 |
| 226 | 121.3 ± 8.2 | 39.9 ± 7.1 | 45.6 ± 3.4 |
| 329 | 133.1 ± 11.5 | 42.3 ± 6.1 | 48.5 ± 7.0 |
FIGURE 1.
Open eye SS PO2. Data are mean ± SD (n = 15); also includes polymacon lenses (Dk/t = 4.2 and 12).11 Solid line is a hyperbolic fit, PO2 = a × (Dk/t)/[b + (Dk/t)], where a = 140.6, b = 32.1. Benjamin curve from (ref. 14). Brennan curve from (ref. 9). Horizontal dashed line represents 155 torr.
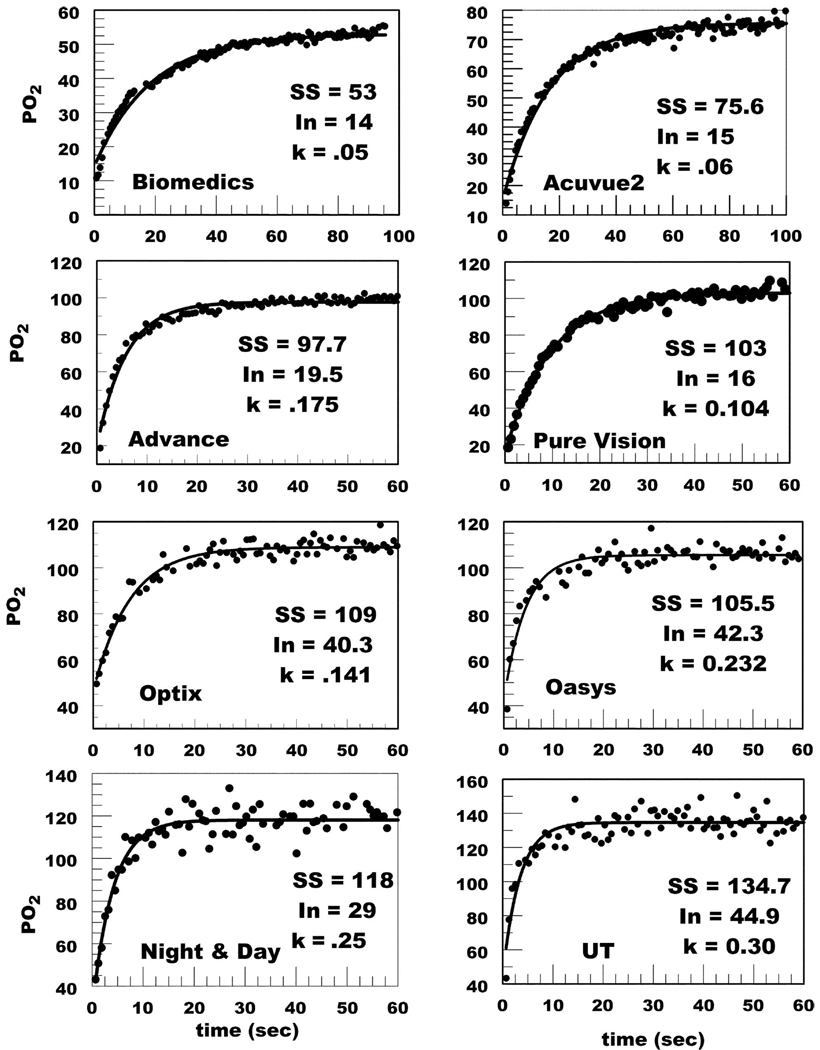
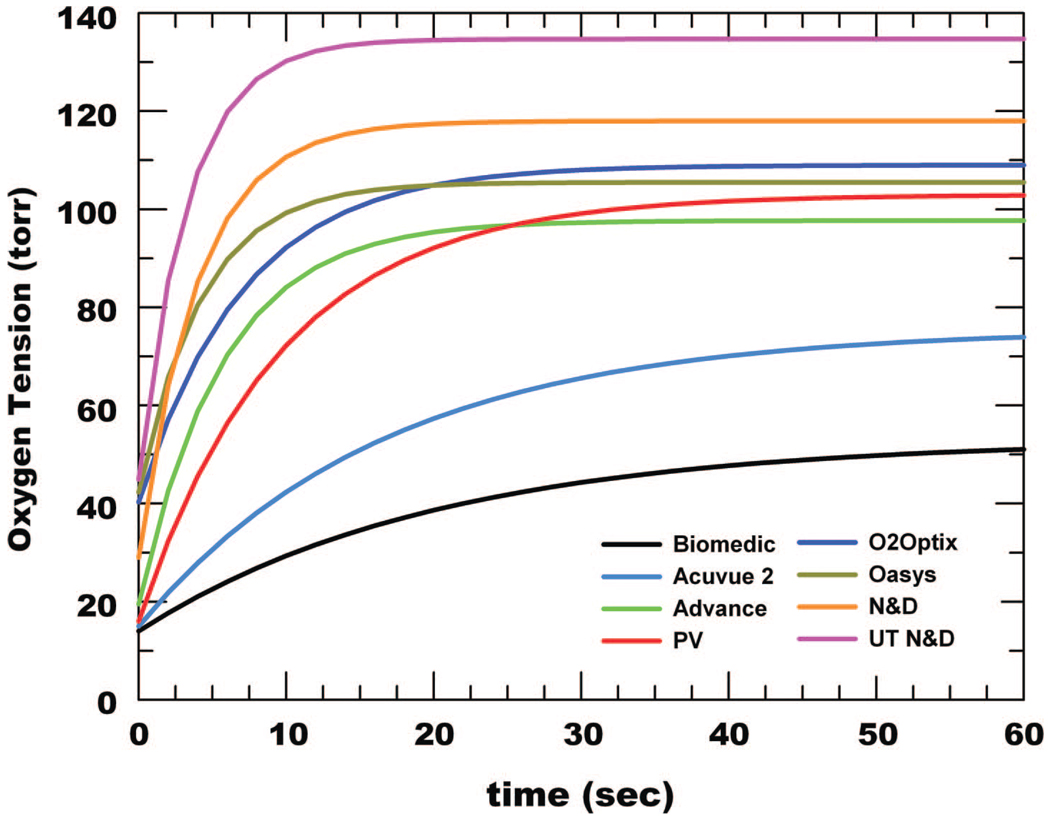
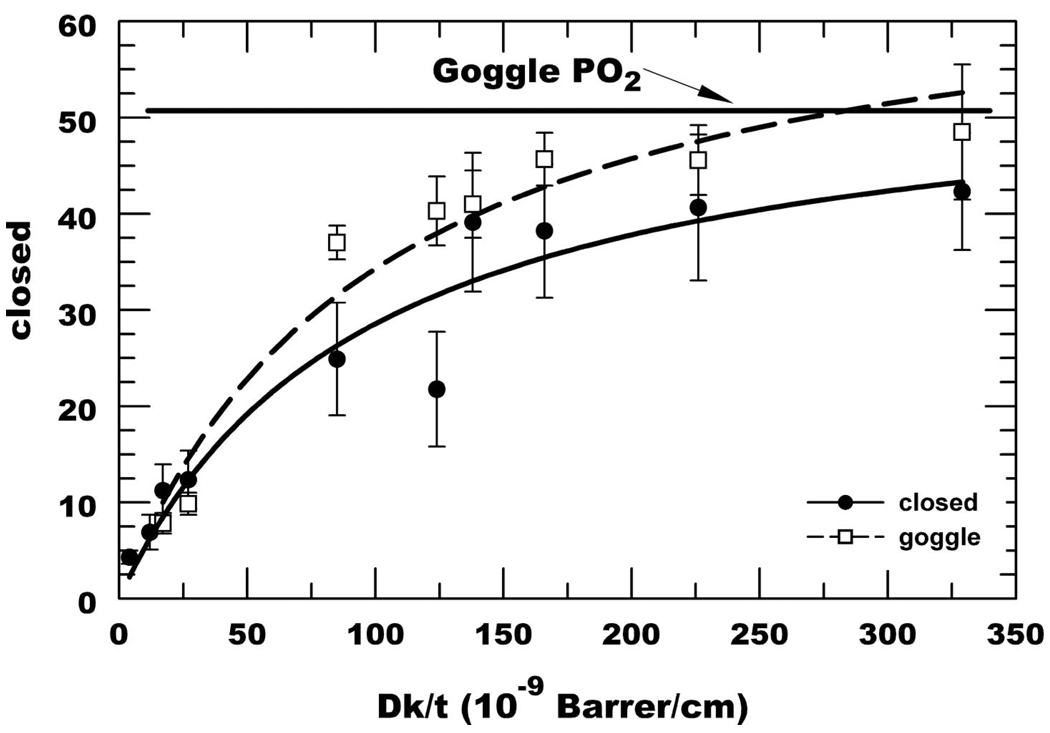
Fig. 2 shows representative data after eye closure for all lenses. The data was fit to a first order exponential model and extrapolated to time 0 to obtain the initial PO2 (In), which was used as the closed eye estimate. At higher PO2 the change in τ for a unit change in PO2 gets smaller, which leads to the observed greater variability in PO2 estimates. Fig. 3 shows summary representative curve fits for all lenses indicating that as the Dk/t increases, the rate of increase in tear PO2 also increases after eye opening. A summary of the averaged closed eye PO2 is listed in Table 2 and plotted in Fig. 4. The closed eye estimates appear to asymptote at 43 to 45 torr.
FIGURE 2.
Examples of PO2 data acquired immediately on eye opening after 5 min of eye closure. In, initial time 0 estimate; k, exponential time constant.
FIGURE 3.
Representative PO2 recovery curves on eye opening after 5 min eye closure for the eight lens types evaluated are overlayed using a common scale.
FIGURE 4.
Summary of posterior surface lens PO2 estimate after eye closure (filled circles) and while using a goggle with 6.7% O2 (open squares). Curve estimates were from fourth order polynomial fits.
Because the closed eye estimate is indirect and requires curve fitting, we devised an alternate method to more directly estimate closed eye tear PO2. A swimmer’s goggle with inflow and outflow tube fittings was placed over the lens-wearing eye. Compressed air at room temperature was hydrated by passing through a water spirging vessel and led to the goggle. The flow rate was adjusted to obtain positive pressure in the goggle. The gas was then switched to a certified gas mixture (6.7% oxygen/balance nitrogen) at the same flow rate. We found that the tear PO2 under the lens with the goggle in air was not significantly different than that without the goggle (data not shown). Fig. 4 shows the summary data using the goggle with 6.7% O2 (51 torr). At Dk/t <30, the closed eye estimates and the goggle estimates were not significantly different. However, at higher Dk/t, the goggle estimate was consistently higher. These data suggest that on average, the anterior lens surface PO2 under the closed lid is <51 torr.
DISCUSSION
This is the first report of direct, in vivo measurements of tear PO2 under a wide range of conventional and SiH contact lenses in human subjects. As expected, SS open eye PO2 increased with increasing Dk/t in a non-linear hyperbolic fashion. What is notable is that the data approaches an asymptote at significantly higher Dk/t than that predicted by previous EOP data and other models.8,9,14 However, our data are very similar to that generated in rabbits using an RGP contact lens Clark-type electrode.10 Furthermore, the early EOP and modeling results8,9,14 predict little oxygen deficiency above Dk/t = 80. However, a recent study using an EOP methodology with high Dk/t RGP lenses indicated significant “oxygen shortfall” with lenses as high as Dk/t = 135.12 These studies, using different methodologies, are consistent with the BSA-porphine data presented here, indicating that our approach provides reasonable PO2 estimates.
For a number of lenses studied here, we needed to use dye quenching constants (qk) that significantly deviated from that in aqueous solution. The cause of this deviation is presumably due to interactions of BSA-porphine with lens surface chemistry. Of note, the Purevision lens avidly binds BSA-porphine to a much greater extent than any of the other lenses. The quenching constant for Purevision was not significantly different from that in solution. This may indicate that if the mole fraction of BSA-porphine significantly exceeds that of the interacting surface chemistry, the quenching constant will tend toward that in solution. Other lenses had significant positive or negative deviations in the quenching constant. Nevertheless, the estimated PO2 for Purevision, positively, and negatively deviating lenses were in a range (100 to 134 torr) and in an order that is internally consistent. This indicates that the calibration procedure was a reasonable estimate of the true quenching constants. Although the calibration lenses were actually on the eye, we were measuring the front surface which could be ~1°C cooler than the back surface. Furthermore, the gas was at room temperature, which could cause further cooling. At 35°C, a 1° drop lowers the quenching constant by 2.3%.13 Thus, cooling could possibly have led to an underestimation of the true quenching constant when the dye is on the posterior surface. Using a quenching constant that is low will lead to an overestimate of PO2 resulting in an under estimate of the corneal oxygen flux. The potential error, however, is small. Considering that SS PO2 under goggles in air was similar to that without goggles suggest that potential cooling is not a significant factor.
PO2 under SiH lenses in the closed eye ranged from 38 to 42 torr. If we assume that the anterior lens surface PO2 is 55 torr in the closed eye, then 42 torr (UT, Table 2) represents about 75% of the potential oxygenation. For the open eye, 155 torr is assumed to be the anterior lens surface PO2 and 134 torr (UT, Table 2) represents 86% of the potential oxygenation. This suggests that the assumed 55 torr in the closed eye is too high. Indeed, the goggle experiments using 51 torr at the anterior lens surface, produced higher PO2 estimates than eye closure, suggesting that the anterior lens surface PO2 in the closed eye is <51 torr. Using several gas mixtures, it should be possible to use this goggle approach to match the closed eye PO2 and get an estimate of the anterior surface PO2 for each individual.
SS oxygen flux into the cornea can be calculated if the Dk/t of the lens and the anterior (Pa) and posterior (Pcl) lens surface PO2 values are known [J = Dk/t × (Pa − Pcl)]. Assuming that Pa = 155 torr, our data suggests that open eye SS flux levels off at ~25 µl/cm2 h for Dk/t > 140. These flux values are higher than those (7 to 9 µl/cm2 h) predicted using models generated from EOP estimates of Pcl behind lenses of relatively lower Dk/t.8,9,14 The open eye flux values calculated from our data are also higher than oxygen flux into the cornea as measured by the oxygen depletion rate from the membrane covering a Clark electrode.15,16 There could be several reasons for this: (1) the BSA-porphine PO2 is an underestimate of the true value. For the reasons described above, this seems unlikely. (2) The Clark electrode PO2 (EOP) is an over estimate. It may be difficult to compare the BSA-porphine estimate of SS flux with the EOP-Clark electrode approach, which is a non-SS measure. (3) The SiH lens Dk/t values are inaccurate. The precision of Dk measures is always a subject of debate, but the variance in Dk measures could not explain the differences in flux. (4) The boundary condition, Pa, is too high. Variations in altitude (Bloomington is 700 to 900 feet above sea level) and relative humidity could reduce Pa. Because the anterior lens surface is coated with water, the relative humidity at that boundary could be considered to be 100%, which would lower Pa to ~146 torr, but this would only reduce the flux estimate from 25 to ~21 µl/cm2 h.
Given the oxygen flux into the cornea it is possible to calculate corneal oxygen consumption, Q. Estimates of Q using our current data are 2 to 4 times higher than direct in vitro estimates of rabbit cornea Q (Harvitt and Bonanno17). However, in vitro estimates are likely to be a lower estimate because of the trauma of handling and dissection, and severing of corneal nerves, activity of which is known to stimulate metabolism.18,19 Mathematical predictions of corneal oxygen flux use in vitro estimates of Dk and thickness of the major corneal layers. The cumulative effect of inaccuracies in these parameters may also result in under estimating the flux and consumption. Indeed, recent modeling analysis of the dynamic changes in tear oxygen tension under lenses worn by human subjects after eye closure suggest that corneal Q and Dk are ~2 and 3 times greater, respectively, than that for in vitro rabbit corneas.20
In summary, our data shows that SiH lenses significantly increase oxygen tension behind the contact lens and oxygen availability to the cornea relative to conventional hydrogels but not to the extent predicted by earlier studies. Our data indicates that increasing Dk/t up to and beyond 140 continues to yield increasing corneal oxygenation. The need to support high levels of oxygen demand in the peripheral cornea is expected to require even higher Dk/t.21 Lastly, the phosphorescence decay methodology is an appropriate technique for future investigations of corneal oxygenation with contact lens wear and potential difference in flux at the central vs. peripheral cornea.
ACKNOWLEDGMENTS
We thank Dr. Courtney F. Morgan for his contributions during discussion of these results.
John Pruitt and Larry Alvord are employees of CIBA Vision.
This work was supported by CIBA Vision Corporation and a grant from the American Optometric Foundation.
Footnotes
This work was presented at the Association for Research in Vision and Ophthalmology meeting, Ft. Lauderdale, FL, May 2008.
REFERENCES
- 1.Keay L, Stapleton F, Schein O. Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens. 2007;33:346–353. doi: 10.1097/ICL.0b013e318157c49d. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 2.Chalmers RL, McNally JJ, Schein OD, Katz J, Tielsch JM, Alfonso E, Bullimore M, O’Day D, Shovlin J. Risk factors for corneal infiltrates with continuous wear of contact lenses. Optom Vis Sci. 2007;84:573–579. doi: 10.1097/OPX.0b013e3180dc9a12. [DOI] [PubMed] [Google Scholar]
- 3.Schein OD, McNally JJ, Katz J, Chalmers RL, Tielsch JM, Alfonso E, Bullimore M, O’Day D, Shovlin J. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112:2172–2179. doi: 10.1016/j.ophtha.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Schein OD, Buehler PO, Stamler JF, Verdier DD, Katz J. The impact of overnight wear on the risk of contact lens-associated ulcerative keratitis. Arch Ophthalmol. 1994;112:186–190. doi: 10.1001/archopht.1994.01090140062024. [DOI] [PubMed] [Google Scholar]
- 5.Young MD, Benjamin WJ. Oxygen permeability of the hypertransmissible contact lenses. Eye Contact Lens. 2003;29:S17–S21. doi: 10.1097/00140068-200301001-00006. [DOI] [PubMed] [Google Scholar]
- 6.Morgan CF, Brennan NA, Alvord L. Comparison of the coulometric and polarographic measurement of a high-Dk hydrogel. Optom Vis Sci. 2001;78:19–29. doi: 10.1097/00006324-200101010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra M, Prausnitz JM, Radke CJ. A single-lens polarographic measurement of oxygen permeability (Dk) for hypertransmissible soft contact lenses. Biomaterials. 2007;28:4331–4342. doi: 10.1016/j.biomaterials.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Brennan NA. Beyond flux: total corneal oxygen consumption as an index of corneal oxygenation during contact lens wear. Optom Vis Sci. 2005;82:467–472. doi: 10.1097/01.opx.0000168560.10861.ae. [DOI] [PubMed] [Google Scholar]
- 9.Brennan NA. A model of oxygen flux through contact lenses. Cornea. 2001;20:104–108. doi: 10.1097/00003226-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Ichijima H, Hayashi T, Mitsunaga S, Hamano H. Determination of oxygen tension on rabbit corneas under contact lenses. CLAO J. 1998;24:220–226. [PubMed] [Google Scholar]
- 11.Bonanno JA, Stickel T, Nguyen T, Biehl T, Carter D, Benjamin WJ, Soni PS. Estimation of human corneal oxygen consumption by non-invasive measurement of tear oxygen tension while wearing hydrogel lenses. Invest Ophthalmol Vis Sci. 2002;43:371–376. [PubMed] [Google Scholar]
- 12.Gardner HP, Fink BA, Mitchell LG, Hill RM. The effects of high-Dk rigid contact lens center thickness, material permeability, and blinking on the oxygen uptake of the human cornea. Optom Vis Sci. 2005;82:459–466. doi: 10.1097/01.opx.0000168562.64251.66. [DOI] [PubMed] [Google Scholar]
- 13.Harvitt DM, Bonanno JA. Direct noninvasive measurement of tear oxygen tension beneath gas-permeable contact lenses in rabbits. Invest Ophthalmol Vis Sci. 1996;37:1026–1036. [PubMed] [Google Scholar]
- 14.Benjamin WJ. EOP and Dk/L: the quest for hyper transmissibility. J Am Optom Assoc. 1993;64:196–200. [PubMed] [Google Scholar]
- 15.Larke JR, Parrish ST, Wigham CG. Apparent human corneal oxygen uptake rate. Am J Optom Physiol Opt. 1981;58:803–805. doi: 10.1097/00006324-198110000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hill RM, Fatt I. Oxygen uptake from a reservoir of limited volume by the human cornea in vivo. Science. 1963;142:1295–1297. doi: 10.1126/science.142.3597.1295. [DOI] [PubMed] [Google Scholar]
- 17.Harvitt DM, Bonanno JA. Oxygen consumption of the rabbit cornea. Invest Ophthalmol Vis Sci. 1998;39:444–448. [PubMed] [Google Scholar]
- 18.Vannas A, Holden BA, Sweeney DF, Polse KA. Surgical incision alters the swelling response of the human cornea. Invest Ophthalmol Vis Sci. 1985;26:864–868. [PubMed] [Google Scholar]
- 19.Cavanagh HD, Colley AM. The molecular basis of neurotrophic keratitis. Acta Ophthalmol Suppl. 1989;192:115–134. doi: 10.1111/j.1755-3768.1989.tb07103.x. [DOI] [PubMed] [Google Scholar]
- 20.Larrea X, Buchler P. A transient diffusion model of the cornea for the assessment of oxygen diffusivity and consumption. Invest Ophthalmol Vis Sci. 2009;50:1076–1080. doi: 10.1167/iovs.08-2479. [DOI] [PubMed] [Google Scholar]
- 21.Alvord LA, Hall WJ, Keyes LD, Morgan CF, Winterton LC. Corneal oxygen distribution with contact lens wear. Cornea. 2007;26:654–664. doi: 10.1097/ICO.0b013e31804f5a22. [DOI] [PubMed] [Google Scholar]