Abstract
Background:
Growth differentiation factor (GDF)-15 is a secreted member of the transforming growth factor-β cytokine superfamily. GDF-15 levels are elevated in the serum of patients with cardiovascular diseases. We hypothesized that GDF-15 levels would also be increased in the plasma and lung tissue of patients with systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH).
Methods:
GDF-15 levels were measured in plasma in subjects with SSc-PAH (n = 30) and compared with subjects with systemic sclerosis (SSc) without pulmonary arterial hypertension (PAH) (n = 24). Patients with idiopathic PAH (IPAH) (n = 44) and normal individuals (n = 13) served as control subjects. Immunohistochemistry and immunofluorescence assay identified GDF-15 protein in lung tissue from patients with SSc-PAH and IPAH.
Results:
Patients with SSc-PAH had significantly higher mean circulating levels of GDF-15 in plasma compared with patients with SSc without PAH (422.3 ± 369.5 pg/mL vs 108.1 ± 192.8 pg/mL, P = .004). GDF-15 levels correlated positively with estimated right ventricular systolic pressure on echocardiogram and plasma levels of the amino terminal propeptide form of brain natriuretic peptide. There was an inverse correlation between circulating GDF-15 and diffusing capacity of the lung for carbon monoxide (Dlco) and a positive correlation with the FVC to Dlco ratio on pulmonary function test. GDF-15 levels > 125 pg/mL were associated with reduced survival. GDF-15 protein expression was increased in lung tissue from patients with SSc-PAH.
Conclusions:
GDF-15 may be a useful biomarker in PAH associated with SSc. Its presence in lung tissue may suggest a role in the pathology of the disease.
Growth differentiation factor (GDF)-15, a member of the transforming growth factor-β superfamily of cytokines, plays an important role in cell growth and differentiation. GDF-15 is normally expressed at high levels in the placenta1‐3 and at lower levels in the prostate, pancreas, and kidney.1 Secreted GDF-15 functions in cell-to-cell signaling,4 signal transduction,5,6 and apoptosis regulation7‐9 in a cell-type-specific manner.
Cardiac stress and tissue damage caused by acute coronary syndrome and chronic left-sided heart failure induce GDF-15 expression in heart tissue.10,11 Arteriosclerotic lesions of human carotid arteries express GDF-15, and this expression colocalizes to macrophages but not to smooth muscle or endothelial cells in atherosclerotic plaques.9 GDF-15 appears to have a protective effect on cultured cardiomyocytes10,11 and in murine models of ischemia and reperfusion injury.10 GDF-15 was identified as an independent predictor of long-term mortality in acute pulmonary embolism12 and idiopathic pulmonary arterial hypertension (IPAH)13 and was proposed as a potential biomarker in these diseases.12,13
Pulmonary arterial hypertension (PAH) is associated with abnormal cellular proliferation in the precapillary pulmonary vasculature. Inflammation is believed to be a major pathogenic component in pulmonary vascular remodeling, with macrophages, lymphocytes, and dendritic cells identified in PAH vascular lesions.14
Approximately 10% to 16% of patients with systemic sclerosis (SSc) develop PAH,15 and nearly 30% of scleroderma-related deaths can be attributed to PAH.16 Patients with SSc-associated PAH (SSc-PAH) are less responsive to therapy17 and are believed to have a worse overall prognosis.18 Patients with SSc would benefit from biomarkers assessing risk for developing PAH and for disease management. We hypothesized that patients with SSc-PAH would have elevated serum levels of GDF-15 protein compared with patients with SSc without PAH and that there would be an increased expression of GDF-15 protein in the lung tissue of patients with SSc-PAH.
Materials and Methods
Subject Recruitment
The Institutional Review Board of the University of Colorado approved this study (COMIRB #06-0247). Written informed consent was obtained from subjects recruited from the Pulmonary Hypertension and Scleroderma clinics at the University of Colorado Denver. One hundred fifty-one subjects were screened; 40 were excluded after failing to meet entry criteria. PAH was confirmed by a resting mean pulmonary artery pressure (mPAP) > 25 mm Hg and a pulmonary capillary wedge pressure < 15 mm Hg by right-sided heart catheterization (RHC). Subjects with SSc without PAH were excluded if they had an estimated right ventricular systolic pressure (RVSP) > 35 mm Hg by echocardiography (ECHO) unless, when follow-up RHC data were available, the RHC results showed an mPAP < 25 mm Hg. Subjects with SSc without PAH were also excluded for abnormal second heart sound, evidence of tricuspid regurgitant (TR) murmur on physical examination, or diffusing capacity of the lung for carbon monoxide (Dlco) < 80% after correction for alveolar volume.19 SSc diagnosis was determined by American College of Rheumatology criteria.20 Normal subjects were self-identified as having neither PAH nor connective tissue disease. Lung tissues were autopsy samples obtained through a tissue bank maintained at the University of Colorado. Tissue sections were examined and characterized by a pathologist blinded to this study.
Subject Baseline Characteristics
The RHC, ECHO, and pulmonary function tests done closest to the blood draw were used. Other laboratory values were obtained within 1 month of the blood draw, and usually on the same day. Survival information was determined by review of medical records and was confirmed by review of the Social Security death index.
Echocardiography
The majority (76%) of ECHOs were performed at the University of Colorado Hospital using M-mode and two-dimensional ECHO with a Philips iE33 ultrasound machine (Philips Healthcare; Andover, Massachusetts). Continuous-wave Doppler signals were recorded with a Philips transducer (Philips Healthcare). RVSP was calculated from peak signal velocity from the TR signal using the modified Bernoulli equation. Right atrial pressure was estimated by evaluating the size and collapsibility of the inferior vena cava and was added to the calculated TR jet velocity to obtain the estimated pulmonary artery systolic pressure. The mean time from ECHO to the blood draw for this study in subjects with SSc-PAH was 91.7 days (± 41.9 SEM).
Right-Sided Heart Catheterization
The majority (84%) of RHCs were performed at the University of Colorado Hospital, using standard techniques. The right internal jugular vein or right femoral vein was used for venous access in all cases. Resting hemodynamics were measured in the supine position. The mean time from RHC to the blood draw for this study in subjects with SSc-PAH was 574.2 days (± 111.6 SEM).
Blood Collection
Peripheral whole blood was collected using a Vacutainer cell preparation tube containing sodium citrate (Becton, Dickinson and Co; Franklin Lakes, New Jersey). RBCs were removed by centrifugation at 1,800g in an Eppendorf 5810R centrifuge (Westbury, New York). Plasma was collected and stored at −80°C.
GDF-15 Enzyme-Linked Immunosorbent Assay and Brain Natriuretic Peptide Fragment Enzyme Immunoassay
The GDF-15 Duoset enzyme-linked immunosorbent assay (ELISA) Development kit (R&D Systems; Rochester, Minnesota) was used to determine plasma levels of GDF-15, following R&D’s protocol. The BNP Fragment enzyme immunoassay (Biomedica; Alpco Diagnostics; Salem, New Hampshire) was used to detect plasma levels of the amino terminal propeptide form of brain natriuretic peptide (NT-proBNP), following Biomedica’s protocol. Plates were read on a VersaMax microplate reader (Molecular Devices; Sunnyvale, California) at 450 nm, with wavelength correction of 540 nm for GDF-15 and 620 nm for NT-proBNP. Results were analyzed using SoftMax Pro v3.1.2 analysis software (MDS Analytical Technologies; Sunnyvale, California).
Immunohistochemistry
Slides of formalin-fixed, paraffin-embedded tissue sections were obtained from a tissue bank maintained at the University of Colorado and were identified by a pathologist as SSc-PAH, SSc without PAH, IPAH, and controls. These tissues were from a cohort separate from the subjects whose serum was used in the ELISA. Antigen retrieval was via the heat-induced epitope retrieval method in pH = 6.0 citrate buffer (Vector Laboratories; Burlingame, California). Rabbit polyclonal α-GDF-15 (Prestige Antibodies, Sigma-Aldrich Co; St. Louis, Missouri) or normal rabbit IgG isotype (Vector Laboratories), and horseradish peroxidase-conjugated goat α-rabbit IgG (Vector Laboratories) were used. Slides were developed with diaminobenzidine (Dako North America, Inc; Carpinteria, California) and counterstained in hematoxylin (Dako North America, Inc).
Immunofluorescence
Slides were blocked with 3% hydrogen peroxide (Sigma-Aldrich Co) and incubated successively with avidin and biotin (Vector Laboratories). The same antibodies for GDF-15 and isotype control used in the immunohistochemistry (IHC) were used in the immunofluorescence assay (IFA). Biotinylated goat α-rabbit IgG antibody was applied, followed by incubation in ready-to-use streptavidin-horseradish peroxidase (both from Vector Laboratories). Tyramide signal amplification reagent (Perkin-Elmer; Waltham, Massachusetts) was applied, followed by streptavidin-Texas Red conjugate (Invitrogen; Carlsbad, California). Slides were then incubated with α-CD68 mouse monoclonal (Dako North America, Inc) and Alexa Fluor 488-conjugated α-mouse (Invitrogen) antibodies. Analysis for IHC and IFA was performed using an Eclipse E800 microscope (Nikon Inc; Melville, New York) with a Photometrics CoolSNAP HQ CCD camera (Roper Scientific; Tucson, Arizona) and NIS Elements AR 3.00 software (Nikon Inc).
Statistical Analysis
Data are presented as absolute numbers, percentages, and mean ± SD. GDF-15 comparisons were performed by Student t test. Relations between GDF-15 and clinical variables were assessed by Spearman rank correlation coefficients. For comparison of the prognostic values of GDF-15 and NT-proBNP, we generated receiver operating characteristic (ROC) curves, the areas under the curves (C statistic) were calculated, and clinically relevant threshold levels were chosen to optimize sensitivity and specificity. Levels of GDF-15 and NT-proBNP identified in the ROC curves were used to examine survival using Kaplan-Meier plots. Univariate and multivariate logistic regression models were used to determine the ability of GDF-15 treated as a continuous variable to classify PAH with the a priori selected covariants, age (years), serum hemoglobin (g/dL), and serum creatinine (mg/dL). A P value < .05 was considered statistically significant. Calculations were performed with JMP, version 7 (SAS Institute Inc; Cary, North Carolina) and GraphPad Prism, version 4.03 for Windows (GraphPad Software; San Diego, California).
Results
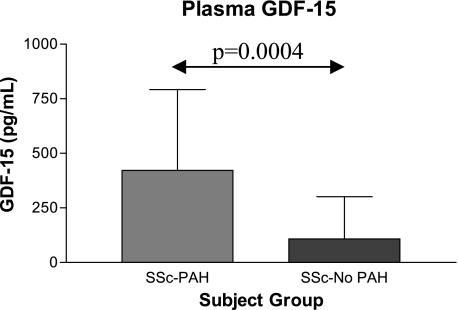
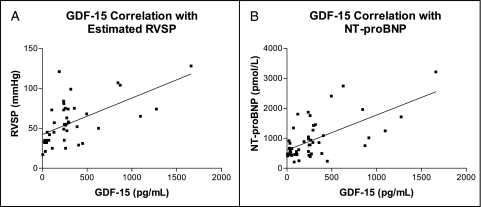
GDF-15 levels were elevated in patients with SSc-PAH compared with patients with SSc without PAH (Fig 1), with patients with IPAH (173.0 ± 193.0 pg/mL, P = .0003), and with control subjects (66.1 ± 46.3 pg/mL, P = .0013). Subjects with SSc were compared with respect to age, sex, race/ethnicity, and clinical laboratory values (Table 1). Pulmonary and hemodynamic features and PAH-specific medications were compared between subjects with SSc-PAH and subjects with IPAH (Table 2). Subjects with IPAH had more severe PAH than those with SSc-PAH in terms of hemodynamics (Table 2). Significant differences existed between subjects with PAH in FVC, Dlco, plasma NT-proBNP level, and age (Table 2). We found a significant positive correlation between GDF-15 levels and estimated RVSP on ECHO in subjects with SSc (Fig 2A), but GDF-15 level did not correlate with other invasively measured cardiac hemodynamics.
Figure 1.
GDF-15 expression in plasma by enzyme-linked immunosorbent assay (ELISA). Plasma levels of GDF-15 are significantly higher in subjects with SSc-PAH (422.3 ± 369.5 pg/mL, n = 30) than in those with SSc-no PAH (108.1 ± 192.8 pg/mL, n = 24). GDF = growth differentiation factor; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SSc-no PAH = systemic sclerosis without pulmonary arterial hypertension.
Table 1.
—Demographic and Clinical Baseline Characteristics of Subjects With SSc With and Without PAH
| SSc-PAH (n = 30) | SSc-No PAH (n = 24) | P Value | |
| Age, y | 61.0 ± 11.0 | 52.0 ± 9.7 | .0029 |
| Female sex | 28 (93.3) | 23 (95.8) | .6969 |
| White race | 26 (86.7) | 18 (75.0) | .1113 |
| Type 2 diabetes | 3 (10.0) | 1 (4.2) | .4256 |
| AST,a U/L | 25.3 ± 8.7 (16) | 24.5 ± 9.0 (15) | .8226 |
| Total bilirubin,a mg/dL | 0.6 ± 0.2 (16) | 0.7 ± 0.3 (15) | .0545 |
| Creatinine,a mg/dL | 1.1 ± 0.5 (19) | 0.9 ± 0.1 (14) | .1246 |
| Hemoglobin,a g/dL | 13.1 ± 1.6 (17) | 14.4 ± 1.0 (15) | .0083 |
| NT-proBNP,a pmol/L | 1130.0 ± 759.1 (29) | 702.4 ± 346.0 (23) | .0158 |
| GDF-15, pg/mL | 422.3 ± 369.5 | 108.1 ± 192.8 | .0004 |
Data are presented as No. (%) or mean ± SD, unless indicated otherwise. AST = aspartate transaminase; GDF = growth differentiation factor; NT-proBNP = amino terminal propeptide form of brain natriuretic peptide; PAH = pulmonary arterial hypertension; SSc = systemic sclerosis; SSc-PAH = systemic sclerosis-associated pulmonary arterial hypertension; SSc-no PAH = systemic sclerosis without pulmonary arterial hypertension.
Values were not available for all subjects. Data are presented as mean ± SD (number available).
Table 2.
—Baseline and Clinical Comparisons of Subjects With SSc-PAH and Idiopathic PAH
| Feature | SSc-PAH (n = 30) | IPAH (n = 44) | P Value |
| Age, y | 61.0 ± 11.0 | 51.0 ± 13.6 | .0013 |
| ECHO | |||
| Estimated RVSP,a mm Hg | 66.4 ± 25.1 | 82.3 ± 28.3 (41) | .0164 |
| Right-sided catheterization | |||
| Mean PAP, mm Hg | 43.4 ± 15.1 | 52.7 ± 14.0 | .0085 |
| Mean RAP,a mm Hg | 5.7 ± 5.7 (28) | 6.8 ± 4.1 (43) | .3305 |
| Mean PCWP, mm Hg | 7.2 ± 3.7 | 8.3 ± 5.0 | .3228 |
| PVR, Wood units | 9.3 ± 5.5 | 12.5 ± 7.6 | .0471 |
| Cardiac output,a L /min | 4.6 ± 1.8 (29) | 4.2 ± 1.7 | .3606 |
| Pulmonary function testa,b | |||
| FVC | 71.6 ± 13.7 (30) | 83.6 ± 19.0 (28) | .0075 |
| Adjusted Dlcoa,b | 53.7 ± 24.7 (23) | 81.3 ± 17.4 (27) | < .0001 |
| NT-proBNP,a pmol/L | 1130.0 ± 759.1 (29) | 663.4 ± 507.0 (40) | .0003 |
| GDF-15, pg/mL | 422.3 ± 369.5 | 173.0 ± 193.0 | .0004 |
| Medicationsc | |||
| Prostanoids | 5 (16.7) | 19 (43.2) | … |
| Endothelin receptor antagonist | 19 (63.3) | 17 (38.6) | … |
| Phosphodiesterase inhibitor | 7 (23.3) | 18 (40.9) | … |
| Calcium channel blocker | 16 (53.3) | 17 (38.6) | … |
Data are presented as No. (%) or mean ± SD, unless indicated otherwise. Dlco = diffusing capacity of the lung for carbon monoxide; ECHO = echocardiogram; IPAH = idiopathic pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; RVSP = right ventricular systolic pressure. See Table 1 for expansion of other abbreviations.
Values were not available for all subjects. Values are then given as mean ± SD (number available).
Data expressed as % predicted values adjusted for age, sex, and race.
Most subjects are on multiple therapies; thus, the total exceeds 100%.
Figure 2.
Echocardiogram and NT-proBNP correlations with GDF-15. A, A significant positive correlation exists between GDF-15 and estimated RVSP on echocardiogram (Spearman r = 0.5557, P = .0001) in subjects with SSc (systemic sclerosis) (n = 43). B, There is a significant correlation between GDF-15 and NT-proBNP plasma levels (Spearman r = 0.4843, P = .0003) in subjects with SSc (n = 52). NT-proBNP = amino terminal propeptide form of brain natriuretic peptide; RVSP = right ventricular systolic pressure. See Figure 1 legend for expansion of other abbreviations.
Plasma NT-proBNP levels correlated significantly with levels of GDF-15 in subjects with SSc (Fig 2B). NT-proBNP levels were significantly higher in patients with SSC-PAH than in those with SSc without PAH (Table 1) and in those with IPAH (Table 2).
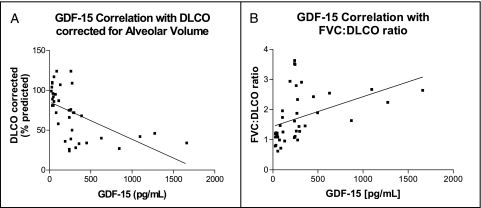
There was a significant negative correlation between GDF-15 plasma levels and Dlco in subjects with SSc (Fig 3A). A significant positive correlation existed between GDF-15 plasma levels and the FVC to Dlco ratio in subjects with SSc (Fig 3B).
Figure 3.
Dlco correlations with GDF-15. A, There is a significant negative correlation between plasma levels of GDF-15 and Dlco (% predicted value, corrected for alveolar volume) in subjects with SSc (Spearman r = −0.6510, P < .0001, n = 38). B, The FVC to Dlco ratio has a significant positive correlation with plasma GDF-15 levels in subjects with SSc (Spearman r = 0.5967, P < .0001, n = 40). Dlco = diffusing capacity of the lung for carbon monoxide. See Figure 1 and 2 legends for expansion of other abbreviations.
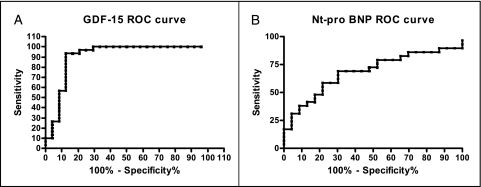
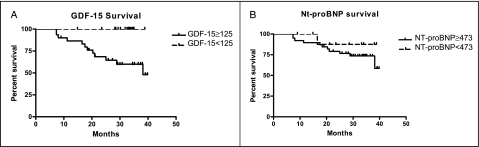
In subjects with SSc, an ROC curve was generated for the diagnosis of PAH using GDF-15 plasma values. The area under the curve was 0.91, with a P value of < .0001 (Fig 4A). A diagnostically relevant value for GDF-15 of 125 pg/mL was derived from the ROC curve, with 93% sensitivity and 88% specificity for the diagnosis of PAH. A Kaplan-Meier survival curve using 125 pg/mL as a cutoff showed survival probabilities of 86.3%, 67.7%, and 58.1% after 1, 2, and 3 years of follow-up, respectively, whereas those with a plasma level < 125 pg/mL were all alive at follow-up (Fig 5A). Mean time to follow-up was 30.0 ± 9.3 months, with a maximum follow-up of 39.9 months and a minimum of 7.3 months.
Figure 4.
ROC curves were generated to determine clinically relevant plasma levels of GDF-15 and NT-proBNP, optimizing sensitivity and specificity. A, An ROC curve for GDF-15 (area under the curve [AUC] = 0.91, P < .0001). B, An ROC curve for NT-proBNP (AUC = 0.69, P = .023). ROC = receiver operating characteristic. See Figure 1 and 2 legends for expansion of other abbreviations.
Figure 5.
A, A Kaplan-Meier survival curve for dichotomous level of GDF-15 (125 pg/mL). B, A Kaplan-Meier survival curve for the dichotomous level of NT-proBNP (473 pmol/L) in subjects with SSc. Elevation of GDF-15 plasma levels above 125 pg/mL was associated with increased mortality in subjects with SSc (n = 54, P = .0021). There was no statistically significant difference in mortality between patients with NT-proBNP plasma levels above 473 pmol/L (n = 52, P = .3813). See Figure 1 and 2 legends for expansion of abbreviations.
A ROC curve was generated for NT-proBNP with an area under the curve of 0.69 and a P value of.0023 (Fig 4B). A diagnostically relevant value for NT-proBNP of 473 pmol/L was derived from the ROC curve, with 86% sensitivity and 30% specificity. No statistically significant difference existed in survival between the SSc groups using the 473 pmol/L value by Kaplan-Meier (Fig 5B).
The relation of GDF-15 plasma levels to the diagnosis of SSc-PAH was statistically significant in a univariate logistic model for odds of PAH when GDF-15 was treated as a continuous variable (OR, 1.09; 95% CI, 1.03-1.15; P = .002). The OR of 1.09 was expressed per 10-U increase in GDF-15 levels, meaning a 9% increase in the odds of PAH for every 10-U increase in GDF-15. Adjusting for covariants of age, serum hemoglobin, and serum creatinine reduced the odds of PAH to 3% for every 10-U increase in GDF-15 and was not statistically significant (OR, 1.03; 95% CI, 0.96-1.10; P = .4).
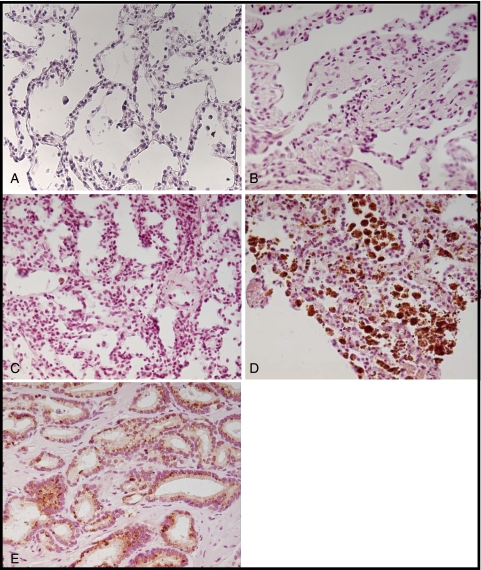
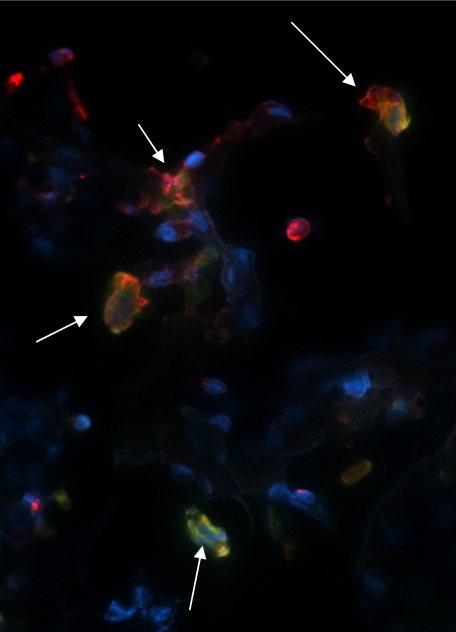
GDF-15 protein levels were increased in the lung tissue of patients with SSc-PAH compared with patients with SSc without PAH or with IPAH by IHC (Fig 6). Lung tissue from patients with IPAH had significantly fewer cells stain positive for GDF-15. Normal lung tissue samples did not express GDF-15. IFA demonstrated colocalization of GDF-15-positive cells with a monocyte/macrophage marker, CD68 (Fig 7).
Figure 6.
Immunohistochemistry for GDF-15 in lung tissue stained with anti-GDF-15 antibody. A, Normal lung tissue. B, SSc-no PAH. C, Idiopathic pulmonary arterial hypertension. D, SSc-PAH. E, Prostate cancer tissue (positive control). (A-E, original magnification × 20). See Figure 1 legend for expansion of the abbreviations.
Figure 7.
Immunofluorescence staining for GDF-15 and CD68 in lung tissue. Lung tissue from a subject with SSc-PAH was stained with both GDF-15 (red) and CD68 (green), a monocyte/macrophage marker, to demonstrate colocalization (arrows). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). See Figure 1 legend for expansion of abbreviations.
Discussion
PAH is a common complication of SSc and is associated with poor prognosis.21 SSc-PAH is generally diagnosed at a late stage of disease, when treatment may be less effective.17,21,22 There is a need for noninvasive biomarkers to help identify and risk stratify this group. Nickel et al13 previously reported elevation of plasma GDF-15 in subjects with IPAH by radioimmunometric assay (RIA). We confirmed elevated plasma concentrations of GDF-15 in our PAH population using a simple ELISA. Notably, GDF-15 concentrations were significantly higher in subjects with SSc-PAH than in subjects with IPAH, even though the IPAH group appeared to have more severe disease based on hemodynamics.
Plasma GDF-15 levels correlated well with other indicators of PAH in subjects with SSc, including a significant positive correlation with estimated RVSP on ECHO, plasma levels of NT-proBNP, and FVC to Dlco ratio, and a significant inverse correlation with Dlco.23 NT-proBNP has been proposed as a potential biomarker for PAH in patients with SSc.24 We found the plasma levels of GDF-15 were better able to identify SSc-PAH and predicted mortality with greater reliability than NT-proBNP.
We did not find significant correlations between GDF-15 and invasively measured hemodynamic parameters such as mPAP and cardiac output. We hypothesized that this was due to the length of time between RHC and the blood draw in this study. Blood samples were drawn during clinic visits, and a significant time lag existed between the blood draw and the most recent RHC in many cases.
Several weaknesses are associated with this study. The SSc-PAH population was older than the population with SSc but no PAH (mean 61.0 ± 11.0 years vs 52.0 ± 9.7 years, P = .0029) and the IPAH cohort (51.0 ± 13.6 years, P = .0013). Therefore, age could have influenced our findings. We note, however, that this discrepancy mirrors the age difference found between SSc populations with and without PAH and may be a difficult confounder to avoid. Another weakness was the time difference between RHC and measurement of GDF-15 levels. However, ECHO results were much closer to the blood draw, and estimated RVSP correlated significantly with GDF-15 levels in plasma. Few patients diagnosed with SSc without PAH underwent RHC to definitively confirm the absence of PAH. However, we carefully excluded PAH in this cohort by setting stringent requirements for ECHO, Dlco, and physical examination. Furthermore, we followed this patient population prospectively for a mean of 2.8 years, confirming the lack of clinical evidence of PAH. SSc-PAH is a progressive disease and we feel confident that our SSc without PAH cohort were, in fact, true PAH-negative at the time of the blood draw. Almost all patients were on multiple PAH treatments (Table 2). Although we cannot exclude treatment as a possible cause of differences in plasma GDF-15, no individual treatment correlated with GDF-15 levels, including prostacyclins (P = .31). GDF-15 measurements were taken at one specific point in time. Future studies could examine GDF-15 levels over multiple time points, or by assessing changes with therapy. We did not exclude subjects with interstitial lung disease in this study, which could have been a potential confounder. Finally, Mathai et al25 recently reported that levels of NT-proBNP predicted survival in subjects with SSc-PAH, but not in those with IPAH. We did not find the same predictive strength for NT-proBNP in our study, possibly because of the larger number of subjects in the Mathai study.
We demonstrated that GDF-15 can be detected in plasma using a commercial ELISA kit, eliminating the requirement for the more technically difficult RIA. Although levels found in the ELISA were significantly lower overall than those found by RIA, the decrease was proportional in all subject groups and we were able to confirm previous correlations made with RIA results.13,26,27 We demonstrated, to our knowledge for the first time, that circulating GDF-15 levels are significantly higher in patients with SSc-PAH than in patients with SSc without PAH.
By IHC, we showed that GDF-15 is detectable in lung tissue from patients with SSc and demonstrated colocalization of GDF-15 with a macrophage marker by IFA. To our knowledge, this is the first time that evidence of GDF-15 protein has been found in lung tissue.
Elevated GDF-15 levels can be found in a variety of tissues when exposed to stress or injury. Inflammatory cells in the lungs of patients with PAH and SSc-PAH could secrete GDF-15, causing an antiapoptotic phenotype and contributing to the proliferation of cells in the pulmonary vasculature characteristic of PAH.
Conclusions
We propose that circulating GDF-15 levels could serve as a valuable biomarker in patients with SSc-PAH. GDF-15 could be useful in risk stratification and might serve to predict disease in patients with SSc in whom PAH is clinically unrecognized, especially in combination with changes in ECHO or Dlco, or combined with NT-proBNP levels. However, the application of GDF-15 in earlier diagnosis of PAH in SSc will require further investigation.
Acknowledgments
Author Contributions: Ms Meadows and Dr Bull had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ms Meadows: contributed to the conception and design of the study, acquisition of the data, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis.
Dr Risbano: contributed to research subject consent, analysis and interpretation of the data, statistical analysis, and drafting of the manuscript.
Ms Zhang: contributed to technical assistance, analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content.
Dr Geraci: contributed to the conception and design of the study; critical revision of the manuscript for important intellectual content; and administrative, technical, or material support.
Dr Tuder: contributed to the analysis and interpretation of the data; critical revision of the manuscript for important intellectual content; and administrative, technical, or material support.
Dr Collier: contributed to the conception and design of the study, research subject consent, and critical revision of the manuscript for important intellectual content.
Dr Bull: contributed to the conception and design of the study; analysis and interpretation of the data; research subject consent; writing of the manuscript; statistical analysis; obtaining of funding; administrative, technical, or material support; supervision of the study; and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Bull has served as a consultant for Actelion. Dr Collier has been on the speakers’ bureau for Gilead, although he has not acted as a speaker at this time. Mss Meadows and Zhang and Drs Risbano, Geraci, and Tuder have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: The authors thank Carlyne Cool, MD, and Jane Parr, BS, HT, for the pathologic assessment and provision of the lung tissues used in this study, and Anne Hines, PhD, for her assistance in recruiting subjects for this study.
Abbreviations
- Dlco
diffusing capacity of the lung for carbon monoxide
- ECHO
echocardiogram
- ELISA
enzyme-linked immunosorbent assay
- GDF
growth differentiation factor
- IFA
immunofluorescence assay
- IHC
immunohistochemistry
- IPAH
idiopathic pulmonary arterial hypertension
- mPAP
mean pulmonary artery pressure
- NT-proBNP
amino terminal propeptide form of brain natriuretic peptide
- PAH
pulmonary arterial hypertension
- RHC
right-sided heart catheterization
- RIA
radioimmunometric assay
- ROC
receiver operating characteristic
- RVSP
right ventricular systolic pressure
- SSc
systemic sclerosis
- SSc-PAH
systemic sclerosis-associated pulmonary arterial hypertension
- TR
tricuspid regurgitant
Funding/Support: This work was supported by a grant from the National Institutes of Health [Grant 5 K08 HL072858-02] and a grant from the Scleroderma Foundation [Grant PN200509-021], both awarded to Dr Bull.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Paralkar VM, Vail AL, Grasser WA, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273(22):13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 2.Hromas R, Hufford M, Sutton J, et al. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997;1354(1):40–44. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 3.Moore AG, Brown DA, Fairlie WD, et al. The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab. 2000;85(12):4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- 4.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamouille S, Mallet C, Feige JJ, et al. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100(13):4495–4501. doi: 10.1182/blood.V100.13.4495. [DOI] [PubMed] [Google Scholar]
- 6.Tan M, Wang Y, Guan K, et al. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci U S A. 2000;97(1):109–114. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari N, Pfeffer U, Dell’Eva R, et al. The transforming growth factor-beta family members bone morphogenetic protein-2 and macrophage inhibitory cytokine-1 as mediators of the antiangiogenic activity of N-(4-hydroxyphenyl)retinamide. Clin Cancer Res. 2005;11(12):4610–4619. doi: 10.1158/1078-0432.CCR-04-2210. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Bauskin AR, Zaunders J, et al. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 2003;63(16):5034–5040. [PubMed] [Google Scholar]
- 9.Schlittenhardt D, Schober A, Strelau J, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318(2):325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 10.Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98(3):351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98(3):342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 12.Lankeit M, Kempf T, Dellas C, et al. Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med. 2008;177(9):1018–1025. doi: 10.1164/rccm.200712-1786OC. [DOI] [PubMed] [Google Scholar]
- 13.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(5):534–541. doi: 10.1164/rccm.200802-235OC. [DOI] [PubMed] [Google Scholar]
- 14.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1) Suppl:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 15.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1) Suppl:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 16.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull TM. Screening and therapy of pulmonary hypertension in systemic sclerosis. Curr Opin Rheumatol. 2007;19(6):598–603. doi: 10.1097/BOR.0b013e3282ec67d4. [DOI] [PubMed] [Google Scholar]
- 18.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179(2):151–157. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 19.Steen VD. The lung in systemic sclerosis. J Clin Rheumatol. 2005;11(1):40–46. doi: 10.1097/01.rhu.0000152147.38706.db. [DOI] [PubMed] [Google Scholar]
- 20.Scussel Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 2001;44(3):735–736. doi: 10.1002/1529-0131(200103)44:3<735::AID-ANR125>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin V, Humbert M, Coghlan G, et al. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii25–iii31. doi: 10.1093/rheumatology/kep107. [DOI] [PubMed] [Google Scholar]
- 22.Kowal-Bielecka O, Delcroix M, Vonk-Noordegraaf A, et al. Outcome measures in pulmonary arterial hypertension associated with systemic sclerosis. Rheumatology (Oxford) 2008;47(Suppl 5):v39–v41. doi: 10.1093/rheumatology/ken308. [DOI] [PubMed] [Google Scholar]
- 23.Hachulla E, Launay D, Mouthon L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009;136(5):1211–1219. doi: 10.1378/chest.08-3042. [DOI] [PubMed] [Google Scholar]
- 24.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48(2):516–522. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 25.Mathai SC, Bueso M, Hummers LK, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35(1):95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 26.Kempf T, von Haehling, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(11):1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 27.Wollert KC, Kempf T, Peter T, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115(8):962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]