Abstract
Oligomerization, conformational changes, and the consequent neurodegeneration of Alzheimer's β-amyloid protein (AβP) play crucial roles in the pathogenesis of Alzheimer's disease (AD). Mounting evidence suggests that oligomeric AβPs cause the disruption of calcium homeostasis, eventually leading to neuronal death. We have demonstrated that oligomeric AβPs directly incorporate into neuronal membranes, form cation-sensitive ion channels (“amyloid channels”), and cause the disruption of calcium homeostasis via the amyloid channels. Other disease-related amyloidogenic proteins, such as prion protein in prion diseases or α-synuclein in dementia with Lewy bodies, exhibit similarities in the incorporation into membranes and the formation of calcium-permeable channels. Here, based on our experimental results and those of numerous other studies, we review the current understanding of the direct binding of AβP into membrane surfaces and the formation of calcium-permeable channels. The implication of composition of membrane lipids and the possible development of new drugs by influencing membrane properties and attenuating amyloid channels for the treatment and prevention of AD is also discussed.
1. Introduction
Alzheimer's disease (AD) is a severe type of senile dementia, affecting a large portion of elderly people worldwide. It is characterized by profound memory loss and inability to form new memories. The pathological hallmarks of AD are the presence of numerous extracellular deposits, termed senile plaques, and intraneuronal neurofibrillary tangles (NFTs). The degeneration of synapses and neurons in the hippocampus or cerebral cortex is also observed [1]. The major components of NFTs are phosphorylated tau proteins, and that of senile plaques are β-amyloid proteins (AβPs). Although the precise cause of AD remains elusive, it is widely accepted that oligomerization of AβP and the consequent neurodegeneration might be the cause of neuronal death in AD patients [2, 3].
There is considerable interest regarding the mechanism by which AβPs cause neurodegeneration. AβPs have been reported to cause various adverse effects on neuronal survivals, such as the production of reactive oxygen species, the induction of cytokines, the induction of endoplasmic reticulum (ER) stresses, and the abnormal increase in intracellular calcium levels ([Ca2+]i) [4]. These adverse effects are complex and may be interwoven. Of these effects, the disruption of calcium homeostasis could be the earliest and primary event, since Ca2+ ions are essential for various neuronal functions. The elevation of [Ca2+]i induces various apoptotic pathways.
There are several mechanisms that account for AβP-induced calcium dyshomeostasis [5–7]. Of these, we focus on the “amyloid channel hypothesis”—direct insertion into membranes of AβP, formation of channels (pores), and disruption of calcium homeostasis via unregulated cytotoxic channels may be the molecular basis of its neurotoxicity [8–10]. Other amyloidogenic disease-related proteins, such as the prion protein or α-synuclein, also exhibit similarities in the formation of amyloid channels and in the disruption of calcium homeostasis.
We review here the current understanding of the “amyloid channel hypothesis” based on our recent results and those of other researchers. It is widely recognized that the composition of membrane lipids influences the formation of amyloid channels by affecting the interaction between peptides and membranes. The possible development of new drugs by influencing membrane lipid properties and attenuating amyloid channels for the treatment and prevention of AD is also discussed.
2. Conformational Changes of AβP and Its Neurotoxicity
AβP is a small peptide with 39–43 amino acid residues. It is secreted by the cleavage of the N-terminal of a large precursor protein (amyloid precursor protein; APP) by β-secretase (β-site APP cleaving enzyme; BACE), followed by the intramembrane cleavage of its C-terminal by γ-secretase. This different C-terminal cleavage of APP causes various truncated AβPs, such as AβP(1–40), the first 40 amino acid residues, or AβP(1–42). Genetic studies of early-onset cases of familial AD indicated that APP mutations and AβP metabolism are associated with AD [11]. It was also revealed that mutations in the presenilin genes account for the majority of cases of early-onset familial AD [12]. Presenilins have been revealed to be γ-secretases [13], and their mutations influence the production of AβP and its neurotoxicity [14].
Yankner et al. reported that AβP(1–40) caused the death of cultured rat hippocampal neurons or neurodegeneration in the brains of experimental animals [15]. However, the neurotoxicity of AβP has been a subject of much debate because of its peculiar characteristics. AβP is a hydrophobic peptide with an intrinsic tendency to self-assemble to form oligomers (aggregates). In the aqueous solution, monomeric form of AβP exhibits a random coil structure. Meanwhile, under incubation at 37۫°C for several days (aging), AβPs form aggregates (oligomers) with β-pleated sheet structures. Pike et al. revealed that aged AβP(1–40) was considerably more toxic to cultured neurons as compared to freshly prepared AβP(1–40) [16]. The neurotoxicity of AβP was correlated with their β-sheet contents, as observed by circular dichroism (CD) spectroscopy [17]. Jarrett and Lansbury demonstrated that AβP forms oligomers by a nucleation-dependent process and that AβP(1–42) becomes “seeds” in the aggregates and enhances the oligomerization of AβP(1–40)—suggesting the significance of intracellular N- and C-terminal heterogeneity [18].
Recent detailed analysis using size-exclusion chromatography, gel electrophoresis, and atomic force microscopy (AFM) has demonstrated that there are several stable types of soluble oligomers: naturally occurring soluble oligomers (dimers or trimers), ADDLs (AβP-derived diffusible ligands), AβP globulomers, or protofibrils. Increasing evidence suggests that soluble amyloid oligomers cause synaptic and neuronal degeneration [19–21]. The identification of toxic AβP spices is crucial and has been a subject of scientific debates. Hartley et al. separated aggregated AβP(1–40) into low-molecular-weight (mainly monomer), protofibrillar, and fibril fractions by size-exclusion chromatography, and found that the protofibrillar fraction caused marked changes in the electrical activity of cultured neurons and neurotoxicity [22]. Walsh et al. reported that the naturally secreted (derived from the cerebrospinal fluid of AD patients), SDS-stable low-molecular-weight oligomers (dimers, trimers, or tetramers), but not AβP monomers or larger aggregates, inhibit long-term potentiation (LTP) and cause the loss of dendritic spines and synapses [23]. Lacor and colleagues reported that AβP-derived diffusible ligands (ADDLs) inhibited LTP and exhibited adverse effects on synaptic plasticity, such as abnormal spine morphology, decreased spine density, and decreased synaptic proteins [24]. Recently, Jan et al. found that mixtures of monomeric and heterogenous oligomers AβP(1–42) were more toxic than monomeric, protofibrillar fractions or fibril [25]. They demonstrated that AβP toxicity depends on the ability to grow and undergo fibril formation of prefibrillar aggregates and monomer. The process of fibril formation and its contribution to toxicity is complicated. Mature fibrils are regarded to be less toxic compared to soluble oligomers [26, 27], although there are some cases fibrils direct cause toxicity [28, 29]. It is possible that the toxicity of mature fibrils can result from the leakage of toxic short protofibrils or oligomers [27] or from its size-dependent mechanical properties of accumulations in the normal tissues [30].
As synaptic plasticity is crucial for the process of memory formation, synaptic degeneration (synaptotoxicity) is involved in the early stages of AD. Indeed, the number of synapses is strongly correlated with the level of memory impairment in AD patients, rather than the number of senile plaques or NFTs, [31]. Considering that AβP is secreted in the cerebrospinal fluid (CSF) of young individuals as well as in aged or dementia patients [32], factors that accelerate or inhibit the oligomerization may play essential roles in the pathogenesis of AD. Various factors, such as the concentration of peptides, the oxidations, mutations, and racemization of AβP, pH, composition of solvents, temperature, and trace elements, can influence the oligomerization processes [33]. Among these factors, Al and other trace elements are of particular interest because of the epidemiological link with AD [34].
3. AβP-Induced Neurotoxicity and the Disruption of Calcium Homeostasis
There is considerable interest regarding in the mechanism by which AβPs cause neurodegeneration. Of various adverse effects caused by AβP, calcium dyshomeostasis could be the earliest and primary adverse event, since Ca2+ ions are essential for various key enzymes such as kinases, phosphatases, and proteases. Once neuronal calcium homeostasis was disrupted and [Ca2+]i was changed, various apoptotic pathways such as calpain and caspase activation occurred, leading to neuronal death. The disruption of calcium homeostasis could trigger the membrane disruption, the formation of reactive oxygen species (ROS), and induce other adverse effects which are often observed after exposure to AβP. It is widely known that the increase in [Ca2+]i induced changes in the number of spines, their morphology, and the number of synapses [35]. Considering that AβP and APP coexist in the synapses [36], calcium imbalances in the synaptic compartment could directly influence neuronal activities and cause synaptic impairment (synaptotoxicity). Ca2+ is also implicated in the phosphorylation of the tau protein [37] or in APP sequestration [38]. Fibroblasts derived from AD patients exhibited different Ca2+ mobilization compared to those derived from age-matched control subjects [39]. Mounting evidence indicates that calcium dysregulation occurs in AD or in AβP-intoxicated neurons [40, 41].
There are several possible mechanisms by which AβPs interact with neurons and disrupt calcium homeostasis. Demuro et al. reviewed the AβP-induced calcium dyshomeostasis and its toxicity in the context of calcium signaling, and outlined three major mechanisms: the activation of some type of cell surface receptors coupled to Ca2+ influx, the disruption of membrane integrity, and the direct incorporation into the membrane to create unregulated cytotoxic channels (pores) [5].
AβPs were reported to bind to NMDA (N-methyl D-aspartate-)type or AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)-type glutamate receptors [42], or nicotinic acetylcholine receptors [43]. All of these receptors were highly Ca2+ permeable. Furthermore, AβP influences voltage gated Ca2+ channels [44] or inositol triphosphate (IP3) receptor [45]. It is widely recognized that presenilins are involved in capacitative Ca2+ entry, in ER Ca2+ signaling, or in mitochondrial Ca2+ signaling, and that their mutations affect the calcium-regulated functions [46–49]. Therefore, disturbances of ER Ca2+ stress or mitochondrial Ca2+ homeostasis may be involved in the pathogenesis of AD.
4. Channel Formation by AβP: Possible Mechanisms of Calcium Dyshomeostasis
In 1993, Arispe et al. first demonstrated that AβP(1–40) directly incorporates into artificial planar lipid bilayer membranes and forms cation selective ion channels [50, 51]. These “amyloid channels” were revealed to be giant multilevel pores and were permeable to Ca2+. Their activity was blocked by Zn2+, which is abundantly present in the brain [52]. Other neurotoxic peptide fragments of AβP, including AβP(25–35) and AβP(1–42), were reported to form calcium-permeable pores on artificial lipid bilayers as well as AβP(1–40) [53, 54]. The characteristics of amyloid channels formed by AβP(1–40) and AβP(1–42) exhibited similarities: multilevel and giant pores (~5 nS) and cation (including Ca2+) selectivity. The activity of both channels could be blocked by Zn2+. Fraser et al. reported that the toxic C-terminal fragment of APP(CT105; containing a full length of AβP) induced channel currents on membranes of Xenopus oocytes [55].
Durell et al. proposed a 3D structural model of amyloid channels obtained from a computer simulation of the secondary structure of AβP(1–40) in membranes, which showed 5 to 8 mers aggregating to form pore-like structures on the membranes [56]. Strodel et al. proposed a model of AβP(1–42) pores which consist of tetrameric and hexameric β-sheet subunits from the observations in NMR [57]. These models are consistent with morphological observations using high-resolution AFM that demonstrated that AβPs form pore-like structures on mica plates or on membranes [58–60].
A large number of studies have demonstrated that AβP directly binds to membranes, causes membrane perturbation or disruption, and induces the increase in permeability to ions (including Ca2+) or large molecules [61–64]. The findings of Demuro et al. are particularly interest in this context [65]. They investigated effects of AβP and other amyloid peptides in various aggregation states, and revealed that oligomeric peptides caused the rapid increase in [Ca2+]i or the membrane disruption, whereas monomers and fibrils did not.
Furthermore, the presence of pore-like structures of AβPs was demonstrated in the neuronal cell membrane of the brains of AD patients and of AD-model mice. Using high-resolution transmission electron microscopy, Inoue observed in situ AβP pores in the neuronal cell membrane in AD brains [66]. Kayed et al. reported that the annular protofibrils (APFs) of AβP exhibit ring-shaped and pore-like structures [67]. The age-dependent accumulation of APFs was observed on the membranes of AD model mice (APP transgenic mice; APP23) [68].
To determine whether or not AβPs form channels on neuronal cell membranes as well as on artificial lipid bilayers, we employed membrane patches from immortalized hypothalamic neurons (GT1-7 cells). GT1-7 cells are derived from murine hypothalamic neurons by site-directed tumorigenesis and exhibit various neuronal characteristics, such as the extension of neuritis, and the expression of various neuron-specific proteins or receptors [69]. Within 3–30 min of the addition of AβP(1–40) to the bath solution, the current derived from the amyloid channels appeared across the excised membrane patches [70]. However, AβP(40–1), a peptide bearing the reversed sequence of AβP(1–40), did not form any channels. The characteristics of amyloid channels formed on the GT1-7 cell membranes were considerably similar to those observed on artificial lipid bilayers: cation selective, multilevel, voltage independent, and long-lasting. Its channel activity was inhibited by the addition of Zn2+, and recovered by a zinc chelator–o-phenanthroline. Furthermore, Sepulveda et al. revealed that AβP(1–40) formed perforations on membranes excised from hippocampal neurons and induced currents [71]. The effect of AβP was similar to that of gramicidin and amphotericin which are commonly used to perforate neuron membranes.
5. Disruption of Calcium Homeostasis Caused by Amyloid Channels
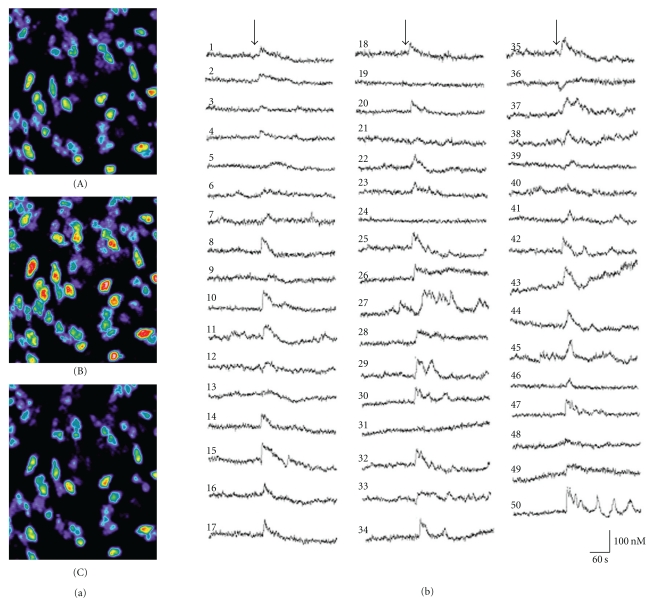
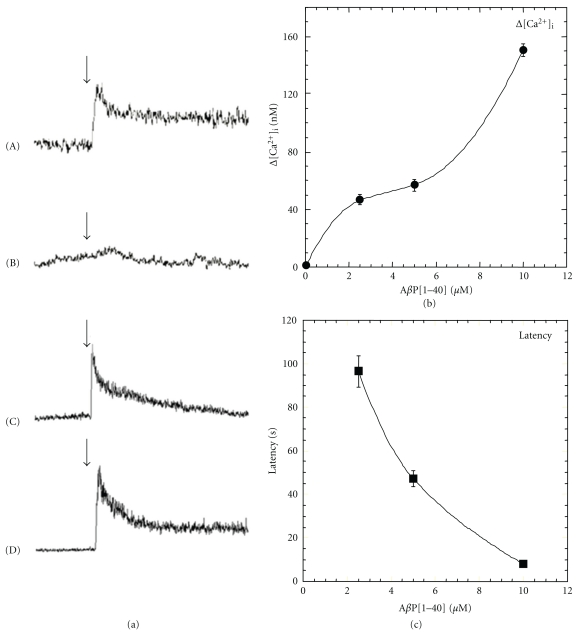
In order to test the validity of the amyloid channel hypothesis, we examined whether AβP alters the [Ca2+]i levels of GT1-7 cells under the same conditions, using a high-resolution multisite video imaging system with calcium-sensitive fluorescent dye, fura-2 [71–74]. Shown in Figure 1(a) are pseudocolor images of levels indicating the [Ca2+]i of GT1-7 cells before and after exposure to AβP(1–40). Shortly after exposure to AβP(1–40), a marked increase in [Ca2+]i occurred among many, but not all GT1-7 cells. Figure 1(b) depicts AβP(1–40)-induced temporal changes of the [Ca2+]i of 50 randomly chosen GT1-7 cells in the same field of view. Furthermore, we compared responses to AβP and the related peptides (Figure 2(a)). Although a marked increase in [Ca2+]i was caused by AβP(1–40) (line (A)) or by AβP(1–42) (line (C)), control peptides such as AβP(40-1) caused no remarkable changes (line (B)).
Figure 1.
Effects of AβP on temporal changes of [Ca2+]i. (a) Pseudocolor images of [Ca2+]i during exposure to AβP(1–40) in GT1-7 cells. A solution of AβP(1–40) (10 μM) was applied onto fura-2-loaded GT1-7 cells. Temporal changes of fluorescence intensities corresponding to increases in [Ca2+]i were analyzed. (A) 1 min before exposure to AβP(1–40); (B) 20 sec after exposure; (C) 5 min after exposure. (b) Temporal changes of randomly chosen 50 GT1-7 cells in the same field of view before and after the exposure to AβP(1–40) are depicted. The arrow indicates the time of peptide addition.
Figure 2.
Characteristics of AβP-induced elevations in [Ca2+]i. (a) Typical time course of [Ca2+]i prior to 2 min and after 3 min of the application of the peptide is depicted. Concentration is 10 μM for all peptides used. (A) AβP(1–40); (B) AβP(40-1); (C) AβP(1–42); (D) D-AβP(1–40). The arrow indicates the time of peptide addition. (b) and (c) Dose-dependence of the increase in [Ca2+]i. Typical responses of [Ca2+]i in cultured neurons following exposure to various concentrations of AβP(1–40) (2.5~10 μM). The peak increase in [Ca2+]i (Δ[Ca2+]i) in each cell (b) and the latency after exposure to AβP(1–40) were analyzed in more than 50 neurons in field of view (360 × 420 μm) cultured neurons (mean ± S.E.M., n = 300).
As previously discussed, there are several mechanisms that could account for the elevations in [Ca2+]i induced by AβP. However, our detailed quantitative analysis of the AβP-induced calcium influx suggests that AβP-induced [Ca2+]i changes occurred via unregulated amyloid channels and not by endogenous receptor-mediated pathways. This is supported by 4 major pieces of evidence.
First, the AβP-induced [Ca2+]i rise was highly heterogeneous among genetically identical GT1-7 cells. Even in the same field of view, exposure to the same peptide solution produced different change patterns in the [Ca2+]i levels as shown in Figure 1(b). Although AβP(1–40) induced an increase in the [Ca2+]i levels either instantly or after some delay, the magnitude and latency differed. Certain other adjacent cells still did not exhibit any responses. It is possible that the membrane binding of AβP is crucial for the cell-to-cell heterogeneity. Simakova and Arispe revealed that the surface phosphatidylserine and the cytosolic ATP levels are important determinants of the binding of AβP to membranes [75]. To analyze AβP-induced calcium influx quantitatively under the cell-to-cell heterogeneous condition, we compared the peak increase in [Ca2+]i (Δ[Ca2+]i) induced by AβPs and its latency (the lag between the [Ca2+]i increase and the time of AβP addition) in each cell. This multisite fluorometry system enables the simultaneous long-term observation of temporal changes in [Ca2+]i of more than 50 neurons. Second, the average Δ[Ca2+]i was increased in a dose-dependent manner of AβP, while the average latency decreased (Figures 2(b) and 2(c)). It is unlikely that the dose-dependent decrease in the latency occurs through the receptor-mediated pathways. These features are considerably similar to those observed in relation to peptide channels formed on membranes [71, 76]. The concentration of AβP required to form amyloid channels is higher (~μM) than the AβP concentration found in the brain. However, it is plausible that it requires a longer period for the lower concentration of AβP to cause changes in [Ca2+]i.
Third, the AβP-induced increase in [Ca2+]i was not influenced by the addition of the Na+ channel blocker (tetrodotoxin), the Ca2+ channel blocker (nifedipine), the antagonist of NMDA-type glutamate receptor (D-APV), or the antagonist of γ-aminobutyric acid (GABA) receptor (bicuculline) [77].
Fourth, D-AβP(1–40), AβP(1–40) composed of all D-amino acid residues, also caused the elevation of [Ca2+]i in a manner similar to AβP(1–40) (Figure 2(a) line (D)). This is consistent with the findings of Cribbs et al. suggesting that all-D-enantiomers of AβP possess the similar toxicity compared to all-L- AβP [78].
Therefore, it is plausible that AβP-induced [Ca2+]i changes occurred through amyloid channels by direct incorporation into membranes, but not through some receptor-mediated pathways.
These results strongly support the hypothetical idea termed “amyloid channel hypothesis,” namely, that the direct incorporation of AβPs and the subsequent imbalances of calcium and other ions through amyloid channels may be the primary event in AβP neurotoxicity [8–10].
6. Channel Formation and [Ca2+]i Influx by Other Amyloidogenic Peptides
Pore formation-induced cytotoxicity, such as in the cases of certain toxins or venoms, is commonly observed in our biological system. For example, the α-toxin of Staphylococcus aureus, which is secreted as a single-chain, water-soluble 33 kDa molecule, nonspecifically binds to membranes to form pore-like structures composed of hexamers with β-sheet structures, causing Ca2+ influx through the pores [79]. Magainin 2, a 26-residue antimicrobial peptide obtained from Xenopus laevis, forms transmembrane Ca2+-permeable pores on bacterial cell membranes [80]. Other antimicrobial peptides such as melitin (a bee venom composed of 28 amino acids), or antibiotics such as amphotericin and gramicidin were also reported to form transmembrane pores and to cause cell lysis [81]. In this respect, AβP and other amyloidogenic proteins might share the similar mechanism with these pore-forming peptides. Indeed, Soscia et al. demonstrated that AβP exerts antimicrobial activity against 8 common and clinically relevant microorganisms [82].
Furthermore, electrophysiological and morphological studies have revealed that other disease-related proteins—termed amyloidogenic proteins—exhibit similarities in the formation of amyloid channels as well as AβP.
Prion diseases, including human kuru, Creutzfeldt-Jakob disease, and bovine spongiform encephalopathy (BSE), are associated with the conversion of a normal prion protein (PrPC) to an abnormal scrapie isoform (PrP SC) [83]. The β-sheet region of PrP SC is suggested to play a crucial role in its transmissible degenerative processes. A peptide fragment of PrP corresponding to residues 106–126 (PrP106–126) coincides with the proposed β-sheet structures and has been reported to cause death in cultured hippocampal neurons [84]. Lin et al. reported that PrP106–126 forms cation permeable pores in artificial lipid bilayers [85]. The activity of PrP channels was also blocked by Zn2+. Kourie and Culverson investigated the detailed characteristics of channels formed by PrP106–126, concluding that it was directly incorporated into lipid bilayers and formed cation selective, copper-sensitive ion channels [86]. They also revealed that quinacrine, a potent therapeutic drug, possibly blocks amyloid channels induced by PrP106–126.
The aggregation and fibrillation of α-synuclein has been implicated in the formation of abnormal inclusions, termed Lewy bodies, and the etiology of dementia with Lewy bodies (DLB) [87]. Nonamyloid component (NAC), a fragment peptide of α-synuclein, accumulates in Alzheimer's senile plaques and causes apoptotic neuronal death [88]. Lashuel et al. demonstrated by electron microscope observation that α-synuclein forms annular pore-like structures [89].
The elongation of a polyglutamine-coding CAG triplet repeat in the responsible genes is based on the pathogenesis of triplet-repeat disease such as Huntington's disease or Machado-Joseph disease [90]. Hirakura et al. reported that polyglutamine formed ion channels in lipid bilayers [91]. Human amylin (IAAP, islet amyloid peptide) forms amyloid fibrils, accumulates in the islet of patients of type 2 diabetes mellitus, and causes cytotoxicity in islet cells or in cultured hippocampal neurons. However, rat amylin did not cause cytotoxicity nor form β-sheet structures, in spite of the 95% similarity in the amino acid sequence [92]. Mirzabeko et al. revealed that human amylin formed ion channels on liposomes, but rat amylin did not [93]. Calcitonin is a 32-amino acid polypeptide hormone, which is produced by the thyroid C-cells. It is involved in calcium homeostasis and is associated with medullary carcinoma of the thyroid [94]. Using transmission electron microscopy (TEM) observation on liposome, Diociaiuti et al. found that calcitonin oligomers exhibit annular pore-like structures [95]. Lal et al. investigated the oligomerization and conformational changes of AβP, synuclein, amylin, and other amyloidogenic proteins using gel electrophoresis and AFM imaging, and demonstrated that these amyloidogenic proteins form annular channel-like structures on bilayer membranes [96].
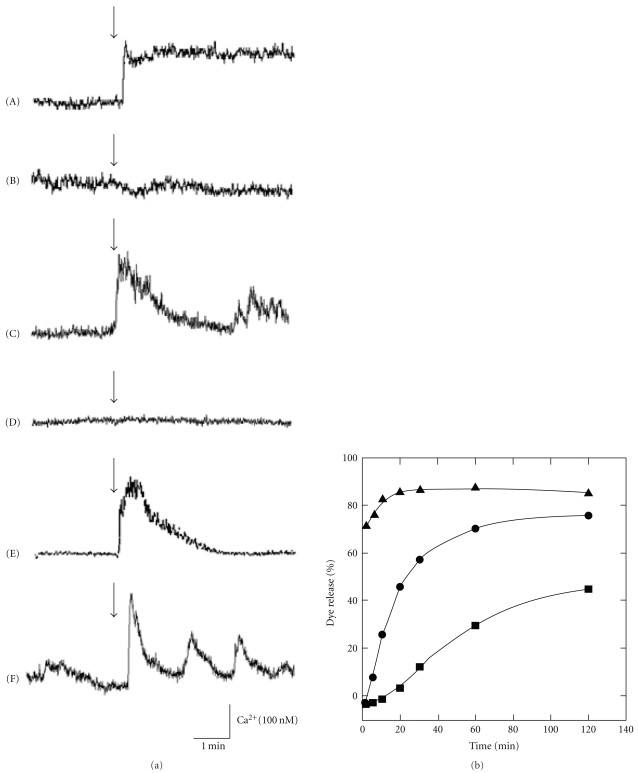
We have demonstrated that these amyloidogenic peptide also cause the elevations in [Ca2+]i as well as AβP (Figure 3(a)). A marked increase in [Ca2+]i was caused by PrP106–126 (line (A)), human amylin (line (C)), NAC (line (E)) and AβP(1–40) (Figure 2(a), line (A)) or pore-forming antimicrobial peptide magainin 2 (line (F)). However, control peptides such as peptide with random sequence of PrP106–126 (scramble PrP106–126) (line (B)) and rat amylin (line (D)) caused no remarkable changes. Furthermore, PrP106–126 and human amylin, as well as AβP(1–40), cause disruption of liposome membranes and induce dye release (Figure 3(b)).
Figure 3.
Effects of amyloidogenic proteins on membrane disruption and [Ca2+]i elevations. (a) Effects of amyloidogenic proteins and their analogues on [Ca2+]i. Typical time course of [Ca2+]i prior to 2 min and after 3 min of the application of the peptide is depicted. Concentration is 10 μM for all peptides used. (A) PrP106–126; (B) scramble PrP106–126; (C) human amylin; (D) rat amylin; (E) NAC; (F) magainin 2. The arrow indicates the time of peptide addition. (b) Membrane disruption by amyloidogenic peptides. AβP(1–40) (closed circle), PrP106–126 (closed square), and human amylin (open circle) (each 10 μM) were added to negatively charged liposomes containing carboxyfluorescein. The ratio of DPPC (dipalmitoil phosphatidyl choline): CHOL (cholesterol): DPPG (dipalmitoil phosphatidyl glycerol) in the liposome was 3 : 4 : 3. The temporal changes of the fluorescence intensity were monitored. The ratio of the released fluorescent dye (carboxy fluoresein; CF) compared to the total amount of CF was described as the percentage of membrane disruption.
These diseases are included in “conformational disease” (protein misfolding disease)—the conformational change of amyloidogenic proteins is suggested to be an important determinant of its toxicity and, consequently, the development of the disease [97]. The disease-related amyloidogenic proteins exhibit similarities in the formation of β-pleated sheet structures, abnormal deposition as amyloid fibrils in the tissues, and introduction of apoptotic degeneration. As shown in Table 1, these amyloidogenic proteins exhibit similarities in the direct incorporation into membranes, formation of calcium-permeable ion channels, and induction of abnormal elevation of [Ca2+]i. It is strongly suggested that disruption of calcium homeostasis via unregulated amyloid channels formed by these disease-related proteins may be the molecular basis of neurotoxicity of these diseases.
Table 1.
Characteristics of amyloidogenic proteins and the related peptides.
| Disease | Amyloidogenic protein or its fragment peptide and the primary sequence | β-sheet formation | Cytotoxicity | Channel formation | [Ca2+]i rise |
|---|---|---|---|---|---|
| Alzheimer's disease | AβP(1–40) | ||||
| DAEFRHDSGYEVHHQKLVFFAE | + | + | + | + | |
| DVGSNKGAIIGLMVGGVV | |||||
| AβP(40-1) | |||||
| VVGGVMLGIIAGKNSGVDEAFFV | – | – | – | – | |
| LKQHHVEYGSDHRFEAD | |||||
| AβP(25-35) | |||||
| DVGSNKGAII | + | + | + | + | |
| AβP(1–42) | |||||
| DAEFRHDSGYEVHHQKLVFFAEDV | + | + | + | + | |
| GSNKGAIIGLMVGGVVIA | |||||
| Prion disease | PrP106–126 (prion protein fragment) | ||||
| KTNMKHMAGAAAAGAVVGGLG | + | + | + | + | |
| Scramble PrP106–126 | |||||
| NGAKALMGGHGATKVMVGAAA | – | – | – | – | |
| Parkinson's disease (DLB; diseases with Lewy bodies) |
α-synuclein NAC (a fragment of α-synuclein) | ||||
| EQVTNVGGAVVTGVTAVAQKTVEGAGSIAAA TGFV | + | + | + | + | |
| Triplet-repeat disease | Polyglutamine | ||||
| QQQQQQQQ— | + | + | + | n.d. | |
| Diabetes mellitus | Human amylin | ||||
| KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY | + | + | + | + | |
| Rat amylin | |||||
| KCNTATCATQRLANFLVRSSNNLGPVLPPTNVGSNTY | – | – | – | – | |
| Medullary carcinoma of the thyroid | Calcitonin | ||||
| CGNLSTCMLGTYTQDFNKFHTFPQTAIGVGAP | + | + | + | + | |
n.d.: not determined.
7. Role of Membrane Lipids in the Formation of Amyloid Channels
It is widely accepted that the direct incorporation of peptides into membranes and consequent channel formation is strongly affected by the membrane lipid composition, particularly the net charges of membrane surfaces and membrane fluidity. Several AβP residues (such as Arg5, Lys16, and Lys28) have a positive charge at neutral pH, and therefore, AβP has an affinity for negatively charged phospholipids, such as phosphatidylserine (PS) or phosphatidylglycerol (PG), but not for neutral phospholipids, such as phosphatidylcholine (PC) [98]. However, membrane phospholipid distribution is asymmetrical in mammals: neutral lipids (PC, etc.) usually exist on the outer surfaces of plasma membranes, whereas negatively charged phospholipids (PS, etc.) exist in the inner surfaces of the membranes. Thus, the binding of AβP to neuronal membrane surface may seldom occur in normal and young brains.
Further influencing the binding of AβP to membranes are gangliosides—sialic-acid-bearing glycophospholipids. Both APP and AβP are localized in detergent-insoluble, cholesterol-, sphingomyelin-, and ganglioside-rich lipid microdomains, termed rafts [99]. Yanagisawa et al. first demonstrated the existence of membrane-bound AβP tightly bound to GM1 gangliosides in the brains of AD patients [100]. AβP binds to GM1 gangliosides in raft-like membranes in vitro, and GM1-bound AβP behave as a “seed” and accelerate the oligomerization of AβP [101]. Numerous studies have indicated the implication of gangliosides in the oligomerization and the binding to the membrane of AβP [102–104].
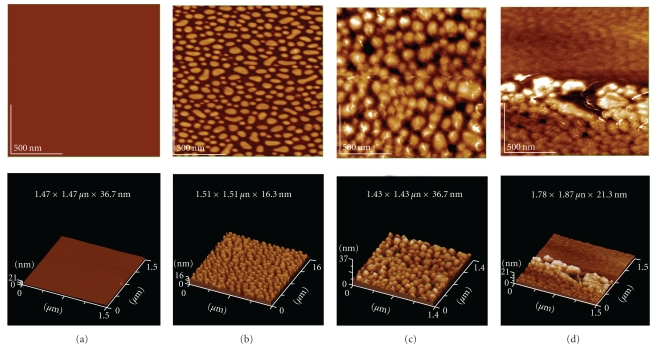
We have observed the deposition of AβP(1–40) on ganglioside (GM1)/phospholipid (dipalmitoil phosphatidyl choline; DPPC) monolayers by AFM imaging (Figure 4). GM1-DPPC membranes exhibit distinctive, island-like GM1 domains embedded in the DPPC matrix [105, 106]. Aged AβP(1–40) deposited and tightly bound to the membrane surfaces and exhibited the damaged structures of membranes, meanwhile freshly prepared AβP showed few changes.
Figure 4.
AFM images of AβP(1–40) on monolayer membranes. Lipid monolayer membranes composed by DPPC (a) or ganglioside GM1-DPPC (dipalmitoil phosphatidyl choline) (b)~(d) were prepared by bath sonication and reconstitution on mica plates. The ratio of GM1:DPPC was 8 : 2. AFM images were obtained after the exposure to freshly prepared AβP(1–40) (c) or aged AβP(1–40) (d). Scale area: 1.5 × 1.5 μm.
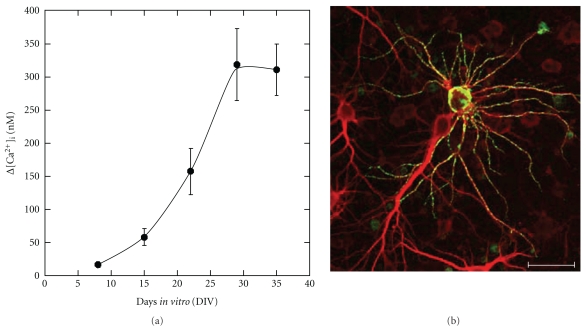
We have previously demonstrated that AβP causes a marked increase in [Ca2+]i in a large proportion of long-term (30–35 days in vitro; DIV) cultured hippocampal neurons. However, few or no changes were observed in [Ca2+]i in short-term (8 DIV) cultures (Figure 5(a)) [107]. After several days of exposure to sublethal levels of AβP(1–40) to long-term cultured neurons, AβP binds to some restricted hippocampal neurons and exhibits dotlike depositions on the somata and dendrites. Meanwhile, there is no detectable AβP deposition on the surfaces of neighboring neurons, despite the morphological similarities of these neurons (Figure 5(b)). Malchiodi-Albedi et al. found that lipid rafts increased during the maturation of culture periods of primary cultured rat hippocampal neurons [108]. They demonstrated that Calcitonin, an amyloidogenic peptide, causes [Ca2+]i changes in mature raft-containing neurons, but not in immature cultured neurons. Williamson et al. found that AβP was not uniformly distributed over the neuronal processes, and was colocalized with GM1 ganglioside [109]. These features are consistent with our results, and it is possible that gangliosides in lipid rafts may regulate the binding of AβP into membranes and its neurotoxicity.
Figure 5.
(a) Maturation-dependent increase in AβP-induced [Ca2+]i changes in primary cultured neurons. (b) Heterogeneous affinity of AβP to mature cultured hippocampal neurons. Long-term cultured rat hippocampal neurons were exposed to 1 μM of AβP(1–40) at 29 DIV and fixed after 4 days. Neurons were double immunostained by anti-MAP2 antibody (Texas Red, red) and anti-AβP antibody (FITC, green), and observed by Laser confocal microscopy. Scale bar represents 50 μm. (modified from [100]).
It is widely accepted that cholesterol enhances membrane stiffness, decreases membrane fluidity, and inhibits pore formation by pore-forming peptides [110]. Lin and Kagan found that cholesterol inhibits channel formation by AβP [111]. Cholesterol blocks AβP-induced elevations in [Ca2+]i [41, 72], aggregation of AβP-containing liposomes [112], and AβP cytotoxicity [112, 113]. Moreover, cholesterol attenuates AβP-induced membrane-disordering effects and calcium increase [114]. Considering that apolipoprotein E, involved in cholesterol transport and metabolism, is present in the senile plaques and NFTs in AD brains and its polymorphism is a risk factor of AD [115], the implication of cholesterol in AD pathogenesis is crucial.
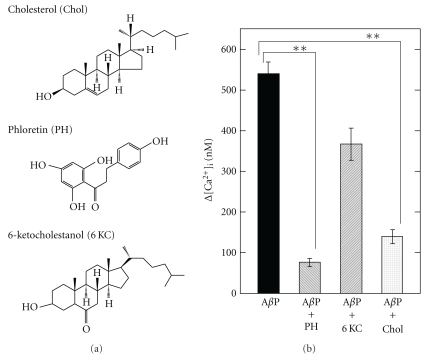
To determine the implications of membrane properties in the formation of amyloid channels, we tested the effects of several lipophilic substances, which modulate membrane properties, on AβP-induced [Ca2+]i elevations [74, 107]. Phloretin, a plant-derived flavonoid, decreases membrane dipole potential, and inhibits the electrostatic interaction between AβP and membrane lipids, and attenuates AβP-induced neurotoxicity [116]. Meanwhile, 6-ketocholestanol increases the magnitude of the membrane dipole potential and decreases membrane fluidity [117]. Figure 6 shows that the preadministration of phloretin and cholesterol markedly inhibited AβP-induced [Ca2+]i elevations; meanwhile, 6-ketocholestanol did not cause significant changes, despite the structural similarity to cholesterol. Therefore, as expected from other findings, the net charges of membrane surfaces and the membrane fluidity play crucial roles in the elevations of [Ca2+]i caused by AβP.
Figure 6.
Effect of membrane charges and fluidity on AβP-induced [Ca2+]i rise. The solutions of phloretin (PH), 6-ketocholestanol (KC), and cholesterol (Chol) were preadministrated on GT1-7 cells; and AβP-induced [Ca2+]i rise was analyzed. Data are mean ± S.E.M., n = 250, **P < .001. (modified from [67]).
Furthermore, numerous studies have demonstrated that gangliosides and cholesterol are implicated in the channel formation of other amyloidogenic proteins. Lipid rafts are considered to be the compartment where the conformational change of PrP occurs [118]. Gangliosides influence the β-sheet formation of PrP106–126 [119] and human amylin [120], or the channel formation of α-synuclein [121]. Cholesterol also inhibits channel formation by human amylin [122].
8. Possible Candidate for the Treatment of AD
The search for protective agents against AβP neurotoxicity is of great importance. Such agents include inhibitors of AβP oligomerization, inhibitors of BACE or γ-secretase, AβP vaccines, and chelators of trace metals; all have been proposed to be effective in the treatment of AD.
Here, we have focused on substances that inhibit the formation of amyloid channels. As discussed, the elevation of [Ca2+]i by permeation through amyloid channels is considered to be the primary event of AβP neurotoxicity; therefore, such compounds could serve as the seed of new effective drugs with fewer adverse effects.
Zn2+ ion, which is abundant in vesicles of presynaptic terminals and is secreted into synaptic clefts with neuronal excitation, inhibits the currents induced by amyloid channels [52, 54, 70]. Zn2+ binds to His residues of AβP: Arispe et al. found that histidine-related peptide derivatives such as His-His or polyhistidine are effective in the inhibition of amyloid channels, the attenuation of AβP-induced [Ca2+]i changes, and the protection of neurons from AβP toxicity [123, 124]. They developed several small amphiphilic pyridinium derivatives which inhibit formation of AβP channels and its neurotoxicity [8, 125].
In line with the search for protective agents, we have screened compounds, which influence membrane properties and inhibit formations of amyloid channels, by observing the AβP-induced Ca2+ influx. Among those tested, we found that several lipophilic substances, such as 17β-estradiol, 17α-estradiol, and neurosteroids (including dehydroepiandrosterone [DHEA], DHEA sulfate [DHEA-S], and pregnenolone) significantly inhibit AβP-induced [Ca2+]i elevation [74, 107]. 17β-estradiol, a female hormone, is neuroprotective and affects membrane fluidity [126]. Considering that both 17β-estradiol and 17α-estradiol inhibit AβP-induced [Ca2+]i elevation, the inhibition may not depend on their genomic actions but on their membrane-modifying effects. Neurosteroids are steroid hormones synthesized de novo in the central nervous system from cholesterol or from peripheral steroid precursors [127]. Several lines of evidence suggest that neurosteroids modulate various functions of the brain and exhibit neuroprotective activities [128]. Considering that concentrations of plasma DHEA are reduced in AD patients [129], the implication of neurosteroids in the pathogenesis of AD may be important.
9. Amyloid Channel Hypothesis
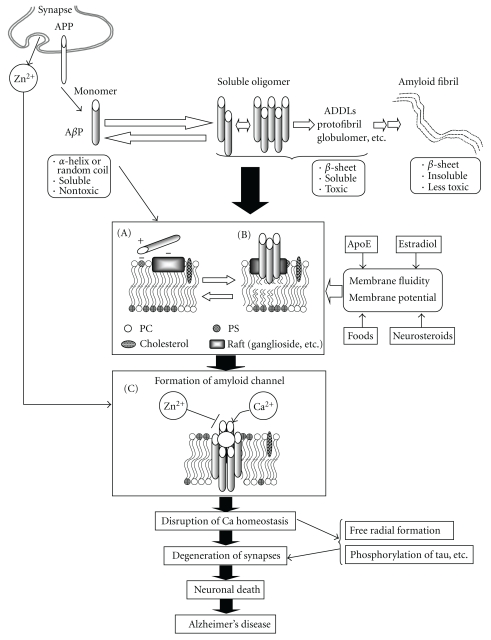
Based on the results of our studies, together with those of other studies, we propose the following hypothetical scheme: that unregulated calcium influx via amyloid channels may underlie the molecular mechanism of AβP neurotoxicity and the pathogenesis of AD (Figure 7).
Figure 7.
Hypothesis concerning amyloid channels and pathogenesis of Alzheimer's disease. AβPs are secreted from APP in synapses, directly incorporated into membranes. The possible hypothetical scheme of the formation of oligomeric amyloid channels is depicted. Details are shown in the text.
AβPs are normally secreted from APP, which exists in the synapse, into the cerebrospinal fluid or synaptic clefts. Secreted AβPs are degraded proteolytically by proteases, such as neprilysin [130], within a short period. However, upregulation of the AβP secretion from APP, or an increased ratio of AβP(1–42) to AβP(1–40) may render AβPs liable to be retained in the brain. Mutations of APP or presenilin gene promote this process. The binding of AβP to neuronal membranes is the important determinant for its neurotoxicity. Since AβP seldom binds to normal neuronal membranes with neutral phospholipids such as PC usually existing on the outer surfaces of plasma membranes, it would be less likely to occur in the brains of normal and young subjects. However, when the asymmetrical distribution is disrupted by apoptotic conditions or aging and negatively charged phospholipids such as PS appear on the outer membrane surfaces, AβPs can bind to membrane surfaces (Figure 7(a)). Furthermore, considering that AβPs have affinity to PS in inner membrane surfaces, the intraneuronal accumulation of AβPs may be more toxic [131]. Gangliosides also contribute to the net charge of the outer membrane surface and to the binding to AβPs (Figure 7(b)). Microcircumstances on the membranes, such as lipid rafts, provide suitable locations which facilitate this process from (A) to (B). After incorporation into the membrane, the conformation of AβPs change and the accumulated AβPs aggregate on the membranes. The ratio of cholesterol to phospholipids in the membrane may alter membrane fluidity, thereby affecting these processes. Finally, aggregated AβP oligomers form ion channels leading to the various neurodegenerative processes (Figure 7(C)).
The velocity of channel formation will be regulated by the binding of AβP on membranes and its concentration. Considering that soluble oligomers are more toxic compared to monomer or fibrils [26, 27, 65], it is provable that AβP oligomerization in vitro accelerates the velocity from (A) to (B), and enhances the formation of tetrameric or hexameric pores on membranes. Indeed, O'Nuallain et al. demonstrated that AβP dimers formed toxic protofibrils more rapidly compared to monomer [132]. However, the proposed structures of AβP channels in membrane mimic conditions are not always similar to the structures formed in the solution such as protofibrils or soluble oligomers. Thus, the conformational changes in membranes may also be significant.
These processes required for channel formation ((A) to (C)) may require a long life span in general and determine the rate of the entire process. Unlike endogenous Ca2+ channels, these AβP channels are not regulated by usual blockers. Thus, once formed on membranes, a continuous flow of [Ca2+]i is initiated.
Disruption of calcium homeostasis triggers several apoptotic pathways and promotes numerous degenerative processes, including free radical formation and tau phosphorylation, thereby accelerating neuronal death. The source of Ca2+ may be from extracellular or intracellular Ca2+ store (ER or mitochondoria). Considering that presenilins are involved in the capacitive calcium entry, in Ca2+ homeostasis in ER or in mitochondoria [46–49] and the implication of ER stress in AD and other neurodegenerative diseases [133], mutations of presenilins may influence these pathways. Free radicals also induce membrane disruption, by which unregulated calcium influx is further amplified. The disruption of calcium homeostasis influences the production and processing of APP. Thus, a vicious spiral of neurodegeneration is initiated. Meanwhile, zinc ions, which are secreted into synaptic clefts in a neuronal activity-dependent manner, inhibit AβP-induced Ca2+ entry, and thus have a protective function in AD.
This hypothesis explains the long delay in AD development; AD occurs only in senile subjects despite the fact that AβPs are also normally secreted in younger or in normal subjects. AD is multifactorial disease. Various environmental factors, such as foods (cholesterol contents) or trace metals, as well as genetic factors will influence these processes and contribute to AD pathogenesis. The amyloid channel hypothesis could explain effects of environmental factors such as cholesterol and other various aspects of AD pathogenesis and may aid in improving a precise understanding of AD and in the development of drugs for AD treatment. Although the findings of channel-like structures in vivo [66, 68], it is difficult to determine whether these amyloid channels really exist in the brains of AD patients. Therefore, further in vivo studies are necessary.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Grant from the Cooperation for Innovative Technology and Advanced Research in Evolutional Area (city area) by the Miyazaki Prefectural Industrial Support Foundation.
Abbreviations
- AD:
Alzheimer's disease,
- AβP:
Alzheimer's β-amyloid protein
- AFM:
Atomic force microscopy
- AMPA:
α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid
- APP:
Amyloid precursor protein
- D-APV:
2-amino-5-phosphonovalerate
- BACE:
β-site APP cleaving enzyme
- ER:
Endoplasmic reticulum
- LTP:
Long-term potentiation
- NFT:
Neurofibrillary tangles
- [Ca2+]i:
Intracellular calcium levels
- NMDA:
N-methyl-D-aspartate
- ROS:
Reactive oxygen species
- SDS:
Sodium dodecyl sulfate
- TTX:
Tetrodotoxin.
References
- 1.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: intraneuronal accumulation of the β-amyloid peptide—the first step of a fatal cascade. Journal of Neurochemistry. 2004;91(3):513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 4.Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Aβ toxicity: from top to bottom. Nature Reviews Neuroscience. 2001;2(8):595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 5.Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. Journal of Biological Chemistry. 2010;285(17):12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium. 2010;47(2):183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camandola S, Mattson MP. Aberrant subcellular neuronal calciumregulation in aging and Alzheimer's disease. doi: 10.1016/j.bbamcr.2010.10.005. Biochimica et Biophysica Acta. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arispe N, Diaz JC, Simakova O. Aβ ion channels. Prospects for treating Alzheimer’s disease with Aβ channel blockers. Biochimica et Biophysica Acta. 2007;1768(8):1952–1965. doi: 10.1016/j.bbamem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Lashuel HA, Lansbury PT., Jr. Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Quarterly Reviews of Biophysics. 2006;39(2):167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara M, Negishi-Kato M, Sadakane Y. Calcium dyshomeostasis and neurotoxicity of Alzheimer’s β-amyloid protein. Expert Review of Neurotherapeutics. 2009;9(5):681–693. doi: 10.1586/ern.09.28. [DOI] [PubMed] [Google Scholar]
- 11.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 12.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131(2):215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nature Reviews Neuroscience. 2008;9(10):768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 15.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 16.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Research. 1991;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 17.Simmons LK, May PC, Tomaselli KJ, et al. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Molecular Pharmacology. 1994;45(3):373–379. [PubMed] [Google Scholar]
- 18.Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 19.Walsh DM, Selkoe DJ. Aβ oligomers—a decade of discovery. Journal of Neurochemistry. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 20.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiology of Aging. 2006;27(4):570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Klein WL, Stine WB, Teplow DB. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiology of Aging. 2004;25(5):569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Hartley DM, Walsh DM, Ye CP, et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. Journal of Neuroscience. 1999;19(20):8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39(35):10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 24.Lacor PN, Buniel MC, Furlow PW, et al. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. Journal of Neuroscience. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jan A, Adolfsson O, Allaman I, et al. Aß42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Aß42 species. Journal of Biological Chemistry. 2011;286(10):8585–8596. doi: 10.1074/jbc.M110.172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annual Review of Neuroscience. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 27.Stefani M. Biochemical and biophysical features of both oligomer/fibril and cell membrane in amyloid cytotoxicity. FEBS Journal. 2010;277(22):4602–4613. doi: 10.1111/j.1742-4658.2010.07889.x. [DOI] [PubMed] [Google Scholar]
- 28.Novitskaya V, Bocharova OV, Bronstein I, Baskakov IV. Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. Journal of Biological Chemistry. 2006;281(19):13828–13836. doi: 10.1074/jbc.M511174200. [DOI] [PubMed] [Google Scholar]
- 29.Engel MFM, Khemtémourian L, Kleijer CC, et al. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Z, Paparcone R, Buehler MJ. Alzheimer’s Aβ(1–40) amyloid fibrils feature size-dependent mechanical properties. Biophysical Journal. 2010;98(10):2053–2062. doi: 10.1016/j.bpj.2009.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 32.Fukuyama R, Mizuno T, Mizuno T, et al. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer’s disease patients. European Neurology. 2000;43(3):155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- 33.Kawahara M. Role of calcium dyshomeostasis via amyloid channels in the pathogenesis of Alzheimer’s disease. Current Pharmaceutical Design. 2010;16:2779–2789. doi: 10.2174/138161210793176545. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. Journal of Alzheimer’s Disease. 2005;8(2):171–182. doi: 10.3233/jad-2005-8210. [DOI] [PubMed] [Google Scholar]
- 35.Kato-Negishi M, Muramoto K, Kawahara M, Hosoda R, Kuroda Y, Ichikawa M. Bicuculline induces synapse formation on primary cultured accessory olfactory bulb neurons. European Journal of Neuroscience. 2003;18(6):1343–1352. doi: 10.1046/j.1460-9568.2003.02901.x. [DOI] [PubMed] [Google Scholar]
- 36.Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The amyloid precursor protein and its regulatory protein, FE65, in growth cones and synapses in vitro and in vivo. Journal of Neuroscience. 2003;23(13):5407–5415. doi: 10.1523/JNEUROSCI.23-13-05407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson GVW. Tau phosphorylation and proteolysis: insights and perspectives. Journal of Alzheimer’s Disease. 2006;9(3):243–250. doi: 10.3233/jad-2006-9s326. [DOI] [PubMed] [Google Scholar]
- 38.Gordon-Krajcer W, Gajkowska B. Excitotoxicity-induced expression of amyloid precursor protein (β-APP) in the hippocampus and cortex of rat brain. An electron-microscopy and biochemical study. Folia Neuropathologica. 2001;39(3):163–173. [PubMed] [Google Scholar]
- 39.Gasparini L, Racchi M, Binetti G, et al. Peripheral markers in testing pathophysiological hypotheses and diagnosing Alzheimer’s disease. FASEB Journal. 1998;12(1):17–34. doi: 10.1096/fasebj.12.1.17. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP, Barger SW, Cheng B, Lieberburg I, Smith-Swintosky VL, Rydel RE. β-Amyloid precursor protein metabolites and loss of neuronal Ca homeostasis in Alzheimer’s disease. Trends in Neurosciences. 1993;16(10):409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann H, Eckert A, Müller WE. Disturbances of the neuronal calcium homeostasis in the aging nervous system. Life Sciences. 1994;55(25-26):2011–2018. doi: 10.1016/0024-3205(94)00381-5. [DOI] [PubMed] [Google Scholar]
- 42.Alberdi E, Sánchez-Gómez MV, Cavaliere F, et al. Amyloid β oligomers induce Ca dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47(3):264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Parri HR, Dineley KT. Nicotinic acetylcholine receptor interaction with β-amyloid: molecular, cellular, and physiological consequences. Current Alzheimer Research. 2010;7(1):27–39. doi: 10.2174/156720510790274464. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JH, Pike CJ, Cotman CW. Ca2+ channel blockers attenuate β-amyloid peptide toxicity to cortical neurons in culture. Journal of Neurochemistry. 1994;62(1):372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheung KH, Mei L, Mak DOD, et al. Gain-of-function enhancement of IP receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Science Signaling. 2010;3(114):p. ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Querfurth HW, LaFerla FM. Alzheimer’s disease. New England Journal of Medicine. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 47.Leuner K, Hauptmann S, Abdel-Kader R, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxidants and Redox Signaling. 2007;9(10):1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 48.Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. Journal of Biological Chemistry. 2003;278(4):2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 49.Green KN, Demuro A, Akbari Y, et al. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid β production. Journal of Cell Biology. 2008;181(7):1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid β- protein [AβP-(1–40)] in bilayer membranes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(22):10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arispe N, Pollard HB, Rojas E. Zn2+ interaction with Alzheimer amyloid β protein calcium channels. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(4):1710–1715. doi: 10.1073/pnas.93.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirzabekov T, Lin MC, Yuan WL, et al. Channel formation in planar lipid bilayers by a neurotoxic fragment of the beta-amyloid peptide. Biochemical and Biophysical Research Communications. 1994;202(2):1142–1148. doi: 10.1006/bbrc.1994.2047. [DOI] [PubMed] [Google Scholar]
- 54.Rhee SK, Quist AP, Lal R. Amyloid β protein-(1–42) forms calcium-permeable, Zn-sensitive channel. Journal of Biological Chemistry. 1998;273(22):13379–13382. doi: 10.1074/jbc.273.22.13379. [DOI] [PubMed] [Google Scholar]
- 55.Fraser SP, Suh YH, Chong YH, Djamgoz MBA. Membrane currents induced in Xenopus oocytes by the C-terminal fragment of the β-amyloid precursor protein. Journal of Neurochemistry. 1996;66(5):2034–2040. doi: 10.1046/j.1471-4159.1996.66052034.x. [DOI] [PubMed] [Google Scholar]
- 56.Durell SR, Guy HR, Arispe N, Rojas E, Pollard HB. Theoretical models of the ion channel structure of amyloid β-protein. Biophysical Journal. 1994;67(6):2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strodel B, Lee JWL, Whittleston CS, Wales DJ. Transmembrane structures for Alzheimer's Aβ1–42 oligomers. Journal of the American Chemical Society. 2010;132(38):13300–13312. doi: 10.1021/ja103725c. [DOI] [PubMed] [Google Scholar]
- 58.Lin H, Bhatia R, Lal R. Amyloid β protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB Journal. 2001;15(13):2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- 59.Jang H, Arce FT, Ramachandran S, Capone R, Lal R, Nussinov R. β-barrel topology of Alzheimer's β-Amyloid ion channels. Journal of Molecular Biology. 2010;404(5):917–934. doi: 10.1016/j.jmb.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang H, Zheng J, Nussinov R. Models of beta-amyloid ion channels in the membrane suggest that channel formation in the bilayer is a dynamic process. Biophysical Journal. 2007;93:1938–1949. doi: 10.1529/biophysj.107.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckert GP, Wood WG, Müller WE. Membrane disordering effects of beta-amyloid peptides. Sub-cellular biochemistry. 2005;38:319–337. doi: 10.1007/0-387-23226-5_16. [DOI] [PubMed] [Google Scholar]
- 62.Mattson MP, Begley JG, Mark RJ, Furukawa K. Aβ25-35 induces rapid lysis of red blood cells: contrast with aβ1–42 and examination of underlying mechanisms. Brain Research. 1997;771(1):147–153. doi: 10.1016/s0006-8993(97)00824-x. [DOI] [PubMed] [Google Scholar]
- 63.McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. Journal of Biological Chemistry. 1996;271(43):26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- 64.Blanc EM, Toborek M, Mark RJ, Hennig B, Mattson MP. Amyloid β-peptide induces cell monolayer albumin permeability, impairs glucose transport, and induces apoptosis in vascular endothelial cells. Journal of Neurochemistry. 1997;68(5):1870–1881. doi: 10.1046/j.1471-4159.1997.68051870.x. [DOI] [PubMed] [Google Scholar]
- 65.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. Journal of Biological Chemistry. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 66.Inoue S. In situ Aβ pores in AD brain are cylindrical assembly of Aβ protofilaments. Amyloid. 2008;15(4):223–233. doi: 10.1080/13506120802524858. [DOI] [PubMed] [Google Scholar]
- 67.Kayed R, Pensalfini A, Margol L, et al. Annular protofibrils area structurally and functionally distinct type of amyloid oligomer. Journal of Biological Chemistry. 2009;284(7):4230–4237. doi: 10.1074/jbc.M808591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kokubo H, Kayed R, Glabe CG, et al. Amyloid ß annular protofibrils in cell processes and synapses accumulate with aging and Alzheimer-associated genetic modification. International Journal of Alzheimer's Disease. 2009;2009:7 pages. doi: 10.4061/2009/689285. Article ID 689285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 70.Kawahara M, Arispe N, Kuroda Y, Rojas E. Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive, cation- selective channels across excised membrane patches from hypothalamic neurons. Biophysical Journal. 1997;73(1):67–75. doi: 10.1016/S0006-3495(97)78048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sepulveda FJ, Parodi J, Peoples RW, Opazo C, Aguayo LG. Synaptotoxicity of Alzheimer ß amyloid can be explained by its membrane perforating property. PLoS One. 2010;27, article e11820 doi: 10.1371/journal.pone.0011820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawahara M, Arispe N, Kuroda Y, Rojas E. Alzheimer's ß-amyloid, human islet amylin and prion protein fragment evoke intracellular free-calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell-line. Journal of Biological Chemistry. 2000;275:14077–14083. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- 73.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Research Bulletin. 2000;53(4):389–397. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 74.Kawahara M, Kuroda Y. Intracellular calcium changes in neuronal cells induced by Alzheimer’s β-amyloid protein are blocked by estradiol and cholesterol. Cellular and Molecular Neurobiology. 2001;21(1):1–13. doi: 10.1023/A:1007168910582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simakova O, Arispe NJ. The cell-selective neurotoxicity of the Alzheimer’s Aβ peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Aβ toxicity. Journal of Neuroscience. 2007;27(50):13719–13729. doi: 10.1523/JNEUROSCI.3006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomita T, Watanabe M, Yasuda T. Influence of membrane fluidity on the assembly of Staphylococcus aureus α-toxin, a channel-forming protein, in liposome membrane. Journal of Biological Chemistry. 1992;267(19):13391–13397. [PubMed] [Google Scholar]
- 77.Kawahara M. Disruption of calcium homeostasis in the pathogenesis of Alzheimer's disease and other conformational diseases. Curr Alzheimer Res. 2004;1(2):87–95. doi: 10.2174/1567205043332234. [DOI] [PubMed] [Google Scholar]
- 78.Cribbs DH, Pike CJ, Weinstein SL, Velazquez P, Cotman CW. All-D-enantiomers of β-amyloid exhibit similar biological properties to all-L-β-amyloids. Journal of Biological Chemistry. 1997;272(11):7431–7436. doi: 10.1074/jbc.272.11.7431. [DOI] [PubMed] [Google Scholar]
- 79.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiological Reviews. 1991;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imura Y, Choda N, Matsuzaki K. Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophysical Journal. 2008;95(12):5757–5765. doi: 10.1529/biophysj.108.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochimica et Biophysica Acta. 2006;1758(9):1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE. 2010;5(3, article e9505) doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prusiner SB. Prions. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forloni G, Angeretti N, Chiesa R, et al. Neurotoxicity of a prion protein fragment. Nature. 1993;362(6420):543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 85.Lin MC, Mirzabekov T, Kagan BL. Channel formation by a neurotoxic prion protein fragment. Journal of Biological Chemistry. 1997;272(1):44–47. doi: 10.1074/jbc.272.1.44. [DOI] [PubMed] [Google Scholar]
- 86.Kourie JI, Culverson A. Prion peptide fragment PrP[106–126] forms distinct cation channel types. Journal of Neuroscience Research. 2000;62(1):120–133. doi: 10.1002/1097-4547(20001001)62:1<120::AID-JNR13>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan B, Ratner V, Haas E. α-synuclein: its biological function and role in neurodegenerative diseases. Journal of Molecular Neuroscience. 2003;20(2):83–92. doi: 10.1385/JMN:20:2:83. [DOI] [PubMed] [Google Scholar]
- 88.El-Agnaf OM, Irvine GB. Aggregation and neurotoxicity of α-synuclein and related peptides. Biochemical Society Transactions. 2002;30(4):559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- 89.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418(6895):p. 291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 90.Ikeda H, Yamaguchi M, Sugai S, Aze Y, Narumiya S, Kakizuka A. Expanded polyglutamine in the Machado-Joseph disease protein induces cell death in vitro and in vivo. Nature Genetics. 1996;13(2):196–202. doi: 10.1038/ng0696-196. [DOI] [PubMed] [Google Scholar]
- 91.Hirakura Y, Azimov R, Azimova R, Kagan BL. Polyglutamine-induced ion channels: a possible mechanism for the neurotoxicity of huntington and other CAG repeat diseases. Journal of Neuroscience Research. 2000;60(4):490–494. doi: 10.1002/(sici)1097-4547(20000515)60:4<490::aid-jnr7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 92.Jaikaran ETAS, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochimica et Biophysica Acta. 2001;1537(3):179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 93.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. Journal of Biological Chemistry. 1996;271(4):1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 94.Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Critical Reviews in Neurobiology. 1997;11(2-3):167–239. doi: 10.1615/critrevneurobiol.v11.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 95.Diociaiuti M, Polzi LZ, Valvo L, Malchiodi-Albedi F, Bombelli C, Gaudiano MC. Calcitonin forms oligomeric pore-like structures in lipid membranes. Biophysical Journal. 2006;91(6):2275–2281. doi: 10.1529/biophysj.105.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lal R, Lin H, Quist AP. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochimica et Biophysica Acta. 2007;1768(8):1966–1975. doi: 10.1016/j.bbamem.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350(9071):134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 98.Seelig J, Lehrmann R, Terzi E. Domain formation induced by lipid-ion and lipid-peptide interactions. Molecular membrane biology. 1995;12(1):51–57. doi: 10.3109/09687689509038495. [DOI] [PubMed] [Google Scholar]
- 99.Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochimica et Biophysica Acta. 2010;1801(8):860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid β-protein (AB): a possible form of preamyloid in Alzheimer’s disease. Nature Medicine. 1995;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 101.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. Journal of Biological Chemistry. 2001;276(27):24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- 102.Matsuzaki K, Kato K, Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochimica et Biophysica Acta. 2010;1801(8):868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 103.Williams TL, Day IJ, Serpell LC. The effect of Alzheimer's Aβ aggregation state on the permeation of biomimetic lipid vesicles. Langmuir. 2010;26(22):17260–17268. doi: 10.1021/la101581g. [DOI] [PubMed] [Google Scholar]
- 104.Peters I, Igbavboa U, Schütt T, et al. The interaction of beta-amyloid protein with cellular membranes stimulates its own production. Biochimica et Biophysica Acta. 2009;1788(5):964–972. doi: 10.1016/j.bbamem.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yokoyama S, Ohta Y, Sakai H, Abe M. Effect of membrane composition on surface states of ganglioside G /dipalmitoylphosphatidylcholine/dioleoylphosphatidylcholine monolayers. Colloids and Surfaces B. 2004;34(1):65–68. doi: 10.1016/j.colsurfb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 106.Ohtsuka I, Yokoyama S. Penetration of bovine serum albumin into dipalmitoylphosphatidylglycerol monolayers: Direct observation by atomic force microscopy. Chemical and Pharmaceutical Bulletin. 2005;53(1):42–47. doi: 10.1248/cpb.53.42. [DOI] [PubMed] [Google Scholar]
- 107.Kato-Negishi M, Kawahara M. Neurosteroids block the increase in intracellular calcium level induced by Alzheimer’s β-amyloid protein in long-term cultured rat hippocampal neurons. Neuropsychiatric Disease and Treatment. 2008;4(1):209–218. doi: 10.2147/ndt.s2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malchiodi-Albedi F, Contrusciere V, Raggi C, et al. Lipid raft disruption protects mature neurons against amyloid oligomer toxicity. Biochimica et Biophysica Acta. 2010;1802(4):406–415. doi: 10.1016/j.bbadis.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 109.Williamson R, Usardi A, Hanger DP, Anderton BH. Membrane-bound β-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB Journal. 2008;22(5):1552–1559. doi: 10.1096/fj.07-9766com. [DOI] [PubMed] [Google Scholar]
- 110.Allende D, Simon SA, McIntosh TJ. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophysical Journal. 2005;88(3):1828–1837. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin MCA, Kagan BL. Electrophysiologic properties of channels induced by Aβ25-35 in planar lipid bilayers. Peptides. 2002;23(7):1215–1228. doi: 10.1016/s0196-9781(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 112.Arispe N, Doh M. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer’s disease AβP (1–40) and (1–42) peptides. FASEB Journal. 2002;16(12):1526–1536. doi: 10.1096/fj.02-0829com. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Y, Richardson JS. Cholesterol protects PC12 cells from β-amyloid induced calcium disordering and cytotoxicity. NeuroReport. 1996;7(15–17):2487–2490. doi: 10.1097/00001756-199611040-00017. [DOI] [PubMed] [Google Scholar]
- 114.Eckert GP, Cairns NJ, Maras A, Gattaz WF, Müller WE. Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2000;11(4):181–186. doi: 10.1159/000017234. [DOI] [PubMed] [Google Scholar]
- 115.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 116.Hertel C, Terzi E, Hauser N, Jakob-Røtne R, Seelig J, Kemp JA. Inhibition of the electrostatic interaction between β-amyloid peptide and membranes prevents β-amyloid-induced toxicity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(17):9412–9416. doi: 10.1073/pnas.94.17.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rokitskaya TI, Antonenko YN, Kotova EA. Effect of the dipole potential of a bilayer lipid membrane on gramicidin channel dissociation kinetics. Biophysical Journal. 1997;73(2):850–854. doi: 10.1016/S0006-3495(97)78117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vey M, Pilkuhn S, Wille H, et al. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14945–14949. doi: 10.1073/pnas.93.25.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miura T, Yoda M, Takaku N, Hirose T, Takeuchi H. Clustered negative charges on the lipid membrane surface induce β-sheet formation of prion protein fragment 106-126. Biochemistry. 2007;46(41):11589–11597. doi: 10.1021/bi700939j. [DOI] [PubMed] [Google Scholar]
- 120.Wakabayashi M, Matsuzaki K. Ganglioside-induced amyloid formation by human islet amyloid polypeptide in lipid rafts. FEBS Letters. 2009;583(17):2854–2858. doi: 10.1016/j.febslet.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 121.Di Pasquale E, Fantini J, Chahinian H, Maresca M, Taïeb N, Yahi N. Altered ion channel formation by the Parkinson’s-disease-linked E46K mutant of α-synuclein is corrected by GM3 but not by GM1 gangliosides. Journal of Molecular Biology. 2010;397(1):202–218. doi: 10.1016/j.jmb.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 122.Cho WJ, Trikha S, Jeremic AM. Cholesterol regulates assembly of human islet amyloid polypeptide on model membranes. Journal of Molecular Biology. 2009;393(3):765–775. doi: 10.1016/j.jmb.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 123.Arispe N, Diaz JC, Flora M. Efficiency of histidine-associating compounds for blocking the Alzheimer’s Aβ channel activity and cytotoxicity. Biophysical Journal. 2008;95(10):4879–4889. doi: 10.1529/biophysj.108.135517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arispe N, Diaz J, Durell SR, Shafrir Y, Guy HR. Polyhistidine peptide inhibitor of the Aβ calcium channel potently blocks the Aβ-induced calcium response in cells. Theoretical modeling suggests a cooperative binding process. Biochemistry. 2010;49(36):7847–7853. doi: 10.1021/bi1006833. [DOI] [PubMed] [Google Scholar]
- 125.Diaz JC, Simakova O, Jacobson KA, Arispe N, Pollard HB. Small molecule blockers of the Alzheimer Aβ calcium channel potently protect neurons from Aβ cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3348–3353. doi: 10.1073/pnas.0813355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sciences. 2000;67(7):743–757. doi: 10.1016/s0024-3205(00)00669-x. [DOI] [PubMed] [Google Scholar]
- 127.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neuroscience Research. 2000;36(4):261–273. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 128.Charalampopoulos I, Alexaki VI, Tsatsanis C, et al. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Annals of the New York Academy of Sciences. 2006;1088:139–152. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- 129.Aldred S, Mecocci P. Decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) concentrations in plasma of Alzheimer’s disease (AD) patients. Archives of Gerontology and Geriatrics. 2010;51(1):e16–e18. doi: 10.1016/j.archger.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 130.Nilsson P, Iwata N, Muramatsu SI, Tjernberg LO, Winblad B, Saido TC. Gene therapy in Alzheimer’s disease—potential for disease modification. Journal of Cellular and Molecular Medicine. 2010;14(4):741–757. doi: 10.1111/j.1582-4934.2010.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathologica. 2010;119(5):523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Nuallain B, Freir DB, Nicoll AJ, et al. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. Journal of Neuroscience. 2010;30(43):14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death and Differentiation. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]