Abstract
Background:
The functional role of dietary carbohydrates in nutrition is one of the most complex and at times controversial areas in nutritional science. In-vitro and in-vivo studies suggest that certain dietary saccharide biopolymers can have bifidogenic and or immunomodulatory effects, and that some could represent preferential substrates or precursors that can impact cellular glycosylation.
Objective:
Examine the impact of oral ingestion of a standardized dietary plant-derived polydisperse polysaccharide supplement (Advanced Ambrotose powder (AA)) on the N-glycosylation status of serum glycoproteins in a cohort of healthy individuals.
Design:
An open-label study was carried out. This study was in two phases: pilot study (n=6 individuals) to assess safety and dose, and a larger study (n=12) to evaluate specific glycosylation changes. Serum N-glycosylation profiles, using mass spectrometry, were monitored at weekly intervals, for 7 weeks, to evaluate baseline levels and normal fluctuations. The individuals were then monitored for a further 7 weeks, during which time increasing doses of AA were ingested (1.3–5.2 g/day).
Results:
No adverse events were encountered. AA supplementation resulted in distinct changes in the relative intensities of seven biantennary N-glycans (P<0.001), and a significant overall shift towards increased sialylation. Regression analysis revealed a dose-dependent decrease in mono- and di-galactosylated structures (coefficient −0.130 decrease/week: P=0.02 and −0.690: P=0.005), and a concomitant increase in disialylated glycans ( × 1.083: P<0.05).
Conclusions:
Supplementation with the dietary plant-derived polysaccharides in AA resulted in significant changes in serum protein N-glycosylation in healthy individuals. How this occurs and whether it has biological significance remains to be evaluated.
Keywords: dietary plant polysaccharides, Serum glycosylation, N-glycans, dietary fiber, glycomodifications, sialylation
Introduction
Fueled by the current interest in the link between diet and health (Ma et al., 2008; Estruch et al., 2009; Lomax and Calder, 2009; Schiffrin et al., 2010), there is now a surge of research by various groups into the biological activities and potential beneficial effects of dietary saccharide biopolymers (Schepetkin and Quinn, 2006; Cumashi et al., 2007; Vos et al., 2007; Rideout et al., 2008; Weickert and Pfeiffer, 2008; Bruzzese et al., 2009; Chan et al., 2009; Courtois, 2009; Graff et al., 2009; Jacobs et al., 2009; Liu et al., 2009). These biopolymers represent a broad range of structurally diverse (Schepetkin and Quinn, 2006), non- or low-digestible, dietary soluble fibers that have been derived from different species of flora including higher plants, fungi, lichens and algae, and have been demonstrated (in-vitro or in-vivo) to affect beneficially one or more target cellular or body functions (Cumashi et al., 2007; Ho et al., 2007; Hua et al., 2007; Vos et al., 2007; Kim and Joo, 2008; Ma et al., 2008; Bruzzese et al., 2009; Chan et al., 2009; Courtois, 2009; Graff et al., 2009; Lomax and Calder, 2009; van den Heuvel et al., 2009).
The physiological effects attributed to these ‘functional' polysaccharides can be divided into six main categories: (1) beneficial effects on colonic microflora (Jacobs et al., 2009) and gastrointestinal physiology; that is functioning as a prebiotic (Macfarlane et al., 2008; de Vrese and Schrezenmeir, 2008; Lomax and Calder, 2009); (2) immunomodulatory effects (Leung et al., 2006; Schepetkin and Quinn, 2006; Cumashi et al., 2007; Hua et al., 2007; Vos et al., 2007; Benyacoub et al., 2008; Kim and Joo, 2008; Bruzzese et al., 2009; Burrows et al., 2009; Graff et al., 2009; Mizuno et al., 2009); (3) anti-angiogenetic (Cumashi et al., 2007) and anti-tumor effects (Chan et al., 2009; Liu et al., 2009); (4) altered lipid metabolism (Rideout et al., 2008; Chen and Huang, 2009); (5) improved bioavailability of essential minerals (Bo et al., 2006; van den Heuvel et al., 2009); and (6) other beneficial health effects such as enhanced production of growth factors involved in re-epithelization and wound healing (Jettanacheawchankit et al., 2009).
In this respect, many of the dietary saccharide polymers studied (isolated from >35 species) (Schepetkin and Quinn, 2006), exhibiting multifunctional physicochemical and physiological characteristics, are now being redefined as secondary metabolites (Vos et al., 2007; Macfarlane et al., 2008; Weickert and Pfeiffer, 2008; Courtois, 2009), or biological response modifiers (BRMs) (Leung et al., 2006).
These BRM polysaccharides (Table 1) have diverse structural complexity, and belong to a wide range of low or high molecular weights, soluble or insoluble, acidic or neutral, homo- or hetero-polymers, which may or may not undergo varying degrees of degradation in the digestive tract either by bacterial enzymes or by specific conditions associated with the digestive process (Sinnott et al., 2007; Macfarlane et al., 2008; van den Broek et al., 2008; Arasaradnam et al., 2009; Courtois, 2009; Jacobs et al., 2009; Schiffrin et al., 2010). Recent evidence suggests that the potential for these polysaccharides to undergo some degree of degradation is highly dependent on the metabolic potential encoded by the combined genomes of the gut microbiota (Arasaradnam et al., 2009; Tuohy et al., 2009).
Table 1. Examples of dietary polysaccharides that have been studied in relation to their biological activities.
| Dietary polysaccharides | Features/examples | Biological functions |
|---|---|---|
| Sulfated polysaccharides; glycosaminoglycans | Heparin-like fucoidans— obtained from edible seaweed. | Potent antioxidant and anticoagulant properties, as well as immunopharmacological properties such as anti-inflammatory activity (Cumashi et al., 2007; Kim and Joo, 2008; Mizuno et al., 2009). |
| Glucomannan polysaccharides | Acemannan—obtained from Aloe vera. | Potent immunostimmulants (Leung et al., 2006) that also exhibit anti-tumor and wound-healing properties via induction of fibroblast proliferation and increased type I collagen expression (Leung et al., 2006; Jettanacheawchankit et al., 2009). |
| β-glucans; wide-spread homo-polysaccharides (-glucose monomers linked by glycosidic bonds) with different molecular weights and degree of branching. | They occur most commonly as the cell wall of yeast, certain fungi and mushrooms (e.g. lentinan). | These exhibit marked anti-tumor (Chan et al., 2009; Liu et al., 2009) and immunostimulatory (Mizuno et al., 2009) activity, affecting both the innate, as well as the adaptive arm (Th1 and Th2) of the immune response (Dalmo and Bogwald, 2008), and may be effective in controlling blood lipids (Chen and Huang, 2009). Generally insoluble (1,3/1,6) β-glucan, has greater biological activity than the soluble (1,3/1,4) β-glucans. |
| Inulin polysaccharides; fructans | Fructose polymers±terminal glucose. Found in many types of plants. Foods naturally high in inulin include garlic, onion and chicory. | These are associated with immunomodulatory anti-inflammatory effects as well as selective mineral absorption. They have also been implicated in controlling blood lipids (Bruzzese et al., 2009; Courtois, 2009; van den Heuvel et al., 2009). |
| Arabinogalactan polysaccharides | Consisting of arabinose and galactose. Major constituents of many gums, including gum gutti and gum tragacanth. | A number of these exhibit immunomodulatory effects via induction of both pro- and anti-inflammatory cytokines (Schepetkin and Quinn, 2006), and have been shown to exhibit potent complement-fixing activity and beneficial prebiotic properties, particularly in gastrointestinal functional disorders such as Irritable bowel syndrome, by increasing the colonic contents of short-chain fatty acids. Guar gum has also been implicated in improving blood lipids (Rideout et al., 2008). |
Current studies into the link between the bioconversion of these dietary polysaccharides, their bioavailability and their downstream effects on the host metabolism and physiology are using metabolomics and metagenomics approaches (Jacobs et al., 2009). These and other innovative approaches in the field of colonic fermentation (Chassard et al., 2007; Arasaradnam et al., 2009) are providing novel insights into gut microbial–human mutualism (Possemiers et al., 2009), its impact on regulating human health and disease, and the importance of dietary modulation (Leung et al., 2006; Macfarlane et al., 2008; Bruzzese et al., 2009; Liu et al., 2009; Lomax and Calder, 2009; Tuohy et al., 2009; Schiffrin et al., 2010).
Although many of the physiological traits attributed to dietary polysaccharides may be secondary, that is, related to their effects on the gut microflora and its biochemical activities (Schiffrin et al., 2010), evidence from various pharmacodynamic and pharmacokinetic studies also indicate microflora-independent immunomodulatory effects (Schepetkin and Quinn, 2006; Ho et al., 2007; Hua et al., 2007).
This ‘direct' immunomodulatory effect is instigated by the binding of certain BRM polysaccharides (for example, β-glucans) (Dalmo and Bogwald, 2008) to specific receptors on immune cells in the gut-associated lymphoid tissues, and results in the intracellular activation of signal transducers and transcription factors that are associated with various effector functions of the immune response (Vetvicka et al., 1996; Schepetkin and Quinn, 2006; Ho et al., 2007; Hua et al., 2007; Vos et al., 2007; Paur et al., 2008; Burrows et al., 2009; Graff et al., 2009; Mizuno et al., 2009).
Although the potential mechanisms of the interaction and the subsequent signal transduction pathways are not fully understood, the evidence supports the direct binding of BRM polysaccharides (or their fragments) to pattern recognition receptors. These receptors are key players in the immune response (receptors for recognition of microbial polysaccharides) and include toll-like receptors (Leung et al., 2006; Hua et al., 2007; Dalmo and Bogwald, 2008; Graff et al., 2009), non-toll pattern recognition receptors (for example, β-glucan receptor or dectin-1), complement receptor type 3 and certain transmembrane lectins (Vetvicka et al., 1996; Vos et al., 2007; Gunning et al., 2009). Recognition by these pattern recognition receptors can result in intracellular signaling cascades for example, activation of protein kinases or nuclear transcription factor kappa B. This can in turn result in subsequent activation or inactivation of a wide spectrum of target genes involved in the regulation of a variety of cellular responses, such as expression of various cell-surface receptors (Kim and Joo, 2008) and cytokine production (Leung et al., 2006; Schepetkin and Quinn, 2006; Cumashi et al., 2007; Dalmo and Bogwald, 2008; Paur et al., 2008; Jettanacheawchankit et al., 2009).
One of the primary immunomodulatory effects of BRM polysaccharides is to promote or alter various leukocyte activities, in particular those of macrophages (Leung et al., 2006; Schepetkin and Quinn, 2006) and immune-regulatory Gamma delta T cells (a sub-population of intraepithelial lymphocytes in the gastrointestinal tract) (Graff et al., 2009), via changes in cytokine expression (Yoshino et al., 2000; Hua et al., 2007; Benyacoub et al., 2008; Kim and Joo, 2008; Burrows et al., 2009; Schiffrin et al., 2010). This in turn can impact both the innate and adaptive arms of the immune response and thus result in activation or dampening of these responses (Schepetkin and Quinn, 2006; Cumashi et al., 2007; Ho et al., 2007; Vos et al., 2007; Graff et al., 2009; Mizuno et al., 2009).
Dietary polysaccharide induced immunomodulatory activities of note include: increased macrophage cytotoxic and phagocytic activities, altered pro- and anti-inflammatory (Leung et al., 2006; Schepetkin and Quinn, 2006; Ho et al., 2007; Benyacoub et al., 2008; Kim and Joo, 2008; Mizuno et al., 2009; Schiffrin et al., 2010) and Th1-Th2 balance (Yoshino et al., 2000; Vos et al., 2007; Burrows et al., 2009), as well as altered expression of certain adhesion molecules (Yoshino et al., 2000; Schepetkin and Quinn, 2006; Cumashi et al., 2007; Ho et al., 2007; Graff et al., 2009). All of which supports the notion that certain dietary plant polysaccharides may have significant immunomodulatory potential (Schepetkin and Quinn, 2006; Cumashi et al., 2007; Ho et al., 2007; Benyacoub et al., 2008; Burrows et al., 2009; Trinchero et al., 2009; Tuohy et al., 2009).
In view of this and given the important link between changes in the immune response and the glycosylation of various effector glycoproteins (Alavi and Axford, 2008), the aim of this study was to examine the possible affects, if any, that oral administration of dietary plant polysaccharides may have on the glycosylation status of serum glycoproteins in a cohort of healthy subjects. We chose a commercial, mixed saccharide dietary supplement (AA), the constituents of which have previously been shown to exhibit prebiotic (Sinnott et al., 2007), as well as possible immunomodulatory activities (Lefkowitz et al., 2000; Koetzner et al., 2010), and for the purposes of this study have no known reported toxicity or negative side effects associated with their use in human subjects (Stancil and Hicks, 2009).
Using AA we carried out an open-label dosing study to first evaluate safety of increased doses, and second to monitor the affect on the N-glycosylation status of serum glycoproteins in healthy subjects.
Materials and methods
Study cohort
Healthy volunteers were recruited from the staff at St George's University of London. The study design was approved by the Ethics Committee and volunteers gave written informed consent.
Supplement
The supplement was Advanced Ambrotose (AA) powder (provided by Mannatech, Inc., Dallas, TX, USA). AA is a plant-derived dietary supplement that contains a standardized mixture of partially purified polydisperse saccharide biopolymers: aloe vera gel powder (containing glucomannan-based polysaccharides), arabinogalactan (high-M.wt soluble arabinose and galactose-containing dietary fiber), gum ghatti and gum tragacanth (mainly soluble mixture of complex galactose polymers; with other saccharide constituents including arabinose, rhamnose, fucose, glucose, mannose, xylose and uronic acids), glucosamine (plant-derived supplemental glucosamine HCl), Undaria pinnatifida fucoidans (sulfated fucose polymer that contains other monosaccharides, galactose and mannose, derived from edible brown seaweed) and rice starch.
Study design
An open-label dosing study was set up. The study was carried out in two phases.
Phase 1
Pilot study was carried out to establish safety and dose necessary to impact serum N-glycosylation profiles. Six individuals (age range 28–55; 3 females), ingested increasing doses of AA supplement (increased by 1.3 g/week) for 7 weeks (starting with 1.3 g/day for week 1, and reaching 9.1 g/day at week 7). From week 7 to week 11, the group was divided into two arms; three stopped their supplement while the other three volunteers continued with supplementation at increasing doses (reaching 14.3 g/day by week 11).
Phase 2
A larger study was performed to evaluate glycosylation changes pre- and during-supplementation. A total of 12 volunteers (age range 22–40; 6 females), were followed for 16 weeks. The first 7 weeks, designated as the pre-supplementation period (week −7 to 0), was to establish baseline serum N-glycosylation patterns and normal fluctuations. Following this period, the individuals ingested increasing doses of AA supplement (week 1–4 at 1.3 g/day; week 5–6 at 2.6 g/day and week 7–8 at 5.2 g/day). This was followed by a final week with no supplement intake (week 9).
Sample collection
Serum was extracted from blood samples and stored at −40 °C. Samples were tested in a blind manner. The codes were not revealed until the study was completed.
Glycosylation analysis
Serum (40 μl) glycoproteins were deglycosylated using a Peptide N-Glycosidase-F (New England BioLabs Inc., Hitchin, Herts, UK) according to the manufacturer's instructions. Released N-glycans were isolated by C18 (Supelco, Poole, Dorset, UK), and graphitized carbon columns, freeze-dried, reconstituted in 100 μl of deionised water, ready for Glycan profiling by Matrix assisted laser desorption ionisation time-of-flight mass spectrometry (MS; Kratos Analytical, Ltd., Manchester, UK), set in positive linear mode and calibrated with a dextran ladder. Dihydroxy-benzoic acid was used as the matrix.
Statistical analysis
Where necessary the data were log transformed. To allow for time trends analysis of covariance with time as a continuous predictor and subject as a categorical factor was used. For each glycan structure, two regression models were fitted. The first ignored AA and fitted a straight-line relationship with time. The second allowed for different regression lines in the pre-supplement and supplement periods. These two models were compared using an F test. The difference between the sums of squares was calculated. The ratio of this to the residual mean square for the larger, two period model=F ratio; used to test effect of AA.
Correction for multiple comparisons
The Bonferroni method was applied and significance was set at P⩽0.006.
Dose-effect
Only the data following the start of AA were used to analyse regression of each glycan on dose and subject as a categorical factor. Time was included as a second covariate (to allow for possible time trends); such that if there was a significant dose trend even after adjustment for time, the effect of dose was fairly secure.
Results
Safety and compliance
Phase 1
All six subjects completed the trial. AA was well tolerated even at high doses, with no adverse reactions.
Phase 2
All six male and five female volunteers completed the trial. However, because of palatability issues at high doses, four of five female volunteers stopped supplementation earlier than planned; two at week 6 and two at week 7. One female volunteer withdrew at week 1 (because of palatability issues), and had data for pre-AA phase only, and was therefore omitted from the analysis.
Serum N-glycan analysis
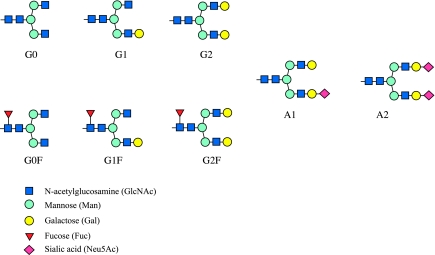
Serum N-glycan profiles for each individual at weekly intervals were examined. Eight main biantennary serum glycoprotein-derived N-glycan structures corresponding to different m/z values were identified (Figure 1). Peak intensities were analyzed.
Figure 1.
Schematic diagram of the serum protein-derived biantennary N-linked glycan structures that were analyzed by MS. Eight main biantennary serum glycoprotein-derived N-glycan structures were identified. These comprised six neutral and two acidic (sialylated) glycans. The glycans are designated as G0, G1 and G2, according to the number of terminal galactose residues, and monosialylated and or A2, according to the number of terminal sialic acid residues that decorate the core pentasaccharide (GlcNAc2, Man3). Three of the glycans are F; giving rise to G0F, G1F and G2F structures. Monosialylation and monogalactosylation may occur on either the α1–3 or α 1–6 arm of the biantennary structures.
Phase 1
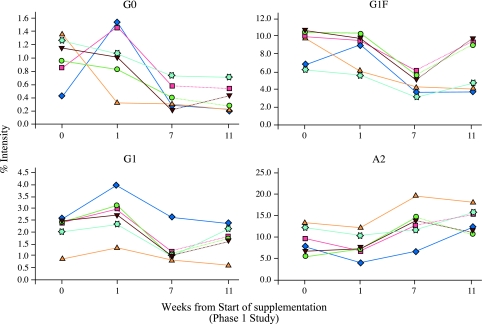
Analysis of the data from the pilot study demonstrated changes in the serum N-glycan profile in response to increasing doses of AA supplementation. Example of the observed changes for each of the six volunteers, at four time points, for four of the glycans, agalactosylated (G0), monogalactosylated (G1), G1fucosylated (G1F) and disialylated (A2), are shown (Figure 2). The time points chosen were time=0 (representing baseline values), time=1 (demonstrating that changes in serum protein glycosylation were evident within 1 week of supplementation at 1.3 g/day;), time=7 (9.1 g/day; time point at which the group were subdivided into two arms; three individuals continued with their oral supplementation at increasing doses, whereas the remaining three discontinued their supplementation) and time=11 (the end of the study demonstrating possible differences between the serum glycosylation profile of the individuals who continued compared with those that discontinued with their AA supplementation).
Figure 2.
Results from the pilot study; examining possible changes in serum protein N-glycosylation in response to different doses of AA. The MS percentage intensity for G0, G1, G1F and A2, for each of the six volunteers, at four time points is shown. Week 0 (baseline), week1 (AA at 1.3 g/day), week 7 (AA at 9.1 g/day) and week 11 (n=3 continued to take increasing doses of AA, reaching a final dose of 14.3 g/day, represented by the solid line; and n=3 discontinued their AA supplementation, represented by dashed line, respectively).
Week 0–7: all apart from the monosialylated glycans (not shown), showed some degree of change in response to AA supplementation.
Week 7–11: comparisons of the glycan profiles in the three individuals that discontinued with those that continued AA supplementation showed differences. These differences were confined mainly to the neutral glycans (G0F, G1, G1F, digalactosylated (G2) and G2F) and were particularly noticeable for G1F, the levels of which reverted (increased) towards baseline (week=0) when AA was discontinued (Figure 2).
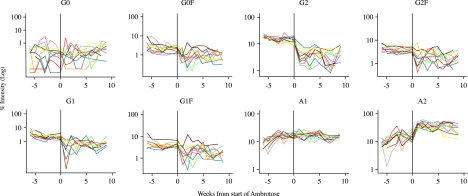
Phase 2
The 7-week pre-supplementation monitoring of serum protein glycan profiles allowed us to evaluate and monitor normal glycosylation fluctuations and to establish statistically meaningful baseline levels. Comparison of the glycan profiles during the supplementation period with the time trends observed during the pre-supplementation period detected distinct changes in the relative intensities of seven of eight glycans studied. These changes were particularly striking within the first 1–2 weeks of supplementation (Figure 3). G0 was the exception, showing major fluctuations in the pre- as well as supplementation period.
Figure 3.
Serum protein N-glycan profiles before and after oral AA supplementation. Plots of MS observations for all subjects for whom there were measurements made before and during AA supplementation, demonstrating the changes in the eight serum protein-derived biantennary N-glycans in response to oral AA supplementation (zero time is the last pre-AA measurement). The changes were significant (F test for the effect of AA; P<0.001) for all the serum protein N-glycans except G0.
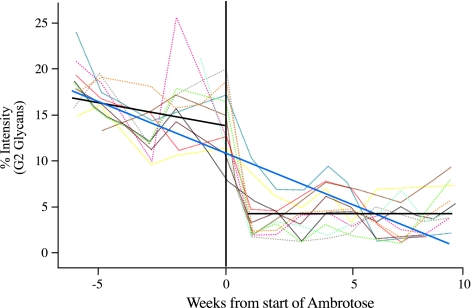
Analysis of covariance using the two regression model was used to assess the strength of evidence that serum glycosylation changes followed AA supplementation. A graphical example of the model as applied to the analysis of the data for G2 glycans is given (Figure 4). Using this analysis, AA supplementation was found to result in significant changes in the levels of all the serum protein-derived N-linked glycans (G0F, G1, G1F, G2, G2F, monosialylated and A2; P<0.001) except G0 (P=0.2).
Figure 4.
Example of the two regression model used for the analysis of covariance showing the change in serum protein G2 glycan levels following AA supplementation (P<0.001). For each glycan structure, two regression models were fitted. The first ignored AA and fitted a straight-line (solid blue line) relationship with time. The second allowed for different regression lines in the pre-supplement and supplement phase (solid black lines; coefficient −0.501 and 0.018, respectively). This was achieved by creating two time variables; the first is time up to AA, but is equal to zero afterwards. The second time variable is time after AA, but is equal to zero before AA.
Dose effect
Regression analysis (with time as a second covariate) following the start of AA was used to assess the strength of evidence that change did occur following supplementation with AA and to examine the effect of dose. The results (Table 2) show dose effects for G1 and G2; the levels of which decreased significantly with increasing AA dose (coefficient factor by which mean glycan levels are multiplied/week −0.130 and −0.690; P=0.02 and 0.005, respectively), and for A2; the levels of which increased significantly with increasing AA dose (Coefficient factor =1.083; P=0.005).
Table 2. Effect of increasing dose of AA supplement on serum protein N-glycosylation.
| Glycans |
Dose alone |
Dose adjusted for time |
||
|---|---|---|---|---|
| Coefficient | P-value | Coefficient | P-value | |
| G0a | 1.124 | 0.1 | 0.993 | 0.09 |
| G0Fa | 0.996 | 0.9 | 1.020 | 0.6 |
| G1b | −0.133 | 0.001 | −0.130 | 0.02 |
| G1Fa | 0.962 | 0.3 | 1.016 | 0.7 |
| G2b | −0.976 | <0.001 | −0.690 | 0.005 |
| G2Fb | −0.226 | 0.001 | −0.170 | 0.2 |
| A1b | 0.532 | 0.007 | 0.669 | 0.1 |
| A2a | 1.082 | 0.002 | 1.083 | 0.005 |
Abbreviations: AA, Advanced Ambrotose powder; A1, monosialylated; A2, disialylated; F, fucosylated; G0, agalactosylated; G1, monogalactosylated; G2, digalactosylated.
For the purposes of statistical analysis, log transformation was applied to those glycans that exhibited a skewed distribution (more variable at high levels than at low levels).
Regression analysis of the data demonstrates significant dose effects for G1, G2 and A2 serum protein N-glycans.
Analysed on logarithmic scale; coefficient=factor by which mean sugar is multiplied per week.
Analysed on natural scale; coefficient=increase in mean sugar per week.
Discussion
Our study reports novel findings showing that the ingestion of a standardized mixture of plant-derived polysaccharides can induce significant changes in the N-glycosylation status of serum glycoproteins in normal healthy individuals. AA supplementation caused an overall, dose dependent, shift towards increased sialylation, resulting in significantly lower levels of neutral glycans (G1 and G2: −0.130 and −0.690 per week, respectively), and increased levels of fully processed, sialylated, glycans (A2: by a factor of 1.083 per week).
Given that glycosylation is a key post-translational modification that can significantly affect the overall biophysical and biochemical functions of proteins (Alavi and Axford, 2008; Biol-N′garagba and Louisot, 2003), the observed shift in the N-glycosylation status of serum glycoproteins in response to AA supplementation may be significant and could reflect changes in one or more physiological parameters.
Interpretation of the biological significance of the observed shift towards more silaylated serum glycoproteins is complex (Alavi and Axford, 2008) and requires further more detailed structure-function examination of given serum glycoproteins (for example, IgG). However, what is known is that increased levels of sialic acid can affect absorption, serum half-life and clearance from the serum, as well as the physical, chemical and immunogenic properties of various key serum glycoproteins (Alavi and Axford, 2008; Bork et al., 2009; Schauer, 2009).
Indeed, sialylation changes can have a key role in many aspects of the immune response, in particular in relation to the regulation of inflammatory processes, as in the case of IgG, where sialylation has been shown to function as a ‘switch'. The presence of sialylation is associated with the steady state, normal, anti-inflammatory function of IgG, whereas its absence is associated with the pro-inflammatory effector function (Alavi and Axford, 2008; Anthony et al., 2008).
However, with this in mind, it is important to note that the elucidation of a specific physiological role for a given glycan modification, such as sialylation, poses formidable challenge. The presence or absence of a particular sugar residue, at a particular glycosylation site on a given protein, may have different roles in different biological settings. In the case of sialic acid, this is further complicated by the fact that sialylation is linkage-specific, and that the presence of the different forms of sialic acid may give rise to very different biological scenarios (Alavi and Axford, 2008; Schauer, 2009). Further research is therefore needed to more clearly elucidate specific effects and their consequent biological significance.
Although the question of whether these serum glycosylation changes are directly linked to the constituents of AA per se, or the affect of these on gut microflora (Sinnott et al., 2007) requires further investigation, the novel finding that small amounts of a polydisperse mixture of dietary polysaccharides can alter the glycosylation of serum proteins is unexpected and intriguing. This is especially true as it provides a potential mechanism through which dietary polysaccharides may alter the effector functions of circulating molecules. This notion is particularly relevant when one considers the fact that, time and again, cross-sectional (Bo et al., 2006) and longitudinal (Estruch et al., 2009) studies indicate an association between dietary-fiber intake and levels of inflammatory serum markers (for example, an inverse correlation with interleukin-6 and tumor necrosis factor-α receptor-2) (Ma et al., 2008). Continued efficacy studies using randomized placebo-controlled trials examining AA and its specific components are warranted to evaluate possible effect(s).
In conclusion, we have demonstrated that supplementation with dietary plant polysaccharides in AA can result in significant serum protein glycomodifications in normal healthy individuals. These finding are interesting and merit further research, where it is hoped that an integrative approach using glycomics together with other—omics such as metabolomics and metagenomics, focusing on the gut associated microbe–host mutualism and its impact on physiology, can help unravel the exogenous and endogenous effects of dietary components and hence, generate novel hypotheses for innovative dietary interventions that may impact health.
Acknowledgments
AA and OF were supported by grant No: RKC0021 from the Research & Development Division of Mannatech, Inc., Dallas, TX, USA.
AA and OF were in receipt of research grants from the R&D Division of Mannatech, Incorporated.
JA was, from 2002–2007, an independent board director, shareholder and consultant for Mannatech, Incorporated.
Mannatech, Incorporated had no role in the conception or design of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to publish.
Footnotes
Contributors: AA, JA and ET conceived and designed the study. AA carried out the study and performed the analysis and interpretation of the data, as well as coordinated and drafted the manuscript. OF and ET carried out the MS analysis. MB performed the statistical analysis. All authors have read and approved the final paper.
References
- Alavi A, Axford JS. Sweet and sour: the impact of sugars on disease. Rheumatology (Oxford) 2008;47:760–770. doi: 10.1093/rheumatology/ken081. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasaradnam RP, Pharaoh MW, Williams GJ, Nwokolo CU, Bardhan KD, Kumar S. Colonic fermentation—more than meets the nose. Med Hypotheses. 2009;73:753–756. doi: 10.1016/j.mehy.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, et al. Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr. 2008;138:123–129. doi: 10.1093/jn/138.1.123. [DOI] [PubMed] [Google Scholar]
- Biol-N′garagba MC, Louisot P. Regulation of the intestinal glycoprotein glycosylation during postnatal development: role of hormonal and nutritional factors. Biochimie. 2003;85:331–352. doi: 10.1016/s0300-9084(03)00039-7. [DOI] [PubMed] [Google Scholar]
- Bo S, Durazzo M, Guidi S, Carello M, Sacerdote C, Silli B, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr. 2006;84:1062–1069. doi: 10.1093/ajcn/84.5.1062. [DOI] [PubMed] [Google Scholar]
- Bork K, Horstkorte R, Weidemann W. Increasing the sialylation of therapeutic glycoproteins: the potential of the sialic acid biosynthetic pathway. J Pharm Sci. 2009;98:3499–3508. doi: 10.1002/jps.21684. [DOI] [PubMed] [Google Scholar]
- Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: an observational study. Clin Nutr. 2009;28:156–161. doi: 10.1016/j.clnu.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Burrows M, Assundani D, Celis E, Tufaro F, Tanaka A, Bradley WG. Oral administration of PPC enhances antigen-specific CD8+ T cell responses while reducing IgE levels in sensitized mice. BMC Complement Altern Med. 2009;9:49. doi: 10.1186/1472-6882-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassard C, Goumy V, Leclerc M, Del′homme C, Bernalier-Donadille A. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol Ecol. 2007;61:121–131. doi: 10.1111/j.1574-6941.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang XF. The effects of diets enriched in beta-glucans on blood lipoprotein concentrations. J Clin Lipidol. 2009;3:154–158. doi: 10.1016/j.jacl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- Courtois J. Oligosaccharides from land plants and algae: production and applications in therapeutics and biotechnology. Curr Opin Microbiol. 2009;12:261–273. doi: 10.1016/j.mib.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D′Incecco A, Piccoli A, Totani L, et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Dalmo RA, Bogwald J. Beta-glucans as conductors of immune symphonies. Fish Shellfish Immunol. 2008;25:384–396. doi: 10.1016/j.fsi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martinez-Gonzalez MA, Corella D, Basora-Gallisa J, Ruiz-Gutierrez V, Covas MI, et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J Epidemiol Community Health. 2009;63:582–588. doi: 10.1136/jech.2008.082214. [DOI] [PubMed] [Google Scholar]
- Graff JC, Kimmel EM, Freedman B, Schepetkin IA, Holderness J, Quinn MT, et al. Polysaccharides derived from Yamoa (Funtumia elastica) prime gammadelta T cells in vitro and enhance innate immune responses in vivo. Int Immunopharmacol. 2009;9:1313–1322. doi: 10.1016/j.intimp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning AP, Bongaerts RJ, Morris VJ. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009;23:415–424. doi: 10.1096/fj.08-106617. [DOI] [PubMed] [Google Scholar]
- Ho YW, Yeung JS, Chiu PK, Tang WM, Lin ZB, Man RY, et al. Ganoderma lucidum polysaccharide peptide reduced the production of proinflammatory cytokines in activated rheumatoid synovial fibroblast. Mol Cell Biochem. 2007;301:173–179. doi: 10.1007/s11010-006-9409-y. [DOI] [PubMed] [Google Scholar]
- Hua KF, Hsu HY, Chao LK, Chen ST, Yang WB, Hsu J, et al. Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J Cell Physiol. 2007;212:537–550. doi: 10.1002/jcp.21050. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Gaudier E, van Duynhoven J, Vaughan EE. Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Curr Drug Metab. 2009;10:41–54. doi: 10.2174/138920009787048383. [DOI] [PubMed] [Google Scholar]
- Jettanacheawchankit S, Sasithanasate S, Sangvanich P, Banlunara W, Thunyakitpisal P. Acemannan stimulates gingival fibroblast proliferation; expressions of keratinocyte growth factor-1, vascular endothelial growth factor, and type I collagen; and wound healing. J Pharmacol Sci. 2009;109:525–531. doi: 10.1254/jphs.08204fp. [DOI] [PubMed] [Google Scholar]
- Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Koetzner L, Grover G, Boulet J, Jacoby HI. Plant-derived polysaccharide supplements inhibit dextran sulfate sodium-induced colitis in the rat. Dig Dis Sci. 2010;55:1278–1285. doi: 10.1007/s10620-009-0848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz DL, Stuart R, Gnade BT, Roberts E, Lefkowitz SS. Effects of a glyconutrient on macrophage functions. Int J Immunopharmacol. 2000;22:299–308. doi: 10.1016/s0192-0561(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Leung MY, Liu C, Koon JC, Fung KP. Polysaccharide biological response modifiers. Immunol Lett. 2006;105:101–114. doi: 10.1016/j.imlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Liu J, Gunn L, Hansen R, Yan J. Yeast-derived beta-glucan in combination with anti-tumor monoclonal antibody therapy in cancer. Recent Pat Anticancer Drug Discov. 2009;4:101–109. doi: 10.2174/157489209788452858. [DOI] [PubMed] [Google Scholar]
- Lomax AR, Calder PC. Prebiotics, immune function, infection and inflammation: a review of the evidence. Br J Nutr. 2009;101:633–658. doi: 10.1017/S0007114508055608. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hebert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008;24:941–949. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104:305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Nishitani Y, Hashimoto T, Kanazawa K. Different suppressive effects of fucoidan and lentinan on IL-8 mRNA expression in in vitro gut inflammation. Biosci Biotechnol Biochem. 2009;73:2324–2325. doi: 10.1271/bbb.90326. [DOI] [PubMed] [Google Scholar]
- Paur I, Austenaa LM, Blomhoff R. Extracts of dietary plants are efficient modulators of nuclear factor kappa B. Food Chem Toxicol. 2008;46:1288–1297. doi: 10.1016/j.fct.2007.09.103. [DOI] [PubMed] [Google Scholar]
- Possemiers S, Grootaert C, Vermeiren J, Gross G, Marzorati M, Verstraete W, et al. The intestinal environment in health and disease - recent insights on the potential of intestinal bacteria to influence human health. Curr Pharm Des. 2009;15:2051–2065. doi: 10.2174/138161209788489159. [DOI] [PubMed] [Google Scholar]
- Rideout TC, Harding SV, Jones PJ, Fan MZ. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vasc Health Risk Manag. 2008;4:1023–1033. doi: 10.2147/vhrm.s3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat Res. 2010;690:50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Sinnott RA, Ramberg J, Kirchner JM, Oubre C, Duncan C, Boyd S, et al. Utilization of arabinogalactan, aloe vera gel polysaccharides, and a mixed saccharide dietary supplement by human colonic bacteria in vitro. Int J Probiotics Prebiotics. 2007;2:97–104. [Google Scholar]
- Stancil AN, Hicks LH. Glyconutrients and perception, cognition, and memory. Percept Mot Skills. 2009;108:259–270. doi: 10.2466/PMS.108.1.259-270. [DOI] [PubMed] [Google Scholar]
- Trinchero J, Ponce NM, Cordoba OL, Flores ML, Pampuro S, Stortz CA, et al. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother Res. 2009;23:707–712. doi: 10.1002/ptr.2723. [DOI] [PubMed] [Google Scholar]
- Tuohy KM, Gougoulias C, Shen Q, Walton G, Fava F, Ramnani P. Studying the human gut microbiota in the trans-omics era—focus on metagenomics and metabonomics. Curr Pharm Des. 2009;15:1415–1427. doi: 10.2174/138161209788168182. [DOI] [PubMed] [Google Scholar]
- van den Broek LA, Hinz SW, Beldman G, Vincken JP, Voragen AG. Bifidobacterium carbohydrases-their role in breakdown and synthesis of (potential) prebiotics. Mol Nutr Food Res. 2008;52:146–163. doi: 10.1002/mnfr.200700121. [DOI] [PubMed] [Google Scholar]
- van den Heuvel EG, Muijs T, Brouns F, Hendriks HF. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr Res. 2009;29:229–237. doi: 10.1016/j.nutres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos AP, M′Rabet L, Stahl B, Boehm G, Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit Rev Immunol. 2007;27:97–140. doi: 10.1615/critrevimmunol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- Yoshino S, Tabata T, Hazama S, Iizuka N, Yamamoto K, Hirayama M, et al. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000;20:4707–4711. [PubMed] [Google Scholar]