Abstract
CD8+ T lymphocytes often play a primary role in adaptive immunity to cytosolic microbial pathogens. Surprisingly, CD8+ T cells are not required for protective immunity to the enteric pathogen Shigella flexneri, despite the ability of Shigella to actively secrete proteins into the host cytoplasm, a location from which antigenic peptides are processed for presentation to CD8+ T cells. To determine why CD8+ T cells fail to play a role in adaptive immunity to S. flexneri, we investigated whether antigen-specific CD8+ T cells are primed during infection but are unable to confer protection or, alternatively, whether T cells fail to be primed. To test whether Shigella is capable of stimulating an antigen-specific CD8+ T-cell response, we created an S. flexneri strain that constitutively secretes a viral CD8+ T-cell epitope via the Shigella type III secretion system and characterized the CD8+ T-cell response to this strain both in mice and in cultured cells. Surprisingly, no T cells specific for the viral epitope were stimulated in mice infected with this strain, and cells infected with the recombinant strain were not targeted by epitope-specific T cells. Additionally, we found that the usually robust T-cell response to antigens artificially introduced into the cytoplasm of cultured cells was significantly reduced when the antigen-presenting cell was infected with Shigella. Collectively, these results suggest that antigen-specific CD8+ T cells are not primed during S. flexneri infection and, as a result, afford little protection to the host during primary or subsequent infection.
INTRODUCTION
Shigella flexneri is a Gram-negative bacterial pathogen that causes severe diarrhea in humans, resulting from a strong inflammatory response and the destruction of the colonic epithelium (38). Following ingestion, Shigella induces its uptake into colonic epithelial cells by using the concerted action of type III secreted effector proteins and subsequently lyses the endosomal membrane, freeing the organisms into the host cell cytoplasm. Once in the cytoplasm, Shigella uses actin-based motility to spread to adjacent cells, thus largely avoiding targeting by opsonizing antibodies and immune cells of the lymphoid follicle (6). In the rectal submucosa, Shigella also infects macrophages and rapidly induces caspase-1 activation via proteins secreted by the type III secretion system (TTSS), resulting in cell death of the macrophage and escape of the bacteria from the phagocytic cell (8, 16, 50).
An age-related decrease in the incidence of shigellosis has been observed for children living in areas where this disease is endemic (37), suggesting that natural protective immunity is induced during Shigella infection. However, acquired immunity against Shigella is apparent only following multiple rounds of exposure to the pathogen and is inefficient in limiting disease progression during subsequent infections (33). Epidemiological studies have demonstrated that the limited protection that arises following infection is serotype specific, indicating that B lymphocytes are important for this protection (19). In contrast, little is known about the role of T-cell-mediated immune responses during shigellosis. There have been reports that T cells infiltrate the rectal mucosa during acute infection (18); however, it is unclear whether these T cells are specific for Shigella antigens. It has also been reported that during acute infection, there are alterations in the T-cell receptor (TCR) Vβ repertoires of peripheral blood CD4+ and CD8+ T cells (20), but again, it is unknown whether the repertoire shift is Shigella specific and whether this shift changes the course of infection.
The difficulty in studying adaptive immune responses to Shigella has partly been that mice do not acquire intestinal disease following intragastric infection with Shigella. Many studies investigating adaptive immunity to S. flexneri have relied on the mouse bronchopulmonary model of infection, in which intranasal inoculation results in an acute inflammatory response characterized by an influx of polymorphonuclear neutrophils into the site of infection. This model recapitulates many elements of human gastrointestinal infection and has been used to investigate the roles of various immune effectors in limiting Shigella infection (31, 44). Similar to the case in natural infection, protective immunity to S. flexneri in the mouse model has been shown to be dependent upon antibody-secreting B lymphocytes, and this protection can be transferred to naïve mice by immune serum (45). While some protective immunity can also be conferred by interleukin-17A (IL-17A)-producing CD4+ Th17 cells (41), evidence suggests that CD8+ T lymphocytes are not required in the adaptive immune response to S. flexneri (45). Evidence that CD8+ T cells are ineffective against Shigella has been demonstrated through studies where the protection afforded by prior infection was not different between wild-type (WT) mice and mice from which CD8+ T cells were depleted prior to challenge (45).
During infection of mice with Listeria monocytogenes, an organism that, like Shigella, escapes from the phagocytic vacuole of cells and replicates in the host cell cytoplasm, CD8+ T cells are stimulated and constitute the major component of adaptive immunity. Therefore, the limited contribution of CD8+ T cells to protection against S. flexneri was unexpected, given the capacity of this organism to secrete proteins into and replicate within the host cytosol in a manner similar to that of Listeria. Shigella proteins are translocated into the host cell cytosol by surface-bound bacteria via the TTSS, and additional proteins are secreted directly into the host cell cytosol once the organisms are present and replicating within the cytoplasm. Because cytosolic proteins are typically processed and then presented by major histocompatibility complex class I (MHC-I) molecules to T cells, we hypothesized that antigen-specific CD8+ T cells should constitute an important component of adaptive immunity to this pathogen. Since previous studies have suggested that CD8+ T cells are not required during the adaptive immune response to S. flexneri, we investigated whether antigen-specific CD8+ T cells are primed during Shigella infection but fail to protect the host upon challenge or whether they initially fail to respond during primary infection. Our findings suggest that antigen-specific CD8+ T cells do not respond during S. flexneri infection and are therefore unable to protect the host. We conclude that limited CD8+ T-cell-mediated protection during natural infection may result from an inability of antigen-specific CD8+ T cells to be primed.
MATERIALS AND METHODS
Bacterial and viral strains.
Shigella flexneri serovar 5 WT strain M90T (39), ipaC mutant SF621 (a derivative of M90T) (30), serovar 2a WT strain 2457T (23), a virulence plasmid-cured derivative of 2457T (BS103) (28), and WT strain 2457T transformed with p-GFPmut2 (M. Goldberg, unpublished data) were grown at 37°C in tryptic soy broth (TSB) and on tryptic soy agar (TSA) plates containing 0.01% (wt/vol) Congo red (Sigma), supplemented with ampicillin (100 μg/ml) where appropriate. Escherichia coli DH5α was grown in LB broth. WT lymphocytic choriomeningitis virus (LCMV) strain Armstrong CA 1371 (12) was propagated in BHK-21 cells (46) and quantitated by plaque assays on Vero cell monolayers as previously described (1). Recombinant vaccinia virus expressing the NP118-126 protein (Vac-NP; a gift from M. Oldstone, The Scripps Institute, San Diego, CA) was purified as described previously (15).
Plasmid and strain construction.
Plasmid p-GFPmut2 (10) was transformed into virulence plasmid-cured Shigella BS103 (this study) by electroporation. Plasmid pAD1 (pIpaC-NP), which carries a fragment containing the NP118-126 epitope sequence ligated into the ipaC gene after codon 57, was constructed as follows. The following primers were annealed to create a fragment encoding the NP118-126 epitope flanked by SalI and HindIII restriction sites: 5′-TCGACCCGCCCGCAAGCGAGCGGCGTGTATATGGAGCTCA-3′ (forward) and 5′-AGCTTGAGCTCCATATACACGCCGCTCGCTTGCGGGCGGG-3′ (reverse). The fragment was cloned in frame into plasmid pIpaC57 (5) (a gift from C. Parsot, Pasteur Institute, Paris, France) after codon 57 of the ipaC gene, between SalI and HindIII restriction sites, to replace the C3 epitope. After verification of the pIpaC-NP nucleotide sequence, the plasmid was transformed into S. flexneri strain SF621, a strain lacking ipaC, by electroporation to form strain ipaC/pIpaC-NP.
Cell lines and cell culture.
B3Z cells (a gift from N. Shastri, University of California, Berkeley, CA), 1308.1 epithelial cells (13), and J774 cells were grown at 37°C and 5% CO2 in RPMI (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, HEPES, 50 μM 2-mercaptoethanol, 50 U/ml penicillin, and 50 μg/ml streptomycin. NP118-126-specific CD8+ T cells (harvested from Vac-NP-immunized mice and stimulated on NP peptide-coated cells) were maintained in the above-described medium supplemented with 5% supernatant from concanavalin A-stimulated rat spleen cells and 50 mM α-methylmannoside. Bone marrow macrophages (BMM) were prepared by harvesting bone marrow from femurs of C57BL/6J and caspase-1−/− mice and culturing the cells for 7 days at 37°C and 5% CO2 in RPMI supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 1 mM pyruvate, and 20% macrophage colony-stimulating factor (M-CSF)-conditioned medium collected from the supernatant of L929 cells.
Mice.
C57BL/6J (used for generating bone marrow-derived macrophages and for isolating T cells for T-cell proliferation assays) and BALB/c (used for all in vivo infections) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Caspase-1-deficient mice (25) were a gift from the Abbott Bioresearch Center (Worcester, MA). All experiments were approved by Harvard's Institutional Animal Care and Use Committee. For in vivo infections, BALB/c mice were each inoculated intranasally (i.n.) at the indicated doses with S. flexneri M90T in a 20-μl volume. For determination of bacterial loads, mice were sacrificed and lungs were homogenized in 10 ml sterile phosphate-buffered saline (PBS) and plated on TSB agar plates for bacterial CFU enumeration. For intraperitoneal (i.p.) infections, mice were injected at the indicated doses with Shigella M90T diluted in PBS to a volume of 200 μl. For LCMV infections, mice were injected i.p. with 4 × 105 PFU diluted in PBS. For infections with recombinant vaccinia virus expressing NP118-126, the virus was first incubated in 0.125% trypsin for 30 min at 37°C and subsequently diluted in PBS before immunization of mice i.p. with 5 × 106 PFU/mouse.
Bacterial infections and virulence assays.
Cells were infected with S. flexneri or E. coli by centrifuging exponential-phase bacteria diluted in PBS onto semiconfluent monolayers of cells at the indicated multiplicities of infection (MOIs) at 700 × g for 10 min. The cells were subsequently incubated for 20 min at 37°C and 5% CO2, washed three times with PBS, and resuspended in medium containing gentamicin at the indicated concentrations. Bacterial invasion of HeLa cells was quantitated using a gentamicin protection assay (14), as follows. HeLa cells were seeded at 2 × 105 cells/well in 24-well tissue culture dishes and grown for 18 h in medium without antibiotics. The cells were then infected with exponential-phase Shigella at an MOI of 10:1 as described above and then incubated for 1.5 h in medium containing gentamicin (50 μg/ml) to kill extracellular bacteria. The cells were then washed in PBS before being lysed in 0.1% Triton X-100–PBS. Enumeration of bacterial CFU was performed by plating lysates on TSA plates and counting bacterial CFU after overnight incubation at 37°C. The ability of Shigella strains to escape from phagosomes was evaluated using a chloroquine resistance assay (14). J774 cells were infected for 30 min as described above and then incubated for 1.5 h at 37°C and 5% CO2 in medium containing gentamicin (50 μg/ml), with or without chloroquine (50 μg/ml). The cells were then washed in PBS and lysed in 0.1% Triton X-100–PBS, and bacterial CFU were enumerated.
Secretion assay and IpaC immunoblotting.
Secretion of IpaC through the TTSS was induced using a Congo red secretion assay. Exponential-phase bacteria were harvested, resuspended in 10 μM Congo red-PBS, and incubated at 37°C for the indicated times. Following incubation, bacteria were pelleted by centrifugation, and supernatants were collected and passed through a 0.22-μm-pore-size filter. Proteins in the supernatants, which represent proteins secreted through the TTSS, were then concentrated by trichloroacetic acid (TCA) precipitation and analyzed by SDS-PAGE and immunoblotting using goat anti-IpaC antibody (bl-15; Santa Cruz Biotech, Santa Cruz, CA).
51Cr release CD8+ T-cell assay.
P815 (H-2d) target cells were incubated with 100 μCi of sodium [51Cr]chromate (PerkinElmer, Waltham, MA), with or without 1 μM NP118-126 peptide, for 1 h at 37°C. Cells were washed three times with PBS, after which effector cells were added to 10,000 radiolabeled target cells at the indicated ratios. Spontaneous or maximum lysis was determined by incubating cells with either medium or 1% Triton X-100, respectively. After 4 h of incubation at 37°C and 5% CO2, release of 51Cr into cell supernatants was quantified on a Wallac 1470 gamma counter.
In vitro assay for IFN-γ production by T cells.
J774 cells were infected at an MOI of 100:1 with S. flexneri M90T or ipaC/pIpaC-NP for 30 min as described above. Uninfected J774 control cells were coated with NP118-126 peptide (100 nM) for 1 h at 37°C. Cells were then incubated in medium with gentamicin (50 μg/ml) for an additional 1.5 h and subsequently irradiated for 35 min (2 × 104 rads). A total of 1 × 104 irradiated infected or uninfected peptide-coated J774 cells were incubated with 1 × 104 NP118-126-specific CD8+ T cells (9 to 11 days poststimulation) and 2.5 × 105 irradiated (2 × 103 rads) syngeneic spleen cells. Cultures were established in medium supplemented with 5% concanavalin A-stimulated rat spleen cells and 50 mM α-methylmannoside. Following 12 h of incubation, the amount of gamma interferon (IFN-γ) present in the culture supernatants was determined using an enzyme-linked immunosorbent assay (ELISA) kit (Endogen).
T-cell proliferation assay.
T cells were isolated from splenocytes of C57BL/6J mice by use of anti-Thy1.2-conjugated MACS microbeads and a magnetic separation column (Miltenyi Biotec) according to the manufacturer's directions. The purified T cells were then labeled with carboxyfluorescein succinimidyl ester (CFSE) as described previously (27) and subsequently infected with Shigella M90T for 3 h as described above. Cells were then washed once in PBS and resuspended in medium supplemented with gentamicin (50 μg/ml), 2% penicillin-streptomycin, and anti-CD28 (1 μg/ml) before being reseeded in wells that had previously been coated with anti-CD3 antibodies (2 μg/ml). After 3 days of stimulation, the cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) using FloJo (Tree Star Industries).
In vitro T-cell stimulation using recombinant anthrax lethal toxin.
LFn-Ova257-264 was engineered and purified as previously described (4). Protective antigen (PA) was generously provided by R. J. Collier (Harvard Medical School, Boston, MA). C57BL/6J BMM, caspase-1−/− BMM, or 1308.1 epithelial cells were infected at the indicated MOIs with Shigella 2457T or E. coli as described above or were left uninfected. Following a 30-min infection, the cells were washed with PBS and incubated in medium containing LFn-Ova257-264 (30 pmol), PA (30 pmol), and gentamicin (100 μg/ml) for 1 h. Cells were washed in PBS, and 1 × 105 B3Z hybridoma T cells were added in medium containing gentamicin (final concentration, 100 μg/ml). At 8 hours postinfection, the medium was replaced with 150 μl lysis buffer (PBS, 100 μM 2-mercaptoethanol, 9 mM MgCl2, 0.125% NP-40, and 0.15 mM chlorophenol red-β-d-galactopyranoside [Calbiochem]), and the absorbance at 570 nm (A570) was determined.
Analysis of MHC-I expression.
For in vitro analysis of MHC-I expression, BMM or 1308.1 epithelial cells were infected at an MOI of 10:1 with Shigella 2457T/p-GFPmut2 or virulence plasmid-cured BS103/p-GFPmut2 as described above and then incubated in medium containing gentamicin (100 μg/ml) for 2.5 additional hours. Cells were lifted nonenzymatically by use of Cellstripper (Mediatech, Inc.) according to the manufacturer's directions and subsequently stained with anti-Kb MHC-I antibody (BD Biosciences) and 7-amino-actinomycin D (7-AAD; eBioscience). Cells were then analyzed by flow cytometry.
Statistical analysis.
Data are expressed as means ± standard deviations (SD). The statistical significance of differences was analyzed using two-tailed Student's t test for paired samples or Fisher's test for nonpaired samples. Where appropriate, analysis of variance (ANOVA) and Bonferroni simultaneous tests were used to determine statistical significance in Minitab and Prism. A P value of <0.05 was considered statistically significant.
RESULTS
Shigella secreting the NP118-126 epitope via the type III secretion system does not prime CD8+ T-cell responses in vivo.
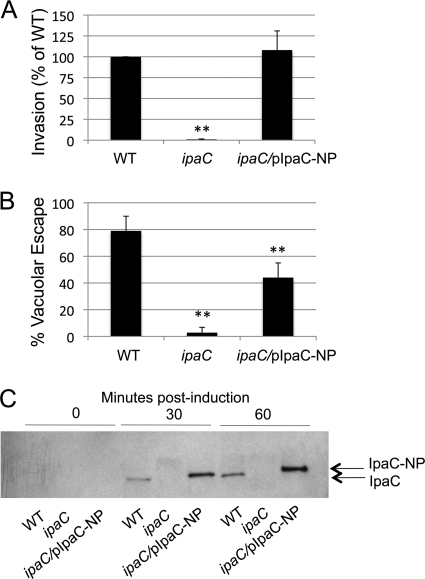
T-cell priming is a critical step in the initiation of a protective T-cell response. The activation, proliferation, and differentiation of naive CD8+ T cells into effector cells confer upon the cells the ability to secrete cytokines and lyse infected antigen-presenting cells. Previously, it was demonstrated that the depletion of CD8+ T lymphocytes from mice does not alter protection against Shigella challenge (45), but it was unclear whether CD8+ T cells are unable to be primed during Shigella infection or whether T cells are primed during infection but fail to protect upon challenge. Since no CD8+ T-cell epitopes from S. flexneri proteins have been identified to date, we constructed a Shigella strain expressing an epitope-tagged IpaC protein to determine if Shigella secreting a well-characterized CD8+ T-cell epitope could stimulate CD8+ T cells in mice. IpaC, which is required for both Shigella invasion and escape into the cytosol of host cells, is secreted by the TTSS into the cytoplasm of host cells upon contact of bacteria with host cells (5, 29); therefore, the secretion of an IpaC-epitope fusion protein into the cytoplasm of cells should allow access of the epitope to the MHC-I processing and presentation pathway. To create a plasmid encoding an IpaC-epitope fusion protein, a fragment encoding the MHC-I-restricted CD8+ T-cell epitope NP118-126 (which is presented by H-2Ld MHC-I) from LCMV was ligated into the ipaC gene in frame after codon 57. The location for the insertion of the epitope into IpaC was chosen based upon a previous study showing that incorporation of a 14-amino-acid fragment at this location did not disrupt the function of IpaC (5). The resulting plasmid, pIpaC-NP, designed to constitutively express the recombinant gene, was then used to transform the ipaC null strain SF621, forming strain ipaC/pIpaC-NP. To assess the ability of plasmid pIpaC-NP to complement an ipaC mutant for cellular invasion and phagosomal escape, the recombinant ipaC/pIpaC-NP strain was tested for the ability to enter cells and escape from phagosomes. A gentamicin protection assay demonstrated that complementation of ipaC with pIpaC-NP restored the invasiveness of the ipaC mutant into HeLa cells to 100% of WT levels (Fig. 1 A). The ability of the strain to escape from phagosomes was evaluated using a chloroquine resistance assay in which chloroquine accumulates in phagosomes and kills bacteria that do not escape to the cytoplasm. As expected, the WT strain escaped from phagosomes with 79% efficiency, while the ipaC mutant largely remained localized in the phagocytic vacuole and demonstrated a basal level of escape to the cytoplasm (3% that of the WT) (Fig. 1B). Complementation of ipaC with pIpaC-NP restored phagosomal escape of the recombinant strain to 44% of that of the WT strain. To confirm that IpaC-NP was secreted from the recombinant strain, the kinetics of the secretion of IpaC into the culture supernatant by the recombinant strain was examined through Congo red induction. IpaC-NP was secreted into the culture supernatant of the recombinant strain as soon as 30 min postinduction and was present at levels slightly higher than the levels of WT IpaC secreted by the WT strain (Fig. 1C) at all time points examined. Collectively, these experiments demonstrate that IpaC expressed by the recombinant strain is functional and, importantly, that the NP epitope is secreted by the bacterium into the host cell cytoplasm early during infection.
Fig. 1.
IpaC-NP is secreted and can functionally complement ΔipaC for invasion and phagosomal escape. (A) HeLa cells were infected with the indicated strains at an MOI of 10:1 and subsequently incubated for 1.5 h in medium containing gentamicin before enumeration of bacterial CFU. Values represent the means of the invasion efficiencies (normalized to the WT) for three independent experiments. (B) J774 cells were infected and then incubated for 1.5 h in medium containing gentamicin, with or without chloroquine, before determination of bacterial CFU. % vacuolar escape was calculated for each strain, and values represent the means for three independent experiments. **, P < 0.01. (C) Type III secretion of bacterial proteins was induced in the presence of Congo red for the indicated times. Supernatants from induced cultures were precipitated with TCA and analyzed by immunoblot analysis for IpaC (bl-15). Data are representative of two independent experiments.
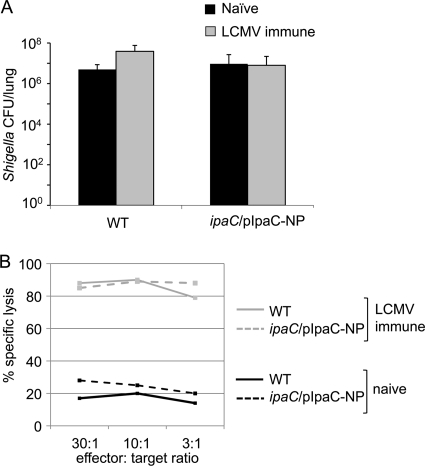
To determine whether NP118-126-specific CD8+ T cells could protect mice against Shigella expressing NP118-126, BALB/c mice, which express H-2d MHC-I, were first vaccinated with LCMV, priming a robust NP118-126-specific CD8+ T-cell response. Four weeks later, naïve or LCMV-immune mice were challenged i.n. with 107 WT S. flexneri organisms or S. flexneri organisms expressing the IpaC-NP fusion protein. If MHC-I molecules on infected cells presented the NP118-126 epitope from the IpaC-NP fusion protein, then NP-specific CD8+ T cells present in the LCMV-immune mice might have a protective effect during challenge with Shigella expressing IpaC-NP. Surprisingly, there was no decrease in the load of ipaC/pIpaC-NP in the lungs of LCMV-immune mice versus naïve mice at 24 h postchallenge (Fig. 2 A), demonstrating that antigen-specific CD8+ T cells did not have a protective effect during S. flexneri infection. A 51Cr release assay confirmed that NP118-126-specific T cells from the LCMV-immune mice were fully cytotoxic and able to lyse peptide-coated target cells in vitro (Fig. 2B), confirming the presence of NP118-126-specific CD8+ T cells in vaccinated mice but their failure to contain infection with Shigella expressing the NP118-126 tag.
Fig. 2.
NP118-126-specific CD8+ T cells do not protect against ipaC/pIpaC-NP challenge. Groups of mice (5 mice/group) were injected i.p. with 4 × 105 PFU of LCMV or with PBS. At 4 weeks postimmunization, mice were challenged i.n. with 1 × 107 CFU of the indicated Shigella strains. (A) Twenty-four hours later, bacterial loads (CFU/lung) were determined. Lysates from ipaC/pIpaC-NP-infected mice were plated on agar plates containing ampicillin (100 μg/ml). Values represent the means of measurements for triplicate samples of a representative experiment of 2 independent experiments, and error bars indicate SD. (B) Splenocytes from these mice were also harvested and restimulated on NP118-126 peptide-coated cells. Following 5 days of in vitro stimulation, the splenocytes were added to target cells that had previously been incubated with 100 μCi 51Cr, with or without 1 μM NP118-126 peptide. After 4 h, release of 51Cr into cell supernatants was quantified.
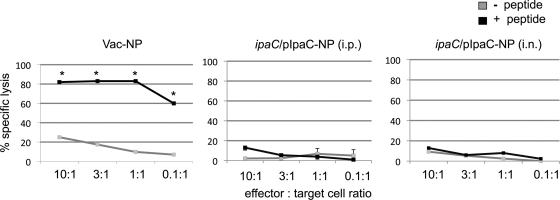
Because our results suggested that NP118-126-specific T cells were unable to protect against Shigella expressing the NP118-126 fusion protein, we next sought to determine whether ipaC/pIpaC-NP could stimulate a detectable local or systemic primary NP118-126-specific CD8+ T-cell response in mice. Mice were infected with ipaC/pIpaC-NP, either i.n. or i.p., or with recombinant vaccinia virus expressing the LCMV NP protein (Vac-NP) as a positive control. At 2 weeks postinfection, draining lymph nodes (from i.n. infected mice) or splenocytes (from i.p. infected mice) were harvested and stimulated on irradiated syngeneic spleen cells that had previously been incubated with NP118-126 peptide. After 5 days of in vitro stimulation, the cells were assayed for the ability to lyse NP118-126 peptide-coated target cells in a 51Cr release assay. Cultures from ipaC/pIpaC-NP-infected mice were not able to lyse peptide-treated cells more efficiently than untreated cells (Fig. 3), clearly demonstrating that this strain was unable to stimulate a cytolytic CD8+ T-cell response. Infection of mice with a different strain of Shigella secreting OVA257-264 similarly failed to induce proliferation or a considerable change in CD62L or CD44 expression (data not shown) of OVA257-264-specific TCR transgenic CD8+ T cells transferred into these mice. Collectively, these results demonstrate that CD8+ T cells not only fail to provide protection against Shigella infection but also remain largely unstimulated during infection.
Fig. 3.
NP118-126-specific CD8+ T cells are not primed in mice infected with Shigella secreting the NP118-126 peptide epitope. Groups of mice (3 mice/group) were infected with 108 CFU ipaC/pIpaC-NP, either i.p. or i.n., or with recombinant vaccinia virus expressing NP118-126 (5 × 106 PFU i.p.). Twelve days later, splenocytes (from i.p. infected mice) or lung draining lymph nodes (from i.n. infected mice) were harvested and stimulated on NP118-126 peptide-coated target cells. Following 5 days of in vitro stimulation, the cells were added to target cells that had previously been incubated with 100 μCi 51Cr, with or without 1 μM NP118-126 peptide. After 4 h, release of 51Cr into cell supernatants was quantified. Data are representative of two independent experiments. *, P < 0.05 compared to nonspecific lysis, as determined by Student's t test.
Antigens delivered into the cytoplasm of Shigella-infected host cells fail to stimulate T cells.
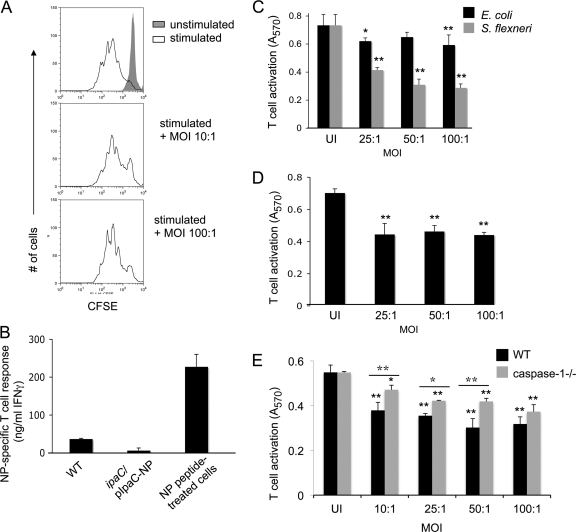
One explanation for the inability of Shigella cells expressing the NP tag to stimulate a T-cell response in vivo is that Shigella regulates the spatial and/or temporal expression of IpaC such that a T-cell response fails to develop against this protein. Additionally, it is possible that Shigella actively inhibits the development of T-cell responses during infection. Our lab has previously shown that Salmonella directly inhibits T-cell proliferation and cytokine secretion in a contact-dependent manner (43). To determine whether Shigella employs a similar immune evasion strategy, T cells were directly infected with Shigella for 3 h to allow for the delivery of bacterial effectors, resuspended in medium containing penicillin-streptomycin to kill the bacteria, and then stimulated for 3 days by CD3ε/CD28 ligation. Shigella did not inhibit the proliferation of T cells in this system (Fig. 4 A); however, it is possible that T-cell proliferation would have been affected in the absence of antibiotic treatment due to Shigella-induced cell death. In an effort to clarify why Shigella was unable to stimulate antigen-specific CD8+ T cells in vivo, we next examined the ability of antigen-presenting cells infected with ipaC/pIpaC-NP to stimulate an NP-specific CD8+ T-cell response in vitro. Murine J774 (H-2d) macrophages, which are of the same genetic background as BALB/c mice, were infected with ipaC/pIpaC-NP and subsequently incubated with NP118-126-specific CD8+ T cells and irradiated syngeneic splenocytes. Twelve hours later, T-cell activation was measured by quantifying the production of IFN-γ by the T cells, using an ELISA. Surprisingly, NP118-126-specific CD8+ T cells were not stimulated by J774 cells infected with ipaC/pIpaC-NP (Fig. 4B), suggesting that the NP118-126 epitope was not presented efficiently for the stimulation of T cells. RAW264.7 cells infected with this strain were also unable to stimulate NP118-126-specific T cells (data not shown). This finding suggests that Shigella blocks MHC-I-restricted presentation of Shigella-specific antigens in infected macrophages. This demonstrates that T cells not only fail to become cytolytic during Shigella infection, as our in vivo experiments suggested, but also remain completely unstimulated when cultured with infected cells.
Fig. 4.
Antigens delivered into the cytoplasm of Shigella-infected cells fail to simulate antigen-specific CD8+ T cells. (A) CFSE-labeled naïve T cells were infected with S. flexneri at the indicated MOIs for 3 h and subsequently stimulated for 3 days by anti-CD3ε/anti-CD28 ligation in the presence of penicillin and streptomycin. Cells were gated on live cells. (B) Infected or NP118-126-coated J774 cells were incubated with NP118-126-specific CD8+ T cells and irradiated syngeneic spleen cells. Following 12 h of incubation, the amounts of IFN-γ present in the culture supernatants were determined by ELISA. (C to E) C57BL/6 BMM (C and E), 1308.1 epithelial cells (D), or caspase 1−/− BMM (E) were infected with S. flexneri or E. coli at the indicated MOIs and subsequently incubated with LFn-Ova257-264 and PA for 1 h. B3Z T cells were added, and at 8 h postinfection, B3Z T-cell activation was determined as described in Materials and Methods. Values represent the means of measurements for triplicate samples of a representative experiment, and error bars indicate SD. UI, uninfected. Data are representative of 3 (A), 2 (B), or at least 5 (C to E) experiments. For panels C to E, ANOVA and Bonferroni simultaneous tests were used for statistical analysis. Unless indicated otherwise, significance is for comparisons to uninfected congenic control cells (*, P < 0.05; **, P < 0.01.) For panel E, values for WT-infected cells were significantly different (P < 0.01) from those for uninfected WT cells at all MOIs tested. Values for caspase-1−/− infected cells were significantly different at all MOIs tested from those for uninfected caspase-1−/− cells. Differences between WT and caspase-1−/− cells were significant at MOIs from 10:1 to 50:1.
Although it is likely that Shigella-induced cell death contributes to the inhibition of antigen presentation in infected cells, it is also possible that a redundant effector mechanism actively interferes with the presentation of antigens at the cell surface and therefore interferes with the development of T-cell-mediated immunity. Because our method for studying immune responses using the IpaC-NP fusion depended upon a functional TTSS, we chose an alternate system for delivering antigens into the host cell cytoplasm that would allow us to determine if the inhibition of antigen presentation was specific to virulent Shigella. Our lab has developed and extensively characterized an antigen delivery system based on the ability of lethal factor (LF) protein from anthrax toxin to bind and enter host cells. LF, which requires PA for its translocation to the cytosol of eukaryotic cells, is endocytosed by cells and translocated to the cytosol following endosomal acidification. By tagging of nontoxic N-terminally truncated lethal factor (LFn) with the MHC-I-restricted OVA257-264 epitope from ovalbumin, OVA257-264 can be delivered into the cytosol of host cells in vitro. Using this system, OVA257-264 enters the antigen processing pathway and OVA-specific CD8+ T cells are stimulated by the resulting OVA257-264–MHC-I complexes (2–4, 11, 26, 49). To determine whether antigens delivered into the cytoplasm of mammalian cells would be processed and presented when the cells were infected with Shigella, BMM were infected with S. flexneri or E. coli at the indicated MOIs and subsequently treated with LFn-OVA257-264 and PA for 1 h to allow for the delivery of OVA257-264 peptide into the host cell cytosol. The use of the LFn delivery system, in which OVA257-264 is presented by H-2Kb MHC-I, required that we use BMM derived from C57BL/6 (H-2b) mice for these experiments. The infected and uninfected cells were then resuspended with B3Z T cells, an OVA257-264-specific CD8+ T-cell hybridoma that synthesizes β-galactosidase upon activation (21, 36), to detect the presentation of the ovalbumin epitope. While a minimal block in antigen presentation occurred when the BMM were infected with E. coli, S. flexneri infection significantly decreased the presentation of OVA257-264 to T cells in a manner that correlated with increasing MOIs (Fig. 4C). The ability of Shigella to decrease antigen presentation in infected cells was dependent upon entry of the bacterium into cells and did not occur when cells were infected with heat-killed WT Shigella (data not shown). The correlation between decreasing presentation and increasing MOIs may have resulted from a larger number of cells being infected in wells with higher MOIs than in those with low MOIs. Additionally, it may be that a larger number of Shigella organisms per individual cell is required to block macrophages from presenting antigen. Because only a portion of the cells were infected in wells to which Shigella was added, it is possible that the B3Z response observed in these wells is completely attributable to uninfected cells. Alternatively, it is possible that Shigella-infected cells are not completely blocked from presenting antigen and therefore make limited contributions to the T-cell response. Because Shigella naturally infects epithelial cells in the rectal submucosa, we repeated these experiments using 1308.1 (H-2b) epithelial cells and again observed a significant decrease in antigen presentation in infected cells (Fig. 4D), suggesting that the presentation of MHC-I-restricted Shigella antigens in the submucosal epithelium might be inhibited similarly.
It is interesting to speculate that Shigella engages an effector mechanism to specifically inhibit antigen presentation. However, we acknowledged that the block in antigen presentation could be due, or partly due, to cell death of infected macrophages. It is well established that Shigella activates caspase-1-mediated pyroptosis in macrophages following binding of the type III secreted effector IpaB to caspase-1 (8, 9, 51, 52). Therefore, the onset of cell death in caspase-1-deficient BMM is significantly delayed and is less robust than that in WT BMM (16, 42). To determine if the ability of Shigella to block antigen presentation was dependent upon caspase-1-mediated cell death, caspase-1−/− BMM were infected and then tested for the ability to present OVA peptide to T cells, using the anthrax toxin antigen delivery system. Although caspase-1 contributed to the inhibition of OVA257-264 presentation during infection for several MOIs tested (by comparing infected WT cells to infected caspase-1−/− cells), presentation was also significantly inhibited in infected caspase-1−/− cells compared to uninfected cells (Fig. 4E). This finding demonstrates that the inability of infected macrophages to present antigen is not entirely dependent upon this major mechanism of cell death.
Cells infected with Shigella present low levels of MHC-I on the cell surface.
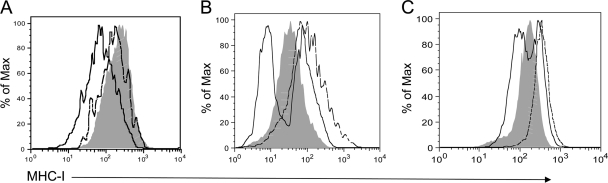
The inability of Shigella-infected cells to present antigen to T cells suggested that MHC-I–antigen complexes might be inhibited from reaching the cell surface or might actively be downregulated during infection, resulting in a lack of T-cell stimulation. To test this hypothesis, cell surface levels of MHC-I on infected cells were analyzed. 1308.1 epithelial cells were infected with green fluorescent protein (GFP)-expressing WT or virulence plasmid-cured Shigella (which lacks the TTSS and therefore remains within the phagocytic vacuole) for 3 h and then analyzed for surface MHC-I expression by flow cytometry. Interestingly, infection of cells with WT Shigella harboring the virulence plasmid reduced the expression of MHC-I molecules on the epithelial cell surface within 3 h of infection (Fig. 5 A). In contrast, infection of cells with plasmid-cured Shigella did not affect the expression of MHC-I relative to that in uninfected cells, indicating that one or more virulence plasmid-encoded proteins or access to the cytoplasm is required for the downregulation of surface MHC-I. We repeated this experiment using WT and caspase-1−/− BMM to determine if a reduction in cell surface MHC-I expression might explain the inability of macrophages to present Shigella-specific antigens to T cells. While infection of WT or caspase-1−/− BMM with plasmid-cured Shigella induced the upregulation of surface MHC-I relative to that in uninfected cells (Fig. 5B and C), infection of cells with WT Shigella reduced surface MHC-I expression on a population of macrophages, similar to the phenotype we observed for epithelial cells. Infection of J774 and RAW264.7 macrophages (which each express H-2d MHC-I) similarly resulted in the reduction of surface MHC-I expression compared to that in uninfected cells (data not shown). Interestingly, a subset of both WT and caspase-1−/− macrophages infected with WT Shigella also displayed high levels of MHC-I, similar to levels induced by virulence plasmid-cured Shigella, at 3 h postinfection. This observation may be explained by the early induction of preformed MHC-I–antigen complexes on the cell surface following stimulation of pathogen recognition receptors during infection. It is also possible that these cells carried different bacterial loads from those of cells that downregulated MHC-I or were uninfected cells carrying outer membrane-associated extracellular bacteria (thus marking the cells as GFP+ “infected” cells). To determine if Shigella-induced cell death could be distinguished from the downregulation of MHC-I on infected cells, infected 1308.1 cells, WT BMM, and caspase-1−/− BMM were stained with 7-AAD, a fluorescent DNA-intercalating agent that penetrates cells with compromised membrane permeability. However, at 3 h postinfection, infected cells that downregulated MHC-I had already incorporated 7-AAD (data not shown). While it is possible that Shigella employs an active mechanism to block MHC-I–antigen complexes from reaching the cell surface, the onset of cell death in Shigella-infected cells impeded our efforts to determine if such a mechanism exists. Interestingly, however, 7-AAD+ cells infected with plasmid-cured Shigella did not downregulate MHC-I at the time points we examined, suggesting that the downregulation of class I molecules by virulent Shigella is not simply the reflection of a general mechanism of bacterium-induced cell death. Whether due to cell death or to a direct effect on antigen presentation, the reduction of class I molecules on the surface of infected cells likely impacts the presentation of microbial antigens to T cells and therefore limits the development of T-cell-mediated immunity during Shigella infection.
Fig. 5.
MHC-I is downregulated on the surfaces of infected cells. 1308.1 epithelial cells (A), WT BMM (B), and caspase-1−/− BMM (C) were infected at an MOI of 10:1 with WT (solid black line) or virulence plasmid-cured (dotted line) Shigella expressing GFP or were mock infected (shaded histogram). Cells were analyzed at 3 h postinfection for cell surface MHC-I expression by flow cytometry. A total of 10,000 cells of the appropriate forward and side scatter for each cell type were collected for each well and were used for subsequent analysis. In the case of cells from infected wells, cells were gated on GFP+ cells to detect infected cells. Data are representative of at least three independent experiments.
DISCUSSION
Previous studies examining the protective role of T cells during S. flexneri infection were limited to analyses of global T cells with undefined antigen specificities. In the present study, we report the first examination of an antigen-specific CD8+ T-cell response to a Shigella strain secreting an MHC-I-restricted peptide epitope via the TTSS. Various pathogens, including Salmonella and Yersinia, have been engineered to secrete heterologous antigenic peptides into cells, resulting in stimulation of both CD4+ and CD8+ T cells (34, 35). Here we report that the delivery of heterologous MHC-I-restricted antigens by Shigella fails to prime CD8+ T cells in vivo or in vitro. We went on to demonstrate that Shigella interferes with MHC-I-restricted antigen presentation of both Shigella-specific and heterologous antigens in infected macrophages, which may contribute to the failure of T cells to be primed. Collectively, these findings suggest a possible mechanism for the inefficient and short-lived natural protective immunity that is generated during human infection.
Surprisingly, we found that CD8+ T cells not only were deficient in controlling bacterial growth when large numbers of antigen-specific T cells were present (Fig. 2) but also failed to become activated during primary infection (Fig. 3). These results are consistent with a recent report by Sellge et al. that demonstrated that unlike CD4+ T cells, CD8+ T cells fail to secrete IFN-γ or to upregulate CD44 following secondary Shigella infection (41). The report went on to show that Shigella infection strongly favors the induction of a Th17/interleukin-17A (IL-17A) response over a Th1/IFN-γ response and that Th17 cells mediate adaptive immunity during infection. Together, these reports establish that Th17 CD4+, but not CD8+, T cells can mediate protective immunity to Shigella flexneri. Interestingly, it has been suggested that CD8+ T cells primed in the presence of Th17-skewing cytokines, conditions such as those present during Shigella infection, develop into IL-17-producing cells with reduced cytolytic functions (17). Therefore, it is possible that these conditions may contribute to the reduced ability of CD8+ T cells to be primed optimally by Shigella in vivo. However, this finding does not entirely explain the inability of T cells that were fully primed by LCMV immunization to be ineffective at controlling Shigella challenge (Fig. 2) or the failure of antigen-specific T cells to respond to infected macrophages in vitro (Fig. 4B).
Our data presented here suggest that one mechanism that may contribute to the lack of CD8+ T-cell priming during infection is a loss of the antigen presentation capacity of Shigella-infected cells, since analysis of the T-cell response to Shigella in vitro revealed that antigen-specific T cells were not activated by macrophages infected with Shigella secreting a well-characterized antigen (Fig. 4B). Although it is likely that Shigella-induced cell death contributes to the inhibition of antigen presentation, it is possible that an effector mechanism actively interferes with the presentation of antigens and therefore interferes with the development of T-cell-mediated immunity. The process of peptide loading onto newly synthesized H-2Db MHC-I molecules and their subsequent presentation at the cell surface requires as few as 2 h (47); therefore, although it is likely that most of the infected macrophages would have undergone Shigella-mediated cell death by the completion of the assay, any NP118-126–MHC-I complexes presented at early time points postinfection (as early as approximately 2 h postinfection) could have generated a detectable T-cell response prior to a general inhibition of molecular trafficking resulting from cell death. The failure of T cells to be stimulated by ipaC/pIpaC-NP suggests that the NP118-126 peptide was never presented for the stimulation of T cells by MHC-I, even at early time points. Because the delivery of CD8+ T-cell antigens requires the ability of bacteria to reach the cell cytoplasm, it was not possible by use of the techniques described here to determine if a mutant with a defect in type III secretion would have been able to stimulate a T-cell response.
We went on to show that Shigella not only blocks presentation of Shigella-specific antigens but also decreases the capacity of macrophages to present heterologous antigens delivered to the host cytosol (Fig. 4C and E). The finding that infected epithelial cells are similarly inhibited from presenting heterologous antigens during infection (Fig. 4D) may also explain the failure of CD8+ T cells to be primed in vivo, since infected epithelial cells of mouse bronchopulmonary tissue should have been able to present Shigella-derived NP118-126 antigen to T cells to stimulate a protective response, even if macrophages were dysfunctional for presentation. The observation that a population of macrophages upregulate MHC-I following Shigella infection (Fig. 5B and C) suggests that trafficking of preformed MHC-I–antigen complexes may not be blocked at early time points postinfection, even though Shigella sufficiently disrupts the function of the host cell to block antigens delivered to the cytosol at the time of infection from ever reaching the cell surface (Fig. 4B). Therefore, it appears that Shigella-infected cells fail to present MHC-I-restricted antigens at the cell surface if those antigens are delivered to the cytosol at any point postinfection. Collectively, these findings suggest that Shigella directly targets antigen-presenting cells to affect the development of T-cell-mediated immunity during infection.
The ability of Shigella to inhibit MHC class I presentation and the presentation of heterologous antigens is not dependent upon caspase-1-mediated cell death (Fig. 4E and 5C). However, it is possible that caspase-1-independent mechanisms of cell death play a role in inhibiting presentation. A recent report by Suzuki et al. demonstrated that Shigella-induced cell death, measured by the release of lactate dehydrogenase (LDH) into the culture supernatant, was delayed by at least 2 h in caspase-1-deficient cells but that LDH release comparable to that in WT cells occurred by 5 h postinfection (42). Therefore, since we analyzed the ability of caspase-1−/− macrophages to stimulate T cells at 8 h postinfection, caspase-1-independent cell death could account for the decrease in the presentation of OVA257-264 peptide. Although it is likely that Shigella-induced cell death contributes to the inhibition of antigen presentation in infected cells, it is also possible that a redundant effector mechanism functions concurrently with Shigella-induced cell death to inhibit antigen presentation. This hypothesis is supported by the observation that macrophages infected with ipaC/pIpaC-NP were unable to stimulate NP118-126-specific CD8+ T cells at any point during the infection (Fig. 4B). Additionally, we found that the presentation of cytoplasmic OVA peptide and the presentation of MHC-I molecules were inhibited significantly in epithelial cells, as early as 8 and 3 h postinfection, respectively, even though cell death is significantly delayed in nonmyeloid cells compared to that in macrophages (7). Collectively, our data suggest that a mechanism other than cell death may function to inhibit CD8+ T-cell responses in vivo as well as in vitro.
The mechanism described here is unlikely to be the only strategy used by Shigella to inhibit CD8+ T-cell responses, since the presentation of antigens by cross-presentation would most likely not be inhibited by the downregulation of MHC-I or by macrophage cell death. In fact, the induction of apoptosis in macrophages by Mycobacterium tuberculosis or Salmonella enterica serovar Typhimurium has been shown to facilitate MHC-I-restricted antigen presentation following the uptake of apoptotic vesicles carrying bacterial antigens by uninfected dendritic cells (40, 48). Therefore, it is likely that the failure of antigen-specific CD8+ T cells to protect against Shigella results from a variety of immune evasion mechanisms, several of which have been described. Phenotypic analysis of apoptotic cells in the lamina propria during acute infection revealed that approximately 40% of T cells undergo cell death during acute infection, suggesting that the number of T cells available to interact with other immune cells is limited during Shigella infection (53). This finding correlated with reduced expression of IL-2 and antiapoptotic Bcl-2 at the site of infection, which may have contributed to the lack of T-cell survival. Additionally, manipulation of innate immunity by Shigella, including the inhibition of NF-κB activation by the effector OspG (22) and the systemic downregulation of IFN-γ during acute infection (32), could contribute to an inefficient CD8+ T-cell response. Therefore, it is likely that Shigella engages several mechanisms to interfere with T-cell priming. Our findings demonstrate that interference with MHC-I-restricted antigen presentation is one mechanism through which this is accomplished.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI055962 to M.N.S. and grant AI043562 to M.B.G.
We thank C. Parsot, M. Oldstone, and N. Shastri for providing various reagents.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballard J. D., Collier R. J., Starnbach M. N. 1998. Anthrax toxin as a molecular tool for stimulation of cytotoxic T lymphocytes: disulfide-linked epitopes, multiple injections, and role of CD4(+) cells. Infect. Immun. 66:4696–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard J. D., Collier R. J., Starnbach M. N. 1996. Anthrax toxin mediated delivery of a cytotoxic T-cell epitope in vivo. Proc. Natl. Acad. Sci. U. S. A. 93:12531–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballard J. D., Doling A. M., Beauregard K., Collier R. J., Starnbach M. N. 1998. Anthrax toxin-mediated delivery in vivo and in vitro of a cytotoxic T-lymphocyte epitope from ovalbumin. Infect. Immun. 66:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barzu S., Benjelloun-Touimi Z., Phalipon A., Sansonetti P., Parsot C. 1997. Functional analysis of the Shigella flexneri IpaC invasin by insertional mutagenesis. Infect. Immun. 65:1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. U. S. A. 86:3867–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carneiro L. A., et al. 2009. Shigella induces mitochondrial dysfunction and cell death in nonmyeloid cells. Cell Host Microbe 5:123–136 [DOI] [PubMed] [Google Scholar]
- 8. Chen Y., Smith M. R., Thirumalai K., Zychlinsky A. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853–3860 [PMC free article] [PubMed] [Google Scholar]
- 9. Clerc P. L., Ryter A., Mounier J., Sansonetti P. J. 1987. Plasmid-mediated early killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect. Immun. 55:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cormack B. P., Valdivia R. H., Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38 [DOI] [PubMed] [Google Scholar]
- 11. Doling A. M., et al. 1999. Cytotoxic T-lymphocyte epitopes fused to anthrax toxin induce protective antiviral immunity. Infect. Immun. 67:3290–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dutko F. J., Oldstone M. B. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689–1698 [DOI] [PubMed] [Google Scholar]
- 13. Faas S. J., Rothstein J. L., Kreider B. L., Rovera G., Knowles B. B. 1993. Phenotypically diverse mouse thymic stromal cell lines which induce proliferation and differentiation of hematopoietic cells. Eur. J. Immunol. 23:1201–1214 [DOI] [PubMed] [Google Scholar]
- 14. Finlay B. B., Falkow S. 1988. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie 70:1089–1099 [DOI] [PubMed] [Google Scholar]
- 15. Fling S. P., et al. 2001. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc. Natl. Acad. Sci. U. S. A. 98:1160–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilbi H., et al. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895–32900 [DOI] [PubMed] [Google Scholar]
- 17. Huber M., et al. 2009. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39:1716–1725 [DOI] [PubMed] [Google Scholar]
- 18. Islam D., Christensson B. 2000. Disease-dependent changes in T-cell populations in patients with shigellosis. APMIS 108:251–260 [DOI] [PubMed] [Google Scholar]
- 19. Islam D., Veress B., Bardhan P. K., Lindberg A. A., Christensson B. 1997. Quantitative assessment of IgG and IgA subclass producing cells in rectal mucosa during shigellosis. J. Clin. Pathol. 50:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Islam D., Wretlind B., Lindberg A. A., Christensson B. 1996. Changes in the peripheral blood T-cell receptor V beta repertoire in vivo and in vitro during shigellosis. Infect. Immun. 64:1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karttunen J., Sanderson S., Shastri N. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 89:6020–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D. W., et al. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. U. S. A. 102:14046–14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labrec E. H., Schneider H., Magnani T. J., Formal S. B. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 88:1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le-Barillec K., et al. 2005. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 175:1735–1740 [DOI] [PubMed] [Google Scholar]
- 25. Li P., et al. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401–411 [DOI] [PubMed] [Google Scholar]
- 26. Lu Y., et al. 2000. Genetically modified anthrax lethal toxin safely delivers whole HIV protein antigens into the cytosol to induce T cell immunity. Proc. Natl. Acad. Sci. U. S. A. 97:8027–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyons A. B., Hasbold J., Hodgkin P. D. 2001. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol. 63:375–398 [DOI] [PubMed] [Google Scholar]
- 28. Maurelli A. T., Blackmon B., Curtiss R., III 1984. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect. Immun. 43:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menard R., Prevost M. C., Gounon P., Sansonetti P., Dehio C. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 93:1254–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menard R., Sansonetti P. J., Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899–5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phalipon A., et al. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raqib R., Gustafsson A., Andersson J., Bakhiet M. 1997. A systemic downregulation of gamma interferon production is associated with acute shigellosis. Infect. Immun. 65:5338–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raqib R., et al. 2002. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand. J. Immunol. 55:414–423 [DOI] [PubMed] [Google Scholar]
- 34. Russmann H., et al. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565–568 [DOI] [PubMed] [Google Scholar]
- 35. Russmann H., et al. 2000. Yersinia enterocolitica-mediated translocation of defined fusion proteins to the cytosol of mammalian cells results in peptide-specific MHC class I-restricted antigen presentation. Eur. J. Immunol. 30:1375–1384 [DOI] [PubMed] [Google Scholar]
- 36. Sanderson S., Shastri N. 1994. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6:369–376 [DOI] [PubMed] [Google Scholar]
- 37. Sansonetti P., Phalipon A. 1996. Shigellosis: from molecular pathogenesis of infection to protective immunity and vaccine development. Res. Immunol. 147:595–602 [DOI] [PubMed] [Google Scholar]
- 38. Sansonetti P. J. 2001. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote-eukaryote cross-talks. FEMS Microbiol. Rev. 25:3–14 [DOI] [PubMed] [Google Scholar]
- 39. Sansonetti P. J., Kopecko D. J., Formal S. B. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaible U. E., et al. 2003. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9:1039–1046 [DOI] [PubMed] [Google Scholar]
- 41. Sellge G., et al. 2010. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J. Immunol. 184:2076–2085 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki T., et al. 2005. A novel caspase-1/Toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J. Biol. Chem. 280:14042–14050 [DOI] [PubMed] [Google Scholar]
- 43. van der Velden A. W., Copass M. K., Starnbach M. N. 2005. Salmonella inhibit T cell proliferation by a direct, contact-dependent immunosuppressive effect. Proc. Natl. Acad. Sci. U. S. A. 102:17769–17774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Voino-Yasenetsky M. V., Voino-Yasenetskaya M. K. 1962. Experimental pneumonia caused by bacteria of the Shigella group. Acta Morphol. Acad. Sci. Hung. 11:439–454 [PubMed] [Google Scholar]
- 45. Way S. S., Borczuk A. C., Goldberg M. B. 1999. Thymic independence of adaptive immunity to the intracellular pathogen Shigella flexneri serotype 2a. Infect. Immun. 67:3970–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welsh R. M., Jr., Lampert P. W., Burner P. A., Oldstone M. B. 1976. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology 73:59–71 [DOI] [PubMed] [Google Scholar]
- 47. Williams D. B., Swiedler S. J., Hart G. W. 1985. Intracellular transport of membrane glycoproteins: two closely related histocompatibility antigens differ in their rates of transit to the cell surface. J. Cell Biol. 101:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yrlid U., Wick M. J. 2000. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191:613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zarozinski C. C., Collier R. J., Starnbach M. N. 2000. Use of anthrax toxin fusions to stimulate immune responses. Methods Enzymol. 326:542–551 [DOI] [PubMed] [Google Scholar]
- 50. Zychlinsky A., et al. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619–627 [DOI] [PubMed] [Google Scholar]
- 51. Zychlinsky A., Kenny B., Prevost M. C., Holland I. B., Sansonetti P. J. 1993. The ipaB gene of Shigella flexneri and macrophage-programmed cell death. Infect. Agents Dis. 2:212–214 [PubMed] [Google Scholar]
- 52. Zychlinsky A., Prevost M. C., Sansonetti P. J. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167–169 [DOI] [PubMed] [Google Scholar]
- 53. Zychlinsky A., et al. 1996. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64:5357–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]