Abstract
Spermatogenesis within the adult testis is an excellent system for studying stem cell renewal and differentiation, which is under the control of testicular somatic cells. In order to understanding spermatogenesis in the half-smooth tongue sole (Cynoglossus semilaevis) as a marine fish model of aquaculture importance, we established a cell line called CSGC from a juvenile gonad of this organism. CSGC is composed of fibroblast-like cells, retains a diploid karyotype of 42 chromosomes, lacks the heterogametic W chromosome, lacks a female specific marker and expresses the dmrt, a marker for testicular somatic cells. Therefore, CSGC appears to consist of testicular somatic cell cells. We show that this cell line is effective for infection by the turbot reddish body iridovirus and flounder lymphocystis disease virus as evidenced by the appearance of cytopathic effect and virus propagation in the virus-infected cells, and most convincingly, the observation of viral particles by electon microscopy, demonstrateing that CSGC is suitable to study interactions between virus and host cells. As a first fish testicular somatic cell line of the ZZ-ZW genetic sex determination system, CSGC will be a useful tool to study sex-related events and interactions between somatic cells and germ cells during spermatogenesis.
Keywords: half-smooth tongue sole, Cynoglossus semilaevis, gonadal cell line, Karyotype, cytopathic effects, virus susceptibility
Introduction
Cell lines provide an important in vitro system for investigations in physiology, virology, toxicology, carcinogenesis and transgenics. So far, many cell lines from freshwater and marine fish species have been reported since the first fish cell line—RTG-2 1-3. These cell lines have been developed from a broad range of tissues such as the fin, kidney, muscle, liver, and embryos 4-8. Gonadal cell lines have not or rarely been developed from marine fish species 9, 10.
Gonadal somatic cell lines are of significance to study how the somatic environment can control the self-renewal of germ stem cells and their differentiation into gametes, namely oogenesis in the ovary and spermatogenesis in the testis. Although in vitro spermatogenesis in mammals has so far ended up with the spermatid stage 11, 12, spermatogenesis in lower vertebrates can proceed fully in vitro. Specifically, sperm production in vitro has been reported in primary testicular cultures from several distantly related fish species. In the eel, all stages of spermatogenesis were established in organ culture of immature testes 13. In the medaka, fertile sperm were obtained during 10 days of primary cultures of spermatocytes at the meiotic prophase 14. In the zebrafish, dissociated testicular cells during 15 days of coculture on a feeder layer of Sertoli-like cells gave rise to fertile sperm 15. Remarkablely, Hong et al have found that spermatogonia from the medaka testis can develop into a stable stem cell line called SG3, which is capable of full recapitulation of spermatogenesis in vitro, including differentiation through meiosis to generate test-tube sperm 16. These results demonstrate that in fish, it is possible to establish germ cell cultures and all stages of spermatogenesis in vitro for test-tube sperma production and for the analysis of interactions between somatic cells and germ cells in these processes. To this end, the availability of various testicular cell lines will provide necessary tools. In addition, gonadal somatic cells are involved in the sex differentiation in germ cells and organisms 17, 18.
The half-smooth tongue sole (Cynoglossus semilaevis) has 42 chromosomes and the ZW sex-determining system 19. In this organism, several cell lines have been established from the heart and embryos 20, 21. The establishment of gonadal cell cultures will provide a potential material to study mechanisms underlying sex determination and sex-related gene expression and function. Here we report the establishment of a cell line from a gonadal cell line, called CSGC, from the testis of the half-smooth tongue sole, which appears to consist of somatic cells in morphology and gene expression. We tested its susceptibility to the flounder lymphocystis disease virus (LCDV) and turbot reddish body iridovirus (TRBIV).
Materials and Methods
Primary cell culture and subculture
A half-smooth tongue-sole weighing 500 g was obtained from the MingBo Fisheries Company in Laizhou, Shandong Province, China, and was disinfected with 75% ethanol. The gonad was taken and transferred to a dish, washed three times with phosphate-buffered saline (PBS) containing antibiotics (penicillin, 1000 IU/ml; streptomycin, 1000 μg/ml), and minced by scissors into small pieces (1 mm3), which were transferred into 25-cm2 tissue culture flasks containing 3 ml of MEM with 20% fetal bovine serum (FBS), 2 ng ml-1 bFGF and 1000 U of penicillin, 1000 U of streptomycin. The primary cells were maintained at 24 ℃. After three days, 2 ml of growth medium was added to the flasks. One half of the growth medium was changed every 3 days for first week. Monolayers of primary cells formed after two weeks of culture.
Primary cultures were dissociated by treatment with 0.25% trypsin-EDTA solution (Sigma) into single cells and transferred into another fresh 25 cm2 flask at a split ratio of 1:2. Dissociation was monitored under an inverted light microscope (Nikon TE2000-S) to ensure that cells had been released from the flask surface. Cells were initially maintained in MEM with 20% FBS. After 45 passages the concentration of FBS in MEM was reduced to 15%. During the first 30 subcultures, a MEM medium containing 20% FBS was used and the cells were split at a ratio of 1:2 every 5-7 days. From passage 30 onwards, the cells were subcultured every 3 or 4 days. To date, CSGC cell line has been subcultured for more than 55 passages.
Effect of different temperature and FBS concentration on cells growth
To analyze the growth requirement of the CSGC cells, 2 x 105 cells at passage 35 were inoculated in three wells of 12-well plate with MEM containing 20% FBS and incubated separately at 10℃, 20 ℃, 24 ℃ and 30℃ for growth curve tests, respectively. Following 1- 4 days post inoculation, cells in one well of different temperature were trypsinized, and cell numbers were measured microscopically via a hemocytometer. The effect of FBS concentration on cell growth at 24°C was evaluated in 6-well microplates for CSGC. The cells were seeded and incubated in MEM containing 5, 15, 20 and 25% FBS and incubated at 24°C. The cells were collected every day for four days and counted for three times in triplicate.
Cryopreservation and recovery of cells
Cells at ~90% confluence were used for cryostorage. Single cell suspension by trypsinization from each flask was collected in a 15 ml centrifuge tube and centrifuged at 1200 g for 3 min (Avanti-26XP, Beckman, USA). The cell pellet was resuspended at a density of 5 × 106 cells/ml in cold medium (4℃) containing 20% FBS, 10% dimethyl sulfoxide (DMSO) and 70% MEM medium. Cells were dispensed into 1.8-ml sterile plastic vial, which were put in a Styrofoam box, incubated at −80°C for 4 hours and transferred into liquid nitrogen for cryostorage. To re-initiate culture from frozen cells, the vial from liquid nitrogen was thawed at 40℃ for 1 min, and centrifuged at 1000 g for 4 min. The cells were suspended in fresh MEM and seeded into a 25 cm2-cell culture flask.
Chromosome analysis
Chromosome preparation for CSGC cells was carried out as described with some modifications 22. In brief, the CSGC cells at passage 25, 35, 50 were inoculated in 25 cm2 culture flasks and incubated at 24℃ for 36 h. Colchicine was added into the cells at 0.1 µg/ml. After 4 h incubation, the cells were treated with 6 ml of hypotonic solution of 0.075 M KCl for 30 min, and then pre-fixed for 3 min by dropping 1 ml of Carnoy's fixative (3:1 methanol:glacial acetic acid) into the above cell suspension. After 5 min centrifugation at 1200 g, the cell pellets were fixed with cold Carnoy's for 30 min. After centrifugation, cells were resuspended in 0.5 ml Carnoy's fixative, dropped and dispersed by blowing on cold glass slides. After air-drying, the slides were stained with 10% Giemsa (in 10 mM potassium phosphate, pH 6.8) for 30 min. The slides were observed and photographed under Nikon Eclipse 80I fluorescence microscope.
Sex genotyping of CSGC
The genetic sex of CSGC was determined by the presence or absence of a female-specific marker developed in our lab 23. Briefly, based on sequences of the female-specific AFLP fragments, a pair of specific PCR primers382 (CseF382N1:5'-ATTCACTGACCCCTGAGAGC-3'; CseF382C1: 5'-AACAACT CACACACGACAAATG-3') was designed. A PCR reaction system (25 μl) consisted of 1 ul of 10 pM of each primer, 1 ul of 2.5 mM of four dNTP each, 1 U of Taq polymerase (Promega, USA), 1.5 μl of 25 mM Mg2+, and 1 to 2.0 μl of 75 ng/μl of DNA as template. DNA was extracted from one female and one male soles as well as from CSGC cells. PCR was run as follows: initial incubation at 95 °C for 5 min, followed by 30 cycles of 95 °C, 30 s; 57 °C, 30 s; and 72 °C, 60 s, with a final extension of 5 min at 72 °C. The PCR products of 2 μl were resolved on 1.5% agarose gel with a DL2000 DNA marker.
RT-PCR analysis of dmrt1 expression in CSGC cells
A pair of specific primer of sex related gene, double sex and mab-3-related transcription factor1 (Dmrt1) (Dmrt-fw: GTCGCTGTGACA AGTGTAACCTC; Dmrt1-rv: TGAGACATCTGCTGGTATTGCTG) was used for PCR reaction system (15 μl). Total RNA was extracted from CSGC cells according to the Trizol R protocols (Invitrogen, Carlsbad, CA, USA) and first strand cDNA synthesized by RT-PCR with M-MLV reverse transcriptase (Promega,USA) was taken as template. PCR was run as follows: initial incubation at 94°C for 5 min, followed by 28 cycles of 94°C, 30 s; 56°C, 30 s; and 72°C, 40 s, with a final extension of 7 min at 72°C24.The amplification products of 12 ml were resolved on 1.5% agarose gel with a DL2000 DNA marker.
Virus challenge Assay
The LCDV and TRBIV were used to detect the CSGC cells susceptibilities to the virus. The titration value of LCDV-C and TRBIV determined based on TCID50 assay was 102 TCID50 ml-1 and 103 TCID50 ml-1. 2×105 cells ml-1 was seeded into a 25-cm2 flask. The infection was carried out by adding virus suspension into the cells, and one hour later, the virus solution was changed with new medium. The cytopathic effect (CPE) was observed and documented under a Nikon eclipse TE2000-U fluorescence microscope every 12 hours. The cells with and without CPE were harvested for PCR analysis and electron microscopy observation.
PCR analysis and electron microscopy
The CSGC cells DNA was extracted from the infected cells after infected with LCDV and TRBIV for 36 h. Two pair of primers specific to the major capsid protein segment of LCDV and TRBIV (LCDVF: 5'-CCGTTGATTCCAA TGGTCA-3'; LCDVR: 5'-CACCGTCAAAGATTACAGGAG-3') (TRBIVF5'-CGTGTTAAGATCCCCTCC-3'; TRBIVR 5'- TCTCGTAAATGAGTGACACC-3') were employed in PCRs. The reactions were performed as the following steps: denaturation at 95℃ for 5 min followed by 30 cycles of denaturation at 94℃ for 40 s, annealing at 57℃for 40 s and elongation at 72℃for 1 min, ending with an additional elongation step of 10 min at 72℃.
The CSGC cells infected with LCDV and TRBIV for 36 h were fixed with 2.5% glutaraldehyde in cacodylate buffer (0.1 M, pH 7.4) for 4 h at 4℃, rinsed in PBS buffer (0.1 M, pH 7.4) for 10 min with three times, then postfixed with 1% osmium tetroxide in cacodylate buffer (0.1 M, pH 7.4) for 2 h. After three rinses in PBS buffer (0.1 M, pH 7.4) for 10 min each, the specimens were dehydrated in an ethanol series (30%, 50%, 70%, 90%, 100%, 10 min every grade) and embedded in Epon812 epoxy resin. Ultra-thin sections were cut on a Reichert-Jung Ultracut-E microtome with a diamond knife, mounted on copper grids, stained with 2% uranyl acetate and lead citrate, and examined and photographed under a JEOL JEM-1200EX transmission electron microscope (TEM).
Results
Primary culture and subculture
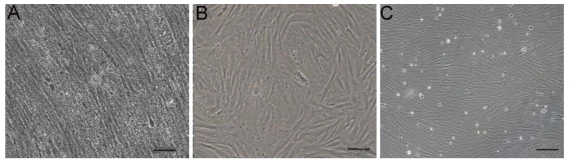
A monolayer of primary cultures was obtained from the gonad of a tongue sole at 15 day after fertilization. Its serical passage led to the establishment of the cell line called CSGC, which consists of fibroblastic cells (Fig. 1).
Fig 1.
Cell line derivation. (A) Gonadal cells primary culture at day 5. (B) Cells at passage 3. (C) Cells at passage 40. Cells in serial culture exhibited a fibroblast-like phenotype. Scale bars, 50 μm in (A and B) and 100 μm in (C).
Growth curve of CSGC cell line in different temperatures and different concentration of FBS
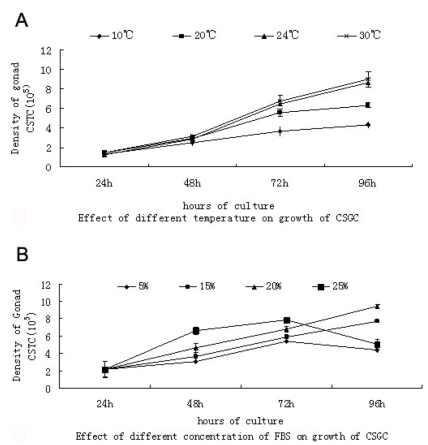
When tested at passage 35 at four different temperatures, CSGC exhibited optimal growth at 24 ℃ and satisfactory growth at 30 ℃, while no obvious cell growth was observed at 10 ℃ (Fig. 2A). Thus, CSGC was maintained at 24 ℃ for subsequent experiments.
Fig 2.
Growth requirement of CSGC cells. (A) Different temperatures. There was no obvious growth at 10℃, and similar growth is seen at 24℃ and 30℃. (B) Different FBS concentration. Optimal growth is seen with 20% FBS.
During the first 72 hour, CSGC cells had a great proliferation in MEM with 25% FBS and 20%FBS.But changes happened in the next 24 hour. Cells in MEM with 25% FBS decreased dramatically probably because the high concentration of FBS was harmful for the cells, cause the death of cells (Fig. 2B). The same effect had not found in other lower concentration of FBS.
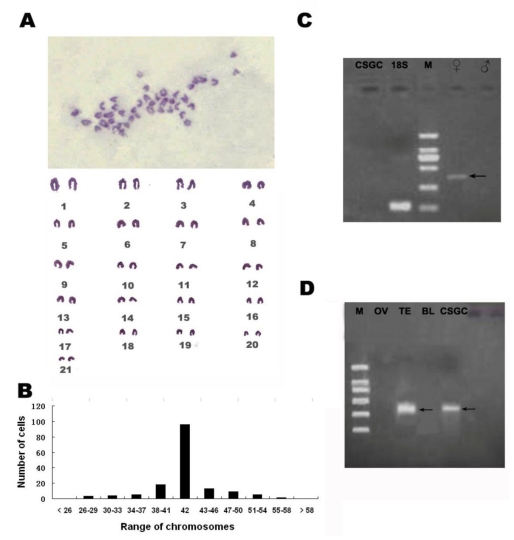
CSGC is genetically male
At passage 25, 35, 50, the chromosome numbers ranged from 26-60, with the majority (75%) having 42 chromosomes of the diploid number in this organism (Fig. 3B)which meant the CSGC cells had high genetic stability. CSGC was initiated from a juvenile gonad whose sex was indeterminate. The half-smooth tongue sole shows a significant sex dimorphism, with its W and Z chromosomes being easily distinguishable. We took this advantage and examined the karyotype of the cell line. This revealed a diploid karyotype consisting of 21 pairs of chromosomes and thus a male karyotype, because the female specific W chromosome was absent (Fig. 3A). Furthermore, a PCR analysis revealed that a female-specific product was absent in the DNA from CSGC cells and testis, although our condition easily detected its presence in the ovary sample (Fig. 3C). Taken together, CSGC is a male cell line from a genetically male gonad, namely the testis.
Fig 3.
Cytogenetic and molecular characterization. (A)Diploid karyotype of CSGC cells at passage 35. The female specific W chromosome is absent. (B) Chromosome number distribution. The main chromosome number was 42. (C) Absence of a female specific DNA marker. Arrow shows female control.(D) Detection of dmrt1 expression. M, BM 2000 DNA marker; OV, ovary tissue; TE, Testis tissue; BL, blood.
Detection of dmrt1 expression in CSGC cells
In several fish species such as medaka, dmrt1 is a marker of testiculat somatic cells, because it shows a preferential expression in the somatic cells but not germ cells of the testis 25, 26. By RT-PCR, a 352-bp specific band of the Dmrt1 gene only appeared in the testis and CSGC cells but not in the ovary and other tissues (Fig. 3D). Therefore, CSGC appears to contain somatic cells of the testis.
Virus challenge assay
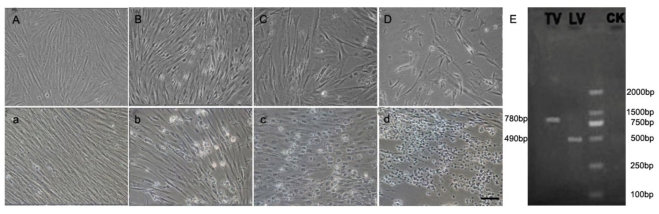
The susceptibility of CSGC to two viruses, namely LCDV and TRBIV, was evaluated by CPE, PCR-analysis and Electron microscopy. Significant CPE was observed obviously in the cells in passage 45 at 36h post infection with LCDV (Fig. 4B-D)and TRBIV (Fig. 4b-d). As predicted, CPE was absent in non-infected control cells (Fig. 4A, a). CPE with typical multiple vacuolation was observed in cells infected with TRBIV (Fig. 4 c) and network degeneration with stellate formation and rounding of cells seen in LCDV infected cells (Fig. 4D). PCR assays detected a prominent product for LCDV and TRBIV (Fig. 4E).
Fig 4.
CPE in CSGC cells after virus infection. CSGC cells were used passage 45. (A, a) Control cells. (B-D) Cells after infection with LCDV. (b-d) Cells after infection with TRBIV. (E) PCR-detection of viruses in infected CSGC cells.
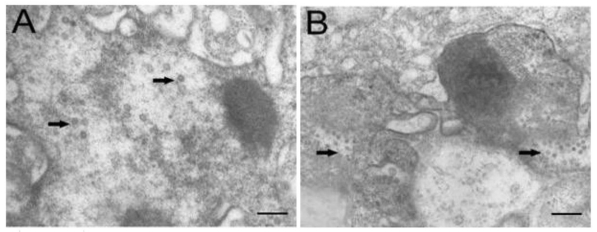
Electron microscopic observation revealed that virus particles were scattered throughout the cytoplasm of cells infected with TRBIV and LCDV (Fig. 5A, B), implying that the multiplication of infected viruses in CSGC cells. The viruses were spherical to icosahedral, and measured 60 to 150 nm in diameter. These results demonstrate a high susceptibility of CSGC to both TRBIV and LCDV.
Fig 5.
Electron microscopic observation. (A) LCDV particles scattering in the cytoplasm of CSGC cells. (B) TRBIV particles scattering in the cytoplasm of CSGC cells. Magnification, 15,000×. Scale bars, 400nm in (A) and 200 nm in (B)
Discussion
In this study, we have obtained a tongue sole gonad cell line designated as CSGC. Four independent lines of evidence suggest that CSGC is a testicular somatic cell line. First, CSGC has a gondal origin. Second, it lacks the heterogametic W chromosome and also does not contain a female specific marker, pointing to the ZZ genetic constitution for the maleness in this organism. Third, CSGC expresses the testicular cell marker dmrt1. Finally, CSGC cells are fibroblast-like in phenotype, compared to round or polygonal shapes of germ cells in culture 16. Although dmrt1 expression in medaka occurs preferentially in Sertoli cells 25, 26, 27, future work is needed to determine whether CSGC essentially consists of Sertoli cells.
Since the half-smooth tongue sole is an important species for marine culture, we have tested the CSGC for potential use in studying host-pathogen interactions. We show that this cell line is highly susceptible for infection by, and propagation of, LCDV and TRBIV viruses. LCDV was firstly isolated from Japanese flounder 28 and can infect also other cell lines of marine fish species 20, 21. Remarkably, CSGC cell line has higher susceptibility to LCDV and TRBIV than other marine fish cell lines 20, 29, 30. For example, typical CPE appears as early as 12 h post infection with LCDV and TRBIV, and dramatic propagation of both viruses in CSGC cells takes place already at 36 h post infection. These data suggest that CSGC can be used as a helpful tool for the isolation, proliferation and investigation of marine fish viruses.
Gonadal somatic cells are involved in differentiation of spermatogonial stem cells (sscs), which is capable of differentiation through meiosis to generate test-tube sperm and also proliferation through mitosis 17. How the gonadal somatic cells effects the spermatogonial stem cells would be a meaningful job for future research.
Sex determination has enormous diversity in fish 31, 32, 33. The half-smooth tongue sole has the ZZ-ZW sex determination system 19. Our work in this study provides CSGC - to our knowledge - as a first testicular somatic cell line in fish. Future work will determine whether this cell line is useful to study sex -related events and interactions between male germ cells and somatic cells in culture.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (31072202), the National 863 High Technology Research Foundation of China (2006AA10A401) and Taishan Scholar Project Fund, Shandong, China.
References
- 1.Wolf K, Quimby MC. Established eurythermic line of fish cells in vitro. Science. 1962;135:1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- 2.Hightower LH, Renfro JL. Recent applications of fish cell culture to biomedical research. J Exp Zool. 1988;248:290–302. doi: 10.1002/jez.1402480307. [DOI] [PubMed] [Google Scholar]
- 3.Fryer JL, Lannon CN. Three decades of fish cell culture: a current listing of cell lines derived from fish. J Tiss Cult Methods. 1994;16:87–94. [Google Scholar]
- 4.Komura J, Mitani H, Shima A. Fish cell culture: establishment of two fibroblast-like cell lines (OL-17 and OL-32) from fins of the medaka, Oryzias shima. In Vitro Cell Dev Biol. 1988;24:294–298. [Google Scholar]
- 5.Watanabe T, Kobayashi N, Sato Y. Continuous cell line derived from the kidney of yamame, Oncorhynchus masou. Bull Japan Soc Sci Fish. 1978;44:415–418. [Google Scholar]
- 6.Frerichs GN, Morgan D, Hart D. Spontaneously productive Ctype retrovirus infection of fish cell lines. J Gen Virol. 1991;72:2537–2539. doi: 10.1099/0022-1317-72-10-2537. [DOI] [PubMed] [Google Scholar]
- 7.Ahne W. Studies on the use of fish tissue cultures for toxicity tests in order to reduce and replace the fish tests. Zbl Bakt Hyg, Abt Orig B. 1985;180:480–504. [PubMed] [Google Scholar]
- 8.Chen SL, Ren GC, Sha ZX. Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus) Aquaculture. 2005;249:63–68. [Google Scholar]
- 9.Fryer J L.Lannan C N. Three decades offish cell culture: A current listing of cell lines derived from fish. Journal of Tissue Culture Methods. 1994;16:87–94. [Google Scholar]
- 10.Lakra WS, Swaminathan TR, Joy KP. Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem. 2010 doi: 10.1007/s10695-010-9411-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Hofmann MC, Hess RA, Goldberg E. Immortalized germ cells undergo meiosis in vitro. Proc Natl Acad Sci USA. 1994;91:5533–5537. doi: 10.1073/pnas.91.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng LX, Chen Y, Dettin L. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- 13.Miura T, Yamauchi K, Takahashi H. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica) Proc Natl Acad Sci USA. 1991;88:5774–5778. doi: 10.1073/pnas.88.13.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai A, Tamura M, Matsumoto M. Establishment of in vitro spermatogenesis from spermatocytes in the medaka, Oryzias latipes. Dev. Growth Differ. 1997;39:337–344. doi: 10.1046/j.1440-169x.1997.t01-2-00009.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakai N. Transmeiotic differentiation of zebrafish germ cells into functional sperm in culture. Development. 2002;129:3359–3365. doi: 10.1242/dev.129.14.3359. [DOI] [PubMed] [Google Scholar]
- 16.Hong YH, Liu TM, Zhao HB. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci USA. 2004;101:8011–8016. doi: 10.1073/pnas.0308668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barroca V, Bruno L, Mathieu C. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nature Cell Biology. 2008;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 18.Sabine C, Markus R, Hennenlotter J. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 19.Chen SL, Deng SP, Ma HY. et al. Molecular marker-assisted sex control in half-smooth tongue sole Cynoglossus semilaevis. Aquaculture. 2008;283:7–12. [Google Scholar]
- 20.Wang XL, Wang N, Chen SL. et al. Establishment, characterization of a new cell line from heart of half-smooth tongue sole (Cynoglossus semilaevis) Fish Physio Biochem. 2010;36:1181–1189. doi: 10.1007/s10695-010-9396-5. [DOI] [PubMed] [Google Scholar]
- 21.Sha ZX, Ren GC, Wang XL. et al. Development and characterization of a cell line from the embryos of half smooth tongue sole (Cynoglossus semilaevis) Acta Oceanol Sin. 2010;29:81–87. [Google Scholar]
- 22.Earley EM. Chromosome preparations from monolayer cell culture. TCA Man. 1975;1:31–35. [Google Scholar]
- 23.Chen SL, Li J, Deng SP. et al. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis) Mar Biotech. 2007;9:273–280. doi: 10.1007/s10126-006-6081-x. [DOI] [PubMed] [Google Scholar]
- 24.Deng S P, Chen SL. Molecular cloning, characterization and RT-PCR expression analysis of Dmrt1α from half-smooth tongue-sole, Cynoglossus semilaevis. Journal of Fishery Sciences of China. 2008;15:577–584. [Google Scholar]
- 25.Brunner B, Hornung U, Nanda I. et al. Genomic organization and expression of the doublesex-related gene cluster in vertebrates and detection of putative regulatory regions for DMRT1. Genomics. 2001;77:8–17. doi: 10.1006/geno.2001.6615. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Yokomizo S, Matsuda M. et al. Gene-centromere mapping of medaka sex chromosomes using triploid hybrids between Oryzias latipes and O. luzonensis. Genetica. 2001;111:71–75. doi: 10.1023/a:1013755701696. [DOI] [PubMed] [Google Scholar]
- 27.Lutfalla G, Crollius HR, Brunet FG. et al. Inventing a Sex-Specific Gene: A Conserved Role of DMRT1 in Teleost Fishes Plus a Recent Duplication in the Medaka Oryzias latipes Resulted in DMY. J Mol Evol. 2003;57:148–153. doi: 10.1007/s00239-003-0021-4. [DOI] [PubMed] [Google Scholar]
- 28.Sun XQ, Qu LY, Zhang JX. et al. Pathogenicity and immunogenicity of lymphocystis virus of Japanese flounder (Paralichthys olivaceus) High Technol Lett. 2000;9:19–21. [Google Scholar]
- 29.Wang N, Wang XL, Sha ZX. et al. Development and characterization of a new marine fish cell line from turbot (Scophthalmus maximus) Fish Physiol Biochem. 2010;36:1227–1234. doi: 10.1007/s10695-010-9402-y. [DOI] [PubMed] [Google Scholar]
- 30.Wang XL, Chen SL, Sha ZX. et al. Establishment and Characterization of a New Cell Line from the kidney of Spotted Halibut Verasper variegates. J Ocean Univ China. 2010;9:162–168. [Google Scholar]
- 31.Kondo M, Nanda I, Schmid M. et al. Sex determination and sex chromosome evolution: insights from medaka. Sex Dev. 2009;3:88–98. doi: 10.1159/000223074. [DOI] [PubMed] [Google Scholar]
- 32.Nagahama Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol Biochem. 2005;31:105–109. doi: 10.1007/s10695-006-7590-2. [DOI] [PubMed] [Google Scholar]
- 33.Sandra GE, Norma MM. Sexual determination and differentiation in teleost fish. Rev Fish Biol Fisheries. 2010;20:101–121. [Google Scholar]