Abstract
Objective
Population studies have shown that age at menopause (AAM) predicts coronary heart disease. It is unknown, however, whether early menopause predicts post–myocardial infarction (MI) angina. We examined whether younger AAM increases risk of post-MI angina.
Methods
In a prospective multicenter MI registry, 493 postmenopausal women were enrolled (mean ± SD age, 65.4 ± 11.3 y, and mean ± SD AAM, 45.2 ± 7.8 y). We categorized AAM into 40 years or younger, 41 to 49 years, and 50 years or older. In the multivariable analysis, we examined whether AAM predicted 1-year post-MI angina and severity of angina after adjusting for angina before MI, demographics, comorbidities, MI severity, and quality of care (QOC).
Results
Women with early AAM (≤40 y; n = 132, 26.8%) were younger and more often smokers but were as likely to have comorbidities as were women with an AAM of 50 years or older. Although there were no differences in pre-MI angina, MI severity, obstructive coronary disease, and QOC based on AAM, the rate of 1-year angina was higher in women with an AAM of 40 years or younger (32.4%) than in women with an AAM of 50 years or older (12.2%). In the multivariable analysis, women with an AAM of 40 years or younger had more than twice the risk of angina (relative risk, 2.09; 95% CI, 1.38–3.17) and a higher severity of angina (odds ratio, 2.65; 95% CI, 1.34–5.22 for a higher severity level) compared with women with an AAM of 50 years or older.
Conclusions
Women with early menopause are at higher risk of angina after MI, independent of comorbidities, severity of MI, and QOC. The use of a simple question regarding AAM may help in the identification of women who need closer follow-up, careful evaluation, and intervention to improve their symptoms and quality of life after MI.
Keywords: Menopause, Myocardial infarction, Sex, Angina
Several epidemiological studies have suggested higher all-cause and coronary heart disease (CHD) mortality for women with early menopause (ie, before 40 y of age) compared with women who were 50 years or older at menopause.1 It is believed that the higher risk associated with early menopause is due to longstanding deprivation of endogenous estrogen, which may influence cardiovascular risk through a variety of effects on metabolism and vascular function.2
Despite the evidence of early menopause being associated with an increased risk for CHD, no descriptions of its prognostic importance among women with known CHD have been reported. After an acute myocardial infarction (MI), longstanding estrogen deficiency due to early menopause may lead to adverse outcomes through the same mechanisms by which it is postulated to be associated with incident CHD. In particular, abnormalities in the microvasculature due to endothelial dysfunction and loss of arterial compliance may increase the risk of angina and other adverse outcomes.3 If early menopause is associated with adverse cardiovascular outcomes in women post-MI, this may help in the risk stratification and management of this patient group.
According to the American Heart Association and the American College of Cardiology guidelines, a primary goal of MI management is the complete or near complete elimination of anginal chest pain.4 Women with established CHD consistently report more angina compared with men after adjusting for comorbidities, clinical characteristics, and MI management.5–7 Women are less likely than men to experience a “classic” pattern of stabbing chest pain in the center or left side of the chest but more often complain of chest heaviness, pressure, tightness, or squeezing.8 Recognition of angina is important because angina symptoms are associated with increased coronary mortality and health-related quality of life, higher rates of hospitalizations, and significant limitation in activities of daily living.9,10 In addition, angina symptom–driven care for women is costly and accounts for most costs associated with CHD care in women.6,11 Thus, it is important to examine additional novel predictors of outcomes that can be directed to exploring new risk assessment paradigms in women with MI. In a prospective registry of MI patients, the Prospective Registry Evaluating Outcomes After Myocardial Infarction: Events and Recovery (PREMIER), we examined whether women with younger age (≤40 y) at menopause have a higher rate of angina and more severe angina at 1 year after MI as compared with women who experienced menopause at an older age (≥50 y).
METHODS
Participants and data collection
The PREMIER study was a prospective cohort study examining patients’ health outcomes, especially post-MI angina, as a function of sociodemographic, clinical, and health status characteristics.12 The methodology of this study has been described previously.12 Briefly, in PREMIER, we screened a consecutive cohort of patients admitted with MI at 17 US study sites between January 2003 and June 2004. Patients were eligible for participation if they were 18 years or older and had increased cardiac biomarkers (troponin or creatine kinase-MB) in addition to having supporting clinical evidence of a MI. Patients who were incarcerated, developed increased cardiac enzymes because of elective coronary revascularization, refused or were unable to provide informed consent, or did not speak English or Spanish were not included. For the purpose of this study, we included only postmenopausal women enrolled in PREMIER who had complete data available regarding age at menopause (AAM) and were alive at discharge. The institutional review board at each participating institution approved the study, and all women provided informed consent forms.
A comprehensive chart abstraction was performed at each participating institution to collect sociodemographic, cardiac, noncardiac, hospital course, and treatment variables. In addition, women were interviewed during their MI admission to collect data on baseline sociodemographic, behavioral, psychosocial, and health status measures. In addition, we assessed depressive symptoms using the Patient Health Questionnaire.13
All sites assessed menopause and other reproductive history using an optional questionnaire. First, women were asked if they were willing to answer questions regarding their menstrual history. If the women were willing, menstrual history and menopause status were obtained by self-report during this interview. If the women responded that they had reached menopause, they were further asked about their AAM, the cause of their menopause (natural or after surgical operation or radiation), and, if they had had a hysterectomy, whether it included bilateral oophorectomy or unilateral oophorectomy. Surgical menopause was defined as cessation of menstrual period after bilateral oophorectomy. We excluded the only woman (n = 1) with menopause resulting from radiation. Women were also asked if they had used estrogen preparations, including birth control pills or postmenopausal hormones.
Outcome variables
Women were interviewed by telephone at 1 year after discharge by a centralized follow-up center to collect comprehensive data on behavioral, treatment, and health status measures. Prespecified primary outcome measures for PREMIER at 1 year were presence of angina and severity of angina.
We used the Seattle Angina Questionnaire (SAQ), a 19-item disease-specific health status instrument, to assess angina symptom burden, that is, frequency of angina (ie, chest pain or chest tightness in the prior 4 wk).14 Responses are scored from 0 to 100, with higher scores indicating less angina.14 Because the desirable outcome is for the woman to be angina free and because of a right skewed distribution of women without any angina symptoms, angina (SAQ angina frequency score) was examined as a dichotomous variable (angina present [SAQ angina frequency score <100] or absent [SAQ angina frequency score = 100] at 1 y). In addition, we further stratified angina into three categories reflecting daily or weekly angina (SAQ angina frequency score, 0–60), angina less than once per week (61–99), and no angina (100).14 All SAQ domains have been validated, and the SAQ predicts mortality and subsequent cardiac events in patients with CHD.14
All-cause mortality was examined as a secondary outcome given the limited number of deaths. We obtained vital status at 1 year postdischarge from contacts with family members and by querying the Social Security Death Master File.
Data analysis
First, we compared differences between women willing versus not willing to discuss their menstrual information using linear trend tests for continuous variables and the Mantel-Haenszel trend test for categorical variables. AAM was categorized into 40 years or younger, 41 to 49 years, and 50 years or older following the classification used in previous studies.1,15 Differences across AAM groups were assessed using linear trend tests for continuous variables and the Mantel-Haenszel trend test for categorical variables. Unadjusted rates of all-cause mortality between menopause age groups are reported as Kaplan-Meier estimates and tested using the log-rank test. No multivariable analysis was carried out for mortality, given the small number of deaths.
For the dichotomous outcome of presence of angina, we used multivariable hierarchical modified Poisson regression models,16 which provide estimates of relative risks (RRs). In addition, we used multivariable hierarchical ordinal logistic regression models for the outcome of severity of angina. The hierarchical structure of these multivariable models accounted for the clustering of women within site of care. Modeling was conducted in subsequent steps: (1) unadjusted, (2) age-adjusted, and (3) fully adjusted multivariable models. We chose our model covariates first based on a priori clinical knowledge about potential confounders and then further balanced the groups by including additional factors where differences were shown between groups based on the data.17 Covariates in the multivariable models included demographic variables (age, white race, insurance status), cardiac history (prior MI, percutaneous coronary intervention or coronary artery bypass grafting, and congestive heart failure [CHF]), medical history (history of diabetes, history of hypertension, smoking status [current vs former], and body mass index), clinical severity of MI (ST-elevation MI [STEMI] and left ventricular ejection fraction [LVEF] <40), and the use of coronary angiography and/or revascularization (thrombolytics, percutaneous coronary intervention, coronary artery bypass grafting). The Centers for Medicare and Medicaid Services and the Joint Commission on Accreditation of Healthcare Organizations have defined the MI quality-of-care indicators usually reported as percentage of MI quality-of-care indicators received.18,19 Because MI quality-of-care indicators influence post-MI outcomes, we adjusted for percentage of MI quality-of-care indicators received and number of MI quality indicators that the women were eligible to receive. We further adjusted for SAQ angina score before MI. Nonlinear relationships were accounted for by including restricted cubic spline terms for all continuous covariates.20 All tests for statistical significance were two tailed, with an α level of 0.05. All analyses were conducted using SAS software, release 9.1 (SAS Institute, Cary, NC), and R version 2.6.0.21
We conducted four sets of secondary analyses. In separate steps, we tested the following interaction terms in fully adjusted models: estrogen use by AAM and cause of menopause by AAM. In addition, in a separate step, we excluded women with surgical menopause to evaluate if the results changed. We also conducted a sensitivity analysis using AAM as a continuous variable similar to the primary analyses as described above. Finally, because of the positive bivariate association of depressive symptoms at MI hospitalization with AAM, we added depressive symptoms in the final model to examine whether it was a confounder in the association of AAM with the angina outcomes.
Some women were missing 1-year angina data because of death (n = 32; 6.5%) or because they were too ill to be interviewed (n = 5; 1.1%), lost to follow-up (n = 31; 6.3%), or refused the 1-year interview (n = 2; 0.4%). We used a propensity model to evaluate potential bias from missing 1-year outcome data due to women who were lost to follow-up or refused the 1-year interview. The propensity model controlled for a wide range of demographic, socioeconomic, and clinical factors.22 In the overall sample including these women, but excluding women who were deceased (n = 32) or too ill to be interview at 1 year follow-up (n = 5), we calculated a propensity score of having a missing 1-year interview using logistic regression. The propensity score was the probability of a person with given characteristics having a missing 1-year interview. The reciprocal of this score was then used to weight the responses of those who provided follow-up, resulting in higher weight given to observations from women that are similar to those with missing outcome data.22
RESULTS
Study population
In the study period, 807 women met MI eligibility criteria, were approached for the study, and were alive at discharge. Of these, 657 women agreed to answer the optional questionnaire pertaining to their reproductive history. From this sample, 84 women were excluded because they were pre-menopausal and 80 women were excluded because they did not recall their AAM, leaving 493 postmenopausal women for this analysis. Women who lacked data on AAM were more likely to be white, to smoke, and to have STEMI compared with women with such data. However, there were no differences in rates of angina either at baseline or at 1 year after discharge. In addition, women who were enrolled in this analysis were of similar age and had similar comorbidities overall compared with women who were screened but not enrolled but were more likely to be white, smokers, and have STEMI. In addition, they were less likely to have a history of renal failure and to have Medicaid as their insurance.
The mean ± SD age of the 493 women included in this analysis was 65.4 ± 11.3 years. Approximately 71% of these women were white, 58.6% had less than high school education, and 44.6% were married. The mean ± SD and median AAM were 45.2 ± 7.8 and 47 years, respectively. Most of the women (n = 412; 83.5%) reported having natural menopause. Almost two thirds of the women reported previous estrogen use (n = 310, 62.8%).
Comparisons of characteristics of women according to AAM
A total of 26.8% (n = 132) of women had experienced menopause by the age of 40 years, 35.5% (n = 175) experienced menopause between 41 and 49 years of age, and 37.7% (n = 186) reached menopause after the age of 49 years. Although most women had a natural menopause, women with an AAM of 40 years or younger were more likely to have gone through surgical menopause than were women in the other AAM groups (Table 1). Younger age was associated with early menopause. More than one third (34%) of women younger than 60 years (n = 63) had reached menopause by the age of 40 years versus only 22.6% (n = 69) of women older than 60 years. Women with an AAM of 40 years or younger were more likely to be smokers and had higher levels of depressive symptoms than did women who reported an older menopause age (Table 1). Sociodemographic factors and comorbidities did not differ significantly by AAM. In addition, there was no significant association between AAM and presentation characteristics such as angina at baseline, LVEF, and type of MI, but women with early AAM tended to have fewer diseased vessels at coronary angiography. In-hospital events, MI quality-of-care indicators, and antianginal medications received also did not differ according to AAM. In addition, there was no difference in cardiac medications received at 1 year after discharge (Table 1).
TABLE 1.
Characteristics of the study population according to age at menopause
| Characteristics | Age at menopause
|
P | ||
|---|---|---|---|---|
| ≤40 y (n = 132) | 41–49 y (n = 175) | ≥50 y (n = 186) | ||
| Sociodemographic factors | ||||
| Age, y | 61.8 ± 11.0 | 65.5 ± 12.1 | 67.8 ± 10.0 | <0.001 |
| African American race | 36 (27.3) | 32 (18.3) | 47 (25.7) | 0.703 |
| Greater than high school education | 55 (42.3) | 68 (39.1) | 78 (42.9) | 0.863 |
| Married | 58 (44.3) | 78 (44.6) | 83 (44.9) | 0.917 |
| Primary medical insurance | ||||
| None/self-pay | 20 (15.7) | 12 (7.0) | 15 (8.2) | 0.045 |
| Medical history | ||||
| Hypercholesterolemia | 58 (43.9) | 93 (53.1) | 96 (51.6) | 0.216 |
| Hypertension | 95 (72.0) | 116 (66.3) | 140 (75.3) | 0.413 |
| Diabetes | 41 (31.1) | 48 (27.4) | 69 (37.1) | 0.195 |
| Prior coronary heart disease (MI/CABG/PCI) | 34 (25.8) | 52 (29.7) | 48 (25.8) | 0.931 |
| History of smoking | 82 (62.1) | 102 (58.3) | 80 (43.0) | <0.001 |
| Congestive heart failure | 14 (10.6) | 23 (13.1) | 26 (14.0) | 0.388 |
| Body mass index ≥30 kg/m2 | 54 (42.5) | 65 (38.5) | 67 (38.3) | 0.480 |
| Presence of angina | 65 (49.2) | 99 (56.6) | 93 (50.0) | 0.986 |
| Severity of angina at baseline | 0.300 | |||
| Daily or weekly | 26 (18.7) | 45 (25.7) | 23 (12.3) | |
| Monthly | 39 (29.5) | 54 (30.9) | 70 (37.6) | |
| None | 67 (50.8) | 76 (43.4) | 93 (50.0) | |
| PHQ depression score | 8.2 ± 6.1 | 6.5 ± 5.8 | 6.3 ± 5.4 | 0.004 |
| Menstrual history | ||||
| Age of menopause, y | 34.7 ± 5.1 | 45.5 ± 2.4 | 52.4 ± 2.6 | <0.001 |
| Cause of menopause | <0.001 | |||
| Natural | 86 (65.2) | 147 (84.0) | 169 (90.9) | |
| Surgery | 41 (31.1) | 25 (14.3) | 15 (8.07) | |
| Number of years taking birth control pills | 6.8 ± 6.7 | 7.1 ± 6.9 | 6.2 ± 7.1 | 0.670 |
| Ever taken birth control pills or HT | 91 (70.5) | 112 (65.1) | 107 (57.8) | 0.019 |
| Clinical characteristics at admission | ||||
| Acute heart rate >100/min | 23 (17.4) | 30 (17.1) | 22 (11.8) | 0.147 |
| Left ventricular ejection fraction ≥40 | 102 (77.3) | 125 (71.8) | 148 (79.6) | 0.520 |
| STEMI | 62 (47.0) | 70 (40.0) | 72 (38.7) | 0.156 |
| QOC | ||||
| Received aspirin at arrival | 122 (94.6) | 166 (97.6) | 173 (97.2) | 0.245 |
| Received β-blocker at arrival | 107 (89.9) | 145 (92.4) | 162 (93.6) | 0.251 |
| Received reperfusion for STEMI/LBBB | 51 (71.8) | 58 (67.4) | 57 (67.1) | 0.534 |
| Received timely reperfusion for STEMI/LBBB | 0.7 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.303 |
| Nitrate | 38 (28.8) | 49 (28.0) | 62 (33.3) | 0.344 |
| Received aspirin at discharge | 122 (94.6) | 157 (91.8) | 166 (92.7) | 0.584 |
| Received ACE inhibitor/ARB for LVSD at discharge | 16 (76.2) | 38 (80.9) | 27 (81.8) | 0.636 |
| Received smoking cessation instructions | 51 (83.6) | 47 (75.8) | 31 (67.4) | 0.051 |
| Received β-blocker at discharge | 112 (90.3) | 149 (93.1) | 158 (88.8) | 0.552 |
| Number of QOC eligible indicators received | 4.7 ± 1.6 | 4.5 ± 1.4 | 4.3 ± 1.4 | 0.053 |
| Eligible QOC indicators received, % | 87.1 ± 19.1 | 88.0 ± 17.8 | 87.1 ± 17.3 | 0.991 |
Values are presented as no. (%) or mean ± SD.
ACE, angiotensin-converting enzyme; ARB, angiotensinogen receptor blockers; CABG, coronary artery bypass grafting; HT, hormone therapy; LVSD, left ventricular systolic dysfunction; LBBB, left bundle branch block; MI, myocardial infarction; PHQ, Patient Health Questionnaire; PCI, percutaneous coronary intervention; QOC, quality of care; STEMI, ST-elevation myocardial infarction.
AAM and MI outcomes
At 1 year, 32 (6.5%) women died; of these, 8 (6.1%) were in the group with an AAM of 40 years or younger, 11 (6.3%) were in the group with an AAM of 41 to 49 years, and 13 (7%) were in the group with an AAM of 50 years or older (P = 0.84).
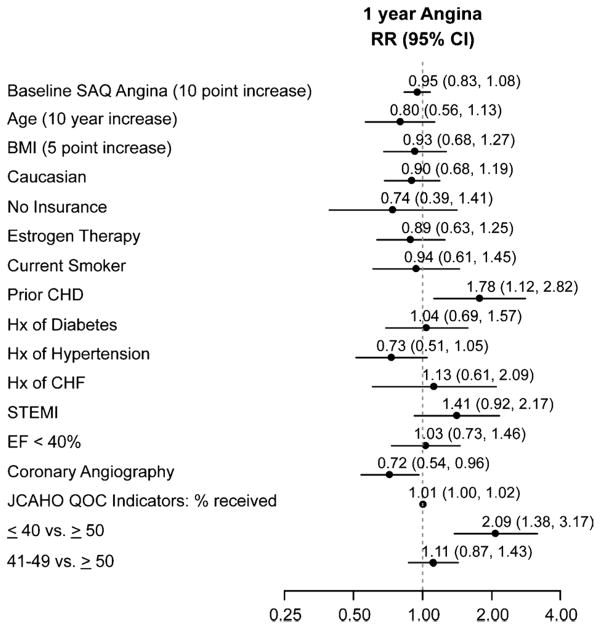
There were no differences in baseline angina among the AAM groups (Table 1). However, at 1 year after their MI, the rate of angina was substantially higher in women with an AAM of 40 years or younger (32.4%) and in women with an AAM between 41 and 49 years (18.9%) than in women with an AAM of 50 years or older (12.2%; Table 2). After adjusting for age, women in either category (AAM ≤40 or 41–49 y) were more likely (2.3 and 1.5 times, respectively) to have angina than were women with an AAM of 50 years or older (Fig. 1). In the fully adjusted model, after controlling for demographics, comorbidities, MI severity, and quality of care, women with an AAM of 40 years or younger had more than twice the risk of angina compared with women with an AAM of 50 years or older (Fig. 1). The intermediate AAM group (41–49 y), in contrast, had only a nonsignificant trend toward having a greater prevalence of angina as compared with those experiencing AAM at 50 years or beyond in the fully adjusted analysis. In the multivariable model of 1-year angina, AAM had the largest magnitude of association of all the risk factors in the model (Fig. 2). Conversely, traditional prognostic markers of angina such as diabetes, hypertension, CHF, ejection fraction, STEMI, or quality of MI care did not predict angina at 1 year in postmenopausal women.
TABLE 2.
Age at menopause and unadjusted outcomes at 1 year after myocardial infarction
| Outcome (number of events) | Age at menopause
|
Pb | ||
|---|---|---|---|---|
| ≤40 y (n = 111)a | 41–49 y (n = 148)a | ≥50 y (n = 164)a | ||
| Presence of angina (n = 84) | 36 (32.4) | 28 (18.9) | 20 (12.2) | <0.001 |
| Angina severity | <0.001 | |||
| None | 75 (67.6) | 120 (81.1) | 144 (87.8) | |
| Monthly | 25 (22.5) | 18 (12.2) | 15 (9.1) | |
| Weekly or daily | 11 (9.9) | 10 (6.8) | 5 (3.0) | |
Number of women available for analysis at 1 year.
P value is adjusted for patient’s age and other covariates.
FIG. 1.
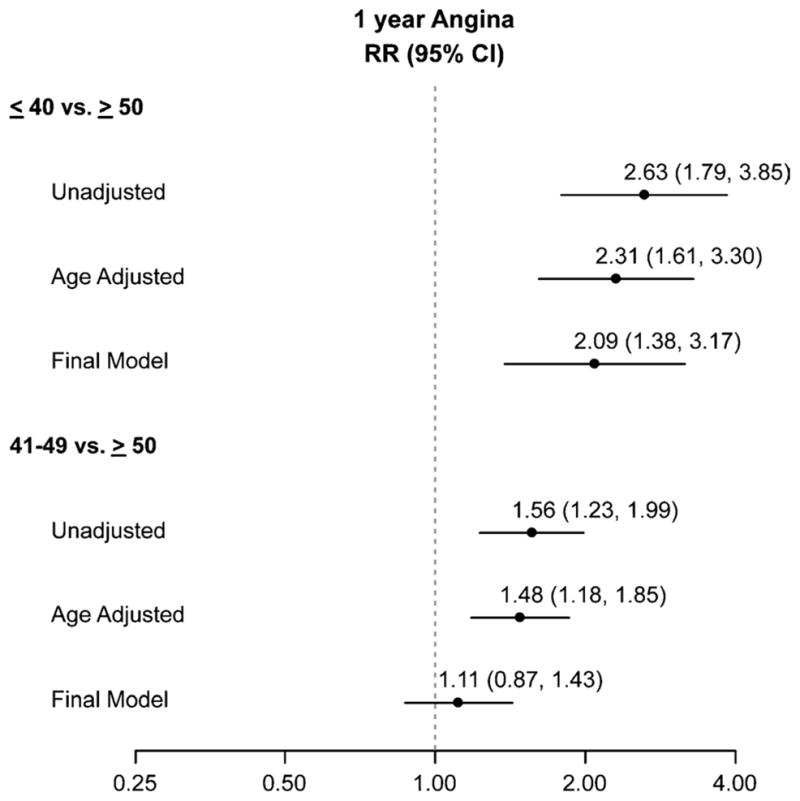
Risk of angina according to age at menopause. RR, relative risk.
FIG. 2.
Predictors of 1-year angina after myocardial infarction in postmenopausal women in the multivariable analysis. SAQ, Seattle Angina Questionnaire; BMI, body mass index; CHD, coronary heart disease; Hx, history; CHF, congestive heart failure; STEMI, ST-elevation myocardial infarction; EF, ejection fraction; JCAHO, Joint Commission on Accreditation of Healthcare Organizations; QOC, quality of care, RR, relative risk.
Ordinal logistic regression results showed that women with an AAM of 40 years or younger were more likely to be at a higher level of angina severity classification (monthly vs none, or weekly/daily vs monthly) at 1 year than were women with an AAM of 50 years or older (Table 2 and Fig. 3). Women with an AAM of 40 years or younger had more than threefold the odds of a higher angina level compared with women with an AAM of 50 years or older (unadjusted odds ratio, 3.37; 95% CI, 1.82–6.26). On multivariable analysis, women with early menopause continued to have an almost threefold higher risk of a more severe angina compared with women who had menopause at age 50 years or beyond (odds ratio, 2.65; 95% CI, 1.34–5.22). There was no difference in angina severity between women with an AAM of 41 to 49 years and those with an AAM of 50 years or older (Fig. 3).
FIG. 3.
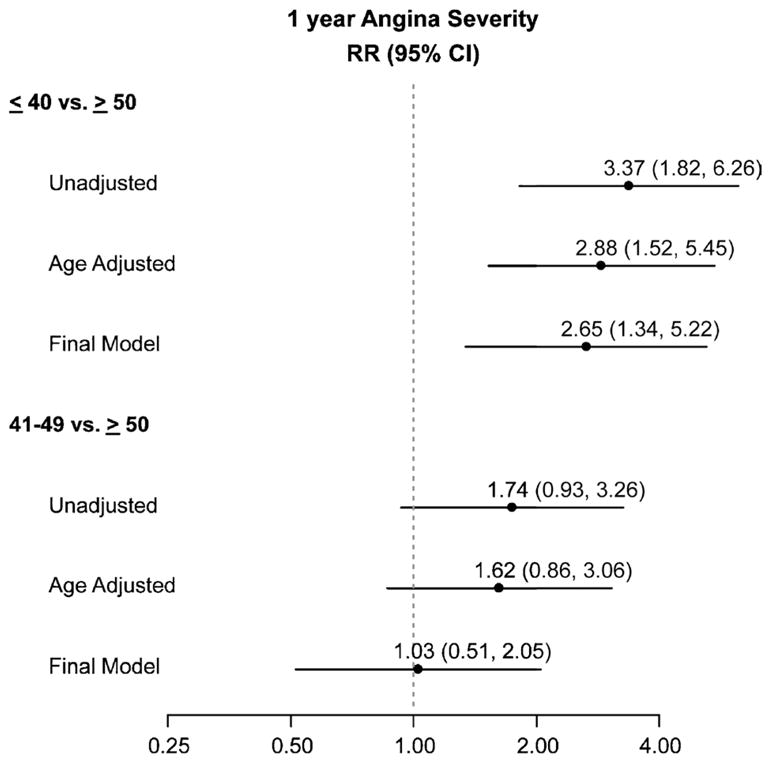
Angina severity according to age at menopause. RR, relative risk.
Sensitivity analyses using AAM as a continuous variable produced similar results. For every 5-year decrease in AAM, the fully adjusted risk of angina increased by 19% (RR, 1.19; 95% CI, 1.07–1.32).
There was no interaction between AAM and previous estrogen use or AAM and cause of menopause (surgical vs natural) for any angina outcomes. Furthermore, the results were similar when we excluded the women with surgical menopause (n = 81) and after adjusting for depressive symptoms. Finally, the results of the sensitivity analyses weighted with propensity scores were also comparable with the primary analyses, suggesting no observable bias due to women lost to follow-up.
DISCUSSION
We found that younger AAM predicts angina after MI, independent of sociodemographic factors, comorbidities, severity of MI, quality of care, and other common prognostic markers. In fact, younger AAM was the strongest predictor of angina in the multivariable models, whereas traditional clinical prognostic indicators such as ejection fraction, STEMI, or presence of CHF did not predict angina. The same relationship with AAM was seen for severity of angina. Women with an AAM of 40 years or younger were almost three times as likely to have a higher severity of angina as women with an AAM of 50 years or older. To our knowledge, this is the first study to demonstrate that AAM is a risk factor for angina in post-MI women. It is important to identify novel predictors of angina after MI in women because this may aid risk stratification and clarification of pathophysiological mechanisms of angina in women. These data are important because women have a disproportionate burden of angina compared with men and angina adversely impacts women’s survival, health status, and societal economic burden of CHD.6,11
The average AAM in our study is lower than what would be expected in the general population (ie, 51 y).23 Almost one third of postmenopausal women with MI in our study had an AAM of 40 years or younger compared with less than 5% in other population studies of women without CHD.15,24 This observation is consistent with early menopause being a risk factor for CHD in women, as shown in previous population studies.1,15 In addition, a recent meta-analysis of 12 population studies compared women with early menopause (with AAM ranging from <35 to ≤43 y) with those whose menopause was at age 50 years or later with respect to CHD risk.1 The pooled CHD RR estimate for early AAM, adjusted for age and smoking status, was 1.38 (95% CI, 1.21–1.58).1 Our results further expand previous literature and suggest that AAM is an important predictor of post-MI angina.
Early AAM, irrespective of the cause of menopause, confers a higher risk for angina in postmenopausal women after MI. Although no previous study has examined this question in women with MI, a recent meta-analysis examining three population studies of early menopause and cardiovascular disease (CVD) risk stratified on type of menopause showed that bilateral oophorectomy before the age of 50 years increased the risk of CVD substantially (RR, 4.44; 95% CI, 2.56–1.81).1 However two of the three population studies in this meta-analysis used premenopausal women as the reference. To our knowledge, only one previous population study in a cohort of postmenopausal women, enrolled in a breast cancer screening project, has examined the effect of AAM on cardiovascular mortality by cause of menopause (natural vs surgical) and did not find a significant interaction between cause of menopause and AAM on CVD risk.24
In our study, there was no significant interaction between AAM and estrogen use for any of the outcomes. In addition, our results remained unchanged after adjusting for estrogen use. Although the results of recent clinical trials have questioned the cardiovascular protective role of hormone therapy in women with CHD and have suggested a tendency for harm,25,26 our study shows that use of estrogen does not modify or confound the association of AAM with adverse outcomes after MI.
Our study provides important new information for understanding the pathophysiological mechanism of post-MI angina in women. Women with early menopause tended to have less obstructive disease in our study and yet had higher angina. It is possible that deprivation of endogenous estrogen, which acts directly and indirectly on the vasculature, leads to higher vascular inflammation, endothelial and micro-vascular dysfunction, coagulation abnormalities, and decreased arterial compliance.2,10 These factors may result in myocardial flow heterogeneity and relatively greater burden of atherosclerosis in relation to the degree of positive arterial remodeling in women with early AAM.10 The resulting microvascular vasculopathy may be manifested by angina even in the setting of nonobstructive coronary artery disease. In addition, estrogen deficiency is associated with clustering of adverse prognostic factors in postmenopausal women, such as obesity, abnormal fat distribution, insulin resistance, metabolic syndrome, and hypertension.3 Moreover, estrogen deficiency with early menopause is a feature of polycystic ovarian syndrome (PCOS), a condition associated with increased CHD mortality in women.27 However, because in our study, AAM was not associated with obesity, hypertension, or diabetes, other major features of PCOS, it is improbable that underlying PCOS explains worse outcomes in post-MI women with early AAM.
We found that women with early AAM have higher depressive symptoms; this finding might suggest that depression mediates, in part, the increased angina rate for these women. In our sensitivity analyses, the results remained unchanged after adjusting for depressive symptoms, confirming that AAM is an independent predictor of post-MI angina. Thus, other risk factors may be mediating the observed relationship between early AAM and post-MI angina. A better clarification of these mechanisms could help in developing effective interventions to prevent or treat angina in these women.
Although a goal of MI management is the complete or near complete elimination of anginal chest pain,4 despite recent advances in diagnosis and treatment, approximately 30% to 40% of MI patients continue to have residual angina.28 After MI, younger and middle-aged women have more angina symptoms and higher mortality despite lower angiographic disease burden and higher rates of preserved left ventricular function as compared with men.29 The current literature has been unable to provide an explanation for this observation. Because the presence of angina symptoms has a considerable impact on women’s’ survival rates, functional status, quality of life, and health-related costs,6,11 it is imperative to examine new risk assessment paradigms in women with MI. In our study, although women with early menopause had a slightly lower angiographic burden and similar comorbidities, MI severity, LVEF, and quality of care in the hospital or at 1 year postdischarge, they were more likely to have angina and a higher severity of post-MI angina. These observations suggest that women with early menopause may have a varying pathophysiological mechanism of CHD. Thus, the use of a simple question about AAM may be helpful in the risk stratification, closer follow-up, and appropriate investigation of angina in post-MI women to guide optimal angina management in women with early menopause to potentially improve their symptoms and quality of life after MI.
Several limitations should be considered in interpreting our study results. First, only postmenopausal women who had complete data available regarding AAM and were alive at discharge were included in this study. Because we were not able to interview the women who chose to not participate in the study and thus did not have information on their AAM, this could potentially introduce bias in our study. However, women enrolled in this analysis were of similar age and had similar overall comorbidities compared with women who were screened but not enrolled, although they were more likely to be white and smokers and have STEMI. Second, there were six deaths in the study before discharge, and only two of these women were willing to discuss their menstrual history. Therefore, there are very few deaths that could possibly introduce bias and not enough deaths to compare AAM for those women who died with those women who were alive at discharge. In addition, not all the women in PREMIER chose to answer the reproductive questionnaire. Although this could theoretically introduce bias in our study, there were no differences in rates of angina either at baseline or at follow-up between the two groups, suggesting that such a bias would be nondifferential. Second, the accuracy of self-reported menopause status and AAM may be a potential concern. However, previous studies have demonstrated that almost 99% of women accurately report their menopause status and 82% accurately report their AAM.30 Even if measurement error occurred in our study, it would most probably be nondifferential and have biased our results to the null. In a busy clinical setting, the use of a simple patient question to determine AAM is probably the only feasible way for this determination; we show that this assessment has prognostic validity. Third, because the number of deaths in our cohort was small, we were unable to assess the effect of AAM on mortality by itself as outcome. The endpoint angina was by self-report. Although this could theoretically lead to bias in the data collection through incomplete or inaccurate patient recall, this bias would be nondifferential according to AAM. In addition, the self-reported angina does not necessarily indicate an ischemic event, and we do not have diagnostic proof that their angina was necessarily of cardiac/coronary origin. Although this is a limitation, angina was assessed by using the SAQ, which has been validated in CHD patients.14
Moreover, we examined the relationship between AAM and outcomes after MI using a prospective design and we took into account the baseline level of reported angina in the analysis. Another limitation of our study is that there was only 1 year of follow-up; it is unclear whether this association would persist over time.
Finally, because this is an observational study, unmeasured confounding is always a potential limitation. Nonetheless, a wide array of variables was available for risk adjustment.
CONCLUSIONS
Early menopause is a significant predictor of angina at 1 year after MI, independent of comorbidities, MI severity, and quality of care. Because angina is a prominent factor driving healthcare costs and disability in women, early AAM may be a useful element for risk stratification of postmenopausal women after MI. A simple, inexpensive, and easily administered question regarding AAM may help identify high-risk women and guide efforts toward improving treatments and quality of life of post-MI women.
Acknowledgments
Funding/support: Cardiovascular Therapeutics and Cardiovascular Outcomes funded the data collection and analysis of the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery Study. This study was also supported by the Emory University General Clinical Research Center (National Institutes of Health [NIH] M01-RR00039) and by NIH grant K12RR17643. Dr. Parashar is supported by Mentored Clinical Scientist Development Award 1K23RR023171. Dr. Vaccarino is supported by grant K24HL077506. Ms. Reid is supported through NIH grant P50-HL077113.
Footnotes
Financial disclosure/conflicts of interest: None reported.
References
- 1.Atsma F, Bartelink M, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn ME, Karas RH. The protective effect of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Merz CNB, Pepine CJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:4S–20S. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Chronic Stable Angina) Circulation. 2003;107:149–158. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 5.Norris CM, Ghali WA, Galbraith DP, et al. Women with coronary artery disease report worse health-related quality of life outcomes compared with men. Health Qual Life Outcomes. 2004;5:21. doi: 10.1186/1477-7525-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 7.Sheps DS, Kaufmann PG, Sheffield D, et al. Sex differences in chest pain in patients with documented coronary artery disease and exercise-induced ischemia: results from the PIMI study. Am Heart J. 2001;142:864–871. doi: 10.1067/mhj.2001.119133. [DOI] [PubMed] [Google Scholar]
- 8.Milner K, Krumholz HM, Vaccarino V, Funk M. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396–399. doi: 10.1016/s0002-9149(99)00322-7. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI–sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 10.Metz CNB, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:21S–29S. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Merz NB, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease—results from the National Institutes of Health-National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Peterson ED, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The PREMIER (Prospective Registry Evaluating Myocardial Infarction: Event and Recovery) Registry—evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Winders JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52:303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 16.Zou G. Modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.Harrell E. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 18.Centers for Medicare and Medicaid Services Hospital Quality Measures. Available at: http://www.cms.hhs.gov/HospitalQualityInits/10_HospitalQualityMeasures.asp#TopOfPage.
- 19.The Centers for Medicare and Medicaid Services and the Joint Commission on Accreditation of Healthcare Organizations. Current specification manual for national hospital quality measures. 2006 Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement?current+NHQM+Manual.htm.
- 20.Harrell FE., Jr . Regression Modeling Strategies. New York, NY: Springer; 2001. [Google Scholar]
- 21.R Development Core Team. R version 2.1.1. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 22.Lunceford J, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 23.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353:571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 24.van der Schouw YT, vanderGraaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 25.Hulley S, Grady D, Bush T. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 26.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy—Heart and Estrogen/Progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Shaw LJ, Azziz R, Stanczyk FZ, et al. Cardiovascular event–free survival in post-menopausal women with polycystic ovary syndrome: results from the Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:902. [Google Scholar]
- 28.Fox KAA, Poole-Wilson P, Clayton TC, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366:914–920. doi: 10.1016/S0140-6736(05)67222-4. [DOI] [PubMed] [Google Scholar]
- 29.Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Circulation. 1997;96:2468–2482. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]