Abstract
Effective immunity to HIV is poorly understood. In particular, a role for antibody-dependent cellular cytotoxicity (ADCC) in controlling HIV is controversial. We hypothesized that significant pressure from HIV-specific ADCC would result in immune-escape variants. A series of ADCC epitopes in HIV-infected subjects to specific consensus strain HIV peptides were mapped using a flow cytometric assay for natural killer cell activation. We then compared the ADCC responses to the same peptide epitope derived from the concurrent HIV sequence(s) expressed in circulating virus. In 9 of 13 epitopes studied, ADCC antibodies were unable to recognize the concurrent HIV sequence. Our studies suggest ADCC responses apply significant immune pressure on the virus. This result has implications for the induction of ADCC responses by HIV vaccines.
Keywords: natural killer cells, viral evolution, neutralizing antibody
There is an urgent need to identify effective immunity to HIV and to use this information to design efficacious vaccines and immunotherapies. The recent Thailand-based RV 144 HIV vaccine trial using a canarypox prime/protein boost approach led to modest but significant protective efficacy (1). There is intense interest in the correlates of immunity in this study. CD8+ cytotoxic T-lymphocyte (CTL) responses generally are weak and infrequently are induced by this regimen, and neutralizing antibody (NAb) responses typically are directed narrowly toward the vaccine strains only. This regimen induces reasonably potent antibody-dependent cellular cytotoxicity (ADCC) antibodies (2), and such responses could be responsible in part for the efficacy observed.
ADCC antibodies often are present at very high titers in HIV-infected subjects, and many, but not all, studies have correlated ADCC responses with slower progression of HIV infection (reviewed in ref. 3). Macaque simian immunodeficiency virus (SIV) vaccine studies and passive antibody transfer studies in recent years strongly suggest a role for ADCC in assisting protective immunity (4–6).
Most HIV ADCC assays measure total responses of all ADCC antibodies to particular whole proteins such as the HIV-1 envelope protein (Env) (7). It now is clear that both CTL and NAb responses against HIV can be directed to particular epitopes that are more effective in controlling viremia than those targeting alternate epitopes (8, 9). Similarly, ADCC responses to specific epitopes may be more effective in controlling HIV replication than others. We recently developed a method to detect and map linear ADCC antibody epitopes by analyzing the ability of ADCC antibodies to activate natural killer (NK) cells in the presence of overlapping HIV peptide sets (10, 11). The HIV antibody-induced expression of degranulation markers by NK cells in this assay correlates with killing of infected cells in cytotoxicity-based ADCC assays. ADCC responses to epitopes identified by this NK cell-activation assay can recognize and lyse HIV-infected cells (12). The NK cell-activation assay allows a finer characterization of epitope-specific ADCC responses.
One measure of the pressure applied by immune responses is their ability to force viral mutations, resulting in escape from immune recognition. Both CTLs and NAb select for immune escape variants during the course of HIV-1 infection (13, 14). Some CTL immune escape variants have reduced replicative capacity (reduced “fitness”) that slows the progression to disease (15, 16). Sequencing single HIV genomes from subjects with acute HIV-1 infection reveals that multiple mutations are acquired during the first months of infection, and most align with sites of CTL or NAb escape mutations (17). However, some mutations do not map clearly to known sites of CTL or NAb escape, suggesting there may be other immune responses, such as ADCC responses, sufficiently potent to select immune escape strains.
We mapped a large series of HIV-specific ADCC epitopes in subjects infected with HIV using a set of consensus HIV peptides. We then cloned and sequenced the subjects’ HIV strains across the relevant epitopes and analyzed whether their ADCC responses were able to recognize their own virus strain. In multiple HIV-specific ADCC responses studied, we found evidence for evolution of immune escape.
Results
Mapping of HIV-Specific ADCC Epitopes.
To study evolution across HIV-specific ADCC epitopes, we first mapped a large series of linear HIV-specific ADCC epitopes. We used an intracellular cytokine staining (ICS)-based ADCC assay which measures NK cell activation by ADCC antibodies in the presence of 15-mer peptide pools corresponding to whole subtype B consensus HIV-1 proteins as previously described (10, 11). Patient plasma samples giving positive responses to peptide pools spanning whole proteins were then tested against subpools of 30 peptides to define the region of the epitope more narrowly. Plasma activating >1% of NK cells in response to subpools were then mapped to individual linear 15-mer peptides (Fig. S1). This epitope-mapping exercise yielded a total of 59 epitopes in 22 subjects (Table S1). Fifty-seven epitopes in Env and three epitopes to Vpu were identified. Interestingly, 14 epitopes were shared by two or more subjects. Commonly targeted epitopes were identified in a sequence within the variable domain 3 (V3) loop region (in nine subjects), and specific sequences were identified within the conserved domain 1 (C1) region (across Env amino acid residues 29–47 in five subjects and Env amino acid residues 89–107 in seven subjects).
Sequencing of Plasma HIV-1 RNA Across ADCC Epitopes.
After identifying a series of ADCC epitopes mapped to consensus subtype B peptides, we investigated how closely the identified viral sequences matched the amino acid sequences of the consensus subtype B peptide to which they responded. The virus sequenced was amplified across 23 epitopes (Table S2). There were many differences between the consensus peptide epitope and the autologous plasma RNA sequence. Only three epitopes had sequences identical to the consensus subtype B peptides, and more than half the epitopes sequenced had three or more amino acid changes.
ADCC Recognition of Autologous Viral Peptides.
Differences in the peptide sequences between the consensus peptides to which the plasma ADCC responses were mapped and the patient's autologous HIV viral sequences could reflect conserved ADCC epitope recognition despite minor amino acid changes. Alternatively, the patient's autologous virus sequence may have evolved to escape the ADCC response. To investigate this possibility, we purchased autologous virus-derived peptides and assayed the ability of plasma from the subjects to activate NK cells in the presence of varying concentrations of the autologous or consensus peptides. An example of apparent escape from an ADCC response as indicated by a loss of reactivity to the autologous peptide compared with the consensus peptide is shown in Fig. 1.
Fig. 1.
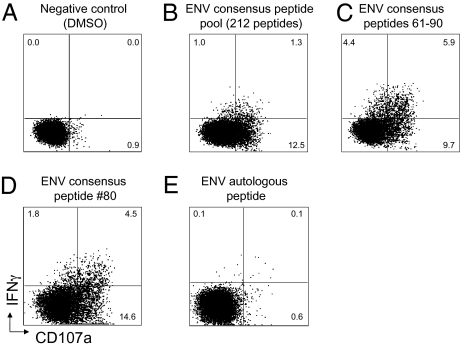
Immune escape at a mapped ADCC epitope. (A) Fresh blood from a HIV+ subject is stimulated with overlapping Env peptides for 6 h and gated CD3-2+ NK cells studied for activation as determined by cytokine (intracellular IFN-γ) expression and degranulation (CD107a expression). (B–D) The process of mapping a response to the 80th peptide in the pool of 212 Env peptides. (E) A peptide based on sequences from the concurrent circulating HIV RNA was assessed and shows minimal activation of NK cells.
Across 9 of the 13 ADCC epitopes studied, we observed substantial loss of recognition of the autologous peptides (Fig. 2). The analyses included studying both IFN-γ and CD107a expression as markers of NK cell activation (an example is shown in Fig. 2A) because these NK cell effector functions can be activated differentially (10). In addition, we studied both CD56+CD3− NK lymphocytes and CD3−CD2+ NK lymphocytes because CD56 expression by NK cells can be down-regulated in subjects with HIV infection (18). We confirmed the escape results from two or more separate blood samples from each subject. We obtained sufficient blood to test replicate samples from most subjects and found significant differences in responses between consensus and autologous peptides.
Fig. 2.
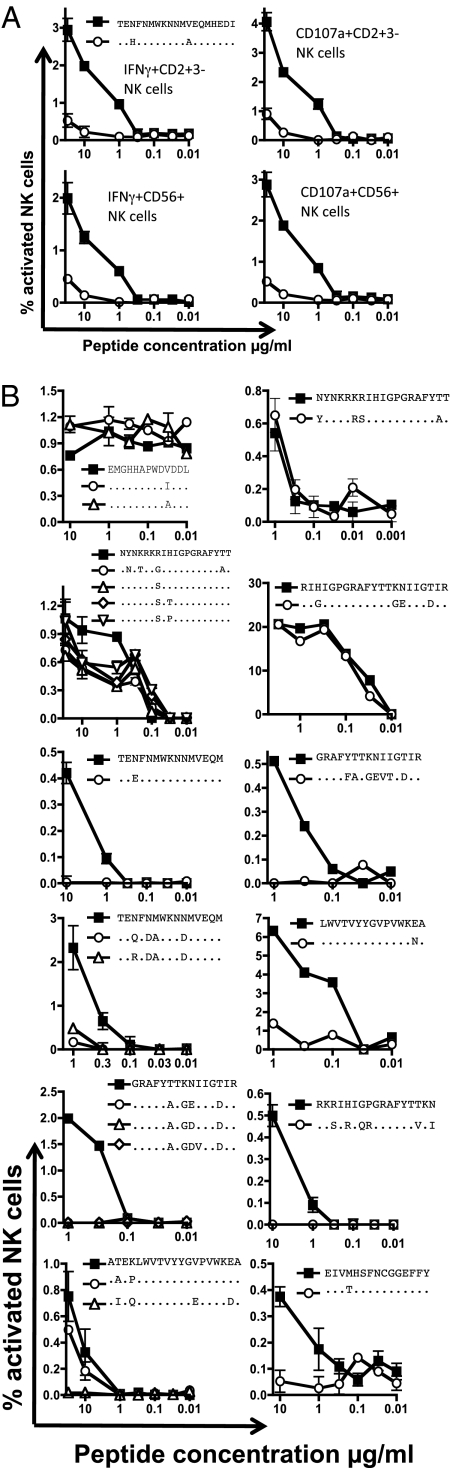
ADCC escape in multiple epitopes. ADCC escape was studied at 12 mapped epitopes in HIV-infected subjects. Epitopes were identified using consensus HIV-1 subtype B peptides from the NIH reagents repository and are shown by solid squares (■). From each subject we cloned and sequenced autologous plasma viral RNA across the epitope and generated the equivalent peptide (or peptides where multiple variants were identified) containing polymorphisms shown by open symbols (△, ○, ◇). (A) One subject in whom ADCC escape was analyzed by either IFN-γ or CD107 from NK cells, which were identified as either CD56+CD3− or CD3−CD2+ gated lymphocytes. (B) In an additional 12 subjects we measured ADCC NK cell activation in the presence of concurrent autologous serum to both consensus and autologous peptides over a series of peptide dilutions. The upper four peptide titration graphs in B show no loss of reactivity of the autologous compared with the consensus peptide; the lower eight panels show substantial immune escape. Most analyses were performed in triplicate (results are shown as mean and SEM), and the differences between consensus and autologous responses were significant in all eight cases (P < 0.01). The top left graph in B is a Vpu peptide epitope; all others are within Env.
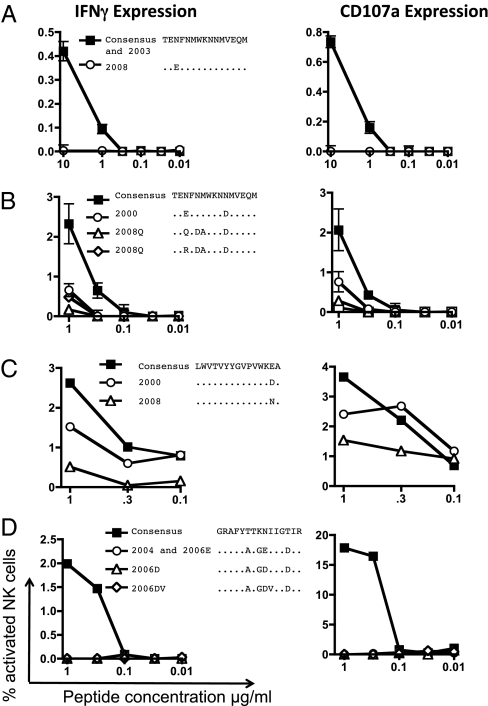
The loss of ADCC activity to autologous sequences presumably reflects evolution over the course of HIV infection away from a sequence more similar to the consensus peptide. To assess this assumption directly, we accessed stored plasma samples taken earlier in the course of HIV infection. For three studies of ADCC response, we accessed samples taken within 2 mo of initial HIV infection, and in one subject we analyzed a sample taken 6 y after initial infection. We sequenced the earlier plasma RNA, purchased additional peptides if necessary, and evaluated ADCC responses across a titration of peptide concentrations (Fig. 3). In one subject, a virus sequence from 5 y earlier (only 6 wk after a seroconversion reaction) exactly matched the consensus B sequence to which the epitope was mapped (Fig. 3A). In two other identified ADCC epitopes, additional viral variants were present 8 y previously, within 2 mo of initial infection. Across both these epitopes, the variants identified 8 y previously had better ADCC recognition than the recent variants isolated from the subject (Fig. 3 B and C). In one subject, a virus sequence taken 2 y previously but 8 y after initial infection had sequence differences but still was fully escaped (Fig. 3D). These results suggest the ADCC recognition of the consensus peptide reflects variants cross-recognized by ADCC responses induced earlier during infection and that the more recent variants have evolved to escape recognition by ADCC responses.
Fig. 3.
Evolution of ADCC escape over time. Peptides corresponding to plasma RNA sequences at different years were assayed for ADCC responses across a titration of peptide concentrations in four subjects. (A–C) Sequences from the earlier time point (within 2 mo of initial infection) show less escape. (D) Sequences from the earlier (2004) time point were 6 y after initial HIV infection, and the earlier time point is still escaped.
Analyses of Sequences Within and Adjacent to ADCC Epitopes.
If ADCC responses specifically selected mutations within the epitope, we might expect to find more mutations within the epitope than random mutations in adjacent sequences. To address this possibility, we cloned and sequenced viral RNA across two epitopes in the C1 region of Env, which has relatively stable sequence. Changes within the epitope were more common than in adjacent sequences (Fig. S2). Because Env sequences are variable, we also analyzed changes in Env that were found only rarely in more than 300 Env clones from different subjects (kindly provided by B. Korber, Los Alamos National Laboratory, Los Alamos, NM). Changes at conserved residues were more common within the ADCC epitopes than outside the epitope.
Another possible reason for the sequence changes observed in the mapped ADCC epitopes is that the epitope was corecognized by a CTL response. To address this possibility, we examined T-cell responses to the ADCC epitopes mapped using the same ICS-based assay used for NK cell activation but gating instead on CD3+ T lymphocytes. We did not find any T-cell responses coinciding with the ADCC epitopes mapped, suggesting T-cell immunity did not result in the mutations observed.
Mapping Mutational Patterns of ADCC Escape.
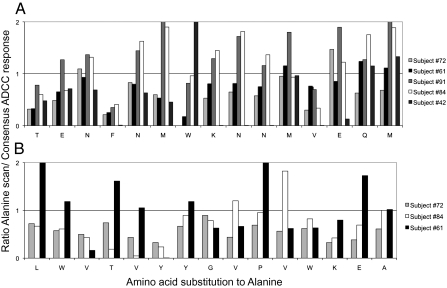
The abrogation of ADCC recognition by amino acid changes in the identified linear epitopes suggests that critical amino acids mutate to escape these responses. Multiple subjects had responses mapped to the peptides comprising Env consensus B amino acids 89–103 (termed “E23”) and 33–47 (termed “E9”). To assess whether there were critical amino acid residues that resulted in loss of activity of the ADCC antibodies, we generated a panel of alanine scan mutant peptides (with alanine replacing the amino acid at each residue in turn) across both epitopes. We tested plasma from several donors to the 15 mutant peptides (five for E23, three for E9), in comparison with the consensus peptide to which the epitope was mapped (Fig. 4). For each subject, there were key residues that resulted in reduced ADCC recognition. For epitope E23 (Fig. 4A), two residues (92F and 100V) when mutated to alanine abrogated ADCC recognition of ADCC antibodies in all five subjects. However, many other residues that substantially influenced ADCC antibody function were different for each subject. For example, at position 95W, subject 1 had almost no recognition of the alanine mutant, whereas subject 5 had enhanced recognition of this mutant; at position 101E, the reverse was true. There were also amino acids critical for recognition of the E9 epitope by all subjects (Fig. 4B), but other mutations showed variable reductions in recognition across the subjects. This result suggests that ADCC antibodies targeting individual HIV-1 peptides are sufficiently different that immune escape can evolve with different patterns.
Fig. 4.
Recognition of alanine scan mutants by ADCC antibodies. A panel of mutated epitopes in which each amino acid is replaced by alanine (or lysine if originally alanine) across two commonly recognized ADCC epitopes was assayed for NK cell activation. The ratio of the ADCC response to the consensus B peptide in comparison with the alanine mutant is shown (1 represents equal recognition). (A) E9 peptide (amino acids 33–47 on consensus B HIV-1 Env). (B) E23 peptide (amino acids 93–107).
Because we sequenced across both the E9 and E23 ADCC epitopes within the C1 region of Env, we could compare sequence variation within the epitopes and the C1 amino acids between and adjacent to the epitopes. We found that mutations between the epitopes were rarer and tended to be more conservative changes than mutations within the epitopes (Fig. S2). This strongly suggests ADCC responses are applying direct immune pressure at these epitopes.
ADCC Escape from Glycoprotein 140 Proteins.
Presentation of linear antibody epitopes within a whole protein can be influenced by conformational and glycosylation changes that affect antibody binding. Additionally, some antibodies recognize conformation-dependent epitopes that are composed of amino acids that may be distal to each other in the linear sequence. To assess ADCC escape in the context of a whole Env protein, we expressed trimeric glycosylated glycoprotein 140 (gp140) proteins that contained patient-derived mutations within one or both Env ADCC epitopes located within E9 and E23, respectively. We chose these mutations because both are commonly targeted Env-specific ADCC epitopes and several subjects had ADCC responses targeting both epitopes. We compared the ability of the wild-type and mutated HIV-1AD8 gp140 proteins to activate NK cells in the presence of whole plasma from one antiretroviral therapy (ART)-naive subject that targeted both epitopes (Fig. 5A). A modest loss of ADCC activity for either singly mutated protein and a more substantial loss of ADCC activity to the Env protein with both mutations were detected.
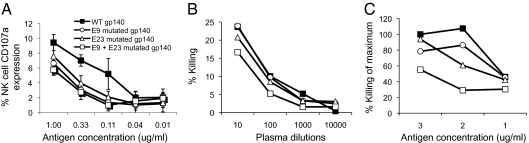
Fig. 5.
ADCC escape from gp140 proteins. Gp140 proteins containing wild-type or mutant forms at one or both of two epitopes (E9 and E23) recognized by the same subject were assayed for ADCC responses by (A) the ICS-ADCC NK cell-activation assay (mean ± SEM of triplicates is shown) and by an RFADCC killing assay using (B) different plasma dilutions and (C) different antigen concentrations.
These studies evaluated NK cell activation as a marker for ADCC activity and immune escape. The generation of the protein reagents also allowed us to evaluate whether mutations result in loss of classical ADCC killing using a cell line pulsed with whole Env protein. We used a rapid fluorescent ADCC (RFADCC) killing assay in which loss of intracellular dye reflects target cell lysis (Fig. 5 B and C). Using whole plasma from subjects, we detected minor loss of ADCC killing activity in cells pulsed with the singly mutated Env proteins. With the protein mutated at both epitopes, we detected a more significant loss of ADCC killing activity. The use of a killing-based ADCC assay confirmed the immune escape identified with the ICS-based assay. The results were consistent across a range of plasma dilutions and antigen dilutions.
Nonneutralizing ADCC Antibodies Can Force Immune Escape.
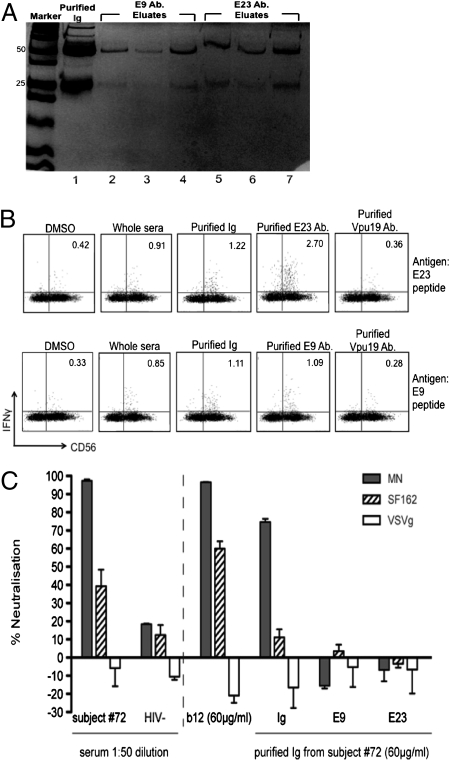
Many previously studied Env-specific ADCC antibodies also are neutralizing (4). When antibodies mediate both neutralization and ADCC functions, it is difficult to dissect the relative contribution of each function to the generation of immune escape. The Env-specific ADCC antibodies E9 and E23 described above recognize relatively conserved regions of the C1 region of Env, a region not commonly a target of characterized NAb. However, to evaluate formally whether these ADCC antibodies also are neutralizing, we purified the E9 and E23 antibodies from serum over columns (first a protein A column to purify total Ig and then peptide-coated CNBr columns to purify the specific Ab) and assessed the function of the purified antibodies in ADCC and neutralization assays. The purified fractions were composed almost entirely of Ig (Fig. 6A) and retained the ability to activate NK cells specifically in the ICS assay (Fig. 6B). Both total serum and purified total Ig from a subject with ADCC responses could mediate neutralization of the HIV-1MN and to a lesser extent HIV-1SF162 strains, as expected because the total antibody response to Env generally contains NAbs targeting multiple epitopes (Fig. 6C). However, the purified E9 and E23 ADCC antibodies did not mediate neutralization of either HIV strain. This result suggests that the generation of immune escape at the E9 and E23 epitopes is mediated by selective pressure to escape ADCC rather than by neutralization.
Fig. 6.
ADCC antibodies to Env C1 E9 and E23 epitopes are nonneutralizing. (A) Antibodies to E9 and E23 Env C1 region peptides from subject #72 were purified using protein A (purifying total Ig) and E9 or E23 peptide-loaded CNBr columns (purifying the specific Ab) and were run on SDS-PAGE gels to illustrate their purity. Lane 1 shows total purified Ig. Lanes 2–4 show three fractions of E9 Abs; lanes 5–7 show three fractions of E23 Abs purified from the Ig sample shown in lane 1. (B) ICS assay for ADCC activity of E23 and E9 purified antibodies. Sera, purified Ig, or purified E9 or E23 antibodies were tested against the E9 and E23 peptide epitopes. Control DMSO stimulation and use of an irrelevant Ab (to a Vpu ADCC epitope purified using the same method) did not activate a significant fraction of CD3−56+ NK cells. (C) Whole serum and purified total Ig from subject #72, but not antibodies purified against the E9 and E23 peptides, mediate neutralization of HIV-1MN and HIV-1SF162 pseudotyped viruses. HIV-negative serum (−) and the patient-derived NAb b12 acted as negative and positive neutralizing antibody controls, respectively, and vesicular stomatitis virus glycoprotein (VSVg) acted as a non-HIV Env-bearing pseudovirus control.
Discussion
This report provides evidence that nonneutralizing ADCC antibodies can force HIV escape mutations. By mapping a large series of ADCC linear epitopes and sequencing autologous HIV RNA across epitopes, we found multiple ADCC antibodies that no longer could recognize autologous viral sequences. Viral sequences from very early in infection were recognized more strongly by recent plasma samples, suggesting evolution of escape over time. Immune escape was identified initially using ADCC peptide epitopes and an NK cell-activation assay and subsequently confirmed using whole Env proteins and a standard killing-based assay.
The HIV genome is able to make multiple changes to avoid CTL, NAb, and antiretroviral drug pressure, and perhaps it should be no surprise that ADCC antibodies also are implicated in viral escape. Nonetheless, the potent immune pressure that can be applied by HIV-specific ADCC antibodies has been brought into sharper focus only recently. In particular, elegant macaque passive transfer studies of NAb mutated in the Fc region so they no longer engage Fc receptors and exert ADCC activity show reduced potency in preventing HIV infection (4). We show that nonneutralizing ADCC antibodies no longer recognize autologous HIV-1 strains, indicating that the selective pressure mediated by ADCC activity is sufficient to drive immunological escape. Passive transfer studies of “ADCC-only” antibodies (without neutralizing activity) will be of interest to evaluate further the potential therapeutic utility of ADCC activity.
ADCC antibodies could be responsible, in part, for the partial efficacy of the recently reported canarypox prime/protein boost vaccine efficacy trial in Thailand (1). Identifying potent ADCC responses that are difficult to escape and inducing these responses by vaccination is a priority for future studies.
This work probably substantially underestimates the number of ADCC epitopes targeted by each HIV+ subject because (i) only linear epitopes are readily mapped and dissected with our ICS technique, and (ii) only consensus B overlapping peptides were used for screening. Conformational ADCC antibodies also are likely to elicit escape, but mapping such responses and identifying escape patterns will be more difficult and will require large numbers of mutant whole Env proteins.
ADCC-forced mutations theoretically could incur some fitness cost to viral replicative capacity, similar to that observed for CTL escape variants (19). Constructing replicating viruses with ADCC-induced mutations will allow testing of this hypothesis. More potent ADCC antibodies are likely to target conserved or functional domains of viral proteins. The Env protein is highly diverse and readily escapes CTL and NAb responses with apparently minimal fitness costs (13, 20–22). It is possible that any fitness cost of ADCC escape in Env could be small. Identifying ADCC antibodies targeting conserved non-Env proteins may reveal more potent ADCC antibodies. For example the Vpu ADCC we described does not appear to undergo immune escape (Fig. 2B). We recently observed that purified ADCC antibodies to this Vpu epitope can recognize and lyse HIV-infected cells (12). Further studies on the patterns of ADCC escape should allow a better understanding of how to limit ADCC escape or force larger fitness costs. Our alanine scan data to date show that even subjects with ADCC antibodies that target the same region of Env undergo divergent patterns of immune escape, suggesting multiple pathways for immune escape at these epitopes are possible.
If escape from ADCC responses does incur a fitness cost, we predict that some ADCC-induced mutations will revert to wild type in the absence of immune pressure following onward transmission of HIV-1. This reversion is observed commonly with CTL mutations (16). Note that this reversion of ADCC-induced mutations may be transient if the recipient subsequently mounts ADCC responses to the same epitope (23). Longitudinal analyses of HIV strains in donor–recipient pairs for reversion of ADCC-induced mutations will be of substantial interest.
In summary, we provide evidence of viral escape from HIV-specific ADCC antibodies. Escape patterns are complex and evolve over time. On the one hand, this result provides evidence of the pressure applied by ADCC antibodies. On the other hand, however, it presents challenges in inducing the most effective ADCC antibodies by vaccination.
Materials and Methods
HIV-Infected Subjects.
Viremic HIV-infected adults who were ART-naïve (n = 80) or who had ART-resistant HIV (n = 12) were recruited from the Melbourne Sexual Health Centre and the Alfred Hospital (Australia) to donate blood samples (10, 24). All subjects provided informed consent. The relevant human research ethics committee approved all studies.
HIV-1 Antigens.
HIV-1 peptides (15 amino acids in length) overlapping by 11 amino acids of consensus B subtype strain were kindly provided by the National Institutes of Health (NIH) AIDS reagent repository. To map ADCC activity across Env, we studied subpools of ∼30 Env peptides and then individual Env peptides as previously described (10). Plasmids for the expression of soluble, uncleaved Env analogs (gp140) were generated by mutating the DNA sequence corresponding to the cleavage site between gp120 and gp41 and inserting a stop codon immediately before the transmembrane domain to produce pN1-AD8-140 as previously described (25). Plasmids encoding Env gp140 proteins with specific mutations corresponding to putative ADCC escape mutants at two epitopes also were generated by PCR-based mutagenesis. All plasmids were transiently transfected into 239T cells, and gp140 was purified from the tissue culture medium using Ni-agarose.
RFADCC Assay.
The RFADCC assay was used as previously described (7, 10). In brief, the CEM-NKr-CCR5 T lymphoblast cell line (kindly provided by the NIH AIDS reagent repository) was labeled with the intracellular dye carboxyfluorescein succinimidyl ester (CFSE) and the membrane dye PKH26 and then pulsed with gp140 proteins (3 μg/1 × 106 cells unless otherwise noted). Healthy donor peripheral blood mononuclear cells (PBMCs) and plasma from the HIV-infected subjects were added to the labeled CEM-NKr-CCR5 cells for 4 h. The proportion of cells that maintained membrane expression of PKH26 but had lost intracellular CFSE (i.e., lysed cells) was analyzed by flow cytometry.
ICS Assay for ADCC Activity.
The ICS-based assay was used to measure HIV antibody-mediated NK cell cytokine expression and degranulation as previously described (10, 11). In brief, 200 μL of fresh whole blood or 50 μL of patient Na-heparin anticoagulated plasma together with 150 μL of healthy donor blood was incubated with either the pool of overlapping 15-mer Env peptides or gp140 Env proteins for 5 h in the presence of Brefeldin A and Monensin (Sigma). At the end of the incubation CD56+ CD3− or CD2+CD3− NK lymphocytes were studied for the expression of intracellular IFN-γ and surface CD107a. Fluorescent antibodies used in the ICS assays were CD3 (catalog no. 347344, fluorescent label PerCP); CD2 (catalog no. 556611, FITC); CD56 [catalog no. 555516, phycoerythrin (PE)]; CD8α (catalog no. 335787 PE-Cy7); CD107a [catalog no. 624078, adenomatous polyposis coli (APC)]; and IFN-γ (catalog no. 557995, Alexa700), all from BD Biosciences. Positive responses were defined as >2 SD above the mean responses to HIV antigens in HIV-1 negative subjects (n = 12).
Sequencing of HIV-1 Clones Across ADCC Epitopes.
Viral sequencing across ADCC epitopes was performed as previously described (21). PCR amplification of ∼500-bp env fragments was performed using env-specific primer pairs as previously described (26). Individual clones were sequenced by BigDye Terminator version 3.1 (Applied Biosystems).
Purification of ADCC Abs.
To assess the neutralizing and ADCC activity of antibodies to specific linear peptide epitopes, we first purified total Ig from sera over a protein A column (GE Healthcare). We then purified the specific Ab using its corresponding peptide bound to cyanogen bromide-activated Sepharose 4B medium (GE Healthcare). The eluted fractions were assessed for protein content during the purification procedure using a NanoDrop spectrophotometer to measure absorbance at 280 nm to determine the Ab-containing fractions and antibody concentration.
Neutralizing Antibody Assay.
The neutralization assay was performed as previously described (25). Briefly, Env-pseudotyped HIV-1 particles were incubated for 1 h at 37 °C in a final volume of 30 μL with varying concentrations of immune sera, nonimmune sera, or antibodies of interest. Target Cf2th-CD4/CCR5/CXCR4 cells were added to the pseudovirus–antibody mixtures, subjected to spinoculation at 1,200 × g for 2 h at room temperature, and cultured for 2 d. Target cells were analyzed for EGFP expression by flow cytometry. The reported % neutralization = (1 − [virus + immune sera or antibody/virus + medium]) ×100, where infection levels observed in the presence and absence of neutralizing antibodies are presented as the mean ± SD of duplicate samples.
Supplementary Material
Acknowledgments
We are grateful to A. Brooks, L. Wren, C. Birch, D. Chibo, J. Silvers, and the subjects studied for assistance with these studies. We thank B. Korber for providing the unique Env sequences. This work was supported by National Health and Medical Research Council Grant 510448, Australian Research Council Grant LP0991498, and National Institutes of Health Grant R21AI081541, and by the Australian Centre for HIV and Hepatitis Virology Research, the Royal Australasian College of Physicians, and the Ramaciotti Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016048108/-/DCSupplemental.
References
- 1.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Karnasuta C, et al. Thai AIDS Vaccine Evaluation Group, Thailand Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2522–2529. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6:515–519. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- 4.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 5.Florese RH, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gómez-Román VR, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Román VR, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Dinges WL, et al. Virus-specific CD8+ T-cell responses better define HIV disease progression than HLA genotype. J Virol. 2010;84:4461–4468. doi: 10.1128/JVI.02438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandi A, et al. NIAID Center for HIV/AIDS Vaccine Immunology Epitopes for broad and potent neutralizing antibody responses during chronic infection with human immunodeficiency virus type 1. Virology. 2010;396:339–348. doi: 10.1016/j.virol.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 11.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isitman G, Navis M, Stratov I, Kent SJ. Purification and Evaluation of HIV-specific ADCC Antibodies, Abstract 329. San Francisco: Conference on Retroviruses and Opportunistic Infections; 2010. [Google Scholar]
- 13.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RE, et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez CS, et al. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79:5721–5731. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie AJ, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 17.Goonetilleke N, et al. CHAVI Clinical Core B The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavilio D, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goepfert PA, et al. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008;205:1009–1017. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troyer RM, et al. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 2009;5:e1000365. doi: 10.1371/journal.ppat.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peut V, Kent SJ. Utility of human immunodeficiency virus type 1 envelope as a T-cell immunogen. J Virol. 2007;81:13125–13134. doi: 10.1128/JVI.01408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peut V, Kent SJ. Substantial envelope-specific CD8 T-cell immunity fails to control SIV disease. Virology. 2009;384:21–27. doi: 10.1016/j.virol.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez CS, et al. Vaccine-induced T cells control reversion of AIDS virus immune escape mutants. J Virol. 2007;81:4137–4144. doi: 10.1128/JVI.02193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stratov I, Dale CJ, Chea S, McCluskey J, Kent SJ. Induction of T-cell immunity to antiretroviral drug-resistant human immunodeficiency virus type 1. J Virol. 2005;79:7728–7737. doi: 10.1128/JVI.79.12.7728-7737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Center RJ, et al. Induction of HIV-1 subtype B and AE-specific neutralizing antibodies in mice and macaques with DNA prime and recombinant gp140 protein boost regimens. Vaccine. 2009;27:6605–6612. doi: 10.1016/j.vaccine.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Peut V, et al. Balancing reversion of cytotoxic T-lymphocyte and neutralizing antibody escape mutations within human immunodeficiency virus type 1 Env upon transmission. J Virol. 2009;83:8986–8992. doi: 10.1128/JVI.00599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.