Abstract
Genetic studies suggest that Zn transporters such as ZnT8 play a role in insulin secretion by pancreatic β-cells; however, little is known about the dynamic roles of Zn trafficking pathways on β-cell physiology. To test the acute effects of the inflammatory cytokines interleukin 1β (IL1β) and tumor necrosis factor α (TNFα) on Zn homeostasis, the mRNA expression profile of Zn transporters of the ZnT and ZIP families was examined. Exposure of MIN6 cells or primary murine islets to IL1β or TNFα altered the mRNA expression profile of Zn transporters; most notable was decreased ZnT8 mRNA levels. siRNA-mediated gene knockdown was used to examine the effects of decreased ZnT8 expression in primary dispersed murine islet cells from C57/BL6 mice and MIN6 cells. ZnT8 knockdown in these murine islets led to reduced glucose stimulated insulin secretion without altering the total cellular insulin content or cell viability at normal or supraphysiological Zn concentrations. The labile Zn content determined by flow cytometry after loading with the Zn-specific sensor FluoZin-3 AM was decreased in MIN6 cells following ZnT8 knockdown or IL1β treatment. These results suggest that an acute decrease in ZnT8 levels impairs β-cell function and Zn homeostasis, and may contribute to inflammatory cytokine-induced alterations in β-cell function.
Introduction
A low level chronic inflammatory state with elevated circulating inflammatory cytokine levels is thought to be an important contributor to the development of type 2 diabetes mellitus (T2DM). Impaired function of insulin secreting β-cells after exposure to the inflammatory cytokines interleukin 1β (IL1β) or tumor necrosis factor α (TNFα) has been shown in human and rodent β-cells as well as in MIN6 cells, a β-cell line. Several mechanisms for this effect have been implicated (Xenos et al. 1992, 1993, 1994, Corbett & McDaniel 1995, Rabinovitch & Suarez-Pinzon 1998, Wu et al. 2001, Donath et al. 2008). Altered expression of several genes involved in β-cell function is thought to be one of the mechanisms underlying this effect of cytokines (Donath et al. 2008).
Zn is the second most abundant transition metal in mammals (Wallwork et al. 1983, Milne et al. 1985, Ward & Mason 1987) and is utilized in a number of biological processes (Cousins et al. 2006). Zn homeostasis is tightly regulated on the cellular and systemic level through various mechanisms (Outten & O’Halloran 2001, Cousins et al. 2006) and is of particular importance to pancreatic β-cell physiology (Chausmer 1998, Dodson & Steiner 1998, Chimienti et al. 2005, 2006, Lemaire et al. 2009). β-Cells have exceedingly high intracellular concentrations of Zn, particularly within secretory vesicles, where Zn facilitates the packaging of insulin into hexamers (Figlewicz et al. 1984, Dodson & Steiner 1998, Chimienti et al. 2006, Lemaire et al. 2009). In order to utilize Zn, the β-cells require the trafficking of Zn across cell and vesicular membranes, mainly through members of the Zn transporter (ZnT, SLC30A) and Zrt–Irt-like protein (ZIP, SLC39A) transporter families. These facilitate Zn diffusion out of or into the cytoplasm respectively. The Zn transporter encoded by SLC30A8, ZnT8, plays a particularly important role in Zn homeostasis in β-cells (Chimienti et al. 2005, 2006, Lemaire et al. 2009). ZnT8 is highly expressed in β-cells (Chimienti et al. 2005, Mocchegiani et al. 2008), and localizes mainly to the membranes of secretory vesicles in β-cells (Chimienti et al. 2006). Other genetic variations in SLC30A8 have been found to be associated with fasting glucose levels (Dupuis et al. 2010). Strong association in humans has been demonstrated between a nonsynonymous single-nucleotide polymorphism rs1326634 in SLC30A8, and increased risk for the development of T2DM or β-cell dysfunction (Saxena et al. 2007, Scott et al. 2007, Sladek et al. 2007, Zeggini et al. 2007, Boesgaard et al. 2008, Hertel et al. 2008, van Hoek et al. 2008, Horikawa et al. 2008, Omori et al. 2008, Wu et al. 2008, Xiang et al. 2008). Other genetic variations in SLC30A8 have been found to be associated with fasting glucose levels (Dupuis et al. 2010). Recently, three studies described impaired Zn accumulation in secretory vesicles of islets isolated from ZnT8 knockout mice. Impaired β-cell function was present under certain age and high-fat dietary conditions (Lemaire et al. 2009, Nicolson et al. 2009, Pound et al. 2009). This highlighted the deleterious effects of a chronic and complete loss of ZnT8 function on islet cell physiology, and the likely role of compensatory and environmental factors in this process.
Given the importance of well-calibrated Zn homeostasis for β-cell physiology, we examined whether the effects of the cytokines IL1β and TNFα on β-cell function may be mediated, in part, through altered expression of Zn transporters. We examined the effect of treating MIN6 cells with these cytokines on the expression of Zn transporters in the ZIP and ZnT families. Additionally, we examined the consequences of the most significant cytokine-induced alteration in Zn transporter expression, decreased expression of ZnT8, in MIN6 cells and primary murine islets.
Materials and Methods
Islet purification
Islets from 10 to 14 weeks old C57BL/6 male mice (Jackson Laboratories, Bar Harbor, ME, USA) were isolated as described previously (Blomeier et al. 2006). After washing, the islets were handpicked and counted under microscopic guidance. All studies were approved by the Northwestern University Animal Care and Use Committee.
MIN6 cell culture
MIN6 cells were a gift from Dr Junichi Miyazaki (Osaka University Medical School, Osaka, Japan), and were grown and maintained as described previously (Miyazaki et al. 1990). Cells between passages 28 and 35 were used.
Cytokine treatment
Cytokine treatment was performed as previously reported with modifications (Chin-Chance et al. 2006). Briefly, MIN6 cells between passages 28 and 34 were plated at a density of 2 × 106 cells per 60-mm dish. Forty-eight hours after plating, the culture medium was exchanged for serum reduced culture medium containing 1% heat inactivated fetal bovine serum (FBS) supplemented with either no cytokines, 5 ng/ml murine IL1β, 10 ng/ml murine TNFα, or a combination of both (R&D Systems, Minneapolis, MN, USA). The cells were treated with cytokines for 6, 24, or 48 h. For studies with murine islets, purified islets were incubated for 24 h in RPMI 1640 (ATCC, Manassas, VA, USA) supplemented with 1% non-heat inactivated FBS. The media were supplemented with either no cytokines, 5 ng/ml IL1β, or 10 ng/ml TNFα.
mRNA extraction and real-time PCR
RNA was extracted from MIN6 and dispersed murine islet cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was extracted from intact murine islets using the RNAeasy Mini kit (Qiagen) according to the manufacturer’s recommendations. The iScript cDNA synthesis kit (Bio-Rad) was used for reverse transcription of 0·5 μg RNA following treatment with DNAse (Turbo DNAse kit, Ambion, Austin, TX, USA) according to the manufacturer’s instructions. Real-time PCR was performed using the SYBR-Green super mix reagent (Bio-Rad) and the Bio-Rad iCycler PCR system. Primers for ZIP1 through 14, ZnT1 through 10, and β-actin, and 18S (idtDNA, Coralville, IA, USA) were used in the PCRs. The sequences for the real-time PCR primers are listed in Supplementary Table 1, see section on supplementary data given at the end of this article. For real-time PCR, an initial denaturation step at 95 °C × 5 min was followed by 40 cycles at 95 °C × 15 s and 60 °C × 30 s. A melt curve analysis was performed by incubating the reaction at 95 °C × 1 min, cooling to 60 °C for 1 min, and then increasing the temperature 0·5 °C every 10 s up to 100 °C. All primers were validated using mRNA from mouse islets, MIN6 cells, and embryoid bodies prepared from mur ine embryonic stem cells. 2% agarose gel electrophoresis demonstrated a product of the expected size for all the ZnTs and ZIPs. Melt curve analyses demonstrated a single PCR product for each reaction. The Δ – ΔCt method was used to calculate the relative level of mRNA of the gene of interest compared with the control condition after normalizing using the level of β-actin mRNA (Livak & Schmittgen 2001). 18S was used as an additional normalizer for experiments comparing MIN6 and primary islet Zn transporter expression profiles.
MIN6 cell imaging
Untreated live MIN6 cells were imaged following incubation for 1 h in phenol red-free DMEM media (Invitrogen) supplemented with the labile Zn-specific fluorescent sensor Fluozin-3-AM (Invitrogen) at a concentration of 5 μM. The images were obtained on an IX2-DSU/BX-DSU spinning disc confocal microscope (Olympus, Center Valley, PA, USA) in the Northwestern University cell imaging facility. Images were analyzed with Metamorph software (Universal Imaging Corp., Downington, PA, USA).
Flow cytometry
Flow cytometry was carried out 72 h after transfection with siRNA or after treatment with IL1β for 24 h. Cells were washed twice with MIN6 cell culture medium containing 1 or 15% heat-inactivated FBS for IL1β or ZnT8 knockdown experiments respectively. Cells were subsequently incubated for 1 h in basic media supplemented with 2 mM FluoZin-3 AM (Invitrogen) and 1 mM CellTrace Red (Invitrogen) at 37 °C in 5% CO2. Cells were then washed twice with cell culture medium and once with PBS. The cells were dispersed using 0·05% trypsin, collected by centrifugation, resuspended in PBS, and passed through a 70-mM Cell Strainer (BD Biosciences, Bedford, MA, USA). Cells were analyzed on an LSR II system (BD, Franklin Lakes, NH, USA). Only the Zn fluorescence of live, single cells, as determined by the CellTrace side scatter and forward scatter fluorescence patterns, was analyzed.
ZnT8 knockdown in dispersed islet cells or MIN6 cells
Islets harvested from C57BL/6 mice were washed with PBS (Invitrogen), pelleted by centrifugation at 0·5 relative centrifugal force (RCF) × 1 min, resuspended in 0·05% trypsin (Invitrogen), and incubated at 37 °C for 4·5 min. The cells were dispersed by pipetting at the 2-min time point and end of the incubation period. The incubation was terminated by adding culture medium (RPMI 1640 supplemented with 10% non-heat inactivated FBS (Hyclone, Logan, UT, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/l glutamine). The dispersed cells were collected by centrifugation (0·5 RCF × 5 min), resuspended in culture medium, and plated onto tissue culture dishes (BD-Falcon, San Jose, CA, USA). MIN6 cells were cultured as previously described (Miyazaki et al. 1990).
MIN6 cells or dispersed murine islet cells were plated in a cell media volume of 1·5 ml at a density of 1·0 × 105 or 2·0 × 105 cells per 35-mm tissue culture dish respectively. Transfection with siRNA was carried out 5 or 24 h after plating for dispersed murine islets or MIN6 cells respectively. Cells were transfected with control (negative control siRNA #3, Dharmacon, Lafayette, CO, USA) or ZnT8 siRNA (Nicolson et al. 2009; see Supplementary Table 1 for sequence of the ZnT8 siRNA) by adding 500 μl of transfection mix (500 μl Opti-MEM-I transfection medium (Invitrogen), 150 pmol siRNA (final concentration 75 nM, Dharmacon), and 7 μl Lipofectamin RNAimax (Invitrogen), prepared according to the manufacturer’s instructions). In MIN6 cell experiments, the cell culture medium was exchanged for antibiotic-free standard cell culture medium immediately prior to transfection. Cells were maintained in this mix of culture and transfection medium until experiments were carried out 72 h later. In each experiment, cells were transfected with ZnT8 siRNA or non-targeting control siRNA in parallel. Decreased ZnT8 mRNA and protein levels were verified using real-time PCR and western blot analysis (see below).
Western blot analysis
Western blot analysis of cell lysates was carried out as described previously (Liu et al. 2001), with modifications as described herein. Samples (20 μg of protein) were suspended in β-mercaptoethanol (β-ME)-containing laemmli buffer without heating and subjected to 10% SDS-PAGE followed by semi dry transfer for 2 h to a polyvinylidene fluoride membrane (Immobilon-P, Millipore, Bedford, MA, USA). The membranes were blocked for 2 h and then hybridized overnight with a 1:2000 dilution of polyclonal rabbit-anti-rat ZnT8 antibody (Mellitech, Grenoble, France). The membranes were then washed × 3 for 10 min in Tris-Buffered Saline Tween-20 (TBS–T) and hybridized with a 1:7500 dilution of secondary antibody (HRP-conjugated goat anti-rabbit IgG, Promega) for 1 h. The membrane was washed × 3 for 10 min in TBS–T. Immunoreactive bands were detected using an ECL detection kit (Amersham). All of the above steps were carried out at 4 °C on ice. The blocking and antibody incubation steps were carried out in TBS-T containing 5% w/v non-fat milk powder (Carnation, Wilkes-Barre, PA, USA). To document equivalent loading of protein, the immunoblots were stripped in 62·5 mM Tris–HCl (pH 6·7), 20% SDS, and 100 mM β-ME for 30 min at 50 °C, rehybridized with a monoclonal mouse anti-mouse GAPDH antibody (Cell Signalling, Danvers, MA, USA), and an HRP-coupled secondary goat anti-mouse antibody.
Glucose-stimulated insulin secretion and total cellular insulin content
To measure glucose-stimulated insulin secretion (GSIS), dispersed murine islet cells were washed three times with PBS 72 h after transfection. Subsequently, the cells were equilibrated in HEPES-buffered Krebs solution (130 mM NaCl, 3·6 mM KCl, 0·5 mM NaH2PO4, 0·5 mM MgSO4, 1·5 mM CaCl2, 1% HEPES, 0.1% BSA, pH 7.4) containing 5·6 mM glucose for 30 min. This was followed by incubation in HEPES-buffered Krebs solution with 5·6 mM glucose for 30 min, and then HEPES-buffered Krebs solution with 16·7 mM glucose for 30 min. After each per iod of incubation, 200 μl of the supernatant was collected. Samples were stored at −80 °C. In separate experiments, cells were incubated for 72 h following transfection and then lysed after washing three times with PBS by adding 100 μl of RIPA lysis buffer supplemented with a cocktail of protease and phosphatase inhibitors (Calbiochem, Madison, WI, USA). Total protein concentration in the lysate was measured using the Micro BCA Protein Assay kit (Pierce, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s recommendations. Measurement of insulin was carried out by ELISA (Alpco Diagnostics, San Francisco, CA, USA) according to the manufacturer’s instructions.
Cell viability
Cell viability experiments were performed in black, opaque 96-well tissue culture plates (BD-Falcon). Dispersed murine islet cells were cultured and transfected with either control or ZnT8 siRNA as described above with proportional adjustment for the decreased culture area. The medium was supplemented with ZnCl2 to final concentrations of Zn of 5, 19, 34, 48, 62, 76, 91, or 105 μM at the time of transfection. Cell viability was assessed 72 h post transfection using the double fluorescence Multitox assay (Promega) according to the manufacturer’s instructions. Briefly, 100 μl of the assay mix was added to each well and incubated at 37 °C for 2 h. Fluorescence was measured on a fluorescence plate reader at excitation wavelength (Ex) 400 nm/emission wavelength (Em) 505 nm and Ex 485/Em 520, which measured fluorescence from proteases in viable and dead cells respectively. Fluorescence values were transformed to dead and live cell readout values using a standard curve obtained using a standardized range of cell densities for the live cell signal and digitonin-induced cell death for the dead cell signal. Readout values were normalized to values obtained from untreated cells. Results were reported as a cell viability index calculated as the ratio of live cell to dead cell readouts.
Measurement of Zn concentration in the media
Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure Zn content in the culture media. The media were diluted with ultra-pure laboratory grade water (Millipore, Billerica, MA, USA), and an internal standard mixture of Sc, Tb, Y, In, and Bi (CPI International, Santa Rosa, CA, USA) was added to this solution. Standards between 0 and 90 ppb were made using a mixed element solution (CPI International). 2% v/v trace metal grade nitric acid (Fisher Scientific, Pittsburgh, PA, USA) was added to the standards and samples. All measurements were performed on an XSeries II ICP-MS instrument (Thermo Scientific, Waltham, MA, USA).
Statistical analysis
Results are reported as means ±S.E.M. unless otherwise stated. The ‘n’ represents the number of independent experiments performed. Changes in parameters normalized to the control condition were tested for statistical significance using a two-sided t-test with unequal variance to account for the normalization to the control. In all other experiments comparing the effects of various treatments on physiological parameters not normalized to the control, groups were compared directly to the control condition using a two-sided t-test with equal variance. P values ≤ 0·05 and 0·005 were considered statistically significant and highly significant respectively (STATA-IC V.10.1, College Station, TX, USA).
Results
Cytokine-induced alterations in expression of Zn transporters
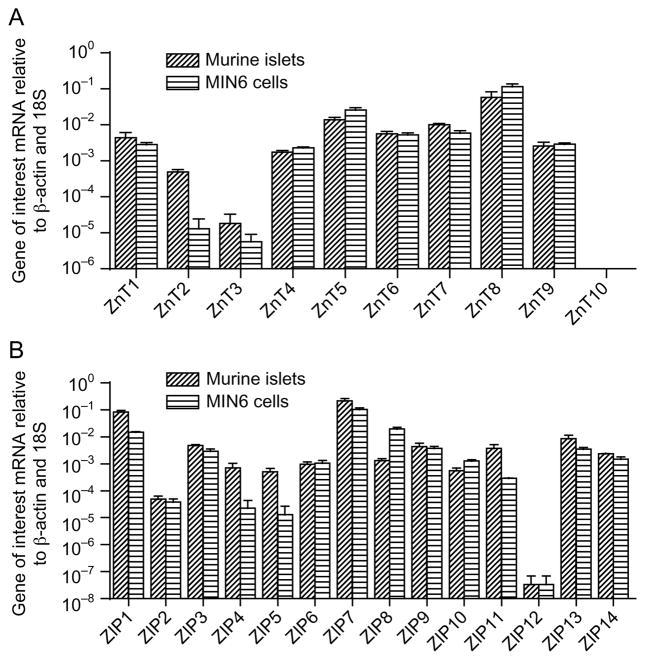
Initial studies compared the expression profile of members of the ZnT and ZIP families in MIN6 cells and primary murine islets. As shown in Fig. 1, mRNAs encoding 14 members of the ZIP family and nine members of the ZnT family are expressed in murine islets. The expression profile of the ZnTs and ZIPs in MIN6 cells was comparable to that in islets with the exception of a markedly lower expression of ZnT2, ZnT3, ZIP4, and ZIP5.
Figure 1.
Relative level of mRNAs encoding ZIP and ZnT transporters in MIN6 cells and murine islets. mRNA was extracted from untreated murine islets and MIN6 cells, and real-time PCR was performed. mRNA levels were normalized to both β-actin and 18S. Values represent the relative level of ZIP or ZnT mRNA normalized to the level of both β-actin and 18S mRNA, and are the mean ±S.E.M. of the results of three independent experiment.
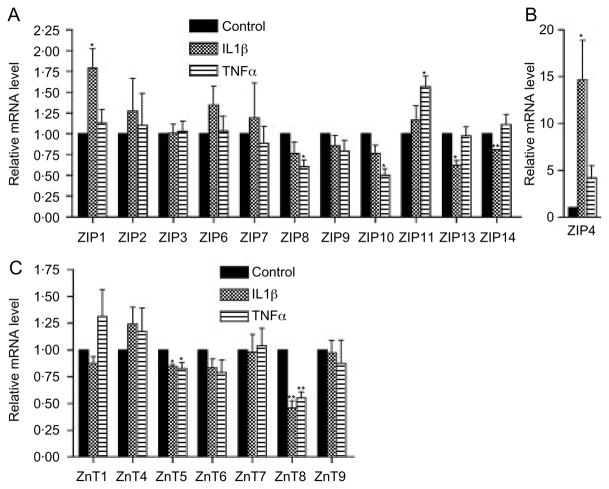
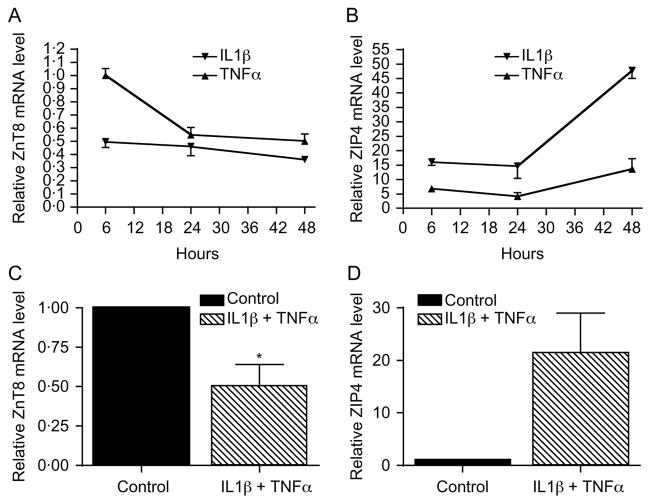
Having established comparable expression profiles of Zn transporters in islets and MIN6 cells, MIN6 cells were used as a model to examine the impact of cytokines on the level of mRNAs encoding the different Zn transporters. Treatment of MIN6 cells with either 5 ng/ml IL1β or 10 ng/ml TNFα for 24 h altered the expression of several Zn transporters (Fig. 2). The transporters with the most marked change in expression were ZnT8 and ZIP4. The level of ZnT8 mRNA was decreased by 54·6 ± 6·9 and 44·7 ± 5·4% by IL1β and TNFα respectively (P = 0·004 and 0·004, Fig. 2C). The level of ZIP4 mRNA was increased 14 ± 4.2-fold after IL1β treatment (P<0·05, Fig. 2B). There was also a trend, albeit not significant, toward increased expression of ZIP4 mRNA following treatment with TNFα (4·1 ± 1·2-fold, P = 0·089, Fig. 2B). Treatment for 48 h with a combination of 5 ng/ml IL1β and 10 ng/ml TNFα decreased ZnT8 mRNA by 50 ± 14% (P = 0·024, Fig. 3C), while ZIP4 mRNA levels showed a clear but statistically non-significant trend toward increased expression (21 ± 7·6-fold, P = 0·057, Fig. 3D). In time course experiments, IL1β treatment decreased ZnT8 mRNA levels by 51 ± 4·2%, 54·6 ± 6·9%, and 64·1 ± 0·6% following treatment for 6, 24, and 48 h respectively (Fig. 3A). Treatment with 10 ng/ml TNFα for 6 h had no effect on ZnT8 mRNA levels but decreased ZnT8 mRNA levels by 44·7 ± 5·4 and 50 ± 5·2% after treatment for 24 and 48 h respectively (Fig. 3A). IL1β treatment for 6, 24, and 48 h increased ZIP4 mRNA levels by 16 ± 1·2-, 14 ± 4·2-, and 47 ± 4·1-fold respectively (Fig. 3B). Treatment with TNFα for 6, 24, and 48 h stimulated a nonsignificant trend toward increased ZIP4 mRNA levels with increases of 6·8 ± 1·2-, 4·1 ± 1·2-, and 13 ± 3·5-fold respectively (Fig. 3B).
Figure 2.
Effect of cytokines on ZIP and ZnT mRNA levels in MIN6 cells. MIN6 cells were treated for 24 h with 5 ng/ml IL1β or 10 ng/ml TNFα, and the level of mRNAs encoding ZIPs (A and B) and ZnTs (C) was determined. Values represent the relative level of mRNA compared with the level in control cells, which was defined as 1·0 and represent the mean ±S.E.M. (n = 4–5 for the different transporters). *P<0·05 compared with control; **P<0·005 compared with control.
Figure 3.
(A and B) Time course of effect of cytokines on ZnT8 and ZIP4 mRNA levels. The level of ZnT8 (A) and ZIP4 (B) mRNA was determined in MIN6 cells after treatment with 5 ng/ml IL1β or 10 ng/ml TNFα for 6 (n = 2), 24 (n = 4), or 48 (n = 2) h. Values represent the relative level of ZnT8 and ZIP4 mRNA compared with the level in control cells, and are the mean ±S.E.M. (C and D) Effect of a combination of cytokines on ZnT8 and ZIP4 mRNA levels. The level of ZnT8 (C) and ZIP4 (D) mRNA was determined in MIN6 cells after treatment with a combination of 5 ng/ml IL1β and 10 ng/ml TNFα for 48 h. Values represent the relative level of ZnT8 and ZIP4 mRNA compared with the level in control cells, and are the mean ±S.E.M. (n = 5). *P<0·05.
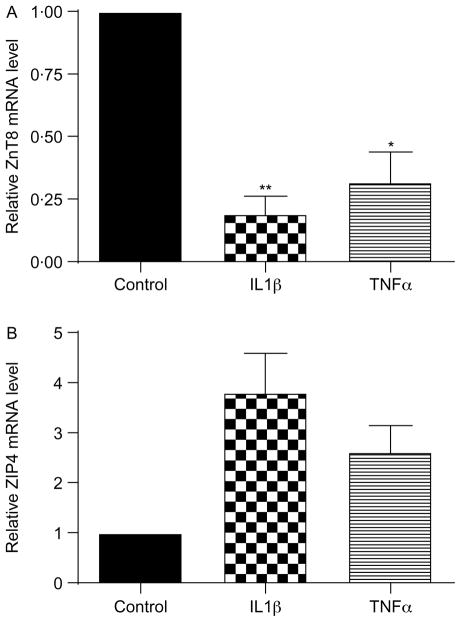
Subsequent experiments determined whether cytokines had a similar effect on ZnT8 and ZIP4 mRNA levels in murine islets. Treatment of islets with 5 ng/ml IL1β for 24 h decreased ZnT8 mRNA levels by 81 ± 7%, while treatment for 24 h with 10 ng/ml TNFα decreased ZnT8 mRNA levels by 68 ± 11% (P = 0·0012 and 0·009, Fig. 4A). Treatment of islets with 5 ng/ml IL1β for 24 h showed a trend toward increased ZIP4 mRNA levels (3·81 ± 0·75-fold, P = 0·064, Fig. 4B). Treatment with 10 ng/ml TNFα for 24 h resulted in a similar nonsignificant trend toward increased ZIP4 mRNA levels (2·62 ± 0·50-fold, P = 0·083, Fig. 4B).
Figure 4.
Effect of cytokines on ZnT8 and ZIP4 mRNA levels in intact murine islets. ZnT8 (A) and ZIP4 (B) mRNA levels were determined in intact murine islets after treatment for 24 h with 5 ng/ml IL1β or 10 ng/ml TNFα. Values are the mean level of ZnT8 and ZIP4 mRNA compared with the level in control cells, which was defined as 1·0 and are the mean ±S.E.M. (n = 4 for ZnT8, n = 3 for ZIP4). *P<0·05, **P<0·005 compared with control.
β-Cell labile Zn distribution and cytokine-induced changes of labile Zn
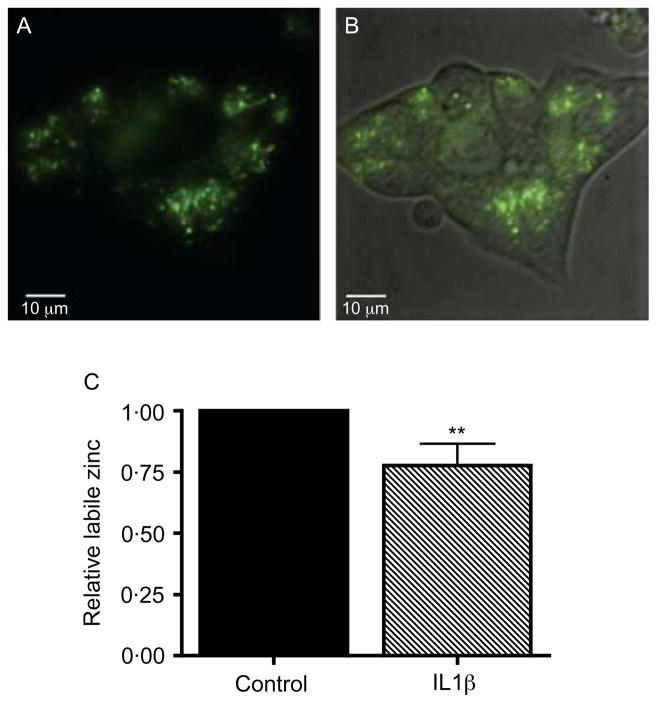
Laser scanning confocal imaging of live MIN6 cells following incubation with Fluozin-3 AM showed a high concentration of labile Zn (unbound or weakly bound intracellular Zn), which appeared to be distributed primarily in secretory vesicles (Fig. 5A). Given the changes in Zn transporter expression induced by cytokine treatment, we examined the changes in labile, FluoZin-3 AM-detectable Zn in β-cells after cytokine treatment which, based upon the live cell imaging, represents primarily Zn present in secretory vesicles. In order to avoid interference by other cell types contained in islets, we used the pancreatic β-cell line MIN6. After 24 h treatment with 5 ng/ml IL1β, the average Zn-related FluoZin-3 AM fluorescence decreased by 22·2 ± 8·8% compared with control cells (P = 0·0012, Fig. 5B).
Figure 5.
(A and B) The distribution of labile Zn in single untreated live MIN6 cells. Labile Zn was imaged following incubation in Fluozin-3 AM in phenol red-free DMEM for 30 min using confocal spinning disc microscopy (100 × magnification, A, Fluozin-3 AM fluorescence alone; B, phase contrast + fluorescence overlay). (C) Effect of IL1β on labile Zn in MIN6 cells. Cells were analyzed using flow cytometry after staining with the Zn-specific fluorescent probe FluoZin-3 AM and live-cell dye CellTrace calcein red-orange, AM. FluoZin-3 AM fluorescence was analyzed in live cells only. In total, 20 000–30 000 live cell events were recorded in each replicate. The change in FluoZin-3 AM detectable Zn was determined in MIN6 cells after treatment or not with 5 ng/ml IL1β for 24 h. Values represent the relative FluoZin-3 AM-related fluorescence in live cells normalized to the control condition, and are the mean ±S.E.M. (n = 3, each in triplicate). **P<0·005 compared with control.
Effect of an acute decrease in ZnT8 expression on GSIS
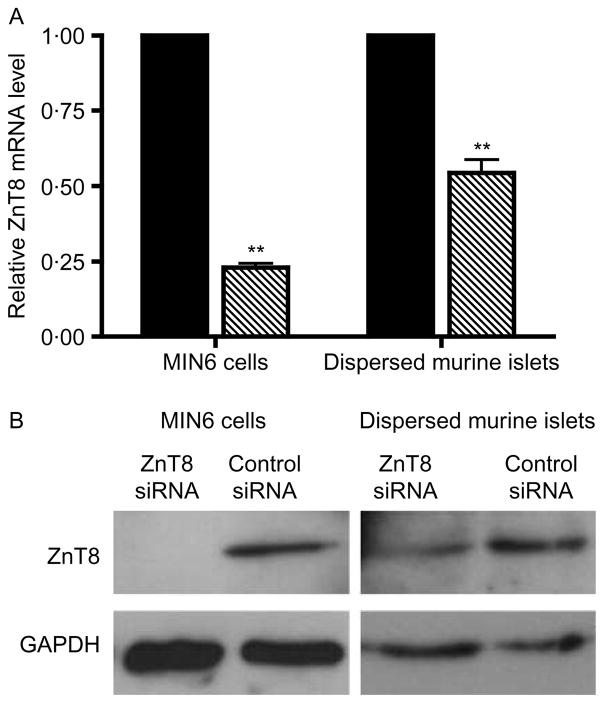
Given the high baseline expression of ZnT8, its localization to insulin secretory granules, and the marked cytokine-induced changes in ZnT8 mRNA levels, we investigated the effect of an acute downregulation of ZnT8 mRNA levels in β-cells using siRNA. Initial studies demonstrated the ability of ZnT8 siRNA to decrease the level of ZnT8 mRNA. Following transfection of MIN6 or primary dispersed murine islet cells with ZnT8 siRNA, the level of ZnT8 mRNA was 23 ± 1·4 and 54·4 ± 4·4% (mean ±S.E.M) of the level in cells transfected with control siRNA respectively (Fig. 6A). A decrease in ZnT8 protein level in dispersed primary murine islets following treatment with ZnT8 siRNA was verified by western blotting (Fig. 6B).
Figure 6.
Effect of ZnT8 siRNA on ZnT8 expression. (A) ZnT8 mRNA levels were determined in MIN6 and dispersed murine islet cells following transfection with either ZnT8 or control siRNA. The values represent the relative level of ZnT8 mRNA in cells treated with ZnT8 siRNA compared with cells treated with control siRNA, which was defined as 1·0, and are the mean ±S.E.M. (n = 3 for MIN6 cells, n = 4 for islet cells). The level of ZnT8 mRNA was normalized to the level of β-actin mRNA. **P<0·005 compared with control siRNA. (B) Western blot analysis comparing ZnT8 in dispersed murine islet cells treated with ZnT8 siRNA and control siRNA. The autoradiograms are representative of the results of three and five independent experiments for MIN6 and dispersed islets respectively.
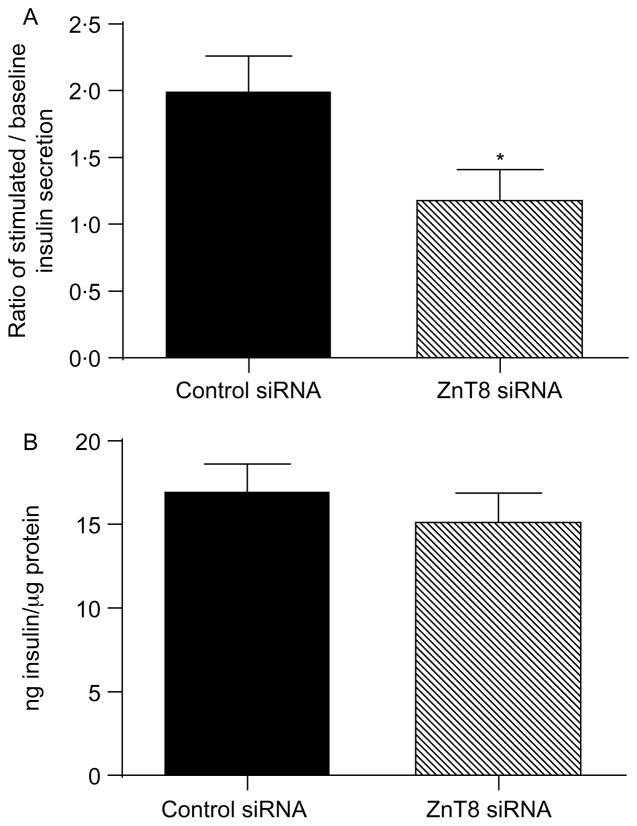
Having established that ZnT8 siRNA was able to decrease ZnT8 expression, GSIS in dispersed murine islet cells following transfection with ZnT8 or control siRNA was determined. The relative ratio of insulin concentration in medium following incubation in 16·7 compared with 5·6 mM glucose was 2·0 ± 0·25 in cells treated with control siRNA (Fig. 7A), while in islet cells treated with ZnT8 siRNA, the ratio was significantly decreased to 1·2 ± 0·21 (P<0·05 compared with the ratio in cells transfected with control siRNA). In contrast to the effect on GSIS, transfection with ZnT8 siRNA did not affect total cellular insulin content. Total cellular insulin content in islet cell lysates was 17·1 ± 1·5 ng insulin/μg protein following transfection with ZnT8 siRNA compared with 15·3 ± 1·6 ng insulin/μg protein following transfection with control siRNA (P = 0·41, Fig. 7B).
Figure 7.
Glucose-stimulated insulin secretion and cellular insulin content in dispersed murine islet cells following transfection with ZnT8 siRNA. (A) 72 h after transfection of dispersed murine islet cells with either ZnT8 or control siRNA, the insulin concentration in the medium was determined following incubation in 5·6 and 16·7 mM glucose. Values represent the ratio of the insulin concentration in medium after incubation for 30 min in 16·7 mM glucose compared with 5·6 mM glucose, and are the mean ±S.E.M. (n = 4). *P<0·05 compared with cells treated with control siRNA. (B) Total cellular content of insulin in dispersed murine islet cells 72 h after transfection with either ZnT8 or control siRNA. Values represent the total insulin content in cell lysates which was determined as described in the Materials and Methods, and are the mean ±S.E.M. (n = 3).
Effects of decreased ZnT8 mRNA levels on labile Zn
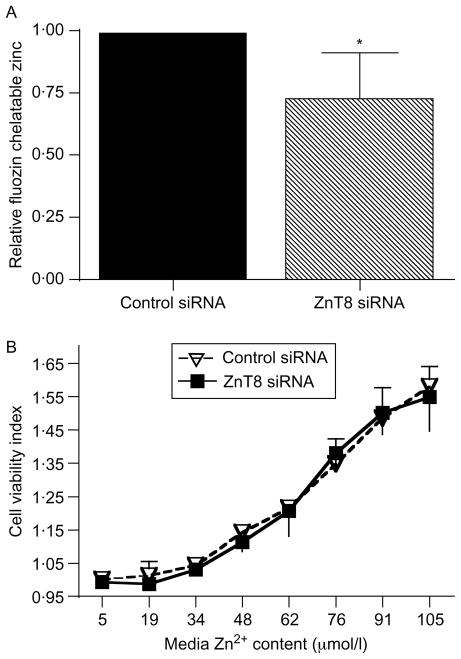
We next examined whether acute downregulation of ZnT8 expression altered Zn homeostasis similar to treatment with ILβ. For these studies, MIN6 cells were transfected with either ZnT8 or control siRNA, and labile Zn was determined using flow cytometry after staining with the Zn-specific fluorescent probe FluoZin-3 AM and the live-cell dye CellTrace calcein red-orange AM. The relative mean labile Zn-related fluorescence from live cells decreased by 26·5 ± 17·6% following transfection with ZnT8 siRNA compared with control siRNA (P<0·05, Fig. 8A).
Figure 8.
Effect of decreased ZnT8 expression on MIN6 cell labile Zn content and dispersed islet cell viability. (A) MIN6 cells were analyzed using flow cytometry after staining with the Zn-specific fluorescent probe FluoZin-3 AM and live-cell dye CellTrace calcein red-orange. FluoZin-3 AM fluorescence was analyzed in live cells only. In total, 20 000–30 000 live cell events were recorded in each replicate. FluoZin-3-chelatable Zn in MIN6 cells transfected with control siRNA or ZnT8 siRNA was then determined. Values represent the relative FluoZin-3-related fluorescence in live cells normalized to the control condition, and are the mean ±S.E.M. (n = 3, each in duplicate or triplicate), *P<0·05. (B) Dispersed islet cells were transfected with either ZnT8 or control siRNA, and then incubated in the presence of increasing concentrations of Zn. Cell viability was determined using the Multitox assay. Changes in the cell viability index correspond to changes in the ratio of the live to dead cell readouts. Values represent the mean cell viability index, and are the mean ±S.D. of the results (n = 4, each in duplicate or triplicate).
Effect of decreased ZnT8 mRNA levels on islet cell viability in various Zn concentrations
Subsequent studies examined the impact of decreased ZnT8 expression and the resulting changes in Zn homeostasis on islet cell viability. Initial studies using ICP-MS demonstrated that the concentration of Zn in the culture medium was 5 μM. Following transfection of dispersed murine islet cells with either ZnT8 or control siRNA, cell viability was determined using the Multitox assay. The viability indices, which are proportional to the ratio of live to dead cells each normalized to the values of control cells with no added Zn, were 1·0002 ± 0·0004 and 0·9937 ± 0·0070 following transfection with control and ZnT8 siRNAs respectively (P = 0·386, n = 4, each performed in duplicate or triplicate, Fig. 8B). Supplementing the medium with ZnCl2 to final Zn concentrations of 19, 34, 48, 62, 76, 91, or 105 μM enhanced cell viability at all concentrations. The viability index increased to 1·5803 ± 0·0608 and 1·5493 ± 0·1017 at 105 μM Zn in cells transfected with control and ZnT8 siRNAs respectively (P = 0·802 for the knockdown versus control comparison, n = 4, each performed in duplicate or triplicate).
Discussion
Impaired function of human and rodent β-cells as well as MIN6 cells after exposure to IL1β or TNFα is well established (Donath et al. 2008). Several potential mechanisms have been suggested for this effect (Xenos et al. 1992, 1993, 1994, Corbett & McDaniel 1995, Rabinovitch & Suarez-Pinzon 1998, Wu et al. 2001, Donath et al. 2008). These include activation of the mitogen-activated protein kinases, c-jun N-terminal kinase, p38, and Extracellular Signal-Regulated-Kinase (ERK) with subsequent activation of the nuclear factor kappa-light-chain-enhancer of activated β-cells (NF-kB) pathway and altered gene expression (Donath et al. 2008). In this study, we examined whether cytokines affect Zn transporter expression as a possible contributing factor to cytokine-induced β-cell dysfunction. We demonstrated that treatment of murine islets or MIN6 cells with IL1β or TNFα alters the expression profile of Zn transporters of the ZnT and ZIP families. One of the observed changes which may be of particular significance is the cytokine-induced decrease in ZnT8 expression. ZnT8 has a high baseline expression level in β-cells, and plays an important role in β-cell function. Moreover, we observed similar alterations in cellular labile Zn content following IL1β exposure and ZnT8 knockdown. This finding and the decrease in GSIS observed following ZnT8 knockdown are consistent with alterations in Zn homeostasis due to decreased ZnT8 expression contributing to cytokine-induced impairment of β-cell function.
Our finding of a cytokine-induced decrease in ZnT8 expression generally agrees with a recent report by Egefjord et al. (2009) which described an IL1β-induced decrease in ZnT8 mRNA levels in INS-1 cells, a rat β-cell line, as well as in rat islets. In contrast to their findings, we did not observe reduced ZnT3, ZnT6, and ZIP6 mRNA levels; however, we do find a significant cytokine-induced increase in ZIP4 mRNA levels. It is likely that these differences relate to differences in the models used.
Zn is critical for the function of mammalian cells. The vast majority of the intracellular Zn is bound to proteins or is accrued in specialized compartments (Suhy et al. 1999, Hambidge 2000, Colvin et al. 2003, Lemire et al. 2007). A vanishingly small amount of free Zn is typically available in the cytosol of most cells (Suhy et al. 1999, Outten & O’Halloran 2001, Bozym et al. 2006). In most cells, Zn is important for the structural integrity and function of transcription factors in the Zn finger family of proteins and for maintaining proper function of Zn-dependent enzymes that protect cells against oxidative stress and apoptosis (Truong-Tran et al. 2001, Prasad et al. 2004). The cellular content of Zn in β-cells is markedly higher than most mammalian cells, which is likely related to its unique roles in β-cells (Chausmer 1998, Dodson & Steiner 1998, Chimienti et al. 2005, Cousins et al. 2006). Estimates suggest that ~50% of Zn in β-cells is present within secretory vesicles (Zalewski et al. 1994), where it has been postulated to play a role in the packaging of proinsulin and insulin as hexamers. In this model, Zn enables storage of insulin at high concentrations and facilitates adequate insulin release in response to a glucose load (Huber & Gershoff 1973, Figlewicz et al. 1984, Huang & Arvan 1995, Dodson & Steiner 1998, Chimienti et al. 2005, 2006).
ZnT8 is highly expressed in β-cells (Chimienti et al. 2005, 2006), and localizes mainly to membranes of secretory vesicles, suggesting that it plays an important role in Zn transport into β-cell secretory vesicles (Chimienti et al. 2006, Mocchegiani et al. 2008). As noted earlier, overexpression of ZnT8 enhanced the insulin secretory capacity of a β-cell line (Chimienti et al. 2006). On the other hand, an R325W mutation in SLC30A8 is associated with type 2 diabetes as well as decreased first phase insulin secretion in non-diabetic subjects bearing at least one copy of the risk allele (Saxena et al. 2007, Scott et al. 2007, Sladek et al. 2007, Zeggini et al. 2007, Boesgaard et al. 2008, Hertel et al. 2008, van Hoek et al. 2008, Horikawa et al. 2008, Omori et al. 2008, Wu et al. 2008, Xiang et al. 2008). Complete loss of ZnT8 expression in mice homozygous for a null mutation of SLC30A8 led to decreased Zn accumulation in islets (Lemaire et al. 2009, Nicolson et al. 2009, Pound et al. 2009). ZnT8 null mice also exhibited decreased GSIS and altered glucose homeostasis (Pound et al. 2009). However, ZnT8 null mice exhibited altered glucose tolerance as well as in vivo and in vitro disturbances in GSIS only under certain age, gender, and dietary conditions (Lemaire et al. 2009, Nicolson et al. 2009). Furthermore, an unexpected increase in weight gain was observed in ZnT8 null mice fed a high fat diet (Nicolson et al. 2009). Given these observations, secondary effects during various developmental stages as well as environmental interactions are likely to have influenced the findings in this global knockout model. In the present study, we have now demonstrated that an acute decrease in ZnT8 expression in dispersed murine islet cells decreases GSIS in cells without altering the total cellular insulin content. In contrast to the global ZnT8 knockout mouse model, our model demonstrates the impact of an isolated, acute decrease in ZnT8 function prior to the induction of compensatory changes during development. This approach furthers our understanding of β-cell physiology by examining the impact of decreased ZnT8 expression independent of potential developmental changes or secondary compensatory mechanisms present in the ZnT8 knockout model.
ZnT8 knockout models demonstrated decreased GSIS under certain age and dietary conditions without changes in total cellular insulin content (Lemaire et al. 2009, Nicolson et al. 2009, Pound et al. 2009). This similarity between the effects of acutely decreased ZnT8 expression and chronic, complete loss of ZnT8 expression in the knockout mice indicates that β-cells have a limited capacity to compensate for changes in Zn homeostasis. Our findings and those in ZnT8 knockout mice in which decreased or absent ZnT8 expression did not impact total cellular insulin content differ from data obtained using the INS-1 β-cell line after an siRNA-mediated decrease in ZnT8 expression (Fu et al. 2009). In that model, both the number of insulin-containing secretory vesicles and total cellular insulin content were decreased after ZnT8 knockdown. These differences may be due to differences in the physiology of primary islet cells and INS cells.
The effect of decreased ZnT8 expression on GSIS in murine islet cells could be due to one or more possible mechanisms. Decreased ZnT8 expression likely results in reduced transport of Zn into secretory vesicles, which would impair the packaging of insulin as hexamers around Zn cores and thereby alter insulin secretion (Huang & Arvan 1995, Dodson & Steiner 1998). Another possible mechanism would be a toxic effect of Zn accumulation in the cytoplasm exceeding the metal-buffering capacity of cytoplasmic proteins (Frederickson et al. 1989, Koh et al. 1996, Kim et al. 1999a,b, Lobner et al. 2000, Frederickson et al. 2005, Lecane et al. 2005, Lemire et al. 2007, Oyama et al. 2007, Park et al. 2007, Zhang et al. 2007, Matsui et al. 2008). To examine this, we studied the changes in labile Zn with decreased ZnT8 expression in MIN6 cells using the fluorescent Zn probe FluoZin-3 AM. This approach has been previously validated for Zn in MIN6 cells (Gee et al. 2002). A significant decrease in intracellular FluoZin-3 AM-detectable Zn, which correlated with decreased ZnT8 expression, was observed. FluoZin-3 AM is reported to have a moderate binding affinity for Zn (Kd of 15 nM per the manufacturer) which is a significantly lower affinity for Zn than that of typical Zn-binding enzymes such as carbonic anhydrase (Bozym et al. 2006). Thus, Zn detected by FluoZin-3 AM represents the fraction of cellular Zn that is loosely bound to assorted intracellular ligands that have a lower affinity for Zn. Most cytoplasmic Zn is reported to be bound to metallothioneins, which have a much higher affinity for Zn than FluoZin-3 AM (Jacob et al. 1998). Therefore, it is unlikely that the FluoZin-3 AM signal reflects cytosolic Zn unless labile Zn concentrations exceed the buffering capacity of the abundant metallothioneins present in the cytoplasm of β-cells (Laychock et al. 2000, Li et al. 2004). FluoZin-3 AM is known to penetrate intracellular vesicles, and, consistent with our imaging of FluoZin-3 AM loaded live β-cells, it is likely that the majority of the labile Zn detected using FluoZin-3 AM is present within secretory vesicles. Taken together, our results suggest that acute downregulation of ZnT8 reduces the capacity of β-cells to accumulate Zn in β-cell insulin secretory vesicles.
The present studies also demonstrated that decreased ZnT8 expression did not have a significant impact on islet cell viability at the physiological Zn concentration of 5 μmol/l or in the presence of supraphysiological concentrations of Zn (up to 105 μmol/l) in the growth medium (Isbir et al. 1994, Sampson et al. 1997, Chausmer 1998, Maret & Sandstead 2006). In contrast to other cell types, β-cells have been shown to tolerate supraphysiological ambient Zn concentrations <200 μmol/l (Huber & Gershoff 1973, Figlewicz et al. 1981, Frederickson et al. 1989, Koh et al. 1996, Kim et al. 1999a,b, 2000, Lobner et al. 2000, Chang et al. 2003, Frederickson et al. 2005, Lecane et al. 2005, Lemire et al. 2007, Oyama et al. 2007, Park et al. 2007, Zhang et al. 2007, Matsui et al. 2008). The absence of toxic effects following ZnT8 knockdown in cells exposed to 105 μmol/l Zn in our study suggests that impaired GSIS with decreased ZnT8 expression is not likely due to toxic effects secondary to free Zn accumulation in the cytoplasm.
Given the low level of ZIP4 mRNA, the physiologic relevance of the increase in ZIP4 expression following exposure to IL1β is uncertain. ZIP4 regulates Zn influx into the cytoplasm of various mammalian cell types. In intestinal enterocytes, ZIP4 is located in the apical cell membrane and facilitates the uptake of sufficient quantities of dietary Zn. ZIP4 expression is reported to increase in Zn-deficient intestinal cells through posttranscriptional mRNA stabilization as well as decreased protein degradation (Dufner-Beattie et al. 2003, Weaver et al. 2007, Andrews 2008). Little is known about the role of ZIP4 in the pancreas, although ZIP4 was reported to be aberrantly expressed in pancreatic cancer (Li et al. 2007). Based upon the real-time PCR assay, the expression of ZIP4 mRNA was relatively low compared with other Zn transporters but increased following cytokine exposure. It is possible that this represents a compensatory response to the IL1β-induced decrease in labile Zn (Fig. 5B). Future studies will be needed to address that issue; specifically, the level of ZIP4 protein and changes in response to cytokine exposure and/or ZnT8 knockdown will need to be examined.
This study highlights the importance of the Zn transporter ZnT8 in pancreatic β-cell physiology. Beyond demonstrating a critical role for ZnT8 in GSIS, our findings suggest that cytokine-mediated β-cell dysfunction may occur, in part, through disruption of Zn metabolism via decreased ZnT8 expression.
Supplementary Material
Acknowledgments
Funding
This study was funded by a Northwestern Memorial Foundation MD-Scientist Fellowship in Genetic Medicine award for ME. The study was also funded by two NIH grants: R37GM038784 (TVO) and U42RR023245 (TVO) and T32 DK007169-27 NRSA Institutional Training Grant (LKB). Imaging was performed at the Northwestern University Cell Imaging Facility generously supported by CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. ICP-MS Metal analysis was performed at the Northwestern University Quantitative Bioelemental Imaging Center generously supported by NASA Ames Research Center NNA04CC36G.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This is linked to the online version of the paper at http://dx.doi.org/10.1677/JOE-09-0420.
References
- Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochemical Society Transactions. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82:452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesgaard TW, Zilinskaite J, Vanttinen M, Laakso M, Jansson PA, Hammarstedt A, Smith U, Stefan N, Fritsche A, Haring H, et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients – the EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chemical Biology. 2006;1:103–111. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- Chang I, Cho N, Koh JY, Lee MS. Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia. 2003;46:1220–1227. doi: 10.1007/s00125-003-1171-z. [DOI] [PubMed] [Google Scholar]
- Chausmer AB. Zinc, insulin and diabetes. Journal of the American College of Nutrition. 1998;17:109–115. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic β-cell-specific zinc transporter. Biometals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. Journal of Cell Science. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- Chin-Chance CV, Newman MV, Aronovitz A, Blomeier H, Kruger J, Lee EJ, Lowe WL., Jr Role of the mitogen-activated protein kinases in cytokine-mediated inhibition of insulin gene expression. Journal of Investigative Medicine. 2006;54:132–142. doi: 10.2310/6650.2006.05035. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Fontaine CP, Laskowski M, Thomas D. Zn2+ transporters and Zn2+ homeostasis in neurons. European Journal of Pharmacology. 2003;479:171–185. doi: 10.1016/j.ejphar.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Corbett JA, McDaniel ML. Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. Journal of Experimental Medicine. 1995;181:559–568. doi: 10.1084/jem.181.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. Journal of Biological Chemistry. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Current Opinion in Structural Biology. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and β-cell biology: from concept to clinical translation. Endocrine Reviews. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. Journal of Biological Chemistry. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature Genetics. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egefjord L, Jensen JL, Bang-Berthelsen CH, Petersen AB, Smidt K, Schmitz O, Karlsen AE, Pociot F, Chimienti F, Rungby J, et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in β-cell apoptosis? BMC Endocrine Disorder. 2009;9:7. doi: 10.1186/1472-6823-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Heldt A, Forhan SE, Grodsky GM. Effect of exogenous zinc on insulin secretion in vitro. Endocrinology. 1981;108:730–732. doi: 10.1210/endo-108-2-730. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Forhan SE, Hodgson AT, Grodsky GM. 65Zinc and endogenous zinc content and distribution in islets in relationship to insulin content. Endocrinology. 1984;115:877–881. doi: 10.1210/endo-115-3-877. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Hernandez MD, McGinty JF. Translocation of zinc may contribute to seizure-induced death of neurons. Brain Research. 1989;480:317–321. doi: 10.1016/0006-8993(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature Reviews. Neuroscience. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tian W, Pratt EB, Dirling LB, Shyng SL, Meshul CK, Cohen DM. Downregulation of ZnT8 expression in INS-1 rat pancreatic β cells reduces insulin content and glucose-inducible insulin secretion. PLoS ONE. 2009;4:e5679. doi: 10.1371/journal.pone.0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic β-cells using a new fluorescent zinc indicator. Journal of the American Chemical Society. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Hambidge M. Human zinc deficiency. Journal of Nutrition. 2000;130:1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- Hertel JK, Johansson S, Raeder H, Midthjell K, Lyssenko V, Groop L, Molven A, Njolstad PR. Genetic analysis of recently identified type 2 diabetes loci in 1,638 unselected patients with type 2 diabetes and 1,858 control participants from a Norwegian population-based cohort (the HUNT study) Diabetologia. 2008;51:971–977. doi: 10.1007/s00125-008-0982-3. [DOI] [PubMed] [Google Scholar]
- van Hoek M, Dehgan A, Witteman JC, van Duijn CM, Uitterlinden AG, Oostra BA, Hofman A, Sijbrands EJ, Janssens AC. Predicting type 2 diabetes based on polymorphisms from genome wide association studies: a population-based study. Diabetes. 2008;57:3122–3128. doi: 10.2337/db08-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa Y, Miyake K, Yasuda K, Enya M, Hirota Y, Yamagata K, Hinokio Y, Oka Y, Iwasaki N, Iwamoto Y, et al. Replication of genome-wide association studies of type 2 diabetes susceptibility in Japan. Journal of Clinical Endocrinology and Metabolism. 2008;93:3136–3141. doi: 10.1210/jc.2008-0452. [DOI] [PubMed] [Google Scholar]
- Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic β-cells. Structural maturation probed by disulfide accessibility. Journal of Biological Chemistry. 1995;270:20417–20423. doi: 10.1074/jbc.270.35.20417. [DOI] [PubMed] [Google Scholar]
- Huber AM, Gershoff SN. Effect of zinc deficiency in rats on insulin release from the pancreas. Journal of Nutrition. 1973;103:1739–1744. doi: 10.1093/jn/103.12.1739. [DOI] [PubMed] [Google Scholar]
- Isbir T, Tamer L, Taylor A, Isbir M. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Research. 1994;26:41–45. [PubMed] [Google Scholar]
- Jacob C, Maret W, Vallee BL. Control of zinc transfer between thionein, metallothionein, and zinc proteins. PNAS. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Koh JY, Kim YH, Sohn S, Joe E, Gwag BJ. Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. European Journal of Neuroscience. 1999a;11:327–334. doi: 10.1046/j.1460-9568.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience. 1999b;89:175–182. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–372. doi: 10.2337/diabetes.49.3.367. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Laychock SG, Duzen J, Simpkins CO. Metallothionein induction in islets of Langerhans and insulinoma cells. Molecular and Cellular Endocrinology. 2000;165:179–187. doi: 10.1016/s0303-7207(00)00247-1. [DOI] [PubMed] [Google Scholar]
- Lecane PS, Karaman MW, Sirisawad M, Naumovski L, Miller RA, Hacia JG, Magda D. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Research. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. PNAS. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J, Mailloux R, Appanna VD. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. Journal of Applied Toxicology. 2007;28:175–182. doi: 10.1002/jat.1263. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. Journal of Biological Chemistry. 2004;279:765–771. doi: 10.1074/jbc.M307907200. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. PNAS. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu Y, Lowe WL., Jr The role of phosphatidylinositol 3-kinase and the mitogen-activated protein kinases in insulin-like growth factor-I-mediated effects in vascular endothelial cells. Endocrinology. 2001;142:1710–1719. doi: 10.1210/endo.142.5.8136. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobner D, Canzoniero LM, Manzerra P, Gottron F, Ying H, Knudson M, Tian M, Dugan LL, Kerchner GA, Sheline CT, et al. Zinc-induced neuronal death in cortical neurons. Cellular and Molecular Biology. 2000;46:797–806. [PubMed] [Google Scholar]
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. Journal of Trace Elements in Medicine and Biology. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Matsui H, Sakanashi Y, Oyama TM, Oyama Y, Yokota S, Ishida S, Okano Y, Oyama TB, Nishimura Y. Imidazole antifungals, but not triazole antifungals, increase membrane Zn2+ permeability in rat thymocytes possible contribution to their cytotoxicity. Toxicology. 2008;248:142–150. doi: 10.1016/j.tox.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Milne DB, Ralston NV, Wallwork JC. Zinc content of blood cellular components and lymph node and spleen lymphocytes in severely zinc-deficient rats. Journal of Nutrition. 1985;115:1073–1078. doi: 10.1093/jn/115.8.1073. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Malavolta M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends in Molecular Medicine. 2008;14:419–428. doi: 10.1016/j.molmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori S, Tanaka Y, Takahashi A, Hirose H, Kashiwagi A, Kaku K, Kawamori R, Nakamura Y, Maeda S. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes. 2008;57:791–795. doi: 10.2337/db07-0979. [DOI] [PubMed] [Google Scholar]
- Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Matsui H, Morimoto M, Sakanashi Y, Nishimura Y, Ishida S, Okano Y. Synergic cytotoxic action induced by simultaneous application of zinc and clotrimazole in rat thymocytes. Toxicology Letters. 2007;171:138–145. doi: 10.1016/j.toxlet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Park SE, Park JW, Cho YS, Ryu JH, Paick JS, Chun YS. HIF-1α promotes survival of prostate cells at a high zinc environment. Prostate. 2007;67:1514–1523. doi: 10.1002/pros.20641. [DOI] [PubMed] [Google Scholar]
- Pound LD, Sarkar S, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochemical Journal. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radical Biology & Medicine. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochemical Pharmacology. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- Sampson B, Kovar IZ, Rauscher A, Fairweather-Tait S, Beattie J, McArdle HJ, Ahmed R, Green C. A case of hyperzincemia with functional zinc depletion: a new disorder? Pediatric Research. 1997;42:219–225. doi: 10.1203/00006450-199708000-00015. [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Suhy DA, Simon KD, Linzer DI, O’Halloran TV. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. Journal of Biological Chemistry. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- Wallwork JC, Johnson LK, Milne DB, Sandstead HH. The effect of interactions between dietary egg white protein and zinc on body weight, bone growth and tissue trace metals in the 30-day-old rat. Journal of Nutrition. 1983;113:1307–1320. doi: 10.1093/jn/113.7.1307. [DOI] [PubMed] [Google Scholar]
- Ward NI, Mason JA. Neuron activation analysis techniques for identifying elemental status in Alzheimer’s disease. Journal of Radioanalytical and Nuclear Chemistry. 1987;113:515–526. [Google Scholar]
- Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biological Chemistry. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Chen X, Cao XC, Baker MS, Kaufman DB. Cytokine-induced metabolic dysfunction of MIN6 β cells is nitric oxide independent. Journal of Surgical Research. 2001;101:190–195. doi: 10.1006/jsre.2001.6285. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li H, Loos RJ, Yu Z, Ye X, Chen L, Pan A, Hu FB, Lin X. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8 and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes. 2008;57:2834–2842. doi: 10.2337/db08-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenos ES, Farney AC, Widmer MB, Casanova D, Stevens RB, Blazar BR, Sutherland DE, Gores PF. Effect of tumor necrosis factor α and of the soluble tumor necrosis factor receptor on insulin secretion of isolated islets of Langerhans. Transplantation Proceedings. 1992;24:2863–2864. [PubMed] [Google Scholar]
- Xenos ES, Stevens RB, Gores PF, Casanova D, Farney AC, Sutherland DE, Platt JL. IL-1β-induced inhibition of β-cell function is mediated through nitric oxide. Transplantation Proceedings. 1993;25:994. [PubMed] [Google Scholar]
- Xenos ES, Stevens RB, Sutherland DE, Lokeh A, Ansite JD, Casanova D, Gores PF, Platt JL. The role of nitric oxide in IL-1β-mediated dysfunction of rodent islets of Langerhans. Implications for the function of intrahepatic islet grafts. Transplantation. 1994;57:1208–1212. doi: 10.1097/00007890-199404270-00012. [DOI] [PubMed] [Google Scholar]
- Xiang J, Li XY, Xu M, Hong J, Huang Y, Tan JR, Lu X, Dai M, Yu B, Ning G. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. Journal of Clinical Endocrinology and Metabolism. 2008;93:4107–4112. doi: 10.1210/jc.2008-0161. [DOI] [PubMed] [Google Scholar]
- Zalewski PD, Millard SH, Forbes IJ, Kapaniris O, Slavotinek A, Betts WH, Ward AD, Lincoln SF, Mahadevan I. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. Journal of Histochemistry and Cytochemistry. 1994;42:877–884. doi: 10.1177/42.7.8014471. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Aizenman E, Defranco DB, Rosenberg PA. Intracellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death. Molecular Medicine. 2007;13:350–355. doi: 10.2119/2007-00042.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.